Abstract
Water-quality indicators and trace elements were measured in the alluvial area of the Kostolac Basin, Serbia. The results revealed that the groundwater was naturally neutral, with a mean conductance of 920.10 μs/cm. The primary factors contributing to the decline in the groundwater quality in the researched area were electrical conductivity (EC), NO3−, SO42−, Cd, and Pb; the water samples were deemed unfit for human consumption and the water was classified as having impaired quality. A Pearson correlation matrix, a principal component analysis (PCA), and a cluster analysis (CA) were applied to identify the pollution source and factors controlling the groundwater quality and the results indicated that both natural and anthropogenic factors influenced the groundwater of the studied area. The Nemerow pollution index indicated medium to high pollution levels along with the degree of contamination. A health risk evaluation was conducted to determine the non-carcinogenic risks posed by nitrates and heavy metals from oral consumption and skin contact in the researched area. Nearly all monitoring sites had hazard quotients (HQs) below 1, suggesting that potential concerns might be negligible. However, children showed the highest exposure levels, with HQs for nitrates at 2.26 and for lead (Pb) at 2.515. No carcinogenic risk from oral lead (Pb) exposure was found in any sample.
1. Introduction
Water is essential for environmental sustainability as well as human health and wellbeing. In the years ahead, numerous challenges will obstruct the global goal of ensuring that humans have access to enough water of adequate quality. According to forecasts, 50% of the global population may face a water shortage by 2025 [1]. Developing nations rely heavily on groundwater as a source of safe drinking water. The quality of groundwater is influenced by both human activities and natural processes [2,3]. Groundwater contamination, particularly with sulfates and nitrates, is increasing due to atmospheric deposition, bacterial production, acid rain, and emissions from household, municipal, and industrial wastewater. International laws and guidelines regulate these ions due to their adverse effects on human health at high concentrations [4,5]. Moreover, urbanization and industrial activities expose groundwater systems to elevated levels of trace metals. Although some of these metals are essential for human health, using low-quality water for irrigation reduces crop yield and can lead to various health problems [6]. According to the World Health Organization (WHO), about 80% of human diseases are related to water-related risk factors. Groundwater serves as a crucial natural resource for drinking and agriculture [7]. However, densely populated areas with intensive land use are particularly prone to groundwater contamination. Almost all activities that release waste or chemicals into the environment, whether intentionally or accidentally, can pollute groundwater. The remediation of contaminated groundwater is costly and time-consuming. The lack of safe and reliable drinking-water sources may lead to desertification, forced migration, hunger, diseases, and domestic or regional conflicts. It is important to note that the impact of water scarcity in developing countries is more challenging than in the developed parts of the world. To create a sustainable water infrastructure, the focus should be on conservation, the protection of water sources, and limitation of pollution. Desalination is considered to be one of the most effective ways to increase water supplies and provide clean and affordable water to millions of people. Nevertheless, it is important to recognize that addressing the global water crisis requires a multifaceted approach. In addition to desalination, other measures should be implemented, such as centralized governance, education, improvements in water catchment and harvesting technologies, irrigation, agricultural practices, distribution infrastructure, prevention of leakage, wastewater reclamation, pollution treatment and prevention, investment in innovative technology, and transboundary water cooperation [8,9].
Evaluation of the health risks associated with groundwater pollution is vital as it links environmental pollution to human health [10,11,12]. The process of evaluating risks to human health typically involves four steps: hazard identification, exposure assessment, dose–response assessment, and risk characterization. Multivariate statistical techniques are employed as powerful tools for data reduction and chemical data interpretation in order to explore variations in groundwater chemistry [13,14].
The Danube River, an international waterway and the second-largest river in Europe, has a total length of 2778 km. It holds significant importance for Serbia, flowing through the country for 558 km from Bezdan in the northwest to Djerdap in the southeast. The section near Kostolac, in Northeast Serbia, is about 17 km long (Kostolac–Kovin). This area is home to industrial and power-generation plants, Kostolac thermal power plants, and farmsteads near the Danube that engage in farming, livestock rearing, and seed production, along with a network of local roads with heavy traffic.
The primary objectives of this investigation were twofold: (1) to analyze groundwater quality in terms of dissolved elements (temperature; pH; electrical conductivity; cations such as Na+, K+, Ca2+, and Mg2+; anions like Cl−, SO42−, NO3−, and PO43−; and trace elements including Pb, As, Cd, Hg, and Zn) using multivariate techniques; and (2) to assess water quality following water standards and guidelines relevant to human health. Numerous groundwater studies have shown that groundwater is being used at rates that globally exceed the natural recharge rates. This paper presents a case study of the pollution of groundwater in the Danube River alluvial area near the Kostolac Basin, Serbia, revealing elevated levels of heavy metals and nitrates and highlighting the need for region-specific studies and remediation strategies. The review results may be used as guidelines for global efforts to protect drinking-water supplies from life-threatening contaminants. Furthermore, these findings help to bridge the knowledge gaps regarding the contamination status, distribution, and sources of chemical pollutants in the surface and groundwater of these areas, ultimately contributing to improved water-management practices.
2. Materials and Methods
2.1. Geographical Location
The Kostolac coal basin covers the area between the Danube River in the north, the Resava River and the town of Svilajnac in the south, the Morava River in the west, and the Golubac Mountains in the east (Figure 1a). Coal deposits have been explored and confirmed in areas of Kostolac as well as in the villages of Drmna, Klenovnik, Ćirikovac, and Poljana. This basin spans an area of 100 km2. The Kostolac Basin represents one of the most economically significant deposits of lignite, which is an essential energy resource in Serbia. The extensive lignite exploitation supports the operation of two thermal power plants, namely, “Kostolac A” and “Kostolac B”. The coal-fired power stations of Kostolac contribute to approximately 14% of the annual electricity production in the Republic of Serbia [15]. The Kostolac power plants and mines are situated in the northeastern part of Serbia, approximately 90 km east of Belgrade [15].
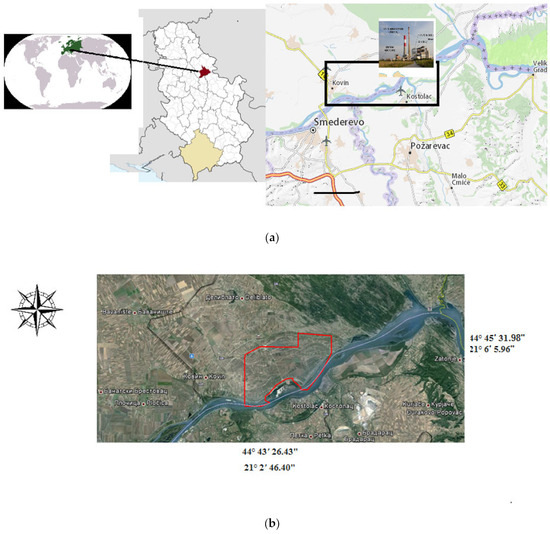
Figure 1.
Study area of Danube River near Kostolac Basin, Serbia (a), and sampling sites (b). The arrow marks a part of the investigated area in Serbia. The red lines marks a part of the sampling sites.
2.2. Sampling and Analytical Procedure
Water samples were collected from 12 monitoring sites (Figure 1) between 29 November and 8 December 2017. Before sampling, groundwater wells were washed and pumped for 15 min to ensure the removal of stagnant water and ensure representative samples were obtained. The samples were collected during the morning, with three repetitions per site, and the average data were used to ensure reliability. Brown glass bottles were utilized for sample collection to prevent contamination, and the samples were subsequently transported to the laboratory in cooled containers within 4 h to maintain the sample integrity. The water samples were filtered using a Whatman No. 40 filter paper and tested according to the methods specified by the standard methods [16,17].
The well water was utilized for both drinking and irrigation purposes.
In this study, 12 well samples were collected and analyzed using the standard procedures for 21 parameters, including temperature (T), pH, electrical conductivity (EC), cations (Na+, K+, Ca2+, and Mg2+), anions (Cl−, NO3, SO42−, and PO43−), and trace elements (Cd, Hg, Pb, As, and Zn) [16,17]. An InoLab720 Multi 720 WTW pH meter was used for the pH measurements. In the first 24 h, the nitrate concentration in the samples was measured with the standard method using a spectrophotometer at a 220 nm wavelength. On-site measurements included EC, pH, and T, while the elemental analysis was conducted using an atomic emission spectrometer (Varian SpectrAA 20-plus). Water samples were digested using concentrated HNO3 for trace metal determination, as described by APHA. Instrument calibration was performed using multielement standard solutions obtained from a certified supplier, ensuring traceability and accuracy. Calibration curves were generated for each analyzed element, with correlation coefficients (R2) greater than 0.999. Recovery rates for the quality control samples were maintained within 95–105% to ensure the accuracy of the analytical procedures. Additionally, reagent blank analyses were conducted to check for any potential contamination, and duplicate samples were analyzed to confirm the precision of the results.
All chemicals used in the analyses were of analytical grade and the glassware was washed with 2% HNO3 before use. Optimizing instrument settings and conducting a sensitivity assessment were integral to the instrument performance evaluation process.
Chemical parameters were expressed in mg/L or μg/L (Table 1), except for pH (units) and EC (μS/cm).

Table 1.
Statistical summary of the analyzed parameters in groundwater from the alluvial area of the Danube River near Kostolac Basin, Serbia.
2.3. Pollution Evaluation Indices
The Heavy Metal Evaluation Index (HEI), Degree of contamination (Cd) and Nemerow pollution index (NI) were investigated to assess the drinking suitability of the groundwater. HEI provides an insight into the overall quality of the groundwater concerning trace metals and is calculated as follows:
where Hc is the monitored value, and Hmac is the maximum admissible concentration of the ith parameter. The degree of heavy metal index was classified into three divisions as follows: HEI ≤ 10: low, HEI (10–20): medium and HEI > 20: high [18].
HEI = ƩHc/Hmac
The degree of contamination (Cd) indicates the overall effects of each contaminant and is calculated using the following equation
where, Cfi = CAi/Ci Ni-1, Cfi = Contamination factor; CAi = analytical value of the ith component, CNi = upper permissible concentration of the ith component. Here, CNi is considered as the Maximum Allowable Concentration (MAC). The pollution level was divided into three categories: Cd < 1: low, Cd (1–3): medium and Cd > 3: high [19].
Cd = ƩC
The Nemerow Pollution Index was applied to determine the extent of heavy metal pollution in the groundwater at the sampling sites in the study area [20,21], and it is calculated as follows:
where Piave and Pimax are the mean and maximum of the single pollution indices for individual trace elements. The pollution in the samples was classified into five levels based on the corresponding PIN values: PIN < 0.7—safety domain; 0.7 ≤ PIN < 1.0—precaution domain; 1.0 ≤ PIN < 2.0-slightly polluted domain; 2.0 ≤ PIN < 3.0—moderately polluted domain and PIN > 3.0—seriously polluted domain.
PIN =√ Piave2 + Pimax2/2
2.4. Human Health Risk Assessment
Most of the residents in the study area use wells to extract groundwater for daily use. As such, a health risk assessment is essential for a comprehensive quality assessment of drinking water. The human health risk models including carcinogenic and non-carcinogenic ones raised by US EPA have proved successful and adopted worldwide [22].
Human health risk due to ingestion of groundwater with various ions-non-carcinogenic risk or chemical toxicity was assessed using hazard index (HI) which is based on the following equations [22]. The calculations for the daily exposure dose of contaminants via various exposure pathways [20,21,22,23].
CDIOral = (CW × IR × EF × ED)/(BW × AT)
CDIDermal = (CW × SA × Kp × ET × EF × ED × CF)/(BW × AT)
CDIDermal = (CW × SA × Kp × ET × EF × ED × CF)/(BW × AT)
Potential non-carcinogenic risks for exposure to contaminants of concern were evaluated by comparing the estimated contaminant intakes from each exposure route (oral, dermal) with the reference dose (RfD) as defined by USEPA, resulting in the calculation of the Hazard Quotient (HQ) [24,25,26,27]. If the value of HQ exceeds 1, there is an unacceptable risk of adverse non-carcinogenic health effects, while an HQ value less than 1 indicates an acceptable level of risk [24,25,26,27].
where, HQ = Hazard Quotient (unitless) and RfD is the reference dose (μg/Kg/day) obtained from the risk-based concentration table. To assess the overall potential for non-carcinogenic effects posed by more than one chemical, the HQs calculated for each chemical are summed and expressed as the Hazard Index (HI).
HQ = CDI/RfD
HI = HQ1 + HQ2+ HQ3 +……Hn
If HI does not exceed unity (HI < 1), no chronic risks are assumed to occur at the site. If HI > 1, it implies a potential non-carcinogenic health risk [28].
Carcinogenic risks were estimated as the incremental probability of an individual developing cancer over a lifetime due to exposure to a potential carcinogen [28]. The following linear dose carcinogenic risk equations, were used for each exposure route.
where “Risk” stands for cancer risk, and “SF” is the slope factor of contaminants (mg/kg/day). Risk in the range of 1 × 10−6 to 1 × 10−4 is typically considered acceptable [29,30].
Cancer risk CR = CDI × CSF
2.5. Multivariate Statistical Analysis
Multivariate statistical methods such as a principal component analysis (PCA), correlation analysis, and cluster analysis (CA) were applied using SPSS (version 22.0) software for Windows to evaluate the analytical data. The principal component analysis identified potential heavy-metal sources. The exploratory factor analysis was performed using the varimax rotation technique, which reduced the number of variables with high loadings on each component, thereby simplifying the interpretation of the PCA results [31]. The principal components (PCs) were employed to pinpoint the most significant values across the entire dataset, enabling data reduction with a minimal loss of baseline information. According to [32], this method reduces the influence of the new set of variables derived from the PCA and the less significant components. A Pearson correlation coefficient was utilized to validate the PCA results and to understand the variations in the components that could be explained by their interactions [33]. The cluster analysis was implemented [34] to identify groups of samples with similar heavy-metal contents, thus providing evidence to support the PCA results. The between-group distance was considered to determine the distance between clusters with similar metal concentrations, and the CA was performed using the average linkage method [35]. The use of these statistical methods allowed for a comprehensive analysis of the groundwater data, identifying underlying patterns and sources of contamination. The PCA helped to reduce the complexity of the dataset by highlighting the most influential factors, while the correlation analysis provided insights into the relationships between different parameters. The cluster analysis grouped the sampling sites based on similarities in heavy-metal content, further supporting the identification of pollution sources.
3. Results
The analysis of the samples included 21 parameters, comprising temperature (T), pH, electrical conductivity (EC), cations (Na+, K+, Ca2+, and Mg2+), anions (Cl−, NO3−, SO42−, and PO43−), and trace elements (Cd, Hg, Pb, As, and Zn). Careful standardization, procedural blank measurements, and duplicate samples ensured the quality of the analytical data. The laboratory also participates in a regular national program of analytical quality control. The basic statistics of the water-quality dataset are summarized in Table 1. The statistical summaries of the parameters analyzed using box and whisker plots are shown in Figure 2. Box and whisker plots are particularly useful when examining summaries of numerical data. They facilitate the identification of potential outliers and abnormal distributions and enable a quick and easy comparison of distributions by displaying the overall range, spread, and center, thus helping to visualize the numerical summary. The recommended mean values of the World Health Organization, European Parliament and Council of the European Union 2006, and MWQ Serbia are presented in Table 2.
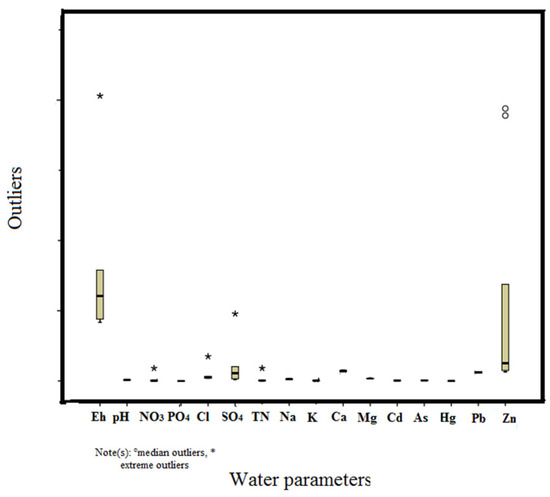
Figure 2.
Box and whisker plot showing the variations in chemical constituents in the studied groundwater samples of Serbia. Note(s): ° median outliers, * extreme outliers.

Table 2.
Comparison of water-quality values from the present study and the recommended guidelines.
The results of the correlations among the investigated groundwater samples are presented in Table 3. The factor loadings, cumulative percentage, and percentage of variance explained by each factor are listed in Table 4. The component plot in the rotated space of the principal component analysis is shown in Figure 3, while the result obtained using HCA in the Q-mode is shown in Figure 4.

Table 3.
Results of the Pearson correlation analysis.

Table 4.
Varimax-rotated principal component analysis of groundwater samples (bold values indicate strong loadings (0.7)).
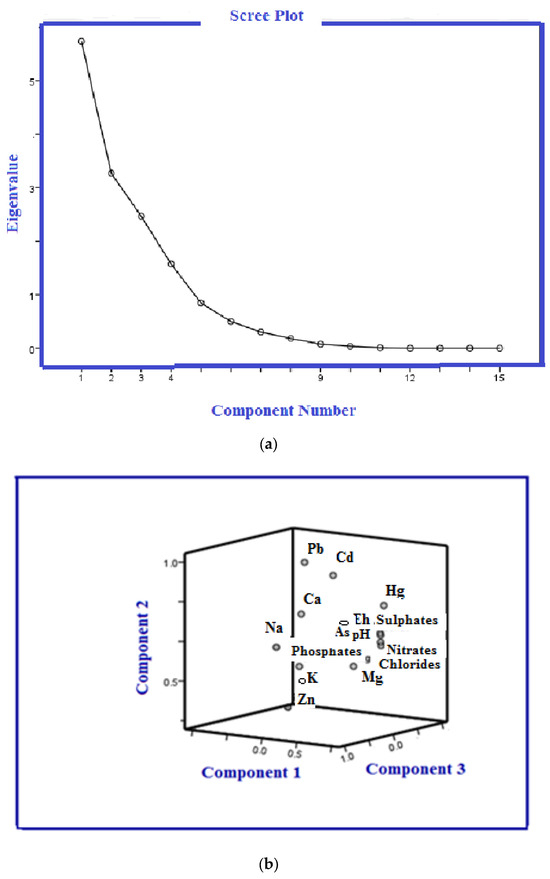
Figure 3.
Principal component analysis (a) scree plot of the characteristic eigenvalues, and (b) component plot in rotated space.
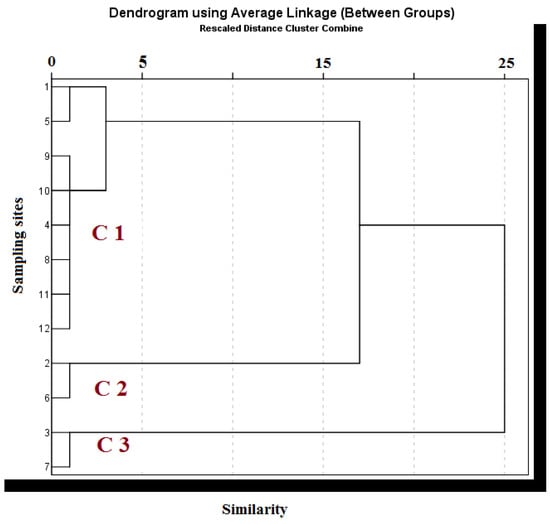
Figure 4.
The dendrogram obtained from a Q-mode hierarchical cluster analysis of groundwater in Serbia. C1, C2 and C3 represent the resulting clusters.
The results of the groundwater pollution evaluation indices are presented in Table 5 and Figure 5. The values of the reference dosage (RfD) used in this study were based on documents from the United States Environmental Protection Agency, shown in Table 6 and Table 7. The chronic human health risks of groundwater (HQ and HI) through oral and dermal exposure routes for both adults and children are summarized in Table 8 and Table 9, while the carcinogenic risk of lead via an oral exposure pathway was calculated for both adults and children and the results are presented in Table 10.

Table 5.
Results of groundwater pollution evaluation indices. Note(s): The high values are in red bold.
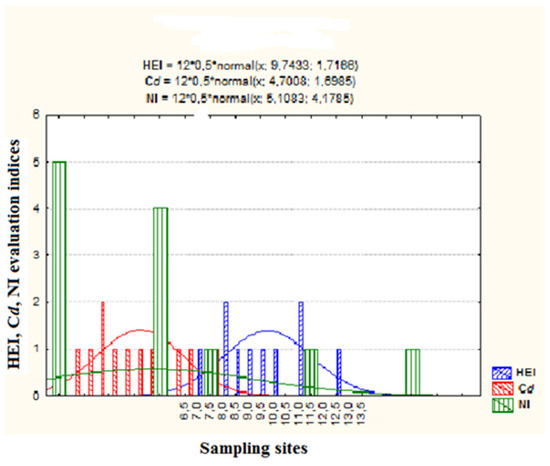
Figure 5.
Plot of HPI, Cd and NI indices versus sampling sites.

Table 6.
Parameters of exposure assessment of oral ingestion and dermal absorption pathways.

Table 7.
The toxicity responses of trace metals for the oral and dermal reference dose (RfD) and oral slope factor (SF).

Table 8.
HQ and H values of adults and children for oral exposure pathway.

Table 9.
HQ and H values of adults and children for dermal exposure pathway.

Table 10.
Cancer risk from lead (Pb) among adults and children for oral exposure pathway.
4. Discussion
4.1. Characteristics of Water Chemistry
Table 1 provides a summary of the statistical information regarding the water-quality data collection. The majority of groundwater samples exhibited a very consistent temperature range of 5.7 to 6.9 °C. The pH of water is one of the most crucial factors in determining its safety for drinking. The pH values of the groundwater samples varied between 6.9 and 7.4, indicating that the water was naturally neutral. Electrical conductivity (EC) is a key indicator used to classify drinking-water quality. Higher EC values correspond with increased concentrations of dissolved solids and ionic strength in the groundwater [35]. Our samples demonstrated positive correlations between EC and both cations and anions. The investigated water samples had an average EC of 920.10 μs/cm, ranging from 417 to 2030 μs/cm. Although the average value was within permissible limits, 16% of the samples exceeded the suggested limits [36,37,38] (Table 2).
The tests used to assess water quality detect naturally occurring cations and anions. The groundwater displayed the following relative abundances of major cations: Ca > Mg > Na > K (mg/L). The relative abundance of anions was SO42− > Cl−>NO3− > PO43−. The study area exhibited a broad concentration range of calcium, with the highest amount among all the cations. Sixty percent of the sampling sites had calcium concentration levels higher than 70 mg/L in general, which is above the WHO-recommended limits. The concentrations of other cations, which are essential to human health, were found to be within their maximum water quality (MWQ) limits. The phosphates and chlorides present were below the acceptable limits.
The findings indicated that the average concentrations of sulfate and nitrate did not exceed the standard determinations set by the MWQ, EU, and WHO [36,37,38], except for two samples in which the values exceeded the suggested criteria. Sulfate in groundwater mainly originates from mineral dissolution, atmospheric deposition, and human activities such as mining and fertilizer use. Sulfate, a common salt necessary for life in certain amounts, is not harmful in the same way as heavy metals, pesticides, or other toxic substances. However, high levels of sulfate in drinking water can cause laxative effects and impair taste [39]. Nitrate is one of the most common pollutants in water. The World Health Organization (WHO) established a guideline value of 50 mg/L for nitrate (as nitrate ion), based on epidemiological studies showing no adverse health effects at concentrations below this value [38]. The primary form of nitrogen pollution was NO3−. Significant correlations would be expected between nitrate and the major cations found in chemical fertilizers if fertilizer use was the main source of nitrate contamination. However, no discernible relationship was found between nitrate and potassium, calcium, or magnesium, indicating that agriculture was not the primary source of the nitrate pollution (Table 3). Furthermore, the regions with elevated nitrate concentrations were primarily industrial districts. High levels of sulfates and nitrates are present in the section of the Danube River passing through this region due to industrial discharges from the Kostolac Basin, although the overall levels are lower than those in globally contaminated surface waters around urban and industrial areas [40]. Significant variations in the EC, sulfate, and zinc values (Figure 2) were primarily caused by geochemical processes such as ion exchange, evaporation, sediment dissolution, and rainwater infiltration, in addition to human sources [41,42]. Heavy metals such as cadmium (Cd) and lead (Pb) have several detrimental effects on human health, including DNA damage, cancer, and central nervous system injuries, as noted in [43]. The levels of Cd and Pb in the samples (67% and 100%, respectively) exceeded the MWQ, EU, and WHO guidelines, indicating a significant impact on the disease burden of the local population in the studied area. The highest trace metal pollution levels of lead (87 μg/L) and cadmium (3.5 μg/L) were found in locations where there were large-scale, uncontrolled metal inputs from human activities such as TEKO Kostolac. The results of the chemical analyses compared with the World Health Organization, the EU Directive, the Water Act, and the Regulations on the Monitoring of Water Quality introduced by the Government of the Republic of Serbia showed that levels of metals like arsenic (As), mercury (Hg), and zinc (Zn) were below or equal to the acceptable limits. This suggested that the groundwater was generally of adequate chemical quality for household or agricultural use, and the water quality at the tested locations could be categorized as class II. Conversely, the water quality fell into class III/IV (polluted) due to the quantities of EC, nitrates, total nitrogen, sulfates, and metals (Pb and Cd) exceeding the MWQ, EU, and WHO criteria. The ranges and means of the nitrates and metal concentrations in the samples from groundwater were compared with data from other countries to evaluate the water quality of the present study area. Generally, except for Pb, Zn, and nitrates, for which high concentrations were observed, the concentrations of the other parameters in the Serbian water were similar or slightly lower than other regions of world [4,8,9,10,17,19,21,22,23]. It should be noted that comparisons between the studies were relative and the reported means and ranges were all based on the methodology of a particular study. Increased pollution control efforts are sorely needed. Industry and agriculture produce a large diversity of pollutants, forming severe threats to groundwater quality. The protection of groundwater quality by effective regulation and strict enforcement is urgently required in all sectors as adequate practices are still rare.
4.2. Statistical Treatment
The source materials of the groundwater were identified using a PCA, a CA, and a Pearson correlation matrix. According to [44], a correlation analysis reveals the relationship between the heavy-metal characteristics of water samples and can provide data regarding the sources of these metals. A high correlation coefficient indicates a strong positive relationship between two variables at a p-value of less than 0.05, while a value close to zero suggests no relationship. References [45,46] explain that a moderate correlation is indicated by an R-value between 0.5 and 0.7, while values of R > 0.7 are considered to be highly correlated. Significant positive and negative correlations between the measured parameters were revealed by the Pearson correlation values presented in Table 3. There was a significant relationship between Pb and Cd, suggesting that they had a similar geological behavior as well as similar sources.
With a Kaiser–Meyer–Olkin test result of 0.684, appropriate sampling adequacy was indicated. To improve the explanation of the potential factors influencing heavy metals, a varimax rotation was employed to maximize the sum of the variance of the factor loadings. Figure 3 displays a scree plot of the eigenvectors as a function of the factor number. Four major components were identified using the Kaiser criterion, which accounted for eigenvalues greater than 1. These components explained 87.63% of the total variance. The scree plot was used to determine the number of PCs. Table 4 lists the component loadings, cumulative percentages, and percentage of variations explained by each factor. Figure 3 displays the component plot in the rotated space of the principal component analysis.
PCA 1, which accounted for 17.89% of the total variance, showed significant correlations for EC, pH, Cl−, NO3−, and SO42−. All these components had levels higher than the baseline except chloride, suggesting that they may have been impacted by human activity rather than natural sources. The anthropogenic toxic pollution sources represented by Component PCA 2 (Cd and Pb) most likely originated from industrial effluents. Component 3 had substantial loadings for Na, K, and Mg and accounted for 15.181% of the total variation. Significant positive correlations (p < 0.01) were found between them in the samples, suggesting that these ions originated from the mineral part of the groundwater (natural sources). The last component featured strong loadings of calcium and phosphate. The Pearson correlation matrix supported the hypothesis for PCA 4 that the behavior of calcium in groundwater may be influenced by other mechanisms. Moreover, a high negative loading by phosphates (with values not exceeding the safe water criteria defined by the MWQ, EU, and WHO [36,37,38]) suggested that this source did not make a major contribution to the other parameters.
Based on the PCA analyses and the similarity of the groundwater-quality features, HCA divided the sample locations into three clusters. A total of eight samples (1, 4, 5, 8, 9, 10, 11, and 12) made up the first cluster (A), which was further separated into two subgroups—A-1 (1, 5, 9, and 10) and A-2 (4, 8, 11, and 12); two samples each made up the second cluster, B (2 and 6) and the third cluster, C (3 and 7) (Figure 4). The hydrochemistry of the A-1 group was dictated by mineralization processes controlled by the lithological composition of the groundwater. Component 3 (all cations) of the PCA dominated in group CA-1, while component PC 2, which had higher indicated contents of Pb and Cd due to human activities (scattered point sources—industrial effluent), was represented in group A-2. Group B samples had higher levels of Eh (2030 μg/L), SO4 (478.50 mg/L), and NO3 (90.40 mg/L) than the other groups. Additionally, they were contaminated with similar pollutants to subgroup A-2. Conversely, the samples from group C had an extremely high loading of Zn (1940 μg/L), which was likely the result of anthropogenic activities or the cation exchange properties of groundwater wells, but these values did not exceed the guidelines set by MWQ, EU, and WHO [36,37,38] for safe water.
4.3. Pollution Level
Table 5 and Figure 5 provide the results of the pollution evaluation levels. The heavy-metal evaluation index had a mean value of 9.041 and it ranged from 7.41 to 12.62. About 41.70 percent of the sample sites exhibited a medium level of pollution, while low and high levels of contamination were found at 58.3% and 0% of the sample sites, respectively. The level of metal pollution at the sampling sites was assessed using the degree of contamination. The Cd results had a mean value of 4.71, with a range from 2.28 to 7.53. The results for the Cd pollution degree showed that 33.3% of the sampling sites fell into the medium pollution category and 66.7% into the high pollution rate category. To determine the extent to which various heavy metals contaminated the groundwater, the Nemerow pollution index was further implemented. Our samples had an NI ranging from 1.43 to 14.85, with a mean value of 5.11. As seen in Table 5, it was found that nearly 58.3% of the sites experienced high pollution levels (severe pollution), with the remaining 41.7% having medium pollution levels. Mercury and lead were identified as the most significant contributors to pollution.
4.4. Human Health Risk
The World Health Organization (WHO) has ranked arsenic, lead, mercury, and cadmium among the top 10 substances of great risk to people worldwide due to their significant toxicity, persistence in the ecosystem, and tendency for bioaccumulation [47]. The United States Environmental Protection Agency (USEPA) defines a human health risk assessment as the process of evaluating the potential for adverse health impacts from human exposure to chemicals in a contaminated environment. This assessment is performed using the following four steps [48]:
Step 1: Hazard Identification—Contaminants are assumed to impact on human health, regardless of whether they meet the toxicity criteria or not. In this paper, eleven specifications were divided into two categories: non-carcinogenic factors such as nitrate (NO3−), Cd, As, Hg, Zn, and Pb; and carcinogenic effects (only Pb was considered in this work because the Pb levels in 100% of the samples exceeded the MWQ, EU, and WHO recommendations). We only considered the non-carcinogenic risk of nitrogen because, currently, there is insufficient evidence to link nitrogen exposure to human cancer [49].
Step 2: Dose–Response Assessment—This step uses the reference dose (RfD) to determine the precise relationship between the contaminant exposure dose and its effects. The RfD values used in this study are listed in Table 6 and Table 7.
Step 3: Exposure Assessment—According to [50], this step involves assessing exposure aspects such as exposure pathways, body parameters, frequency of exposure, and duration of exposure, either quantitatively or qualitatively. There are three potential routes of exposure: direct ingestion of drinking water, inhalation, and skin absorption. In this study, groundwater exposure was quantified using an oral exposure pathway and dermal absorption. By comparing the estimated contaminant exposures from each pathway (oral and dermal) with the RfD using Equation (4), potential non-carcinogenic concerns were identified. This article focused on nitrogen exposure from a drinking-water intake route because skin absorption is considered to be less significant (less than one-thousandth of the drinking-water intake) [51,52,53]. Table 8 and Table 9 summarize the chronic human health risks associated with groundwater exposure through oral and dermal routes for both adults and children. It was found that the HQ values for Cd, As, Hg, and Zn were less than 1, indicating that these elements might not pose serious hazards when present alone. However, the highest HQ oral values for Pb and nitrates were found to be 1.776 and 2.260 for adults and 2.515 and 2.260 for children, respectively. According to USEPA (Table 8), most of the samples indicated a medium category of chronic risk for both adults and children. Table 9 provides additional results of dermal HI for adults and children, showing that dermal exposure is typically less hazardous than oral exposure. In both the adult and the child populations, 100% of the samples were associated with low levels of chronic risk. In all cases (adults and children), sampling site Gw 6 exhibited the highest potential chronic risk.
Carcinogenic Risk—Equation (6) and Table 7 illustrate how the carcinogenic risk (CR) associated with Pb exposure was calculated as the total daily intake [mg/(kg day)−1] multiplied by the cancer slope factor (SF). As noted earlier, the only carcinogenicity risk established in this study originated from lead. Based on the guidelines suggested by some authors [54,55], all of the samples fell into the low-risk category concerning the carcinogenic risk of lead exposure via the oral pathway (Table 10 provides estimates for both adults and children).
5. Conclusions
Multivariate statistical and health risk analyses of water-quality parameters were applied to determine the source of contaminants and their effects on human health in the alluvial area of the Danube River near the Kostolac Basin, Serbia. A total of 21 parameters, including temperature (T), pH, electrical conductivity (EC), cations (Na+, K+, Ca2+, and Mg2+), anions (Cl−, NO3−, SO42−, and PO43−), and trace elements (Cd, Hg, Pb, As, and Zn), in water samples collected from 12 groundwater wells were analyzed. The levels of nitrates (90.40 μg/L), Pb (87 μg/L), and cadmium (3.5 μg/L) in 17%, 67%, and 100% of the samples, respectively, exceeded the Serbian Water Act criteria (MWQ), European Parliament and Council of the European Union (EU), and the World Health Organization (WHO) groundwater-quality guidelines, indicating a significant impact on the disease burden of the local population in the studied area. There was a significant relationship between Pb and Cd, suggesting that they had a similar geological behavior as well as similar sources. These samples were categorized as class III/IV, based on the Serbian Water Act criteria, indicating that they required sanitation due to unacceptable quality. A principal component analysis (PCA), a cluster analysis (CA), and a Pearson correlation matrix identified the dominant and potential sources of contaminants in the groundwater wells, including natural geogenic processes and industrial activities. According to the HEI results, around 50% of the samples exhibited a medium level of contamination, while 83% and 58.3% of the samples showed a high level of pollution regarding Cd and Ni, respectively. Pb and nitrates reported the highest HQ oral values for adults (1.980 and 1.776, respectively) and children (2.515 and 2.260, respectively). Generally, there is a lower risk associated with dermal exposure than oral exposure. As the HQ values at other sites were less than 1, it was considered probable that any potential problems could be disregarded. However, HQ values greater than 1 for lead and nitrates at some sample sites raised concerns regarding both chronic and carcinogenic health risks. According to the lead carcinogenic risk via the oral exposure pathway, there was a minimal risk for both adults and children associated with all of the samples.
Most groundwater pollution is anthropocentric and can be prevented through intensive health education. If the improvement of groundwater quality and future scenarios are not considered, this problem may become even more serious. Therefore, this study provides relevant information to residents and public authorities concerned with drinking-water quality and the potential effects of contamination. This article also provides an insight into the groundwater contamination issues that are affecting low- and middle-income countries and countries with emerging economies in the Eastern Hemisphere. Researchers from Europe, North America, and other high-income countries often do not grasp the extent of groundwater contamination from geogenic and anthropogenic sources in other regions. The co-authors of this paper anticipate that this article will facilitate an understanding of the origins and extent of groundwater contamination and its consequences, providing examples of possible approaches to the remediation of groundwater contamination and the protection of groundwater quality. In the future, “big data” analyses, drone surveys, and molecular and stable isotope analysis technologies should be applied to groundwater research.
Author Contributions
Conceptualization, G.D. and M.P. (Marija Pergal); methodology, M.P. (Miodrag Pergal); software, G.D.; validation and investigation, M.P. (Miodrag Pergal); data curation M.P. (Marija Pergal) and M.P. (Miodrag Pergal); Writing—review and editing, G.D. and M.P. (Marija Pergal). All authors have read and agreed to the published version of the manuscript.
Funding
The authors would like to thank the The Ministry of Science, Technological Development and Innovation (Grant No: 451-03-66/2024-03/200026).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors would like to thank Katarina Spasic for useful advice and English proofreading. Also, the authors thank the reviewers of this paper for their useful advice.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kumar, S.; Tripathi, V.R.; Garg, S.K. Physicochemical and microbiological assessment of recreational and drinking waters. Environ. Monitt Assess. 2012, 184, 2691–2698. [Google Scholar] [CrossRef] [PubMed]
- Herms, I.; Jódar, J.; Soler, A.; Lambán, L.J.; Custodio, E.; Núñez, J.A.; Arnó, G.; Parcerisa, D.; Jorge-Sánchez, J. Identification of Natural and Anthropogenic Geochemical Processes Determining the Groundwater Quality in Port del Comte High Mountain Karst Aquifer (SE, Pyrenees). Water 2021, 13, 2891. [Google Scholar] [CrossRef]
- Sunkari, E.D.; Seidu, J.; Ewusi, A. Hydrogeochemical evolution and assessment of groundwater quality in the Togo and Dahomeyan aquifers, Greater Accra Region, Ghana. Environ. Res. 2022, 208, 112679. [Google Scholar] [CrossRef] [PubMed]
- Torres-Martínez, J.A.; Mora, A.; Knappett, P.S.K.; Ornelas-Soto, N.; Mahlknecht, J. Tracking nitrate and sulfate sources in groundwater of an urbanized valley using a multi-tracer approach combined with a Bayesian isotope mixing model. Water Res. 2020, 182, 115962. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Chakraborty, A.J.; Tareq, A.M.; Emran, B.T.; Nainu, F.; Khusro, A.; Idris, A.M.; Khandaker, M.U.; Osman, H.; Alhumaydhi, F.A.; et al. Impact of heavy metals on the environment and human health: Novel therapeutic insights to counter the toxicity. J. King Saud Univ. Sci. 2022, 34, 101865. [Google Scholar] [CrossRef]
- Dotaniya, M.L.; Meena, V.D.; Saha, J.K.; Dotaniya, C.K.; Mahmoud, A.D.; Meena, B.L.; Meena, M.D.; Sanwal, R.C.; Meena, S.R.; Doutaniya, R.K.; et al. Reuse of poor-quality water for sustainable crop production in the changing scenario of climate. Environ. Dev. Sustain. 2023, 25, 7345–7376. [Google Scholar] [CrossRef] [PubMed]
- Carrard, N.; Foster, T.; Willetts, J. Groundwater as a Source of Drinking Water in Southeast Asia and the Pacific: A Multi-Country Review of Current Reliance and Resource Concerns. Water 2019, 11, 1605. [Google Scholar] [CrossRef]
- Borpatra Gohain, M.; Karki, S.; Yadav, D.; Yadav, A.; Thakare, N.R.; Hazarika, S.; Lee, H.K.; Ingole, P.G. Development of Antifouling Thin-Film Composite/Nanocomposite Membranes for Removal of Phosphate and Malachite Green Dye. Membranes 2022, 12, 768. [Google Scholar] [CrossRef]
- Shemer, H.; Wald, S.; Semiat, R. Challenges and Solutions for Global Water Scarcity. Membranes 2023, 13, 612. [Google Scholar] [CrossRef]
- Doza, B.; Islam, S.D.-U.; Hasan, T.; Alam, F.; Haque, M.; Rakib, M.; Asad, A.; Rahman, A. Groundwater pollution by trace metals and human health risk assessment in central west part of Bangladesh. Groundw. Sustain. Dev. 2019, 9, 100219. [Google Scholar] [CrossRef]
- Krishna, A.K.; Mohan, K.R.; Dasaram, B. Assessment of groundwater quality, toxicity and health risk in an industrial area using multivariate statistical methods. Environ. Syst. Res. 2019, 8, 26. [Google Scholar] [CrossRef]
- Chakraborty, M.; Tejankar, A.; Coppola, G.; Chakroborthy, S. Assessment of groundwater quality using statistical methods: A case study. Arab. J. Geosci. 2022, 15, 1136. [Google Scholar] [CrossRef]
- Belkhiri, L.; Boudoukha, A.; Mouni, L. A multivariate Statistical Analysis of Groundwater Chemistry Data. Int. J. Environ. Res. 2011, 5, 537–544. [Google Scholar]
- Ielp, P.; Leardi, R.; Pappagallo, G.; Uricchio, V.F. Tools based on multivariate statistical analysis for classification of soil and groundwater in Apulian agricultural sites. Environ Sci Pollut Res. 2017, 24, 13967–13978. [Google Scholar] [CrossRef] [PubMed]
- Gojkovic, Z.; Kilibarda, M.; Brajovic, L.; Marjanovic, M.; Milutinovic, A.; Ganic, A. Ground Surface Subsidence Monitoring Using Sentinel-1 in the “Kostolac” Open Pit Coal Mine. Remote Sens. 2023, 15, 2519. [Google Scholar] [CrossRef]
- APHA. WEF Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association (APHA): Washington, DC, USA; American Water Works Association (AWWA): Washington, DC, USA; WPCR, Water Environment Federation: Washington DC, USA, 2005. [Google Scholar]
- APHA. Standard Methods for the Examination of Water and Wastewater, 18th ed.; American Public Health Association (APHA): Washington, DC, USA; American Water Works Association (AWWA): Washington, DC, USA; Water Pollution Control Federation (WPCF): Washington DC, USA, 1992. [Google Scholar]
- Rajkumar, H.; Naik, P.K.; Rishi, M.S. A new indexing approach for evaluating heavy metal contamination in groundwater. Chemosphere 2020, 245, 125598. [Google Scholar] [CrossRef]
- Rahman, M.; Khan, M.R.; Hossain, M.S.; Hossain, M.I.S.; Hasan, M.; Hamli, H.; Mustafa, M.G. Groundwater Contamination and Health Risk Evaluation of Naturally Occurring Potential Toxic Metals of Hatiya Island, Bangladesh. J. Ecol. Eng. 2022, 23, 223–236. [Google Scholar] [CrossRef]
- Su, K.; Wang, Q.; Li, L.; Cao, R.; Xi, Y.; Li, G. Water quality assessment based on Nemerow pollution index method: A case study of Heilongtan reservoir in central Sichuan province, China. PLoS ONE 2022, 17, e0273305. [Google Scholar] [CrossRef] [PubMed]
- Ramos, E.; Bux, R.K.; Medina, D.I.; Barrios-Piña, H.; Mahlknecht, J. Spatial and Multivariate Statistical Analyses of Human Health Risk Associated with the Consumption of Heavy Metals in Groundwater of Monterrey Metropolitan Area, Mexico. Water 2023, 15, 1243. [Google Scholar] [CrossRef]
- U.S. EPA (U.S. Environmental Protection Agency). Guidelines for Human Exposure Assessment; (EPA/100/B-19/001); Risk Assessment Forum; U.S. EPA: Washington, DC, USA, 2019. Available online: https://www.epa.gov/sites/default/files/2020-01/documents/ (accessed on 6 May 2023).
- Saha, N.; Rahman, M.S. Groundwater hydrogeochemistry and probabilistic health risk assessment through exposure to arsenic-contaminated groundwater of Meghna floodplain, central-east Bangladesh. Ecotoxicol. Environ. Saf. 2020, 206, 111349. [Google Scholar] [CrossRef]
- He, X.; Li, P.; Wu, J.; Wei, M.; Reu, X.; Wang, D. Poor groundwater quality and high potential health risks in the Datong Basin, northern China: Research from published data. Environ. Geochem. Health 2021, 43, 791–812. [Google Scholar] [CrossRef] [PubMed]
- USEPA. National Recommended Water Quality Criteria; United States Environmental Protection Agency, Office of Water, Office of Science and Technology: Washington, DC, USA, 2006. [Google Scholar]
- Yang, X.; Duan, J.; Wang, L.; Li, W.; Guan, J.; Beecheam, S.; Mulcahy, D. Heavy metal pollution and health risk assessment in the Wei River in China. Environ. Monit. Assess. 2015, 187, 111. [Google Scholar] [CrossRef] [PubMed]
- Proshad, R.; Islam, S.; Tusher, T.R.; Zhang, D.; Khadka, S.; Gao, J.; Kundu, S. Appraisal of heavy metal toxicity in surface water with human health risk by a novel approach: A study on an urban river in vicinity to industrial areas of Bangladesh. Toxin Rev. 2021, 40, 803–819. [Google Scholar] [CrossRef]
- Boum-Nkot, S.M.; Nlend, B.; Komba, D.; Nkoue Ndondo, G.R.; Bello, M.; Fongoh, E.J.; Ntamak Nida, M.J.; Etame, J. Hydrochemistry and assessment of heavy metals groundwater contamination in an industrialized city of sub-Saharan Africa (Douala, Cameroon). Implic. Hum. Health HydroResearch 2023, 6, 52–64. [Google Scholar] [CrossRef]
- Rahman, M.M.; Ng, J.C.; Naidu, R. Chronic exposure of arsenic via drinking water and its adverse health impacts on humans. Environ. Geochem. Health 2009, 31, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Osborne, J.W. What is Rotating in Exploratory Factor Analysis? Pract. Assess. Res. Eval. 2015, 20, 2. [Google Scholar] [CrossRef]
- Jolliffe, I.T.; Cadima, J. Principal component analysis: A review and recent developments. Phil. Trans. R Soc. 2016, 374, 20150202. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Lo, S. Effects of data normalization and inherent-factor on decision of optimal coagulant dosage in water treatment by artificial neural network. Expert Syst. Appl. 2010, 37, 4974–4983. [Google Scholar] [CrossRef]
- Unmesh, P.; Sundaray, S.K.; Rath, P.; Nauk, B.; Bhatta, D.P. Application of factor and cluster analysis for characterization of river and estuarine water systems A case study: Mahanadi River (India). J. Hydrol. 2006, 331, 434–445. [Google Scholar] [CrossRef]
- Ahmed, N.; Bodrud-Doza, M.; Islam, D.U.; Choudhry, M.A.; Muhib, I.M.; Zahid, A.; Hossain, S.; Moniruzzaman, M.; Deb, N.; Bhuiyan, A.Q.M. Hydrogeochemical evaluation and statistical analysis of groundwater of Sylhet, north-eastern Bangladesh. Acta Geochim. 2018, 38, 440–455. [Google Scholar] [CrossRef]
- Marandi, A.; Polikarpus, M.; Jõeleht, A. A new approach for describing the relationship between electrical conductivity and major anion concentration in natural waters. Appl. Geochem. 2013, 38, 103–109. [Google Scholar] [CrossRef]
- Water Act (Official Gazette of the Republic of Serbia nos. 46/91, 53/93, 67/93, and 48/94) and the Regulations on the Monitoring of Water Quality introduced by the Government of the Republic of Serbia.
- European Commission (EC). Directive 2006/118/EC of the European Parliament and the Council of 12th of December 2006 on the protection of ground water against pollution and deterioration. Off. J. Eur. Union 2006, [L 372/19. 27/12]. XLIII. [Google Scholar]
- WHO. Guidelines for Drinking-Water Quality: First Addendum to the Fourth Edition; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Wang, H.; Zhang, Q. Research Advances in Identifying Sulfate Contamination Sources of Water Environment by Using Stable Isotopes. Int. J. Environ. Res. Public Health 2019, 16, 1914. [Google Scholar] [CrossRef] [PubMed]
- Torres-Martínez, J.A.; Mora, A.; Mahlknecht, J.; Kaown, D.; Barcelo, D. Determining nitrate and sulfate pollution sources and transformations in a coastal aquifer impacted by seawater intrusion—A multi-isotopic approach combined with self-organizing maps and a Bayesian mixing model. J. Hazard. Mater. 2021, 417, 126103. [Google Scholar] [CrossRef] [PubMed]
- Ehya, F.; Marbouti, Z. Hydrochemistry and contamination of groundwater resources in the Behbahan plain, SW Iran. Environ. Earth Sci. 2016, 75, 455. [Google Scholar] [CrossRef]
- Hosseinifard, S.J.; Aminiyan, M.M. Hydrochemical characterization of groundwater quality for drinking and agricultural purposes: A case study in Rafsanjan plain, Iran. Water Qual. Expo. Health 2015, 7, 531–544. [Google Scholar] [CrossRef] [PubMed]
- Michalczyk, K.; Kupnicka, P.; Witczak, G.; Tousty, P.; Bosiacki, M.; Kurzawski, M.; Chlubek, D.; Cymbaluk-Płoska, A. Assessment of Cadmium (Cd) and Lead (Pb) Blood Concentration on the Risk of Endometrial Cancer. Biology 2023, 12, 717. [Google Scholar] [CrossRef] [PubMed]
- Barakat, A.; El Baghdadi, M.; Rais, J.; Aghezzaf, B.; Slassi, M. Assessment of spatial and seasonal water quality variation of Oum Er Rbia River (Morocco) using multivariate statistical techniques. Int. Soil Water Conserv. Res. 2016, 4, 284–292. [Google Scholar] [CrossRef]
- Varol, S.; Davraz, A.; Şener, Ş.; Şener, E.; Aksever, F.; Kırkan, B.; Tokgözlü, A. Assessment of groundwater quality and usability of Salda Lake Basin (Burdur/Turkey) and health risk related to arsenic pollution. J. Environ. Health Sci. Eng. 2021, 11, 681–706. [Google Scholar] [CrossRef]
- Boateng, T.K.; Opoku, F.; Acquaah, S.O.; Akoto, O. Pollution evaluation, sources and risk assessment of heavy metals in hand-dug wells from Ejisu-Juaben Municipality, Ghana. Environ. Syst. Res. 2015, 4, 18. [Google Scholar] [CrossRef]
- Witkowska, D.; Słowik, J.; Chilicka, K. Heavy Metals and Human Health: Possible Exposure Pathways and the Competition for Protein Binding Sites. Molecules 2021, 7, 6060. [Google Scholar] [CrossRef] [PubMed]
- USEPA (US Environmental Protection Agency). Risk Assessment Guidance for Superfund Volume I: Human Health Evaluation Manual (Part E). 2004. Available online: http://www.epa.gov/oswer/riskassessment/ragse/pdf/introduction.pdf (accessed on 12 May 2023).
- USEPA (US Environmental Protection Agency). Baseline Human Health Risk Assessment Vasquez Boulevard and I-70 Superfund Site, Denver CO. 2001. Available online: http://www.epa.gov/region8/superfund/sites/VB-170-Risk.pdf (accessed on 24 May 2023).
- USEPA. National Primary and Secondary Drinking Water Standards. 2009. Available online: http://www.epa.gov/safewater/consumer/pdf/mcl.pdf (accessed on 25 May 2023).
- Kaur, L.; Rishi, M.S.; Siddiqui, A.U. Deterministic and probabilistic health risk assessment techniques to evaluate non-carcinogenic human health risk (NHHR) due to fluoride and nitrate in groundwater of Panipat, Haryana, India. Environ. Pollut. 2019, 259, 113711. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Bian, J.; Wan, H.; Ma, Y.; Sun, X. Health risk assessment of groundwater nitrogen pollution in Songnen Plain. Ecotoxicol. Environ. Saf. 2021, 207, 111245. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Ma, Y.; Qi, Y.; Zhong, Y.; Sha, X. Health risk assessment of groundwater nitrogen pollution in Yinchuan plain. J. Contam. Hydrol. 2022, 249, 104031. [Google Scholar] [CrossRef] [PubMed]
- Giri, S.; Singh, A.K. Human health risk assessment via drinking water pathway due to metal contamination in the groundwater of Subarnarekha River Basin, India. Environ. Monit. Assess. 2015, 187, 6. [Google Scholar] [CrossRef]
- Triassi, M.; Cerino, P.; Montuori, P.; Pizzolante, A.; Trama, U.; Nicodemo, F.; D’Auria, J.L.; De Vita, S.; De Rosa, E.; Limone, A. Heavy Metals in Groundwater of Southern Italy: Occurrence and Potential Adverse Effects on the Environment and Human Health. Int. J. Environ. Res. Public Health 2023, 20, 1693. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).