Abstract
S-metolachlor is one of the most frequently used herbicides worldwide. However, toxicity studies of this herbicide to aquatic organisms are scarce. In the present study, two experiments were conducted to test the effects of S-metolachlor on common carp, one of the most economically important fish species, with a distribution throughout the world: (1) 96 h acute semi-static toxicity test, aiming to determine LC50; (2) a subchronic semi-static test that lasted 28 days, in which juvenile carp were exposed to 3%, 8%, and 25% of previously determined LC50—0.5 mg·L−1, 1.4 mg·L−1, and 4.1 mg·L−1, respectively. Several biomarkers were employed to assess fish responses to toxicants. Blood biochemistry analysis and nuclear alterations of erythrocytes did not show any difference among experimental groups. Semi-quantitative histopathological analysis revealed mild alterations in the gills and liver, where oedema of secondary epithelium of gills and leukocyte infiltration in liver were significantly higher in fish exposed to 1.4 mg·L−1 and 4.1 mg·L−1. Histopathological indices in liver, as well as the total histopathological index, also showed significantly higher scores in the same groups. Bioconcentration factors of S-metolachlor ranged from 3.2 to 9.4, depending on the experimental group.
1. Introduction
S-metolachlor (S-MET) is an S-isomer pair of metolachlor, a selective pre-emergent and early post-emergent herbicide used for dicotyledonous and grass weed control in agriculture. Its predecessor, metolachlor, was one of the most widely used herbicides, with registered use for more than 70 crops all over the world. In 2008, metolachlor was one of the four most widely used active substances of pesticides in the United States [1]. The herbicidal activity of metolachlor mostly derives from S conformation. With equivalent efficacy at application rates lower than metolachlor (65%), highly concentrated formulations (960 g/L), low risk for developing weed resistance, and reduced risks to humans and the environment, S-MET is an appropriate choice for sustainable weed management [2]. After the ban on metolachlor in the European Union in 2002, the use of S-MET has increased. In 2003, S-MET was one of the ten active substances of pesticides most commonly used in the EU [3], and amounted to 4.2% of the global pesticide usage [4].
As a selective herbicide from the chloroacetamide group, S-MET reduces seed germination by inhibiting mitosis, gibberellin, and both cell division and cell enlargement [5,6,7]. The site of action is a very long chain fatty acid (VLCFA), FAE1-synthase, an enzyme required for the elongation of C16 and C18 to C20 fatty acids. Losses of cell membrane permeability and rigidity occur as a result of the imbalance in the fatty acid composition of cell membranes after binding of the S-MET to FAE1-synthase [8,9]. Effects of S-MET on soil enzymes and environmental fate have also been demonstrated [10,11,12]. S-MET is classified by the K3 Herbicide Resistance Action Committee (HRAC) group as a VLCFA synthesis inhibitor [13].
The half-life of S-MET under field conditions amounts to 11–30 days, depending on the soil type; no accumulation occurs [2]. In line with that, the potential for the contamination of groundwater is low, as confirmed by recent investigation [14]. However, it can regularly be found in surface waters in Europe and North America as a result of spray drift during application and runoff from agricultural areas, after rain events in crop-growing seasons. The maximum detected concentration from the literature was 143 μg·L−1 [15], although maximum concentrations in fishponds are much lower—3.4 μg·L−1 [16]. S-MET has also been detected in Western Mediterranean Sea surface water [17]. Studies of S-MET toxicity to aquatic organisms are scarce; however, it is established that S-MET and its metabolites have an impact on embryo development, exert endocrine-disrupting properties, decrease survival and the hatching percentage, and impair the swim bladder, which have implications on the behaviour and locomotion of zebrafish (Danio rerio) [18,19,20,21,22].
With the relatively high solubility of S-MET (480 mg·L−1), and thus its presence in surface waters and bioavailability to fish and other non-target aquatic organisms, its potential toxic effects need to be evaluated. Therefore, the aim of the study was to expose common carp to S-MET in both acute and subchronic tests and establish its LC50 value (during acute exposure), as well as to evaluate its effects on various blood and tissue markers (during subchronic exposure). The research hypothesis was that dose-dependent exposure to S-MET also leads to a dose-dependent response to selected biomarkers that were analysed in the study. The fish species was chosen due to its presence in the waters of Europe and Asia (therefore, it represented a non-target organism), and because it was not possible to find results of exposure tests on common carp in the literature. To the best of the authors’ knowledge, only one study has been published, in which pesticide formulation was tested, with common carp exposed to a mixture of terbuthylazine and S-MET, but not solely to S-MET [23].
2. Materials and Methods
2.1. Test Chemicals
S-MET was purchased from the Galenika—Fitofarmacija company (Belgrade, Serbia) with >97% purity. Acetonitrile was purchased from Fisher Chemical (Loughborough, UK), and methanol (Ultra Gradient HPLC Grade) was purchased from J.T. Baker (Deventer, The Netherlands). Water was deionized (>18 M·cm−1) using an Elga Maxima system. QuEChERS Extraction Packets, adopting the EN Method (BondElute, P/N 5982-5650, Agilent Technologies, Santa Clara, CA, USA), were used for extraction and dispersive SPE; fatty samples (BondElute, P/N 5982-5121, Agilent Technologies, Santa Clara, CA, USA) represented cleanup samples.
The stock solution (412 mg·L−1) for the experiment was prepared by dissolving 8.248 g S-MET in 16 L deionized water. The concentration of S-MET was evaluated using liquid chromatography with tandem mass spectrometry (LC-MS/MS). Standard stock solutions for the calibration were prepared in acetonitrile (1.0 mg·mL−1); the working standards were in concentrations of 10 µg·mL−1 and 50 µg·mL−1. These solutions were used for spiking samples and also to prepare the calibration standards of 0.001, 0.005, 0.010, 0.025, 0.050, and 0.100 µg·mL−1 in the mobile phase. For HPLC water analysis, calibration standards were 1, 5, 10, 25, and 50 mg·L−1 in water.
2.2. Experimental Design
Juvenile common carp (Cyprinus carpio L.) used in this trial were obtained from the Center for Fishery and Applied Hydrobiology “Mali Dunav” (CEFAH) of the Experimental school estate of the University of Belgrade—Faculty of Agriculture “Radmilovac”. Two trials were conducted: (1) 96 h acute exposure test, in order to determine LC50 values of S-MET in the common carp; (2) 28-day subchronic exposure test, in order to determine chronic/sublethal effects of the exposure of S-MET on the common carp. Both experiments were carried out at the CEFAH in 6 (acute test) or 12 (subchronic test) glass aquaria measuring 60 × 40 × 40 cm (length × height × width), equipped with water heaters and aerated with air stones. In both trials, 40 L of dechlorinated tap water per aquarium was used, while fish were maintained on a 12 h light/12 h dark photoperiod. Fish were fed during the adaptation period and subchronic test with commercial extruded feed “Soprofish” containing 25% proteins and 7% lipids (Veterinarski Zavod, Subotica, Serbia) ad libitum.
2.3. Acute Toxicity Test
The acute test was conducted using a modified acute toxicity test protocol provided by OECD [24]. One-month-old common carp juveniles were artificially spawned, with a mean weight of 0.63 ± 0.09 g. Ten fish were allocated to each of the six test aquaria 10 days prior to the start of the experiment for an adaptation period. In a semi-static test, fish were exposed to six nominal concentrations of S-MET for 96 h: 0 mg·L−1 (A0), 3.1 mg·L−1 (A1), 5.3 mg·L−1 (A2), 9 mg·L−1 (A3), 15.3 mg·L−1 (A4), and 26 mg·L−1 (A5). Each day, half of the water in each aquarium was renewed with corresponding concentrations of S-MET, and water samples were taken before and after every water change. Water samples were analysed on an Agilent 1260 HPLC system with a diode array detector (DAD) (Agilent Technologies, Waldron, Germany) for actual concentrations of S-MET, and the results were as follows: 0.0 ± 0.0 mg·L−1 (A0); 2.8 ± 0.2 mg·L−1 (A1); 5.0 ± 0.2 mg·L−1 (A2); 8.5 ± 0.3 mg·L−1 (A3); 14.6 ± 0.6 mg·L−1 (A4); 24.4 ± 0.8 mg·L−1 (A5). Since differences between actual and nominal concentrations did not exceed 10%, nominal concentrations were used in this study. Basic water quality parameters were monitored daily using a water field kit, MULTI 340i/SET (WTW, Weilheim, Germany), and values did not significantly differ between aquaria: temperature, 21.9 ± 0.9 °C; pH, 8.4 ± 0.1; dissolved oxygen, 8.2 ± 0.2 mg·L−1. Fish were not fed during the acute exposure test. Mortality and behaviour changes (qualitative analysis, such as responses to mechanical stimuli, ataxia, akinesia, lethargy, and erratic swimming) were observed daily. For the determination of LC50 values, Graph Pad Prism V5.0 (Graph Pad Software, Boston, MA, USA) was used. The LC50 was calculated from nonlinear logarithmic regression of the concentration–response curve, based on measured concentrations.
2.4. Subchronic Toxicity Test
For subchronic exposure tests, the modified juvenile growth test protocol provided by OECD [25] was used. Fish were juveniles of common carp obtained from one of the ponds of the CEFAH. Fish were of the same age as in a previous trial, but larger, since they were reared in the pond and fed on zooplankton; the start weight and total length were 3.51 ± 0.48 g and 7.83 ± 0.31 cm, respectively. An average start value of condition factor (CF) of the fish used in the subchronic test was 0.70 ± 0.02. Based on the LC50 results from the acute test, a control and three nominal concentrations were defined for subchronic exposure test: 0 mg·L−1 (C0); 0.5 mg·L−1 (C1); 1.4 mg·L−1 (C2); and 4.1 mg·L−1 (C3). The concentration value in C3 corresponded to 25% of 96 h LC50, while subsequent concentrations were calculated as 3 times lower than the previous group (C2 = C3 × 0.33; C1 = C2 × 0.33). Ten fish were randomly placed in each aquarium, and after an adaptation period of 10 days, the trial lasted 28 days. Each concentration was tested in triplicate. Half of the water in each aquarium was renewed with corresponding concentrations every second day, while water samples were taken weekly to determine the concentrations of S-MET, ammonia, nitrates, and total hardness of the water. Basic water quality parameters were measured every other day, using the same device as in the acute tests. The water parameter results were as follows: temperature, 22.28 ± 0.89 °C; pH, 8.14 ± 0.06; dissolved oxygen, 7.81 ± 0.21 mg·L−1. Three additional parameters were determined once per week using standard laboratory methods: ammonia, 0.37 ± 0.12 mg·L−1; nitrates, 0.66 ± 0.16 mg·L−1; total hardness, 21.90 ± 1.18 dH. None of the water parameters were significantly different between aquaria. Water samples were analysed on an Agilent 1260 HPLC system with a diode array detector (DAD) (Agilent Technologies, Waldron, Germany) for actual concentrations of S-MET with the following results: 0.00 ± 0.00 mg·L−1; 0.49 ± 0.04 mg·L−1; 1.33 ± 0.12 mg·L−1; and 3.55 ± 0.40 mg·L−1. Fish were fed every second day, and the amount of feed given corresponded to 2.5% of the total fish body mass per aquarium. The un-ionized fraction of ammonia was calculated according to the levels of pH and temperature in the water [26]; the values never exceeded 0.05 mg·L−1. The condition factor (CF) was calculated using the following formula: CF = (W·TL−3) × 100, where W is the body weight (g) and TL is the total length (cm).
2.5. Sampling
At the end of the subchronic exposure test, two fish from each aquarium were sampled, making a total of 6 per concentration. Fish were anaesthetized with clove oil and 0.5 mL of blood from v. caudalis was taken from each fish. Samples were taken using heparinised syringes and needles and placed into 1.5 mL microcentrifuge tubes. Blood plasma was isolated by centrifugation (1400× g, 10 min) and stored at −20 °C prior to measurements. Following blood sampling, the same fish were sacrificed with a quick blow to the head. Fish were carefully dissected, the gills and the liver were removed, and immediately transferred to 4% formaldehyde (Lach-Ner, Neratovice, Czech Republic) for histological processing. Part of the dorsal muscle was cleaned from the skin, placed inside plastic bags, sealed, and stored at −20 °C before preparing samples for measuring the concentrations of S-MET.
2.6. Blood Biochemistry Analyses
The following blood biochemical parameters were measured using a Roche Hitachi 911 Chemistry Analyser (Roche Diagnostics GmbH, Mannheim, Germany): total protein (TP) using the Biuret test; alanine transaminase (ALT) and aspartate transaminase (AST) using the kinetic UV test; alkaline phosphatase (ALP) using the hydrolysis of p-nitrophenyl phosphate test; lactate dehydrogenase (LDH) by the conversion of l-lactate into pyruvate; and acid phosphatase (ACP) by the hydrolysis of 1-naphthyl phosphate [27].
2.7. Histological Analyses
After fixation, tissue was placed in a Leica TP 1020 automatic tissue processor (Leica, Nussloch, Germany) and dehydrated in ethanol series, treated with xylene, and embedded in paraffin. Paraffin blocks were serially sectioned at 5 µm on a Leica SM 2000R microtome (Leica, Nussloch, Germany); sections were dewaxed, and gills and livers were stained with haematoxylin and eosin (HE). Micrographs were taken using a Leica DM LB (Leica, Mannheim, Germany) microscope equipped with a Leica DFC 295 camera (Leica, Mannheim, Germany).
For the description of histopathological lesions and exposure assessments, Bernet’s method was used [28]. According to Bernet et al. [28], histopathological (HP) changes are classified into five reaction patterns: circulatory, regressive, progressive, inflammatory, and neoplastic. An importance factor (IF) ranging from 1 (minimal alteration) to 3 (marked importance) was assigned to each alteration; the relevance of a lesion and its pathological importance and the value for each alteration are presented in Table 1. A score value ranging from 0 (unchanged) to 6 (severe occurrence) was specified depending on the degree and extent of lesions. By multiplying the importance factor and score value, an organ index of each organ was determined. For the gills, score values were obtained by calculating the percentage of primary lamellae containing lesions (Score = N (primary lamellae with lesion) × N (primary lamellae)−1 × 6). The following formulas were used [28]:

Table 1.
List of tissue alterations in gills and liver found in the study and respective importance factors.
(a) For the histopathological index of the organ:
(b) For the sum of the indices of both organs assessed results in a total index for the individual fish:
where org represents organ (IL, liver; IG, gills), rp represents reaction pattern, alt represents alteration, a is the score value, and w is the importance factor.
2.8. Nuclear Abnormalities of Erythrocytes
A drop of blood was placed onto a glass slide and blood smears were prepared, fixed with methanol, air-dried, and later stained with May–Grünwald–Giemsa. One hundred micrographs were collected from each glass slide using the same microscope setup as in the histopathological analyses, and were then analysed at 1000× magnification. Four different types of nuclear alterations of erythrocytes were quantified: (1) micronucleus; (2) nuclear buds; (3) fragmented nuclei; and (4) bi-nucleated cells. For each nuclear alteration, approximately 4000 erythrocytes were counted (40 per micrograph); the results are presented as N·1000−1 erythrocytes for each blood smear. The definitions of nuclear abnormalities were as follows: (1) micronucleus, represented by an ovoid body clearly separated from the nucleus with a size 10–33% of that of the nucleus, with the same (or slightly lighter) colour and positioned in the same plane as the nucleus; (2) nuclear bud, resembling the micronucleus in appearance, but remaining attached to the nucleus; (3) cells containing fragmented nuclei, characterized by pale cytoplasm and the presence of numerous vacuoles; (4) bi-nucleated cells possessing two nuclei with intact nuclear envelopes which are similar in size and sometimes connected with a nucleoplasmic bridge [29]. Only alterations that were indubitably distinguishable from normal cells and had intact cell membranes were counted.
2.9. Determination of S-MET Concentrations in Water and Common Carp Muscles
2.9.1. Chemicals and Apparatus
In water samples, S-MET was determined by HPLC analysis on the Agilent 1200 HPLC system (Agilent Technologies, Waldron, Germany) with a degasser, a binary pump, an autosampler, and a DAD detector (Agilent Technologies, Santa Clara, CA, USA). Chromatographic separation was performed on the Zorbax XDB C18 analytical column of 150 × 4.6 mm and 1.8 µm particle size (Agilent Technologies, Santa Clara, CA, USA). The LC flow was maintained at 1 mL·min−1, with an injection volume of 5 μL. The mobile phase was (0.1% HCOOH) H2O (pH 2.8):AcCN = 20:80. The DAD wavelength was 210 nm. Water samples were filtered through syringe filters (RCC; 0.45 µm) into autosampler vials (2 mL) vials and analysed.
For the determination of S-MET in common carp muscles, LC-MS/MS analysis was performed on the Agilent 1200 HPLC system (Agilent Technologies, Waldron, Germany) with a degasser, a binary pump, and an autosampler connected to the Agilent TripleQuad mass spectrometer (Agilent Technologies, Santa Clara, CA, USA). Chromatographic separation was performed on a Zorbax XDB C18 analytical column of 50 × 4.6 mm and 1.8 µm particle size (Agilent Technologies, Santa Clara, CA, USA). The LC flow was maintained at 0.4 mL·min−1; the injection volume was 2 µL. The mobile phase gradient program started at 5% of B (methanol with 0.1% formic acid), was held for 2 min, decreased to 70% at 15 min, then to 98% at 23 min, and held for 2 min. The mass spectrometer was operated in the positive ESI mode with MRM at unit resolution. Two precursor-to-product ion transitions were simultaneously monitored: m/z 284.14/252.1 (Frag.100 V, CE 12 V) and m/z 284.14/176.1 (Frag.100 V, CE 26 V). The data were processed in MassHunter software (version B.06.00 Agilent Technologies).
2.9.2. Sample Preparation for LC-MS/MS Analyses
The fish samples were homogenized by grinding them into fine pasta in a porcelain lab mortar. Then, 2 g was weighed in a 50 mL centrifuge tube, 8 mL of water was added, and the mixture was vortexed for 1 min. Internal standard (carbofurane–D3) spiking solution (10 µg·mL−1) was added to all the samples to yield a concentration of 0.050 µg·mL−1. The samples were extracted using 10 mL of 1% glacial acetic acid in acetonitrile as an extraction solvent, followed by the addition of QuEChERS citrate buffer salts (BondElute, P/N 5982-5650). The tube was shaken for 2 min on a vortex and centrifuged at 5000 RPM for 5 min. An aliquot (1 mL) of the supernatant was cleaned by dSPE (BondElute, P/N 5982-5121), and 0.5 mL of the extract was collected and dried under a stream of nitrogen. The dried residue was then dissolved in 0.5 mL mixture of water–methanol (50:50, v/v) and filtered through a syringe (0.45 µm) before LC-MS/MS analysis was performed.
2.10. Bioconcentration Factor
The bioconcentration factor (BCF) was calculated at the end of the exposure using the following formula:
where CM (ng·g−1) and CW (µg·L−1) are the chemical concentrations in common carp muscles (wet weight) and exposure solution, respectively. In previous studies, it was determined that S-MET reached a steady-state concentration within approximately 7 days in zebrafish and bluegill sunfish (Lepomis macrochirus) [19,30]; thus, the abovementioned formula could be used [31].
2.11. Statistical Analyses
Comparisons of the HP scores, nuclear alterations of erythrocytes, and concentrations of S-MET in muscles among the groups were performed using the non-parametric Kruskal–Wallis test, because the variables did not meet the normality (Kolmogorov–Smirnov test) and homoscedasticity of variance (Levene’s test) assumptions. Comparisons of body mass, length, and condition factor, as well as blood biochemistry data, were performed using one-way ANOVA, followed by a Tukey’s post hoc test. Data are presented as the mean ± standard deviation, and the statistical evaluations were performed using Statistica 12.0 software (Statsoft, Tulsa, OK, USA). The significance level was set at the standard confidence level of 95%.
3. Results
3.1. Acute Toxicity Test
During acute toxicity tests, fish subjected to the highest concentrations started to alter their behaviour after merely couple of hours. Five hours after the start of exposure, half of the fish from A5 exhibited ataxia and lethargy; they were swimming on their side, very slowly, at the bottom of the aquarium. After the first 24 h of the experiment, all fish in A5 exhibited akinesia, which continued until their death. Half of the fish from A4 showed lethargy after 24 h, while after 48 h, all fish were lethargic, with 2–5 out of 10 swimming or lying on their side. No apparent changes in fish behaviour were observed in A0–A3 during the experimental period. Fish survival during the acute toxicity test is shown in Figure 1. The first mortality in A4 and A5 was observed after 72 h; after 96 h, not a single fish remained alive in the highest concentration, while in A4, one fish was dead. The estimated acute S-MET toxicity (96 h LC50) to common carp was determined to be 16.31 mg·L−1.
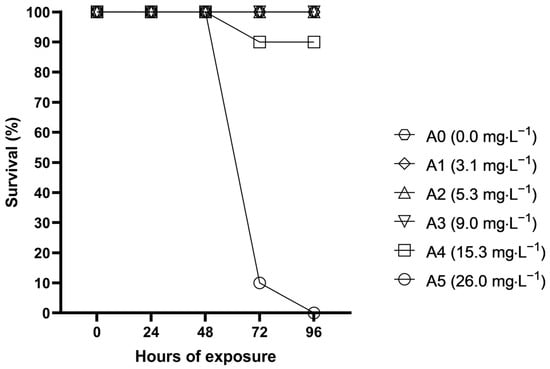
Figure 1.
Fish survival during 96 h of acute toxicity exposure of S-metolachlor to common carp.
3.2. Subchronic Toxicity Test
No mortality of fish occurred during the 28-day chronic toxicity test. No altered fish behaviour was noticed in any of the groups. Fish from all groups gained body mass compared with the start of the experiment, but no significant difference or trend was observed between them (Table 2). However, a trend was observed in fish length, where it seems that values were enhanced with increased concentrations. This affected the condition coefficient of fish, with the reverse trend present in that parameter. Compared with the start of the experiment, only fish from the control group exhibited improved CF values.

Table 2.
Weights, lengths, and condition factors of fish exposed to S-metolachlor in the subchronic test after 28 days (mean ± SD; C0—control; C1—0.5 mg·L−1; C2—1.4 mg·L−1; C3—4.1 mg·L−1).
Blood biochemistry parameters are presented in Table 3. No significant difference among groups was found in any of blood biochemistry parameters investigated in this study.

Table 3.
Blood biochemistry parameters of fish exposed to S-metolachlor in subchronic exposure after 28 days (mean ± SD; C0—control; C1—0.5 mg·L−1; C2—1.4 mg·L−1; C3—4.1 mg·L−1).
Histopathological changes in experimental animals were mild (Table 4 and Figure 2). Highest HP scores in all groups were recorded for hyperaemia and oedema of secondary epithelium (Figure 2a). The scores for secondary epithelium oedema were significantly higher in the C3 group compared with C1 and C2 (p < 0.05). The presence of eosinophilic granular cells (EGC Figure 2b) and the hyperplasia of epithelial cells (Figure 2b) showed higher scores in groups C2 and C3; however, neither of these alterations were statistically significant. A higher number and intensity of HP alterations was observed in the gills compared with the liver. The dominant HP alteration in the liver was leukocyte infiltration (Figure 2d). This change resulted in the highest HP scores, with group C3 significantly higher than C0 (p < 0.01). Necrosis, as an alteration with the highest negative significance in histopathology, was rare and focal in both the gills (Figure 2c) and the liver (Figure 2e), and was not observed in any control group. In the liver, leukocyte infiltration and necrosis usually appeared in portal and periportal areas (Figure 2d,f). Hepatocytes in the vicinity of large blood vessels appeared necrotic, while nuclei of those hepatocytes were weakly stained with haematoxylin; some could not be seen at all (Figure 2e). Concerning organ HP indices, no significance was found in IG; however, in IL, C2 was significantly higher than C0; and in terms of the total organ index, group C3 was significantly higher (p < 0.05) compared with the control group.

Table 4.
The frequency of histopathological alterations found in the subchronic exposure trial (mean ± SD; C0—control; C1—0.5 mg·L−1; C2—1.4 mg·L−1; C3—4.1 mg·L−1).
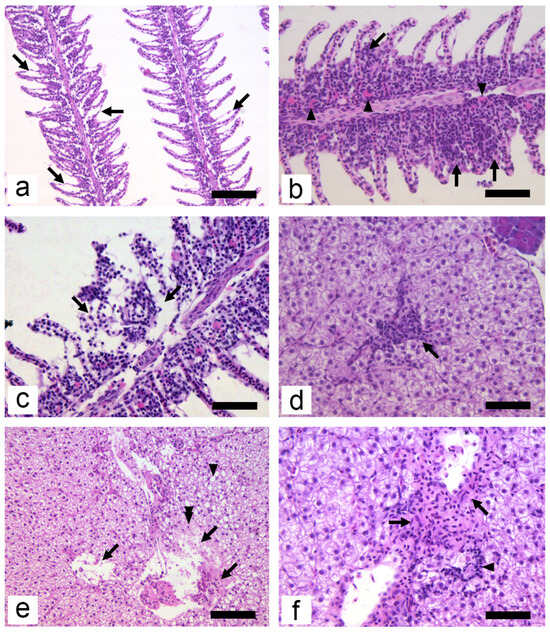
Figure 2.
Histopathological alterations of gills (a–c) and liver (d–f) in the common carp at the end of the subchronic exposure period: (a) oedema of secondary epithelium (arrows) (HE, 200×, bar = 100 µm); (b) focal hyperplasia of interlamellar cell mass (arrows) and increased presence of EGC (arrowheads) (HE, 400×, bar = 50 µm); (c) focal necrosis of secondary lamellae and primary epithelium (arrows) (HE, 400×, bar = 50 µm); (d) infiltration of leucocytes into liver parenchyma (HE, 400×, bar = 50 µm); (e) pyknosis of hepatocyte (double arrowhead) and necrosis of liver parenchyma and portal areas (arrows). Note the presence of vacuolated hepatocytes (arrowhead) in the micrograph (HE, 200×, bar = 100 µm); (f) fibrosis of portal areas (arrows) and infiltration of leucocytes (arrowhead) (HE, 400×, bar = 50 µm).
The accumulation of pesticide in common carp muscles was determined at the end of the exposure and, as expected, values increased with the concentration of exposure solution (Figure 3a). Groups C2 and C3 were significantly higher compared with C0 and C1 groups. The BCF results in fish muscles are presented in Figure 4b for each experimental group.
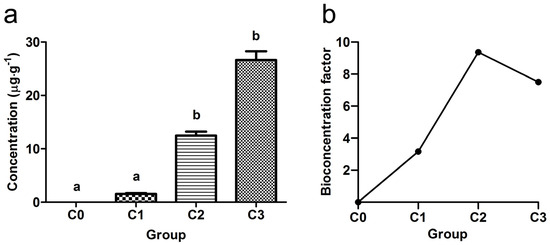
Figure 3.
(a) Concentrations of S-metolachlor determined in the muscles of experimental fish at the end of the trial; (b) bioconcentration factor in muscles at the end of the experimental period (mean ± SD; C0—control; C1—0.5 mg·L−1; C2—1.4 mg·L−1; C3—4.1 mg·L−1). Different letters denote significant differences among groups (Kruskal–Wallis test, followed by Mann–Whitney U test p < 0.05).
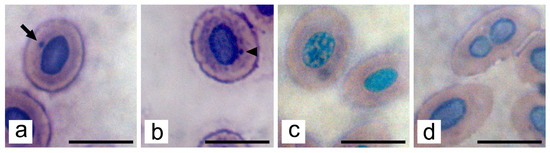
Figure 4.
Illustration of nuclear alterations in erythrocytes from common carp after 28 days exposure trial to S-metolachlor: (a) micronucleus (arrow); (b) nuclear bud (arrowhead); (c) fragmented nucleus; (d) bi-nucleated cell; Grünwald–Giemsa staining; bar = 10 µm.
Four nuclear alterations were observed in studied erythrocytes (Figure 4): micronucleus, nuclear bud, fragmented nucleus, and bi-nucleated erythrocytes. The frequencies of their appearance are presented in Table 5. No statistical significance among experimental groups was established.

Table 5.
Frequency (‰) of nuclear alterations of erythrocytes in peripheral blood of fish exposed to S-metolachlor in subchronic test after 28 days (mean ± SD; C0—control; C1—0.5 mg·L−1; C2—1.4 mg·L−1; C3—4.1 mg·L−1).
4. Discussion
Acute exposure tests of the common carp revealed high LC50 values (16.31 mg·L−1), which pose a low risk to this fish species, due to the current low environmental concentrations of S-MET. However, in comparison to other aquatic animals, common carp showed higher sensitivity to S-MET: LC50 values for clams Scrobicularia plana [32], embryos of zebrafish [20], or the Perez’s frog (Pelophylax perezi) [33] were all higher compared with the common carp LC50 values observed in this study. As previously mentioned, there is a knowledge gap regarding LC50 values in fish, at least in the scientific literature. Some 96 h LC50 values available via Canadian Council of Ministers of the Environment were reviewed and presented by Anderson et al. [34]; these ranged from 3.9 to 45.2 mg·L−1, depending on fish species, which is in line with the results of the present study. From the same source, the No Observed Effect Concentration (NOEC) for fish was reported at a concentration of 30 µg·L−1 [34].
The purpose of the subchronic exposure test was to evaluate the status of biomarkers after exposure, as well as to explore the degree of accumulation, based on the pesticide concentrations in the muscles. Both blood biochemistry parameters and histopathology are routinely used in fish toxicological assays. These biomarkers are non-specific in their responses towards chemical stressors, but they provide very useful information about general fish health status [35]. Exposing common carp to pesticides affects liver enzyme activity, i.e., increases ALT, AST, LDH, ACP, and ALP [36,37,38,39,40]. Higher levels of ALT and AST in fish blood are usually linked to hepatocellular injuries and are used as an important marker of liver status. In the same vein, LDH, ALP, and ACP are used as general markers of tissue damage [41]. As previously identified, published data are scarce in terms of studies of the exposure of aquatic animals to S-MET. The exposure of marbled crayfish (Procambarus virginalis) to 42 μg·L−1 of S-MET in chronic test (28 days) increased the concentrations of ALT and AST in haemolymphs [42]. In another study, African catfish (Clarias gariepinus) were exposed in a subchronic test (15 days) to 470 μg·L−1 pesticide containing two active substances (S-MET and atrazine), resulting in increased concentrations of ALT and AST in liver tissue [43]. A significantly higher concentration of 13 mg·L−1 was used when common carp were exposed in an acute (96 h) test to another pesticide with two active ingredients (terbuthylazine and S-metolachlor); AST and LDH concentrations were increased compared with the control group [23]. However, in the present study, no significant differences in blood biochemical parameters were present among the exposed groups. The majority of screened blood biochemistry parameters are markers of cellular necrosis; therefore, it could be concluded that a low degree of necrosis was present in experimental fish. This was confirmed by the histological results presented below, where only focal necroses were observed in the gills and the liver.
Common carp HP responses to pesticides in subchronic and chronic assays vary according to the concentration and type of pesticide. When common carp were exposed to simazine, terbutryn, alachlor, or prometryne in subchronic and chronic tests, apart from mild steatosis in the liver, no effects on the gills and liver were observed [44,45,46,47]. On the other hand, similar HP changes to those found in the present study were reported in experiments in which common carp were exposed to glyphosate [48], trifluralin [38], fipronil, buprofezin [49] atrazine, chlorpyrifos, and cypermethrin [50,51,52] in subchronic and subacute tests. In the present study, more alterations, as well as higher-intensity scores, were present in the gills compared with the liver, which was in line with the fact that gills are in direct contact with contaminants in aquatic environments [53]. Therefore, gills represent the most sensitive fish organ regarding tissue responses to xenobiotics [54] or changes in water quality parameters [55,56]. Two tissue alterations that contributed the most to the respective organ indices were hyperplasia of the epithelium in the gills and leukocyte infiltration in the liver. Epithelial hyperplasia is one of the basic defensive mechanisms in fish response to xenobiotics [57], and its quantification sometimes occurs in a dose-dependent manner according to increasing experimental concentrations [58,59], similar to the present study. Leukocyte infiltration in the liver is a frequent lesion reported in fish (including common carp) exposed to pesticides and represents a non-specific cellular response to xenobiotics [60,61,62].
Oedema of secondary epithelium and hyperaemia were also present in the gills of fish in the control group, even though fish were kept in well-aerated tap water. A probable reason for this phenomenon is the time needed for blood sampling of the fish, when these lesions could form [63,64]. Another factor could be the use of clove oil as an anaesthetic, which results in the appearance of the capillary ectasia of gill filaments in common carp [65]. Total HP index values showed that the C3 group had a higher incidence of HP lesions compared with controls, indicating the adverse effect of S-MET. This clearly supports the assessment of multiple organs in fish toxicological assays, because low levels of HP lesions in a single organ do not always provide a complete overview of changes that occur in different organ systems [28]; however, this approach was recently questioned by Wolf [66]. The absence of severe necrotic changes in both the gills and the liver, as well as low levels of other lesions, confirmed the mild response to tissue architecture of those two organs.
The numbers of micronuclei in healthy fish differed not only among species, but also among individuals of the same species [67]. The frequency of micronuclei in common carp from control groups in various experimental setups showed large variations: 0.01–0.02‰ [68]; 0.17‰ [69]; 0.02–0.06‰ [70]; 0.52‰ [71]; 0.03–0.98‰ [72]; and 6.2‰ [73]. Numbers of micronuclei observed in this experiment ranged from 0‰ to 0.23‰, and thus, can partially or fully fit into the control results presented by the aforementioned authors. This reveals that S-MET exerted no significant genotoxic effect on common carp erythrocytes in the present study.
Concentrations of S-MET in the muscles of experimental fish after the exposure period of 28 days were high, especially compared with the maximum allowable concentration of S-MET in meat in the European Union—0.01 µg·g−1 [74]. There are no available published data of S-MET concentrations in aquatic animals in environmental studies for comparison. Ou-Yang et al. [14] exposed zebrafish to 300 µg·L−1 of S-MET for 28 days to study the bioaccumulation and metabolism of herbicides; concentrations in the whole bodies of zebrafish at the end of exposure were 0.8 µg·g−1. This is comparable to the results obtained in the present study (1.5 µg·g−1), taking into consideration that the exposed concentration was 500 µg·L−1. The BCF in the same study was 2.18, but comparison among different species is difficult, because the value depends on various factors [75]. Exposure tests of different pesticides in common carp muscle showed variations in BCF from 2.4 to 26 [76], which represents a low bioaccumulation potential for this species [75]. When exposure was ended and if fish were subjected to depuration period, the concentrations of S-MET rapidly decreased [19].
In conclusion, the results of the present study indicate that S-MET has a low toxicity to common carp upon acute exposure. Exposure to sublethal concentrations of S-MET over a 28-day period resulted in slight histopathological changes in the gills and liver at the two highest concentrations, although the effects on fish body mass, condition factor, erythrocyte nuclear abnormalities, and blood biochemistry were not significant. The research hypothesis was therefore rejected because no dose-dependent relationship was observed. However, we must emphasise that the chemical tested was an active substance and not a pesticide formulation; pesticide formulations may have different toxicological properties compared with active ingredients. Nevertheless, the data obtained fulfil the need for assessments of potential toxicity, and contribute to existing knowledge of the effects of S-MET.
Author Contributions
Conceptualization, D.B., B.R. and V.P.; methodology, D.B., B.R., G.B., G.V. and V.P.; formal analysis, B.R. and G.B.; investigation, B.R., G.B., D.Ć.M. and B.Š.T.; resources, Z.M., G.V. and D.B.; writing—original draft preparation, B.R., D.B., G.B. and B.Š.T.; writing—review and editing, V.P., Z.M. and G.V.; visualization, B.R.; funding acquisition, Z.M. and D.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science, Technological Development and Innovation of the Republic of Serbia, grant number 451-03-47/2023-01/200116.
Institutional Review Board Statement
The experiments were performed in accordance with the ethical conditions approved by the Serbian Ministry of Agriculture, Forestry and Water Management (approval number: 323-07-12222/2023-05).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, B.R., upon reasonable request.
Acknowledgments
The authors wish to express their gratitude to Zorica Radović for preparing samples for histological analysis, and to Marko Stanković for performing blood sampling.
Conflicts of Interest
Gavrilo Božić is an employee of MDPI; however, he does not work for the journal Water at the time of submission and publication. The other authors declare no conflict of interest.
References
- Jorge, F.-C.; Nehring, R.; Osteen, C.; Wechsler, S.; Martin, A.; Vialou, A. Pesticide Use in U.S. Agriculture: 21 Selected Crops, 1960–2008; U.S. Department of Agriculture, Economic Research Service: Washington, DC, USA, 2014. [Google Scholar]
- O’Connell, P.J.; Harms, C.T.; Allen, J.R.F. Metolachlor, S-metolachlor and their role within sustainable weed-management. Crop Prot. 1998, 17, 207–212. [Google Scholar] [CrossRef]
- Eurostat. The Use of Plant Protection Products in the European Union (Data 1992–2003); European Communities: Luxembourg, 2007; p. 222. [Google Scholar]
- Fenner, K.; Canonica, S.; Wackett, L.P.; Elsner, M. Evaluating Pesticide Degradation in the Environment: Blind Spots and Emerging Opportunities. Science 2013, 341, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Vallotton, N.; Moser, D.; Eggen, R.I.; Junghans, M.; Chevre, N. S-metolachlor pulse exposure on the alga Scenedesmus vacuolatus: Effects during exposure and the subsequent recovery. Chemosphere 2008, 73, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Lanasa, S.; Niedzwiecki, M.; Reber, K.P.; East, A.; Sivey, J.D.; Salice, C.J. Comparative Toxicity of Herbicide Active Ingredients, Safener Additives, and Commercial Formulations to the Nontarget Alga Raphidocelis subcapitata. Environ. Toxicol. Chem. 2022, 41, 1466–1476. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Q.; Su, W.; Sun, L.; Xu, H.; Xue, F.; Lu, C.; Wu, R. The mechanism of exogenous gibberellin A3 protecting sorghum shoots from S-metolachlor Phytotoxicity. Pest Manag. Sci. 2022, 78, 4497–4506. [Google Scholar] [CrossRef]
- Götz, T.; Böger, P. The Very-Long-Chain Fatty Acid Synthase Is Inhibited by Chloroacetamides. Z. Naturforsch. C 2004, 59, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Schmalfuß, J.; Matthes, B.; Mayer, P.; Böger, P. Chloroacetamide Mode of Action, I: Inhibition of Very Long Chain Fatty Acid Synthesis in Scenedesmus acutus. Z. Naturforsch. C 1998, 53, 995–1003. [Google Scholar] [CrossRef][Green Version]
- Filimon, M.N.; Roman, D.L.; Caraba, I.V.; Isvoran, A. Assessment of the Effect of Application of the Herbicide S-Metolachlor on the Activity of Some Enzymes Found in Soil. Agriculture 2021, 11, 469. [Google Scholar] [CrossRef]
- Lunn, R.D.J.; Tocher, D.A.; Sidebottom, P.J.; Montgomery, M.G.; Keates, A.C.; Carmalt, C.J. Applying the Crystalline Sponge Method to Agrochemicals: Obtaining X-ray Structures of the Fungicide Metalaxyl-M and Herbicide S-Metolachlor. Cryst. Growth Des. 2021, 21, 3024–3036. [Google Scholar] [CrossRef]
- Marín-Benito, J.M.; Herrero-Hernández, E.; Ordax, J.M.; Sánchez-Martín, M.J.; Rodríguez-Cruz, M.S. The role of two organic amendments to modify the environmental fate of S-metolachlor in agricultural soils. Environ. Res. 2021, 195, 110871. [Google Scholar] [CrossRef]
- HRAC. Global Herbicide Classification Lookup. Available online: Hracglobal.com/tools/classification-lookup (accessed on 12 October 2023).
- Mottes, C.; Lesueur Jannoyer, M.; Le Bail, M.; Guene, M.; Carles, C.; Malezieux, E. Relationships between past and present pesticide applications and pollution at a watershed outlet: The case of a horticultural catchment in Martinique, French West Indies. Chemosphere 2017, 184, 762–773. [Google Scholar] [CrossRef] [PubMed]
- Battaglin, W.A.; Furlong, E.T.; Burkhardt, M.R.; Peter, C.J. Occurrence of sulfonylurea, sulfonamide, imidazolinone, and other herbicides in rivers, reservoirs and ground water in the Midwestern United States, 1998. Sci. Total Environ. 2000, 248, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Sarrazin, B.; Wezel, A.; Guerin, M.; Robin, J. Pesticide contamination of fish ponds in relation to crop area in a mixed farmland-pond landscape (Dombes area, France). Environ. Sci. Pollut. Res. 2022, 29, 66858–66873. [Google Scholar] [CrossRef] [PubMed]
- Brumovsky, M.; Becanova, J.; Kohoutek, J.; Borghini, M.; Nizzetto, L. Contaminants of emerging concern in the open sea waters of the Western Mediterranean. Environ. Pollut. 2017, 229, 976–983. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, L.; Chen, K.; Yang, H.; Ling, M.; Wu, L.; Zhou, X.; Ma, G.; Bai, L. Combined effects of S-metolachlor and benoxacor on embryo development in zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2022, 238, 113565. [Google Scholar] [CrossRef] [PubMed]
- Ou-Yang, K.; Feng, T.; Han, Y.; Li, G.; Li, J.; Ma, H. Bioaccumulation, metabolism and endocrine-reproductive effects of metolachlor and its S-enantiomer in adult zebrafish (Danio rerio). Sci. Total Environ. 2022, 802, 149826. [Google Scholar] [CrossRef] [PubMed]
- Quintaneiro, C.; Patricio, D.; Novais, S.C.; Soares, A.; Monteiro, M.S. Endocrine and physiological effects of linuron and S-metolachlor in zebrafish developing embryos. Sci. Total Environ. 2017, 586, 390–400. [Google Scholar] [CrossRef]
- Rozmankova, E.; Pipal, M.; Blahova, L.; Njattuvetty Chandran, N.; Morin, B.; Gonzalez, P.; Blaha, L. Environmentally relevant mixture of S-metolachlor and its two metabolites affects thyroid metabolism in zebrafish embryos. Aquat. Toxicol. 2020, 221, 105444. [Google Scholar] [CrossRef]
- Yang, L.; Ivantsova, E.; Souders, C.L., 2nd; Martyniuk, C.J. The agrochemical S-metolachlor disrupts molecular mediators and morphology of the swim bladder: Implications for locomotor activity in zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2021, 208, 111641. [Google Scholar] [CrossRef]
- Dobšíková, R.; Blahová, J.; Modrá, H.; Škorič, M.; Svobodová, Z. The effect of acute exposure to herbicide Gardoprim Plus Gold 500 SC on haematological and biochemical indicators and histopathological changes in common carp (Cyprinus carpio L.). Acta Vet. Brno 2011, 80, 359–363. [Google Scholar] [CrossRef]
- OECD. Test No. 203: Fish, Acute Toxicity Test; OECD: Paris, France, 2019. [Google Scholar]
- OECD. Test No. 215: Fish, Juvenile Growth Test; OECD: Paris, France, 2000. [Google Scholar]
- Svobodová, Z.; Lloyd, R.; Máchová, J.; Vykusová, B. Water Quality and Fish Health; Food and Agriculture Organization of the United Nations: Rome, Italy, 1993; p. 59. [Google Scholar]
- Chen, C.-Y.; Wooster, G.A.; Getchell, R.G.; Bowser, P.R.; Timmons, M.B. Blood chemistry of healthy, nephrocalcinosis-affected and ozone-treated tilapia in a recirculation system, with application of discriminant analysis. Aquaculture 2003, 218, 89–102. [Google Scholar] [CrossRef]
- Bernet, D.; Schmidt, H.; Meier, W.; Burkhardt-Holm, P.; Wahli, T. Histopathology in fish: Proposal for a protocol to assess aquatic pollution. J. Fish Dis. 1999, 22, 25–34. [Google Scholar] [CrossRef]
- Fenech, M.; Chang, W.P.; Kirsch-Volders, M.; Holland, N.; Bonassi, S.; Zeiger, E. HUMN project: Detailed description of the scoring criteria for the cytokinesis-block micronucleus assay using isolated human lymphocyte cultures. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2003, 534, 65–75. [Google Scholar] [CrossRef] [PubMed]
- RAC. Proposing Harmonised Classification and Labelling at EU Level of S-Metolachlor; RAC: Walsall, UK, 2022; p. 34. [Google Scholar]
- Arnot, J.A.; Gobas, F.A.P.C. A review of bioconcentration factor (BCF) and bioaccumulation factor (BAF) assessments for organic chemicals in aquatic organisms. Environ. Rev. 2006, 14, 257–297. [Google Scholar] [CrossRef]
- Gutiérrez, I.B.; Mesquita, A.F.; Gonçalves, F.J.M.; Marques, J.C.; Gonçalves, A.M.M. Biomarkers’ responses of the benthic clam Scrobicularia plana to the main active ingredients (S-metolachlor and Terbuthylazine) of a common herbicide. Ecol. Indic. 2019, 96, 611–619. [Google Scholar] [CrossRef]
- Quintaneiro, C.; Soares, A.; Monteiro, M.S. Effects of the herbicides linuron and S-metolachlor on Perez’s frog embryos. Chemosphere 2018, 194, 595–601. [Google Scholar] [CrossRef]
- Anderson, J.C.; Marteinson, S.C.; Prosser, R.S. Prioritization of Pesticides for Assessment of Risk to Aquatic Ecosystems in Canada and Identification of Knowledge Gaps. In Reviews of Environmental Contamination and Toxicology; de Voogt, P., Ed.; Springer International Publishing: Cham, Switzerland, 2021; Volume 259, pp. 171–231. [Google Scholar]
- van der Oost, R.; Beyer, J.; Vermeulen, N.P.E. Fish bioaccumulation and biomarkers in environmental risk assessment: A review. Environ. Toxicol. Pharmacol. 2003, 13, 57–149. [Google Scholar] [CrossRef]
- Banaee, M.; Mirvagefei, A.R.; Rafei, G.R.; Majazi Amiri, B. Effect of sub-lethal diazinon concentrations on blood plasma biochemistry. Int. J. Environ. Res. 2008, 2, 189–198. [Google Scholar]
- Neskovic, N.K.; Elezovic, I.; Karan, V.; Poleksic, V.; Budimir, M. Acute and subacute toxicity of atrazine to carp (Cyprinus carpio L.). Ecotoxicol. Environ. Saf. 1993, 25, 173–182. [Google Scholar] [CrossRef]
- Poleksić, V.; Karan, V. Effects of trifluralin on carp: Biochemical and histological evaluation. Ecotoxicol. Environ. Saf. 1999, 43, 213–221. [Google Scholar] [CrossRef]
- Velisek, J.; Stara, A.; Zuskova, E.; Svobodova, Z. Use of biometric, hematologic, and plasma biochemical variables, and histopathology to assess the chronic effects of the herbicide prometryn on Common Carp. Vet. Clin. Pathol. 2013, 42, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Velisek, J.; Sudova, E.; Machova, J.; Svobodova, Z. Effects of sub-chronic exposure to terbutryn in common carp (Cyprinus carpio L.). Ecotoxicol. Environ. Saf. 2010, 73, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Banaee, M.; Sureda, A.; Mirvaghefi, A.R.; Ahmadi, K. Effects of diazinon on biochemical parameters of blood in rainbow trout (Oncorhynchus mykiss). Pestic. Biochem. Physiol. 2011, 99, 1–6. [Google Scholar] [CrossRef]
- Stara, A.; Kubec, J.; Zuskova, E.; Buric, M.; Faggio, C.; Kouba, A.; Velisek, J. Effects of S-metolachlor and its degradation product metolachlor OA on marbled crayfish (Procambarus virginalis). Chemosphere 2019, 224, 616–625. [Google Scholar] [CrossRef] [PubMed]
- Nwani, C.D.; Ifo, C.T.; Nwamba, H.O.; Ejere, V.C.; Onyishi, G.C.; Oluah, S.N.; Ikwuagwu, O.E.; Odo, G.E. Oxidative stress and biochemical responses in the tissues of African catfish Clarias gariepinus juvenile following exposure to primextra herbicide. Drug Chem. Toxicol. 2015, 38, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Mikula, P.; Modra, H.; Nemethova, D.; Groch, L.; Svobodova, Z. Effects of subchronic exposure to LASSO MTX® (Alachlor 42% W/V) on hematological indices and histology of the common carp, Cyprinus carpio L. Bull. Environ. Contam. Toxicol. 2008, 81, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Velisek, J.; Stara, A.; Koutnik, D.; Machova, J. Effects of prometryne on early life stages of common carp (Cyprinus carpio L.). Pestic. Biochem. Physiol. 2015, 118, 58–63. [Google Scholar] [CrossRef]
- Velisek, J.; Stara, A.; Machova, J.; Dvorak, P.; Zuskova, E.; Prokes, M.; Svobodova, Z. Effect of terbutryn at environmental concentrations on early life stages of common carp (Cyprinus carpio L.). Pestic. Biochem. Physiol. 2012, 102, 102–108. [Google Scholar] [CrossRef]
- Velisek, J.; Stara, A.; Machova, J.; Svobodova, Z. Effects of long-term exposure to simazine in real concentrations on common carp (Cyprinus carpio L.). Ecotoxicol. Environ. Saf. 2012, 76, 79–86. [Google Scholar] [CrossRef]
- Nešković, N.K.; Poleksić, V.; Elezović, I.; Karan, V.; Budimir, M. Biochemical and histopathological effects of glyphosate on carp, Cyprinus carpio L. Bull. Environ. Contam. Toxicol. 1996, 56, 295–302. [Google Scholar] [CrossRef]
- Qureshi, I.Z.; Bibi, A.; Shahid, S.; Ghazanfar, M. Exposure to sub-acute doses of fipronil and buprofezin in combination or alone induces biochemical, hematological, histopathological and genotoxic damage in common carp (Cyprinus carpio L.). Aquat. Toxicol. 2016, 179, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Kokushi, E.; Koyama, J.; Uno, S.; Ghosh, A.R. Histopathological alterations in gill, liver and kidney of common carp exposed to chlorpyrifos. J. Environ. Sci. Health B 2012, 47, 180–195. [Google Scholar] [CrossRef]
- Xing, H.; Li, S.; Wang, Z.; Gao, X.; Xu, S.; Wang, X. Oxidative stress response and histopathological changes due to atrazine and chlorpyrifos exposure in common carp. Pestic. Biochem. Physiol. 2012, 103, 74–80. [Google Scholar] [CrossRef]
- Yancheva, V.; Georgieva, E.; Velcheva, I.; Iliev, I.; Stoyanova, S.; Vasileva, T.; Bivolarski, V.; Todorova-Bambaldokova, D.; Zulkipli, N.; Antal, L.; et al. Assessment of the exposure of two pesticides on common carp (Cyprinus carpio Linnaeus, 1758): Are the prolonged biomarker responses adaptive or destructive? Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2022, 261, 109446. [Google Scholar] [CrossRef] [PubMed]
- Au, D.W.T. The application of histo-cytopathological biomarkers in marine pollution monitoring: A review. Mar. Pollut. Bull. 2004, 48, 817–834. [Google Scholar] [CrossRef] [PubMed]
- Bernet, D.; Schmidt-Posthaus, H.; Wahli, T.; Burkhardt-Holm, P. Evaluation of two monitoring approaches to assess effects of waste water disposal on histological alterations in fish. Hydrobiologia 2004, 524, 53–66. [Google Scholar] [CrossRef]
- Nikolić, D.; Skorić, S.; Rašković, B.; Lenhardt, M.; Krpo-Ćetković, J. Impact of reservoir properties on elemental accumulation and histopathology of European perch (Perca fluviatilis). Chemosphere 2020, 244, 125503. [Google Scholar] [CrossRef]
- Rašković, B.; Jarić, I.; Koko, V.; Spasić, M.; Dulić, Z.; Marković, Z.; Poleksić, V. Histopathological indicators: A useful fish health monitoring tool in common carp (Cyprinus carpio Linnaeus, 1758) culture. Cent. Eur. J. Biol. 2013, 8, 975–985. [Google Scholar] [CrossRef]
- Harper, C.; Wolf, J.C. Morphologic effects of the stress response in fish. ILAR J. 2009, 50, 387–396. [Google Scholar] [CrossRef]
- Monteiro, S.M.; Rocha, E.; Mancera, J.M.; Fontaínhas-Fernandes, A.; Sousa, M. A stereological study of copper toxicity in gills of Oreochromis niloticus. Ecotoxicol. Environ. Saf. 2009, 72, 213–223. [Google Scholar] [CrossRef]
- Mueller, M.E.; Sanchez, D.A.; Bergman, H.L.; McDonald, D.G.; Rhem, R.G.; Wood, C.M. Nature and time course of acclimation to aluminum in juvenile brook trout (Salvelinus fontinalis). II. Gill histology. Can. J. Fish. Aquat. Sci. 1991, 48, 2016–2027. [Google Scholar] [CrossRef]
- Destro, A.L.F.; Silva, S.B.; Gregorio, K.P.; de Oliveira, J.M.; Lozi, A.A.; Zuanon, J.A.S.; Salaro, A.L.; da Matta, S.L.P.; Goncalves, R.V.; Freitas, M.B. Effects of subchronic exposure to environmentally relevant concentrations of the herbicide atrazine in the Neotropical fish Astyanax altiparanae. Ecotoxicol. Environ. Saf. 2021, 208, 111601. [Google Scholar] [CrossRef] [PubMed]
- Ghelichpour, M.; Taheri Mirghaed, A.; Mirzargar, S.S.; Joshaghani, H.; Ebrahimzadeh Mousavi, H. Plasma proteins, hepatic enzymes, thyroid hormones and liver histopathology of Cyprinus carpio (Linnaeus, 1758) exposed to an oxadiazin pesticide, indoxacarb. Aquac. Res. 2017, 48, 5666–5676. [Google Scholar] [CrossRef]
- Jiraungkoorskula, W.; Upathama, E.S.; Kruatrachue, M.; Sahaphong, S.; Vichasri-Gramsa, S.; Pokethitiyooka, P. Histopathological effects of Roundup, a glyphosate herbicide, on Nile tilapia (Oreochromis niloticus). Sci. Asia 2002, 28, 121–127. [Google Scholar] [CrossRef]
- Speare, D.J.; Ferguson, H.W. Fixation artifacts in rainbow trout (Salmo gairdneri) gills: A morphometric evaluation. Can. J. Fish. Aquat. Sci. 1989, 46, 780–785. [Google Scholar] [CrossRef]
- Wolf, J.C.; Baumgartner, W.A.; Blazer, V.S.; Camus, A.C.; Engelhardt, J.A.; Fournie, J.W.; Frasca, S.; Groman, D.B.; Kent, M.L.; Khoo, L.H.; et al. Nonlesions, misdiagnoses, missed diagnoses, and other interpretive challenges in fish histopathology studies: A guide for investigators, authors, reviewers, and readers. Toxicol. Pathol. 2014, 43, 297–325. [Google Scholar] [CrossRef]
- Velisek, J.; Svobodova, Z.; Piackova, V.; Groch, L.; Nepejchalova, L. Effects of clove oil anaesthesia on common carp (Cyprinus carpio L.). Vet. Med. 2012, 50, 269–275. [Google Scholar] [CrossRef]
- Wolf, J.C. Comparing apples and oranges and pears and kumquats: The misuse of index systems for processing histopathology data in fish toxicological bioassays. Environ. Toxicol. Chem. 2018, 37, 1688–1695. [Google Scholar] [CrossRef]
- Bolognesi, C.; Perrone, E.; Roggieri, P.; Pampanin, D.M.; Sciutto, A. Assessment of micronuclei induction in peripheral erythrocytes of fish exposed to xenobiotics under controlled conditions. Aquat. Toxicol. 2006, 78, S93–S98. [Google Scholar] [CrossRef]
- Buschini, A.; Martino, A.; Gustavino, B.; Monfrinotti, M.; Poli, P.; Rossi, C.; Santoro, M.; Dörr, A.J.M.; Rizzoni, M. Comet assay and micronucleus test in circulating erythrocytes of Cyprinus carpio specimens exposed in situ to lake waters treated with disinfectants for potabilization. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2004, 557, 119–129. [Google Scholar] [CrossRef]
- Grisolia, C.K.; Starling, F.L.R.M. Micronuclei monitoring of fishes from Lake Paranoá, under influence of sewage treatment plant discharges. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2001, 491, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Gustavino, B.; Scornajenghi, K.A.; Minissi, S.; Ciccotti, E. Micronuclei induced in erythrocytes of Cyprinus carpio (teleostei, pisces) by X-rays and colchicine. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2001, 494, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Llorente, M.T.; Martos, A.; Castaño, A. Detection of Cytogenetic Alterations and Blood Cell Changes in Natural Populations of Carp. Ecotoxicology 2002, 11, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Nepomuceno, J.C.; Ferrari, Í.; Spanó, M.A.; Centeno, A.J. Detection of micronuclei in peripheral erythrocytes of Cyprinus carpio exposed to metallic mercury. Environ. Mol. Mutagen. 1997, 30, 293–297. [Google Scholar] [CrossRef]
- Al-Sabti, K. Clastogenic effects of five carcinogenic-mutagenic chemicals on the cells of the common carp, Cyprinus carpio L. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 1986, 85, 5–9. [Google Scholar] [CrossRef]
- EC. Commision Regulation (EU) No 1317/2013 Amending Annexes II, III and V to Regulation (EC) No 396/2005 of the European Parliament and of the Council as Regards Maximum Residue Levels for 2,4-D, Beflubutamid, Cyclanilide, Diniconazole, Florasulam, Metolachlor and S-Metolachlor, and Milbemectin in or on Certain Products. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32013R1317 (accessed on 30 November 2023).
- Wassenaar, P.N.H.; Verbruggen, E.M.J.; Cieraad, E.; Peijnenburg, W.; Vijver, M.G. Variability in fish bioconcentration factors: Influences of study design and consequences for regulation. Chemosphere 2020, 239, 124731. [Google Scholar] [CrossRef]
- Tsuda, T. Bioconcentration of Pesticides in Fish from Rivers and Lakes. In Pesticides; Stoytcheva, M., Ed.; IntechOpen: Rijeka, Croatia, 2011; pp. 333–350. ISBN 978-953-307-532-7. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).