A Framework to Evaluate Groundwater Quality and the Relationship between Rock Weathering and Groundwater Hydrogeochemistry in the Tropical Zone: A Case Study of Coastal Aquifer Arroyo Grande, in the Caribbean Region of Colombia
Abstract
1. Introduction
2. Review of Hydrochemical Studies of Coastal Aquifers
3. Methods and Materials
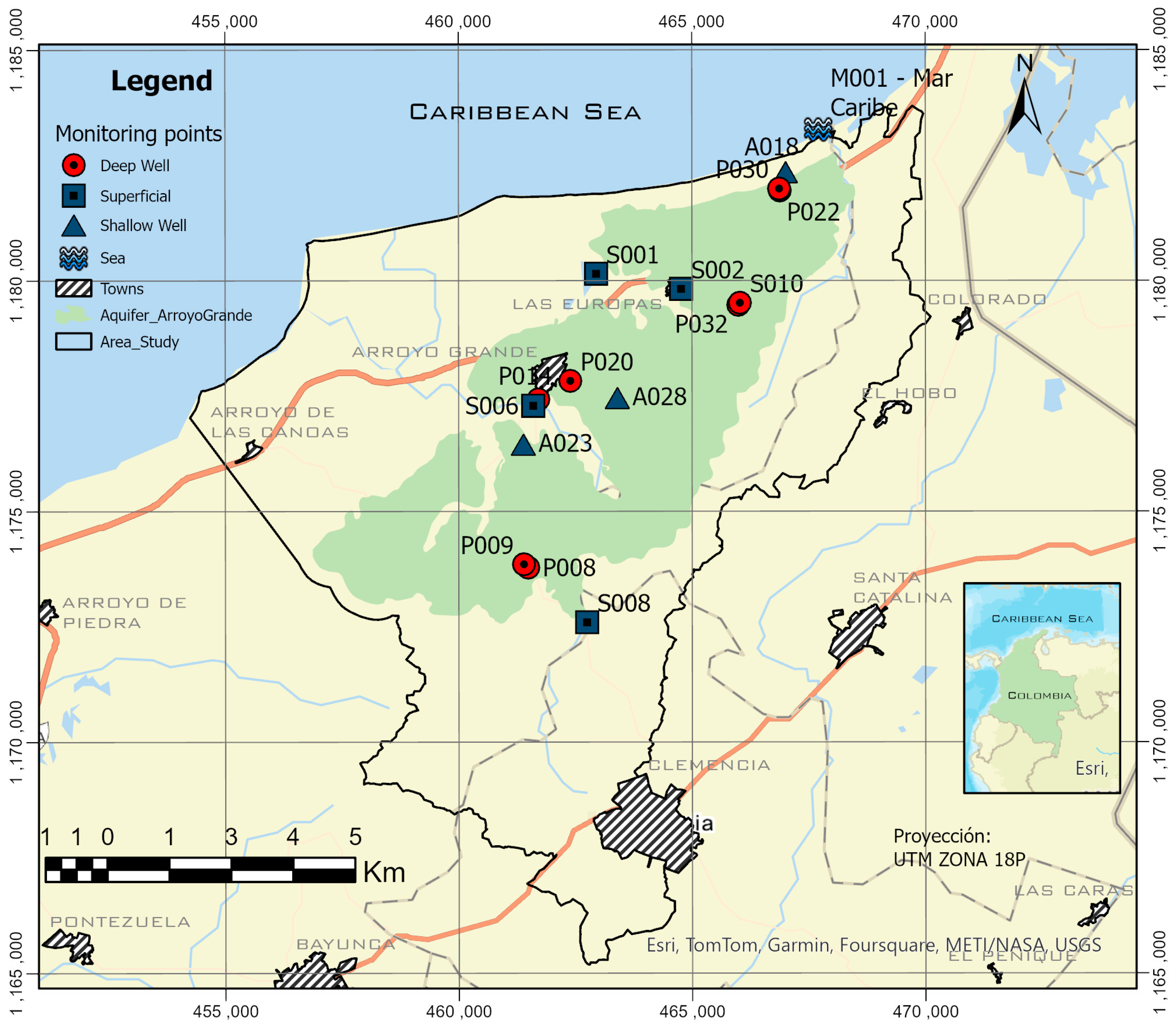
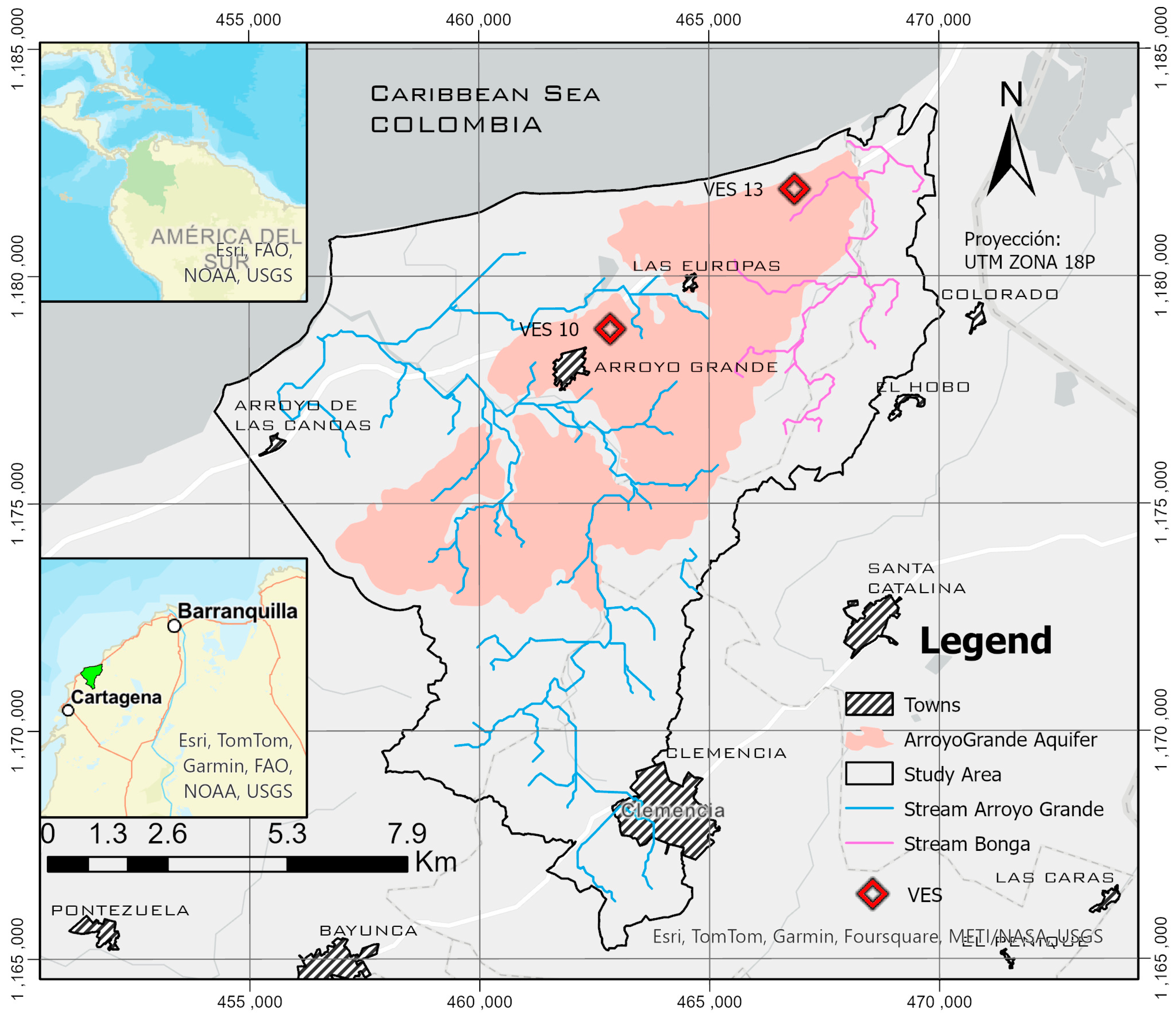
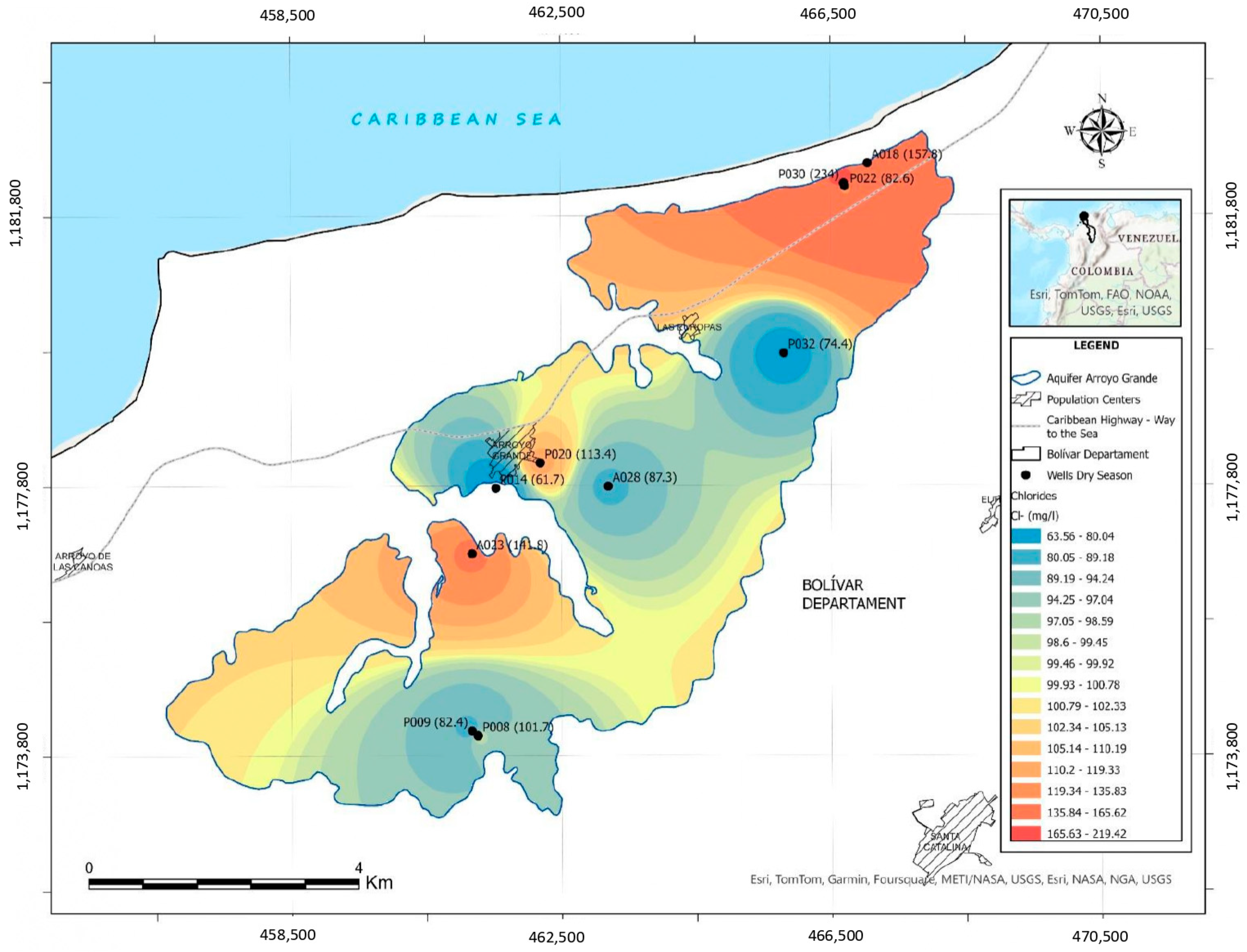
3.1. Study Area
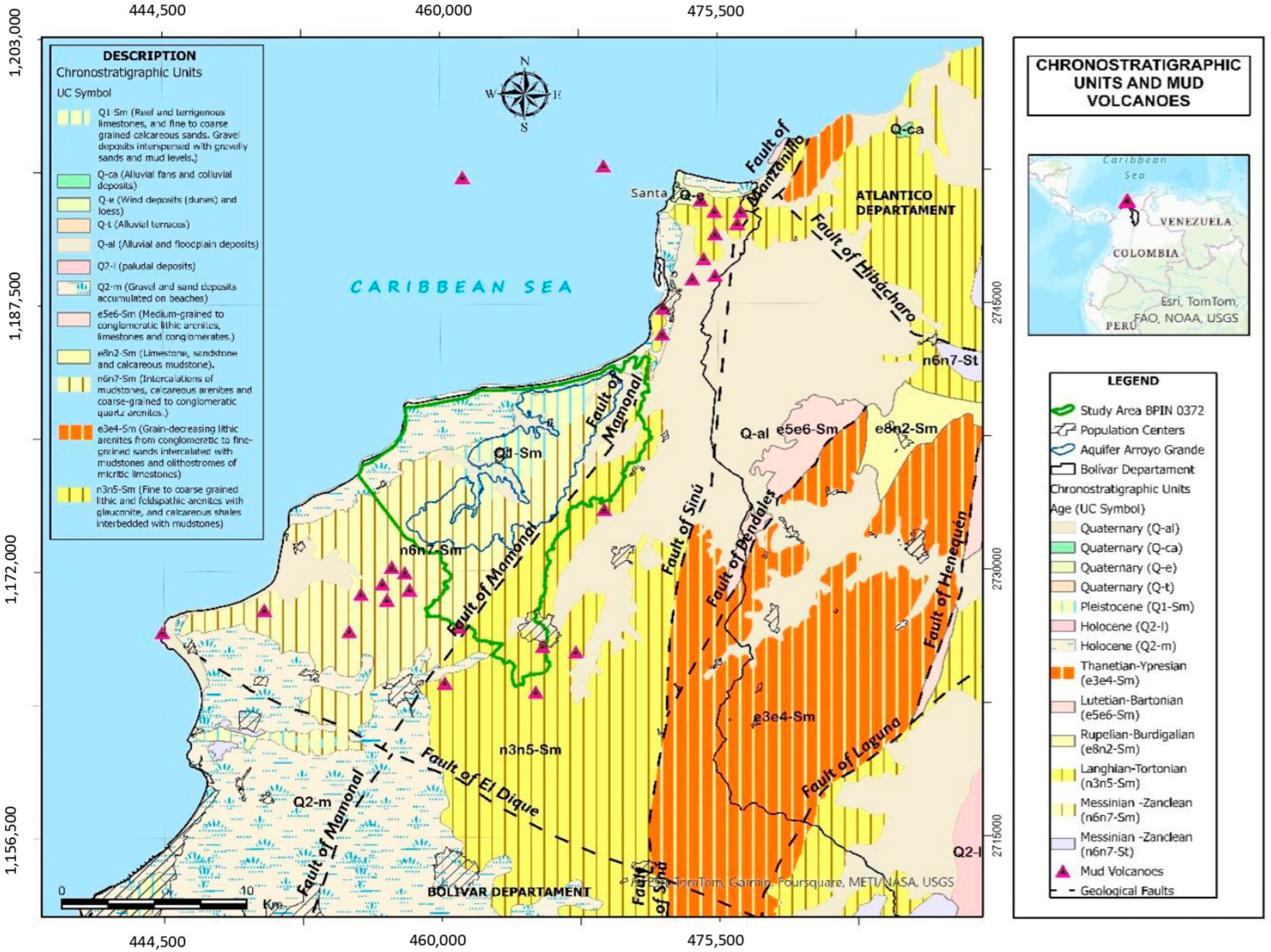
Geology and Hydrogeology
3.2. Proposed Integrated Framework
3.2.1. Physicochemical Characterization
3.2.2. Identification of Water Constituents and Types of Water
Descriptive Statistics
Hydrochemical Diagrams and Indices
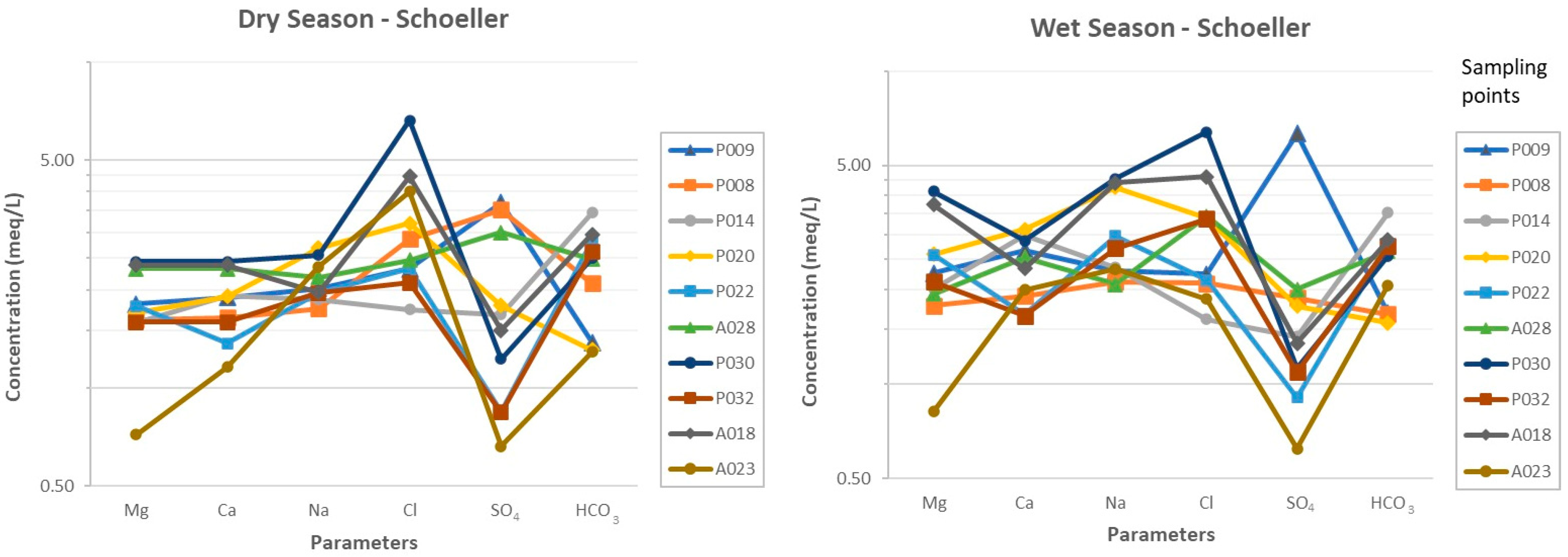
Schoeller Plot
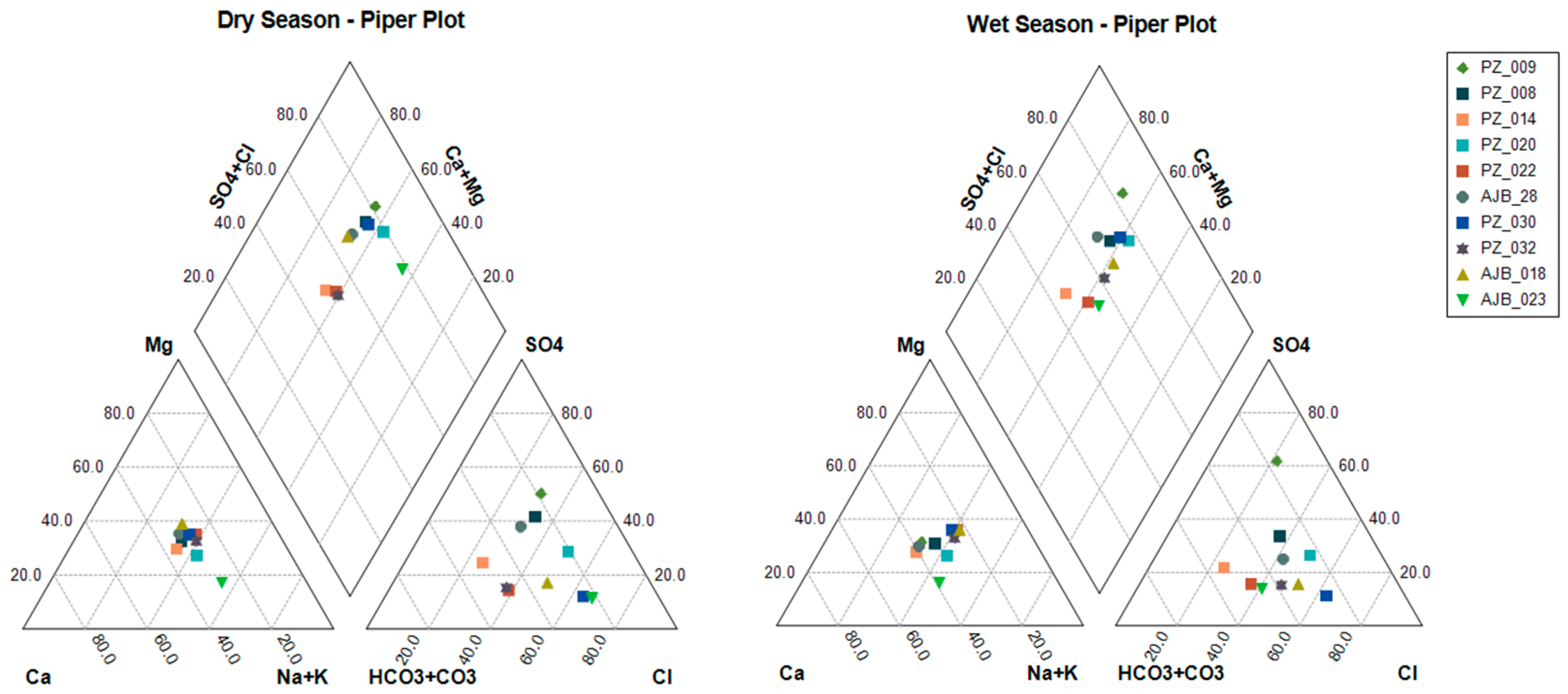
Piper Diagram
3.2.3. Identification of Dominant Mechanisms in Water Composition
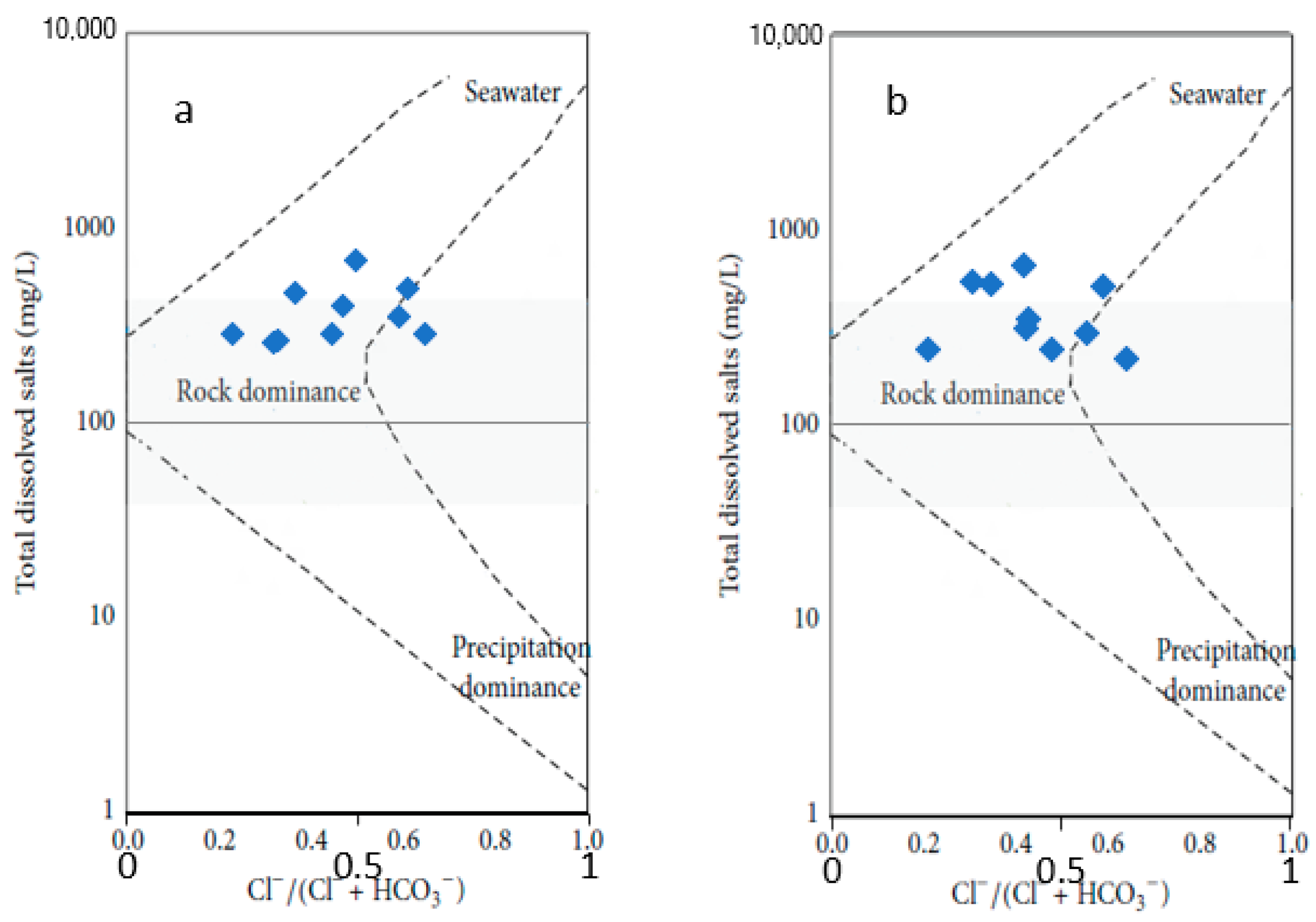
Gibbs Plot
3.2.4. Evaluation of Ion Exchange Processes
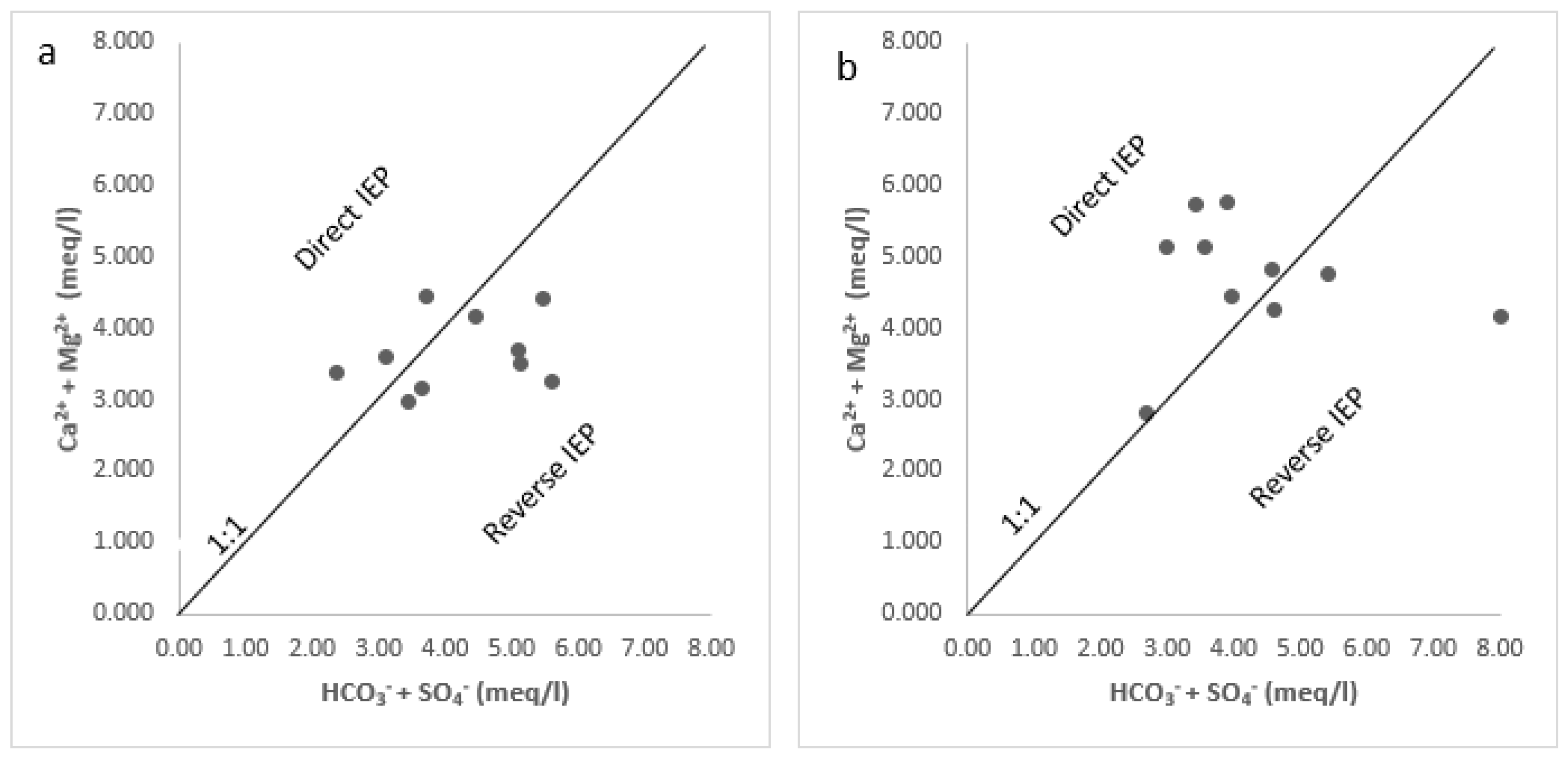
Scatter Diagrams of Ionic Ratios
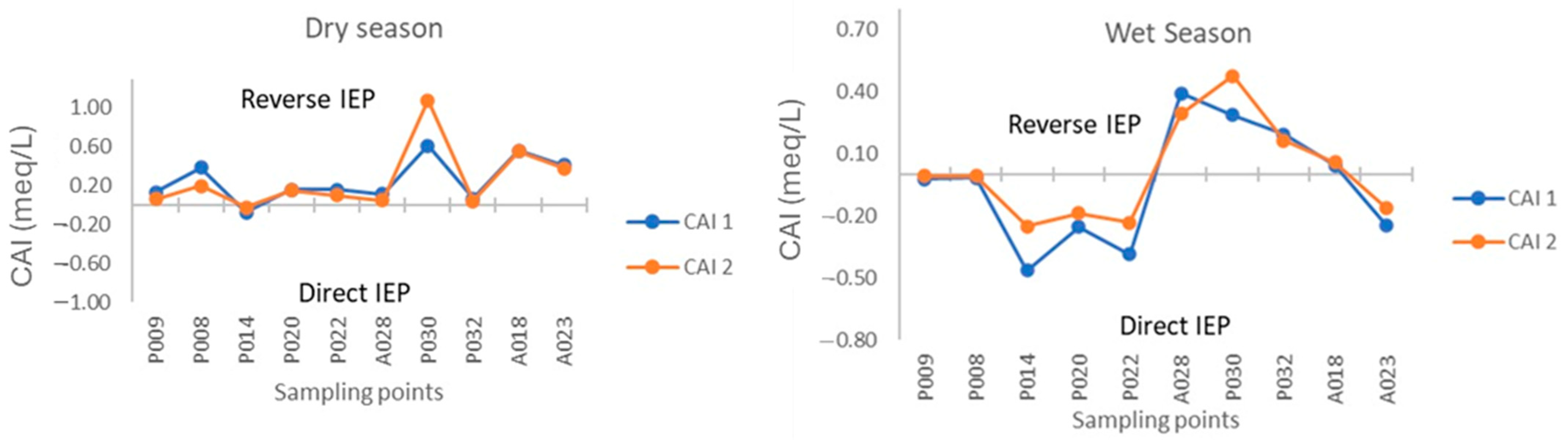
CAI Index
3.2.5. Identification of Processes Impacting Water Composition and Interrelationship between Chemical Parameters
Multivariate Statistical Analysis
3.2.6. Drinking Water Quality Assessment
4. Results and Discussion
4.1. Groundwater Hydrochemistry
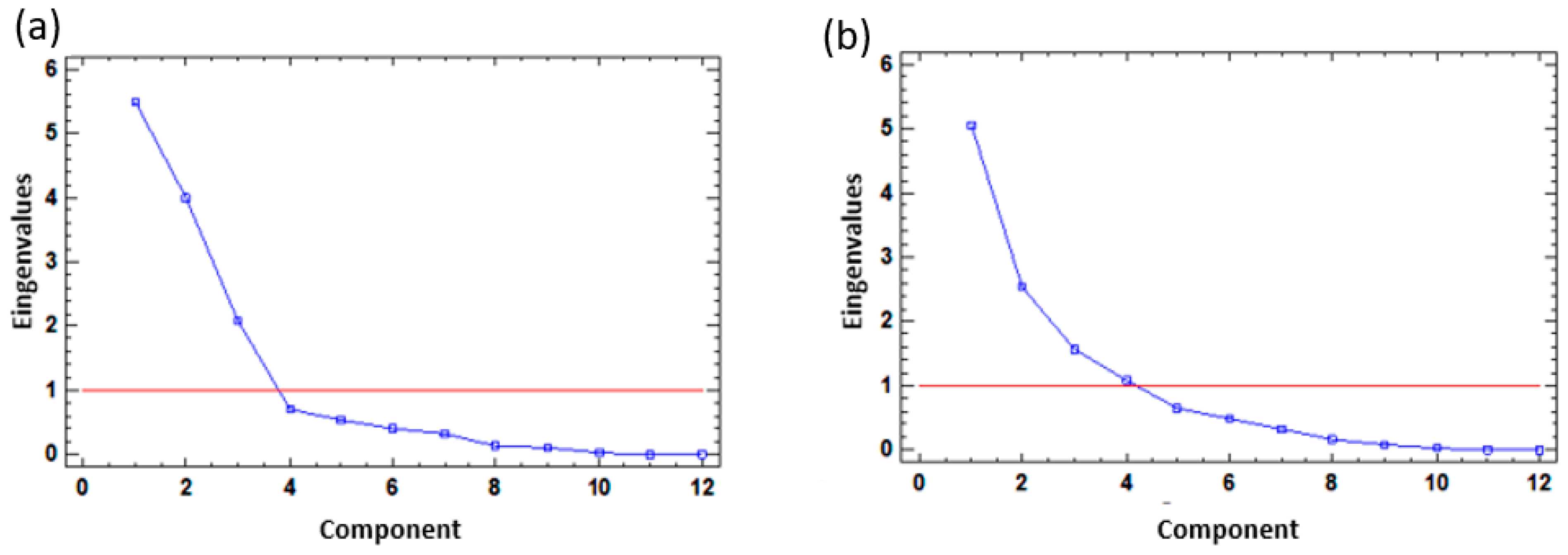
4.2. Principal Component Analysis (PCA)
4.3. Water Quality Index (WQI)
5. Conclusions
- The groundwater and surface waters of the aquifer evidenced distinct levels of mineralization due to the occurrence of mechanisms such as mineral dissolution, ion exchange, seawater intrusion, and anthropogenic contamination.
- The characteristics acquired by the water affected the availability of freshwater suitable for drinking for the communities near the area, to the point that in the dry and wet seasons, at least 50% of the sampled groundwater points are inadequate for drinking and fish farming.
- The groundwater of the Arroyo Grande hydrogeological unit exhibits high spatial and temporal variability in its main components. The heterogeneity and dynamics of this aquifer are evidenced in the several types of water found, which correspond to sodium sulfate, calcium bicarbonate, sodium chloride, sodium bicarbonate, magnesium sulfate, and magnesium chloride.
- The dominant elements in the Arroyo Grande water chemistry are the anions bicarbonate, chloride, and sulfate, in addition to cations sodium, calcium, and magnesium. The rock–water interaction mechanism is the most relevant in defining the chemical signature of the groundwater, through mineral dissolution and ion exchange processes related to the geological information of the area. The identification of the exact minerals that are contributing to the presence of Fe in the groundwater of the Arroyo Grande aquifer requires further mineralogical studies.
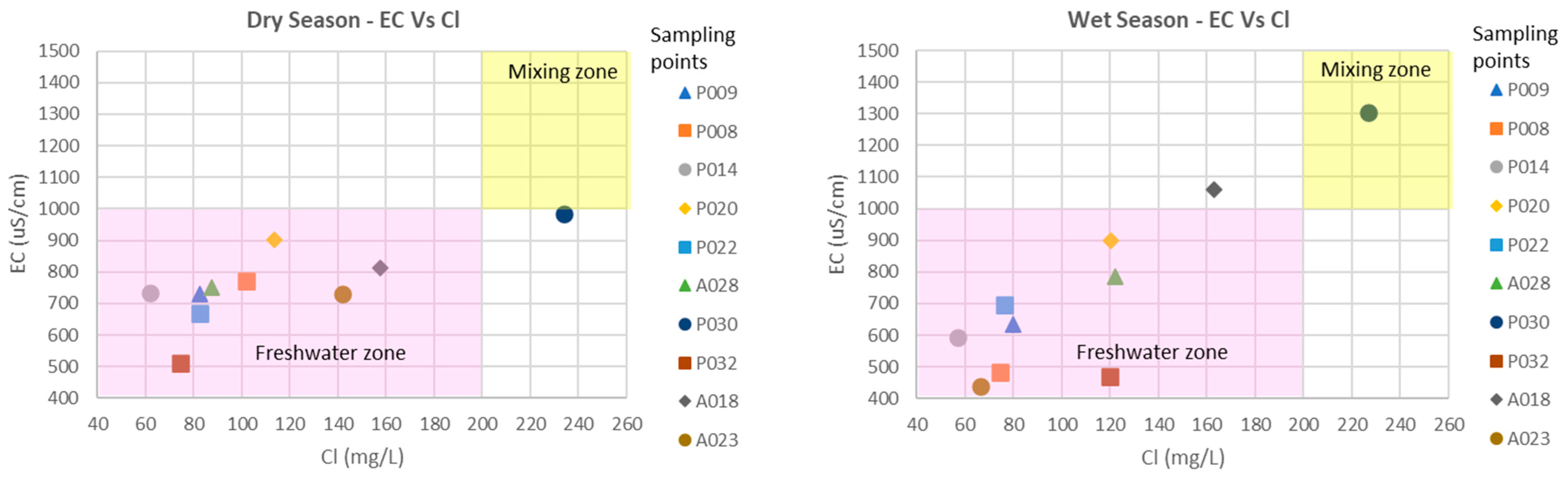
- Other factors affecting the chemical composition are the intrusion of seawater and anthropogenic activities. An increase in the saline intrusion phenomenon has been observed, currently affecting the extraction points near the coastal strip, which have high values of electrical conductivity and chlorides. Concentrations of iron and manganese are higher than drinking water standards, which requires constant monitoring of these parameters.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| System | Geology | Study Season | Types of Water Identified | Dominant Ions | Dominant Hydrochemical Processes a | Applied Hydrochemical Analysis b | Ref. | |
|---|---|---|---|---|---|---|---|---|
| Rock | Minerals | |||||||
| Morrosquillo Aquifer, Colombia | Sedimentary rock and sedimentary deposits | Calcite Dolomite Quartz Cast Halita | Dry and wet season | HCO3-Ca-Mg Cl-Na HCO3-Na | Dry season: Cation: Na+ > Ca2+ > Mg2+ > K+ Anion: HCO3− > Cl− > SO42− Wet season: Cation: Na+ > Ca2+ > Mg2+ > K+ Anion: HCO3− > Cl− > SO42− | R, I, S | PD, SP, SF | [6] |
| Recife metropolitan region aquifer, Brasil | Sedimentary rock | Calcite Dolomite Cast | Not indicated | Cl-Na HCO3-Na HCO3-Ca SO42-Ca | Cation: Na+ > Mg2+ > Ca2+ > K+ Anion: Cl− > HCO3− > SO42− > NO3− | R, I, W, P, A | G, IR, PD, ST, SF | [17] |
| Southwest Indian coastal aquifer, India | Igneous and metamorphic rocks (laterite, migmatite, granodiorite, and peninsular gneiss) | Quartz, feldspar, micas, muscovite, biotite, and amphibole | Pre-monsoon (may), monsoon, post (Sept) monsoon (January) | HCO3-Ca-Mg Cl-Ca Cl-SO42-Na Cl-SO42-Ca | Pre-monsoon Cation: Na+ > Ca2+ Anion: Cl− > HCO3− Monsoon Cation: Ca2+ > Na+ Anion: HCO3− > Cl− Post monsoon Cation: Ca2+ > Na+ Anion: HCO3− > Cl− | R, I | G, IR, PD, ST, CAI | [20] |
| Gaza coastal aquifer, Palestine | Sedimentary rock and sedimentary deposits | Calcite Dolomite Halite | Not indicated | Cl-Na HCO3-Na mixta Cl-Ca-Mg Ca/Mg–NO3/HCO3 | Cation: Ca2+ > Na+ > Mg2+ Anion: HCO3− > Cl− >SO42− | R, I, S, W, P | IR, PD, ST, CAI, CH, SP | [21] |
| Multilayered aquifers, Lower Kelantan Basin, Malaysia | Sedimentary rock and sedimentary deposits (shale, sandstone, phyllite, and slate) | Quartz Muscovite Sericite Dolomite Mica Goethite Hematite | Not indicated | HCO3-Ca HCO3-Na | Cation: Na+ > Ca2+ > Mg2+ > K+ Anion: HCO3− > Cl− > SO42− > CO3− | R, I, S, W, F | IR, PD, ST, SP | [22] |
| Lagos coastal belt aquifer, Nigeria | Sedimentary rock and sedimentary deposits | Calcite Dolomite Cast | Dry and wet season | Dry season: HCO3-Ca HCO3-Ca-Mg Wet season: HCO3-Ca-Mg Cl-SO42-Ca–Mg Cl-Ca-Mg Cl-Ca | Dry season: Cation: Ca2+ > K+ > Na+ > Mg2+ Anion: HCO3− > Cl− Wet season: Cation: Ca2+ > Mg2+ > K+ > Na+ Anion: Cl− > HCO3− > SO42− | R, I, S, M | IR, PD, SP, D, GM | [23] |
| Various mediterranean coast aquifers * | Sedimentary rock and sedimentary deposits | Calcite Dolomite Yeso anhydrite | Not indicated | Cl-Na Cl-ca HCO3-Ca HCO3-Na SO42-Ca-Mg | Cation: Na+ > Ca2+ > Mg2+ Anion: Cl− > SO42− > HCO3− | R, I, S, A | IR, PD, ST, SI | [24] |
| Quintana Roo southern zone aquifer, Mexico | Sedimentary rocks (Limestone-dolimias-evapotites) | Dolomite Aragonite Cast Halite | Wet season | HCO3-Ca Cl-Ca-Mg SO42-Ca Cl-Na | Cation: Ca2+ > Na+ > Mg2+ > K+ Anion: HCO3− > SO42− > Cl− > NO3− | R, I, S, A | PD, ST | [25] |
| Arroyo Grande aquifer, Colombia | Sedimentary rock and sedimentary deposits | Calcite Dolomite Quartz Halita | Dry and wet season | HCO3-Ca HCO3-Na Cl-Na SO42-Na Cl-Mg SO42-Mg | Dry season: Cation: Na+ > Ca2+ > Mg2+ > Fe2+ Anion: HCO3− > Cl− > SO42− > NO3− Wet season: Cation: Na+ > Ca2+ > Mg2+ > Fe2+ Anion: HCO3− > Cl− > SO42− > NO3− | R, I, S, A | G, IR, PD, CAI, ST, SP | This study |
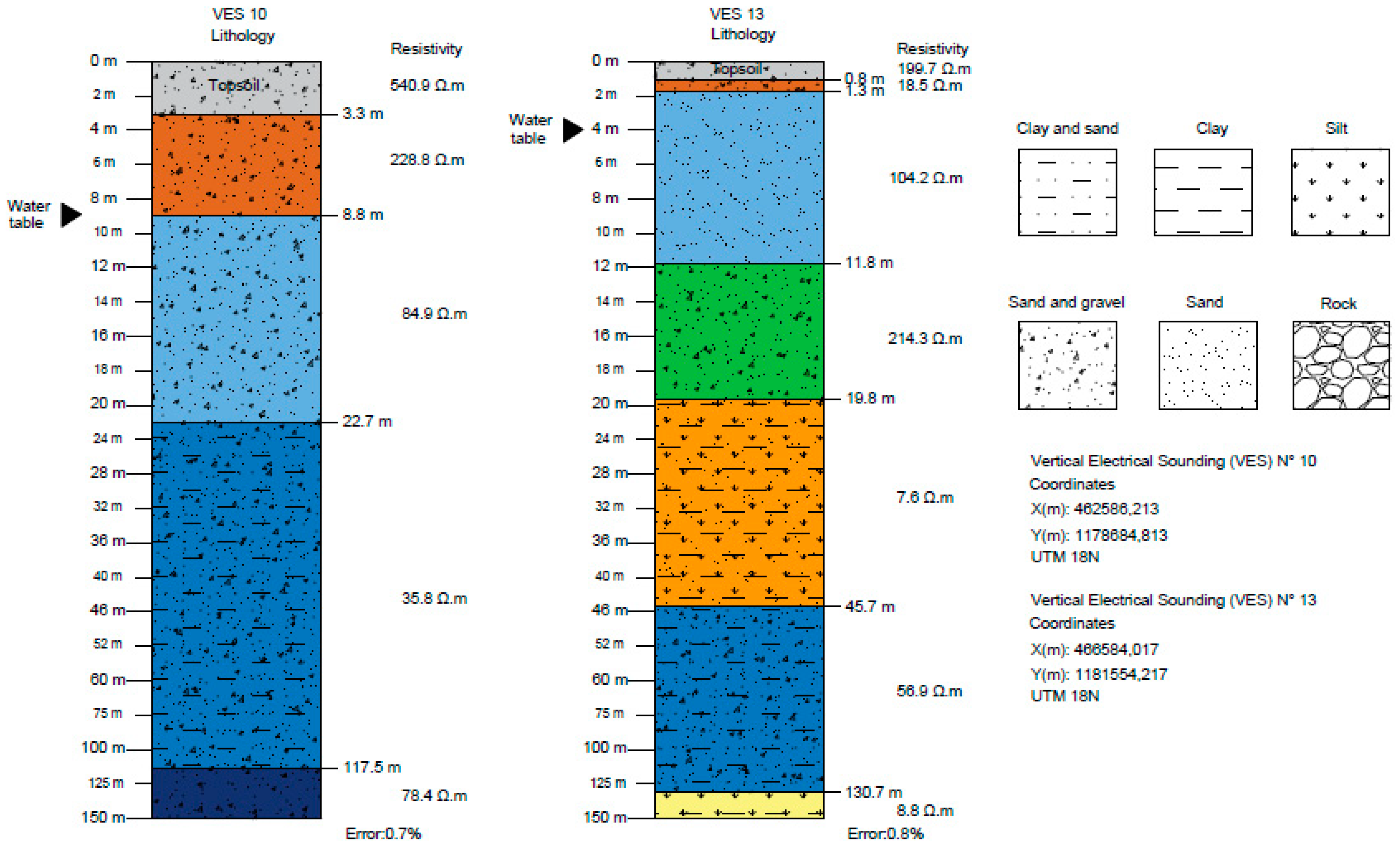
| Layer | Lithology SEV 10 | Resistivity (Ω.m) | Thickness (m) | Depth (m) |
|---|---|---|---|---|
| 1 | Topsoil covered by sand and gravel | 540.9 | 3.4 | 3.4 |
| 2 | Dry granular material (sands and gravels) | 228.8 | 5.4 | 8.8 |
| 3 | Saturated granular material (sands and gravels) | 84.9 | 13.9 | 22.7 |
| 4 | Saturated fine granular materials (sands, gravels, clays, and silts) | 35.8 | 94.8 | 117.5 |
| 5 | Saturated granular material (sands and gravels) | 78.4 | - | - |
| Layer | Lithology SEV 13 | Resistivity (Ω.m) | Thickness (m) | Depth (m) |
|---|---|---|---|---|
| 1 | Vegetation covered by sand, gravel, and silt | 199.7 | 0.8 | 0.8 |
| 2 | Fine to dry granular materials (sands, clays, and silt) | 18.5 | 0.5 | 1.3 |
| 3 | Saturated granular material (sands) | 104.2 | 10.5 | 11.8 |
| 4 | Saturated granular material (sands and gravels) | 214.3 | 8.0 | 19.8 |
| 5 | Fine to saturated granular materials (few sands, clays, and silt) | 7.6 | 25.9 | 45.7 |
| 6 | Saturated granular materials (sands, gravels, and silt) | 56.9 | 85 | 130.7 |
| 7 | Fine to saturated granular materials (few sands, clays, and silt) | 8.8 | ? | ? |
| Parameter | Analysis | Uncertainty (±) |
|---|---|---|
| Anions | Nitrates | 0.033 |
| Anions | Fluorides | 0.039 |
| Anions | Nitrites | 0.033 |
| Anions | Sulphate | 0.071 |
| Principal ions or minerals | Total alkalinity | 0.05 |
| Principal ions or minerals | Sulphate | 0.04 |
| Principal ions or minerals | Carbonates | 0.057 |
| Principal ions or minerals | Bicarbonates | 0.057 |
| Nutrients | Nitrite | 0.001 |
| Nutrients | Total nitrogen Kjeldahl | 0.01 |
| Nutrients | Total phosphorus | 0.03 |
| Principal ions or minerals | Total calcium | 0.03 |
| Principal ions or minerals | Total magnesium | 0.13 |
| Principal ions or minerals | Total potassium | 0.04 |
| Principal ions or minerals | Total sodium | 0.05 |
| Trace metals | Total arsenic | 0.13 |
| Trace metals | Total boro | 0.03 |
| Trace metals | Total iron | 0.05 |
| Trace metals | Total manganese | 0.13 |
| Trace metals | Total mercury | 0.13 |
| Trace metals | Total lead | 0.14 |
| Trace metals | Total selenio | 0.16 |
References
- Post, V.; Eichholz, M.; Brentfuerher, R. Groundwater management in Coastal Zones; Bundesanstalt für Geowissenschaften und Rohstoffe (BGR): Hannover, Germany, 2018. [Google Scholar]
- Custodio, E. Trends in Groundwater Pollution: Loss of Groundwater Quality and Related Services; Groundwater Governance: Delft, The Netherlands, 2011. [Google Scholar]
- IDEAM—Instituto de Hidrología, Meteorología y Estudios Ambientales. Estudio Nacional del Agua; IDEAM: Bogota, Colombia, 2022. [Google Scholar]
- Chala, D.C.; Quiñones-Bolaños, E.; Mehrvar, M. An integrated framework to model salinity intrusion in coastal unconfined aquifers considering intrinsic vulnerability factors, driving forces, and land subsidence. J. Environ. Chem. Eng. 2022, 10, 106873. [Google Scholar] [CrossRef]
- UNESCO. UN-Water: United Nations World Water Development Report 2020: Water and Climate Change; UNESCO: Paris, France, 2020. [Google Scholar]
- Martinez, D.C.; Betancur, T.; Herrera, H.M. Hydrogeochemical assessment and modeling of Morrosquillo Coastal Aquifer (Sucre-Colombia). Rev. Fac. Ing. 2014, 71, 126–140. [Google Scholar] [CrossRef]
- Gomez Arevalo, E.D. A Groundwater Flow Model to Aid in Water Resource Management for the Carraipia Basin in the Coastal Semi-Arid Region of La Guajira State (Colombia); The University of Maine: Orono, ME, USA, 2020; p. 83. [Google Scholar]
- Cardique, Identificacion de la Vulnerabilidad del Acuifero Costero de Arroyo Grande Ante la Intrusion Salina; Cardique: Cartagena, Colombia, 2006.
- Restrepo-Ángel, J.D.; Mora-Páez, H.; Díaz, F.; Govorcin, M.; Wdowinski, S.; Giraldo-Londoño, L.; Tosic, M.; Fernández, I.; Paniagua-Arroyave, J.F.; Duque-Trujillo, J.F. Coastal subsidence increases vulnerability to sea level rise over twenty first century in Cartagena, Caribbean Colombia. Sci. Rep. 2021, 11, 18873. [Google Scholar] [CrossRef]
- Hincapie, G.; Hueguett, A. Memoria Tecnica de la Plancha 5-04-Atlas de Aguas Subterraneas de Colombia pdf; Bogotá, Colombia, 2003. Available online: https://recordcenter.sgc.gov.co/B3/12006050020005/documento/Pdf/29.MemoriaPL5-04.pdf (accessed on 10 July 2022).
- CARDIQUE. Hidrogeologos Estudio Hidrogeologico del Acuifero de Arroyo Grande; CARDIQUE: Bolivar, Colombia, 1999. [Google Scholar]
- Chala, D.C.; Jiménez, T.; Rodríguez, D.; Quinoñes, E.; Mehrvar, M.; St, V.; Mb, O.N.; Carolina, D.; Diaz, C.; Bolaños, E.Q. Groundwater governance of coastal aquifers in the Colombian Caribbean Region, South America: Call to action to strengthen aquifers resilience and groundwater use against climate change effects. In Proceedings of the International Young Water Professionals Conference, Toronto, ON, Canada, 23 June 2019. [Google Scholar]
- Machiwal, D.; Jha, M.K. Identifying sources of groundwater contamination in a hard-rock aquifer system using multivariate statistical analyses and GIS-based geostatistical modeling techniques. J. Hydrol. Reg. Stud. 2015, 4, 80–110. [Google Scholar] [CrossRef]
- Aldhyani, T.H.H.; Al-Yaari, M.; Alkahtani, H.; Maashi, M. Water Quality Prediction Using Artificial Intelligence Algorithms. Appl. Bionics Biomech. 2020, 2020, 6659314. [Google Scholar] [CrossRef]
- Malik, A. Sugandh Groundwater hydro-geochemistry, irrigation and drinking quality, and source apportionment in the intensively cultivated area of Sutlej sub-basin of main Indus basin. Environ. Earth Sci. 2022, 81, 456. [Google Scholar] [CrossRef]
- Islam, A.R.M.T.; Kabir, M.M.; Faruk, S.; Al Jahin, J.; Bodrud-Doza, M.; Didar-ul-Alam, M.; Bahadur, N.M.; Mohinuzzaman, M.; Fatema, K.J.; Safiur Rahman, M.; et al. Sustainable groundwater quality in southeast coastal Bangladesh: Co-dispersions, sources, and probabilistic health risk assessment. Environ. Dev. Sustain. 2021, 23, 18394–18423. [Google Scholar] [CrossRef]
- Silva, T.R.; Leitão, T.E.; Lima, M.M.C.; Martins, T.N.; Oliveira, M.M.; Albuquerque, M.S.C.; Costa, W.D. Hydrogeochemistry and isotope compositions of multi-layered aquifer systems in the Recife Metropolitan Region, Pernambuco (NE Brazil): An integrated approach using multivariate statistical analyses. J. S. Am. Earth Sci. 2021, 109, 103323. [Google Scholar] [CrossRef]
- Pohl, C. From science to policy through transdisciplinary research. Environ. Sci. Policy 2008, 11, 46–53. [Google Scholar] [CrossRef]
- Pahl-Wostl, C.; Holtz, G.; Kastens, B.; Knieper, C. Analyzing complex water governance regimes: The Management and Transition Framework. Environ. Sci. Policy 2010, 13, 571–581. [Google Scholar] [CrossRef]
- Akshitha, V.; Balakrishna, K.; Udayashankar, H.N. Assessment of hydrogeochemical characteristics and saltwater intrusion in selected coastal aquifers of southwestern India. Mar. Pollut. Bull. 2021, 173, 112989. [Google Scholar] [CrossRef]
- Alnaeem, M.A.; Yusoff, I.; Ng, T.; Alias, Y.; May, R.; Haniffa, M. An integrated multi-techniques approach for hydrogeochemical evaluation of ion exchange processes and identification of water types based on statistical analysis: Application to the Gaza coastal aquifer, Gaza Strip, Palestine. Groundw. Sustain. Dev. 2019, 9, 100227. [Google Scholar] [CrossRef]
- Sefie, A.; Aris, A.Z.; Ramli, M.F.; Narany, T.S.; Shamsuddin, M.K.N.; Saadudin, S.B.; Zali, M.A. Hydrogeochemistry and groundwater quality assessment of the multilayered aquifer in Lower Kelantan Basin, Kelantan, Malaysia. Environ. Earth Sci. 2018, 77, 397. [Google Scholar] [CrossRef]
- Yusuf, M.A.; Abiye, T.A.; Ibrahim, K.O.; Abubakar, H.O. Hydrogeochemical and salinity appraisal of surficial lens of freshwater aquifer along Lagos coastal belt, South West, Nigeria. Heliyon 2021, 7, e08231. [Google Scholar] [CrossRef] [PubMed]
- Telahigue, F.; Mejri, H.; Mansouri, B.; Souid, F.; Agoubi, B.; Chahlaoui, A.; Kharroubi, A. Assessing seawater intrusion in arid and semi-arid Mediterranean coastal aquifers using geochemical approaches. Phys. Chem. Earth 2020, 115, 102811. [Google Scholar] [CrossRef]
- Sánchez-Sánchez, J.A.; Álvarez-Legorreta, T.; Pacheco-Ávila, J.G.; González-Herrera, R.A.; Carrillo-Bribiezca, L. Caracterización hidrogeoquímica de las aguas subterráneas del sur del Estado de Quintana Roo, México. Rev. Mex. Ciencias Geol. 2015, 32, 62–76. [Google Scholar]
- Cao, T.; Han, D.; Song, X. Past, present, and future of global seawater intrusion research: A bibliometric analysis. J. Hydrol. 2021, 603, 126844. [Google Scholar] [CrossRef]
- Sánchez, J.A.; Álvarez, T.; Pacheco, J.G.; Carrillo, L.; González, R.A. Calidad del agua subterránea: Acuífero sur de Quintana Roo, México. Tecnol. Ciencias Agua 2016, 7, 75–96. [Google Scholar]
- Abascal, E.; Gómez-Coma, L.; Ortiz, I.; Ortiz, A. Global diagnosis of nitrate pollution in groundwater and review of removal technologies. Sci. Total Environ. 2022, 810, 152233. [Google Scholar] [CrossRef]
- IDEAM. Consulta y Descarga de Datos Hidrometeorológicos. 2023. Available online: http://dhime.ideam.gov.co/atencionciudadano (accessed on 27 July 2022).
- Briceño, A.M. Dinámica del Crecimiento y Relación con el Clima de Especies Arbóreas de los Bosques de la Región Caribe, Colombia. Ph.D. Thesis, Universidad Nacional de Colombia, Bogotá, DC, Colombia, 2017. [Google Scholar]
- Murtinho, F.; Tague, C.; de Bievre, B.; Eakin, H.; Lopez-Carr, D. Water Scarcity in the Andes: A Comparison of Local Perceptions and Observed Climate, Land Use and Socioeconomic Changes. Hum. Ecol. 2013, 41, 667–681. [Google Scholar] [CrossRef]
- IDEAM. Estudio Nacional del Agua 2014; IDEAM: Bogota, Colombia, 2015. [Google Scholar]
- Guzmán, G.; Gómez, E.; Serrano, B. Geología de los Cinturones Del Sinú, San Jacinto y Borde Occidental del Valle Inferior del Magdalena Caribe Colombiano; Servicio Geológico Colombiano: Bogota, Colombia, 2004. [Google Scholar]
- Reyes, G.; Guzmán, O.; Gonzalo, B.; Zapata, G. Geología de las Planchas 23 Cartagena y 29–30 Arjona-Memoria Explicativa; Bogotá D.C. 2001. Available online: https://www2.sgc.gov.co/MGC/Documents/MGC_2023/Memoria_mgc_gmc_agc_2023.pdf (accessed on 26 July 2022).
- Gomez, J.; Montes, N.E.; Marín, E. Mapa Geologico de Colombia 2023 Escala 1:1 500 000; Servicio Geológico Colombiano: Bogota, Colombia, 2023. [Google Scholar]
- Alcaldia de Cartagena de Indias. Consultorias y Servicios Juridicos y Comerciales Consultoría para la Elaboración de los Estudios, Análisis y Mediciones Necesarios para la Administración, Adquisición y Mantenimiento de Predios que Integran las Áreas de Interés Estratégico que Surten al Acueducto de Cartagena, Contrato no. c. 2018; Alcaldia de Cartagena de Indias: Bolívar, Colombia, 2018. [Google Scholar]
- Waterloo Hydrogeologic. AquaChem 11.0 Student License, Canada. Computacional Program 2022. Canada, 2022. Available online: http://www.waterloohydrogeologic.com/AquaChem (accessed on 27 July 2022).
- Leite, C.M.; Wendland, E.; Gastmans, D. Caracterização hidrogeoquímica de águas subterrâneas utilizadas para abastecimento público na porção nordeste do Sistema Aquífero Guarani. Eng. Sanit. Ambient. 2021, 26, 29–43. [Google Scholar] [CrossRef]
- APHA; AWWA; WEF. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Whashington, DC, USA, 2017. [Google Scholar]
- NTC-ISO 5667-1; Enviromental Management Water Quality Sampling Huidance on the Desing of Sampling Programmes. ICONTEC: Bogotá, Colombia, 1995.
- Pfaff, J.D. Determination of Inorganic Anions By Ion Chromatography. Methods Determ. Met. Environ. Samples 1996, 388–417. [Google Scholar] [CrossRef]
- USEPA. USEPA Method 200.8. US Environ. Prot. Agency 1994, 4, 1–57. [Google Scholar]
- IDEAM. Principios Básicos para el Conocimiento y Monitoreo de las AGUAS subterráneas-Contenidos del Taller de Formación; IDEAM: Bogota, Colombia, 2015. [Google Scholar]
- University of Amsterdam. JASP 9.2 Free Software, Amsterdan. Computacional Program 2018. Available online: https://jasp-stats.org/ (accessed on 27 July 2022).
- Gopal, V.; Achyuthan, H.; Jayaprakash, M. Hydrogeochemical characterization of Yercaud lake southern India: Implications on lake water chemistry through multivariate statistics. Acta Ecol. Sin. 2018, 38, 200–209. [Google Scholar] [CrossRef]
- Omonona, O.V.; Okogbue, C.O. Geochemistry of rare earth elements in groundwater from different aquifers in the Gboko area, central Benue Trough, Nigeria. Environ. Earth Sci. 2017, 76, 8. [Google Scholar] [CrossRef]
- Heikhy Narany, T.; Sefie, A.; Aris, A.Z. The long-term impacts of anthropogenic and natural processes on groundwater deterioration in a multilayered aquifer. Sci. Total Environ. 2018, 630, 931–942. [Google Scholar] [CrossRef] [PubMed]
- Cloutier, V.; Lefebvre, R.; Therrien, R.; Savard, M.M. Multivariate statistical analysis of geochemical data as indicative of the hydrogeochemical evolution of groundwater in a sedimentary rock aquifer system. J. Hydrol. 2008, 353, 294–313. [Google Scholar] [CrossRef]
- Zakaria, N.; Anornu, G.; Adomako, D.; Owusu-nimo, F.; Gibrilla, A. Evolution of groundwater hydrogeochemistry and assessment of groundwater quality in the Anayari catchment. Groundw. Sustain. Dev. 2020, 12, 100489. [Google Scholar] [CrossRef]
- Ghosh, A.; Bera, B. Hydrogeochemical assessment of groundwater quality for drinking and irrigation applying groundwater quality index (GWQI) and irrigation water quality index (IWQI). Groundw. Sustain. Dev. 2023, 22, 100958. [Google Scholar] [CrossRef]
- Varol, S.; Davraz, A. Evaluation of the groundwater quality with WQI (Water Quality Index) and multivariate analysis: A case study of the Tefenni plain (Burdur/Turkey). Environ. Earth Sci. 2015, 73, 1725–1744. [Google Scholar] [CrossRef]
- Kouassy Kalédjé, P.S.; Mfonka, Z.; Ntsama, I.S.B.; Kpoumié, A.; Fouépé Takounjou, A.; Ndam Ngoupayou, J.R. Groundwater quality assessment in the catchment area of Kadey (East-Cameroon): Water quality index approach. Sustain. Water Resour. Manag. 2023, 9, 137. [Google Scholar] [CrossRef]
- Rushdi, M.I.; Basak, R.; Das, P.; Ahamed, T.; Bhattacharjee, S. Assessing the health risks associated with elevated manganese and iron in groundwater in Sreemangal and Moulvibazar Sadar, Bangladesh. J. Hazard. Mater. Adv. 2023, 10, 100287. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First Addendum; SWISS: Geneva, Switzerland, 2017. [Google Scholar]
- Sunkari, E.D.; Seidu, J.; Ewusi, A. Hydrogeochemical evolution and assessment of groundwater quality in the Togo and Dahomeyan aquifers, Greater Accra Region, Ghana. Environ. Res. 2022, 208, 112679. [Google Scholar] [CrossRef] [PubMed]
- Malagon, J.P. Análisis Hidrogeoquímico Multivariado del Agua Subterránea del Sistema Acuífero del Valle Medio del Magdalena–Colombia. Ph.D. Thesis, Universidad Nacional de Colombia, Medellin, Colombia, 2017. [Google Scholar]
- Abu-alnaeem, M.F.; Yusoff, I.; Ng, T.F.; Alias, Y.; Raksmey, M. Assessment of groundwater salinity and quality in Gaza coastal aquifer, Gaza Strip, Palestine: An integrated statistical, geostatistical and hydrogeochemical approaches study. Sci. Total Environ. 2018, 615, 972–989. [Google Scholar] [CrossRef] [PubMed]
- Abdelshafy, M.; Saber, M.; Abdelhaleem, A.; Abdelrazek, S.M.; Seleem, E.M. Hydrogeochemical processes and evaluation of groundwater aquifer at Sohag city, Egypt. Sci. Afr. 2019, 6, e00196. [Google Scholar] [CrossRef]
- Guillen-rivas, J.R.; Jaramillo-Cedeño, A.R.; Baquerizo-Crespo, R.J.; Córdova-Mosquera, R.A. Estudio de los procesos de remoción de hierro y manganeso en aguas subterráneas: Una revisión. Polo Conoc. 2021, 6, 1384–1407. [Google Scholar]
- Usman, U.A.; Yusoff, I.; Raoov, M.; Hodgkinson, J. Trace metals geochemistry for health assessment coupled with adsorption remediation method for the groundwater of Lorong Serai 4, Hulu Langat, west coast of Peninsular Malaysia. Environ. Geochem. Health 2020, 42, 3079–3099. [Google Scholar] [CrossRef] [PubMed]
- Hounsinou, S.P. Assessment of potential seawater intrusion in a coastal aquifer system at Abomey-Calavi, Benin. Heliyon 2020, 6, e03173. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, M.; Sridhar, S.G.D.; Ayyamperumal, R.; Karuppannan, S.; Gopalakrishnan, G.; Chakraborty, M.; Huang, X. Isotopic signatures, hydrochemical and multivariate statistical analysis of seawater intrusion in the coastal aquifers of Chennai and Tiruvallur District, Tamil Nadu, India. Mar. Pollut. Bull. 2022, 174, 113232. [Google Scholar] [CrossRef]
- Chaurasia, A.K.; Pandey, H.K.; Tiwari, S.K.; Pandey, P.; Ram, A. Groundwater vulnerability assessment using water quality index (WQI) under geographic information system (GIS) framework in parts of Uttar Pradesh, India. Sustain. Water Resour. Manag. 2021, 7, 40. [Google Scholar] [CrossRef]
| Majority Ion and Highly Relevant | Dominant Hydrochemical Processes | Type of Aquifer | Applied Hydrochemical Analysis | Refs. |
|---|---|---|---|---|
| HCO3− | Rock dissolution | Igneous | Gibbs plot | [20] |
| Cl− | Seawater mixing | Sedimentary | Ionic ratios | [17,21,24] |
| HCO3− | Rock dissolution | Sedimentary | Gibbs plot | [22,24] |
| HCO3− | Rock dissolution | Sedimentary | Piper diagram | [6,25] |
| HCO3−—Fe2+ | Rock dissolution | Sedimentary | Gibbs plot—Ionic ratios | This study |
| N° | Step | Data | Technique | Considerations/Relationship with Other Steps |
|---|---|---|---|---|
| 1 | Physicochemical characterization |
|
| Consider sampling in different climatic seasons to identify the variation generated in the composition of the groundwater, due to rainfall regimes. |
| 2 | Identification of water constituents and types of water |
|
| Establish the minor or trace ions of interest for quality assessment and identification of processes of composition. |
| 3 | Identification of dominant mechanisms in water composition |
|
| Set between rock–water interaction, rainfall, and evaporation. |
| 4 | Evaluation of ion exchange processes |
|
| Of great relevance in systems with domain of rock–water interaction. |
| 5 | Analysis of spatial distribution of components |
|
| Implement in parameters of greatest relevance for the evaluation of drinking water. |
| 6 | Identification of processes impacting water composition and interrelationship between chemical parameters |
| Multivariate statistics: principal component analysis (PCA) | Perform comparative analysis with results of hydrochemical diagrams and indices (Step 2-3-4) |
| 7 | Drinking water quality assessment |
| Water quality index (WQI) | Associate with spatial analysis results (step 5) and processes identified in step 6. |
| Parameters | WHO (2017) Standards [54] | ) | |
|---|---|---|---|
| pH | 7.5 | 4 | 0.100 |
| TDS | 600 | 4 | 0.100 |
| Ca2+ | 200 | 3 | 0.075 |
| Mg2+ | 150 | 3 | 0.075 |
| Na2+ | 200 | 4 | 0.100 |
| Fe2+ | 0.3 | 4 | 0.100 |
| Mn+ | 0.1 | 4 | 0.100 |
| HCO3− | 300 | 5 | 0.125 |
| NO3− | 50 | 2 | 0.050 |
| SO42− | 250 | 3 | 0.075 |
| Cl− | 250 | 4 | 0.100 |
| 40 | 1.0 |
| WQI | Water Quality Status | Usage |
|---|---|---|
| 0–25 | Excellent | Drinking (Requires basic treatment), irrigation, and industrial |
| 26–50 | Good | Drinking (Requires basic treatment), irrigation, and industrial |
| 51–75 | Poor | Irrigation and industrial |
| 76–100 | Very poor | Irrigation |
| >100 | Not recommended for drinking | Requires advanced treatment |
| T | pH | EC | Salinity | OD | ORP | TDS | ρ | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Units | °C | µS/cm | UPS | mgO2/L | mV | mg/L | ohms.m | |||||||||
| Campaigns | Dry | Wet | Dry | Wet | Dry | Wet | Dry | Wet | Dry | Wet | Dry | Wet | Dry | Wet | Dry | Wet |
| Average | 29.60 | 29.36 | 6.80 | 6.91 | 848.20 | 924.07 | 0.39 | 0.45 | 3.91 | 2.96 | 151.38 | 124.04 | 415.80 | 475.80 | 13.02 | 13.37 |
| Standard deviation | 0.99 | 1.38 | 0.45 | 0.66 | 333.98 | 531.44 | 0.18 | 0.28 | 2.70 | 2.03 | 120.25 | 129.38 | 185.77 | 264.91 | 3.69 | 5.54 |
| Coefficient of variation | 0.03 | 0.05 | 0.07 | 0.10 | 0.39 | 0.58 | 0.46 | 0.62 | 0.69 | 0.69 | 0.79 | 1.04 | 0.45 | 0.56 | 0.28 | 0.42 |
| Minimum | 27.9 | 27.6 | 6.2 | 6.3 | 510 | 438 | 0.24 | 0.21 | 0.18 | 0.57 | −46.6 | −50.2 | 261 | 219 | 5.4 | 3.97 |
| Maximum | 31.2 | 32.2 | 7.74 | 8.57 | 1842 | 2518 | 0.93 | 1.28 | 8.43 | 8.5 | 287.4 | 306.1 | 922 | 1258 | 19.6 | 22.83 |
| Cl− | HCO3− | PO4− | NO3− | SO42− | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Campaign | Dry | Wet | Dry | Wet | Dry | Wet | Dry | Wet | Dry | Wet |
| Average | 113.71 | 110.61 | 139.709 | 147.798 | 0.111 | 0.218 | 14.688 | 1.281 | 90.15 | 88.86 |
| Standard deviation | 51.9 | 52.559 | 45.8 | 39.305 | 0.117 | 0.253 | 38.533 | 2.789 | 54.296 | 78.385 |
| Coefficient of variation | 0.456 | 0.475 | 0.328 | 0.266 | 1.05 | 1.162 | 2.623 | 2.177 | 0.602 | 0.882 |
| Minimum | 61.7 | 57.1 | 78.77 | 95.85 | 0.05 | 0.05 | 0.05 | 0.05 | 31.8 | 29.86 |
| Maximum | 234 | 226.9 | 210.26 | 215.02 | 0.34 | 0.83 | 123.42 | 8.96 | 177.9 | 303.65 |
| Fe2+ | Na2+ | NH4+ | Ca2+ | Mg2+ | Mn+ | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Campaign | Dry | Wet | Dry | Wet | Dry | Wet | Dry | Wet | Dry | Wet | Dry | Wet |
| Average | 3.451 | 1.887 | 48.965 | 69.169 | 0.203 | 0.197 | 34.439 | 47.645 | 21.829 | 29.163 | 0.383 | 0.486 |
| Standard deviation | 4.181 | 1.446 | 7.063 | 22.9 | 0.317 | 0.288 | 6.413 | 10.892 | 6.156 | 11.593 | 0.26 | 0.515 |
| Coefficient of variation | 1.212 | 0.766 | 0.144 | 0.331 | 1.559 | 1.464 | 0.186 | 0.229 | 0.282 | 0.398 | 0.679 | 1.059 |
| Minimum | 0.14 | 0.148 | 40.3 | 47.977 | 0.03 | 0.03 | 23.22 | 33.131 | 8.76 | 9.941 | 0.003 | 0.003 |
| Maximum | 14.21 | 3.794 | 61.68 | 104.402 | 1.065 | 0.844 | 42.25 | 62.43 | 29.63 | 50.111 | 0.837 | 1.497 |
| . | Component Loadings Sampling Dry Season | Component Loadings Sampling Wet Season | |||||
|---|---|---|---|---|---|---|---|
| C1 | C2 | C3 | C1 | C2 | C3 | C4 | |
| pH | 0.282424 | 0.126286 | −0.530711 | 0.323275 | −0.181528 | 0.265716 | −0.129277 |
| EC | 0.374609 | 0.169896 | 0.267494 | 0.396742 | 0.0258248 | −0.328585 | 0.0979372 |
| TDS | 0.348535 | −0.0938908 | 0.280064 | 0.391211 | −0.0001663 | −0.340062 | 0.0430268 |
| Cl− | 0.171016 | 0.25692 | 0.472877 | 0.385045 | −0.044183 | −0.292593 | 0.17366 |
| HCO3− | 0.310495 | 0.137331 | −0.458393 | 0.218109 | 0.169418 | 0.556459 | 0.217662 |
| NO3− | −0.215621 | 0.386586 | 0.244289 | 0.0604122 | −0.445081 | 0.418031 | 0.245162 |
| SO42− | 0.188917 | −0.369393 | 0.225704 | 0.23876 | 0.107972 | −0.003213 | −0.704989 |
| Fe2+ | 0.128403 | −0.43039 | 0.0425605 | −0.16577 | 0.530845 | 0.0225487 | 0.0814132 |
| Na+ | 0.207884 | 0.409104 | −0.0493622 | 0.392562 | −0.0602683 | 0.0680221 | 0.216773 |
| Ca2+ | 0.428083 | 0.0181953 | 0.126786 | 0.236632 | 0.222878 | 0.331526 | −0.429069 |
| Mg2+ | 0.427494 | 0.0834325 | −0.0460756 | 0.299241 | 0.36061 | 0.143884 | 0.213799 |
| Mn+ | 0.157742 | −0.464446 | −0.0394322 | −0.0378293 | 0.510713 | 0.0043489 | 0.231876 |
| Eigenvalue | 5.48597 | 3.99511 | 2.08157 | 5.04561 | 2.55318 | 1.56551 | 1.07587 |
| Variance (%) | 39.619 | 28.852 | 15.033 | 42.047 | 21.276 | 13.046 | 8.966 |
| Var. accumulated (%) | 39.619 | 68.472 | 83.505 | 42.047 | 63.323 | 76.369 | 85.335 |
| WQI | Water Quality Status (WQS) | Percentage of Samples (%) | |
|---|---|---|---|
| Dry Season | Wet Season | ||
| 0–25 | Excellent | 0.0% | 0.0% |
| 26–50 | Good | 21.43% | 28.57% |
| 51–75 | Poor | 0.00% | 14.29% |
| 76–100 | Very poor | 21.43% | 7.14% |
| Above one hundred | Unsuitable for drinking and fish culture | 57.14% | 50.00% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arroyo-Figueroa, C.; Chalá, D.C.; Gutiérrez-Ribon, G.; Quiñones-Bolaños, E. A Framework to Evaluate Groundwater Quality and the Relationship between Rock Weathering and Groundwater Hydrogeochemistry in the Tropical Zone: A Case Study of Coastal Aquifer Arroyo Grande, in the Caribbean Region of Colombia. Water 2024, 16, 1650. https://doi.org/10.3390/w16121650
Arroyo-Figueroa C, Chalá DC, Gutiérrez-Ribon G, Quiñones-Bolaños E. A Framework to Evaluate Groundwater Quality and the Relationship between Rock Weathering and Groundwater Hydrogeochemistry in the Tropical Zone: A Case Study of Coastal Aquifer Arroyo Grande, in the Caribbean Region of Colombia. Water. 2024; 16(12):1650. https://doi.org/10.3390/w16121650
Chicago/Turabian StyleArroyo-Figueroa, Carlos, Dayana Carolina Chalá, Guillermo Gutiérrez-Ribon, and Edgar Quiñones-Bolaños. 2024. "A Framework to Evaluate Groundwater Quality and the Relationship between Rock Weathering and Groundwater Hydrogeochemistry in the Tropical Zone: A Case Study of Coastal Aquifer Arroyo Grande, in the Caribbean Region of Colombia" Water 16, no. 12: 1650. https://doi.org/10.3390/w16121650
APA StyleArroyo-Figueroa, C., Chalá, D. C., Gutiérrez-Ribon, G., & Quiñones-Bolaños, E. (2024). A Framework to Evaluate Groundwater Quality and the Relationship between Rock Weathering and Groundwater Hydrogeochemistry in the Tropical Zone: A Case Study of Coastal Aquifer Arroyo Grande, in the Caribbean Region of Colombia. Water, 16(12), 1650. https://doi.org/10.3390/w16121650








