In Vitro Potential of Antioxidant Extracts from Gracilaria gracilis Cultivated in Integrated Multi-Trophic Aquaculture (IMTA) for Marine Biobased Sector
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Processing
2.1.1. Extraction by Solvents
2.1.2. Total Polyphenol Contents
2.1.3. DPPH Radical Scavenging Activity
2.2. Protective Effect of G. gracilis Extract against the Pro-Oxidant BDE-47 in the SAF-1 Cell Line
SAF-1 Cell Culture and Treatment
2.3. Gene Expression Assay
Quantitative Reverse Transcription PCR
2.4. Statistical Analysis
3. Results and Discussion
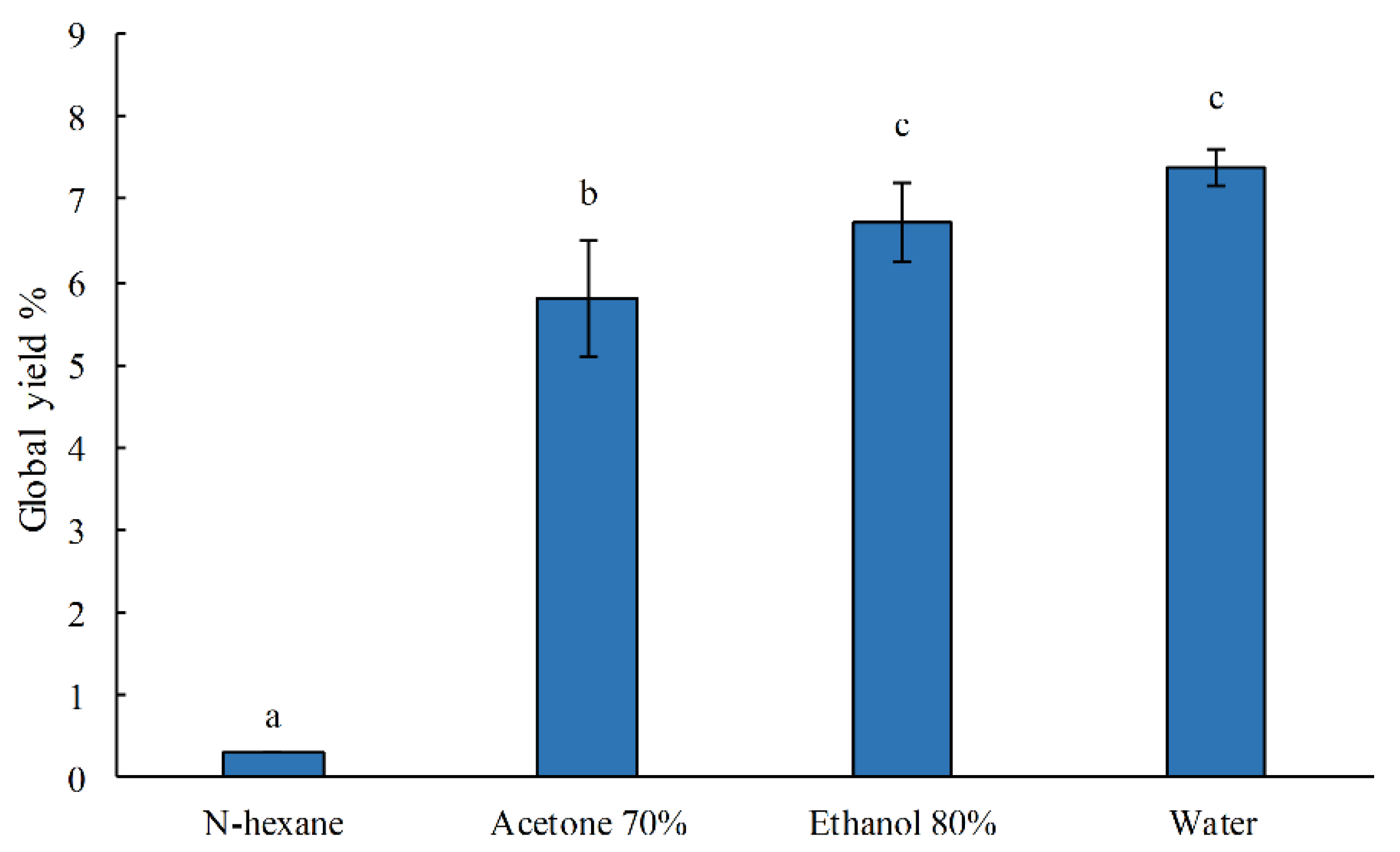
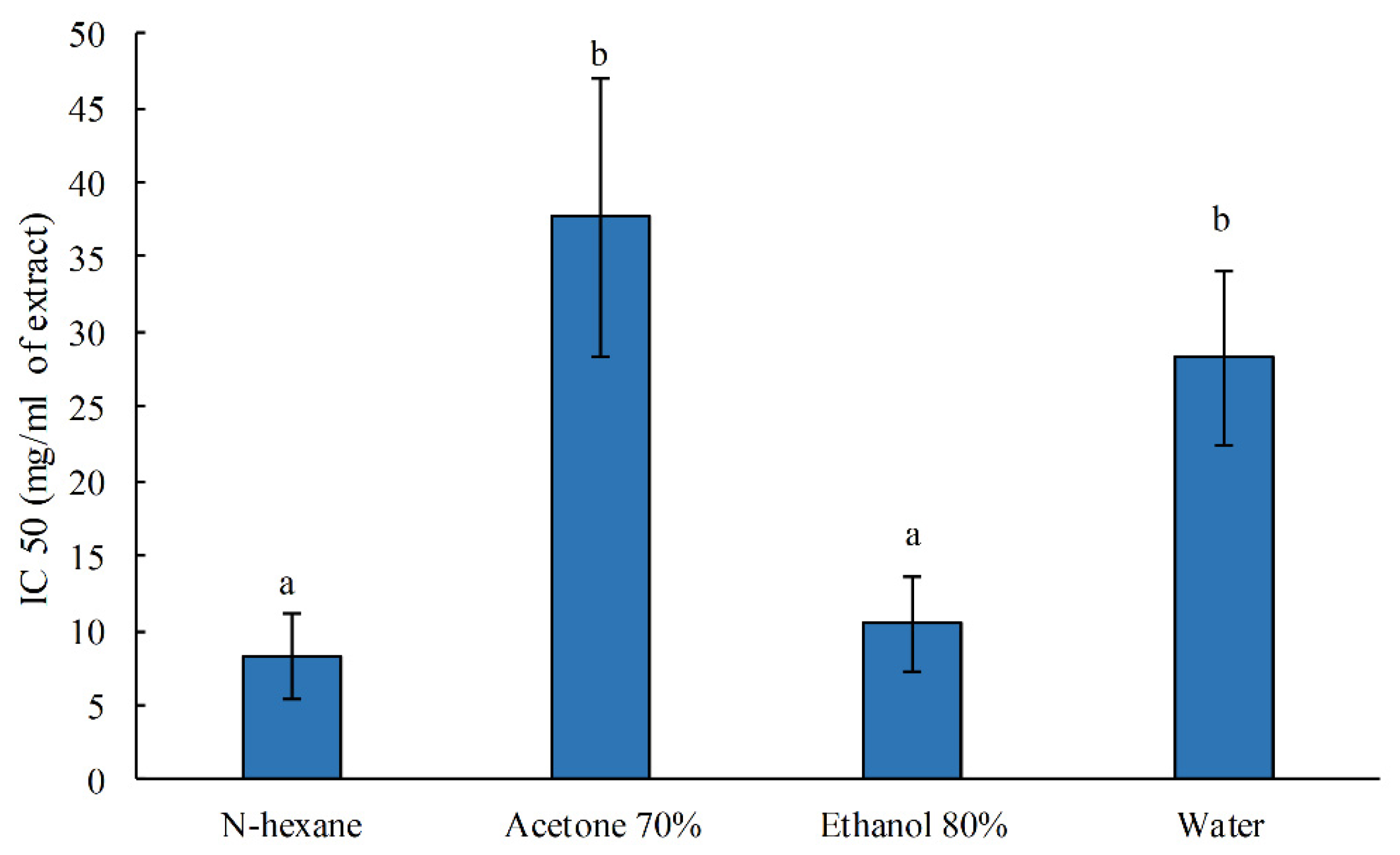
3.1. Characterization of the Antioxidant Power
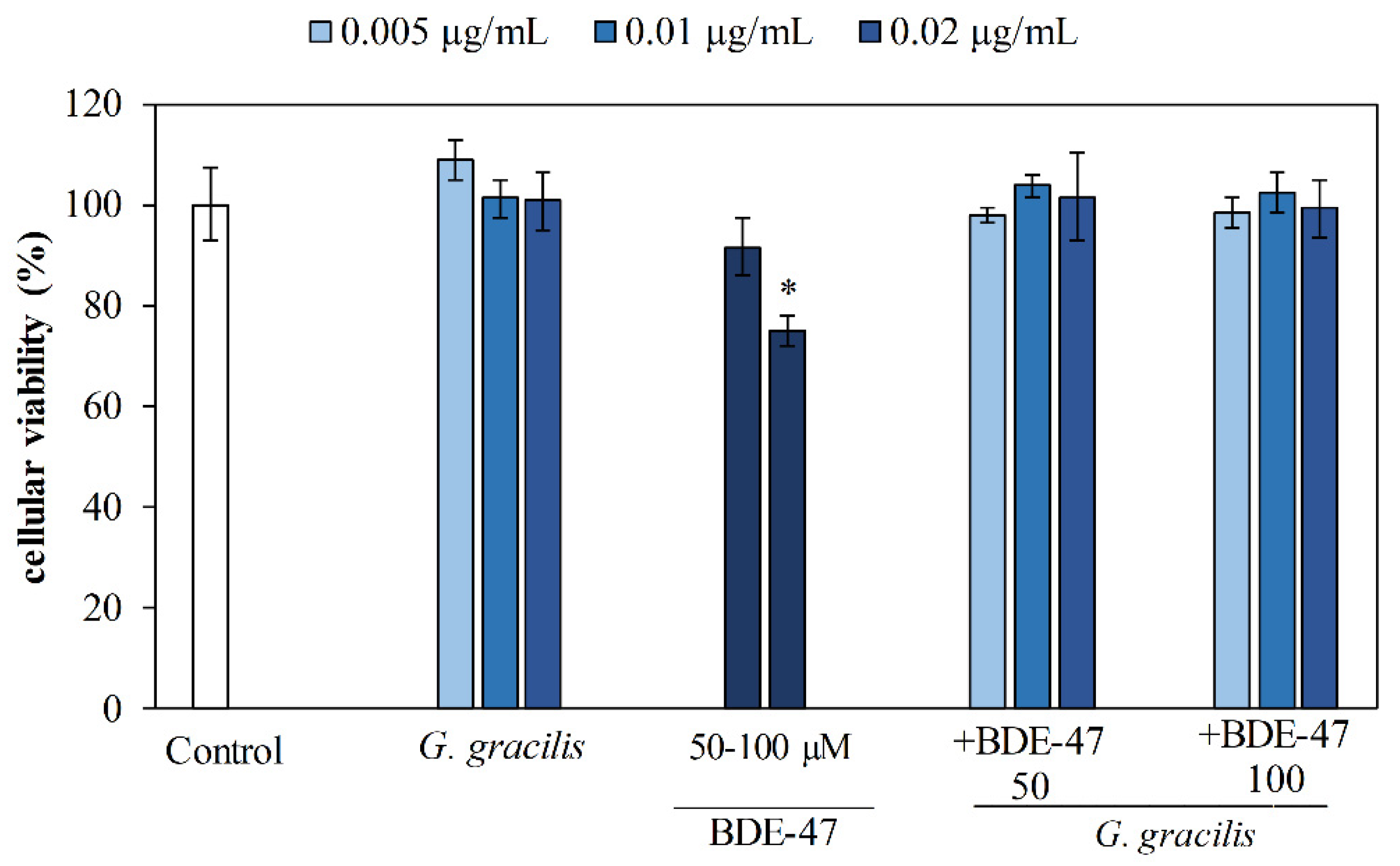
3.2. Cytoprotective Potential of Ethanol Extract of G. gracilis in SAF-1 Cells Exposed to BDE-47
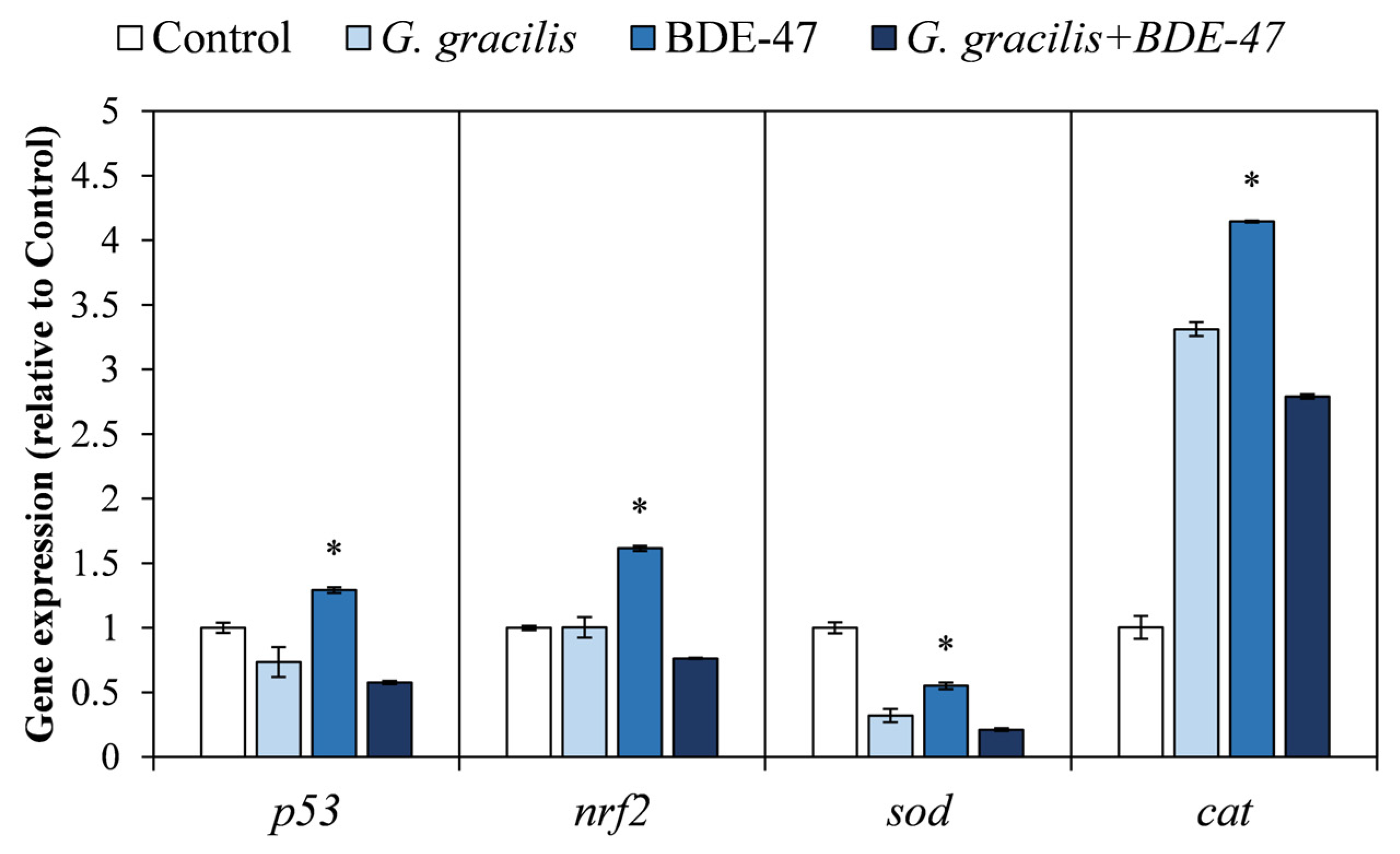
Evaluation of Molecular Biomarkers Related to Cell Cycle and Oxidative Stress by Gene Expression Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization. In Brief to The State of World Fisheries and Aquaculture 2022; FAO: Rome, Italy, 2022; ISBN 978-92-5-136367-6. [Google Scholar]
- Lal, J.; Singh, S.K.; Pawar, L.; Biswas, P.; Meitei, M.M.; Meena, D.K. Integrated Multi-Trophic Aquaculture: A Balanced Ecosystem Approach to Blue Revolution. In Organic Farming: Global Perspectives and Methods, 2nd ed.; Woodhead Publishing: Sawston, UK, 2023; pp. 513–535. ISBN 9780323991452. [Google Scholar]
- Lothmann, R.; Sewilam, H. Potential of Innovative Marine Aquaculture Techniques to Close Nutrient Cycles. Rev. Aquac. 2023, 15, 947–964. [Google Scholar] [CrossRef]
- Food and Agriculture Organization. The State of Food Security and Nutrition in the World 2023; FAO: Rome, Italy, 2023; ISBN 9789251372265. [Google Scholar]
- Papageorgiou, N.; Dimitriou, P.D.; Chatzivasileiou, D.; Tsapakis, M.; Karakassis, I. Can IMTA Provide Added Ecosystem Value Services in the Fish Farms of Greece? Front. Mar. Sci. 2023, 9, 1083099. [Google Scholar] [CrossRef]
- Luo, G. Review of Waste Phosphorus from Aquaculture: Source, Removal and Recovery. Rev. Aquac. 2023, 15, 1058–1082. [Google Scholar] [CrossRef]
- Troell, M.; Joyce, A.; Chopin, T.; Neori, A.; Buschmann, A.H.; Fang, J.G. Ecological Engineering in Aquaculture—Potential for Integrated Multi-Trophic Aquaculture (IMTA) in Marine Offshore Systems. Aquaculture 2009, 297, 1–9. [Google Scholar] [CrossRef]
- Chopin, T. Aquaculture, Integrated Multi-Trophic (IMTA). In Sustainable Food Production; Springer: New York, NY, USA, 2013; pp. 184–205. [Google Scholar]
- Giangrande, A.; Licciano, M.; Arduini, D.; Borghese, J.; Pierri, C.; Trani, R.; Longo, C.; Petrocelli, A.; Ricci, P.; Alabiso, G.; et al. An Integrated Monitoring Approach to the Evaluation of the Environmental Impact of an Inshore Mariculture Plant (Mar Grande of Taranto, Ionian Sea). Biology 2022, 11, 617. [Google Scholar] [CrossRef]
- Vladkova, T.; Georgieva, N.; Staneva, A.; Gospodinova, D. Recent Progress in Antioxidant Active Substances from Marine Biota. Antioxidants 2022, 11, 439. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, D.J. Highlights of Marine Natural Products Chemistry (1972–1999). Nat. Prod. Rep. 2000, 17, 1–6. [Google Scholar] [CrossRef]
- Lushchak, V.I. Environmentally Induced Oxidative Stress in Aquatic Animals. Aquat. Toxicol. 2011, 101, 13–30. [Google Scholar] [CrossRef]
- Herrero, M.; del Pilar Sánchez-Camargo, A.; Cifuentes, A.; Ibáñez, E. Plants, Seaweeds, Microalgae and Food by-Products as Natural Sources of Functional Ingredients Obtained Using Pressurized Liquid Extraction and Supercritical Fluid Extraction. TrAC Trends Anal. Chem. 2015, 71, 26–38. [Google Scholar] [CrossRef]
- Pangestuti, R.; Kim, S.K. Biological Activities and Health Benefit Effects of Natural Pigments Derived from Marine Algae. J. Funct. Foods 2011, 3, 255–266. [Google Scholar] [CrossRef]
- Ebrahimzadeh, M.A.; Khalili, M.; Dehpour, A.A. Antioxidant Activity of Ethyl Acetate and Methanolic Extracts of Two Marine Algae, Nannochloropsis Oculata and Gracilaria Gracilis—An in Vitro Assay. Brazilian J. Pharm. Sci. 2018, 54, 1–6. [Google Scholar] [CrossRef]
- Vega, J.; Álvarez-Gómez, F.; Güenaga, L.; Figueroa, F.L.; Gómez-Pinchetti, J.L. Antioxidant Activity of Extracts from Marine Macroalgae, Wild-Collected and Cultivated, in an Integrated Multi-Trophic Aquaculture System. Aquaculture 2020, 522, 735088. [Google Scholar] [CrossRef]
- Knowler, D.; Chopin, T.; Martínez-Espiñeira, R.; Neori, A.; Nobre, A.; Noce, A.; Reid, G. The Economics of Integrated Multi-Trophic Aquaculture: Where Are We Now and Where Do We Need to Go? Rev. Aquac. 2020, 12, 1579–1594. [Google Scholar] [CrossRef]
- Torres, P.; Santos, J.P.; Chow, F.; dos Santos, D.Y.A.C. A Comprehensive Review of Traditional Uses, Bioactivity Potential, and Chemical Diversity of the Genus Gracilaria (Gracilariales, Rhodophyta). Algal Res. 2019, 37, 288–306. [Google Scholar] [CrossRef]
- De Almeida, C.L.F.; De S. Falcão, H.; De M. Lima, G.R.; De A. Montenegro, C.; Lira, N.S.; De Athayde-Filho, P.F.; Rodrigues, L.C.; De Souza, M.d.F.V.; Barbosa-Filho, J.M.; Batista, L.M. Bioactivities from Marine Algae of the Genus Gracilaria. Int. J. Mol. Sci. 2011, 12, 4550–4573. [Google Scholar] [CrossRef]
- Silva-Brito, F.; Cardoso, A.; Machado, M.; Ramos-Pinto, L.; Hinzmann, M.; Abreu, H.; Costas, B.; Magnoni, L. Dietary Supplementation with Gracilaria Gracilis By-Products Modulates the Immune Status and Oxidative Stress Response of Gilthead Seabream (Sparus aurata) Stimulated with Photobacterium Damselae Subsp. Piscicida. Fish Shellfish Immunol. 2022, 126, 164–177. [Google Scholar] [CrossRef]
- Gioele, C.; Marilena, S.; Valbona, A.; Nunziacarla, S.; Andrea, S.; Antonio, M. Gracilaria Gracilis, Source of Agar: A Short Review. Curr. Org. Chem. 2017, 21, 380–386. [Google Scholar] [CrossRef]
- Kazir, M.; Abuhassira, Y.; Robin, A.; Nahor, O.; Luo, J.; Israel, A.; Golberg, A.; Livney, Y.D. Extraction of Proteins from Two Marine Macroalgae, Ulva Sp. and Gracilaria Sp., for Food Application, and Evaluating Digestibility, Amino Acid Composition and Antioxidant Properties of the Protein Concentrates. Food Hydrocoll. 2019, 87, 194–203. [Google Scholar] [CrossRef]
- Pradhan, B.; Bhuyan, P.P.; Patra, S.; Nayak, R.; Behera, P.K.; Behera, C.; Behera, A.K.; Ki, J.-S.; Jena, M. Beneficial Effects of Seaweeds and Seaweed-Derived Bioactive Compounds: Current Evidence and Future Prospective. Biocatal. Agric. Biotechnol. 2022, 39, 102242. [Google Scholar] [CrossRef]
- Passos, R.; Correia, A.P.; Ferreira, I.; Pires, P.; Pires, D.; Gomes, E.; do Carmo, B.; Santos, P.; Simões, M.; Afonso, C.; et al. Effect on Health Status and Pathogen Resistance of Gilthead Seabream (Sparus aurata) Fed with Diets Supplemented with Gracilaria Gracilis. Aquaculture 2021, 531, 735888. [Google Scholar] [CrossRef]
- Bonsignore, M.; Messina, C.; Bellante, A.; Manuguerra, S.; Arena, R.; Santulli, A.; Maricchiolo, G.; Del Core, M.; Sprovieri, M. Chemical and Biochemical Responses to Sub—Lethal Doses of Mercury and Cadmium in Gilthead Seabream (Sparus aurata). Chemosphere 2022, 307, 135822. [Google Scholar] [CrossRef]
- Ruiz, C.E.; Manuguerra, S.; Cuesta, A.; Esteban, M.A.; Santulli, A.; Messina, C.M. Sub-Lethal Doses of Polybrominated Diphenyl Ethers Affect Some Biomarkers Involved in Energy Balance and Cell Cycle, via Oxidative Stress in the Marine Fish Cell Line SAF-1. Aquat. Toxicol. 2019, 210, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Espinosa Ruiz, C.; Manuguerra, S.; Curcuraci, E.; Santulli, A.; Messina, C.M. Carbamazepine, Cadmium Chloride and Polybrominated Diphenyl Ether-47, Synergistically Modulate the Expression of Antioxidants and Cell Cycle Biomarkers, in the Marine Fish Cell Line SAF-1. Mar. Environ. Res. 2020, 154, 104844. [Google Scholar] [CrossRef]
- Manuguerra, S.; Ruiz, C.E.; Santulli, A.; Messina, C.M. Sub-Lethal Doses of Polybrominated Diphenyl Ethers, in Vitro, Promote Oxidative Stress and Modulate Molecular Markers Related to Cell Cycle, Antioxidant Balance and Cellular Energy Management. Int. J. Environ. Res. Public Health 2019, 16, 588. [Google Scholar] [CrossRef] [PubMed]
- Sureda, A.; Box, A.; Terrados, J.; Deudero, S.; Pons, A. Antioxidant Response of the Seagrass Posidonia Oceanica When Epiphytized by the Invasive Macroalgae Lophocladia Lallemandii. Mar. Environ. Res. 2008, 66, 359–363. [Google Scholar] [CrossRef]
- Espinosa, C.; Pérez-Llamas, F.; Guardiola, F.A.; Esteban, M.A.; Arnao, M.B.; Zamora, S.; López-Jiménez, J.A. Molecular Mechanisms by Which White Tea Prevents Oxidative Stress. J. Physiol. Biochem. 2014, 70, 891–900. [Google Scholar] [CrossRef]
- Tanaka, M.; Kishimoto, Y.; Sasaki, M.; Sato, A.; Kamiya, T.; Kondo, K.; Iida, K. Terminalia bellirica (Gaertn.) Roxb. Extract and Gallic Acid Attenuate LPS-Induced Inflammation and Oxidative Stress via MAPK/NF-κ B and Akt/AMPK/Nrf2 Pathways. Oxid. Med. Cell. Longev. 2018, 2018, 9364364. [Google Scholar] [CrossRef]
- Yang, J.I.; Yeh, C.C.; Lee, J.C.; Yi, S.C.; Huang, H.W.; Tseng, C.N.; Chang, H.W. Aqueous Extracts of the Edible Gracilaria Tenuistipitata Are Protective against H2O2-Induced DNA Damage, Growth Inhibition, and Cell Cycle Arrest. Molecules 2012, 17, 7241–7254. [Google Scholar] [CrossRef]
- Zhao, Y.; Tang, X.; Quigg, A.; Lv, M.; Zhao, Y. The Toxic Mechanisms of BDE-47 to the Marine Diatom Thalassiosira Pseudonana-a Study Based on Multiple Physiological Processes. Aquat. Toxicol. 2019, 212, 20–27. [Google Scholar] [CrossRef]
- Wang, X.; Hales, B.F.; Robaire, B. Effects of Flame Retardants on Ovarian Function. Reprod. Toxicol. 2021, 102, 10–23. [Google Scholar] [CrossRef]
- Tornero, V.; Hanke, G. Chemical Contaminants Entering the Marine Environment from Sea-Based Sources: A Review with a Focus on European Seas. Mar. Pollut. Bull. 2016, 112, 17–38. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Muñoz, D.; Llorca, M.; Blasco, J.; Barceló, D. Contaminants in the Marine Environment. In Marine Ecotoxicology; Academic Press: Cambridge, MA, USA, 2016; pp. 1–34. [Google Scholar]
- Nasri, A.; Allouche, M.; Hannachi, A.; Barhoumi, B.; Wahbi, A.; Harrath, A.H.; Mahmoudi, E.; Beyrem, H.; Boufahja, F. Ecotoxicity of Polybrominated Diphenyl Ether (BDE-47) on a Meiobenthic Community with Special Emphasis on Nematodes: Taxonomic and Trophic Diversity Assessment. Environ. Pollut. 2021, 277, 116727. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Tang, X.; Sun, T.; Wang, Y. BDE-47 Exposure Changed the Immune Function of Haemocytes in Mytilus Edulis: An Explanation Based on ROS-Mediated Pathway. Aquat. Toxicol. 2017, 182, 58–66. [Google Scholar] [CrossRef]
- Herrera, A.; Acosta-Dacal, A.; Luzardo, O.P.; Martínez, I.; Rapp, J.; Reinold, S.; Montesdeoca-Esponda, S.; Montero, D.; Gómez, M. Bioaccumulation of Additives and Chemical Contaminants from Environmental Microplastics in European Seabass (Dicentrarchus labrax). Sci. Total Environ. 2022, 822, 153396. [Google Scholar] [CrossRef]
- Harmelin-Vivien, M.; Bodiguel, X.; Charmasson, S.; Loizeau, V.; Mellon-Duval, C.; Tronczyński, J.; Cossa, D. Differential Biomagnification of PCB, PBDE, Hg and Radiocesium in the Food Web of the European Hake from the NW Mediterranean. Mar. Pollut. Bull. 2012, 64, 974–983. [Google Scholar] [CrossRef] [PubMed]
- Bodiguel, X.; Tronczynski, J.; Loizeau, V.; Munschy, C.; Guiot, N.; Le Guellec, A.M.; Olivier, N.; Roupsard, F.; Mellon, C. Classical and Novel Organohalogen Compounds (PCBs and PBDEs) in Hake (M. merluccius, L.) from the Mediterranean and Atlantic Coasts (France). WIT Trans. Ecol. Environ. 2008, 110, 157–166. [Google Scholar] [CrossRef]
- UNEP. Chemicals Listed in Annex A; Stockholm Convention for Persistent Organic Pollutants Chatelaine: Geneva, Switzerland, 2019. [Google Scholar]
- Geng, Q.; Guo, M.; Wu, H.; Peng, J.; Zheng, G.; Liu, X.; Zhai, Y.; Tan, Z. Effects of Single and Combined Exposure to BDE-47 and PFOA on Distribution, Bioaccumulation, and Toxicity in Blue Mussel (Mytilus galloprovincialis). Ecotoxicol. Environ. Saf. 2021, 228, 113014. [Google Scholar] [CrossRef]
- Espinosa Ruiz, C.; Manuguerra, S.; Cuesta, A.; Santulli, A.; Messina, C. Oxidative Stress, Induced by Sub-Lethal Doses of BDE 209, Promotes Energy Management and Cell Cycle Modulation in the Marine Fish Cell Line SAF-1. Int. J. Environ. Res. Public Health 2019, 16, 474. [Google Scholar] [CrossRef]
- Messina, C.M.; Troia, A.; Arena, R.; Manuguerra, S.; Ioannou, T.; Curcuraci, E.; Renda, G.; Hellio, C.; Santulli, A. Species-Specific Antioxidant Power and Bioactive Properties of the Extracts Obtained from Wild Mediterranean calendula Spp. (Asteraceae). Appl. Sci. 2019, 9, 4627. [Google Scholar] [CrossRef]
- Folin, O.; Ciocalteu, V. On Tyrosine and Tryptophane Determinations in Proteins. J. Biol. Chem. 1927, 73, 627–650. [Google Scholar] [CrossRef]
- Dhouibi, N.; Manuguerra, S.; Arena, R.; Mahdhi, A.; Messina, C.M.; Santulli, A.; Dhaouadi, H. Screening of Antioxidant Potentials and Bioactive Properties of the Extracts Obtained from Two Centaurea L. Species (C. kroumirensis Coss. and C. sicula L. Subsp Sicula). Appl. Sci. 2020, 10, 2267. [Google Scholar] [CrossRef]
- Bernatoniene, J.; Masteikova, R.; Davalgiene, J.; Peciura, R.; Gauryliene, R.; Bernatoniene, R.; Majiene, D.; Lazauskas, R.; Civinskiene, G.; Velziene, S.; et al. Topical Application of Calendula officinalis (L.): Formulation and Evaluation of Hydrophilic Cream with Antioxidant Activity. J. Med. Plants Res. 2011, 5, 868–877. [Google Scholar]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Chan, P.T.; Matanjun, P.; Yasir, S.M.; Tan, T.S. Antioxidant Activities and Polyphenolics of Various Solvent Extracts of Red Seaweed, Gracilaria Changii. J. Appl. Phycol. 2015, 27, 2377–2386. [Google Scholar] [CrossRef]
- Messina, C.M.; Renda, G.; Laudicella, V.A.; Trepos, R.; Fauchon, M.; Hellio, C.; Santulli, A. From Ecology to Biotechnology, Study of the Defense Strategies of Algae and Halophytes (from Trapani Saltworks, NW Sicily) with a Focus on Antioxidants and Antimicrobial Properties. Int. J. Mol. Sci. 2019, 20, 881. [Google Scholar] [CrossRef]
- Hulkko, L.S.S.; Chaturvedi, T.; Thomsen, M.H. Extraction and Quantification of Chlorophylls, Carotenoids, Phenolic Compounds, and Vitamins from Halophyte Biomasses. Appl. Sci. 2022, 12, 840. [Google Scholar] [CrossRef]
- Foo, S.C.; Yusoff, F.M.; Ismail, M.; Basri, M.; Yau, S.K.; Khong, N.M.H.; Chan, K.W.; Ebrahimi, M. Antioxidant Capacities of Fucoxanthin-Producing Algae as Influenced by Their Carotenoid and Phenolic Contents. J. Biotechnol. 2017, 241, 175–183. [Google Scholar] [CrossRef]
- Arena, R.; Manuguerra, S.; Collins, E.; Mahdhi, A.; Renda, G.; Messina, C.M.; Santulli, A. Antioxidant Properties of a Supercritical Fluid Extract of the Halophyte Mesembryanthemum nodiflorum L. from Sicilian Coasts: Nutraceutical and Cosmeceutical Applications. Appl. Sci. 2020, 10, 2374. [Google Scholar] [CrossRef]
- Yang, J.; Chan, K.M. Evaluation of the Toxic Effects of Brominated Compounds (BDE-47, 99, 209, TBBPA) and Bisphenol a (BPA) Using a Zebrafish Liver Cell Line, ZFL. Aquat. Toxicol. 2015, 159, 138–147. [Google Scholar] [CrossRef]
- Afonso, C.; Correia, A.P.; Freitas, M.V.; Mouga, T.; Baptista, T. In Vitro Evaluation of the Antibacterial and Antioxidant Activities of Extracts of Gracilaria Gracilis with a View into Its Potential Use as an Additive in Fish Feed. Appl. Sci. 2021, 11, 6642. [Google Scholar] [CrossRef]
- Ashkenazi, D.Y.; Figueroa, F.L.; Korbee, N.; García-Sánchez, M.; Vega, J.; Ben-Valid, S.; Paz, G.; Salomon, E.; Israel, Á.; Abelson, A. Enhancing Bioproducts in Seaweeds via Sustainable Aquaculture: Antioxidant and Sun-Protection Compounds. Mar. Drugs 2022, 20, 767. [Google Scholar] [CrossRef] [PubMed]
- Klinčić, D.; Dvoršćak, M.; Jagić, K.; Mendaš, G.; Herceg Romanić, S. Levels and Distribution of Polybrominated Diphenyl Ethers in Humans and Environmental Compartments: A Comprehensive Review of the Last Five Years of Research. Environ. Sci. Pollut. Res. 2020, 27, 5744–5758. [Google Scholar] [CrossRef]
- De Oro-Carretero, P.; Landaluze, J.S. Bioaccumulation And Biotransformation of BDE-47 Using Zebrash Eleutheroembryos (Danio rerio). Environ Toxicol Chem. 2023, 42, 835–845. [Google Scholar] [CrossRef]
- Regoli, F.; Giuliani, M.E. Oxidative Pathways of Chemical Toxicity and Oxidative Stress Biomarkers in Marine Organisms. Mar. Environ. Res. 2014, 93, 106–117. [Google Scholar] [CrossRef]
- Lee, H.Y.; Oh, S.H. Autophagy-Mediated Cytoplasmic Accumulation of P53 Leads to Apoptosis through DRAM-BAX in Cadmium-Exposed Human Proximal Tubular Cells. Biochem. Biophys. Res. Commun. 2021, 534, 128–133. [Google Scholar] [CrossRef]
- Yee, K.S.; Vousden, K.H. Complicating the Complexity of P53. Carcinogenesis 2005, 26, 1317–1322. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, N.; Wang, X.; Jin, X.; Du, H.; Peng, G.; Xue, J. Benzo(a)Pyrene-7,8-Diol-9,10-Epoxide Induced P53-Independent Necrosis via the Mitochondria-Associated Pathway Involving Bax and Bak Activation. Hum. Exp. Toxicol. 2015, 34, 179–190. [Google Scholar] [CrossRef]
- Giuliani, M.E.; Regoli, F. Identification of the Nrf2–Keap1 Pathway in the European Eel Anguilla Anguilla: Role for a Transcriptional Regulation of Antioxidant Genes in Aquatic Organisms. Aquat. Toxicol. 2014, 150, 117–123. [Google Scholar] [CrossRef]
- Chen, H.; Tang, X.; Zhou, B.; Zhou, Z.; Xu, N.; Wang, Y. A ROS-Mediated Mitochondrial Pathway and Nrf2 Pathway Activation Are Involved in BDE-47 Induced Apoptosis in Neuro-2a Cells. Chemosphere 2017, 184, 679–686. [Google Scholar] [CrossRef]
- Younus, H. Therapeutic Potentials of Superoxide Dismutase. Int. J. Health Sci. 2018, 12, 88. [Google Scholar]
- Sharma, I.; Ahmad, P. Catalase: A Versatile Antioxidant in Plants. In Oxidative Damage to Plants Antioxid. Networks Signal; Elsevier: Amsterdam, The Netherlands, 2014; pp. 131–148. [Google Scholar] [CrossRef]
- Khan, M.A.; Tania, M.; Zhang, D.Z.; Chen, H.C. Antioxidant Enzymes and Cancer. Chinese J. Cancer Res. 2010, 22, 87–92. [Google Scholar] [CrossRef]
- Fuchs, P.; Perez-Pinzon, M.A.; Dave, K.R. Cerebral Ischemia in Diabetics and Oxidative Stress. In Diabetes: Oxidative Stress and Dietary Antioxidants; Academic Press: Cambridge, MA, USA, 2013; pp. 15–23. ISBN 9780124058859. [Google Scholar]

| Genes | F/R Primer Sequence (5′–3′) |
|---|---|
| p53 | CCTCATCCTCATCATCGCCT |
| AGCTCGTTGAATTTGCAGGG | |
| nrf2 | GTTCAGTCGGTGCTTTGACA |
| CTCTGATGTGCGTCTCTCCA | |
| sod | CCATGGTAAGAATCATGGCGG |
| CGTGGATCACCATGGTTCTG | |
| cat | TTCCCGTCCTTCATTCACTC |
| CTCCAGAAGTCCCACACCAT | |
| β-actin | GTCATGGATTCCGGTGATGG |
| TGGTGAAGGAGTAGCCACGC | |
| 18s | TGTGCCGCTAGAGGTGAAATT |
| GCAAATGCTTTCGCTTTCG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manuguerra, S.; Arena, R.; Curcuraci, E.; Renda, G.; Rannou, M.; Hellio, C.; Messina, C.M.; Santulli, A. In Vitro Potential of Antioxidant Extracts from Gracilaria gracilis Cultivated in Integrated Multi-Trophic Aquaculture (IMTA) for Marine Biobased Sector. Water 2024, 16, 2667. https://doi.org/10.3390/w16182667
Manuguerra S, Arena R, Curcuraci E, Renda G, Rannou M, Hellio C, Messina CM, Santulli A. In Vitro Potential of Antioxidant Extracts from Gracilaria gracilis Cultivated in Integrated Multi-Trophic Aquaculture (IMTA) for Marine Biobased Sector. Water. 2024; 16(18):2667. https://doi.org/10.3390/w16182667
Chicago/Turabian StyleManuguerra, Simona, Rosaria Arena, Eleonora Curcuraci, Giuseppe Renda, Maxime Rannou, Claire Hellio, Concetta Maria Messina, and Andrea Santulli. 2024. "In Vitro Potential of Antioxidant Extracts from Gracilaria gracilis Cultivated in Integrated Multi-Trophic Aquaculture (IMTA) for Marine Biobased Sector" Water 16, no. 18: 2667. https://doi.org/10.3390/w16182667
APA StyleManuguerra, S., Arena, R., Curcuraci, E., Renda, G., Rannou, M., Hellio, C., Messina, C. M., & Santulli, A. (2024). In Vitro Potential of Antioxidant Extracts from Gracilaria gracilis Cultivated in Integrated Multi-Trophic Aquaculture (IMTA) for Marine Biobased Sector. Water, 16(18), 2667. https://doi.org/10.3390/w16182667






