Abstract
The employment of granular ferric iron-(oxy)hydroxides, a well-known economic and effective method, lowers arsenic concentrations in different water types. However, for direct application in polluted groundwaters, there is a need to develop new injectable adsorbents for aquifers that could also neutralize acidic media. In this context, a granular ferric hydroxide-calcite (GFH-C) adsorbent was size-reduced to 0.4–50 µm by sonication with the aim of improving (i) the adsorption of As(III) and As(V) at different pHs and (ii) the pH control through the dissolution of calcite. Batch experiments were conducted to determine As(III) and As(V) adsorption isotherms and kinetics, as well as calcite dissolution kinetics, using GFH-C of two sizes (granular and sonicated). Results showed that the arsenic binding capacity of sonicated adsorbents did not improve significantly. On the contrary, the As(III) and As(V) adsorption kinetics improved with the sonication, as in the case of calcite dissolution kinetics. The dissolution of calcite from the adsorbent made the water pH increase to around 9.2–9.4. The sonicated adsorbent offers an advantage in depolluting As-containing groundwater due to its smaller size, which is linked with faster arsenic adsorption and effective acidic water neutralization.
1. Introduction
Arsenic (As) is present in groundwater from all over the world, with concentrations exceeding the drinking water guideline value of 10 µg/L [1,2,3] due to both natural and anthropogenic origins. In groundwaters, the speciation of arsenic is diverse, with a ratio of As(III) to As(V) that has a broad range as a result of the different redox-active solids [2]. Arsenic in groundwater is frequently found at very acidic pH (2–4), because is linked to pyrite oxidation [4,5,6] and acid mine drainage (AMD) formation [7,8,9]. The low pH of groundwater increases the dissolution of metals and corrosion problems, and limits the application of some remediation reactants (e.g., iron hydroxides and zerovalent iron) that could be dissolved.
In order to solve the acidic problem, classical passive remediation solutions such as permeable reactive barriers (PRB) combine remediation reactants with calcite limestone [5,7,8] to increase the pH and to promote metal and sulphate precipitation.
In recent years, a lot of research has been carried out to find adsorbents to efficiently remove arsenic from polluted aquifers [10,11]. The main traditionally employed materials have been iron and alumina-based compounds and minerals, activated carbon, silica, clays and biosorbents. Adsorption of arsenic onto granular ferric hydroxide (GFH), based on iron-(oxy)hydroxides, is an economic and effective well-known method of lowering the concentrations of arsenic in oxidation states (III) and (V) [12,13,14] in several kind of waters. The use of these granular sorbents in columns operating in continuous mode is a known alternative [15] that limits the mass transfer of arsenic from the groundwater to the adsorbent due to intraparticle diffusion limitations [16,17,18], and thus could limit the remediation of groundwater with techniques such as PRB.
A recent in situ approach for the remediation of groundwater consists of the use of so-called reactive zone barriers (RZB), which use suspensions of small-sized materials to treat arsenic and other metal in groundwater [19,20,21]. To perform RZB groundwater treatments, powdered adsorbents and neutralizing agents are needed, and thus the reduction of size of a granular adsorbent is a good alternative.
Studies conducted so far have compared powdered product with the parent granular ferric hydroxides and determined the adsorption isotherms and kinetics. Kumar et al. [22] and Martí et al. [23] used this adsorbent to study the effect of different particle size on phosphate adsorption equilibrium and kinetics, and showed that phosphate is removed more rapidly with small size particles, while the total adsorption capacity remained similar. Martí et al. [14] found same conclusions for arsenate adsorption.
Ferrosorp® Plus is a commercial granular ferric hydroxide-calcite (GFH-C) adsorbent obtained from drinking water treatment plant sludge composed of Fe(III) hydroxides along with calcite impurities [24]. The recycling of water treatment sludges as adsorbents for environmental remediation is advantageous from a circular economy perspective, since it extends the life of materials contributing to sustainable development. Some studies have successfully treated polluted waters or soils with similar GFH obtained from different water treatments [25,26], which demonstrates the potential of this kind of adsorbent. Here, the size of commercial GFH-C was reduced using ultrasonication, as in previous works [14,23,27], with the aim of improving the kinetics of arsenic adsorption and calcite dissolution, as well as extending its applicability to make it injectable into the aquifer on affected groundwater, following the remediation idea of RZB. This research also explores the ability of this added-value adsorbent to treat acidic groundwater polluted with As(III) or As(V). Calcite dissolution from this GFH-C needs to be better understood to determine its role in pH control and as an acid-neutralizing agent. The objectives of this article were to study how the reduction of the particle size affects (i) the adsorption of arsenic ((As(III) and As(V)) and (ii) the dissolution of calcite and pH control.
2. Materials and Methods
2.1. Adsorbent Preparation
Granular Ferrosorp® Plus was purchased from HeGo Biotec GmbH (Berlin, Germany). Granular Ferrosorp (here, OF-G) was sieved to 1000 µm for application on adsorption experiments. In parallel, the size of the original material was reduced with a customized sonicator developed by Eurecat (Cerdanyola del Vallès, Spain). A multi-stepped ultrasonic horn was designed to maximize the energy-emitting area, as this type of geometry has been shown to improve the sonochemical performance of the sonotrode [28]. The equipment used in the experiments was a Branson® DCX ultrasonic generator, working at a frequency of 30 kHz. Sonication was conducted applying a 7.5 µm peak-to-peak ultrasonic amplitude for 5 min over 0.5 g of OF-G in 40 mL MiliQ water solution. The obtained product was designated OF-S. This process was repeated twenty times to obtain enough OF-S for the whole experimentation.
2.2. Adsorbent Characterization
The granulometric characterization of OF-G was performed with an RP09 digital sieve shaker from CISA, using test sieves of 500, 250, 100, 50, 20 and 10 μm, while the granulometric analysis for OF-S was made with a Beckman Coulter LS Particle Size Analyzer.
Nitrogen Brunauer–Emmett–Teller (BET) specific surface area and micropore area analyses on dry samples were performed with Micromeritics ASAP 2020 equipment (Aachen, Germany) with N2 at −196 °C. Prior samples’ degassing was carried out for several hours at a maximum temperature of 100 °C.
A scanning electron microscope (SEM) (FE-SEM, Zeiss, Jena, Germany) was used to investigate the homogeneity of the sample and the morphology of the surface.
Thermogravimetric analysis was performed on OF-G with TGA/DSC STARe (Mettler Toledo, Cornellà de Llobregat, Spain) equipment. Thermic decomposition of 16.2 g of the sample was evaluated from 0 to 1000 °C with a heating rate of 10 °C/min in an inert N2 atmosphere. Thermal decomposition of carbonates was determined between 600 and 850 °C, and initial calcium carbonate content was calculated accordingly.
X-ray diffraction (XRD) was performed on OF-G sample with a Bruker (Billerica, MA, USA) D8 A25 Advance, θ-θ diffractometer, with CuKα1 radiation, Bragg–Brentano geometry, and a position-sensitive LynxEyeXE detector. The diffractograms were obtained at 40 kV and 40 mA, scanning from 4° to 60° of 2θ with a step size of 0.019° and a counting time of 0.1 s/step, maintaining the sample in rotation (15/min). The crystalline phase identification was carried out by using EVA software (Version 8.5) (Bruker) with an ICDD database.
2.3. Batch Experiment Perfomance
Three kind of batch experiments were carried out: (1) pH evolution due to calcite dissolution, (2) arsenic adsorption isotherms, and (3) arsenic adsorption and calcite dissolution kinetics.
In the first experiment, pH evolution in water samples with different initial pH was studied. Briefly, 50 mL tubes were filled with 50 mL of synthetic water and 0.025 g of adsorbent (OF-G), and placed in a tube rotator at 15–20 rpm (Reax 2 Heidoph, Schwabach, Germany) for 72 h in thermostated chambers at 25 °C. Synthetic water consisted of 0.1 M NaCl (Scharlau, Barcelona, Spain) prepared in MiliQ, previously adjusted to the desired pH (2.0–9.2) with the addition of HCl or NaOH (Scharlau).
Arsenic adsorption isotherms tests followed a similar procedure to the pH evolution experiment. Here, the tubes were filled with synthetic water and the required amounts of standard As(III) or As(V) solutions (TraceCERT®, Sigma-Aldrich, St. Louis, MI, USA) in contact with the OF-G or OF-S adsorbents. Initial arsenic concentrations were 2.5–34 mg/L for As(III) and 0.6–15 mg/L for As(V). The initial equilibrium pH was 9.2–9.4, which was the equilibrium pH determined after calcite dissolution in the pH evolution experiment. Due to calcite buffer, it was not possible to determine the adsorption isotherms at lower pHs (between 4–8). The experiments were stopped at 96 h, once the adsorption equilibrium was reached, to proceed with the measurement of pH, temperature and As in water.
Kinetics experiments were performed with 25 mg/L of As(III) or As(V) at an adjusted initial acidic pH of 4.0 or alkaline pH of 8.0–9.2 in contact with OF-G or OF-S. Batch tests were sacrificed at 0.2, 0.5, 1, 2, 5, 8, 24, 48, 72 and 96 h. For each batch test, one sample of 10 mL was taken to measure pH and temperature, and another one for dissolved As, Ca and trace metals (Fe, Zn, Pb, Ni, Cr, Cu, Mn) analyses.
All employed chemicals in three experiments were of analytical grade. Experiments with As(III) were carried out in a glove box (Jacomex GP(concept)-II-P) under a N2 atmosphere to prevent any arsenic oxidation. Every experimental set was accompanied by an adsorbent blank (without OF-G/OF-S and with As(III) or As(V)) to check the adsorption of arsenic on the wall tubes or the increase in concentration due to evaporation. In addition, a control test with OF-G/OF-S and without arsenic was also carried out to check the impurities that could be released by the adsorbent.
2.4. Aqueous Samples Characterization
pH and temperature were directly measured with a pH probe (sensION+ Hach). All the aqueous samples were filtered with disposable syringe filters (cellulose acetate, 0.45 µm) and acidified with 100 µL of suprapur® HNO3 before analysis. One aliquot was used for As, Zn, Pb, Ni, Cr, Cu, Mn determination by ICP-MS (inductively coupled plasma-mass spectrometry) (Agilent 7500cx) and another one for calcium quantification by AAS (atomic absorption spectroscopy) (ContrAA Analitik Jena, Jena, Germany) and Fe determination by the ferrozine method. Metal(loid)s’ and calcium quantification was carried out based on the average of at least two different dilutions. Detection limits in µg/L were 0.1 for As, Pb and Cr, 0.5 for Ni and Mn, 1 for Cu and 10 for Zn and Fe, while the uncertainty was ±10%. The detection limit for Ca was 100 µg/L and the uncertainty was ±5%. The analytical accuracy in both methods was determined by spiking a given amount of standard reference solutions on some samples and calculating the relative standard deviation (RSD) with respect to the certified value. The RSD was always lower than 5%. The blanks used in every experimental set were always in the range of ±10% with respect to the initial arsenic concentration.
The concentration of arsenic attached to the solid was calculated with Equation (1):
where q is the amount of arsenic adsorbed onto the solid (As mg/g) and at time t, Co and C are the arsenic concentration in water (mg/L) at the initial time and at time t, respectively, V is the liquid volume (L), and m is the mass of solid material (g).
2.5. Isotherm Modelling
The experimental arsenic equilibrium data were fitted to three different adsorption isotherm models: Freundlich, Langmuir and surface complexation. The first two are empirical models, while the third one is a mechanistic model implemented here through numerical simulations.
2.5.1. Freundlich and Langmuir Models
The Freundlich equation is an empirical model that assumes that the adsorption sites on the surface are heterogeneous, and that the adsorption intensity varies with coverage. It is described according to Equation (2):
where qe is the arsenic adsorbed at the equilibrium (As mg/g), KF is the Freundlich adsorption constant (As mg1−1/n ·L1/n/g), Ce is the dissolved arsenic concentration (mg/L) at the equilibrium, and 1/n is a constant related to the adsorption intensity. KF and 1/n were obtained by representing log qe vs. log Ce and fitting the experimental data with linearization of the Freundlich equation (Equation (3)):
The Langmuir isotherm is also an empirical model that assumes that solute molecules are adsorbed onto a limited number of sites on the surface, and that each site can only accommodate one solute molecule. It is described by Equation (4):
where qmax is the maximum amount of arsenic that can be adsorbed onto the surface at saturation (As mg/g), and b is the measure of the strength of the interaction between the adsorbate and the surface (L/mg). qe and Ce have been described in Equation (2). qmax and b were obtained by representing Ce/qe vs. qe and fitting the experimental data with linearization of the Langmuir equation (Equation (5)):
2.5.2. Surface Complexation Model
The surface complexation model describes the sorption based on surface reaction equilibrium. The model employed here is based on the Dzombak and Morel [29] database for the complexation of heavy metal ions on hydrous ferric oxide (Hfo) or ferrihydrite. This model considers a double diffusion layer; the first layer comprises ions sorbed onto the solid due to chemical interactions, and the second one is composed of counter ions attracted to the surface charges via the coulomb force. Ferrihydrite, like many other oxyhydroxides, binds metals and protons on strong (Hfo_s) and weak sites (Hfo_w), and develops a charge depending on the ions sorbed. Arsenic adsorption equilibrium on feryhydrite was simulated with the PHREEQC (version 3) chemical reaction code [30], assuming both types of sites were available on the oxide surface and without specifiyng the composition of the double diffusion layer. The minteqv4 thermodynamic database, which include the significant surface complexation reactions (Table 1), was employed with the addition of carbonate adsorption constants on ferrihydrite, determined by Appelo et al. [31].

Table 1.
Surface complexation reactions of protons, hydroxide, arsenic, calcium, and carbonate onto Hfo (assimilated to ferrihydrite) and its logaritimic equilibrium constants (Log K).
Details about the numerical simulation input can be found in Table S1. Briefly, serial simulations were run with different initial As(III) and As(V) concentrations (2–35 mg/L) with the rest of the parameters kept constant. The Hfo were assimilated to ferrihydrite with a surface area of 24,901 m2/mol for OF-G and 22,550 m2/mol, according to BET results and assuming that the whole surface area corresponded to the ferrihydrite. The calcite surface area was negligible, since it has reported values in mainly in the range of 1–2000 m2/mol, depending on its origins [32,33,34]. Simulations were fitted to the experimental results by modifying the strong and weak sites’ density. Models were adjusted by finding the strong and weak site densities that minimized the sum of squared errors (SSE).
2.6. Kinetics Modelling
2.6.1. Arsenic Adsorption
Two different approaches were applied to determine the kinetics of arsenic adsorption. The first approach consisted of fitting an empirical pseudo second-order model (Equation (6)) [35]
where q is the amount of adsorbed arsenic (As mg/g), qe is the arsenic adsorbed at the equilibrium (As mg/g), k2 is the second-order kinetic constant (g/mg·h), and t is the time (h). The model parameters (qe and k2) were adjusted with a non-linear fitting using the Solver complement in Microsoft Excel™ software (Version 16), and using a GRG nonlinear solving method to minimize sum of squared errors.
The second approach consisted of applying the film diffusion mass transfer mechanistic model [36] to determine the predominant rate-controlling step (i.e., pore diffusion or film diffusion) during arsenic adsorption. The equation form of this model is (Equation (7)):
where F is the fractional attainment of equilibrium (q/qe), Kfd is the liquid diffusion rate constant (h−1), and C is a constant related to the boundary layer effect. The first experimental points were adjusted to a straight line corresponding to the film diffusion stage (Kfd1), and the rest of the points to a second straight line corresponding to the pore diffusion stage (Kfd2). The points were assigned to one stage maximizing the coefficient determination (R2) of adjusted straight lines.
2.6.2. Calcite Dissolution
Calcite dissolution kinetics present in the sorbent was simulated with PHREEQC (version 3) chemical reaction code, as for arsenic isotherm determination. The employed calcite dissolution rate corresponded to Plummer et al. [37]’s model and was retrieved from the wateq4f database (Equation (8)).
R is the dissolution rate (mol/s); A corresponds to the surface area (cm2); k1, k2 and k3 are the reaction rate constants for the three involved parallel reactions (mol/cm2/s); , and are the activities of hydrogen ion, carbon dioxide and water, respectively; IAP is the ion activity product of calcite; and Kcalcite is the equilibrium constant of the calcite dissolution reaction.
Numerical simulations were run with 25 mg/L of As(III)/As(V) in contact with calcite at two initial pH levels of 4.0/9.2. Calcite was added as a kinetic phase. Calcium concentration and pH were fitted to experimental data by modifying the reactive area in the calcite dissolution kinetics and finding the value that minimized the sum of squared errors. Calcium adsorption onto GFH-C was not considered, since the kinetics of this reaction has not ben assessed. Details of the input numerical simulations can be found in Table S2.
3. Results and Discussion
3.1. Adsorbent Characterization
In the present experiments, the employed OF-G size ranged from 250–1000 µm, while the OF-S exhibited a size about 100 times lower, according to the median value (Table 2; Figure S1). The range of size obtained for OF-S is very similar to previous references (1.9–50.2 µm) [23]. These authors used 20 kHz for 5 min, with concentrations of Ferrosorp ranging from 10 to 20 g/L of deionized water. The surface area of the OF-G adsorbent was 218 m2/g (Figure S2). This was similar to the surface measured by Martí et al. [23] (199.7 m2/g) and Kumar et al. [22] (179 m2/g), but was lower than stated by the manufacturer (~300 m2/g) or found by Janneck et al. [38] (321 m2/g). The micropores’ area, <2 nm, was similar to that in the work of Martí et al. [23] (20.9 m2/g), but low compared with that of Kumar et al. [22] (82 m2/g) or Janneck et al. [38] (276.6 m2/g), showing that this product is highly heterogeneous, due to its origin as a recovered waste. When sonication was applied, the total surface area remained almost the same (6% increase), but the micropore area decreased (46% decrease). This is in line with a previous study that determined that in sonicated samples the intraparticle porosity of initial grains, represented with the micropores, was lost during sonication, thus resulting in a total surface area decrease [23], or only a slight increase, like in this study.

Table 2.
Adsorbent characteristics.
SEM images showed similar types of surfaces in granular and sonicated samples (Figure S3); both solids showed pores of different sizes, which are responsible for the high surface area. Calcite crystals could not be identified with this technique, although calcium was detected with the EDX. Thermogravimetric analysis determined a calcite content of 8.5% in OF-G samples (Figure S4). This value was of the same order of magnitude as the 15.6% found in the work of Martí et al. [23], using another analytical method (ICP). Moreover, XRD also identified calcite as a crystalline phase, while the rest of components of GFH-C could be linked to amorphous phases, corresponding to iron oxyhydroxides (Figure S5). According to the FerroSorp® Plus product information sheet, these amorphous phases can be attributed to ferrihydrite. In numerical simulations, 8.5% calcite and 91.5% (wt.) ferrihydrite were assumed as input phases.
3.2. pH Evolution due to Calcite Dissolution
An experiment was conducted to determine the equilibrium pH of the adsorbent in contact with synthetic water at different starting pHs (Figure S6). For the initial pH equal to or higher than 4, the equilibrium pH rose to 9.2–9.4 in 24 h due to calcite dissolution and CO32−/HCO3− buffering reactions. Under the studied conditions, at an initial pH lower than 4, the calcite was completely dissolved, and the adsorbent was not able to buffer the acidity of the system. Therefore, we decided to study arsenic adsorption and calcite dissolution for a pH range wherein neutralization occurred, i.e., at an initial pH equal or higher than 4.
3.3. Arsenic Adsorption Equilibrium
Figure 1 presents the As(III) and As(V) adsorption experimental isotherm results and three adjusted models. The fitting parameters for the Freundlich, Langmuir and surface complexation models applied to the experimental points are displayed in Table 3. The fitting and the coefficient of determination for the experimental values using linearization of Freundlich and Langmuir models is shown in Figures S7 and S8. The coefficient of determination (R2) value was higher than 0.95 in all cases, which implies a high goodness of fit. According to SSE, the Freundlich model is the one that has the best fit for As(III) adsorption, while the Langmuir model better describes the As(V) adsorption.
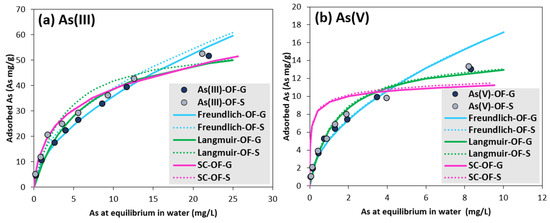
Figure 1.
(a) As(III) and (b) As(V) adsorption isotherms and adjusted Freundlich, Langmuir and SC (“surface complexation”) models at 25 °C.

Table 3.
Adsorption equilibrium isotherm parameters and calculated errors for the Freundlich, Langmuir and surface complexation models.
In the As(III) Freundlich models, the “n” constant, related to the sorption intensity and distribution coefficient, respectively, was slightly higher for OF-S than for OF-G. This constant was of the same order of magnitude, but lower than in other studies (2.66 and 3.96 for GFH and dust ferric hydroxide (DFH), respectively, in the work of Usman et al. [39]) with similar adsorbents. KF showed a slightly higher As(III) adsorption capacity with OF-S samples, probably related to small differences in the surface chemistry, although the reason for this is unclear. Moreover, it could not be compared with the literature, since its unit depends on “n”.
The “b” constant, from Langmuir model, predicted a slightly higher adsorption intensity for OF-S than for OF-G in As(V) models. The qmax values reported here were higher than in other studies with similar OF-G for As(V) adsorption (6.56 mg/g in the work of Martí et al. [14] and 9.5 mg/g in that of Usman et al. [39]). However, in the work of Usman et al. [39], the authors reported a qmax for DFH of 18.9 mg/g, and found that the adsorption capacity notably increased with the small-power-size OF. Here, the As(V) adsorption capacity was independent of the adsorbent size.
The surface complexation model reproduced the adsorption of As(III) well, while for As(V) adsorption, the model curve did not mimic the same trend as the experiments. The number of strong and weak adsorption sites were not modified with the adsorbent size, as they depend on their chemical structure. Dzombak and Morel [29] estimated a median strong site density for hydrous ferric oxides of 0.005 mol/mol Fe, which here corresponds to 0.005 As mol/mol OF, which is close to the figures obtained in the present study. Numerical simulations determined that the strong sites were mainly occupied by calcium in the form of Hfo_sOHCa2+ (74–100%, depending on arsenic concentrations), and there was only As(III) adsorption at the higher concentrations, accounting for a molar fraction of 19% in the form of Hfo_sH2AsO3. No As(V) was adsorbed on strong sites. For weak sites, the authors estimated a median value of 0.2 As mol/mol Fe for sorption of different compounds. However, the values reported here are slightly lower, but in the range of those found by Pierce and Moore [40] for As(III) (0.05–0.18) and As(V) (0.1–0.13) adsorption. Numerical simulations showed that at lower As concentrations, the main speciation of weak sites was Hfo_wO- (37%), while Hfo_wCa2+ represented 25 to 36%. At higher As concentrations, 66% of Hfo_w was in the form of Hfo_WH2AsO3, and 33% was Hfo_wOHAsO4−3, for As(III) and As(V) experiments, respectively. The predominant arsenic reactions in this case were the following (Equations (9) and (10)):
Hfo_wOH + H2AsO3− + H+ = Hfo_wH2AsO3 + H2O for As(III)
Hfo_wOH + HAsO4−2 = Hfo_wOHAsO4−3 + H+ for As(V)
In these conditions, calcium was the main adsorbed element on Hfo_w in As(V) experiments (60%). Regarding the adsorption mechanism, the employed surface complexation model was limited to monodentate complexes, while according to the literature, other complexation reactions may take place. Some studies have suggested that the main binding mechanism of arsenic onto ferrihydrite or GFH is the formation of bidentate binuclear complexes, for As(III) [41] and As(V) [42]. Considering the qmax from the Langmuir models, if 1 mol of adsorbed arsenic was adsorbed to 1 Hfo_w, the calculated moles of the weak sites would be 0.085 and 0.021, for As(III) and As(V), respectively. Here, the number of adjusted weak sites was approximately twice or three times these figures (Table 3), thus suggesting that complexes other than monodentate may occur. To confirm this hypothesis, complexation reactions with other coordination complexes, such as bidentate binuclear, should be incorporated to the numerical model in future research.
For a determined equilibrium arsenic concentration in water, the amount of adsorbed As(III) was higher than the amount of As(V) due to its speciation. According to PHREEQC simulations, at the equilibrium pH of 9.2–9.4, 54% of As(III) was in the form of H2AsO3− and 46% in the form of H3AsO3, while 97% As(V) was in the form of HAsO42−. The point of zero charge (PZC) of this solid was 8.4 [23]. This value is similar to the reported value of 9.1 measured for Ferrosorp© [43], and also to several ferric hydroxides and ferrihydrite reviewed by Hlavay and Polyak [44], with a pH range of 7.9 to 8.5. At a pH lower than that value, the surface was predominantly positively charged, and thus the negatively charged arsenic species were more electrostatically attracted. At higher pHs, the surface is predominantly negatively charged, and consequently, a greater repulsion with negative species is generated. According to numerical simulations, the surface charge density at the equilibrium pH was between −1.7 × 10−2–−1.8 × 10−2 C/m2 for As(III) and −2 × 10−2–−7 × 10−2 C/m2 for As(V).
3.4. Arsenic Adsorption Kinetics
Figure 2 presents the arsenic adsorption kinetics experimental results and the pseudo-second-order adjusted models. Arsenic adsorption reached the equilibrium after 24 h in kinetic experiments. Adjusted qe values were similar for OF-S and OF-G, but were higher for As(III) than for As(V), similar to qmax in the Langmuir isotherm model, adjusted under different initial As concentrations.
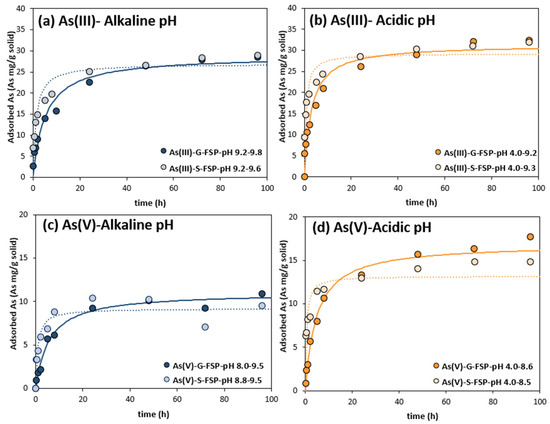
Figure 2.
As(III) and As(V) adsorption onto OF-G and OF-S at different initial pH levels during kinetic experiments. Straight lines represent the adjusted pseudo-second-order kinetics for OF-G experiments, and dotted lines the OF-S ones. The pH range in the legend corresponds to the initial and the final experimental pH. (a) As(III) adsorption at alkaline pH, (b) As(III) adsorption at acidic pH, (c) As(V) adsorption at alkaline pH, (d) As(V) adsorption at acidic pH.
As(III) and As(V) were adsorbed faster in OF-S than in OF-G, for both alkaline and acid pH. At alkaline pH, the non-linear pseudo-second-order adsorption constant “K2” (Table 4) was higher by approximately 4-fold and 8-fold for As(III) and As(V), respectively. Size reduction of GFH resulted in an enhanced As(III) and As(V) adsorption rate, as already found in other studies [14,39].

Table 4.
Fitted parameters for the non-linear pseudo-second-order adsorption kinetic models.
At acid pH, the “K2” increment was greater, by 5-fold and 13-fold, respectively. In addition, “K2” was higher for As(V) than As(III) in all conditions, but initial rates (calculated as K2 × qe2) were higher for As(III). Acidic pH generally resulted in higher adsorption rates, which is probably related with the pH of point of zero charge (pHPZC) of GFH and the lower electrostatic repulsion at low pH, as explained above.
The adsorption of arsenite onto calcite at acidic pH was discarded, since it has already been reported it is not well adsorbed on this mineral phase [45]. On the contrary, arsenate could be adsorbed onto calcite at acidic pH and enter in competition with carbonates for sorption sites. However, the complexation constants for arsenate adsorption onto iron hydroxides (log K = −10.12 to 8.61, Table 1) are much higher than for adsorption onto calcite (log K = −9.81 to −7.22) [45]. Carbonates’ adsorption onto GFH and competition with arsenic at alkaline pH could also explain to some extent the lower rates, as has been reported before [46]. According to arsenic adsorption surface complexation simulations, up to 0.53 carbonate mg/g OF and 0.13 carbonate mg/g OF were adsorbed at pH > 9.5 in the presence of As(III) and As(V), respectively.
The film diffusion mass transfer model was adjusted to determine the control mechanisms of the reaction at given times (Figure S8). Generally, the goodness of fit was greater than 0.9 (R2) (Table S3). Model showed that film diffusion dominated during the first 5 h, while the rest of the time, the pore diffusion mechanism controlled the adsorption (Figure S9). The Kfd1 was higher in sonicated samples than in granular ones, which means the film diffusion was more effective in the first case. The pore diffusion rate (Kfd2) was similar in all the experiments. Size reduction contributed to a loss of intraparticle porosity, thus resulting in an increase in film diffusion mass transfer in OF-U and As arrival onto the external surface of the adsorbent. This idea has already been explained in the work of Martí et al. [14], who mentioned that the intraparticle diffusion effect, expressed here as “pore diffusion”, was less significant in size-reduced adsorbents
3.5. Calcite Dissolution Kinetics
Figure 3 presents the calcite dissolution kinetics results and the adjusted numerical simulations. In all the experiments, either at an initial pH of 4.0 or 9.2, calcium was released into the medium, due to calcite dissolution, at a faster rate with OF-S than with OF-G. In addition, the experiments performed at an initial pH of 4.0 released more calcium than the ones that started at pH 9.2. Between 48 and 96 h, the equilibrium calcium concentration was 10 ± 1 mg/L for the experiments performed at initial pH of ~9.2, while it was 15 ± 4 for the ones at an initial pH of 4.0. pH varied accordingly (Figure 4). Considering the composition of 8.5% of calcite in OF and the released calcium, we calculated that 88.2% of the calcite was dissolved in experiments at pH 4.0 to 9.3, while 58.8% was dissolved in experiments at pH 9.2 to 9.8. No noticeable differences were observed in the calcite dissolution rates between As(III) and As(V) tests.
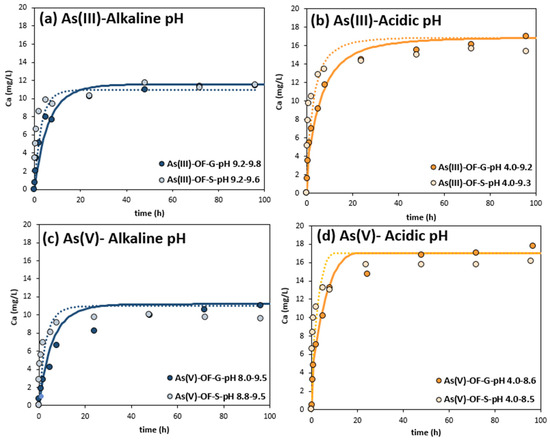
Figure 3.
Calcium released into the medium at different initial pH with either OF-G or OF-S in As(III) and As(V) adsorption kinetic experiments. Straight lines represent the adjusted calcite dissolution numerical simulation for OF-G experiments, and dotted lines for the OF-S ones. The pH range in the legend corresponds to the initial and the final pH. (a) Ca released in As(III) adsorption experiments at alkaline pH, (b) Ca released in As(III) adsorption experiment at initial acidic pH, (c) Ca released in As(V) adsorption experiment at alkaline pH, (d) Ca released in As(V) adsorption experiment at initial acidic pH.
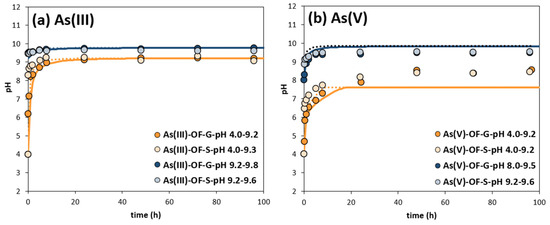
Figure 4.
pH variation due to calcite dissolution at different initial pH levels with either OF-G or OF-S in (a) As(III) and (b) As(V) kinetic experiments. Straight lines represent the adjusted calcite dissolution numerical simulation for OF-G experiments, and dotted lines for the OF-S ones.
The best calcite surface area fitting corresponded to 500,000 cm2/mol (0.5 m2/g) for OF-S and 250,000 cm2/mol (0.25 m2/g) for OF-G, indicating that the sonication process resulted in an increase in the calcite area. These figures are in the same order of magnitude as those found for precipitated calcite from aqueous solutions [34]. Calcite surface area increased with sonication, making it more accessible and thereby increasing the calcite dissolution rate. Since calcite is made of crystals with negligible internal porosity, when its size is reduced by sonication, surface area increases with the lower particle size [47]. In OF-S, although the calcite content is the same as in OF-G, there is a loss of internal porosity, and calcite is also more exposed to the medium, resulting in higher dissolution rates in the first 5 h (Table S4).
It was also noticed that the rates were higher at acidic pH than alkaline pH, as for arsenic adsorption. This has already been described in previous research wherein calcite dissolution under different pH levels, CO2 pressures, ionic solution strengths and temperatures were determined in detail [37,48]. At pH 4, calcite is far from equilibrium, and thus the reaction is faster according to the transition-state theory kinetic law for mineral dissolution. This may be advantageous when neutralizing acidic waters, since 1 h would be enough to raise the pH above 6 if calcite is in excess.
The equilibrium pH (Figure 4) was lower for experiments starting at pH 4 with As(V) (8.5) than for As(III) (9.2), because the corresponding dissociation constant (Ka) of As(V) species (H2AsO4− ↔ HAsO42− + H+; pKa: 6.96) is higher than that of As(III) species (H3AsO3 ↔ H2AsO3− + H+; pKa: 9.22), thus meaning that As(V) species deprotonate at lower pHs, contributing to the acidification of the medium. In addition, Figure 4 shows that the equilibrium pH is close to PZC, and that this is independent of the size of adsorbent for the four experiments carried out with As(III) or As(V), supporting the independence of the PZC with the size.
3.6. Environmental Significance
One issue that could be controversial is the release of metal impurities contained in the recycled adsorbent into the groundwater. The safety of the adsorbent was assessed here in terms of metals’ release during arsenic adsorption; no Ni, Zn, or Pb were detected, and only traces (<10 µg/L) of Cr, Cu and Mn were determined, in concentrations far below the standards of drinking water regulations (Figure S10), meaning its use in drinking water treatment may be permitted.
Another remarkable feature of the employed GFH is its calcite content. This characteristic makes the adsorbent appropriate for remediating acidic groundwaters reported worldwide, since water is neutralized concomitantly to metal(loid) adsorption. Calcium materials have been traditionally employed to treat this kind of water, since they raise the pH, generating iron precipitates with the coprecipitation of metal(loid)s or their adsorption on the surface [49]. The application of the present adsorbent could be of particular interest in low-iron-content acidic waters.
Finally, performance enhancement with the implementation of size reduction is an asset in groundwater treatments. The use of size-reduced adsorbents could be advantageous, since the adsorbents can be directly injected into the affected zone. Besides, the kinetics of adsorption and pH increase were faster in the smaller adsorbents, and this could be beneficial when groundwater fluxes are high, such as in sandy aquifers. Future research should be focused on testing this kind of adsorbent to treat polluted groundwater in a field environment, as well as investigating the possibility of reusing the OF-S sorbents in a pump-and-treat approach.
4. Conclusions
The application of sonication to a granular ferric hydroxide-calcite (FHC) adsorbent resulted in a size reduction that implied a slightly higher BET area but a lower intraparticle porosity. The arsenic binding capacity of sonicated adsorbents did not improve significantly. The surface complexation model reproduced the adsorption of As(III) well.
On the contrary, the As(III)/As(V) adsorption kinetics improved with the sonication, as in the case of calcite dissolution kinetics. The dissolution of calcite from the adsorbent made the water pH increase from values of around 4 up to 9.2–9.4.
The advantage of sonicated adsorbents for the depollution of acidic As-containing groundwater is that its size is adequate for aquifer injection in an RZB that uses suspensions of small-sized materials; it can also adsorb arsenic faster than a granular adsorbent, while neutralizing acidic waters.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/w16010030/s1, Table S1: Initial solution characteristics, equilibrium phases, and surface parameters in arsenic adsorption isotherm simulations. As(III) and As(V) simulations were run for different initial concentrations (0.0338–0.693 mol/L for As(III) and 0.0076–0.266 mol/L for As(V)) to reproduce the isotherm batch experiment, with the rest of parameters kept constant; Table S2: Initial solution characteristics, kinetics, equilibrium phases, and surface parameters in calcite dissolution; Figure S1: Particle size distribution of OF-G and OF-S; Figure S2: BET isotherms and surface area reports for (a) OF-G and (b) OF-S; Figure S3: SEM images of (a,b) OF-G and (c,d) OF-S at different magnifications; Figure S4: Thermogravimetric analysis of OF-G; Figure S5: XRD diffractogram of OF-G sample; Figure S6: pH variation in batch experiments with OF-G samples and synthetic water at an initial pH from 2 to 9; Figure S7: Fitting of experimental data with the linearization of the Freundlich equation for (a) As(III) and (b) As(V); Figure S8: Fitting of experimental data with the linearization of the Langmuir equation for (a) As(III) and (b) As(V); Figure S9: Film diffusion mass transfer models and linear adjustments for As(III) and As(V) experiments. Solid lines correspond to OF-G, and dotted lines to OF-S linear adjustments. (a) As(III) adsorption at alkaline pH, (b) As(III) adsorption at acidic pH, (c) As(V) adsorption at alkaline pH, (d) As(V) adsorption at acidic pH; Table S3: Absolute values of constants of the adjusted film diffusion mass transfer models; Table S4: Measured calcium release rates in the medium for the first 5 h (data from Figure 3); Figure S10: Traces released during the arsenic adsorption experiments.
Author Contributions
Conceptualization, L.F.-R., I.J., M.R. and V.M.; methodology, L.F.-R. and I.J.; software, L.F.-R. and M.R.; investigation, L.F.-R., N.B. and M.J.; resources, M.R., X.M.-L. and I.J.; writing—original draft preparation, L.F.-R.; writing—review and editing, L.F.-R., I.J., V.M. and M.R.; supervision, M.R. and X.M.-L.; project administration, M.R. and I.J.; funding acquisition, M.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a Torres Quevedo grant (PTQ-2019-010503) to L.F.R., and by the Catalan Government through the funding grant ACCIÓ- Eurecat (Project PRIV2019-20-MICONANO) to I.J., N.B. and M.R.
Data Availability Statement
The data presented in this study are available on request from the corresponding authors.
Acknowledgments
The authors thank Samuel Rodríguez from the University of Barcelona for his contribution to the adsorbent characterization during his master’s thesis, as well as Cristina González and Marta González from Eurecat for their support in performing the experiments. The authors thank HeGo Biotec GmbH for supplying the Ferrosorp Plus® samples.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Shaji, E.; Santosh, M.; Sarath, K.V.; Prakash, P.; Deepchand, V.; Divya, B.V. Arsenic Contamination of Groundwater: A Global Synopsis with Focus on the Indian Peninsula. Geosci. Front. 2021, 12, 101079. [Google Scholar] [CrossRef]
- Smedley, P.L.; Kinniburgh, D.G. A Review of the Source, Behaviour and Distribution of Arsenic in Natural Waters. Appl. Geochem. 2002, 17, 517–568. [Google Scholar] [CrossRef]
- Mukherjee, A.; Sengupta, M.K.; Hossain, M.A.; Ahamed, S.; Das, B.; Nayak, B.; Lodh, D.; Rahman, M.M.; Chakraborti, D. Arsenic Contamination in Groundwater: A Global Perspective with Emphasis on the Asian Scenario. J. Health Popul. Nutr. 2006, 24, 142–163. [Google Scholar]
- Baragaño, D.; Boente, C.; Rodríguez-Valdés, E.; Fernández-Braña, A.; Jiménez, A.; Gallego, J.L.R.; González-Fernández, B. Arsenic Release from Pyrite Ash Waste over an Active Hydrogeological System and Its Effects on Water Quality. Environ. Sci. Pollut. Res. 2020, 27, 10672–10684. [Google Scholar] [CrossRef]
- Golab, A.; Peterson, M.; Indraratna, B. Selection of Permeable Reactive Barrier Materials for Treating Acidic Groundwater in Acid Sulphate Soil Terrains Based on Laboratory Column Tests. Environ. Earth Sci. 2009, 59, 241–254. [Google Scholar] [CrossRef]
- Ha, Q.K.; Choi, S.; Phan, N.L.; Kim, K.; Phan, C.N.; Nguyen, V.K.; Ko, K.S. Occurrence of Metal-Rich Acidic Groundwaters around the Mekong Delta (Vietnam): A Phenomenon Linked to Well Installation. Sci. Total Environ. 2019, 654, 1100–1109. [Google Scholar] [CrossRef]
- Angai, J.U.; Ptacek, C.J.; Pakostova, E.; Bain, J.G.; Verbuyst, B.R.; Blowes, D.W. Removal of Arsenic and Metals from Groundwater Impacted by Mine Waste Using Zero-Valent Iron and Organic Carbon: Laboratory Column Experiments. J. Hazard. Mater. 2022, 424, 127295. [Google Scholar] [CrossRef]
- Gibert, O.; Rötting, T.; Cortina, J.L.; de Pablo, J.; Ayora, C.; Carrera, J.; Bolzicco, J. In-Situ Remediation of Acid Mine Drainage Using a Permeable Reactive Barrier in Aznalcóllar (Sw Spain). J. Hazard. Mater. 2011, 191, 287–295. [Google Scholar] [CrossRef]
- Casiot, C.; Leblanc, M.; Bruneel, O.; Personné, J.-C.; Koffi, K.; Elbaz-Poulichet, F. Geochemical Processes Controlling the Formation of As-Rich Waters within a Tailings Impoundment (Carnoulès, France). Aquat. Geochem. 2003, 9, 273–290. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U. Arsenic Removal from Water/Wastewater Using Adsorbents-A Critical Review. J. Hazard. Mater. 2007, 142, 1–53. [Google Scholar] [CrossRef] [PubMed]
- Asere, T.G.; Stevens, C.V.; Du Laing, G. Use of (Modified) Natural Adsorbents for Arsenic Remediation: A Review. Sci. Total Environ. 2019, 676, 706–720. [Google Scholar] [CrossRef] [PubMed]
- Thirunavukkarasu, O.S.; Viraraghavan, T.; Subramanian, K.S. Arsenic Removal from Drinking Water Using Granular Ferric Hydroxide. Water SA 2003, 29, 161–170. [Google Scholar] [CrossRef]
- Szlachta, M.; Wójtowicz, P. Treatment of Arsenic-Rich Waters Using Granular Iron Hydroxides. Desalin. Water Treat. 2016, 57, 26376–26381. [Google Scholar] [CrossRef]
- Martí, V.; Jubany, I.; Fernández-Rojo, L.; Ribas, D.; Benito, J.A.; Diéguez, B.; Ginesta, A. Improvement of As(V) Adsorption by Reduction of Granular to Micro-Sized Ferric Hydroxide. Processes 2022, 10, 1029. [Google Scholar] [CrossRef]
- Maity, J.P.; Chen, C.Y.; Bhattacharya, P.; Sharma, R.K.; Ahmad, A.; Patnaik, S.; Bundschuh, J. Advanced Application of Nano-Technological and Biological Processes as Well as Mitigation Options for Arsenic Removal. J. Hazard. Mater. 2021, 405, 123885. [Google Scholar] [CrossRef]
- Usman, M.; Zarebanadkouki, M.; Waseem, M.; Katsoyiannis, I.A.; Ernst, M. Mathematical Modeling of Arsenic(V) Adsorption onto Iron Oxyhydroxides in an Adsorption-Submerged Membrane Hybrid System. J. Hazard. Mater. 2020, 400, 123221. [Google Scholar] [CrossRef]
- Sperlich, A.; Schimmelpfennig, S.; Baumgarten, B.; Genz, A.; Amy, G.; Worch, E.; Jekel, M. Predicting Anion Breakthrough in Granular Ferric Hydroxide (GFH) Adsorption Filters. Water Res. 2008, 42, 2073–2082. [Google Scholar] [CrossRef]
- Badruzzaman, M.; Westerhoff, P.; Knappe, D.R.U. Intraparticle Diffusion and Adsorption of Arsenate onto Granular Ferric Hydroxide (GFH). Water Res. 2004, 38, 4002–4012. [Google Scholar] [CrossRef]
- Mohammadian, S.; Krok, B.; Fritzsche, A.; Bianco, C.; Tosco, T.; Cagigal, E.; Mata, B.; Gonzalez, V.; Diez-Ortiz, M.; Ramos, V.; et al. Field-Scale Demonstration of in Situ Immobilization of Heavy Metals by Injecting Iron Oxide Nanoparticle Adsorption Barriers in Groundwater. J. Contam. Hydrol. 2021, 237, 103741. [Google Scholar] [CrossRef]
- Castaño, A.; Prosenkov, A.; Baragaño, D.; Otaegui, N.; Sastre, H.; Rodríguez-Valdés, E.; Gallego, J.L.R.; Peláez, A.I. Effects of in Situ Remediation with Nanoscale Zero Valence Iron on the Physicochemical Conditions and Bacterial Communities of Groundwater Contaminated with Arsenic. Front. Microbiol. 2021, 12, 643589. [Google Scholar] [CrossRef]
- Montalvo, D.; Vanderschueren, R.; Fritzsche, A.; Meckenstock, R.U.; Smolders, E. Efficient Removal of Arsenate from Oxic Contaminated Water by Colloidal Humic Acid-Coated Goethite: Batch and Column Experiments. J. Clean. Prod. 2018, 189, 510–518. [Google Scholar] [CrossRef]
- Kumar, P.S.; Korving, L.; Keesman, K.J.; van Loosdrecht, M.C.M.; Witkamp, G.J. Effect of Pore Size Distribution and Particle Size of Porous Metal Oxides on Phosphate Adsorption Capacity and Kinetics. Chem. Eng. J. 2019, 358, 160–169. [Google Scholar] [CrossRef]
- Martí, V.; Jubany, I.; Ribas, D.; Benito, J.A.; Ferrer, B. Improvement of Phosphate Adsorption Kinetics onto Ferric Hydroxide by Size Reduction. Water 2021, 13, 1558. [Google Scholar] [CrossRef]
- Kunaschk, M.; Schmalz, V.; Dietrich, N.; Dittmar, T.; Worch, E. Novel Regeneration Method for Phosphate Loaded Granular Ferric (Hydr)Oxide—A Contribution to Phosphorus Recycling. Water Res. 2015, 71, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, K.; Amy, G.L.; Prevost, M.; Nour, S.; Jekel, M.; Gallagher, P.M.; Blumenschein, C.D. Kinetic and Thermodynamic Aspects of Adsorption of Arsenic onto Granular Ferric Hydroxide (GFH). Water Res. 2008, 42, 3371–3378. [Google Scholar] [CrossRef] [PubMed]
- Garau, G.; Silvetti, M.; Castaldi, P.; Mele, E.; Deiana, P.; Deiana, S. Stabilising Metal(Loid)s in Soil with Iron and Aluminium-Based Products: Microbial, Biochemical and Plant Growth Impact. J. Environ. Manage. 2014, 139, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Torres-Rivero, K.; Bastos-Arrieta, J.; Florido, A.; Martí, V. Potential Use of Precipitates from Acid Mine Drainage (AMD) as Arsenic Adsorbents. Water 2023, 15, 3179. [Google Scholar] [CrossRef]
- Wei, Z.; Kosterman, J.A.; Xiao, R.; Pee, G.Y.; Cai, M.; Weavers, L.K. Designing and Characterizing a Multi-Stepped Ultrasonic Horn for Enhanced Sonochemical Performance. Ultrason. Sonochem. 2015, 27, 325–333. [Google Scholar] [CrossRef]
- Dzombak, D.A.; Morel, F.M.M. Surface Complexation Modeling: Hydrous Ferric Oxide; John Wiley & Sons: Hoboken, NJ, USA, 1990. [Google Scholar]
- Parkhurst, D.L.; Appelo, C.A.J. Description of Input and Examples for PHREEQC Version 3—A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations; U.S. Geological Survey Techniques and Methods, Book 6; U.S. Geological Survey: Reston, VA, USA, 2013; Chapter A43; 497p. [CrossRef]
- Appelo, C.A.J.; Van Der Weiden, M.J.J.; Tournassat, C.; Charlet, L. Surface Complexation of Ferrous Iron and Carbonate on Ferrihydrite and the Mobilization of Arsenic. Environ. Sci. Technol. 2002, 36, 3096–3103. [Google Scholar] [CrossRef]
- Suarez, D.L.; Wood, J.D. Division S-2-Soil Chemistry Simultaneous Determination of Calcite Surface Area and Content in Soils 1. Soil Sci. Soc. Am. J. 1984, 48, 1232–1235. [Google Scholar] [CrossRef]
- Yang, J.H.; Shih, S.M.; Wu, C.I.; Tai, C.Y. Der Preparation of High Surface Area CaCO3 for SO2 Removal by Absorption of CO2 in Aqueous Suspensions of Ca(OH)2. Powder Technol. 2010, 202, 101–110. [Google Scholar] [CrossRef]
- Noiriel, C.; Steefel, C.I.; Yang, L.; Ajo-Franklin, J. Upscaling Calcium Carbonate Precipitation Rates from Pore to Continuum Scale. Chem. Geol. 2012, 318–319, 60–74. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-Second Order Model for Sorption Processes Y.S. Org. Process Res. Dev. 2017, 21, 866–870. [Google Scholar] [CrossRef]
- Podder, M.S.; Majumder, C.B. Biosorption of As(III) and As(V) on the Surface of TW/MnFe2O4 Composite from Wastewater: Kinetics, Mechanistic and Thermodynamics. Appl. Water Sci. 2017, 7, 2689–2715. [Google Scholar] [CrossRef]
- Plummer, L.N.; Wigley, T.M.L.; Parkhurst, D.L. The kinetics of calcite dissolution in CO2-water systems at 5 °C to 60 °C and 0.0 to 1.0 atm CO2. Am. J. Sci. 1978, 278, 179–216. [Google Scholar] [CrossRef]
- Janneck, E.; Burghardt, D.; Simon, E.; Pfeiffer, S.; Paul, M.; Koch, T. Development of an Adsorbent Comprising Schwertmannite and Its Utilization in Mine Water Treatment. In Proceedings of the International Mine Water Association, Santiago de Chile, Chile, 21–24 April 2015; Paper 215. pp. 1–10. [Google Scholar]
- Usman, M.; Katsoyiannis, I.; Mitrakas, M.; Zouboulis, A.; Ernst, M. Performance Evaluation of Small Sized Powdered Ferric Hydroxide as Arsenic Adsorbent. Water 2018, 10, 957. [Google Scholar] [CrossRef]
- Pierce, M.L.; Moore, C.B. Adsorption of Arsenite and Arsenate on Amorphous Iron Hydroxide. Water Res. 1982, 16, 1247–1253. [Google Scholar] [CrossRef]
- Kim, S.O.; Lee, W.C.; Cho, H.G.; Lee, B.T.; Lee, P.K.; Choi, S.H. Equilibria, Kinetics, and Spectroscopic Analyses on the Uptake of Aqueous Arsenite by Two-Line Ferrihydrite. Environ. Technol. 2014, 35, 251–261. [Google Scholar] [CrossRef]
- Guan, X.H.; Wang, J.; Chusuei, C.C. Removal of Arsenic from Water Using Granular Ferric Hydroxide: Macroscopic and Microscopic Studies. J. Hazard. Mater. 2008, 156, 178–185. [Google Scholar] [CrossRef]
- Karn, S.K.; Pan, X. Simultaneous Application Arsenic Oxidising Bacteria and Biochar for the Reclamation of Arsenic Contaminated Soil. Int. J. Environ. Waste Manag. 2018, 21, 155. [Google Scholar] [CrossRef]
- Hlavay, J.; Polyák, K. Determination of Surface Properties of Iron Hydroxide-Coated Alumina Adsorbent Prepared for Removal of Arsenic from Drinking Water. J. Colloid Interface Sci. 2005, 284, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Sø, H.U.; Postma, D.; Jakobsen, R.; Larsen, F. Sorption and Desorption of Arsenate and Arsenite on Calcite. Geochim. Cosmochim. Acta 2008, 72, 5871–5884. [Google Scholar] [CrossRef]
- Radu, T.; Subacz, J.L.; Phillippi, J.M.; Barnett, M.O. Effects of Dissolved Carbonate on Arsenic Adsorption and Mobility. Environ. Sci. Technol. 2005, 39, 7875–7882. [Google Scholar] [CrossRef] [PubMed]
- Kojima, Y.; Yamaguchi, K.; Nishimiya, N. Effect of Amplitude and Frequency of Ultrasonic Irradiation on Morphological Characteristics Control of Calcium Carbonate. Ultrason. Sonochem. 2010, 17, 617–620. [Google Scholar] [CrossRef]
- Pokrovsky, O.S.; Golubev, S.V.; Schott, J.; Castillo, A. Calcite, Dolomite and Magnesite Dissolution Kinetics in Aqueous Solutions at Acid to Circumneutral PH, 25 to 150 °C and 1 to 55 Atm PCO2: New Constraints on CO2 Sequestration in Sedimentary Basins. Chem. Geol. 2009, 265, 20–32. [Google Scholar] [CrossRef]
- Maree, J.P.; Du Plessis, P. Neutralization of Acid Mine Water with Calcium Carbonate. Water Sci. Technol. 1994, 29, 285–296. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).