Carcinogenic and Non-Carcinogenic Health Risk Evaluation of Heavy Metals in Water Sources of the Nubian Sandstone Aquifer in the El-Farafra Oasis (Egypt)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Samples Collection and Analysis
2.3. Statistical Analyses
2.4. Human Health Risk Evaluation
2.4.1. Non-Carcinogenic Health Hazard
2.4.2. Carcinogenic Health Risk Assessment
3. Results
4. Discussion
4.1. Geogenic Origin of the Heavy-Metal Enrichment of the Wells and of the Medium-High Solute Content of the Springs
4.2. Health-Risk Assessment
4.3. Non-Carcinogenic Health Impacts
4.4. Carcinogenic Risk Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ravindra, K.; Mor, S. Distribution and health risk assessment of arsenic and selected heavy metals in groundwater of Chandigarh, India. Environ. Pollut. 2019, 250, 820–830. [Google Scholar] [CrossRef]
- Zakir, H.M.; Sharmin, S.; Akter, A.; Rahman, M.S. Assessment of health risk of heavy metals and water quality indices for irrigation and drinking suitability of waters: A case study of Jamalpur Sadar area, Bangladesh. Environ. Adv. 2020, 2, 100005. [Google Scholar] [CrossRef]
- Shah, R.A.; Shafi, A.; Andrabi, S.M.A.; Bhat, S.U.; Hamid, A.; Mondal, N.C. Spatial distribution and health risk assessment of heavy metals in the groundwater of selected watersheds of Srinagar and Pulwama districts of Kashmir Himalaya. Water Air Soil Pollut. 2023, 234, 665. [Google Scholar] [CrossRef]
- Ustaoğlu, F.; Islam, M.S. Potential toxic elements in sediment of some rivers at Giresun, Northeast Turkey: A preliminary assessment for ecotoxicological status and health risk. Ecol. Indic. 2020, 113, 106237. [Google Scholar] [CrossRef]
- Rahman, Z.; Singh, V.P. The relative impact of toxic heavy metals (THMs) (arsenic (As), cadmium (Cd), chromium (Cr)(VI), mercury (Hg), and lead (Pb)) on the total environment: An overview. Environ. Monit. Assess. 2019, 191, 419. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, S.; Mehra, R.; Kanwar, P.; Mishra, R.; Kaur, I. Assessment of radon concentration and heavy metal contamination in groundwater of Udhampur district, Jammu & Kashmir India. Environ. Geochem. Health 2018, 40, 815–831. [Google Scholar] [CrossRef]
- Laniyan, T.A.; Adewumi, A.J. Health risk assessment of heavy metal pollution in groundwater around an exposed dumpsite in southwestern Nigeria. J. Health Pollut. 2019, 9, 191210. [Google Scholar] [CrossRef]
- Lu, Y.; Khan, H.; Zakir, S.; Ihsanullah, S.; Khan, S.; Khan, A.A.; Wei, L.; Wang, T. Health risks associated with heavy metals in the drinking water of Swat, northern Pakistan. J. Environ. Sci. 2013, 25, 2003–2013. [Google Scholar] [CrossRef]
- Rezaei, A.; Hassani, H.; Jabbari, N. Evaluation of groundwater quality and assessment of pollution indices for heavy metals in North of Isfahan Province Iran. Sustain. Water Resour. Manag. 2019, 5, 491–512. [Google Scholar] [CrossRef]
- Tang, W.; Ao, L.; Zhang, H.; Shan, B. Accumulation and risk of heavy metals in relation to agricultural intensification in the river sediments of agricultural regions. Environ. Earth Sci. 2014, 71, 3945–3951. [Google Scholar] [CrossRef]
- Jabbari, N.; Aminzadeh, F.; de Barros, F.P.J. Hydraulic fracturing and the environment: Risk assessment for groundwater contamination from well casing failure. Stoch. Environ. Res. Risk Assess. 2017, 31, 1527–1542. [Google Scholar] [CrossRef]
- Fazlzadeh, M.; Rahmani, K.; Zarei, A.; Abdoallahzadeh, H.; Nasiri, F.; Khosravi, R. A novel green synthesis of zero valent iron nanoparticles (NZVI) using three plant extracts and their efficient application for removal of Cr (VI) from aqueous solutions. Adv. Powder Technol. 2017, 28, 122–130. [Google Scholar] [CrossRef]
- Fakhri, Y.; Saha, N.; Ghanbari, S.; Rasouli, M.; Miri, A.; Avazpour, M.; Rahimizadeh, A.; Riahi, S.-M.; Ghaderpoori, M.; Keramati, H.; et al. Carcinogenic and non-carcinogenic health risks of metal (oid)s in tap water from Ilam city Iran. Food Chem. Toxicol. 2018, 118, 204–211. [Google Scholar] [CrossRef]
- Mohammadi, A.A.; Zarei, A.; Majidi, S.; Ghaderpoury, A.; Hashempour, Y.; Saghi, M.H.; Alinejad, A.; Yousefi, M.; Hosseingholizadeh, N.; Ghaderpoori, M. Carcinogenic and non-carcinogenic health risk assessment of heavy metals in drinking water of Khorramabad Iran. MethodsX 2019, 6, 1642–1651. [Google Scholar] [CrossRef]
- Wang, X.; Sato, T.; Xing, B.; Tao, S. Health risks of heavy metals to the general public in Tianjin, China via consumption of vegetables and fish. Sci. Total Environ. 2005, 350, 28–37. [Google Scholar] [CrossRef]
- Xiao, M.; Li, F.; Zhang, J.; Lin, S.; Zhuang, Z.; Wu, Z. Investigation and health risk assessment of heavy metals in soils from partial areas of Daye city, China. IOP Conf. Ser. Earth Environ. Sci. 2017, 64, 012066. [Google Scholar] [CrossRef]
- Radfard, M.; Yunesian, M.; Nabizadeh, R.; Biglari, H.; Nazmara, S.; Hadi, M.; Yousefi, N.; Yousefi, M.; Abbasnia, A.; Mahvi, A.H. Drinking water quality and arsenic health risk assessment in Sistan and Baluchestan, Southeastern Province, Iran. Hum. Ecol. Risk Assess. Int. J. 2019, 25, 949–965. [Google Scholar] [CrossRef]
- Shams, M.; Nezhad, N.T.; Dehghan, A.; Alidadi, H.; Paydar, M.; Mohammadi, A.A.; Zarei, A. Heavy metals exposure, carcinogenic and non-carcinogenic human health risks assessment of groundwater around mines in Joghatai Iran. Int. J. Environ. Anal. Chem. 2022, 102, 1884–1899. [Google Scholar] [CrossRef]
- Saber, A.A.; Bhat, S.U.; Hamid, A.; Gabrieli, J.; Garamoon, H.; Gargini, A.; Cantonati, M. Chemical quality and hydrogeological settings of the El-Farafra Oasis (Western Desert of Egypt) groundwater resources in relation to human uses. Appl. Sci. 2022, 12, 5606. [Google Scholar] [CrossRef]
- Bastawesy, M.E.; Ali, R.R. The use of GIS and remote sensing for the assessment of waterlogging in the dryland irrigated catchments of Farafra Oasis, Egypt. Hydrol. Process. 2013, 27, 206–216. [Google Scholar] [CrossRef]
- Elsheikh, A.E. Mitigation of groundwater level deterioration of the Nubian Sandstone aquifer in Farafra Oasis, Western Desert, Egypt. Environ. Earth Sci. 2015, 74, 2351–2367. [Google Scholar] [CrossRef]
- Powell, N.; Fensham, R. The history and fate of the Nubian Sandstone Aquifer springs in the oasis depressions of the Western Desert, Egypt. Hydrogeol. J. 2016, 24, 395–406. [Google Scholar] [CrossRef]
- Voss, C.I.; Soliman, S.M. The transboundary non-renewable Nubian Aquifer System of Chad, Egypt, Libya and Sudan: Classical groundwater questions and parsimonious hydrogeologic analysis and modeling. Hydrogeol. J. 2014, 22, 441–468. [Google Scholar] [CrossRef]
- Dabous, A.; Osmond, J. Uranium isotopic study of artesian and pluvial contributions to the Nubian Aquifer, Western Desert, Egypt. J. Hydrol. 2001, 243, 242–253. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington DC, USA, 2017. [Google Scholar]
- Bempah, C.K.; Ewusi, A. Heavy metals contamination and human health risk assessment around Obuasi gold mine in Ghana. Environ. Monit. Assess. 2016, 188, 261. [Google Scholar] [CrossRef]
- Wongsasuluk, P.; Chotpantarat, S.; Siriwong, W.; Robson, M. Heavy metal contamination and human health risk assessment in drinking water from shallow groundwater wells in an agricultural area in Ubon Ratchathani province, Thailand. Environ. Geochem. Health 2014, 36, 169–182. [Google Scholar] [CrossRef]
- Wu, B.; Zhao, D.; Jia, H.; Zhang, Y.; Zhang, X.; Cheng, S. Preliminary risk assessment of trace metal pollution in surface water from Yangtze River in Nanjing Section, China. Bull. Environ. Contam. Toxicol. 2009, 82, 405–409. [Google Scholar] [CrossRef]
- USEPA. Risk Assessment Guidance for Superfund. Volume 1, Human Health Evaluation Manual (Part E, Supplemental Guidance for Dermal Risk Assessment); EPA/540/R/99/005; U.S. Environmental Protection Agency: Washington, DC, USA, 2004.
- USDOE. The Risk Assessment Information System (RAIS); U.S. Department of Energy’s Oak Ridge Operations Office (ORO): Oak Ridge, TN, USA, 2011.
- Luo, X.S.; Ding, J.; Xu, B. Incorporating bio accessibility into human health risk assessments of heavy metals in urban park soils. Sci. Total Environ. 2012, 424, 88–96. [Google Scholar] [CrossRef]
- Awuchi, C.G.; Igwe, V.S.; Amagwula, I.O. Ready-to-use therapeutic foods (RUTFs) for remedying malnutrition and preventable nutritional diseases. Int. J. Adv. Acad. Res. 2020, 6, 47–81. [Google Scholar]
- California Office of Environmental Health Hazard Assessment. Technical Support Document for Cancer Potency Factors 2009. Hot Spots Unit Risk and Cancer Potency Values; Updated May 2019, Appendix A; OEHHA: Sacramento, CA, USA, 2019.
- Khalili, F.; Mahvi, A.H.; Nasseri, S.; Yunesian, M.; Yaseri, M.; Djahed, B. Health risk assessment of dermal exposure to heavy metals content of chemical hair dyes. Iran. J. Public Health 2019, 48, 902. [Google Scholar] [CrossRef]
- Kamunda, C.; Mathuthu, M.; Madhuku, M. Potential human risk of dissolved heavy metals in gold mine waters of Gauteng Province, South Africa. J. Toxicol. Environ. Health Sci. 2018, 10, 56–63. [Google Scholar] [CrossRef]
- Guerra, F.; Trevizam, A.R.; Muraoka, T.; Marcante, N.C.; Canniatti-Brazaca, S.G. Heavy metals in vegetables and potential risk for human health. Sci. Agric. 2012, 69, 54–60. [Google Scholar] [CrossRef]
- Sultana, M.S.; Rana, S.; Yamazaki, S.; Aono, T.; Yoshida, S. Health risk assessment for carcinogenic and non-carcinogenic heavy metal exposures from vegetables and fruits of Bangladesh. Cogent Environ. Sci. 2017, 3, 1291107. [Google Scholar] [CrossRef]
- Gržetić, I.; Ghariani, R.H.A. Potential health risk assessment for soil heavy metal contamination in the central zone of Belgrade (Serbia). J. Serb. Chem. Soc. 2008, 73, 923–934. [Google Scholar] [CrossRef]
- Tepanosyan, G.; Maghakyan, N.; Sahakyan, L.; Saghatelyan, A. Heavy metals pollution levels and children health risk assessment of Yerevan kindergartens soils. Ecotoxicol. Environ. Saf. 2017, 142, 257–265. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First Addendum, 2017. Available online: https://iris.who.int/bitstream/handle/10665/254637/9789241549950-eng.pdf?sequence=1 (accessed on 15 January 2024).
- Sherif, M.I.; Sturchio, N.C. Elevated radium levels in Nubian Aquifer groundwater of Northeastern Africa. Sci. Rep. 2021, 11, 78. [Google Scholar] [CrossRef]
- Kotp, Y.H.; Ali, M.E.; Gomaah, M. Impact of the geothermal activities on physico-chemical characteristics of the groundwater in Nubian sandstone aquifer in Farafra Oases, Western Desert, Egypt. Middle East J. Appl. Sci. 2018, 8, 1112–1130. [Google Scholar]
- Abdel Zaher, M.; Elbarbary, S.; El-Shahat, A.; Mesbah, H.; Embaby, A. Geothermal resources in Egypt integrated with GIS-based analysis. J. Volcanol. Geother. Res. 2018, 365, 1–12. [Google Scholar] [CrossRef]
- USEPA. Human Health Risk Assessment: Risk-Based Concentration Table. 2010. Available online: https://www.epa.gov/risk/regional-screening-levels-rsls-generic-tables (accessed on 8 December 2023).
- Díaz-Somoano, M.; Kylander, M.E.; López-Antón, M.A.; Suárez-Ruiz, I.; Martínez-Tarazona, M.R.; Ferrat, M.; Kober, B.; Weiss, D.J. Stable lead isotope compositions in selected coals from around the world and implications for present day aerosol source tracing. Environ. Sci. Technol. 2009, 43, 1078–1085. [Google Scholar] [CrossRef]
- U.E.P. Agency. Guidelines for the Health Risk Assessment of Chemical Mixtures; Federal Register; U.S. Environmental Protection Agency: Washington, DC, USA, 1986; Volume 51, pp. 34014–34025.
- Tani, F.; Barrington, S. Zinc and copper uptake by plants under two transpiration rates. Part II. Buckwheat (Fagopyrum esculentum L.). Environ. Pollut. 2005, 138, 548–558. [Google Scholar] [CrossRef]
- Cao, S.; Duan, X.; Zhao, X.; Ma, J.; Dong, T.; Huang, N.; Sun, C.; He, B.; Wei, F. Health risks from the exposure of children to As, Se, Pb and other heavy metals near the largest coking plant in China. Sci. Total Environ. 2014, 472, 1001–1009. [Google Scholar] [CrossRef]
- Wcisło, E.; Ioven, D.; Kucharski, R.; Szdzuj, J. Human health risk assessment case study: An abandoned metal smelter site in Poland. Chemosphere 2002, 47, 507–515. [Google Scholar] [CrossRef]
- Yang, G.; Li, Y.; Wu, L.; Xie, L.; Wu, J. Concentration and health risk of heavy metals in topsoil of paddy field of Chengdu Plain. Environ. Chem. 2014, 33, 269–275. [Google Scholar]
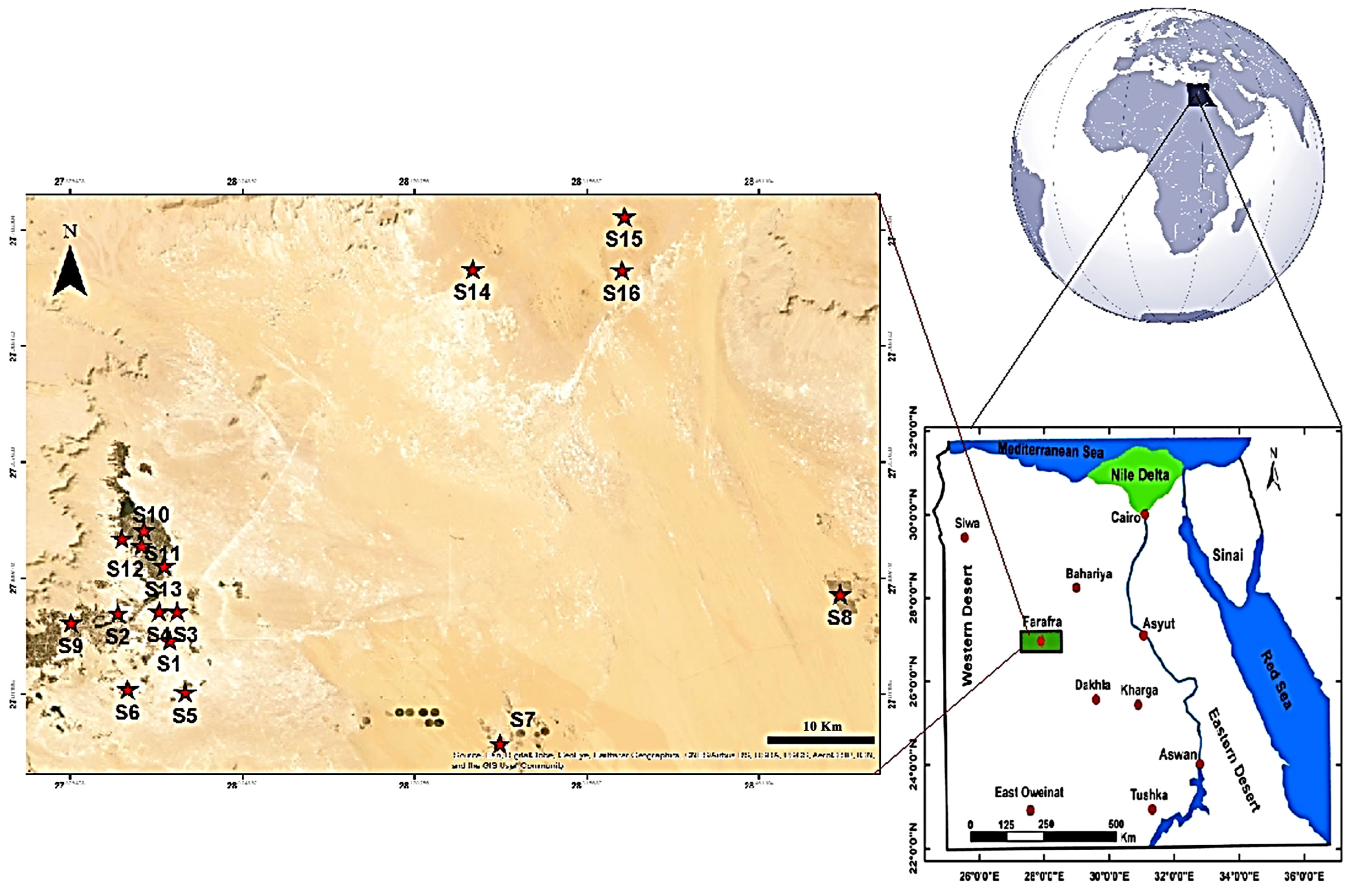


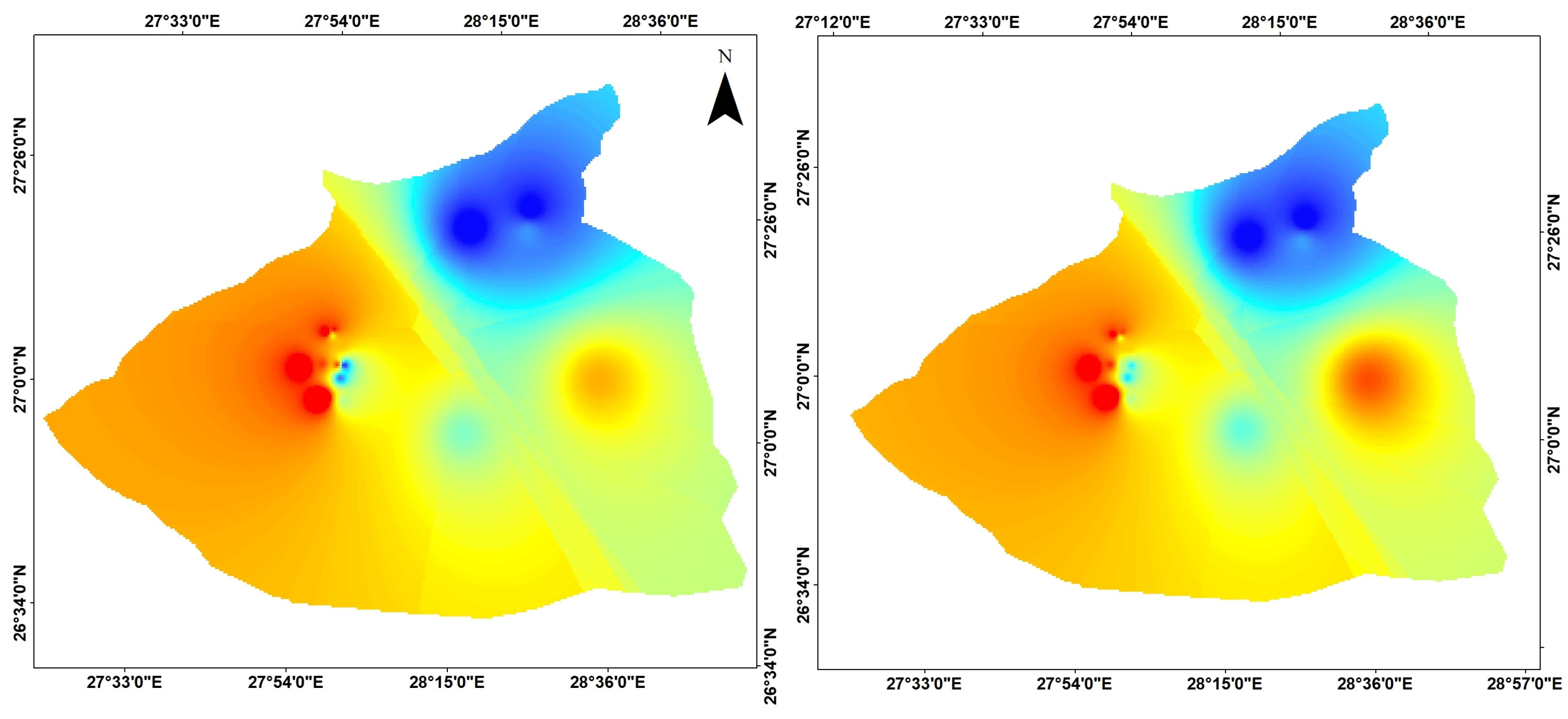
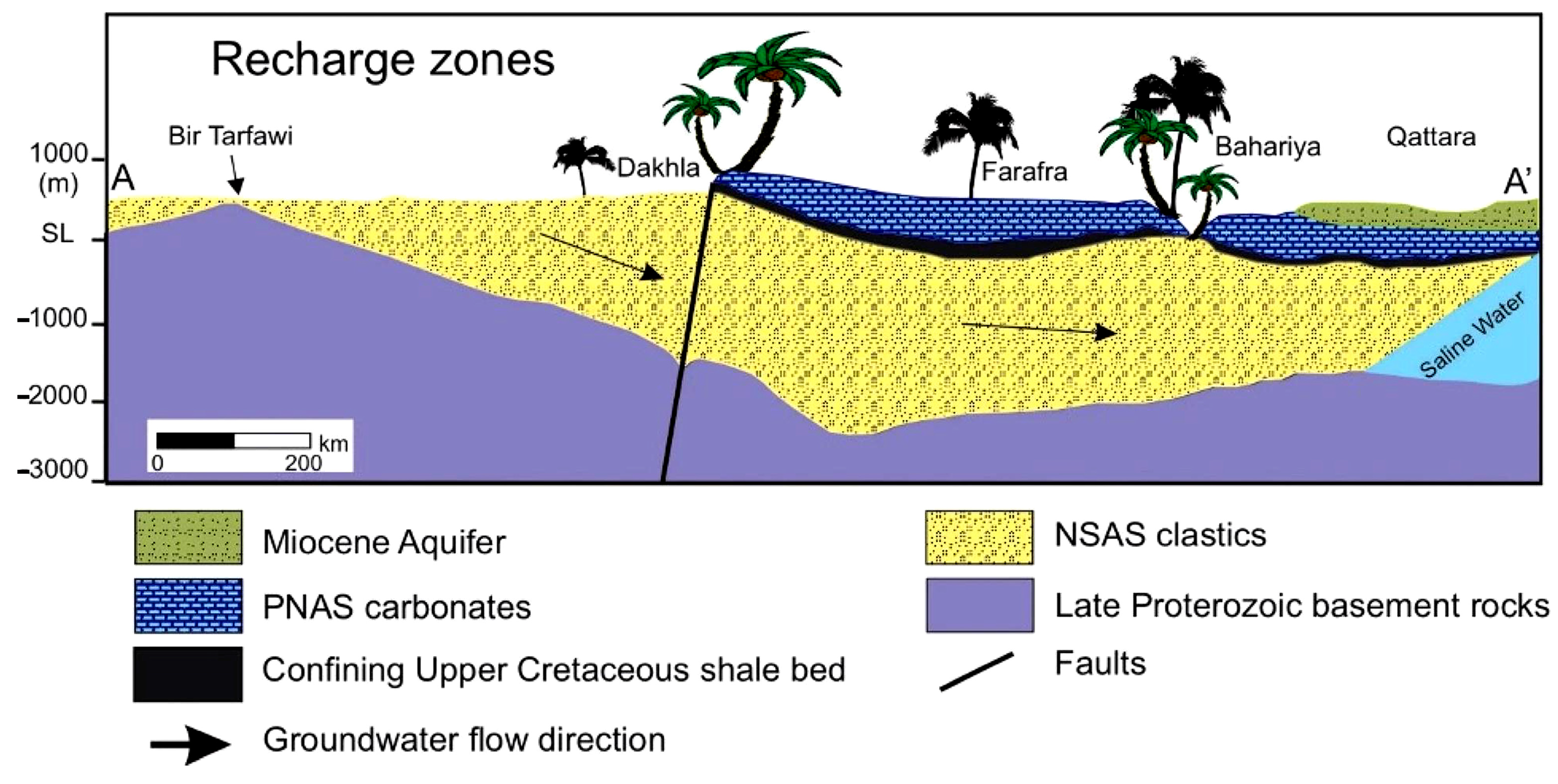
| Samples No. | Name | Latitude | Longitude | Elevation | Temp. | pH | E.C. | T.D.S. |
|---|---|---|---|---|---|---|---|---|
| (N) | (E) | m (a.s.l.) | °C | µS·cm−1 | mg·L−1 | |||
| S1 | Ain El-Balad (Qasr El-Farafra) | 27°03′24.6″ | 27°57′49.3″ | 117 ± 5.7 | 36.3 | 6.74 | 430 | 291 |
| S2 | Bir Sitta (Qasr El-Farafra) | 27°04′49.6″ | 27°55′11.6″ | 74.2 ± 8.1 | 37 | 6.19 | 390 | 254 |
| S3 | Ain Bishwa (Qasr El-Farafra) | 27°04′53.8″ | 27°58′10.3″ | 83 ± 3.9 | 23.8 | 7.78 | 1220 | 864 |
| S4 | Ain El-Hateyya (Qasr El-Farafra | 27°04′54.2″ | 27°57′17.4″ | 82.1 ± 6.4 | 26.3 | 6.60 | 1290 | 901 |
| S5 | Bir Gelaw (Qasr El-Farafra) | 27°00′46.8″ | 27°58′34.8″ | 107.7 ± 3.4 | 33.7 | 6.33 | 320 | 227 |
| S6 | Bir Felao (Qasr El-Farafra) | 27°00′58.2″ | 27°55′41.1″ | 100.6 ± 5 | 38.4 | 6.85 | 330 | 227 |
| S7 | Bir at El-Saad Company for Land Reclamation (Sahl Baraka) | 26°58′13.7″ | 28°14′31.3″ | 103 ± 3.8 | 34.6 | 6.15 | 530 | 322 |
| S8 | Bir 10 at Qarawein Company for Land Reclamation (Qarawein) | 27°05′46.2″ | 28°31′44.7″ | 98 ± 4.1 | 34.4 | 6.20 | 650 | 419 |
| S9 | Bir 7 (Lewa Soubah village) | 27°04′18.7″ | 27°52′47.3″ | 63.7 ± 3.5 | 39 | 6.19 | 320 | 216 |
| S10 | Bir 4 (El-Nahda village) | 27°08′35.6″ | 27°55′23.3″ | 54.8 ± 7.6 | 40.6 | 6.31 | 300 | 200 |
| S11 | Bir 150 (El-Nahda village) | 27°09′00.5″ | 27°56′28.3″ | 58.2 ± 3.7 | 42 | 6.20 | 280 | 157 |
| S12 | Bir Kamel (El-Nahda village) | 27°08′14.3″ | 27°56′21.4″ | 59.1 ± 3.7 | 39 | 6.20 | 230 | 143 |
| S13 | Bir Abd El-Azem (Esha Abd El-Rahman village) | 27°07′12.5″ | 27°57′31.6″ | 63.7 ± 3.9 | 36.6 | 6.19 | 300 | 177 |
| S14 | Ain Khadra, also called “Ain El-Wadi” (the White Desert National Park) | 27°22′15″ | 28°13′08.8″ | 31.3 ± 4.3 | 24 | 7.25 | 330 | 200 |
| S15 | Ain Maqfi, also called “Ain Abu Hawas” (the White Desert National Park) | 27°24′54.9″ | 28°20′50.6″ | 39.3 ± 5.4 | 24.1 | 7.09 | 440 | 260 |
| S16 | Ain El-Serw (the White Desert National Park) | 27°22′11.6″ | 28°20′43.3″ | 72.7 ± 4.3 | 29.3 | 6.86 | 520 | 292 |
| Values | ||||
|---|---|---|---|---|
| Parameter | Unit | Ingestion | Dermal Absorption | References |
| Heavy Metal Concentration (Cw) | mg·L−1 | − | − | [14] |
| Daily Average Intake (DI) | L·day−1 | 2.2 | [29] | |
| Skin Surface Area (SA) | cm2 | − | 18,000 | [29] |
| Permeability Coefficient (Kp) | cm·h−1 | Fe, Cd, Cu, Mn, Co & Cr = 0.001, | [29] | |
| Ni = 0.0002, Zn= 0.0006, Pb = 0.0001 | ||||
| Exposure Time (ET) | h/event | − | 0.58 | [29] |
| Exposure Frequency (EF) | days·year−1 | 365 | 350 | [29] |
| Exposure Duration (ED) | year | 71.8 | 30 | [29,30,31] |
| Conversion Factor (CF) | L·cm−3 | − | 0.001 | [30,31,32] |
| Average Body Weight (ABW) | kg | 70 | 70 | [29,32] |
| Absorption Factor (ABS) | 0.001 | 0.001 | [29,33] | |
| Average Time (AT) | day | 25,550 | 25550 | [29] |
| Element * | UTIL (Upper Tolerable Intake Level) (mg·day−1·person−1) | RfDoral(mg·kg−1·day−1) | RfDdermal (mg·kg−1·day−1) | CSF (mg·kg−1·day−1) | References |
|---|---|---|---|---|---|
| Cr | NE | 3.00 × 10−3 | 0.015 | 41 | |
| Mn | 11 | 0.014 | 0.014 | − | * [34] * [35] * [36] * [37] * [38] * [39] |
| Fe | 45 | 7.00 × 10−1 | 7.00 × 10−1 | − | |
| Co | NE | 2.00 × 10−2 | 5.70 × 10−6 | ||
| Ni | 1 | 2.00 × 10−2 | 5.60 × 10−3 | 0.84 | |
| Cu | 10 | 3.700 × 10−2 | 2.40 × 10−2 | − | |
| Zn | 40 | 3.00 × 10−1 | 7.50 × 10−2 | − | |
| As | <0.14 | 3.00 × 10−4 | 3.00 × 10−4 | − | |
| Cd | 0.064 | 5.00 × 10−4 | 5.00 × 10−4 | 6.1 | |
| Pb | 0.24 | 3.60 × 10−3 | 3.60 × 10−3 | 8.5 |
| Sites | Cr | Mn | Fe | Co | Ni | Cu | Zn | As | Cd | Pb | T | CE | TDS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drilled wells | |||||||||||||
| S2 | 0.119372 | 177.2177 | 545.5475 | 0.026926 | 0.286247 | 0.11249 | 14.15531 | 0.103312 | 0.001771 | 0.009463 | 37 | 390 | 254 |
| S5 | 0.159037 | 103.2946 | 90.1865 | 0.02282 | 0.129873 | 0.382748 | 10.73514 | 0.138157 | 0.003134 | 0.01907 | 33.7 | 320 | 227 |
| S6 | 0.125202 | 229.8951 | 1679.592 | 0.027751 | 0.248687 | 0.854854 | 6.741019 | 0.098807 | 0.001226 | 0.090233 | 38.4 | 330 | 227 |
| S7 | 0.129763 | 87.02292 | 245.137 | 0.016241 | 0.134799 | 0.071018 | 5.539477 | 0.088233 | 0.001226 | 0.00338 | 34.6 | 530 | 322 |
| S8 | 0.146351 | 130.0505 | 1377.297 | 0.045411 | 0.153779 | 0.124949 | 8.305639 | 0.182359 | 0.002862 | 0.139771 | 34.4 | 650 | 419 |
| S9 | 0.103199 | 220.8075 | 697.2878 | 0.023991 | 0.271787 | 0.149543 | 8.339093 | 0.065525 | 0.002315 | 0.002274 | 39 | 320 | 216 |
| S10 | 0.180237 | 205.5416 | 441.0293 | 0.017329 | 0.161211 | 0.114995 | 9.021342 | 0.07159 | 0.001498 | 0.004076 | 40.6 | 300 | 200 |
| S11 | 0.088197 | 166.1444 | 1602.556 | 0.014526 | 0.10241 | 0.077896 | 7.320414 | 0.05755 | 0.003134 | 0.007558 | 42 | 280 | 157 |
| S12 | 0.125882 | 125.8638 | 534.9328 | 0.018039 | 0.119695 | 0.616044 | 9.478833 | 0.072629 | 0.001771 | 0.013662 | 39 | 230 | 143 |
| S13 | 0.095393 | 149.6548 | 236.9866 | 0.018682 | 0.122119 | 0.095456 | 8.477634 | 0.075057 | 0.000817 | 0.005715 | 36.6 | 300 | 177 |
| Springs | |||||||||||||
| S1 | 0.110907 | 37.13324 | 242.2767 | 0.046912 | 0.274857 | 0.078209 | 9.453508 | 0.100885 | 0.002452 | 0.012761 | 36.3 | 430 | 291 |
| S3 | 0.417823 | 5.549176 | 88.5735 | 0.080239 | 0.49829 | 0.711812 | 2.220363 | 0.328666 | 0.004495 | 0.027879 | 23.8 | 1220 | 864 |
| S4 | 0.3819 | 4.786005 | 60.77042 | 0.101349 | 1.538941 | 5.788521 | 4.928506 | 0.43216 | 0.005177 | 0.054179 | 26.3 | 1290 | 901 |
| S14 | 0.107467 | 4.634081 | 29.42385 | 0.025508 | 0.125834 | 0.121665 | 7.550634 | 0.091699 | 0.001226 | 0.006083 | 24 | 330 | 200 |
| S15 | 0.119226 | 8.042004 | 23.45446 | 0.021633 | 0.114364 | 0.114475 | 7.323754 | 0.07783 | 0.001908 | 0.004711 | 24.1 | 440 | 260 |
| S16 | 0.13674 | 49.73614 | 29.94921 | 0.025805 | 0.143926 | 0.172211 | 10.87517 | 0.084417 | 0.001771 | 0.004998 | 29.3 | 520 | 292 |
| Metals | Heavy Metal Concentrations (µg·L−1) | Drinking Water Standards (µg·L−1) | |||
|---|---|---|---|---|---|
| Mean | Min | Max | SD (±) | [41] | |
| Cr | 0.16 | 0.09 | 0.41 | 0.09 | 50 |
| Mn | 106.6 | 4.6 | 229.89 | 81.0 | 100 |
| Fe | 495.3 | 23.4 | 1679.59 | 566.7 | 300 |
| Co | 0.03 | 0.014 | 0.101 | 0.02 | 2000 |
| Ni | 0.28 | 0.10 | 1.54 | 0.35 | 20 |
| Cu | 0.6 | 0.07 | 5.79 | 1.4 | 50 |
| Zn | 8.1 | 2.2 | 14.15 | 2.7 | 5000 |
| As | 0.13 | 0.057 | 0.43 | 0.10 | 10 |
| Cd | 0.002 | 0.00081 | 0.0052 | 0.001 | 3 |
| Pb | 0.025 | 0.0023 | 0.14 | 0.04 | 10 |
| Parameters | Statistics | ||
|---|---|---|---|
| Drilled Wells | Springs | ||
| T (°C) | |||
| Min | 33.7 | 23.8 | |
| Max | 42.0 | 36.3 | |
| Average | 37.5 | 27.3 | F = 29.22, p = 0.00009275 |
| E.C. (µS·cm−1) | |||
| Min | 230.0 | 330.0 | |
| Max | 650.0 | 1290.0 | |
| Average | 365.0 | 705.0 | F = 5.638, p = 0.03241 |
| Mn (µg·L−1) | |||
| Min | 87.0 | 4.6 | |
| Max | 229.9 | 49.7 | |
| Average | 159.5 | 18.3 | F = 44.23, p = 0.00001096 |
| Fe (µg·L−1) | |||
| Min | 90.2 | 23.4 | |
| Max | 1679.6 | 242.3 | |
| Average | 744.0 | 79.0 | F = 7.371, p = 0.01676 |
| T.D.S. (mg·L−1) | |||
| Min | 143.0 | 200.0 | |
| Max | 419.0 | 901.0 | |
| Average | 234.2 | 468.0 | F = 4.922, p = 0.04356 |
| Metals | CDIoral | CDIdermal | CDItotal | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Min | Max | Mean | Min | Max | Mean | Min | Max | |
| Cr | 4.59339 × 10−9 | 2.99807 × 10−9 | 1.31316 × 10−8 | 9.18183 × 10−12 | 5.41 × 10−12 | 2.56 × 10−11 | 2.30129 × 10−9 | 5.41 × 10−12 | 1.31 × 10−8 |
| Mn | 3.57951 × 10−6 | 1.45643 × 10−7 | 7.22527 × 10−6 | 6.14853 × 10−9 | 2.84 × 10−10 | 1.41 × 10−8 | 1.79283 × 10−6 | 2.84 × 10−10 | 7.23 × 10−6 |
| Fe | 1.41904 × 10−5 | 7.3714 × 10−7 | 5.27872 × 10−5 | 2.85727 × 10−8 | 1.44 × 10−9 | 1.03 × 10−7 | 7.10949 × 10−6 | 1.44 × 10−9 | 5.28 × 10−5 |
| Co | 9.28348 × 10−10 | 5.10437 × 10−10 | 2.52181 × 10−9 | 7.68902 × 10−13 | 3.56 × 10−13 | 2.48 × 10−12 | 4.64558 × 10−10 | 3.56 × 10−13 | 2.52 × 10−9 |
| Ni | 6.4279 × 10−9 | 3.59431 × 10−9 | 1.56605 × 10−8 | 3.19208 × 10−12 | 1.26 × 10−12 | 1.89 × 10−11 | 3.21554 × 10−9 | 1.26 × 10−12 | 1.57 × 10−8 |
| Cu | 8.0244 × 10−9 | 2.23198 × 10−9 | 2.68668 × 10−8 | 3.45644 × 10−11 | 4.35 × 10−12 | 3.55 × 10−10 | 4.02948 × 10−9 | 4.35 × 10−12 | 2.69 × 10−8 |
| Zn | 2.76915 × 10−7 | 6.97828 × 10−8 | 4.44881 × 10−7 | 2.82228 × 10−10 | 8.17 × 10−11 | 5.21 × 10−10 | 1.38599 × 10−7 | 8.17 × 10−11 | 4.45 × 10−7 |
| As | 3.51562 × 10−9 | 2.05935 × 10−9 | 1.03295 × 10−8 | 7.4591 × 10−12 | 3.53 × 10−12 | 2.65 × 10−11 | 1.76154 × 10−9 | 3.53 × 10−12 | 1.03 × 10−8 |
| Cd | 6.32119 × 10−11 | 2.56645 × 10−11 | 1.4127 × 10−10 | 1.3261 × 10−13 | 5.01 × 10−14 | 3.17 × 10−13 | 3.16723 × 10−11 | 5.01 × 10−14 | 1.41 × 10−10 |
| Pb | 7.33402 × 10−10 | 7.14574 × 10−11 | 4.28267 × 10−9 | 1.46311 × 10−13 | 1.39 × 10−14 | 8.57 × 10−13 | 3.66774 × 10−10 | 1.39 × 10−14 | 4.28 × 10−9 |
| Metals | HQoral | HQdermal | HQtotal | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Max | Min | Mean | Max | Min | Mean | Max | Min | |
| Cr | 4.48 × 10−6 | 1.31 × 10−5 | 2.77 × 10−6 | 6.50379 × 10−10 | 1.70727 × 10−9 | 3.60383 × 10−10 | 2.2401 × 10−6 | 6.56665 × 10−6 | 1.38614 × 10−6 |
| Mn | 3.68 × 10−3 | 7.23 × 10−3 | 1.46 × 10−4 | 6.53282 × 10−6 | 1.40906 × 10−5 | 2.8403 × 10−7 | 0.00184434 | 0.003619682 | 7.29633 × 10−5 |
| Fe | 1.65 × 10−2 | 5.28 × 10−2 | 7.37 × 10−4 | 3.03585 × 10−5 | 0.000102945 | 1.43756 × 10−6 | 0.00824087 | 0.026445058 | 0.000369289 |
| Co | 2.25 × 10−6 | 6.30 × 10−6 | 1.14 × 10−6 | 1.63392 × 10−7 | 4.96945 × 10−7 | 7.12275 × 10−8 | 1.2053 × 10−6 | 3.40073 × 10−6 | 6.06293 × 10−7 |
| Ni | 3.11 × 10−5 | 7.83 × 10−5 | 1.61 × 10−5 | 6.28071 × 10−13 | 3.49349 × 10−12 | 2.32478 × 10−13 | 1.5568 × 10−5 | 3.91514 × 10−5 | 8.04654 × 10−6 |
| Cu | 7.68 × 10−6 | 2.69 × 10−5 | 2.23 × 10−6 | 3.06039 × 10−12 | 2.95656 × 10−11 | 3.62731 × 10−13 | 3.8379 × 10−6 | 1.34334 × 10−5 | 1.11599 × 10−6 |
| Zn | 4.57 × 10−4 | 7.41 × 10−4 | 1.16 × 10−4 | 4.99779 × 10−12 | 8.67602 × 10−12 | 1.3609 × 10−12 | 0.00022832 | 0.000370734 | 5.81524 × 10−5 |
| As | 3.41 × 10−6 | 1.03 × 10−5 | 1.81 × 10−6 | 2.64177 × 10−9 | 8.82925 × 10−9 | 1.17578 × 10−9 | 1.7058 × 10−6 | 5.16917 × 10−6 | 9.04944 × 10−7 |
| Cd | 6.54 × 10−8 | 1.41 × 10−7 | 2.57 × 10−8 | 2.81797 × 10−10 | 6.34575 × 10−10 | 1.00101 × 10−10 | 3.2849 × 10−8 | 7.09524 × 10−8 | 1.28823 × 10−8 |
| Pb | 7.02 × 10−6 | 4.28 × 10−5 | 7.15 × 10−7 | 3.70131 × 10−13 | 2.03971 × 10−12 | 3.31798 × 10−14 | 3.5121 × 10−6 | 2.14133 × 10−5 | 3.57287 × 10−7 |
| HI | 2.06 × 10−2 | 6.05 × 10−2 | 1.40 × 10−3 | 3.70583 × 10−5 | 0.000117174 | 2.03886 × 10−6 | 0.01034163 | 0.030293143 | 0.000703278 |
| Metals | ILCR | ||
|---|---|---|---|
| Mean | Min | Max | |
| Cr | 9.43528 × 10−8 | 2.22 × 10−10 | 1.239 × 10−15 |
| Ni | 2.70106 × 10−9 | 1.05 × 10−12 | 4.23 × 10−17 |
| Cd | 1.93201 × 10−10 | 3.05 × 10−13 | 2.72935 × 10−20 |
| Pb | 3.11758 × 10−9 | 1.18 × 10−13 | 1.33516 × 10−17 |
| Σ ILCR | 1.00365 × 10−7 | 2.23 × 10−10 | 1.29468 × 10−15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saber, A.A.; Al-Mashhadany, M.F.M.; Hamid, A.; Gabrieli, J.; Tockner, K.; Alsaif, S.S.A.; Al-Marakeby, A.A.M.; Segadelli, S.; Cantonati, M.; Bhat, S.U. Carcinogenic and Non-Carcinogenic Health Risk Evaluation of Heavy Metals in Water Sources of the Nubian Sandstone Aquifer in the El-Farafra Oasis (Egypt). Water 2024, 16, 1649. https://doi.org/10.3390/w16121649
Saber AA, Al-Mashhadany MFM, Hamid A, Gabrieli J, Tockner K, Alsaif SSA, Al-Marakeby AAM, Segadelli S, Cantonati M, Bhat SU. Carcinogenic and Non-Carcinogenic Health Risk Evaluation of Heavy Metals in Water Sources of the Nubian Sandstone Aquifer in the El-Farafra Oasis (Egypt). Water. 2024; 16(12):1649. https://doi.org/10.3390/w16121649
Chicago/Turabian StyleSaber, Abdullah A., Mahmood Fayz M. Al-Mashhadany, Aadil Hamid, Jacopo Gabrieli, Klement Tockner, Sarah S. A. Alsaif, Ali A. M. Al-Marakeby, Stefano Segadelli, Marco Cantonati, and Sami Ullah Bhat. 2024. "Carcinogenic and Non-Carcinogenic Health Risk Evaluation of Heavy Metals in Water Sources of the Nubian Sandstone Aquifer in the El-Farafra Oasis (Egypt)" Water 16, no. 12: 1649. https://doi.org/10.3390/w16121649
APA StyleSaber, A. A., Al-Mashhadany, M. F. M., Hamid, A., Gabrieli, J., Tockner, K., Alsaif, S. S. A., Al-Marakeby, A. A. M., Segadelli, S., Cantonati, M., & Bhat, S. U. (2024). Carcinogenic and Non-Carcinogenic Health Risk Evaluation of Heavy Metals in Water Sources of the Nubian Sandstone Aquifer in the El-Farafra Oasis (Egypt). Water, 16(12), 1649. https://doi.org/10.3390/w16121649









