Optimization of Secondary Chlorination in Water Distribution Systems for Enhanced Disinfection and Reduced Chlorine Odor Using Deep Belief Network and NSGA-II
Abstract
1. Introduction
2. Methods
2.1. Chloric Odor Intensity Assessment
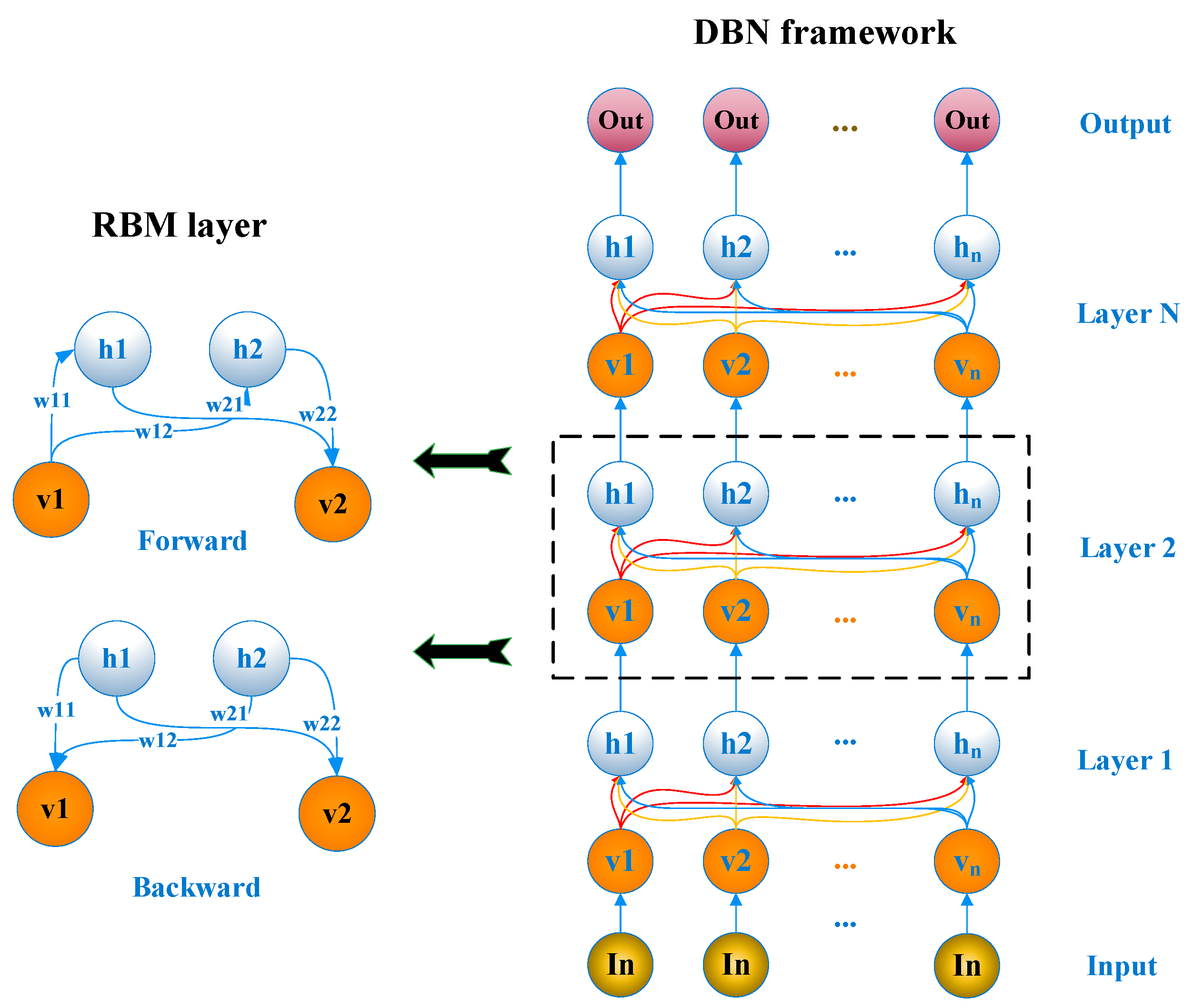
2.2. Chloric Odor Prediction
2.3. Kinetic Models of Multiple Components
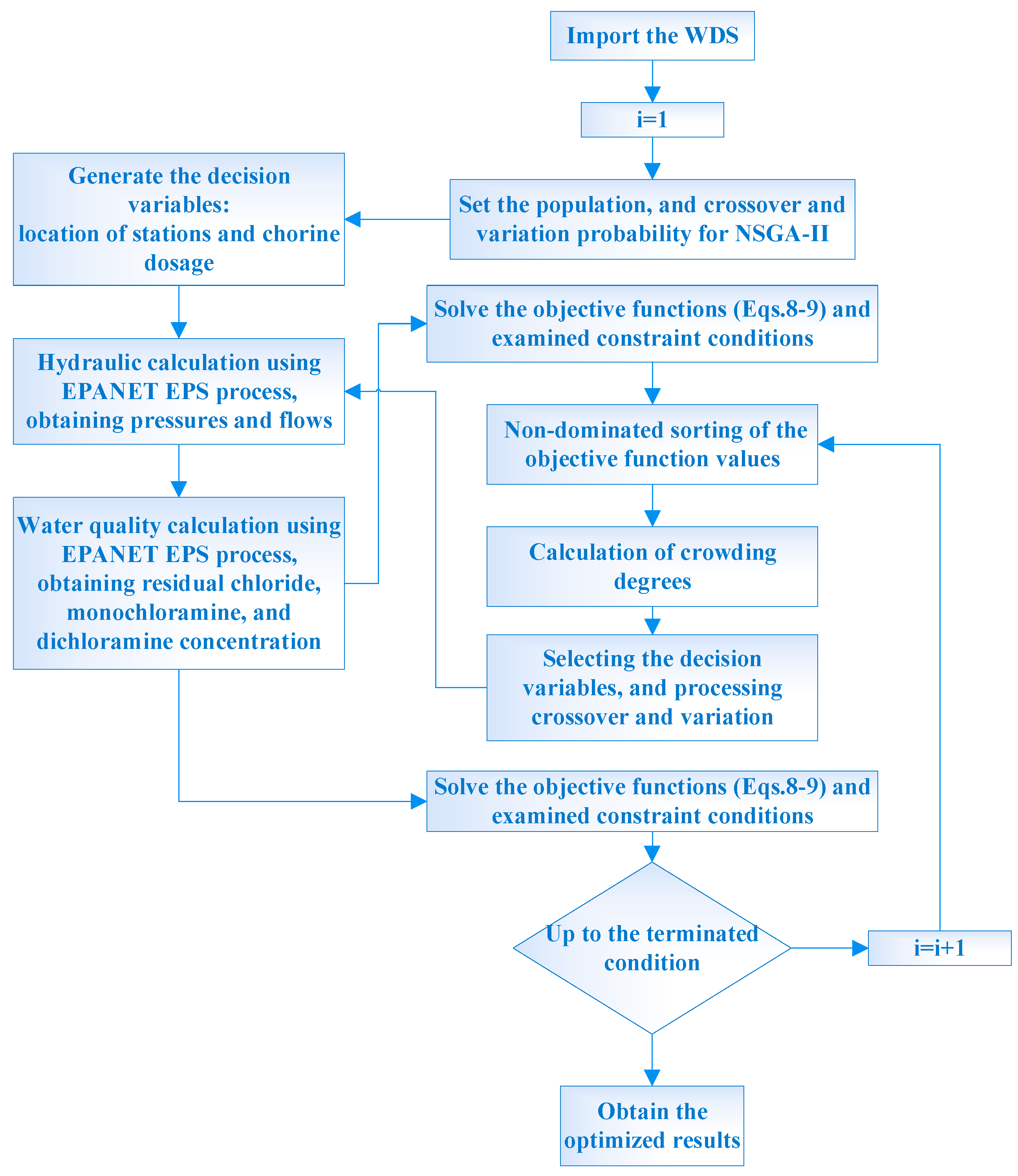
2.4. Secondary Chlorination Dosage and Station Optimization
3. Case Studies
3.1. Chloric Odor Intensity Experiment
3.2. Simulation Environment
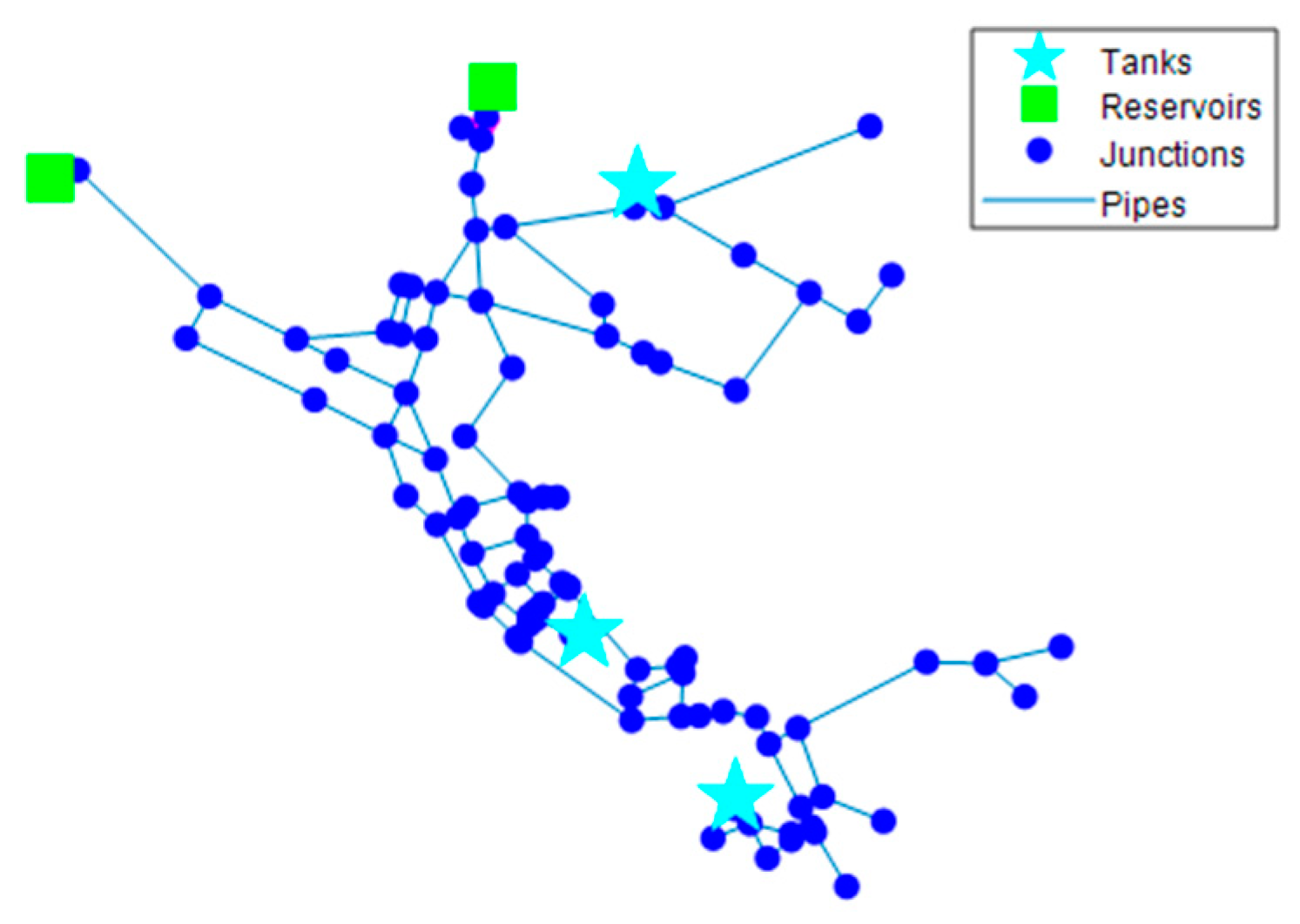
3.3. Network A
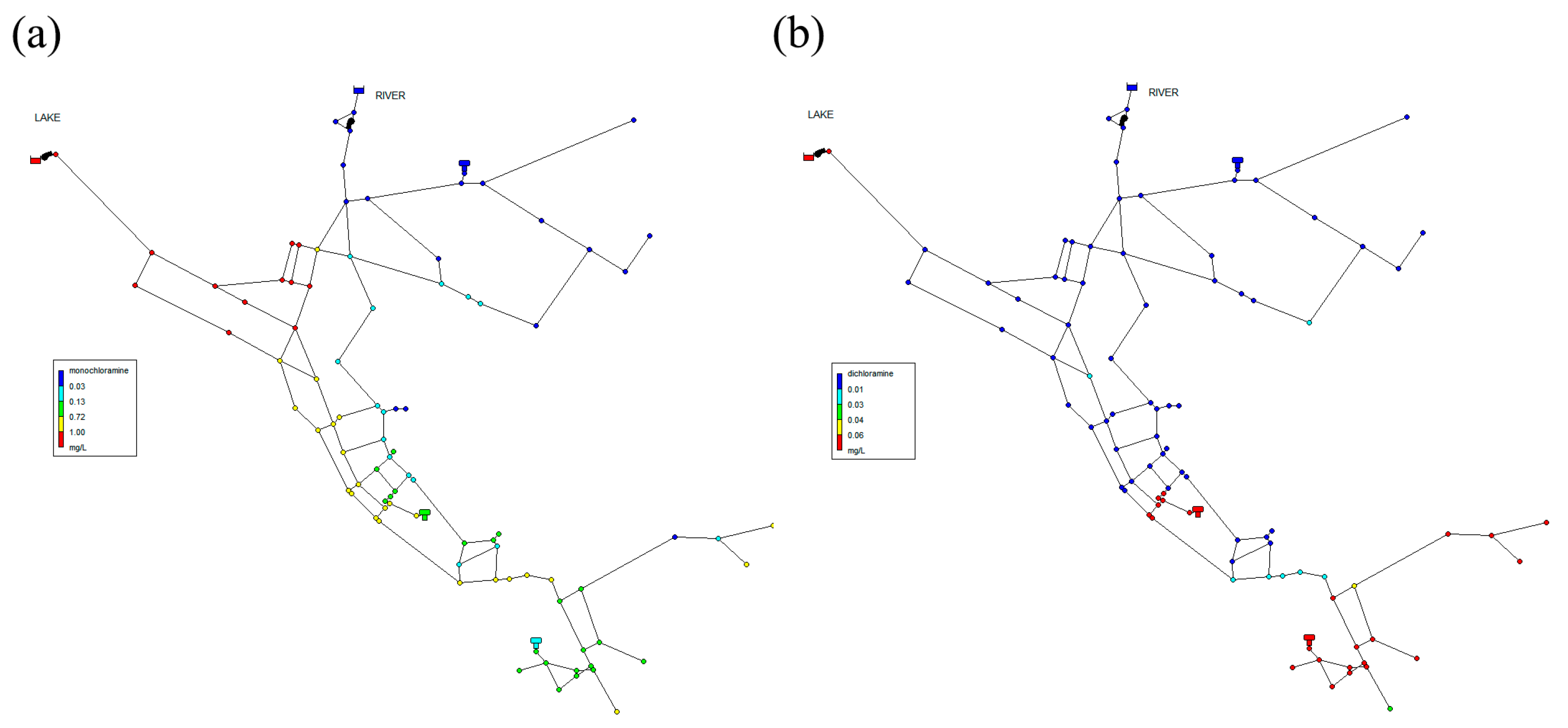
3.4. Network B
4. Discussion
- (1)
- The NSGA-II algorithm allows for a cost-effective approach to secondary chlorination. Through optimizing the locations and dosages of secondary chlorination stations, it ensures that adequate disinfectant residuals are maintained throughout the network, which is crucial for sustainable water management, while balancing economic investment with the need for effective disinfection and odor control. Thereby, the algorithm can help to maintain high water quality standards, underscoring its potential as a sustainable solution for water utilities;
- (2)
- The proposed method leverages advanced algorithms, including EPANET-MSX simulations for water hydraulic and quality modeling and the DBN in deep learning frameworks. This combination enhances the accuracy of chloric odor predictions and optimizes chlorination strategies;
- (3)
- The proposed method significantly improves the distribution and effectiveness of chlorine in WDSs. Through carefully balancing the levels of chlorine, monochloramine, and dichloramine, the method mitigates unpleasant odors without compromising disinfection efficacy. This leads to an improved sensory quality of drinking water.
- (1)
- The training intensity of the DBN network poses a challenge in terms of the availability of extensive and accurate data. The data from chloric odor intensity experiments are only produced in the laboratory. Thus, while the method is effective in specific conditions, such as cases A and B, adapting it to larger or more complex network systems may present challenges related to scalability;
- (2)
- The accuracy of this method could be influenced by variations in input parameters such as chlorine decay rates, temperature, and flow dynamics. Sensitivity to these parameters necessitates careful calibration and validation using sensors in real WDSs to ensure that reliable results are obtained;
- (3)
- The integration of deep learning models and multi-objective optimization algorithms requires substantial computational resources. This can be a limiting factor, especially for utilities with limited access to high-performance computing facilities.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, Q.; Zheng, F.; Jia, Y.; Savic, D.; Kapelan, Z. Real-time foul sewer hydraulic modelling driven by water consumption data from water distribution systems. Water Res. 2021, 188, 116544. [Google Scholar] [CrossRef]
- Kennedy, A.; Flint, L.; Aligata, A.; Hoffman, C.; Arias-Paić, M. Regulated disinfection byproduct formation over long residence times. Water Res. 2021, 188, 116523. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, K.; Zhang, T.; Li, C.; Mao, X. An ignored and potential source of taste and odor (T&O) issues—Biofilms in drinking water distribution system (DWDS). Appl. Microbiol. Biotechnol. 2017, 101, 3537–3550. [Google Scholar]
- Liu, M.; Graham, N.; Wang, W.; Zhao, R.; Lu, Y.; Elimelech, M.; Yu, W. Spatial assessment of tap-water safety in China. Nat. Sustain. 2022, 5, 689–698. [Google Scholar] [CrossRef]
- Doederer, K.; Gernjak, W.; Weinberg, H.S.; Farré, M.J. Factors affecting the formation of disinfection by-products during chlorination and chloramination of secondary effluent for the production of high quality recycled water. Water Res. 2014, 48, 218–228. [Google Scholar] [CrossRef]
- Lee, W.H.; Wahman, D.G.; Bishop, P.L.; Pressman, J.G. Free chlorine and monochloramine application to nitrifying biofilm: Comparison of biofilm penetration, activity, and viability. Environ. Sci. Technol. 2011, 45, 1412–1419. [Google Scholar] [CrossRef]
- Goyal, R.V.; Patel, H.M. Optimal location and scheduling of booster chlorination stations using EPANET and PSO for drinking water distribution system. ISH J. Hydraul. Eng. 2018, 24, 157–164. [Google Scholar] [CrossRef]
- Geng, B.; Fan, J.; Shi, M.; Zhang, S.; Li, J. Control of maximum water age based on total chlorine decay in secondary water supply system. Chemosphere 2022, 287, 132198. [Google Scholar] [CrossRef]
- Tabesh, M.; Azadi, B.; Roozbahani, A. Quality management of water distribution networks by optimizing dosage and location of chlorine injection. Int. J. Environ. Res. 2011, 5, 321–332. [Google Scholar]
- Shen, L.; Wu, J.-Y.; Lin, G.-F.; Shen, J.-H.; Westendorf, J.; Huehnerfuss, H. The mutagenic potentials of tap water samples in Shanghai. Chemosphere 2003, 52, 1641–1646. [Google Scholar] [CrossRef]
- Mao, R.; Zhang, K.; Zhang, Q.; Xu, J.; Cen, C.; Pan, R.; Zhang, T. Joint majorization of waterworks and secondary chlorination points considering the chloric odor and economic investment in the DWDS using machine learning and optimization algorithms. Water Res. 2022, 220, 118595. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kweon, J.H.; Kim, D.I. Prediction of residual chlorine using two-component second-order decay model in water distribution network. J. Korean Soc. Water Wastewater 2014, 28, 287–297. [Google Scholar] [CrossRef]
- Ohar, Z.; Ostfeld, A. Alternative formulation for DBP’s minimization by optimal design of booster chlorination stations. In World Environmental and Water Resources Congress 2010: Challenges of Change; American Society of Civil Engineers: Reston, VA, USA, 2010; pp. 4260–4269. [Google Scholar]
- Wang, H.; Guo, W.; Xu, J.; Gu, H. A Hybrid PSO for Optimizing Locations of Booster Chlorination Stations in Water Distribution Systems. In Proceedings of the 2010 International Conference on Intelligent Computation Technology and Automation, Changsha, China, 11–12 May 2010; pp. 126–129. [Google Scholar]
- Loutfi, A.; Coradeschi, S. Smell, think and act: A cognitive robot discriminating odours. Auton. Robot. 2006, 20, 239–249. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Rahman, A. Optimized Deep Learning Framework for Water Distribution Data-Driven Modeling. Procedia Eng. 2017, 186, 261–268. [Google Scholar] [CrossRef]
- Hinton, G.E.; Osindero, S.; Teh, Y.-W. A Fast Learning Algorithm for Deep Belief Nets. Neural Comput. 2006, 18, 1527–1554. [Google Scholar] [CrossRef]
- Rossman, L.A. EPANET 2.0: Users Manual; Water Supply and Water Resources Division, National Risk management Laboratory, USEPA: Cincinnati, OH, USA, 2000. [Google Scholar]
- Maheshwari, A.; Abokifa, A.; Gudi, R.D.; Biswas, P. Optimization of disinfectant dosage for simultaneous control of lead and disinfection-byproducts in water distribution networks. J. Environ. Manag. 2020, 276, 111186. [Google Scholar] [CrossRef]
- Ramani, K.; Rudraswamy, G.K.; Umamahesh, N.V. Optimal Design of Intermittent Water Distribution Network Considering Network Resilience and Equity in Water Supply. Water 2023, 15, 3265. [Google Scholar] [CrossRef]
- Xu, J.; Bai, D. Multi-Objective Optimal Operation of the Inter-Basin Water Transfer Project Considering the Unknown Shapes of Pareto Fronts. Water 2019, 11, 2644. [Google Scholar] [CrossRef]
- Mu, T.; Huang, M.; Tang, S.; Zhang, R.; Chen, G.; Jiang, B. Sensor Partitioning Placements via Random Walk and Water Quality and Leakage Detection Models within Water Distribution Systems. Water Resour. Manag. 2022, 36, 5297–5311. [Google Scholar] [CrossRef]
- Mu, T.; Lu, Y.; Tan, H.; Zhang, H.; Zheng, C. Random Walks Partitioning and Network Reliability Assessing in Water Distribution System. Water Resour. Manag. 2021, 35, 2325–2341. [Google Scholar] [CrossRef]
- Sirsant, S.; Reddy, M.J. Assessing the Performance of Surrogate Measures for Water Distribution Network Reliability. J. Water Resour. Plan. Manag. 2020, 146, 04020048. [Google Scholar] [CrossRef]
- Deb, K.; Pratap, A.; Agarwal, S.; Meyarivan, T. A fast and elitist multiobjective genetic algorithm: NSGA-II. IEEE Trans. Evol. Comput. 2002, 6, 182–197. [Google Scholar] [CrossRef]
- Mu, T.; Huang, M.; Tan, H.; Chen, G.; Zhang, R. Pressure and Water Quality Integrated Sensor Placement Considering Leakage and Contamination Intrusion within Water Distribution Systems. ACS ES&T Water 2021, 1, 2348–2358. [Google Scholar] [CrossRef]
- Zheng, S.; Lin, T.; Chen, H.; Zhang, X.; Jiang, F. Impact of changes in biofilm composition response following chlorine and chloramine disinfection on nitrogenous disinfection byproduct formation and toxicity risk in drinking water distribution systems. Water Res. 2024, 253, 121331. [Google Scholar] [CrossRef]
- Bryant EMcDonnell, S.E.; Uber, J.; Klise, K. Open Water Analytics (OWA); KIOS Research Center for Intelligent Systems and Networks of the University of Cyprus: Nicosia, Cyprus, 2007. [Google Scholar]
- Rossman, L.A.; Clark, R.M.; Grayman, W.M. Modeling Chlorine Residuals in Drinking-Water Distribution Systems. J. Environ. Eng. 1994, 120, 803–820. [Google Scholar] [CrossRef]
- Ostfeld, A.; Uber James, G.; Salomons, E.; Berry Jonathan, W.; Hart William, E.; Phillips Cindy, A.; Watson, J.-P.; Dorini, G.; Jonkergouw, P.; Kapelan, Z.; et al. The Battle of the Water Sensor Networks (BWSN): A Design Challenge for Engineers and Algorithms. J. Water Resour. Plan. Manag. 2008, 134, 556–568. [Google Scholar] [CrossRef]
- Ostfeld, A.; Salomons, E.; Ormsbee, L.; Uber, J.G.; Bros, C.M.; Kalungi, P.; Burd, R.; Zazula-Coetzee, B.; Belrain, T.; Kang, D. Battle of the water calibration networks. J. Water Resour. Plan. Manag. 2012, 138, 523–532. [Google Scholar] [CrossRef]
- Hwang, H.; Lansey, K. Water Distribution System Classification Using System Characteristics and Graph-Theory Metrics. J. Water Resour. Plan. Manag. 2017, 143, 04017071. [Google Scholar] [CrossRef]








| Intensities | Levels of Odor |
|---|---|
| [0, 2) | None |
| [2, 4) | Minimal detection |
| [4, 6) | Subtle |
| [6, 8) | Mild |
| [8, 10) | Mild to intermediate |
| [10, 12) | Intermediate |
| [12, 14) | Intermediate to pronounced |
| 14 | Pronounced |
| Parameters | Values L/(mg·h) |
|---|---|
| 1.5 × 1010 | |
| 2.3 × 10−3 | |
| 2.2 × 108 | |
| 1.0 × 106 | |
| 1.0 | |
| 7.6 × 10−2 | |
| 55.0 | |
| 4.0 × 105 | |
| 6.5 × 105 |
| Models | Accuracy % | Precision % | Recall % | F1-Score % |
|---|---|---|---|---|
| DBN | 96.67 | 96.67 | 100.00 | 98.33 |
| BP | 76.67 | 80.00 | 90.91 | 85.11 |
| Linear | 66.67 | 72.00 | 85.71 | 78.17 |
| Index of Node | Chlorine Dosage (mg/L) | (USD/Year) | ||
|---|---|---|---|---|
| 2 | [26, 32] | [0.15, 0.24] | 2.72 | 16,020.37 |
| 3 | [33, 27, 35] | [0.13, 0.32, 0.33] | 2.04 | 24,002.27 |
| 4 | [33, 26, 22, 31] | [0.25, 2.96, 1.07, 0.30] | 1.75 | 32,002.81 |
| 5 | [21, 27, 10, 22, 32] | [1.44, 0.63, 0.33, 0.48, 0.34] | 1.20 | 40,020.77 |
| 6 | [14, 18, 28, 30, 31, 10] | [0.50, 1.40, 0.63, 1.49, 1.25, 0.20] | 1.08 | 48,172.92 |
| 7 | [18, 31, 35, 32, 28, 8, 10] | [2.60, 1.43, 0.86, 1.08, 0.53, 0.61, 1.88] | 0.94 | 56,035.67 |
| 8 | [34, 3, 21, 20, 26, 31, 30, 22] | [0.68, 0.94, 0.57, 1.00, 0.77, 1.96, 1.68, 0.79] | 0.85 | 64,109.10 |
| 9 | [27, 21, 22, 10, 28, 17, 32, 36, 33] | [1.30, 2.02, 0.83, 0.47, 0.36, 1.38, 2.58, 2.29, 1.50] | 0.72 | 72,154.92 |
| 10 | [26, 29, 36, 35, 18, 32, 16, 10, 3, 19] | [2.03, 0.53, 0.46, 2.22, 0.91, 0.58, 1.25, 1.45 0.72, 2.82] | 0.66 | 80,204.68 |
| Index of Node | Chlorine Dosage (mg/L) | Monochloramine (mg/L) | Dichloramine (mg/L) | (USD/Year) | ||
|---|---|---|---|---|---|---|
| 2 | [17, 38] | [0.10, 0.12] | 0.31–0.54 | 0.01–0.03 | 1.85 | 18,050.87 |
| 3 | [2, 38, 68] | [0.08, 0.10, 0.12] | 0.33–0.59 | 0.01–0.04 | 1.60 | 27,075.81 |
| 4 | [2, 17, 40, 68] | [0.06, 0.08, 0.10, 0.12] | 0.33–0.64 | 0.01–0.04 | 1.42 | 36,100.41 |
| 5 | [17, 38, 34, 45, 68] | [0.05, 0.06, 0.08, 0.10, 0.12] | 0.41–0.66 | 0.01–0.05 | 1.25 | 45,125.68 |
| 6 | [17, 26, 29, 34, 40, 68] | [0.04, 0.05, 0.06, 0.08, 0.10, 0.12] | 0.40–0.70 | 0.02–0.05 | 1.10 | 54,150.93 |
| 7 | [2, 29, 34, 40, 45, 68, 77] | [0.03, 0.04, 0.05, 0.06, 0.08, 0.10, 0.12] | 0.44–0.71 | 0.02–0.05 | 0.98 | 63,175.59 |
| 8 | [2, 17, 34, 38, 40, 45, 68, 77] | [0.02, 0.03, 0.04, 0.05, 0.06, 0.08, 0.10, 0.12] | 0.48–0.75 | 0.02–0.06 | 0.88 | 71,405.38 |
| 9 | [17, 34, 20, 26, 40, 48, 59, 71, 77] | [0.02, 0.02, 0.03, 0.04, 0.05, 0.06, 0.08, 0.10, 0.12] | 0.46–0.77 | 0.03–0.06 | 0.80 | 81,225.14 |
| 10 | [2, 17, 26, 29, 34, 40, 45, 48, 71, 77] | [0.01, 0.02, 0.02, 0.03, 0.04, 0.05, 0.06, 0.08, 0.10, 0.12] | 0.51–0.79 | 0.03–0.06 | 0.72 | 90,250.65 |
| 11 | [2, 16, 26, 29, 34, 40, 45, 59, 68, 71, 77] | [0.01, 0.02, 0.02, 0.03, 0.04, 0.05, 0.06, 0.08, 0.10, 0.12, 0.12] | 0.55–0.82 | 0.03–0.06 | 0.65 | 99,275.54 |
| 12 | [2, 17, 20, 26, 29, 34, 40, 45, 59, 65, 68, 77] | [0.01, 0.01, 0.02, 0.02, 0.03, 0.04, 0.05, 0.06, 0.08, 0.10, 0.12, 0.12] | 0.56–0.85 | 0.04–0.07 | 0.59 | 108,300.22 |
| 13 | [2, 16, 20, 26, 29, 34, 40, 45, 48, 59, 68, 71, 77] | [0.01, 0.01, 0.02, 0.02, 0.03, 0.04, 0.05, 0.06, 0.08, 0.10, 0.12, 0.12, 0.13] | 0.62–0.89 | 0.04–0.08 | 0.54 | 117,325.21 |
| 14 | [2, 16,17, 20, 26, 29, 40, 45, 48, 59, 65, 68, 71, 77] | [0.01, 0.01, 0.02, 0.02, 0.03, 0.04, 0.05, 0.06, 0.08, 0.10, 0.12, 0.12, 0.13, 0.13] | 0.65–0.87 | 0.05–0.09 | 0.49 | 126,350.76 |
| 15 | [2, 17,20, 21, 26, 29, 40, 45, 48, 59, 65, 68, 70, 71, 77] | [0.01, 0.01, 0.02, 0.02, 0.03, 0.04, 0.05, 0.06, 0.08, 0.10, 0.12, 0.12, 0.13, 0.13, 0.14] | 0.65–0.92 | 0.05–0.11 | 0.45 | 135,375.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, B.; Shu, S.; Li, D. Optimization of Secondary Chlorination in Water Distribution Systems for Enhanced Disinfection and Reduced Chlorine Odor Using Deep Belief Network and NSGA-II. Water 2024, 16, 2666. https://doi.org/10.3390/w16182666
Dong B, Shu S, Li D. Optimization of Secondary Chlorination in Water Distribution Systems for Enhanced Disinfection and Reduced Chlorine Odor Using Deep Belief Network and NSGA-II. Water. 2024; 16(18):2666. https://doi.org/10.3390/w16182666
Chicago/Turabian StyleDong, Bo, Shihu Shu, and Dengxin Li. 2024. "Optimization of Secondary Chlorination in Water Distribution Systems for Enhanced Disinfection and Reduced Chlorine Odor Using Deep Belief Network and NSGA-II" Water 16, no. 18: 2666. https://doi.org/10.3390/w16182666
APA StyleDong, B., Shu, S., & Li, D. (2024). Optimization of Secondary Chlorination in Water Distribution Systems for Enhanced Disinfection and Reduced Chlorine Odor Using Deep Belief Network and NSGA-II. Water, 16(18), 2666. https://doi.org/10.3390/w16182666




