Abstract
This paper explores how water and water-based systems change their structure under different conditions, such as pH, temperature, and electric fields. These changes affect the properties and performance of living and non-living systems that use water or water-based systems in various technologies. We can use pH, temperature, and electric fields to measure and control the structural changes in water and water-based systems and improve the outcomes of different technologies in biology and medicine. More research is needed to understand how various factors influence the structure of water and water-based systems and how this affects living and non-living systems.
1. Introduction
Water quality for drinking and other uses is usually evaluated based on four criteria: epidemiological, organoleptic, sanitary/toxicological, and radiation [1]. However, recent studies have shown that another criterion should be considered: the structure of drinking and mineral water [1,2,3,4]. Water was traditionally seen as a passive substance that acted as a solvent or heat storage for chemical reactions [2,5,6]. However, water can also have different structures that affect its properties and functions.
The liquid state of water is characterised by a three-dimensional network of water molecules held together by hydrogen bonds and van der Waals forces. This network is dynamic and flexible, as water molecules can break and form new bonds with other molecules [6]. The structure of water is influenced by both types of intermolecular interactions, as [7] suggests.
Water molecules can form and break bonds with each other, creating groups of molecules called clusters [2,8,9,10,11]. The smallest cluster has six water molecules [12]. Using laser interference, researchers have found much larger clusters of up to 100 µm that last from 10−11 s to 1 s or more, called giant water clusters (GWCs) [13].
Water is essential for the structure and function of living cells [2]. Water within biological tissues exists in both “bound” and “free” states. The orientation of bound water molecules on a protein’s surface leads to the formation of an aqueous shell. The structure of bound water affects the properties and functions of protein macromolecules, enzyme activity, structure, and biological membranes [3]. Additionally, when structured water combines with organic compounds, it creates a polymer–liquid pseudocrystal matrix, which includes RNA and DNA [2]. This conditionally supports the idea that the sizes and shapes of globular proteins correspond to water clusters [3].
Some researchers claim that water-based systems have GWCs with similar dimensions and properties to the cells of living beings. GWCs also exist in plant sap [14]. A GWC has a “membrane” and a trans-membrane potential (around 100 mV), like the cells of living beings. Moreover, smaller water clusters can exist inside larger ones [8,13], resembling the organelles inside a cell. These clusters can form structures that look like multicellular organisms [8,15,16]. The “cellular” structure of water suggests that water already has a differentiation that can explain the cellular structure of living beings. The organic components of future cells in biosystems may occupy the pre-existing “cellular” structure [16].
Various factors, such as magnetic field, solution composition, temperature, and others, can affect the structure of water and alter the sizes of water clusters [3,15,17,18,19,20]. The structure of aqueous solutions also changes in the near-wall layer, depending on the material of the surface and the substance dissolved in the water [21,22]. Many publications have reported these changes in water structure under different conditions.
Water and water-containing systems can undergo structural modifications due to various low-energy effects [2,3,15,18,19,20,23,24,25,26]. These structural changes can alter water’s biological, therapeutic, and other properties and applications [17]. Experimental and clinical evidence supports that water’s structure influences biological activity and other characteristics.
A more comprehensive understanding of how water with different initial structures affects the processes of living and non-living systems is still lacking [2,17,27]. The relationship between various modifications in the structure of water, water-containing systems, and changes in their properties has scarcely been investigated.
One of the research objectives in this area is to understand how water affects the susceptibility of living systems to various weak influences, which is relevant to ecology and medicine [27]. Several authors have emphasized the practical significance of investigating the primary and secondary mechanisms of water and water-containing systems’ responses to low-energy therapeutic physical factors, including at the molecular level [20,23,24,28,29]. In this context, changes in the structure and properties of water, especially intracellular water, are worth investigating [20,23,24,25,28,29,30,31]. As [23] stated, water is a critical molecule in the action of therapeutic physical factors.
Changes in the properties of cement stone, concrete, and plants were observed when exposed to water by a magnetic field, ultrasound, or electric current [32,33]. However, these studies did not explain how these effects are related to changes in water structure under these influences.
In the rest of this article, we will present new data, representing the first step in formulating methods for assessing the structure of water and water-containing systems (for example, pH values, thermometry parameters, parameters of electrophysical methods, and other methods) to improve living and non-living systems. The relevance of research in this direction is confirmed in the works of recent years [34,35,36,37].
This review aims to show the possibilities of methods for assessing the composition and structure of water and water-containing systems to improve the properties of living and non-living systems.
2. Assessment of the Possibility of Using pH, Thermometry and Electrophysical Methods to Evaluate the Structure of Water and Water-Containing Systems
Currently, several studies aimed at studying changes in the structure and other properties of water and water-containing systems with various properties, including low-energy (“informational”) influences [14,17,18,38,39], in which the energy from exposure is much less than the energy released as the result of exposure [40]. With such influences, several orders of magnitude lower than the energy of Brownian movement and not causing temperature changes, significant changes in the properties of living and non-living systems occur, comparable to those with powerful energy influences.
To justify methods for studying the structure of water, the following data should be taken into account. It is known that water molecules are in continuous thermal movement. With this in mind, when studying the structure of water and aqueous solutions, it is possible to talk about the specific position of individual molecules (dipoles) of water relative to each other within a time interval of less than 10−13 s. It is also important to note that under constant thermodynamic conditions, the structure of water is reproduced.
This allows the use of non-destructive research methods for various influences: evaluation of the structure of water, which is due to a change in the ratio and mobility of the “free” dipoles of water, water dipoles located in water clusters and in hydrated formations of ions, etc. [17,22].
The use of electrophysical and other methods to evaluate the structure is based firstly on the fact that despite the brief existence of associates (clusters) of water, with constant thermodynamic parameters, the structure of water and water-containing systems is reproduced [2,8,9,10,11,13].
Secondly, when using electrophysical methods in measuring cells through water and water-containing systems, the minimum possible current values from 10 nA/cm2 to 780 nA/cm2 are used. At the same time, the research results in various papers did not contradict each other [17,21,22,26,29,40,41,42,43,44,45,46,47,48,49,50].
Thirdly, when evaluating the results, the influence of the layers of the walls was taken into account. It was shown that for research with distilled water and water-containing systems, it is advisable to use measuring cells in which the capacitor plates are located at a distance of 5 cm to 10 cm from each other [17,21,22].
Fourth, taking into account the dynamics of the formation and destruction of water clusters, the study of the effect of temperature on the structure of water and water-containing systems was carried out at a rate of temperature change per 1 degree of at least 50 s.
When studying the structure of water and water-containing systems using pH, thermometry, and electrophysical parameters and when following the aforementioned methodological conditions, the results of the research did not contradict each other and were consistent with known ideas about the properties and structural features of water and water-containing systems.
3. Using pH to Assess the Structure of Water and Water-Containing Systems to Improve the Properties of Living and Non-Living Systems
The impacts of various factors on water and water-containing systems have produced exciting and unexpected results. The pH of water, mineral water, and water-containing systems increases under a constant magnetic field, indicating a change in the water structure [31,40,41,51,52,53]. The pH value varies depending on the magnetisation time [52], the number of magnetic activation cycles [38], the magnetic field induction [53,54], the water flow rate [54], and the water temperature [53,55].
Magnetised water has been used in construction to improve the properties of concrete. The concrete strength is affected by the number of times the water is treated with magnets, the duration between the treatment and the use of water, and the characteristics of the magnets [38,56,57,58]. Previous studies have demonstrated that altering the structure and energy of water and water–salt solutions can control their reactivity and enhance the quality of building materials [58]. Concrete products can be strengthened by 18% using magnetised water [59], and cement consumption can be reduced by up to 20% without compromising the quality of the concrete mixes or concrete [57].
The authors of [40] demonstrated that magnetised mineral water has a higher pH and more significant therapeutic potential. Previous studies [33,60,61] have shown that the factors that increase the pH of water and water-based systems also enhance the healing properties of mineral water and the productivity of plants. Moreover, the plant yield is directly related to the pH level [33,39].
The authors of [30] investigated how water properties were affected by a magnetic field and light radiation. They found that tap water’s pH increased after 30 s of He–Ne laser exposure. Distilled water showed less response to the He–Ne laser. The combined effect of these factors was more pronounced than their individual effects. Distilled water was also less responsive to the magnetic field and the He–Ne laser [30]. Similar findings were reported in [31]. The authors suggested a synergistic effect of a constant magnetic field and laser radiation on tap water activation, resulting in a more considerable pH change than each factor alone.
Water’s role in the mechanism of action of therapeutic physical factors is vital. According to [30], water is a crucial molecule in the action of therapeutic physical factors. According to [28], EHF therapy’s primary mechanism of action is the change in water structure. According to [62], the positive effect of laser exposure is due to a shift in water structure and properties in the body.
Photomagnetic therapy devices have been proven effective in preventing and treating various diseases in clinical settings [26]. Photomagnetotherapy can provide benefits, such as reducing inflammation, swelling, and pain and improving immune function. Moreover, ongoing research on developing new technologies and devices that combine physiotherapy and photomagnetotherapy is being conducted.
4. Using Thermometry to Assess the Structure of Water and Water-Containing Systems to Improve the Properties of Living and Non-Living Systems
The effect of temperature on the properties of water and its solutions is well established in the literature. Temperature influences the rate and direction of chemical and biochemical processes that occur in water [43,44,63,64]. Moreover, thermometry can also reveal the structural features of water and its solutions, as shown in thermometric studies [44]. These studies found that the relative changes in the temperature of distilled water (Ti%) during cooling from 46 °C to 29 °C and heating from 29 °C to 46 °C exhibited peaks at 32 °C, 39 °C, and 42 °C (Figure 1). The cooling and warming curves follow the same path. The water structure was relatively stable when the temperature changed from 33 °C to 38 °C [17,44,45]. Similar findings were reported in other works [17,45].
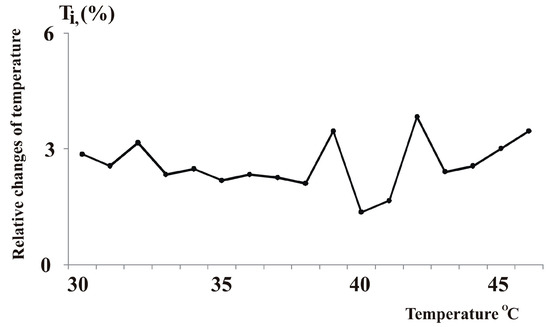
Figure 1.
The curve of arithmetic mean values of the relative time of temperature change by 1 degree (Ti%) when cooling distilled water from 46 °C to 29 °C and heating from 29 °C to 46 °C in a 100 mL container [17].
The relative time of the change in the temperature of liquids by 1 degree (Ti%) was calculated using Formula (1):
Ti (%) measures how quickly the temperature changes at a certain level. It is calculated by dividing the difference between two consecutive time intervals by the initial time interval. For example, if the temperature drops from 42 °C to 41 °C in 10 min and then from 41 °C to 40 °C in 8 min, the relative time of the temperature change at 42 °C is (10 − 8)/10 = 0.2 or 20%. The temperature change rate at 42 °C is 20% faster than the average rate of those two intervals.
In a previous study [14], the researchers found that heating pure water and salt water to 40 °C breaks up clusters of water molecules that range from 2 to 40 µm in size. That means that breaking up these clusters requires energy. Based on this finding, the authors explained the local changes in the curve as the result of structural changes in water that happen when the liquid cools down from 46 °C to 29 °C, releasing energy, or when it warms up from 29 °C to 46 °C, absorbing energy [43]. Water has the unique property of changing its heat exchange rate at different temperatures. For example, water at 32 °C, 39 °C, and 42 °C has different rates of cooling or heating when exposed to the same environment. This property may help warm-blooded animals adapt to various environmental changes, such as temperature fluctuations, electromagnetic fields, and so on [43].
Studies [65,66] have demonstrated that warm-blooded animals must keep their body temperature at approximately 37 °C (with a variation of 36 °C to 42 °C) to ensure the stability of their vital functions. That helps them minimize the energy expenditure for maintaining homeostasis. Body temperature is a factor that influences the survival and adaptation of living beings. Organisms with a body temperature between 35 °C and 41 °C are more likely to cope with environmental challenges. That may be related to how water systems are organized and how stable the body structures are at these temperatures. These organisms also have enough flexibility in their vital processes to adjust to various changes in their surroundings and body conditions [43].
That implies that the chemical and biochemical reactions in water-based solutions at 35 °C–41 °C have similar rates and directions as the reactions of warm-blooded animals, such as humans. That is important for various studies in biology and medicine [67].
Collected data have helped create the best methods of using therapeutic mud for treatment [40]. The best temperature range for mud is from 33 °C to 38 °C. In [40], therapeutic mud at 35–36 degrees effectively helped treat patients with osteoarthrosis.
The authors of [46] investigated how the temperature in a measurement cell changed when they cooled down distilled water and salt water from 10 °C to 1 °C. They found that the temperature change curves exhibited some local fluctuations that were more noticeable than in [44] (see Figure 2).
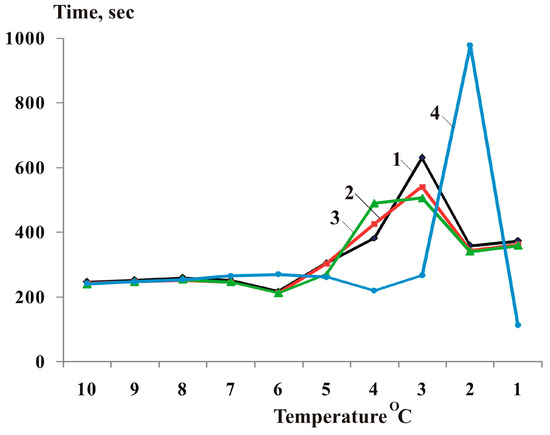
Figure 2.
Dynamics of time (seconds) of temperature decrease by 1 °C upon cooling from 10 °C to 1 °C: 1—distilled water; 2, 3, and 4—solution of NaCl at concentrations of 1 × 10−4 M, 1 × 10−2 M, and 1.5 × 10−1 M.
The time curves of the temperature decrease in distilled water, a 1 × 10−4 M NaCl solution, and a 10−2 M NaCl solution showed some compelling features when the liquids were cooled from 7 °C to 6 °C and from 4 °C to 3 °C. The cooling rate changed significantly in these temperature intervals, resulting in local minima and maxima on the curves. Specifically, the cooling rate increased by 14% when the temperature dropped from 7 °C to 6 °C, creating local minima on the curves. Conversely, the cooling rate decreased when the temperature dropped from 4 °C to 3 °C, creating local maxima on the curves. These phenomena indicate some factors affecting the heat transfer process of these liquids at these temperatures.
The cooling process of a 0.15 M NaCl solution was studied by measuring the temperature change over time. The results showed that the cooling rate varied depending on the temperature range. Specifically, a 9% increase in the cooling rate was detected when the solution temperature dropped from 5 °C to 4 °C, indicating a faster heat loss. Conversely, a 246% decrease in the cooling rate was observed when the solution temperature fell from 3 °C to 2 °C, suggesting a slower heat transfer.
The effect of temperature and concentration on the structure of water in distilled water and salt solutions has been studied. Previous research [14] showed that water clusters of 2 to 40 μm in size are disrupted by heat absorption when the temperature reaches 40 °C. Moreover, higher concentrations of solutions reduce the number of water clusters [68,69]. Therefore, we hypothesized that the observed local minima in the solution cooling time curves are caused by a structural change in the water with heat absorption (and hence, with the breakdown of smaller clusters). Conversely, the local maxima are caused by a structural shift in the water with heat release (and, therefore, with the formation of larger clusters). In a more concentrated solution of sodium chloride (1.5 × 10−1 M), larger clusters may form with a more noticeable drop in temperature (from 3 °C to 2 °C) [46].
Thermometry revealed that water and water-containing systems undergo significant structural changes at temperatures of 2 °C, 7 °C, 32 °C, 39 °C, and 42 °C. These results are significant for researching and creating various technologies, such as in biology and medicine.
5. Using Electrophysical Parameters to Assess the Structure of Water and Water-Containing Systems to Improve the Properties of Living and Non-Living Systems
Water structure can be altered by various factors, as reported by many studies [3,15,17,18,19,20]. For instance, the authors of [47] demonstrated that different salts at the same concentrations had different effects on water structure, as measured via electric capacitance (Figure 3).
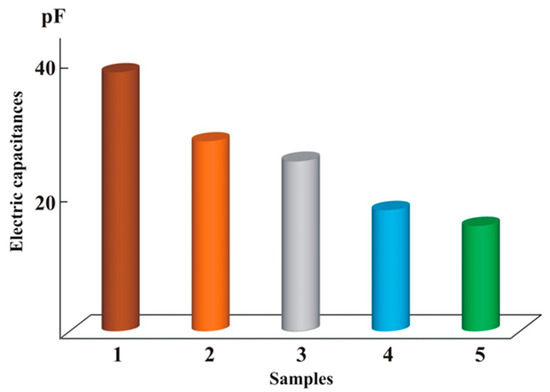
Figure 3.
The electric capacitance (pF) values of salt solutions at a reactive current frequency of 3 MHz: 1—1 × 10−2 M FeCl3, 2—1 × 10−2 M CaCl2, 3—1 × 10−2 M MgCl2, 4—1 × 10−2 M KCl, and 5—1 × 10−2 M NaCl.
Another study demonstrated that the electric capacitance of distilled water steadily dropped to 37% of its original value as the reactive current frequency increased from 1 to 100 kHz [45] (Figure 4).
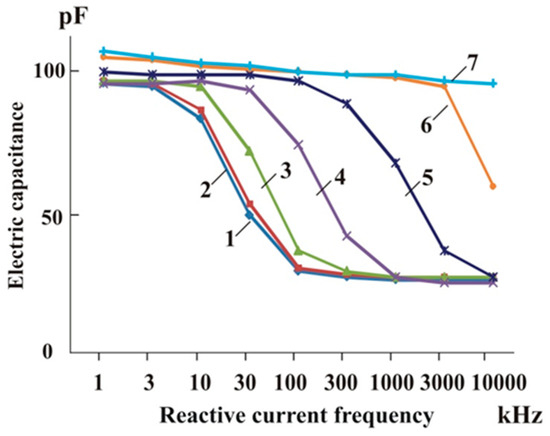
Figure 4.
Dependence of the electrical capacitance of distilled water and aqueous solutions on varying frequencies of reactive current and concentrations of NaCl solution: 1—distilled water; 2, 3, 3, 4, 5, 6, and 7—solutions of sodium chloride salt at concentrations of 1 × 10−6 M, 1 × 10−5 M, 1 × 10−4 M, 1 × 10−3 M, 1 × 10−2 M, and 1 × 10−1 M, respectively [17].
The electric capacitance of distilled water is almost constant when the frequency changes from 100 to 10,000 kHz. The electric capacitance of an aqueous solution of NaCl gradually increases as the concentration and the frequency increase. That indicates that the cluster structure of water is partially disrupted by sodium chloride and other salts, and the water molecules become more mobile [14,68]. That means that the water-containing system undergoes a supramolecular rearrangement that enhances the mobility of water dipoles and consequently the electrical capacitance of aqueous salt solutions [48].
In [70], the structural features of water and aqueous solutions in the measuring cell were assessed by measuring their electrical capacitance in the frequency range from 1 to 300 kHz and similar results were obtained. At a temperature of 20 °C, with an increase in the current frequency from 1 to 100 kHz, there was a decrease in the electrical capacity of distilled water, which, according to the authors, is due to the existence of structural formations (water clusters) in water, in which the oscillation frequencies of water dipoles are lower than the frequencies of the external current. In this study, based on experimental data [13,14,15] and calculations, the possibility of combining individual molecules into associates in quantities of up to 1012 is shown, and calculations of the natural frequencies of vibrations of associates of equal sizes during their interaction at distances equal to the diameter of the kinetic formations are given.
It turned out that with an increase in the number of water molecules in associates, the frequencies of their vibrations significantly decrease to 1 kHz and the orientation of kinetic formations relative to the electric field lines will become more difficult with increasing frequency, which is observed in the study.
Considering that interactions between associates can occur between associates of different sizes and at different distances, the spectrum of natural frequencies of oscillations of kinetic formations is quite wide and is determined by the thermodynamic conditions of the system’s existence.
In solutions of NaCl, KCl, CaCl2 and MgCl2, the electrical capacity of the solutions increased. This may be due to the destruction of clusters, an increase in the number of unconnected water dipoles, and ion hydration processes, which led to an increase in the mobility of water dipoles.
A water layer with a thickness of approximately 300 µm near a solid surface (boundary-layer water) has different properties from the water in the rest of the volume (“bulk”), as shown in [18,19,22]. The mobility of water dipoles and the electric capacitance of distilled water and solutions are lower in the near-wall layer, as demonstrated in [21,22]. The reduction in electric capacitance is nonlinearly related to the distance to the solid surface, the concentration and type of solute, and the surface material. In sodium chloride solutions, the reduction in electric capacitance is more noticeable when the distance to the solid surface decreases from 150 to 50 μm [21] (Figure 5).
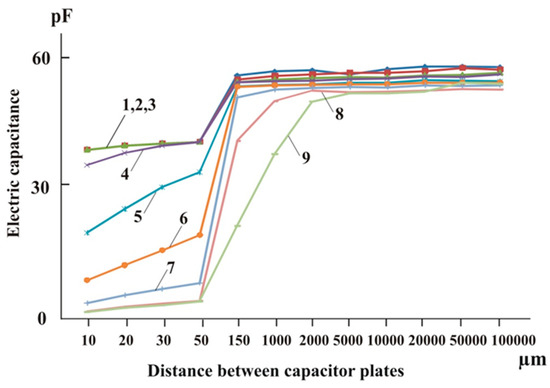
Figure 5.
Dependence of the electrical capacitance (C, pF) of a sodium chloride solution with a concentration of 1.5 × 10−1 M on the distance between the capacitor plates (μm) at different frequencies of reactive current: 1—1 kHz, 2—3 kHz, 3—10 kHz, 4—30 kHz, 5—100 kHz, 6—300 kHz, 7—1000 kHz, 8—3000 kHz, and 9—10,000 kHz [17].
According to the electrophysical properties of water and water-based systems and the findings of [14,50,68], we can infer that increasing the concentration of sodium chloride solutions and other salts causes the breakdown of distilled water’s supramolecular structures (clusters). This results in higher mobility of the water dipoles and the formation of hydrate structures.
Conversely, the near-wall layer lowers the mobility of water dipoles and increases the structuredness of distilled water and its solutions [21,22]. This is important for selecting the best parameters for different technologies that use water-containing systems and hard surfaces.
The electrophysical parameters of water, such as its capacitance and resistance, can also indicate the influence of magnetic fields on water. In [49], a measuring cell with distilled water was connected to an oscillatory circuit with a sinusoidal generator. The results showed that the oscillation amplitudes decreased after the water was exposed to a magnetic field. This confirms the previous findings on how a magnetic field changes the properties of water [30,31].
6. Conclusions
Water has a unique molecular structure, consisting of two hydrogen atoms bonded to an oxygen atom. Water has remarkable properties that are crucial for life on Earth. Moreover, water’s ability to exist in different states—solid, liquid, and gas—and its high heat capacity make it integral to Earth’s climate and the existence of diverse ecosystems.
Water acts as a universal solvent and plays a key role in biological systems and various technological processes. The composition and structure of water and its interactions with other substances can significantly affect the properties and functions of living and non-living systems. Based on this, it is essential to develop and apply new methods for assessing the composition, structure, and other properties of water and water-containing systems under various influences.
The structure of water and water-containing systems can be evaluated using pH, thermometry, and electrophysical parameters, but only when performing the above methodological conditions. The results of such studies do not contradict each other and correspond to the known data on the properties and structural features of water and water-containing systems. On the other hand, the structure of water and water-containing systems depends on pH, temperature, and various influences. Based on this, it is possible to change pH, temperature, and electrophysical parameters in various ways to find the best way to influence non-living systems and living systems (organisms) to improve their quality and health.
It should also be noted that the influence of solid surfaces on the mobility of water dipoles is a significant factor in the function of living systems and non-living systems. Within a 50 μm range, the presence of a solid surface can change the orientation and movement of water molecules, which in turn can affect the supramolecular organization of biological ends and other structures. The reorientation of water dipoles near solid surfaces can impact the function of membrane-bound enzymes, potentially affecting their catalytic efficiency. These structural changes can affect the structure of proteins, DNA, other biomolecules, disease development, and treatment outcomes.
The pronounced relationship between water’s structure and its influence on different processes is a burgeoning field of research in biology, medicine, and other technologies. Studies have shown that factors such as temperature, magnetic fields, light radiation, and near-wall effects can alter the structure of water and water-containing systems, leading to significant changes in their biological, therapeutic, and other properties.
The development of research in these areas can lead to breakthroughs in the fields of medical science, biotechnology, and other technologies.
Author Contributions
G.S.: methodology and investigation; B.L.: methodology and investigation; M.B.: conceptualisation, resources, and writing—original draft preparation; N.G.: methodology and conceptualisation; L.A.: investigation and data curation; A.V.: conceptualisation and writing—original draft preparation; A.K.: conceptualisation and writing—original draft preparation. All authors have read and agreed to the published version of the manuscript.
Funding
This research received support from the Slovenian Research Agency (research funding P2-0180).
Institutional Review Board Statement
The director of the research firm Novetehnologije d.o.o. G. Sidorenko considers it possible to publish this article.
Data Availability Statement
All data specified in this work are available online.
Acknowledgments
The authors acknowledge the financial support from the Slovenian Research Agency (research core funding P2-0180).
Conflicts of Interest
Author Galina Sidorenko and Boris Laptev were employed by the company Nove Tehnologije d.o.o. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Savostikova, O.N. Hygienic assessment of the effect of structural changes in water on its physicochemical and biological properties. In Abstract of the Dissertation for the Degree of Candidate of Medical Sciences; Research Institute of Human Ecology and Environmental Hygiene, Russian Academy of Medical Sciences: Moscow, Russia, 2008; p. 26. [Google Scholar]
- Rakhmanin, Y.A.; Kondratov, V.K. Water Is a Cosmic Phenomenon; Russian Academy of Medical Sciences: Moscow, Russia, 2002; p. 427. [Google Scholar]
- Farashchuk, N.F.; Rakhmanin, Y.A. Water Is the Structural Basis for Adaptation; Russian Academy of Medical Sciences: Smolensk, Russia; Moscow, Russia, 2004; p. 172. [Google Scholar]
- Lindinger Michael, I. Structured water: Effects on animals. J. Anim. Sci. 2021, 99, skab063. [Google Scholar] [CrossRef] [PubMed]
- Kordonskaya, M.A.; Kondakov, A.M.; Egorov, V.V. Influence of water structure on the chemical reactions rate. Biotechnology 2014, 4, 43–45. [Google Scholar]
- Malenkov, G.G. Structure and dynamics of liquid water. J. Struct. Chem. 2006, 47, 5–35. [Google Scholar] [CrossRef]
- Roy, R.; Tiller, W.A.; Bell, I.; Hoover, M.R. The structure of liquid water; novel insights from materials research; potential relevance to homoeopathy. Mater. Res. Innov. 2005, 9, 577–608. [Google Scholar] [CrossRef]
- Syroeshkin, A.V.; Smirnov, A.N.; Goncharuk, V.V.; Uspenskaya, E.V.; Nikolaev, G.M.; Popov, P.I.; Karamzina, T.V.; Samsoni-Todorov, A.O.; Lapshin, V.B. Water as a heterogeneous structure. Electron. J. Investig. Russ. 2006, 843–854. Available online: http://zhurnal.ape.relarn.ru/articles/2006/088.pdf (accessed on 7 July 2021).
- Ho, M.-W. Large Supramolecular Water Clusters Caught on Camera. A Review. Water 2013, 6, 1–12. [Google Scholar] [CrossRef]
- Lo, S.-Y.; Geng, X.; Gann, D. Evidence for the existence of stable-water-clusters at room temperature and normal pressure. Phys. Lett. A 2009, 373, 3872–3876. [Google Scholar] [CrossRef]
- Oka, K.; Shibue, T.; Sugimura, N.; Watabe, Y.; Winther-Jensen, B.; Nishide, H. Long-lived water clusters in hydrophobic solvents investigated by standard NMR techniques. Sci. Rep. 2019, 9, 223. [Google Scholar] [CrossRef] [PubMed]
- Michaelides, A.; Morgenstern, K. Ice nanoclusters at hydrophobic metal surfaces. Nat. Mater. 2007, 6, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Goncharuk, V.V.; Smirnov, V.N.; Syroeshkin, A.V. Clusters and giant geterophasic clusters of water. Chem. Technol. Water 2007, 29, 3–17. [Google Scholar]
- Goncharuk, V.V.; Orekhova, E.A.; Malyarenko, V.V. Influence of temperature on water clusters. Chem. Technol. Water 2008, 30, 150–158. [Google Scholar] [CrossRef]
- Smirnov, A.N.; Syroeshkin, A.V. Supranadmolecular complexes of water. Ros. Chem. J. 2004, 48, 125–135. [Google Scholar]
- Zenin, S.V. Biological and energy-informational properties of water. Water Sci. 2007, 25. [Google Scholar]
- Sidorenko, G.; Brilly, M.; Laptev, B.; Gorlenko, N.; Antoshkin, L.; Vidmar, A.; Kryžanowski, A. The Role of Modification of the Structure of Water and Water-Containing Systems in Changing Their Biological, Therapeutic, and Other Properties Overview. Water 2021, 13, 2441. [Google Scholar] [CrossRef]
- Postnov, S.E.; Podchernyaeva, R.Y.; Mezentseva, M.V. Unusual properties of the boundary layer water. Bull. Russ. Acad. Nat. Sci. 2009, 3, 12–15. [Google Scholar]
- Postnov, S.E.; Mezentseva, M.V.; Podchernyaeva, R.Y.; Danlybaeva, G.A.; Surgucheva, I.M.; Grinkevich, O.M.; Lopatina, O.A.; Baklanova, O.V. New approaches in biomedical technology based on boundary layer water. Biomed. Radio Eng. 2009, 1, 3–15. [Google Scholar]
- Ulashchik, V.S. Molecular aspects of the action of therapeutic physical factors (introduction to the problem). Med. News 2003, 1, 30–38. [Google Scholar]
- Sidorenko, G.N.; Laptev, B.I.; Gorlenko, N.P.; Antoshkin, L.V. Assessment of changes in the structure of water and aqueous solutions at a distance of 5–50,000 microns from a solid surface (in the wall layer) using dielectrometry and resonance methods. Water Purif. Water Treat. Water Supply 2020, 7, 16–21. [Google Scholar]
- Laptev, B.I.; Sidorenko, G.N.; Gorlenko, N.P.; SarkisovYSAntoshkin, L.V. Assessment of changes in the structure of aqueous solutions in near-wall layers using dielectrometry and resonance methods. Bull. New Med. Technol. Electron. Period. 2015, 2, 2–9. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ulashchik, B.C. Water is a key molecule in the action of therapeutic physical factors. Vopr. Kurortol. Physiother. Exerc. Ther. 2002, 1, 3–9. [Google Scholar]
- Ulashchik, B.C. Combined physiotherapy: New methods and devices. Health 2011, 2, 25–30. [Google Scholar]
- Ulashchik, B.C. Physiotherapy. Universal Medical Encyclopedia. Moskow Knizhnyy Dom 2008, 640, 493–496. [Google Scholar]
- Sidorenko, G.N.; Kuzmenko, O.V.; Laptev, B.I.; Gorlenko, N.P.; Antoshkin, L.V. Assessment of the mechanisms of action and the effectiveness of the combined action of photo- and magnetic therapy. Bull. New Med. Technol. Electron. Period. 2020, 14, 100–109. [Google Scholar] [CrossRef]
- Aksenov, S.I. Water and Its Role in the Regulation of Biological Processes; Institute for Computer Research: Moscow, Russia, 2008; p. 212. [Google Scholar]
- Lukyanitsa, V.V. Primary mechanism of action in EHF-therapy. Med. J. 2013, 43, 94–99. [Google Scholar]
- Sidorenko, G.N.; Konovalov, A.I.; Laptev, B.I. On the role of water structure in the mechanism of complex action of the magnetic field, natural healing factors and highly diluted solutions. Bull. New Med. Technol. 2017, 1, 71–81. [Google Scholar] [CrossRef]
- Britova, A.A.; Adamko, I.V.; Bachurina, V.L. Water activation by laser radiation, magnetic field and their combination. Bull. Novgorod State Univ. 1998, 7, 11–14. [Google Scholar]
- Veprikov, Y.V. Influence of laser and magnetic activation of water on the value of the hydrogen index. Izvestiyavuzov. N. Cauc. Reg. Nat. Sci. 2014, 3, 44–49. [Google Scholar]
- Gorlenko, N.P.; Sarkisov, Y.S.; Syryamkin, V.I.; Naumova, L.B.; Pavlova, A.N.; Laptev, B.I. Wave mechanism of structure formation in cement compositions. IOP Conf. Ser. Mater. Sci. Eng. 2019, 597, 1–5. [Google Scholar] [CrossRef]
- Pasko, O.A. Influence of pre-sowing stimulation of cucumber seeds on yield. Agric. Sci. 2011, 8, 20–22. [Google Scholar]
- Ramsey, C.L. Magnetized Seeds and Structured Water: Effects on Resilience of Velvet Bean Seedlings (Mucuna pruriens) under Deficit Irrigation. J. Basic Appl. Sci. 2023, 19, 249. [Google Scholar] [CrossRef]
- Stemler, A.J. Rotational Direction of a Weak Magnetic Field Selectively Targets Chiral Clusters in Liquid Water and Modifies Its Chemical Reactivity. J. Water Chem. Technol. 2023, 45, 544–551. [Google Scholar] [CrossRef]
- Gorlenko, N.P.; Laptev, B.I.; Sarkisov, Y.S.; Zhuravlev, V.A.; Sidorenko, G.N.; Prishchepa, I.A. The Role of Water and Aqueous Solutions in the Formation of Induction Periods of Hydration and Structure Formation of Cement Stone. Phys. Wave Phen. 2023, 31, 206–215. [Google Scholar] [CrossRef]
- Alattar, E.; Radwan, E.; Elwasife, K. Improvement in growth of plants under the effect of magnetized water. AIMS Biophys. 2022, 9, 346–387. [Google Scholar] [CrossRef]
- Safronov, V.N.; Kugaevskaya, S.A. Optimization of the properties of cement composites with various technological methods of preparation of cyclic magnetic activation of mixing water. Vestn. TSASU 2014, 1, 85–99. [Google Scholar]
- Pasko, O.A. Physical and chemical changes in tap water during its treatment in various ways. Water Chem. Ecol. 2010, 7, 40–45. [Google Scholar]
- Levitsky, E.F.; Laptev, B.I.; Sidorenko, G.N. Electromagnetic Fields in Balneology and Physiotherapy. Tomsk Russia 2000, 113, 202–214. [Google Scholar]
- Laptev, B.I.; Sidorenko, G.N.; Gorlenko, N.P.; Sarkisov, Y.S.; Antoshkin, L.V.; Kulchenko, A.K. Electrical properties of water under external influences. Water Purif. Water Treat. Water Supply 2014, 9, 20–27. [Google Scholar]
- Sidorenko, G.N.; Laptev, B.I.; Gorlenko, N.P.; Sarkisov, Y.S.; Antoshkin, L.V. Assessment of structure formation processes in water and water-containing media using electrophysical methods and thermometry. Bull. TGASU 2019, 2, 202–214. [Google Scholar] [CrossRef]
- Laptev, B.I.; Sidorenko, G.N.; Gorlenko, N.P.; Sarkisov, Y.S.; Antoshkin, L.V. Assessment of the structure of water using thermometry and electrophysical research methods. Bull. New Med. Technol. 2016, 23, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Sidorenko, G.N.; Laptev, B.I.; Gorlenko, N.P.; Sarkisov, Y.S.; Antoshkin, L.V. Possibilities of electrophysical research methods and thermometry for assessing the structure of water-containing media (solutions, plant and animal objects). Bull. New Med. Technol. Electron. J. 2016, 2, 11. [Google Scholar] [CrossRef] [PubMed]
- Sidorenko, G.N.; Laptev, B.I.; Gorlenko, N.P.; Kochetkova, T.D.; Antoshkin, L.V. Variability of properties of water and water-containing systems under various external influences. Bull. of TSU Chem. 2020, 51–68. [Google Scholar] [CrossRef]
- Sidorenko, G.N.; Laptev, B.I.; Gorlenko, N.P.; Antoshkin, L.V. Evaluation of changes in the structure of aqueous solutions of sodium chloride with a decrease in temperature from 10 °C to 1 °C. In Proceedings of the Collection of Articles of the XXIV International Scientific and Practical Conference, Petrozavodsk, Russia, 7–24 April 2023; pp. 201–211. [Google Scholar]
- Sidorenko, G.N.; Laptev, B.I.; Gorlenko, N.P.; Antoshkin, L.V. The use of low-energy effects to improve the properties of water. Water Purif. Water Treat. Water Supply 2018, 4, 18–21. [Google Scholar]
- Sidorenko, G.N.; Laptev, B.I.; Gorlenko, N.P.; Antoshkin, L.V. Comparative evaluation of the structure of solutions of sodium chloride, potassium chloride, magnesium chloride, calcium chloride, iron chloride, and iron hydroxide sols using dielectrometry and resonance methods. In Proceedings of the Collection of Articles Based on Materials of the IX International Scientific and Practical Conference, St. Petersburg, Russia, 16–18 November 2017; pp. 8–14. [Google Scholar] [CrossRef]
- Sidorenko, G.N.; Laptev, B.I.; Gorlenko, N.P.; Antoshkin, L.V. A new method for assessing the structure of water and aqueous solutions when the measuring cell is connected to the oscillatory circuit of the generator of sinusoidal oscillations. Water Purif. Water Treat. Water Supply 2020, 11, 12–18. [Google Scholar]
- Sidorenko, G.N.; Laptev, B.I.; Gorlenko, N.P.; Sarkisov, Y.S.; Antoshkin, L.V. Assessment of changes in the structure of water and water systems under various influences. Bull. New Med. Technol. 2016, 23, 203–212. [Google Scholar] [CrossRef]
- Musienko, K.S.; Ignatova, T.M.; Glazkova, V.V. Study of the influence of physical fields on the physical and chemical properties of water. Biomed. Eng. Electron. 2014, 2, 1–7. [Google Scholar]
- Matvievsky, A.A. Cement composites based on magnetically and electrochemically activated mixing water. In Abstract of the Dissertation for the Degree of Candidate of Technological Sciences; Mordovia Publishing House: Saransk, Russia, 2010; p. 24. [Google Scholar]
- Sarkisov, Y.S.; Gorlenko, N.P.; Safronov, V.N.; Kovaleva, M.A.; Rakhmanova, I.A.; Tsvetkov, N.A. Geonics: From water geochemistry to the creation of high-quality mixing fluid for cement systems. Fundam. Res. 2017, 7, 71–76. [Google Scholar]
- Epstein, E.A.; Rybakov, V.A. Magnetic activation of water in the building materials industry. The use of magnetic water in the production of tongue-and-groove plates. Eng. Constr. Mag. 2009, 4, 32–38. [Google Scholar]
- Sarkisov, Y.S.; Gorlenko, N.P.; Safronov, V.N.; Kugaevskaya, S.A.; Kovaleva, M.A.; Ermilova, T.A.; Afanasiev, D.A. Temperature responses of water and aqueous solutions to the external influence of a magnetic field. Bull. TGASU Chem. 2015, 2, 20–29. [Google Scholar] [CrossRef]
- Safronov, V.N.; Kugaevskaya, S.A. Time factor in the technology of cyclic magnetic activation of water mixing mineral binders. Bull. TGASU 2013, 1, 163–171. [Google Scholar]
- Safronov, V.N.; Petrov, G.G.; Kugaevskaya, S.A.; Shchetinov, E.Y.; Gorlenko, N.P. The influence of the holding time before the closure of magnetised water on the properties of cement composites. Bull. TSASU Chem. 2010, 4, 139–149. [Google Scholar]
- Gorlenko, N.P.; Laptev, B.I.; Sarkisov, Y.S.; Sidorenko, G.N.; Kulchenko, A.K. Influence of electromagnetic fields on the properties of mixing fluid of cement systems. In Proceedings of the International Scientific Conference of Young Scientists, Tomsk, Russia, 15–16 October 2014; pp. 137–145. [Google Scholar]
- Pomazkin, V.A.; Makaeeva, A.A. Magnetically activated water in construction technologies. Bull. OSU 2001, 1, 109–114. [Google Scholar]
- Abdelaziz, A.M.; Elshokali, A.M. Abdelbagi Impact of magnetised water on elements contents in plants seeds. Int. J. Sci. Res. Innov. Technol. 2014, 1, 12–21. [Google Scholar]
- Hozayn, M.; Abdallah, M.M.; Abd El-Monem, A.A.; El-Saady, A.A.; Darwish, M.A. Applications of magnetic technology in agriculture: A novel tool for improving crop productivity (1): Canola. Afr. J. Agric. Res. 2016, 11, 441–449. [Google Scholar] [CrossRef]
- Lukyanitsa, V.V. Influence of laser radiation on the optical density and structure of water. The main component of the human body. In Proceedings of the International Scientific and Technical Conference, Minsk, Belarus, 11–13 June 2014; pp. 46–48. [Google Scholar]
- Baturov, L.N.; Govor, I.N.; Obukhov, A.S.; Plotnichenko, V.G.; Dianov, E.M. Detection of nonequilibrium phase transitions in water. JETP Lett. 2011, 93, 92–94. [Google Scholar] [CrossRef]
- Kuznetsov DM Smirnov, A.N.; Syroeshkin, A.V. Acoustic emission during phase transformations in the aquatic environment. Ros. Chem. Mag. 2008, 52, 114–121. [Google Scholar]
- Belyanin, V.; Romanova, E. Life, the water molecule and the golden proportion. Sci. Life 2004, 10, 2–9. [Google Scholar]
- Trincher, K. About water and warm-bloodedness. SANUM-Post 1991, 15, 21–26. [Google Scholar]
- Sidorenko, G.N.; Laptev, B.I.; Gorlenko, N.P.; Sarkisov, Y.S.; Antoshkin, L.V. Assessment of changes in the structural and energy state of water during cooling and heating using thermometry and electrophysical research methods. Bull. Tomsk State Univ. Chem. 2017, 7, 80–93. [Google Scholar] [CrossRef]
- Uspenskaya, E.V. Study of the Structure of Water at the Supramolecular Level for the Development of New Methods of Standardisation and Quality Control of Mineral Waters and Liquid Dosage Forms. Chemical Sciences. Ph.D. Dissertation, RUDN University, Moscow, Russia, 2007; 27p. [Google Scholar]
- Baranov, A.V.; Petrov, V.I.; Fedorov, A.V. Investigation of NaCl microimpurities on the dynamics of cluster formation in liquid water: Low-frequency Raman spectroscopy. JETP Lett. 1993, 57, 356–359. [Google Scholar]
- Laptev, B.I.; Sidorenko, G.N.; Gorlenko, N.P.; Sarkisov, Y.S.; Antoshkin, L.V. The processes of structural formation in water and aqueous solutions. Water Ecol. Probl. Solut. 2012, 50, 26–33. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).