Allantoin and Tissue Specific Redox Regulation in Mud Crab Scylla serrata under Varied Natural Water Physico-Chemical Parameters
Abstract
1. Introduction
2. Material and Methods
2.1. Sampling of Crabs and Analysis of Physico-Chemical Properties of Water and Sediment
2.2. Tissue Collection and Processing
2.3. Determination of Allantoin
2.4. Determination of OS Parameter
2.5. Enzymatic Antioxidant Assays
2.6. Assay of Total Antioxidant Capacity and Small Antioxidant Molecules
2.7. Statistical Analysis
3. Results
3.1. Physico-Chemical Properties of Water and Sediment Factors
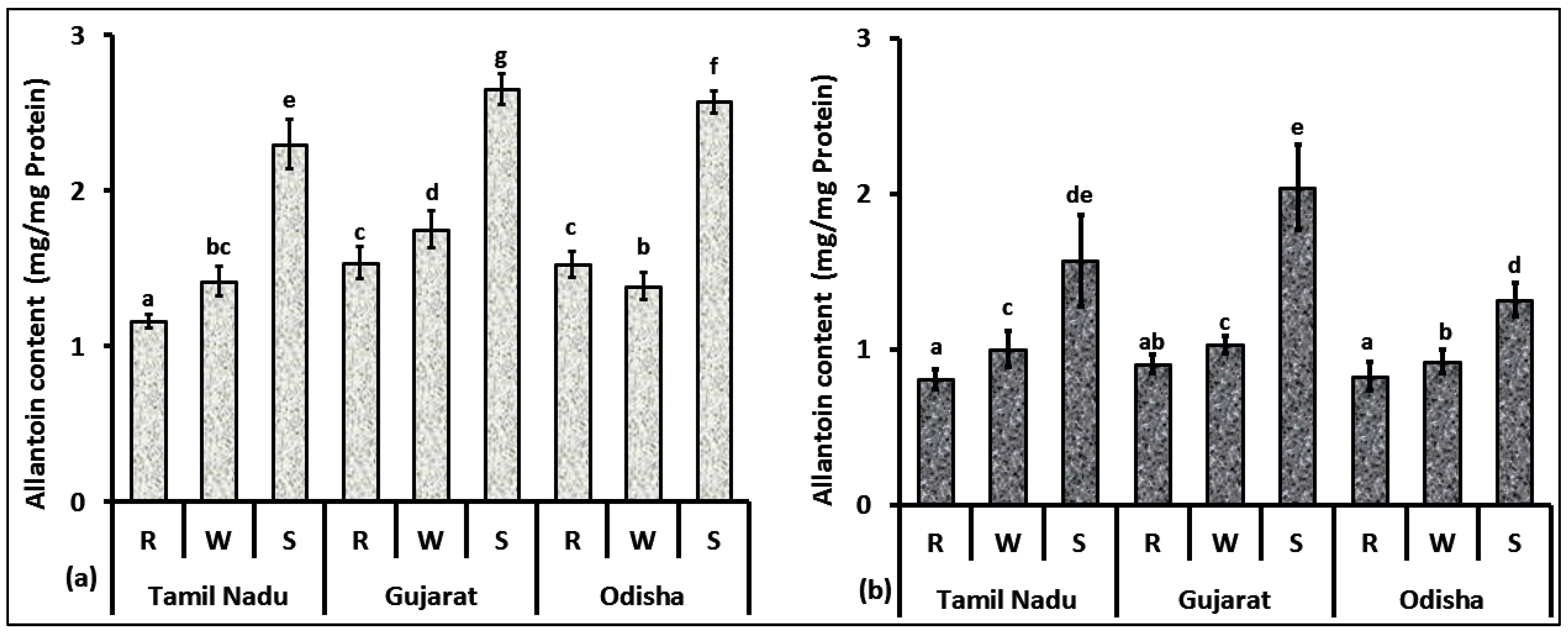
3.2. Allantoin
3.3. Oxidative Stress, ROS, and Antioxidant Parameters
3.4. Correlation of Allantoin and Environmental Factors
3.5. Correlation between Allantoin and Oxidative Stress Physiology Parameters
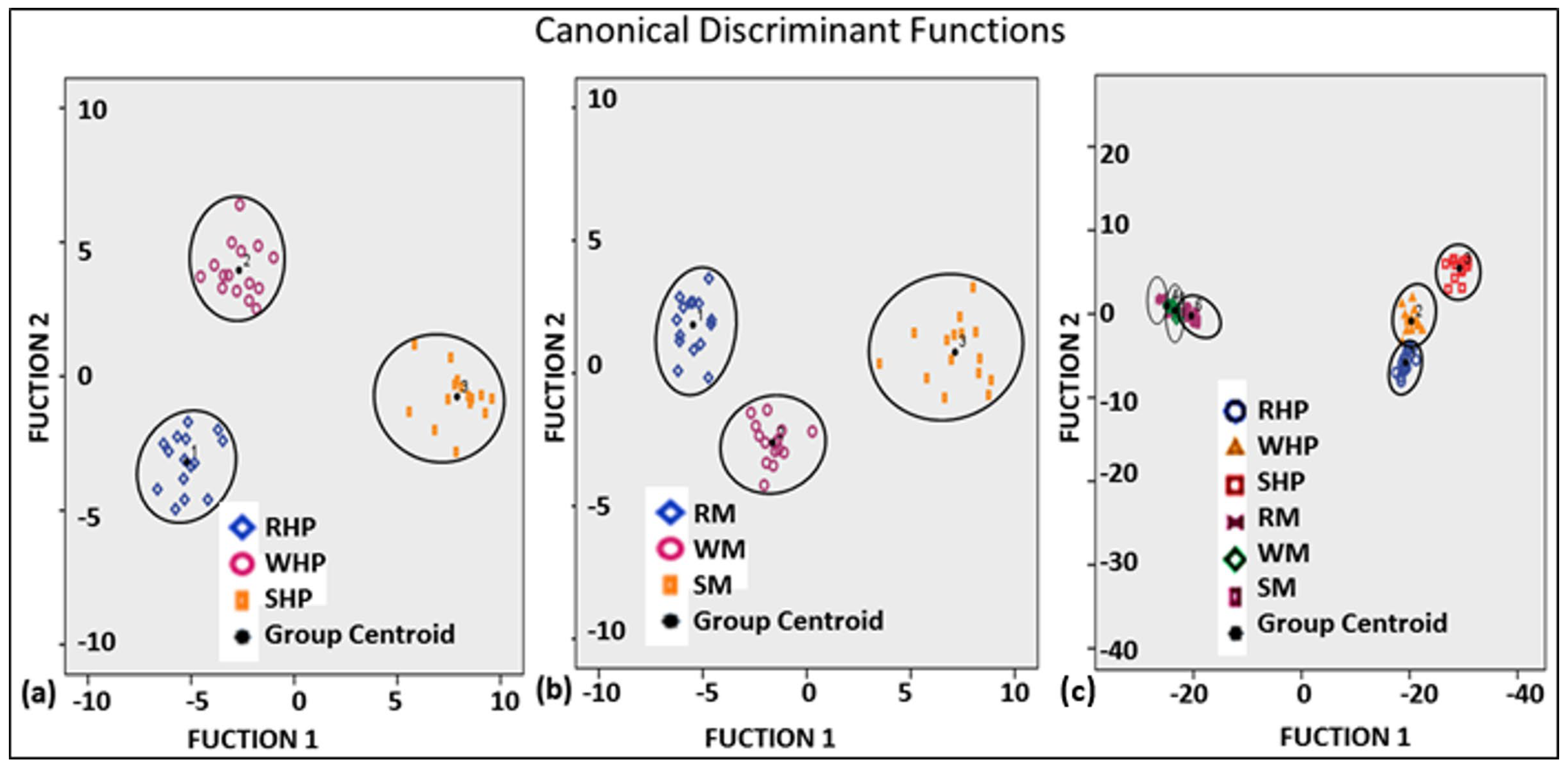
3.6. Multivariate Statistical Analysis
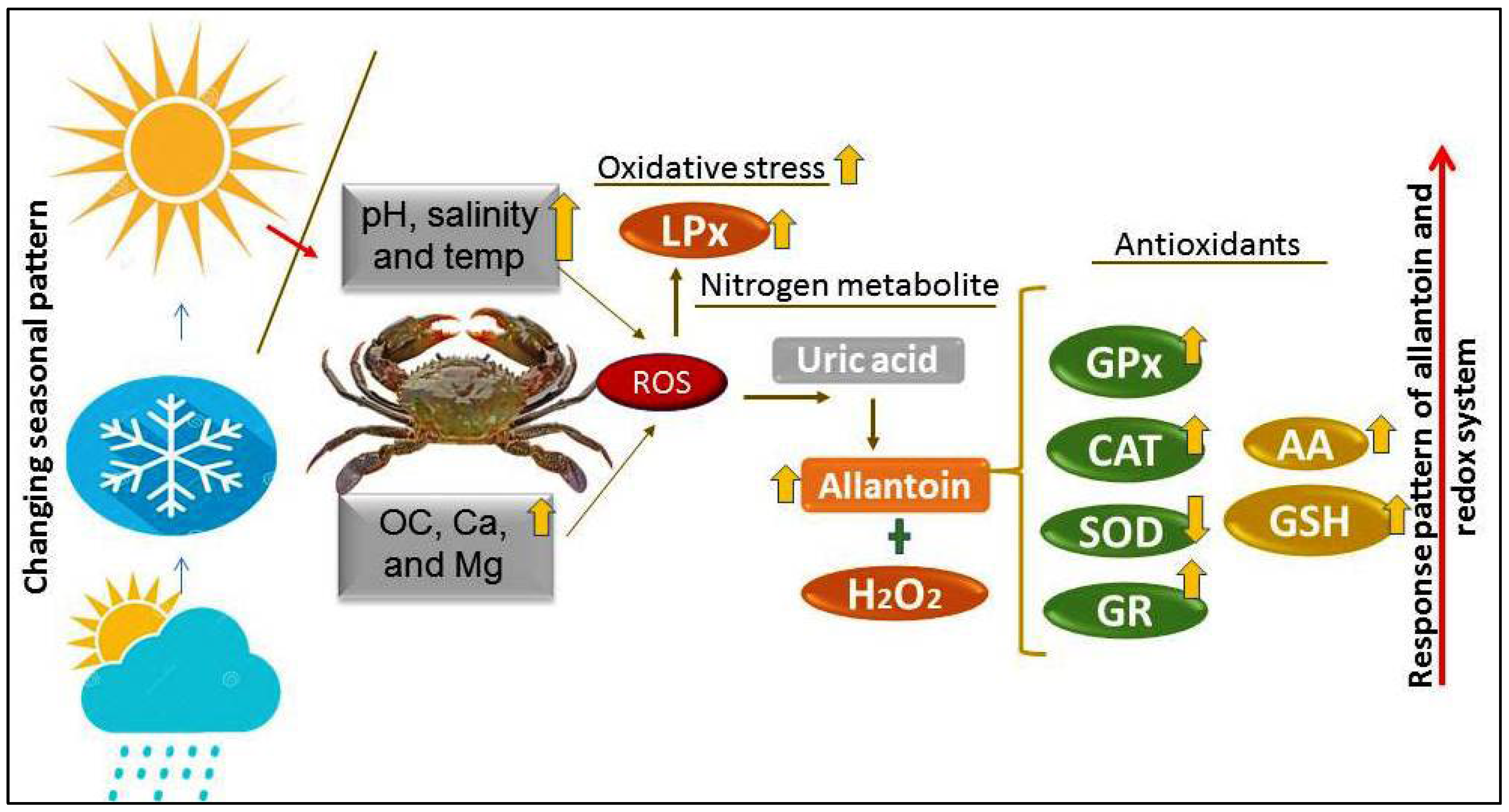
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Igile, G.; Essiet, G.; Uboh, F.; Edet, E. Rapid method for the identification and quantification of allantoin in body creams and lotions for regulatory activities. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 552–557. [Google Scholar]
- Tsahar, E.; Arad, Z.; Izhaki, I.; Guglielmo, C.G. The relationship between uric acid and its oxidative product allantoin: A potential indicator for the evaluation of oxidative stress in birds. J. Comp. Physiol. B 2006, 176, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Case, L.P.; Daristotle, L.; Hayek, M.G.; Raasch, M.F. Chapter 27—Inherited Disorders of Nutrient Metabolism. In Canine and Feline Nutrition, 3rd ed.; Mosby: St. Louis, MI, USA, 2011; pp. 297–311. [Google Scholar]
- Savić, V.L.; Nikolić, V.D.; Arsić, I.A.; Stanojević, L.P.; Najman, S.J.; Stojanović, S.; Mladenović-Ranisavljević, I.I. Comparative study of the biological activity of allantoin and aqueous extract of the comfrey root. Phytother. Res. 2015, 29, 1117–1122. [Google Scholar] [CrossRef]
- Sayıt, G.; Tanrıverdi, S.T.; Özer, Ö.; Özdoğan, E. Preparation of allantoin loaded liposome formulations and application for cosmetic textile production. J. Text. Inst. 2022, 113, 725–736. [Google Scholar] [CrossRef]
- Alvarez-Lario, B.; Macarron-Vicente, J. Is there anything good in uric acid? QJM Int. J. Med. 2011, 104, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Marzook, F.; Marzook, E.; El-Sonbaty, S. Allantoin may modulate aging impairments, symptoms and cancers. Pak. J. Pharm. Sci. 2021, 34, 1377–1384. [Google Scholar] [PubMed]
- Pompelli, M.F.; Pompelli, G.M.; de Oliveira, A.F.; Antunes, W.C. The effect of light and nitrogen availability on the caffeine, theophylline and allantoin contents in the leaves of Coffea arabica L. AIMS Environ. Sci. 2013, 1, 1–11. [Google Scholar] [CrossRef]
- Dresler, S.; Kováčik, J.; Sowa, I.; Wójciak, M.; Strzemski, M.; Rysiak, A.; Babula, P.; Todd, C.D. Allantoin overaccumulation enhances production of metabolites under excess of metals but is not tightly regulated by nitric oxide. J. Hazard. Mater. 2022, 436, 129138. [Google Scholar] [CrossRef]
- Gus’kov, E.P.; Prokof’ev, V.N.; Kletskii, M.E.; Kornienko, I.V.; Gapurenko, O.A.; Olekhnovich, L.P.; Chistyakov, V.A.; Shestopalov, A.V.; Sazykina, M.A.; Markeev, A.V.; et al. Allantoin as a vitamin. Dokl. Biochem. Biophys. 2004, 398, 320–324. [Google Scholar] [CrossRef]
- Inazawa, K.; Yamaguchi, S.; Hosoyamada, M.; Fukuuchi, T.; Tomioka, N.H.; Yamaoka, N.; Kaneko, K. Urinary excretion of uric acid, allantoin, and 8-OH-Deoxyguanosine in uricase-knockout mice. Nucleosides Nucleotides Nucleic Acids 2016, 35, 559–565. [Google Scholar] [CrossRef]
- Santana, M.S.; Nascimento, K.P.; Lotufo, P.A.; Benseãor, I.M.; Meotti, F.C. Allantoin as an independent marker associated with carotid intima-media thickness in subclinical atherosclerosis. Braz. J. Med. Biol. Res. 2018, 51, e7543. [Google Scholar] [CrossRef] [PubMed]
- Komeili, M.T.; Moslehi, A.; Golchoob, M.; Ababzadeh, S. Allantoin improves methionine-choline deficient diet-induced nonalcoholic steatohepatitis in mice through involvement in endoplasmic reticulum stress and hepatocytes apoptosis-related genes expressions. Iran. J. Basic Med. Sci. 2019, 22, 736–744. [Google Scholar] [CrossRef]
- Tzeng, C.Y.; Lee, W.S.; Liu, K.F.; Tsou, H.K.; Chen, C.J.; Peng, W.H.; Tsai, J.C. Allantoin ameliorates amyloid β-peptide-induced memory impairment by regulating the PI3K/Akt/GSK-3β signaling pathway in rats. Biomed. Pharmacother. 2022, 153, 113389. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Bai, Q.; Kong, F.; Li, Y.; Zha, X.; Zhang, L.; Zhao, Y.; Gao, S.; Li, P.; Jiang, Q. Allantoin-functionalized silk fibroin/sodium alginate transparent scaffold for cutaneous wound healing. Int. J. Biol. Macromol. 2022, 207, 859–872. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Sun, Y.; Zhu, C.; Shen, X.; Sun, J.; Jing, T.; Jun, S.; Wang, C.; Yu, G.; Dong, X.; et al. Allantoin induces pruritus by activating MrgprD in chronic kidney disease. J. Cell. Physiol. 2023, 238, 813–828. [Google Scholar] [CrossRef]
- Nishinami, S.; Yoshizawa, S.; Arakawa, T.; Shiraki, K. Allantoin and hydantoin as new protein aggregation suppressors. Int. J. Biol. Macromol. 2018, 114, 497–503. [Google Scholar] [CrossRef]
- Wang, P.; Kong, C.H.; Sun, B.; Xu, X.H. Distribution and function of allantoin (5-ureidohydantoin) in rice grains. J. Agric. Food Chem. 2012, 60, 2793–2798. [Google Scholar] [CrossRef]
- Harms, L.; Frickenhaus, S.; Schiffer, M.; Mark, F.C.; Storch, D.; Held, C.; Pörtner, H.-O.; Lucassen, M. Gene expression profiling in gills of the great spider crab Hyas araneus in response to ocean acidification and warming. BMC Genom. 2014, 15, 789. [Google Scholar] [CrossRef]
- Kand’ár, R.; Žáková, P.; Mužáková, V. Monitoring of antioxidant properties of uric acid in humans for a consideration measuring of levels of allantoin in plasma by liquid chromatography. Clin. Chim. Acta 2006, 365, 249–256. [Google Scholar] [CrossRef]
- da Silva, D.M.; Martins, J.L.R.; de Oliveira, D.R.; Florentino, I.F.; da Silva, D.P.B.; Dos Santos, F.C.A.; Costa, E.A. Effect of allantoin on experimentally induced gastric ulcers: Pathways of gastroprotection. Eur. J. Pharmacol. 2018, 821, 68–78. [Google Scholar] [CrossRef]
- Dinica, R.M.; Sandu, C.; Dediu Botezatu, A.V.; Cazanevscaia Busuioc, A.; Balanescu, F.; Ionica Mihaila, M.D.; Dumitru, C.N.; Furdui, B.; Iancu, A.V. Allantoin from valuable romanian animal and plant sources with promising anti-inflammatory activity as a nutricosmetic ingredient. Sustainability 2021, 13, 10170. [Google Scholar] [CrossRef]
- Chainy, G.B.N.; Paital, B.; Dandpat, J. An overview of seasonal changes in oxidative stress and antioxidant defence parameters in some invertebrate and vertebrate species. Scientifica 2016, 2016, 6126570. [Google Scholar] [CrossRef] [PubMed]
- Pati, S.G.; Paital, B.; Panda, F.; Jena, S.; Sahoo, D.K. Impacts of Habitat Quality on the Physiology, Ecology, and Economical Value of Mud Crab Scylla sp.: A Comprehensive Review. Water 2023, 15, 2029. [Google Scholar] [CrossRef]
- Pati, S.G.; Panda, F.; Paita, B.; Sahoo, D.K.; Jena, S. Oxidative stress physiology in Scylla serrata for environmental health assessment. Front. Environ. Sci. 2023, 11, 1142495. [Google Scholar] [CrossRef]
- Paital, B.; Chainy, G.B.N. Effects of temperature on complex I and II mediated mitochondrial respiration, ROS generation and oxidative stress status in gills of the mud crab Scylla serrata. J. Therm. Biol. 2014, 41, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Mikami, T.; Sorimachi, M. Uric acid contributes greatly to hepatic antioxidant capacity besides protein. Physiol. Res. 2017, 66, 1001–1007. [Google Scholar] [CrossRef]
- Wang, M.; Wu, J.; Jiao, H.; Oluwabiyi, C.; Li, H.; Zhao, J.; Zhou, Y.; Wang, X.; Lin, H. Enterocyte synthesizes and secrets uric acid as antioxidant to protect against oxidative stress via the involvement of Nrf pathway. Free Radic. Biol. Med. 2022, 179, 95–108. [Google Scholar] [CrossRef]
- Zhang, F.; Sun, X.; Shen, X.; Yan, Y.; Wang, J.; Yuan, Q. Biosynthesis of allantoin in Escherichia coli via screening a highly effective urate oxidase. Biotechnol. Bioeng. 2022, 119, 2518–2528. [Google Scholar] [CrossRef]
- Pati, S.G.; Panda, F.; Jena, S.; Sahoo, D.K.; Paital, B. Effects of soil trace metals, organic carbon load and physicochemical stressors on active oxygen species metabolism in Scylla serrata sampled along the Bay of Bengal in Odisha state, India. Front. Environ. Sci. 2022, 10, 994773. [Google Scholar] [CrossRef]
- Paital, B.; Chainy, G.B.N. Seasonal variability of antioxidant biomarkers in mud crabs (Scylla serrata). Ecotoxicol. Environ. Saf. 2013, 87, 33–41. [Google Scholar] [CrossRef]
- Walkley, A.; Black, I.A. An examination of the degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 1, 29–38. [Google Scholar] [CrossRef]
- Schofield, R.K.; Taylor, A.W. The Measurement of Soil pH. Soil Sci. Soc. Am. J. 1955, 19, 164–167. [Google Scholar] [CrossRef]
- Paital, B.; Chainy, G.B.N. Antioxidant defenses and oxidative stress parameters in tissues of mud crab (Scylla serrata) with reference to changing salinity. Comp. Biochem. Physiol. C 2010, 151, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Young, E.G.; Conway, C.F. On the estimation of allantoin by the Rimini-Schryver reaction. J. Biol. Chem. 1942, 142, 839–853. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Samanta, L.; Chainy, G.B.N. A modified spectrophotometric assay of superoxide dismutase using nitrite formation by superoxide radicals. Ind. J. Biochem. Biophys. 2000, 37, 201–204. [Google Scholar]
- Cohen, G.; Kim, M.; Ogwu, V. A modified catalase assay suitable for a plate reader and for the analysis of brain cell cultures. J. Neurosci. Methods 1996, 67, 53–56. [Google Scholar] [CrossRef]
- Aebi, H. Catalase. In Methods of Enzymatic Analysis; Academic Press: New York, NY, USA, 1974; Volume 2, pp. 673–683. [Google Scholar] [CrossRef]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar]
- Massey, V.; Williams, C.H. On the reaction mechanism of yeast glutathione reductase. J. Biol. Chem. 1965, 240, 4470–4480. [Google Scholar] [CrossRef]
- Sedlak, J.; Lindsay, R.H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal. Biochem. 1968, 25, 192–205. [Google Scholar] [CrossRef]
- Mitsui, A.; Ohta, T. Photooxidative consumption and photoreductive formation of ascorbic acid in green leaves. Plant Cell. Physiol. 1961, 2, 31–44. [Google Scholar] [CrossRef]
- Mishra, P.; Paital, B.; Jena, S.; Samanta, L.; Kumar, S.; Swain, S. Possible activation of NRF2 by Vitamin E/Curcumin against altered thyroid hormone induced oxidative stress via NFĸB/AKT/mTOR/KEAP1 signaling in rat heart. Sci. Rep. 2019, 9, 7408. [Google Scholar] [CrossRef]
- Gus’kov, E.P.; Shkurat, T.P.; Milyutina, N.P.; Prokof’ev, V.N.; Pokudina, I.O.; Mashkina, E.V.; Timofeeva, I.V. Effect of allantoin on the activity of enzymes providing regulation of the ROS-dependent status of an organism. Dokl. Biochem. Biophys. 2001, 379, 239–242. [Google Scholar] [CrossRef]
- Il’yasova, D.; Scarbrough, P.; Spasojevic, I. Urinary biomarkers of oxidative status. Clin. Chim. Acta 2012, 413, 1446–1453. [Google Scholar] [CrossRef]
- Huang, C.; Jiao, H.; Song, Z.; Zhao, J.; Wang, X.; Lin, H. Heat stress impairs mitochondria functions and induces oxidative injury in broiler chickens. J. Anim. Sci. 2015, 93, 2144–2153. [Google Scholar] [CrossRef]
- Aman, R.M.; Zaghloul, R.A.; El-Dahhan, M.S. Formulation, optimization and characterization of allantoin-loaded chitosan nanoparticles to alleviate ethanol-induced gastric ulcer: In-vitro and in-vivo studies. Sci. Rep. 2021, 11, 2216. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.F.; Fuhrman, F.A.; Fuhrman, G.J.; Mosher, H.S. Isolation of allantoin and adenosine from the marine sponge Tethya aurantia. Comp. Biochem. Physiol. Part B 1981, 70, 799–801. [Google Scholar] [CrossRef]
- Gu, F.; Luo, X.; Jin, X.; Cai, C.; Zhao, W. Association of total calcium with serum uric acid levels among united states adolescents aged 12–19 years: A cross-sectional study. Front. Med. 2022, 9, 915371. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.K.; Patterson, P.H. Effect of minerals on activity of microbial uricase to reduce ammonia volatilization in poultry manure. Poult. Sci. 2003, 82, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, D.; Wang, Z.; Zou, C.; Wang, B.; Zhang, H.; Gai, Z.; Zhang, P.; Wang, Y.; Li, C. Exogenous allantoin improves the salt tolerance of sugar beet by increasing putrescine metabolism and antioxidant activities. Plant Physiol. Biochem. 2020, 154, 699–713. [Google Scholar] [CrossRef]
- Kand’ár, R.; Záková, P. Allantoin as a marker of oxidative stress in human erythrocytes. Clin. Chem. Lab. Med. 2008, 46, 1270–1274. [Google Scholar] [CrossRef]
- Tolun, A.A.; Scarbrough, P.M.; Zhang, H.; McKillop, J.A.; Wang, F.; Kishnani, P.S.; Millington, D.S.; Young, S.P.; Il’yasova, D. Systemic oxidative stress, as measured by urinary allantoin and F(2)-isoprostanes, is not increased in Down syndrome. Ann. Epidemiol. 2012, 22, 892–894. [Google Scholar] [CrossRef]
- Raihan, M.R.H.; Rahman, M.; Rastogi, A.; Fujita, M.; Hasanuzzaman, M. Exogenous Allantoin Confers Rapeseed (Brassica campestris) Tolerance to Simulated Drought by Improving Antioxidant Metabolism and Physiology. Antioxidants 2023, 12, 1508. [Google Scholar] [CrossRef]
- Hamidi-Zad, Z.; Moslehi, A.; Rastegarpanah, M. Attenuating effects of allantoin on oxidative stress in a mouse model of nonalcoholic steatohepatitis: Role of SIRT1/Nrf2 pathway. Res. Pharm. Sci. 2021, 16, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Nelson, S.K.; Bose, S.K.; Grunwald, G.K.; Myhill, P.; McCord, J.M. The induction of human superoxide dismutase and catalase in vivo: A fundamentally new approach to antioxidant therapy. Free Radic. Biol. Med. 2006, 40, 341–347. [Google Scholar] [CrossRef]
- Chen, J.C.; Chia, P.G. Hemolymph ammonia and urea and nitrogenous excretions of Scylla serrata at different temperature and salinity levels. Mar. Ecol. Prog. Ser. 1996, 139, 119–125. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-Mediated Cellular Signaling. Oxid. Med. Cell. Longev. 2016, 2016, 4350965. [Google Scholar] [CrossRef] [PubMed]
- Selamoglu, Z.; Dusgun, C.; Akgul, H.; Gulhan, M.F. In-vitro antioxidant activities of the ethanolic extracts of some contained-allantoin plants. Iran. J. Pharm. Res. 2017, 16, 92–98. [Google Scholar] [PubMed]
- Kaur, R.; Chandra, J.; Varghese, B.; Keshavkant, S. Allantoin: A Potential Compound for the Mitigation of Adverse Effects of Abiotic Stresses in Plants. Plants 2023, 12, 3059. [Google Scholar] [CrossRef] [PubMed]
| Water Quality of S. serrata Sampling Sites | Soil Quality of S. serrata Sampling Sites | ||||||
|---|---|---|---|---|---|---|---|
| Location | Season | pH | Temp | Salinity | Organic Carbon % | Ca | Mg (mg kg−1) |
| (°C) | (ppt) | (mg kg−1) | |||||
| Odisha | R | 7.94 ± 0.1 a | 21.3 ± 0.85 a | 17.48 ± 0.71 a | 0.84 ± 0.004 a | 445.28 ± 11 a | 88.3 ± 3.5 a |
| W | 7.85 ± 0.06 b | 17.2 ± 0.77 b | 22.66 ± 0.88 b | 1.80 ± 0.05 b | 497.3 ± 11 b | 96.8 ± 2.1 b | |
| S | 8.22 ± 0.03 c | 28.1 ± 0.94 c | 25.58 ± 0.97 c | 2.98 ± 0.03 c | 503.62 ± 13 c | 104.1 ± 2.2 c | |
| Gujarat | R | 7.5 ± 0.06 a | 23.6 ± 0.7 a | 12.32 ± 0.65 a | 0.6 ± 0.002 a | 512.5 ± 15 a | 94 ± 1.2 a |
| W | 7.53 ± 0.05 b | 12.5 ± 0.8 b | 22.78 ± 0.2 b | 1.92 ± 0.006 b | 546 ± 16 b | 97.5 ± 2.3 b | |
| S | 7.92 ± 0.1 c | 27.3 ± 0.67 c | 23.8 ± 0.9 c | 2.46 ± 0.08 c | 568.17 ± 14 c | 103.5 ± 3.2 c | |
| Tamil Nadu | R | 7.13 ± 0.04 a | 22.22 ± 0.1 a | 17.68 ± 0.6 a | 0.96 ± 0.004 a | 447.5 ± 16 a | 97.6 ± 2.2 a |
| W | 7.22 ± 0.3 b | 17.34 ± 0.91 b | 23.22 ± 0.9 b | 1.80 ± 0.001 b | 495.8 ± 13 b | 102.9 ± 3.1 b | |
| S | 7.84 ± 0.2 c | 30.51 ± 0.93 c | 35.49 ± 1.8 c | 2.54 ± 0.04 c | 528.15 ± 13 c | 128.5 ± 1.5 c | |
| Locations | T | Sn | LPx | SOD | CAT | GPx | GR | GST | AA | GSH | DPPH |
|---|---|---|---|---|---|---|---|---|---|---|---|
| GJ | HP | R | 3.43 ± 0.035 d | 6.23 ± 0.247 b | 7654.63 ± 25.7 b | 2.4 ± 0.106 c | 117.49 ± 3.14 c | 1.85 ± 0.026 d | 122.1 ± 4.09 b | 0.121 ± 0.0038 a | 12.36 ± 0.48 b |
| W | 3.28 ± 0.052 b | 7.85 ± 0.381 c | 7059.57 ± 17.8 a | 2.7 ± 0.063 d | 135.28 ± 1.79 d | 1.87 ± 0.031 e | 103.9 ± 3.8 a | 0.259 ± 0.0062 d | 14.84 ± 0.48 c | ||
| S | 4.35 ± 0.066 e | 5.21 ± 0.25 a | 8123.94 ± 24.8 c | 3.6 ± 0.072 e | 180.132 ± 1.96 f | 2.66 ± 0.039 f | 111.86 ± 3.7 a | 0.196 ± 0.0043 c | 16.66 ± 0.56 e | ||
| Muscle | R | 0.162 ± 0.007 c | 1.18 ± 0.060 a | 567.97 ± 5 e | 0.47 ± 0.0091 b | 23.944 ± 0.26 b | 0.155 ± 0.001 b | 69.66 ± 0.69 c | 0.405 ± 0.0038 c | 5.71 ± 0.22 b | |
| W | 0.131 ± 0.003 a | 1.75 ± 0.091 c | 500.74 ± 5.2 d | 0.84 ± 0.0076 d | 42.7 ± 0.21 c | 0.169 ± 0.003 c | 57.056 ± 4.44 a | 0.406 ± 0.0029 c | 6.54 ± 0.42 c | ||
| S | 0.18 ± 0.004 d | 1.05 ± 0.138 a | 640.97 ± 6.3 f | 1.24 ± 0.0375 e | 63.484 ± 1.07 d | 0.191 ± 0.003 e | 71.52 ± 4.4 c | 0.334 ± 0.0072 b | 7.42 ± 0.25 d | ||
| TN | HP | R | 3.55 ± 0.024 d | 8.65 ± 0.219 d | 8271.15 ± 20.6 d | 1.3 ± 0.0353 a | 69.636 ± 0.89 a | 1.86 ± 0.060 d | 125.26 ± 4.4 b | 0.143 ± 0.0019 a | 11.24 ± 0.63 a |
| W | 3.22 ± 0.02 b | 10.65 ± 0.191 f | 8097.94 ± 35.1 c | 1.6 ± 0.0403 b | 83.33 ± 1.48 b | 2.23 ± 0.034 ef | 114.15 ± 2.7 ab | 0.232 ± 0.0036 d | 16.41 ± 0.47 e | ||
| S | 4.15 ± 0.04 de | 7.81 ± 0.251 cd | 8994.07 ± 32 e | 1.7 ± 0.0626 b | 88.742 ± 2.12 b | 3.18 ± 0.056 g | 123.57 ± 3.96 a | 0.167 ± 0.0063 b | 17.85 ± 0.45 f | ||
| Muscle | R | 0.179 ± 0.004 d | 1.71 ± 0.088 c | 234.76 ± 9.4 a | 0.45 ± 0.0065 b | 23.07 ± 0.16 b | 0.156 ± 0.0043 b | 56.49 ± 5.14 a | 0.327 ± 0.0026 b | 4.78 ± 0.61 a | |
| W | 0.132 ± 0.003 a | 2.66 ± 0.188 d | 250.96 ± 8.2 b | 0.81 ± 0.03 d | 41.89 ± 0.83 c | 0.187 ± 0.0035 d | 56.08 ± 4.71 a | 0.288 ± 0.0026 a | 6.28 ± 0.64 c | ||
| S | 0.229 ± 0.009 f | 1.49 ± 0.074 b | 364.6 ± 11.2 c | 1.80 ± 0.026 f | 90.56 ± 1.18 f | 0.287 ± 0.0044 f | 65.2 ± 4.45 b | 0.32 ± 0.0022 b | 7.51 ± 0.52 d | ||
| OD | HP | R | 3.33 ± 0.034 c | 9.74 ± 0.308 f | 8412.42 ± 23.6 d | 2.24 ± 0.040 c | 114.52 ± 1.32 c | 1.245 ± 0.035 a | 123.8 ± 7.19 b | 0.181 ± 0.0025 b | 12.73 ± 0.35 b |
| W | 3.05 ± 0.052 a | 13.19 ± 0.329 g | 7889.27 ± 23.2 b | 3.20 ± 0.244 e | 168.32 ± 9.42 e | 1.415 ± 0.0253 b | 131.4 ± 3.56 c | 0.192 ± 0.0024 c | 14.49 ± 0.6 d | ||
| S | 4.61 ± 0.042 d | 9.043 ± 0.269 e | 9827.03 ± 26.7 f | 4.2 ± 0.209 f | 220.15 ± 6.57 g | 1.781 ± 0.045 c | 137.4 ± 4.46 c | 0.171 ± 0.0021 b | 17.70 ± 0.65 f | ||
| Muscle | R | 0.163 ± 0.0022 c | 2.94035 ± 0.22 e | 803.04 ± 6.07 h | 0.4 ± 0.0045 a | 20.62 ± 0.32 a | 0.14 ± 0.0026 a | 62.7 ± 4.1 b | 0.404 ± 0.0022 c | 5.87 ± 0.23 b | |
| W | 0.145 ± 0.0026 b | 3.483 ± 0.035 f | 707.09 ± 10.98 g | 0.7 ± 0.021 c | 39.08 ± 0.88 c | 0.18 ± 0.002 cd | 59.9 ± 3.4 a | 0.584 ± 0.0029 f | 7.61 ± 0.29 d | ||
| S | 0.187 ± 0.0035 e | 2.7823 ± 0.182 d | 889.53 ± 8.47 h | 1.2 ± 0.041 e | 64.19 ± 1.3 e | 0.21 ± 0.0039 e | 74 ± 3.28 d | 0.421 ± 0.0023 d | 6.82 ± 0.27 cd |
| Parameters | Gujarat | Odisha | Tamil Nadu | |||
|---|---|---|---|---|---|---|
| HP | Muscle | HP | Muscle | HP | Muscle | |
| pH | 0.96 | 0.95 | 0.97 | 0.84 | 0.97 | 0.88 |
| Temp | 0.53 | 0.59 | 0.95 | 0.78 | 0.81 | 0.72 |
| Salinity | 0.69 | 0.61 | 0.69 | 0.81 | 0.97 | 0.88 |
| OC | 0.81 | 0.75 | 0.83 | 0.88 | 0.92 | 0.84 |
| Ca | 0.87 | 0.81 | 0.47 | 0.66 | 0.88 | 0.81 |
| Mg | 0.95 | 0.92 | 0.76 | 0.86 | 0.97 | 0.88 |
| Parameters | Gujarat | Odisha | Tamil Nadu | |||
|---|---|---|---|---|---|---|
| HP | Muscle | HP | Muscle | HP | Muscle | |
| LPx | 0.90 | 0.54 | 0.98 | 0.65 | 0.80 | 0.60 |
| SOD | −0.59 | −0.46 | −0.66 | −0.14 | −0.48 | −0.26 |
| CAT | 0.69 | 0.77 | 0.97 | 0.66 | 0.90 | 0.78 |
| GPx | 0.97 | 0.88 | 0.76 | 0.90 | 0.72 | 0.90 |
| GR | 0.95 | 0.88 | 0.75 | 0.91 | 0.62 | 0.90 |
| GST | 0.99 | 0.86 | 0.83 | 0.79 | 0.95 | 0.88 |
| GSH | 0.20 | −0.92 | −0.78 | −0.23 | −0.03 | −0.15 |
| AA | 0.18 | 0.28 | 0.31 | 0.63 | 0.05 | 0.26 |
| DPPH | 0.76 | 0.71 | 0.75 | 0.07 | 0.70 | 0.55 |
| Function | Eigenvalue | % of Variance | Cumulative % | Canonical Correlation |
|---|---|---|---|---|
| 1 | 576.148 | 97.3 | 97.3 | 0.999 |
| 2 | 11.694 | 2 | 99.2 | 0.96 |
| 3 | 3.448 | 0.6 | 99.8 | 0.88 |
| 4 | 0.866 | 0.1 | 100 | 0.681 |
| 5 | 0.269 | 0 | 100 | 0.46 |
| Parameters | Function | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| AA | −0.118 | 0.318 | 0.12 | 0.174 | −0.816 |
| GSH | −0.124 | 0.065 | −0.188 | 0.081 | 0.428 |
| CAT | 1.281 | −1.391 | 0.172 | −0.06 | 0.74 |
| SOD | 0.985 | 0.103 | −0.324 | 0.506 | 0.044 |
| GST | 0.365 | 0.507 | −0.503 | 0.201 | −0.155 |
| DPPH | 0.133 | −0.06 | 0.456 | 0.641 | 0.609 |
| GR | 1.013 | 1.494 | 1.065 | −1.656 | 0.787 |
| GPx | −0.425 | −1.416 | −1.409 | 1.964 | −1.213 |
| LPx | 0.124 | 1.615 | −0.124 | −0.295 | −0.055 |
| Allantoin | 0.75 | −0.354 | 0.791 | 0.108 | −0.048 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pati, S.G.; Paital, B.; Sahoo, D.K. Allantoin and Tissue Specific Redox Regulation in Mud Crab Scylla serrata under Varied Natural Water Physico-Chemical Parameters. Water 2024, 16, 480. https://doi.org/10.3390/w16030480
Pati SG, Paital B, Sahoo DK. Allantoin and Tissue Specific Redox Regulation in Mud Crab Scylla serrata under Varied Natural Water Physico-Chemical Parameters. Water. 2024; 16(3):480. https://doi.org/10.3390/w16030480
Chicago/Turabian StylePati, Samar Gourav, Biswaranjan Paital, and Dipak Kumar Sahoo. 2024. "Allantoin and Tissue Specific Redox Regulation in Mud Crab Scylla serrata under Varied Natural Water Physico-Chemical Parameters" Water 16, no. 3: 480. https://doi.org/10.3390/w16030480
APA StylePati, S. G., Paital, B., & Sahoo, D. K. (2024). Allantoin and Tissue Specific Redox Regulation in Mud Crab Scylla serrata under Varied Natural Water Physico-Chemical Parameters. Water, 16(3), 480. https://doi.org/10.3390/w16030480








