Plant-Based Flocculants as Sustainable Conditioners for Enhanced Sewage Sludge Dewatering
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Plant-Based Flocculants
2.2. Physicochemical Characterization of Plant-Based Flocculants
2.2.1. Preparation and pH Measurement of Plant Solutions
2.2.2. Determination of Organic Matter, Ash and Mineral Composition
2.2.3. Analysis of Bioactive Sugars
2.2.4. Determination of Crude Protein Content
2.2.5. FTIR Spectra and Zeta Potential Measurement
2.3. Sewage Sludge Sampling
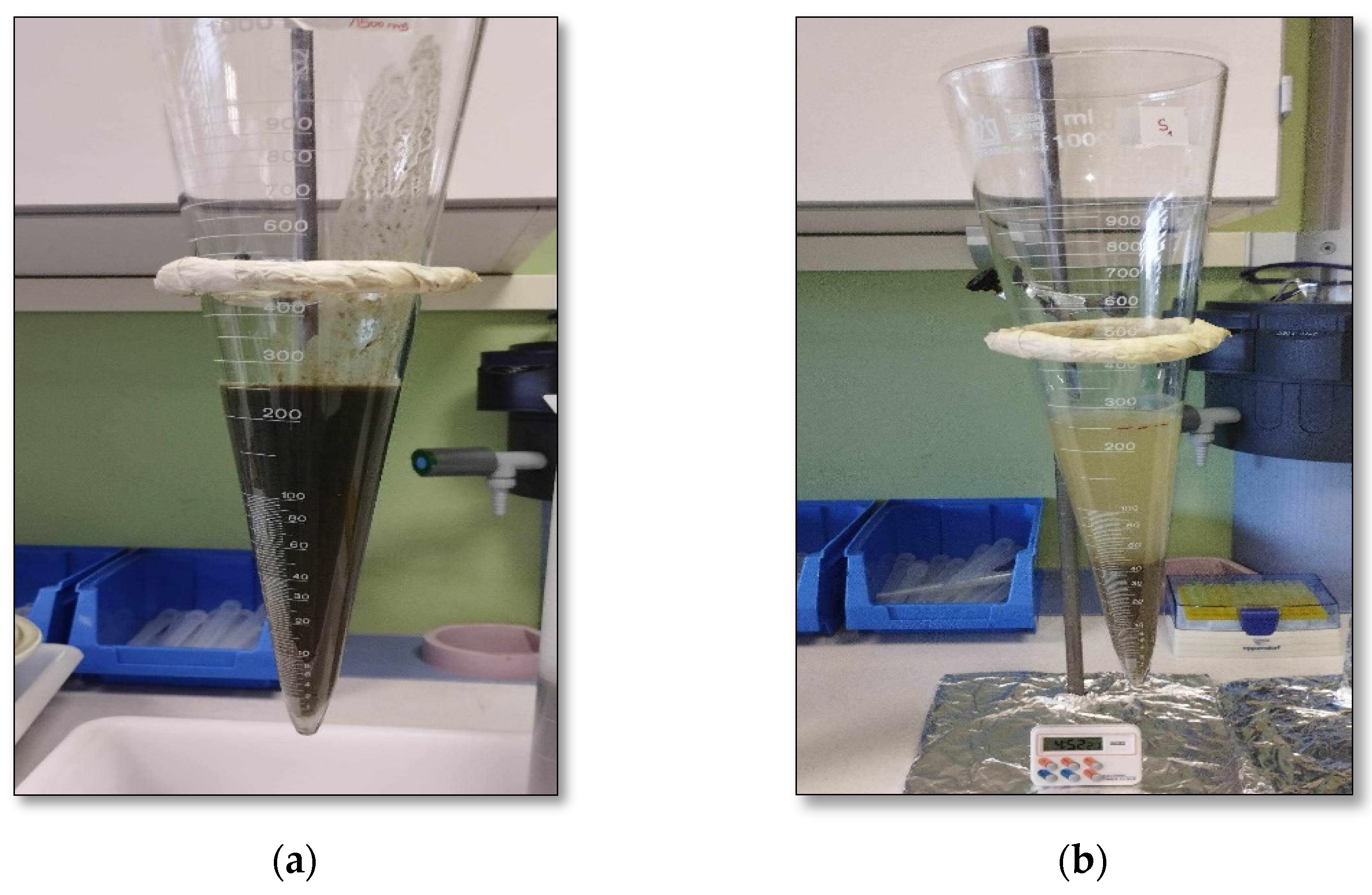
2.4. Conditioning Experiments and Sludge Dewaterability Assessments
3. Results and Discussion
3.1. Physico-Chemical Characterization of the Plant-Based Flocculants
3.1.1. Biochemical Composition of the Plant Extracts
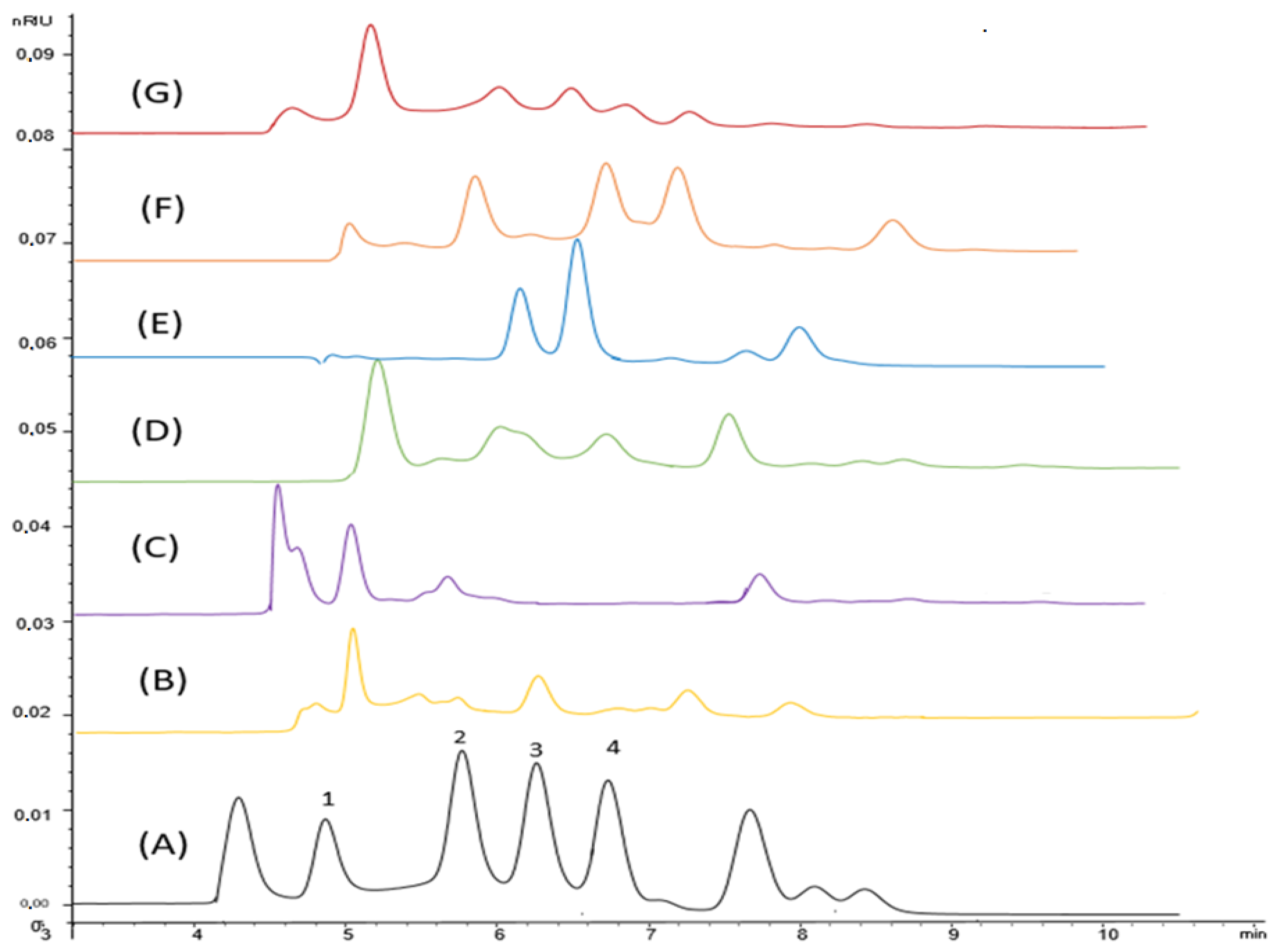
3.1.2. HPLC Analysis
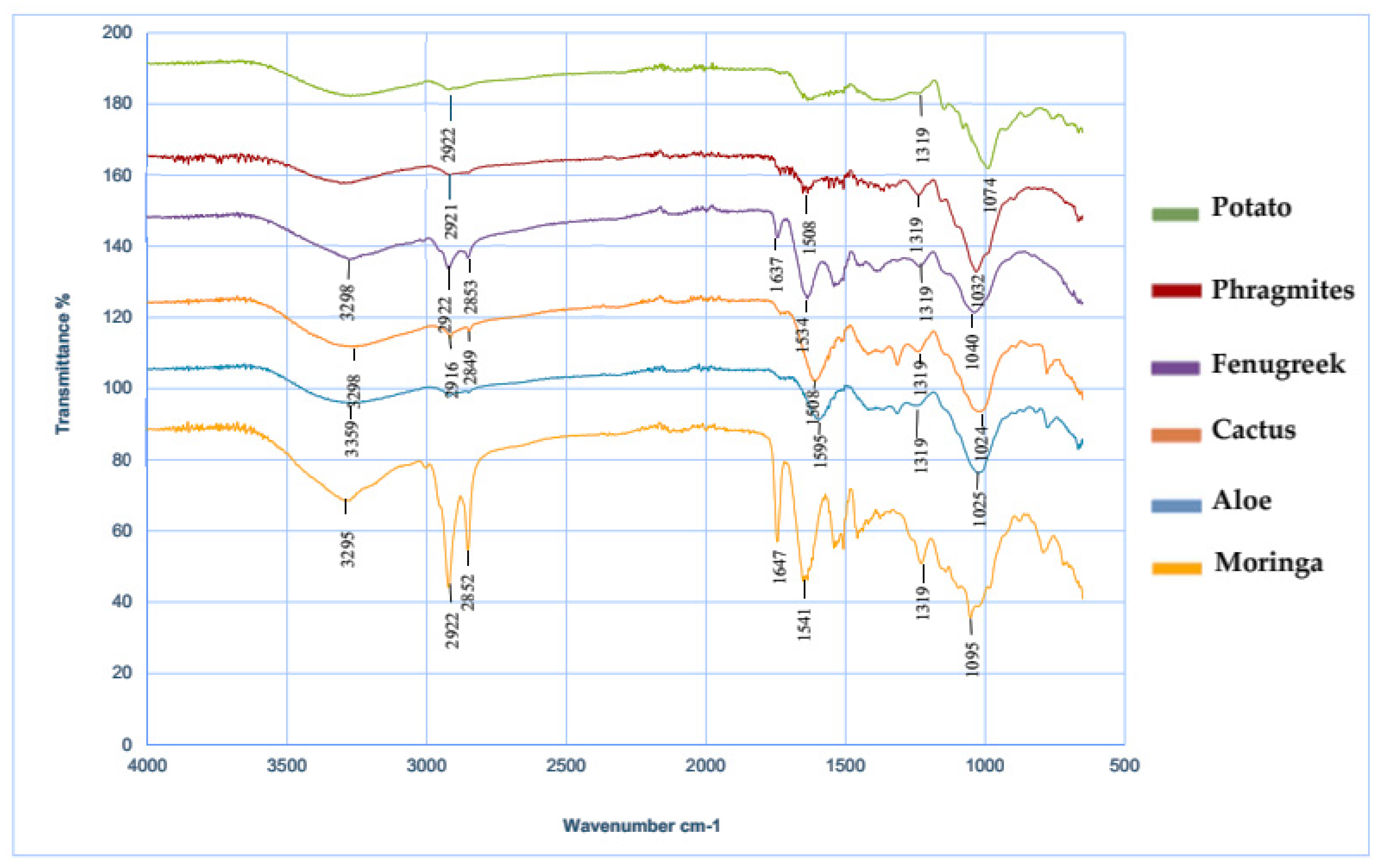
3.1.3. Fourier-Transform Infrared (FTIR) Spectroscopy
3.1.4. Zeta Potential Analysis
3.2. Raw Sewage Sludge Characterization
3.3. Evaluation of the Dewatering Performance of the Plant-Based Flocculants
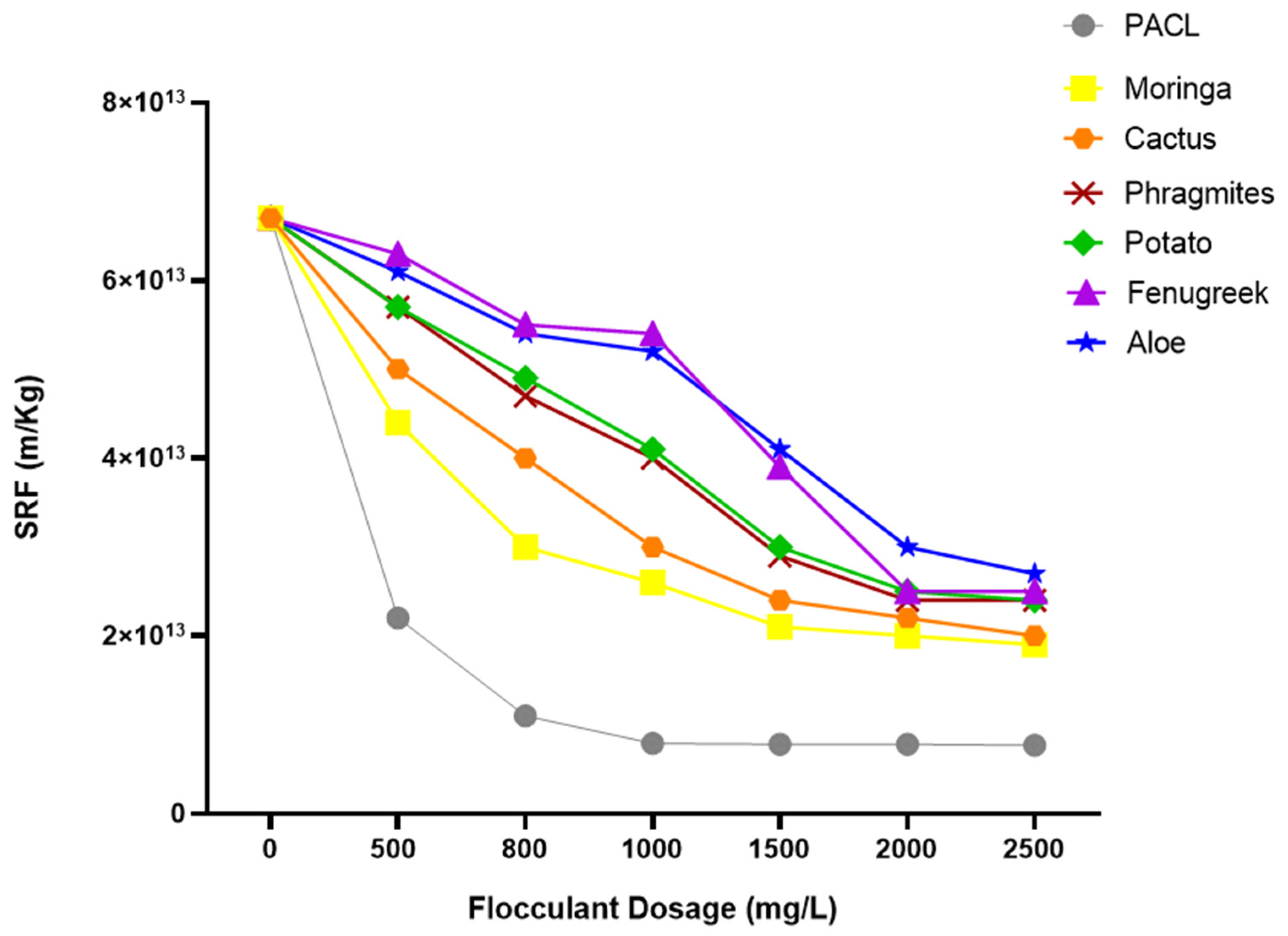
3.3.1. Effect of Sludge Conditioning on Specific Resistance to Filtration (SRF)
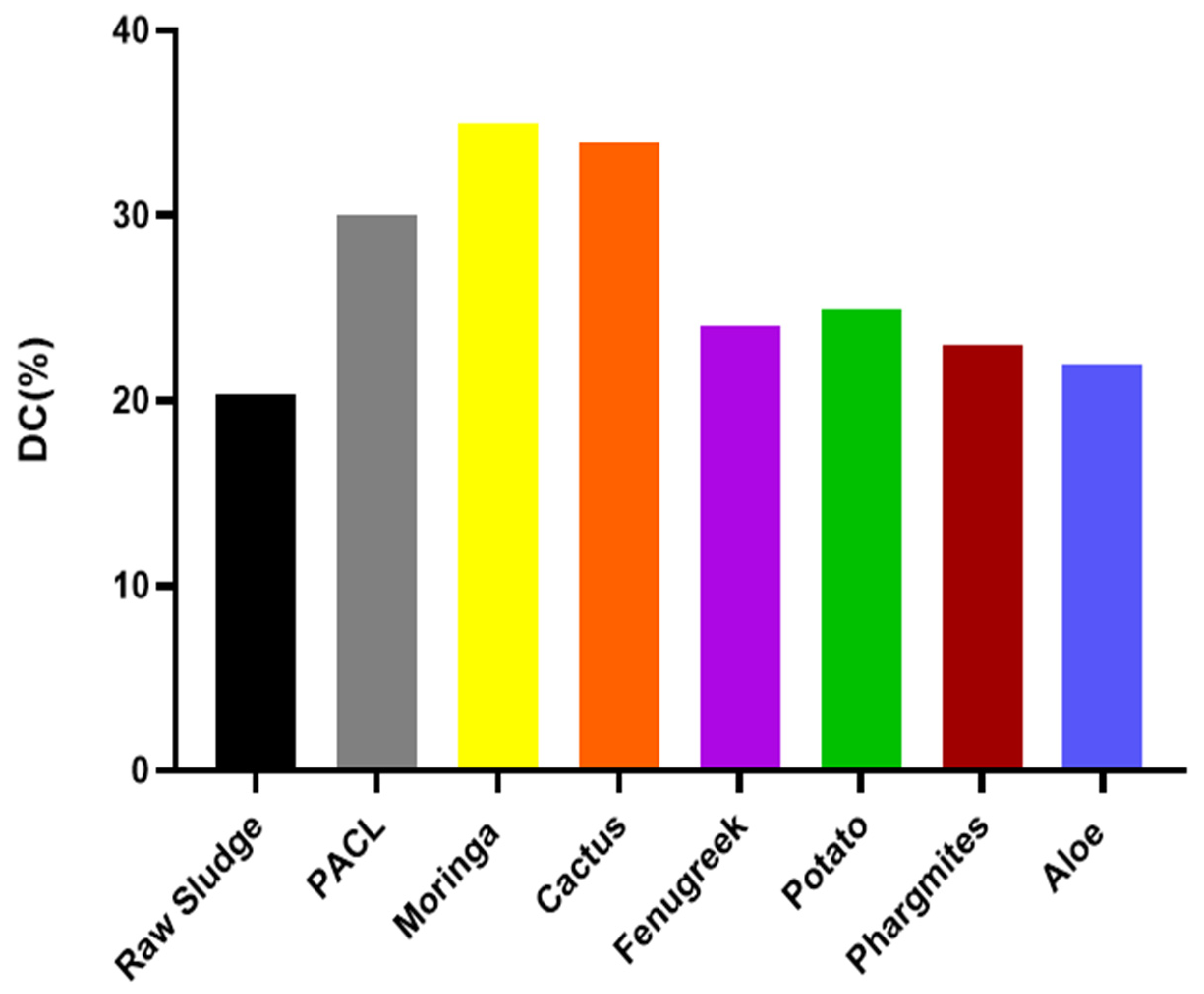
3.3.2. Variation of Dryness of Filtration Cake upon Sludge Dewatering
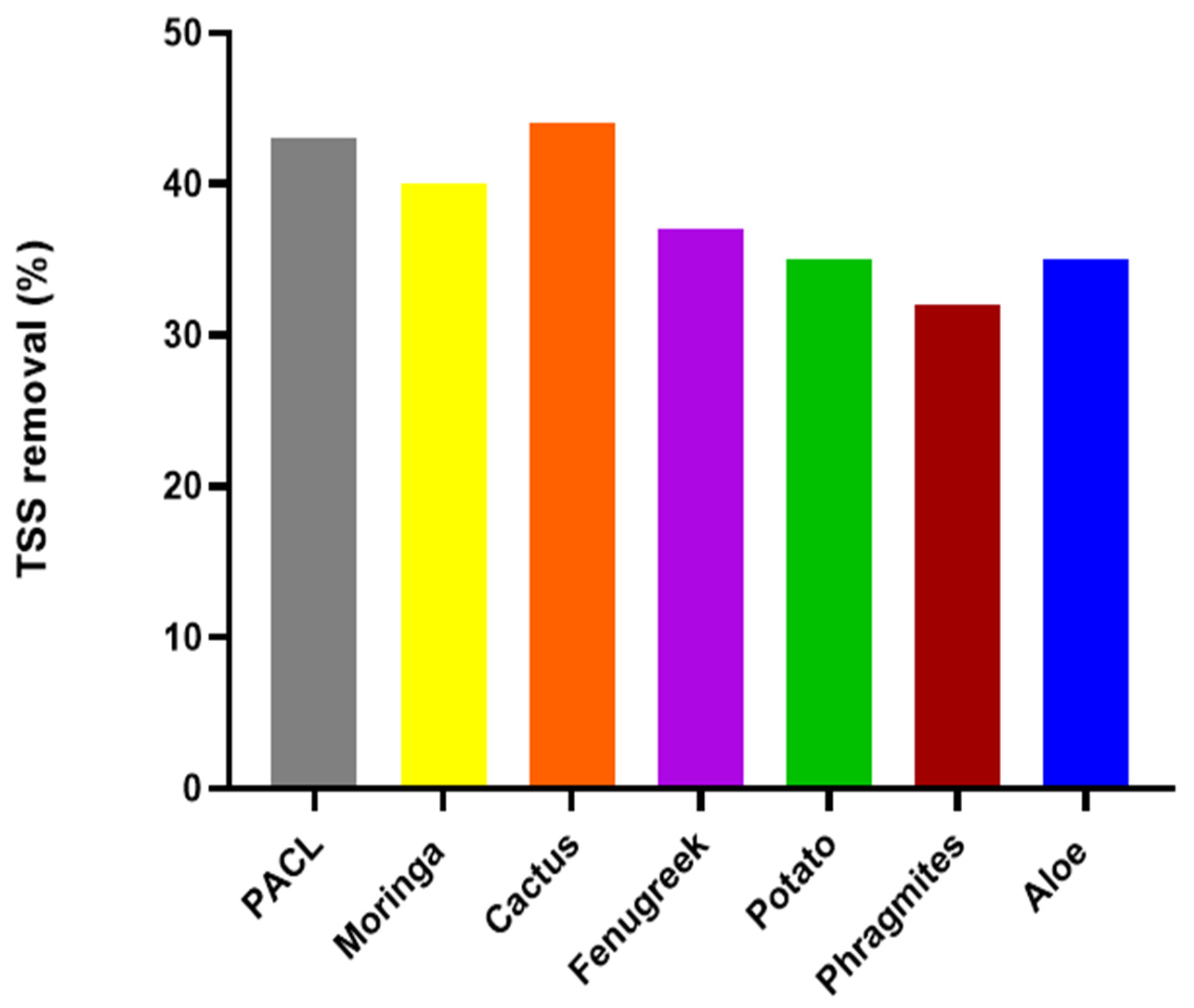
3.3.3. Variation of Total Suspended Solids Removal during Sludge Conditioning
3.4. Flocculation Mechanisms in Sludge Dewatering Using Plant-Based Flocculants
4. Conclusions
- Flocculants like Moringa and Cactus exhibited a high content of carbohydrates (mainly glucose and sucrose), along with proteins and minerals such as calcium and potassium.
- All the tested plant-based flocculants had similar FTIR spectra, showing characteristic peaks with the presence of carboxyl (–COOH), hydroxyl (–OH), and amino or amine (–NH2) functional groups.
- Compared to the synthetic chemical flocculant (PACl), Moringa and Cactus demonstrated the most significant reduction in specific resistance to filtration (1.9 × 1013 m/Kg and (2 × 1013 m/Kg, respectively) and the highest dryness of filtration cake (35% and 34%, respectively) among the tested plants. However, the other plant materials also showed notable improvements in dewatering performance.
- Based on FTIR data and chemical characterization, the flocculation mechanism of these plant-based flocculants is supposed to involve both bridging and charge neutralization.
- This investigation contributes to the protection of the environment and human health while promoting sustainable practices in sewage sludge treatment and its safe valorization.
- Perspectives and Future Works
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aleluia, J.; Ferrão, P. Assessing the costs of municipal solid waste treatment technologies in developing Asian countries. Waste Manag. 2017, 69, 592–608. [Google Scholar] [CrossRef] [PubMed]
- Bień, B.; Bień, J.D. Conditioning of Sewage Sludge with Physical, Chemical and Dual Methods to Improve Sewage Sludge Dewatering. Energies 2021, 14, 5079. [Google Scholar] [CrossRef]
- Hadj Mansour, Y.; Othmani, B.; Rebah, F.B.; Mnif, W.; Saoudi, M.; Khadhraoui, M. Could Plant-Based Flocculants Substitute the Conventional Synthetic Chemicals in the Sludge Dewatering Process? Water 2023, 15, 2602. [Google Scholar] [CrossRef]
- Shi, C.; Sun, W.; Sun, Y.; Chen, L.; Xu, Y.; Tang, M. Synthesis, Characterization, and Sludge Dewaterability Evaluation of the Chitosan-Based Flocculant CCPAD. Polymers 2019, 11, 95. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Torrellas, S.; Peres, J.A.; Gil-Álvarez, V.; Ovejero, G.; García, J. Effective adsorption of non-biodegradable pharmaceuticals from hospital wastewater with different carbon materials. Chem. Eng. J. 2017, 320, 319–329. [Google Scholar] [CrossRef]
- Egea-Corbacho Lopera, A.; Ruiz, S.G.; Alonso, J.M.Q. Removal of emerging contaminants from wastewater using reverse osmosis for its subsequent reuse: Pilot plant. J. Water Process Eng. 2019, 29, 100800. [Google Scholar] [CrossRef]
- Sousa, J.M.; Macedo, G.; Pedrosa, M.; Becerra-Castro, C.; Castro-Silva, S.; Pereira, M.F.R.; Silva, A.M.T.; Nunes, O.C.; Manaia, C.M. Ozonation and UV254nm radiation for the removal of microorganisms and antibiotic resistance genes from urban wastewater. J. Hazard. Mater. 2017, 323, 434–441. [Google Scholar] [CrossRef]
- Mnif, W.; Ben Rebah, F. Bioflocculants as Alternative to Synthetic Polymers to Enhance Wastewater Sludge Dewaterability: A Review. Energies 2023, 16, 3392. [Google Scholar] [CrossRef]
- Lin, Q.; Fan, M.; Peng, X.; Ma, J.; Zhang, Y.; Yu, F.; Wu, Z.; Liu, B. Response of Vallisneria natans to aluminum phytotoxicity and their synergistic effect on nitrogen, phosphorus change in sediments. J. Hazard. Mater. 2020, 400, 123167. [Google Scholar] [CrossRef]
- Oladoja, N.A. Headway on natural polymeric coagulants in water and wastewater treatment operations. J. Water Process Eng. 2015, 6, 174–192. [Google Scholar] [CrossRef]
- Ismail, N.; Abdullah, S.R.S.; Idris, M.; Kurniawan, S.B.; Halmi, M.I.E.; Al Sbani, N.H.; Jehawi, O.H.; Hasan, H.A. Applying rhizobacteria consortium for the enhancement of Scirpus grossus growth and phytoaccumulation of Fe and Al in pilot constructed wetlands. J. Environ. Manag. 2020, 267, 110643. [Google Scholar] [CrossRef] [PubMed]
- Exley, C.; Mold, M.J. Aluminium in human brain tissue: How much is too much? JBIC J. Biol. Inorg. Chem. 2019, 24, 1279–1282. [Google Scholar] [CrossRef] [PubMed]
- Mold, M.; Linhart, C.; Gómez-Ramírez, J.; Villegas-Lanau, A.; Exley, C. Aluminum and Amyloid-β in Familial Alzheimer’s Disease. J. Alzheimer’s Dis. 2020, 73, 1627–1635. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, T.A.; Noor, M.J.M.M.; Ghazali, A.H. Assessment of using synthetic polymers in dewatering of sewage sludge. Desalination Water Treat. 2016, 57, 23308–23317. [Google Scholar] [CrossRef]
- Wan, C.; Alam, M.A.; Zhao, X.-Q.; Zhang, X.-Y.; Guo, S.-L.; Ho, S.-H.; Chang, J.-S.; Bai, F.-W. Current progress and future prospect of microalgal biomass harvest using various flocculation technologies. Bioresour. Technol. 2015, 184, 251–257. [Google Scholar] [CrossRef]
- Ge, D.; Bian, C.; Yuan, H.; Zhu, N. An in-depth study on the deep-dewatering mechanism of waste activated sludge by ozonation pre-oxidation and chitosan re-flocculation conditioning. Sci. Total Environ. 2020, 714, 136627. [Google Scholar] [CrossRef]
- Saleem, M.; Bachmann, R.T. A contemporary review on plant-based coagulants for applications in water treatment. J. Ind. Eng. Chem. 2019, 72, 281–297. [Google Scholar] [CrossRef]
- Abdulazeez, Q.; Jami, M.; Alam, M.; Iwata, M. Analysis of the Efficiency of Sludge Dewatering Using Moringa oleifera as Natural Phytocoagulant. Int. J. Res. Chem. Metall. Civ. Eng. 2015, 2, 111–117. [Google Scholar]
- Betatache, H.; Aouabed, A.; Drouiche, N.; Lounici, H. Conditioning of sewage sludge by prickly pear cactus (Opuntia ficus Indica) juice. Ecol. Eng. 2014, 70, 465–469. [Google Scholar] [CrossRef]
- Jaouadi, T.; Hajji, M.; Kasmi, M.; Kallel, A.; Chatti, A.; Hamzaoui, H.; Mnif, A.; Tizaoui, C.; Trabelsi, I. Aloe sp. leaf gel and water glass for municipal wastewater sludge treatment and odour removal. Water Sci. Technol. 2020, 81, 479–490. [Google Scholar] [CrossRef]
- Othmani, B.; Gamelas, J.A.F.; Rasteiro, M.G.; Khadhraoui, M. Characterization of Two Cactus Formulation-Based Flocculants and Investigation on Their Flocculating Ability for Cationic and Anionic Dyes Removal. Polymers 2020, 12, 1964. [Google Scholar] [CrossRef] [PubMed]
- Eichhorn, C.; Weckmüller, S.; Urban, W. Natural Flocculant from a Combination of Moringa oleifera Seeds and Cactus Cladodes (Opuntia ficus-indica) to Optimize Flocculation Properties. Water 2022, 14, 3570. [Google Scholar] [CrossRef]
- Hussain, G.; Haydar, S. Comparative Evaluation of Glycine max L. and Alum for Turbid Water Treatment. Water Air Soil Pollut. 2020, 231, 57. [Google Scholar] [CrossRef]
- Nkurunziza, T.; Nduwayezu, J.B.; Banadda, E.N.; Nhapi, I. The effect of turbidity levels and Moringa oleifera concentration on the effectiveness of coagulation in water treatment. Water Sci. Technol. 2009, 59, 1551–1558. [Google Scholar] [CrossRef] [PubMed]
- Xiong, B.; Piechowicz, B.; Wang, Z.; Marinaro, R.; Clement, E.; Carlin, T.; Uliana, A.; Kumar, M.; Velegol, S.B. Moringa oleifera f-sand Filters for Sustainable Water Purification. Environ. Sci. Technol. Lett. 2018, 5, 38–42. [Google Scholar] [CrossRef]
- Lanan, F.A.B.M.; Selvarajoo, A.; Sethu, V.; Arumugasamy, S.K. Utilisation of natural plant-based fenugreek (Trigonella foenum-graecum) coagulant and okra (Abelmoschus escluentus) flocculant for palm oil mill effluent (POME) treatment. J. Environ. Chem. Eng. 2021, 9, 104667. [Google Scholar] [CrossRef]
- Lim, K.S.; Sethu, V.; Selvarajoo, A. Natural plant materials as coagulant and flocculants for the treatment of palm oil mill effluent. Mater. Today Proc. 2022, 48, 871–887. [Google Scholar] [CrossRef]
- Zhang, H.; Guan, G.; Lou, T.; Wang, X. High performance, cost-effective and ecofriendly flocculant synthesized by grafting carboxymethyl cellulose and alginate with itaconic acid. Int. J. Biol. Macromol. 2023, 231, 123305. [Google Scholar] [CrossRef]
- Khattabi, L.; Boudiar, T.; Bouhenna, M.M.; Chettoum, A.; Chebrouk, F.; Chader, H.; Lozano-Sánchez, J.; Segura-Carretero, A.; Nieto, G.; Akkal, S. RP-HPLC-ESI-QTOF-MS Qualitative Profiling, Antioxidant, Anti-Enzymatic, Anti-Inflammatory, and Non-Cytotoxic Properties of Ephedra alata Monjauzeana. Foods 2022, 11, 145. [Google Scholar] [CrossRef]
- Zaky, A.S.; Pensupa, N.; Andrade-Eiroa, Á.; Tucker, G.A.; Du, C. A new HPLC method for simultaneously measuring chloride, sugars, organic acids and alcohols in food samples. J. Food Compos. Anal. 2017, 56, 25–33. [Google Scholar] [CrossRef]
- Chapman, H.D.; Pratt, P.F. Methods of analysis for soils, plants and waters. Soil Sci. 1962, 93, 68. [Google Scholar] [CrossRef]
- Rice, E.W.; Bridgewater, L.; American Public Health Association. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2012. [Google Scholar]
- Owodunni, A.A.; Ismail, S. Revolutionary technique for sustainable plant-based green coagulants in industrial wastewater treatment—A review. J. Water Process Eng. 2021, 42, 102096. [Google Scholar] [CrossRef]
- Olivier, J.; Vaxelaire, J.; Vorobiev, E. Modelling of Cake Filtration: An Overview. Sep. Sci. Technol. 2007, 42, 1667–1700. [Google Scholar] [CrossRef]
- Khinchi, R. Proximate analysis and chemical composition of Moringa oleifera seeds and its use in broilers diet. Int. J. Chem. Stud. 2018, 6, 563–566. [Google Scholar]
- Maghfiroh, W.; Saefurahman, G.; Hidayatuloh, S.; Kawaroe, M. Harvesting effectiveness of Chlorella sp. biomass using different flocculation treatments of Moringa oleifera extract and pH conditions. IOP Conf. Ser. Earth Environ. Sci. 2018, 209, 012014. [Google Scholar] [CrossRef]
- Mabrouki, S.; Omri, B.; Abdouli, H.; Hiar, K.; Gamaoun, W.; Triki, M.A. Chemical, Functional and Nutritional Characteristics of raw, autoclaved and germinated fenugreek seeds. J. New Sci. 2015, 16, 541–551. [Google Scholar]
- Chu, R.L.; Vasanthi, S.; Anurita, S. Aloe vera as a natural flocculant for palm oil mill effluent (POME) treatment—Characterisation and optimisation studies. IOP Conf. Series. Mater. Sci. Eng. 2021, 1195, 012035. [Google Scholar] [CrossRef]
- Javed, A.; Ahmad, A.; Tahir, A.; Shabbir, U.; Nouman, M.; Hameed, A. Potato peel waste—Its nutraceutical, industrial and biotechnological applacations. AIMS Agri. Food 2019, 4, 807–823. [Google Scholar] [CrossRef]
- Anhwange, B.; Ajibola, V.O.; Oniye, S. Amino Acids compositions of the Seeds of Moringa oleifera (LAM) Detarium mirocarpum (Guill & Perr) Bauhinia monandra (Linn). ChemClass J. 2004, 93, 9–13. [Google Scholar]
- Tat, W.K.; Idris, A.; Noor, M.J.M.M.; Mohamed, T.A.; Ghazali, A.H.; Muyibi, S.A. Optimization study on sewage sludge conditioning using Moringa oleifera seeds. Desalination Water Treat. 2010, 16, 402–410. [Google Scholar] [CrossRef]
- Lee, C.; Chong, M.; Robinson, J.; Binner, E. A Review on Development and Application of Plant-Based Bioflocculants and Grafted Bioflocculants. Ind. Eng. Chem. Res. 2014, 53, 18357–18369. [Google Scholar] [CrossRef]
- Zafar, M.S.; Tausif, M.; Mohsin, M.; Ahmad, S.W.; Zia-ul-Haq, M. Potato Starch as a Coagulant for Dye Removal from Textile Wastewater. Water Air Soil Pollut. 2015, 226, 244. [Google Scholar] [CrossRef]
- El-Mostafa, K.; El Kharrassi, Y.; Badreddine, A.; Andreoletti, P.; Vamecq, J.; El Kebbaj, M.; Latruffe, N.; Lizard, G.; Nasser, B.; Cherkaoui-Malki, M. Nopal Cactus (Opuntia ficus-indica) as a Source of Bioactive Compounds for Nutrition, Health and Disease. Molecules 2014, 19, 14879–14901. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Shen, L.; Zhuang, X.; Shi, J.; Wang, Y.; He, N.; Chang, Y.-I. Flocculation Characterization of a Bioflocculant from Bacillus licheniformis. Ind. Eng. Chem. Res. 2015, 54, 2894–2901. [Google Scholar] [CrossRef]
- Hou, J.; Hong, C.; Ling, W.; Hu, J.; Feng, W.; Xing, Y.; Wang, Y.; Zhao, C.; Feng, L. Research progress in improving sludge dewaterability: Sludge characteristics, chemical conditioning and influencing factors. J. Environ. Manag. 2024, 351, 119863. [Google Scholar] [CrossRef]
- Sultana, B.; Anwar, F.; Ashraf, M. Effect of Extraction Solvent/Technique on the Antioxidant Activity of Selected Medicinal Plant Extracts. Molecules 2009, 14, 2167–2180. [Google Scholar] [CrossRef]
- Zhang, Y.; Bao, Z.; Ye, X.; Xie, Z.; He, K.; Mergens, B.; Li, W.; Yatcilla, M.; Zheng, Q. Chemical Investigation of Major Constituents in Aloe vera Leaves and Several Commercial Aloe Juice Powders. J. AOAC Int. 2018, 101, 1741–1751. [Google Scholar] [CrossRef] [PubMed]
- Kunyanga, C.N.; Vellingiri, V.; Imungi, K.J. Nutritional quality, phytochemical composition and health protective effects of an under-utilized prickly cactus fruit (Opuntia stricta Haw.) collected from Kenya. Afr. J. Food Agric. Nutr. Dev. 2014, 14, 9561–9577. [Google Scholar] [CrossRef]
- Tursun, N.; Seyithanoglu, M.; Uygur, F.N.; Elibuyuk, I.O.; Elibuyuk, E.A. Seasonal dynamics of soluble carbohydrates in rhizomes of Phragmites australis and Typha latifolia. Flora Morphol. Distrib. Funct. Ecol. Plants 2011, 206, 731–735. [Google Scholar] [CrossRef]
- Compaoré, W.R.; Nikièma, P.A.; Bassolé, H.I.N.; Savadogo, A.; Mouecoucou, J. Chemical composition and antioxidative properties of seeds of Moringa oleifera and pulps of Parkia biglobosa and Adansonia digitata commonly used in food fortification in Burkina Faso. Curr. Res. J. Biol. Sci. 2011, 3, 64–72. [Google Scholar]
- Lahuta, L.B.; Szablińska, J.; Ciak, M.; Górecki, R.J. The occurrence and accumulation of d-pinitol in fenugreek (Trigonella foenum graecum L.). Acta Physiol. Plant 2018, 40, 155. [Google Scholar] [CrossRef]
- Choudhary, M.; Neogi, S. A natural coagulant protein from Moringa oleifera: Isolation, characterization, and potential use for water treatment. Mater. Res. Express 2017, 4, 105502. [Google Scholar] [CrossRef]
- Ramlee, A.; Som, A.M.; Puasa, W.; Amani, H.; Hamid, A. Coagulation-flocculation mechanism and characterisation of Hylocereus undatus foliage as a natural coagulant in industrial wastewater treatment. Chem. Pap. 2023, 77, 6083–6093. [Google Scholar] [CrossRef]
- Murdock, R.C.; Braydich-Stolle, L.; Schrand, A.M.; Schlager, J.J.; Hussain, S.M. Characterization of Nanomaterial Dispersion in Solution Prior to In Vitro Exposure Using Dynamic Light Scattering Technique. Toxicol. Sci. 2008, 101, 239–253. [Google Scholar] [CrossRef]
- Junior, A.T.; Din, S.; Sebastien, N.Y. Optimization of Coagulation/Flocculation Treatment of Brewery Wastewater Employing Organic Flocculant Based of Vegetable Tannin. Water Air Soil Pollut. 2019, 230, 202. [Google Scholar] [CrossRef]
- Freitas, T.K.F.S.; Oliveira, V.M.; De Souza, M.T.F.; Geraldino, H.C.L.; Almeida, V.C.; Fávaro, S.L.; Garcia, J.C. Optimization of coagulation-flocculation process for treatment of industrial textile wastewater using okra (A. esculentus) mucilage as natural coagulant. Ind. Crops Prod. 2015, 76, 538–544. [Google Scholar] [CrossRef]
- Choudhary, M.; Ray, M.B.; Neogi, S. Evaluation of the potential application of cactus (Opuntia ficus-indica) as a bio-coagulant for pre-treatment of oil sands process-affected water. Sep. Purif. Technol. 2019, 209, 714–724. [Google Scholar] [CrossRef]
- Pöykiö, R.; Watkins, G.; Dahl, O. Characterisation of Municipal Sewage Sludge as a Soil Improver and a Fertilizer Product. Ecol. Chem. Eng. S 2019, 26, 547–557. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, K.; Tian, G.; Liu, B.; Jiang, Z.; Bian, B. Feasibility of improving wastewater sludge dewaterability by combination of cationic polyacrylamide and synthetic fibers for resource utilization. Sep. Purif. Technol. 2023, 306, 122620. [Google Scholar] [CrossRef]
- Wai, K.T.; Idris, A.; Johari, M.M.N.M.; Mohammad, T.A.; Ghazali, A.H.; Muyibi, S.A. Evaluation on different forms of Moringa oleifera seeds dosing on sewage sludge conditioning. Desalination Water Treat. 2009, 10, 87–94. [Google Scholar] [CrossRef]
- Li, L.; Peng, C.; Zhan, Z.; Ma, F.; Zhang, J. A novel treatment for amelioration of sludge dewaterability using green starch-grafted flocculant and realized mechanism. Sep. Purif. Technol. 2022, 301, 122060. [Google Scholar] [CrossRef]
- Hamman, J.H. Composition and applications of Aloe veraleaf gel. Molecules 2008, 13, 1599–1616. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraghavan, G.; Sivakumar, T.; Kumar, A.V. Application of plant based coagulants for waste water treatment. Int. J. Adv. Eng. Res. Stud. 2011, 1, 88–92. [Google Scholar]
- Sepúlveda, E.; Sáenz, C.; Aliaga, E.; Aceituno, C. Extraction and characterization of mucilage in Opuntia spp. J. Arid Environ. 2007, 68, 534–545. [Google Scholar] [CrossRef]
- Shebek, K.; Schantz, A.B.; Sines, I.; Lauser, K.; Velegol, S.; Kumar, M. The flocculating cationic polypetide from Moringa oleifera seeds damages bacterial cell membranes by causing membrane fusion. Langmuir 2015, 31, 4496–4502. [Google Scholar] [CrossRef]
- Pambou, Y.B.; Fraikin, L.; Salmon, T.; Crine, M.; Léonard, A. Enhanced sludge dewatering and drying comparison of two linear polyelectrolytes co-conditioning with polyaluminum chloride. Desalination Water Treat. 2016, 57, 27989–28006. [Google Scholar] [CrossRef]
- Katsiris, N.; Kouzeli-Katsiri, A. Bound water content of biological sludges in relation to filtration and dewatering. Water Res. 1987, 21, 1319–1327. [Google Scholar] [CrossRef]
- Zemmouri, H.; Mameri, N.; Lounici, H. Chitosan use in chemical conditioning for dewatering municipal-activated sludge. Water Sci. Technol. 2015, 71, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Katrivesis, F.K.; Karela, A.D.; Papadakis, V.G.; Paraskeva, C.A. Revisiting of coagulation-flocculation processes in the production of potable water. J. Water Process Eng. 2019, 27, 193–204. [Google Scholar] [CrossRef]
- Gold, M.; Dayer, P.; Faye, M.C.A.S.; Clair, G.; Seck, A.; Niang, S.; Morgenroth, E.; Strande, L. Locally produced natural conditioners for dewatering of fecal sludge. Environ. Technol. 2016, 37, 2802–2814. [Google Scholar] [CrossRef]
- Sethu, V.; Selvarajoo, A.; Lee, C.; Ganesan, P.; Goh, L.; Mok, X. Opuntia cactus as a novel bio-coagulant for the treatment of Palm Oil Mill Effluent (POME). Prog. Energy Environ. 2019, 9, 11–26. [Google Scholar]
- Lai, H.; Fang, H.; Huang, L.; He, G.; Reible, D. A review on sediment bioflocculation: Dynamics, influencing factors and modeling. Sci. Total Environ. 2018, 642, 1184–1200. [Google Scholar] [CrossRef] [PubMed]
- Muyibi, S.; Alfugara, A. Treatment of surface water with Moringa Oleifera seed extract and alum—A comparative study using a pilot scale water treatment plant. Int. J. Environ. Stud. 2003, 60, 617–626. [Google Scholar] [CrossRef]
- Ndabigengesere, A.; Narasiah, K.S.; Talbot, B.G. Active agents and mechanism of coagulation of turbid waters using Moringa oleifera. Water Res. 1995, 29, 703–710. [Google Scholar] [CrossRef]
- Bratby, J. Coagulation and Flocculation in Water and Wastewater Treatment; IWA Publishing: London, UK, 2006; 421p. [Google Scholar]


| Plants | pH | Organic Matter (%) | Proteins (%) | Ca (mg/g dw) | Mg (mg/g dw) | Na (mg/g dw) | K (mg/g dw) |
|---|---|---|---|---|---|---|---|
| Fenugreek | 6.5 | 97.0 | 27.65 | 0.60 | 1.40 | 15.05 | 2.46 |
| Moringa | 5.9 | 95.0 | 25.33 | 0.32 | 4.71 | 0.34 | 12.90 |
| Potato | 6.5 | 94.2 | 10.19 | 0.11 | 2.25 | 1.80 | 35.10 |
| Phragmites | 6.2 | 90.7 | 8.18 | 0.72 | 0.71 | 0.73 | 34.10 |
| Cactus | 5.7 | 89.0 | 7.3 | 56.0 | 24.9 | 0.90 | 11.95 |
| Aloe | 5.8 | 87.6 | 3.76 | 22.5 | 9.30 | 0.78 | 12.75 |
| Peak Retention Time (min) | Identified Compound in the Plant Extract | Cactus | Aloe | Phragmites | Moringa | Fenugreek | Potato |
|---|---|---|---|---|---|---|---|
| 4.9 | Sucrose | 4.66 | 0.88 | 4.85 | 3.97 | 1.18 | 0.85 |
| 5.8 | Glucose | 10.24 | 15.12 | 1.36 | 0.90 | 0.24 | nd |
| 6.3 | Fructose | 10.59 | 29.8 | 1.18 | 0.56 | nd | 0.23 |
| 6.8 | Arabinose | nd | nd | 0.80 | nd | nd | nd |
| Wave Number (cm−1) | Functional Chemical Groups |
|---|---|
| 3298 | Hydroxyl group OH/N-H bands |
| 2916–2921 | C–H symmetric stretching in CH2 |
| 1637–1647 | Carbonyl function C=O of the COO– ionic form of carboxylic acids/Ionized COOH (COO– symmetric stretching) |
| 1508–1595 | C=C groups of carboxylic acids and ketones |
| 1319 | CH3 primary aromatic amines/Ionized COOH (COO– symmetric stretching) |
| 1025 | C–O–C/C–N stretching vibration of amine groups |
| Plants | Cactus | Moringa | Aloe | Potato | Fenugreek | Phragmites |
|---|---|---|---|---|---|---|
| ζ (mV) | −7.62 | −8.03 | −12.4 | −26.8 | −25.4 | −24.8 |
| pH | 6.05 | 6.05 | 5.95 | 6.5 | 6.5 | 6.37 |
| Parameter | Unit | Present Study | Betatache et al., 2014 [19] | Pöykiö et al., 2019 [59] | Yang et al., 2023 [60] |
|---|---|---|---|---|---|
| WC | % | ~86 | 95.38 | 76.4 | 96.2 |
| pH | - | 5.6 | 7.16 | 6.1 | nd |
| TS | g/L | 36 | 45.4 | 23.6 | nd |
| TSS | mg/L | 51 | nd | nd | nd |
| Zeta Potential (ζ) | mV | −13.9 | nd | nd | −12.5 |
| SRF | m/kg | 6.7 × 1013 | 1.03 × 1013 | nd | nd |
| DC | % | 20.3 | 4.62 | nd | nd |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hadj Mansour, Y.; Othmani, B.; Ben Rebah, F.; Mnif, W.; Khadhraoui, M.; Saoudi, M. Plant-Based Flocculants as Sustainable Conditioners for Enhanced Sewage Sludge Dewatering. Water 2024, 16, 2949. https://doi.org/10.3390/w16202949
Hadj Mansour Y, Othmani B, Ben Rebah F, Mnif W, Khadhraoui M, Saoudi M. Plant-Based Flocculants as Sustainable Conditioners for Enhanced Sewage Sludge Dewatering. Water. 2024; 16(20):2949. https://doi.org/10.3390/w16202949
Chicago/Turabian StyleHadj Mansour, Yosra, Bouthaina Othmani, Faouzi Ben Rebah, Wissem Mnif, Moncef Khadhraoui, and Mongi Saoudi. 2024. "Plant-Based Flocculants as Sustainable Conditioners for Enhanced Sewage Sludge Dewatering" Water 16, no. 20: 2949. https://doi.org/10.3390/w16202949
APA StyleHadj Mansour, Y., Othmani, B., Ben Rebah, F., Mnif, W., Khadhraoui, M., & Saoudi, M. (2024). Plant-Based Flocculants as Sustainable Conditioners for Enhanced Sewage Sludge Dewatering. Water, 16(20), 2949. https://doi.org/10.3390/w16202949








