PFOA and PFOS Pollution in Surface Waters and Surface Water Fish
Abstract
1. Introduction
2. Material and Method
2.1. Standards and Chemicals
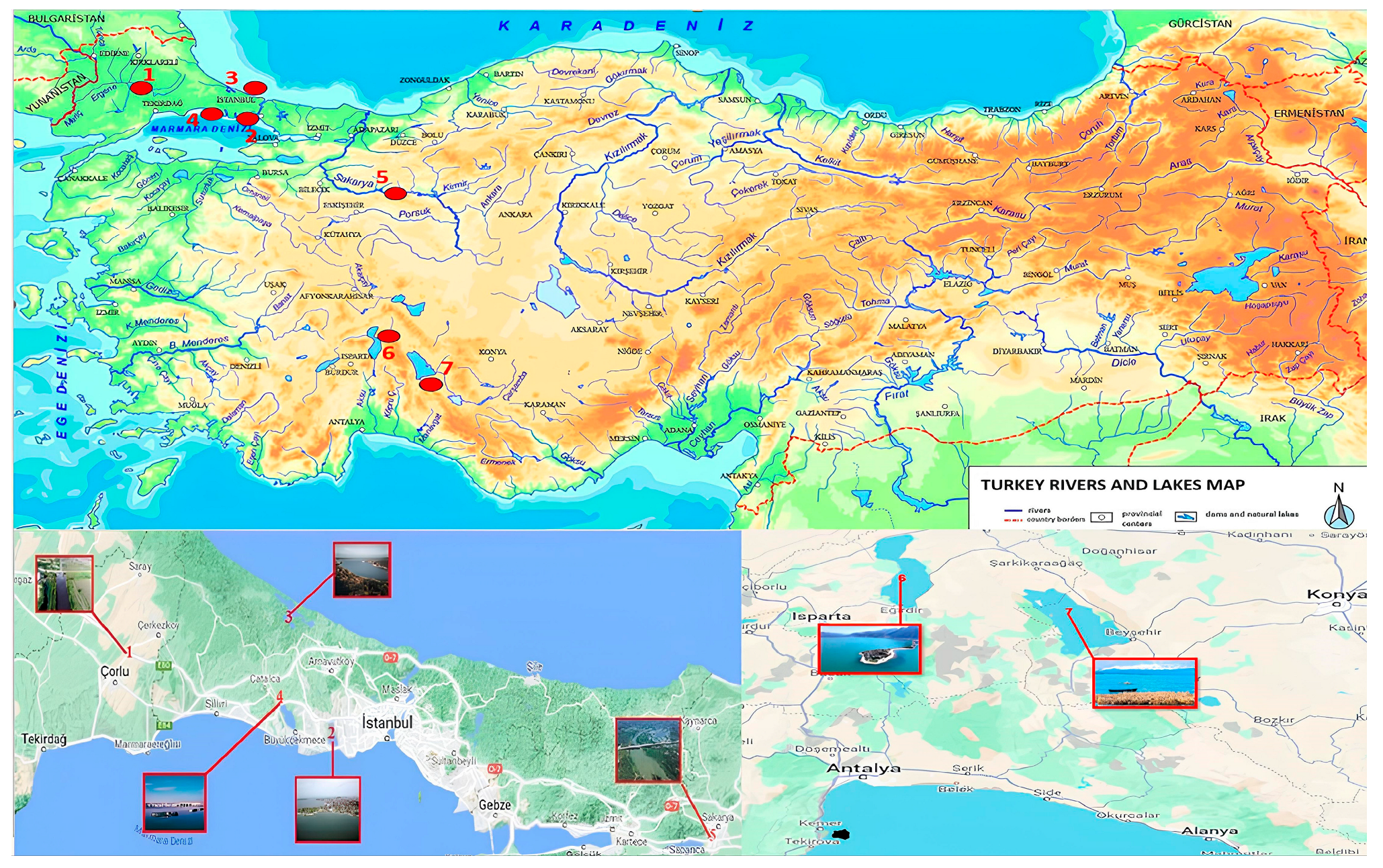
2.2. Sample Collection, Pre-Treatment, and Extraction Procedures
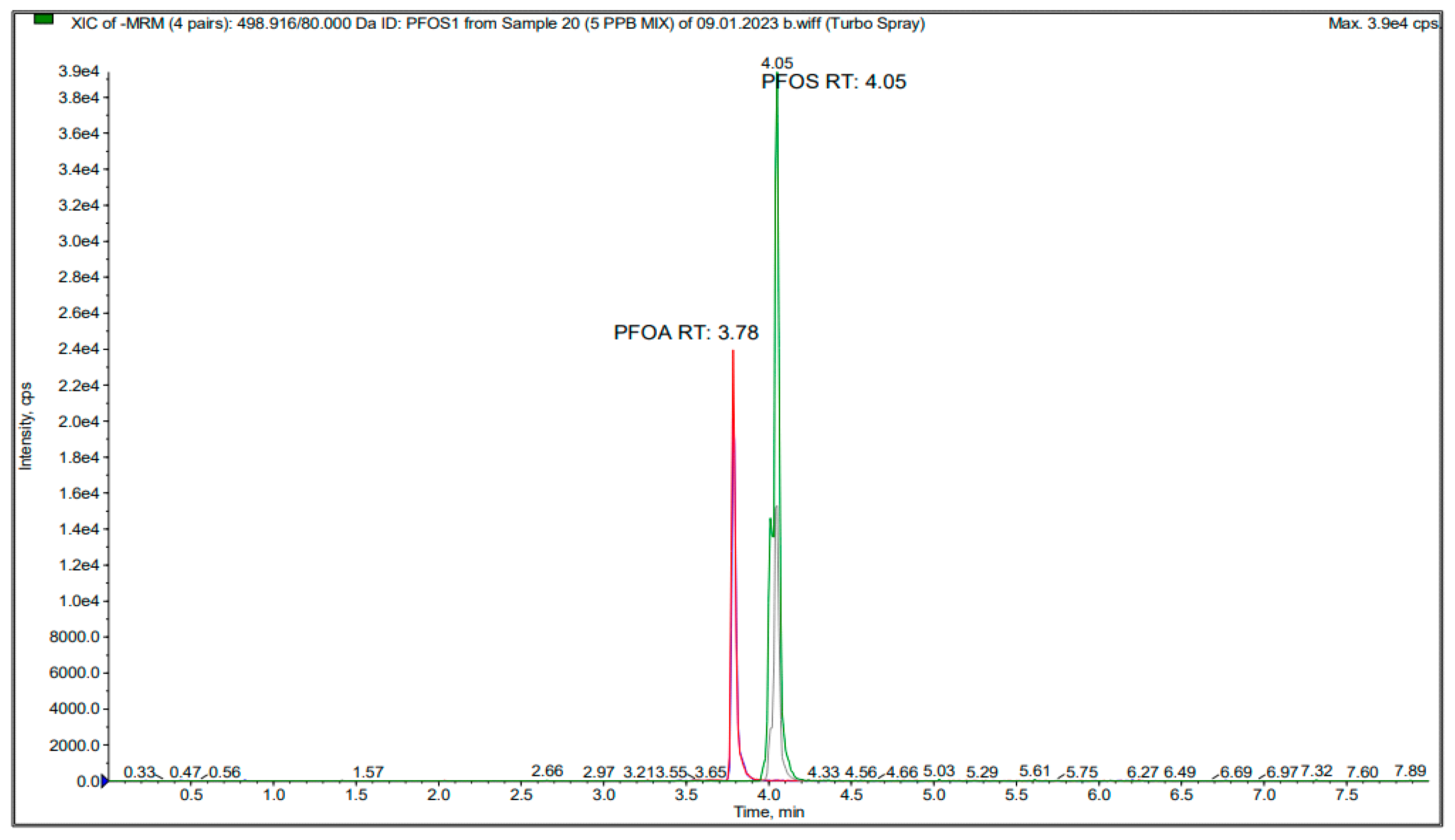
2.3. Ultra-High-Performance (QTRAP® LC-MS/MS) Analysis
3. Findings and Discussion
3.1. Methodological Performance
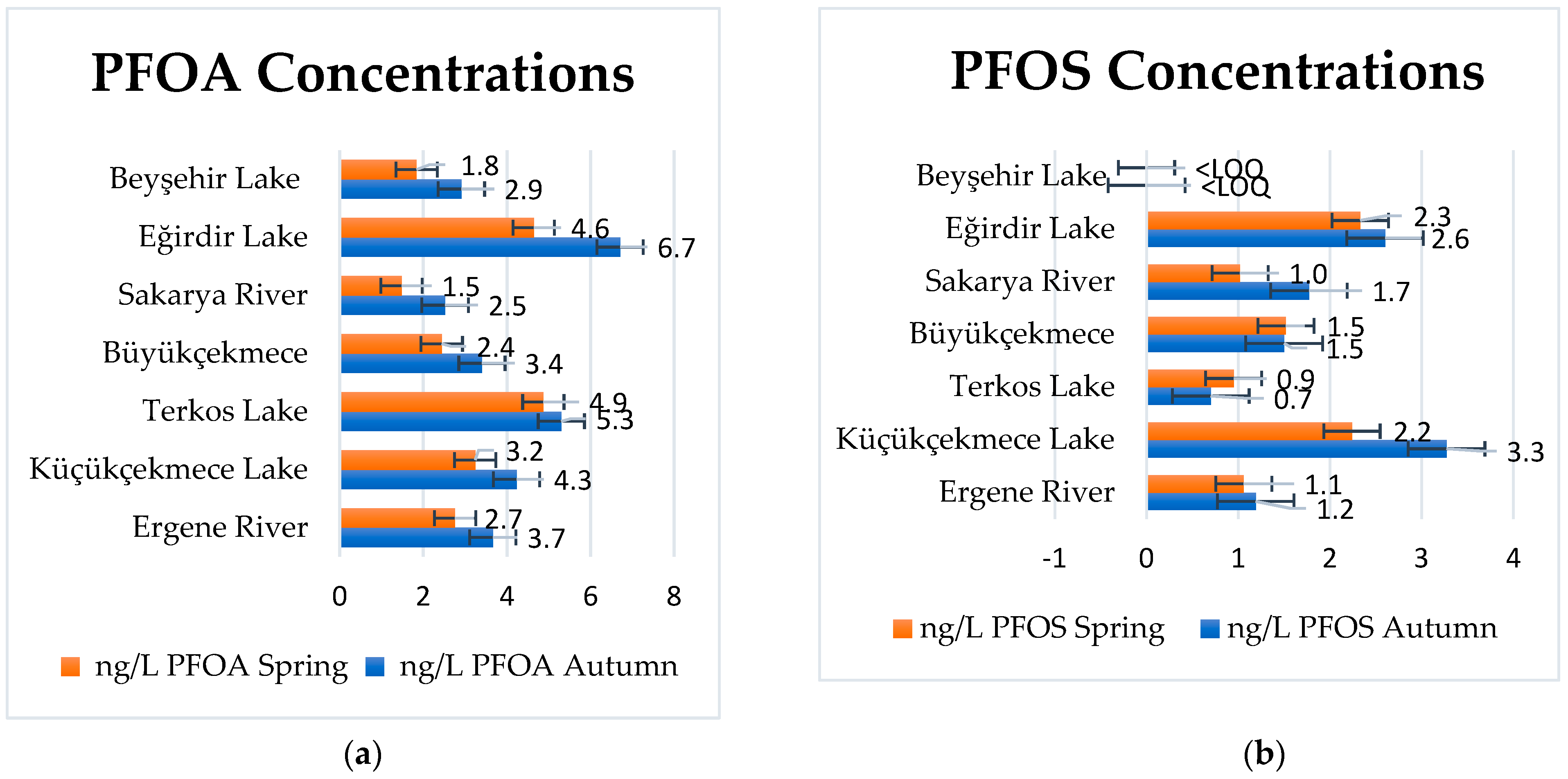
3.2. PFOA and PFOS in Surface Waters
3.3. PFOA and PFOS in Fish Tissue
4. Discussion
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fuertes, I.; Gomez-Lavin, S.; Elizalde, M.P.; Urtiaga, A. Perfluorinated alkyl substances (PFASs) in northern Spain municipal solid waste landfill leachates. Chemosphere 2017, 168, 399–407. [Google Scholar] [CrossRef]
- Blake, B.E.; Fenton, S.E. Early life exposure to per- and polyfluoroalkyl substances (PFAS) and latent health outcomes: A review including the placenta as a target tissue and possible driver of peri- and postnatal effects. Toxicology 2020, 443, 152565. [Google Scholar] [CrossRef]
- Glüge, J.; Scheringer, M.; Cousins, I.T.; DeWitt, J.C.; Goldenman, G.; Herzke, D.; Lohmann, R.; Ng, C.A.; Trier, X.; Wang, Z. An overview of the uses of per- and polyfluoroalkyl substances (PFAS). Environ. Sci. Process. Impacts 2020, 22, 2345–2373. [Google Scholar] [CrossRef]
- Cordner, A.; Goldenman, G.; Birnbaum, L.S.; Brown, P.; Miller, M.F.; Mueller, R.; Patton, S.; Salvatore, D.H.; Leonardo, L. The true cost of PFAS and the benefits of acting now. Environ. Sci. Technol. 2021, 55, 9630–9633. [Google Scholar] [CrossRef]
- Cousins, I.T.; DeWitt, J.C.; Glüge, J.; Goldenman, G.; Herzke, D.; Lohmann, R.; Miller, M.; Ng, C.A.; Scheringer, M.; Vierke, L.; et al. Strategies for group in per-and polyfluoroalkyl substances (PFAS) to protect human and environmental health. Environ. Sci. Process. Impacts 2020, 22, 1444–1460. [Google Scholar] [CrossRef]
- Sunderland, E.M.; Hu, X.C.; Dassuncao, C.; Tokranov, A.K.; Wagner, C.C.; Allen, J.G. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 131–147. [Google Scholar] [CrossRef]
- Lee, Y.M.; Lee, J.Y.; Kim, M.K.; Yang, H.; Lee, J.E.; Son, Y.; Kho, Y.; Choi, K.; Zoh, K.D. Concentration and distribution of per- and polyfluoroalkyl substances (PFAS) in the Asan Lake area of South Korea. J. Hazard. Mater. 2020, 381, 120909. [Google Scholar] [CrossRef]
- Gaines, L.G.T. Historical and current usage of per-and polyfluoroalkylsubstances (PFAS): A literature review. Am. J. Ind. Med. 2023, 66, 353–378. [Google Scholar] [CrossRef]
- Gerardu, T.; Dijkstra, J.; Beeltje, H.; Duivenbode, A.V.; Griffioen, J. Accumulation and transport of atmospherically deposited PFOA and PFOS in undisturbed soils downwind from a fluoropolymers factory. Environ. Adv. 2023, 11, 100332. [Google Scholar] [CrossRef]
- Podder, A.; Anwar-Sadmani, A.H.M.; Reinhart, D.; Chang, N.B.; Goel, R. Per and poly-fluoroalkyl substances (PFAS) as a contaminant of emerging concern in surface water: A transboundary review of their occurrences and toxicity effects. J. Hazard. Mater. 2021, 419, 126361. [Google Scholar] [CrossRef]
- Pontus, F. Regulation of Perfluorooctanoic Acid (PFOA) and Perfluorooctane Sulfonic Acid (PFOS) in Drinking Water: A Comprehensive Review. Water 2019, 11, 2003. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Shi, G.; Liu, C.; Hao, Q.; Wu, L. Occurrence and Risk Assessment of Perfluorooctanoate (PFOA) and Perfluorooctane Sulfonate (PFOS) in Surface Water, Groundwater and Sediments of the Jin River Basin, Southeastern China. Bull. Environ. Contam. Toxicol. 2022, 108, 1026–1032. [Google Scholar] [CrossRef]
- Vo, P.H.N.; Ngo, H.H.; Guo, W.; Nguyen, T.M.H.; Li, J.; Liang, H.; Deng, L.; Chen, Z.; Nguyen, T.A.H. Poly-and perfluoroalkyl substances in water and wastewater: A comprehensive review from sources to remediation. J. Water Process Eng. 2020, 36, 101393. [Google Scholar] [CrossRef]
- U.S Environmental Protection Agency. Proposed PFAS National Primary Drinking Water Regulation. Available online: https://www.epa.gov/sdwa/and-polyfluoroalkyl-substances-pfas (accessed on 10 August 2024).
- Flores, C.; Ventura, F.; Alonso, J.M.; Caixach, J. Occurrence of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in N.E. Spanish surface waters and their removal in a drinking water treatment plant that combines conventional and advanced treatments in parallel lines. Sci. Total Environ. 2013, 461–462, 618–626. [Google Scholar] [CrossRef]
- ISO 25101. 2009 Water Quality. Available online: https://www.iso.org/standard/42742.html (accessed on 29 October 2023).
- Ciccotelli, V.; Abete, C.M.; Stefania Squadrone, S. PFOS and PFOA in cereals and fish: Development and validation of a high-performance liquid chromatography-tandem mass spectrometry method. Food Control 2016, 59, 46–52. [Google Scholar] [CrossRef]
- Ikizoglu, B.; Turkdogan, F.I.; Kanat, G.; Aydıner, C. Seasonal analysis of commonly prescribed antibiotics in Istanbul city. Environ. Monit. Assess. 2023, 195, 566. [Google Scholar] [CrossRef]
- Llorca, M.; Farré, M.; Tavano, M.S.; Alonso, B.; Koremblitn, G.; Barceló, D. Fate of a broad spectrum of perfluorinated compounds in soils and biota from Tierra del Fuego Antarctica. Environ. Pollut. 2012, 163, 158–166. [Google Scholar] [CrossRef]
- Ozvardarlı, A. Quantitative Determination of Perfluorooctane Sulfonate (PFOS) and Perfluorooctanoic Acid (PFOA) Compounds in Surface Waters in Ergene Waterbasin and Evaluation of the Treatability of These Compounds. Ph.D. Thesis, Namık Kemal University, Graduate School of Natural and Applied Sciences, Tekirdağ, Türkiye, 2020. [Google Scholar]
- Huset, C.A.; Chiaia, A.C.; Barofsky, D.F.; Jonkers, N.; Kohler, H.P.E.; Ort, C.; Giger, W.; Field, J.A. Occurrence and mass flows of fluorochemicals in the Glatt valley watershed, Switzerland. Environ. Sci. Technol. 2008, 42, 6369–6377. [Google Scholar] [CrossRef]
- Liu, C.; Gin, K.Y.H.; Chang, V.W.C.; Goh, B.P.L. Novel perspectives on the bioaccumulation of PFCs–The concentration dependency. Environ. Sci. Technol. 2011, 45, 9758–9764. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Kannan, K. Distribution and partitioning of perfluoroalkyl carboxylic acids in surface soil, plants, and earthworms at a contaminated site. Sci. Total Environ. 2019, 647, 647–961. [Google Scholar] [CrossRef]
- Awad, E.; Xianming Zhang, X.; Bhavsar, S.P.; Steve Petro, S.; Patrick, W.; Crozier, P.W.; Reiner, E.J.; Fletcher, R.; Tittlemier, S.A.; Braekevelt, E. Long-Term Environmental Fate of Perfluorinated Compounds after Accidental Release at Toronto Airport. Environ. Sci. Technol. 2011, 45, 8081–8089. [Google Scholar] [CrossRef] [PubMed]
- Furdui, V.I.; Crozier, P.W.; Reiner, E.J.; Mabury, S.A. Trace level determination of perfluorinated compounds in water by direct injection. Chemosphere 2008, 73, 524–530. [Google Scholar] [CrossRef]
- Moody, C.A.; Martin, J.W.; Kwan, W.C.; Derek, C.G.; Muir, D.G.C.; Mabury, S. Monitoring Perfluorinated Surfactants in Biota and Surface Water Samples Following an Accidental Release of Fire-Fighting Foam into Etobicoke Creek. Environ. Sci. Technol. 2002, 36, 545–555. [Google Scholar] [CrossRef]
- Sinclair, E.; Mayack, D.T.; Kenneth Roblee, K.; Nobuyoshi Yamashita, N.; Kannan, K. Occurrence of Perfluoroalkyl Surfactants in Water, Fish, and Birds from New York State. Arch. Environ. Contam. Toxicol. 2006, 50, 398–410. [Google Scholar] [CrossRef] [PubMed]
- Boulanger, B.; Wargo, J.; Schnoor, J.S.; Hornbuckle, K.C. Detection of Perfluorooctane Surfactants in Great Lakes Water. Environ. Sci. Technol. 2004, 38, 4064–4070. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.H.; Liu, W.; Sato, I.; Nakayama, S.F.; Sasaki, K.; Saito, N.; Tsuda, S. PFOS and PFOA in environmental and tap water in China. Chemosphere 2009, 77, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Yeung, L.W.Y.; Yamashita, Y.; Taniyasu, N.; Lam, P.K.S.; Sinha, R.K.; Borole, D.V.; Kannan, K. A survey of perfluorinated compounds in surface water and biota including dolphins from the Ganges River and in other waterbodies in India. Chemosphere 2009, 76, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Zushi, Y.; Takeda, T.; Masunaga, S. Existence of nonpoint source of perfluorinated compounds and their loads in the Tsurumi River basin, Japan. Chemosphere 2008, 71, 1566–1573. [Google Scholar] [CrossRef]
- Senthilkumar, K.; Ohi, E.; Sajwan, K.; Takasuga, T.; Kannan, K. Perfluorinated compounds in river water, river sediment, market fish, and wildlife samples from Japan. Bull. Environ. Contam. Toxicol. 2007, 79, 427–431. [Google Scholar] [CrossRef]
- Sánchez-Avila, J.; Meyer, J.; Lacorte, S. Spatial distribution and sources of perfluorochemicals in the NW Mediterranean coastal waters (Catalonia, Spain). Environ. Pollut. 2010, 158, 2833–2840. [Google Scholar] [CrossRef]
- Pico, Y.; Blasco, C.; Farré, M.; Barceló, D. Occurrence of perfluorinated compounds in water and sediment of L’Albufera Natural Park (València, Spain). Environ. Sci. Pollut. Res. 2012, 19, 946–957. [Google Scholar] [CrossRef]
- Lin, A.Y.C.; Panchangam, S.C.; Lo, C.C. The impact of semiconductor, electronics and optoelectronic industries on downstream perfluorinated chemical contamination in Taiwan rivers. Environ. Pollut. 2009, 157, 1365–1372. [Google Scholar] [CrossRef]
- Takagi, S.; Adachi, F.; Miyano, K.; Koizumi, Y.; Tanaka, H.; Watanabe, I.; Tanabe, S.; Kannan, K. Fate of perfluorooctanesulfonate and perfluorooctanoate in drinking water treatment processes. Water Res. 2011, 45, 3925–3932. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Contaminants in the Food Chain (EFSA CONTAM Panel). Risk to human health related to the presence of perfluoroalkyl substances in food. EFSA J. 2020, 18, 6223. [Google Scholar] [CrossRef]
- Paiano, V.; Fattore, E.; Carra, A.; Generoso, C.; Fanelli, R.; Bagnati, R. Liquid chromatography–tandem mass spectrometry analysis of perfluorooctane sulfonate and perfluorooctanoic acid in fish fillet samples. J. Anal. Methods Chem. 2012, 719010. [Google Scholar] [CrossRef]
- Squadrone, S.; Ciccotelli, V.; Favoro, L.; Scanzio, T.; Prearo, M.; Abete, M.C. Fish consumption as a source of human exposure to perfluorinated alkyl substances in Italy: Analysis of two edible fish from Lake Maggiore. Chemosphere 2014, 114, 181–186. [Google Scholar] [CrossRef]
- Costopoulou, D.; Vassiliadou, I.; Leondiadis, L. PFASs intake from fish, eggs and drinking water in Greece in relation to the safety limits for weekly intake proposed in the EFSA scientific opinion of 2020. Chemosphere 2022, 286, 131851. [Google Scholar] [CrossRef]
- Augustsson, T.; Lennqvist, C.M.G.; Osbeck, P.; Tibblin, A.; Glynn, M.A.; Nguyen, E.; Westberg, R. Consumption of freshwater fish: A variable but significant risk factor for PFOS exposure. Environ. Res. 2021, 192, 110284. [Google Scholar] [CrossRef]
- Goodrow, S.M.; Ruppel, B.; Lippincott, R.L.; Post, G.B.; Procopio, N.A. Investigation of levels of perfluoroalkyl substances in surface water, sediment and fish tissue in New Jersey, USA. Sci. Total Environ. 2020, 729, 138839. [Google Scholar] [CrossRef]
- Fenton, S.E.; Ducatman, A.; Boobis, A.; DeWitt, J.C.; Lau, C.; Ng, C.; Smith, J.S.; Roberts, S.M. Per- and Polyfluoroalkyl Substance Toxicity and Human Health Review: Current State of Knowledge and Strategies for Informing Future Research. Environ. Toxicol. Chem. 2020, 40, 606–630. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.W.; Hamid, F.S.; Yusoff, I.; Chan, V. A review of PFAS research in Asia and occurrence of PFOA and PFOS in groundwater, surface water and coastal water in Asia. Groundw. Sustain. Dev. 2023, 22, 100947. [Google Scholar] [CrossRef]
- Toptancı, I.; Ketenoglu, O.; Kıralan, M. Assessment of the migration of perfuorinated compounds and primary aromatic amines from PTFE-coated non-stick cookware marketed in Turkey. Environ. Sci. Pollut. Res. 2022, 29, 38535–38549. [Google Scholar] [CrossRef]
- Sungur, Ş.; Köroğlu, M.; Turgut, F. Determination of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS) in food and beverages. Int. J. Environ. Anal. Chem. 2018, 98, 360–368. [Google Scholar] [CrossRef]
- Ünlü-Endirlik, B.; Bakır, E.; Boşgelmez, İ.İ.; Eken, A.; Narin, İ.; Gürbay, A. Assessment of perfluoroalkyl substances levels in tap and bottled water samples from Turkey. Chemosphere 2019, 235, 1162–1171. [Google Scholar] [CrossRef]
| PFC Molecule | Mass Q1 (Da) | Mass Q3 (Da) | DP (Volt) | EP (Volt) | CEP (Volt) | CE (Volt) | CXP (Volt) |
|---|---|---|---|---|---|---|---|
| PFOA 1 | 412.852 | 169 | −25 | −4.5 | −30 | −24 | −2 |
| PFOA 2 | 412.852 | 169 | −25 | −4.5 | −30 | −12 | −4 |
| PFOS 1 | 498.916 | 80 | −80 | −10.5 | −20 | −74 | 0 |
| PFOS 2 | 498.916 | 98.9 | −80 | −10.5 | −20 | −62 | 0 |
| Surface Water | Sample Point Coordinates | Autumn ng/L | Spring ng/L | |||
|---|---|---|---|---|---|---|
| Latitude (N) | Longitude (E) | PFOA | PFOS | PFOA | PFOS | |
| Ergene River | 41°21′31.92 | 27°81′39.82 | 3.7 ± 0.3 | 1.1 ± 0.1 | 2.7 ± 0.2 | 1.1 ± 0.1 |
| Sakarya River | 40°47′45.18 | 30°26′13.56 | 2.5 ± 0.3 | 1.4 ± 0.2 | 1.8 ± 0.1 | 1.0 ± 0.1 |
| Küçükçekmece Lake | 41°0′2.61 | 28°45′53.28 | 4.2 ± 0.1 | 3.3 ± 0.2 | 3.2 ± 0.7 | 2.2 ± 0.2 |
| Büyükçekmece Lake | 41°2′13.92 | 28°33′40.83 | 3.4 ± 0.2 | 1.5 ± 0.2 | 2.4 ± 0.1 | 1.5 ± 0.2 |
| Terkos Lake | 41°19′19.59 | 28°37′15.84 | 5.3 ± 1.1 | 0.7 ± 0.1 | 4.9 ± 0.3 | 0.9 ± 0.2 |
| Eğirdir Lake | 37°53′45.71 | 30°50′45.04 | 6.7 ± 2.9 | 2.6 ± 0.8 | 4.6 ± 0.2 | 2.3 ± 1.2 |
| Beyşehir Lake | 37°54′7.43 | 31°28′37.66 | 2.9 ± 0.3 | <LOQ | 1.8 ± 0.1 | <LOQ |
| Country | Water Body | PFOA (ng/L) | PFOS (ng/L) | References |
|---|---|---|---|---|
| Canada | Lake Ontorio | 4.4–44 | 3.1–37 | [24] |
| Grand River | 6.5–9.4 | 10.2–20.0 | [25] | |
| Lake Ontorio | <0.25–33 | <10 | [26] | |
| USA | Hudson River | 22–173 | 1.5–3.4 | [27] |
| Lake Champlain | 10–46 | 0.8–7.7 | ||
| Lake Erie | 21–47 | 11–39 | [28] | |
| China | Hong Lake | 12.2 | 6.0 | [29] |
| Dong Lake | 3.3 | 3.8 | ||
| Shenzhen River | 30.8 | 10.2 | ||
| Pearl River | 6.2–14.3 | 5.7–14.1 | ||
| India | Gangs River | 0.033–2.0 | 0.04–8.4 | [30] |
| Japan | Tsurumi River | 13.4–15.9 | 179.6–179.9 | [31] |
| Uji River | 100–110 | 8.7–10 | [32] | |
| Spain | Catalan Rivers | 0.79–9.63 | 1.09–9.56 | [33] |
| L’Albufera lake | 0.03–10.90 | 0.10–4.80 | [34] | |
| Taiwan | Xioali River | 17.3 | 82 | [35] |
| Tauchien River | 10.9 | 48.9 | ||
| Keya River | 310 | 5440 |
| Surface Waters | ng/g | |
|---|---|---|
| PFOA | PFOS | |
| Lake Küçükçekmece Pike (Esox Lucius) | <LOQ | 7.5 ± 0.3 |
| Lake Eğridir Trout (Salmo Trutta) | 2.5 ± 0.1 | <LOQ |
| Lake Eğridir 1st season (Cyprinus Carpio) | <LOQ | <LOQ |
| Lake Eğirdir 2nd season (Cyprinus Carpio) | <LOQ | <LOQ |
| Lake Beysehir Carp 1st season (Cyprinus Carpio) | <LOQ | <LOQ |
| Lake Beyşehir Carp 2nd season (Cyprinus Carpio) | <LOQ | <LOQ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikizoglu, B. PFOA and PFOS Pollution in Surface Waters and Surface Water Fish. Water 2024, 16, 2342. https://doi.org/10.3390/w16162342
Ikizoglu B. PFOA and PFOS Pollution in Surface Waters and Surface Water Fish. Water. 2024; 16(16):2342. https://doi.org/10.3390/w16162342
Chicago/Turabian StyleIkizoglu, Bahar. 2024. "PFOA and PFOS Pollution in Surface Waters and Surface Water Fish" Water 16, no. 16: 2342. https://doi.org/10.3390/w16162342
APA StyleIkizoglu, B. (2024). PFOA and PFOS Pollution in Surface Waters and Surface Water Fish. Water, 16(16), 2342. https://doi.org/10.3390/w16162342






