Abstract
Adenoviruses are considered one of the most persistent enteric pathogens that can penetrate entire aquifer matrices. An ongoing monitoring of irrigation water is essential to mitigate potential public health risks. We investigated the prevalence of human adenoviruses (HAdV) in the groundwater discharge basins of Aluraiji (AW-DB) and Diriyah (DW-DB) and in the irrigation water of Al Harriq (H-IW) and Al Hayer (AH-IW) from January to December 2021. The meteorological impact (temperature, relative humidity, and wind speed) on HAdV prevalence and molecular diversity was investigated by targeting a selected region of the Hexon gene. The samples were concentrated using the polyethylene glycol precipitation (PEG) method. HAdVs were detected using PCR and sequenced by Sanger sequencing, and phylogenetic analysis was performed. The highest HAdV prevalence was recorded in H-IW, AH-IW, and DW-DB (100%). HAdV sequences were found to be closely related to species F (type 41) with a predominance of the 00-2B64 sequence (86.4%). Phylogenetic analysis depicted a close relationship of this study’s isolate 00-2B64 to a Brazilian and Saudi isolate, whereas 08-2B64 was found to be related to a sequence from an AnNazim landfill (LF1; d = 0.00) from Riyadh, Saudi Arabia. A high HAdV prevalence was recorded at a temperature range of 19–28 °C, wind speed was recorded at a range of (16–20 km/h), and relative humidity was recorded at a range of (15–25%). Meteorological variations exhibited no significant effect on the prevalence of HAdVs. The findings provided data on HAdV prevalence and predominant species in the irrigation water of Riyadh, Saudi Arabia and presented information regarding the environmental effects of HAdV persistence. In public health contexts, this will help in the planning of disease control strategies.
1. Introduction
Groundwater, revered as an invaluable natural resource, plays a pivotal role across various sectors, including agriculture, industry, and domestic water supply. Its significance stems from its reliability, quality, and extensive use in irrigation systems. When compared to surface water, treated water, and other alternative sources, groundwater is widely acknowledged for its inherent microbiological safety, owing to natural filtration processes as it percolates through layers of soil and rock. This filtration mechanism helps to reduce the presence of pathogenic microorganisms, rendering groundwater a relatively safer water source for human consumption and irrigation purposes. However, it is susceptible to contamination by environmental enteric viruses [1]. Additionally, the abundance and survival rates of viruses in a water environment are incredibly high in feces (including human and animal enteric viruses). As a result of their persistence and prevalence, enteric viruses become pollutants in environmental waters. They have the potential to contaminate water utilized for irrigation, aquaculture, recreation, and drinking purposes [2]. Moreover, irrigation water is a crucial factor for the virus contamination of fresh produce, and is, therefore, a critical control point to be integrated into food safety management [3]. There are seven HAdV species that are currently characterized, ranging from A to G [4]. Adenoviruses infections may lead to clinical symptoms, such as gastroenteritis in children, which is linked to HAdV serotypes 40 and 41 [5]. Many human adenoviruses are responsible for respiratory diseases such as upper respiratory tract infection and pneumonia [6]. A HAdV species B infection is related to severe illness, including urinary tract infections [7]. In 2022, a total of 650 cases of emerging hepatitis was reported from 33 countries suspected to have links to HAdV-F41, based on laboratory findings. So, precautionary measures including the surveillance and establishment of backward linkages should be adopted to prevent its spread [8]. Adenoviruses have been a significant pathogen after the rotaviruses that cause diarrhea in children in Saudi Arabia [9,10,11]. Due to adenoviruses’ high stability and resistance to environmental conditions, their occurrence and spreading have increased worldwide; for this reason, a wide variety of HAdV serotypes have been found in the matrices of water such as surface, treated, river, recreational, and groundwater throughout the world [3,12,13,14,15]. Adenoviruses can be spread through contact with contaminated surfaces or objects, as well as through the air in the form of small droplets; however, viral transmission between contaminated water from environmental sources and humans is crucial. Direct sequencing of PCR amplicon and sequence analysis of cloned PCR amplicon are two methods commonly used to study adenoviruses in aquatic environments [16]. Few previous studies highlight the prevalence and seasonal differences of aquatic HAdVs in the Kingdom of Saudi Arabia [17]. Various studies reported utilizing adenoviruses as an indicator for quality in different types of water reservoirs [12,18,19]. In addition, the WHO recommended the detection of more than one of the enteric viruses to ascertain a high level of quality in water. These enteric viruses include enteroviruses, adenoviruses, rotaviruses, caliciviruses, HAVs, HEVs, and astroviruses. Recently, the Environmental Protection Agency (EPA/USA) has published the Contaminant Candidate List 5 (CCL 5), which contains the most important microbes for water pollution, and HAdVs was reported at the top of the list [20]. Consequently, the importance of studying and monitoring the seasonal changes of adenoviruses in irrigation water becomes apparent as a detector for the presence of contaminations by humans [21]. There are five primary water resources in Saudi Arabia including water desalination, renewable and non-renewable groundwater, surface water, and treated wastewater. The kingdom mainly depends on the water of groundwater aquifers. A majority of the water resources are used for agricultural purposes. The total demand for water in Saudi Arabia during the year 2021 reached more than 14,264 million cubic meters (m3), of which 25% were for urban purposes, 5% for industrial purposes, and 70% for agricultural purposes, according to the latest statistics from the Ministry of Environment, Water and Agriculture. Among the limited water resources that are available, the KSA mainly depends on the water of groundwater aquifers. The agricultural sector consumes a large amount of water, which mainly comes from deep groundwater aquifers. Consequently, the number of wells increased dramatically to 155,324, of which 72,073 wells were established in the Riyadh region alone followed by 50,000 wells in the Northern region including the Qassim region, 12,597 wells in the Southern region, 11,510 wells in the Eastern region, and, lastly, 9066 in the Western region, as stated in the statistical data of the Ministry of Environment, Water and Agriculture, Saudi Arabia [22]. Waterborne infectious diseases pose a silent threat to public health. Therefore, we must continue to expand our investigation of their presence in environmental waters to fully understand and appropriately prepare for any expected outbreaks [23]. This study is of particular interest, since the regional burden of waterborne infectious diseases poses ongoing public health concerns [24]. In Saudi Arabia, the presence and extent of enteric virus contaminations in groundwater are unexplored. This study aimed to evaluate the prevalence and genotyping of HAdVs for a period of one year from different irrigation water sources in the Riyadh region. By continually monitoring HAdV prevalence, conducting molecular serotyping, and investigating the influence of meteorological factors, we can better comprehend the epidemiology and transmission patterns of these viruses in different water environments. Overall, understanding the viral contamination of water is expected to provide an effective system for achieving the new sustainable development goals of Saudi Vision 2030 and a national shift towards progress in the safety of irrigation water sources.
2. Materials and Methods
2.1. Samples Collection
Forty-eight samples of irrigation water were collected in sterile polypropylene bottles from 4 different locations in the Riyadh region, Saudi Arabia, including Al Harriq (H-IW) (23°36′57.6″ N 46°31′15.3″ E), which is located 250 km south-west of Riyadh; Al Hayer (AH-IW) (24°23′09.6″ N 46°51′28.7″ E), which is located south of Riyadh; Aluraiji (AW-DB) (24°43′46.9″ N 46°34′24.7″ E); and Diriyah (DW-DB) (24°43′42.4″ N 46°34′29.5″ E), as presented in Figure 1. From January to December 2021, four samples were taken on a monthly basis. Sampling points included Al Harriq and Al Hayer reservoir discharge endpoints, Aluraiji and Diriyah well-dependent discharge basins. Water samples, measured at 200 mL for each, were collected by washing the bottle twice from the same source before taking the sample. The samples were immediately transferred to the laboratory using a cooler box at approximately ≈4 °C. On the sampling day, the meteorological data of the temperature, relative humidity, and wind speed was recorded from the online website AccuWeather (https://accuweather.com, accessed on 7 August 2023).
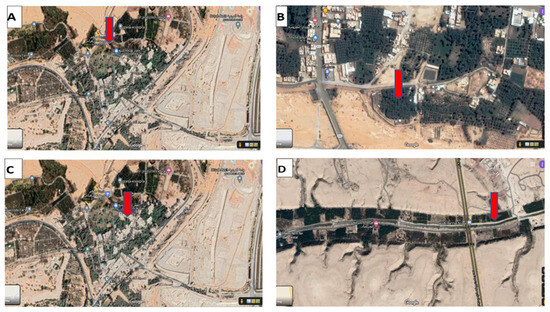
Figure 1.
The locations of collected samples from the Riyadh region: (A,C) Aluraiji (AW-DB) and Diriyah (DW-DB), 13711, groundwater well-dependent basins; (B) Al Harriq (H-IW) 16564, groundwater water reservoir; (D) Al Hayer (AH-IW), Riyadh, 14582. Google Maps, 2021.
2.2. Virus Concentration
The water samples (200 mL) were concentrated using polyethylene glycol precipitation [25]. In 200 mL of the water sample, a glycine buffer (0.05 M glycine, 0.3 g/L beef extract, PH = 9.6) was added and centrifuged at 8000× g for 30 min. A 0.2-μm syringe filter was used to filter the supernatant (Corning, NY 14831, USA). NaCl 17.5 g/L and PEG 80 g/L were combined, and the mixture was agitated at 100 rpm for two hours at room temperature. The mixture was centrifuged at 10,000× g for 20 min. The pellet was resuspended in 1 mL of Phosphate Buffer Saline [26] and stored at −80 °C until the next application.
2.3. The Nucleic Acid Extraction and Amplification of the Specific Hexon Gene
Nucleic Acid from 200-μL of a sample was extracted by using the PowerViral® Environmental RNA/DNA Isolation Kit (MO BIO, Carlsbad, CA, USA), and the Hexon target gene sequence of HAdV was amplified using 2x Phusion Master Mix (Thermo Fisher Scientific, Waltham, MA, USA). The 50-μL PCR mixture consisted of 25 μL of Master Mix, 5 μL DNA template, 1-μL (10-pmol) forward primer (AdFhex-F; 5′-GCCACCGATACCTACTTCAGCCTG-3′), and 1-μL (10-pmol) reverse primer (AdFhex-R; 5′-GGCAGTGCCGGAGTAGGGTTTAAA-3′) [27]; 18-μL RNase-DNase-free water were added, and PCR were carried out at 98 °C for 30 s, led by 40 cycles of 98 °C for 10 s, 72 °C for 30 s, and a final extension at 72 °C for 10 min and stored at 4 °C until the next application.
2.4. Purification and Sanger Sequencing
The amplicons were analyzed by gel electrophoresis using a 1.5% agarose gel (Cleaver, UK). A PCR product of 261 bp was cleaned up using Wizard SV Gel and the PCR Clean-Up System (Promega Co., Madison, WI, USA) and sequenced both forward and in a reverse direction using the BigDye Terminator Kit by ABI genetic analyzer 3130Xl (Applied Biosystems®, Carlsbad, CA, USA), following the manufacturer’s instructions. Two μL of 5 X Sequencing Buffer, 4 μL of BigDye Terminator, the bidirectional of 1 μL of primer (Forward/Reverse), 1.5 μL of sample, and 11.5 μL of RNase-DNase-free water were used. The cycle sequence was carried out at 96 °C for 1 min, followed by 35 cycles of 96 °C for 20 s, 72 °C for 15 s, and 60 °C for 4 min. Sanger sequences were then obtained by using the ABI genetic analyzer 3130 Xl (Applied Biosystems).
2.5. Phylogenetic Analysis
The Clean-Up and assembly of both directions of obtained sequences were done using Bioedit software, whereas the MEGA 11was used to analyze HAdV nucleotide sequences [28]. HAdV sequences were compared with closet sequences using the Basic Local Alignment Search Tool (BLAST) server from GenBank. A total of 86 related sequences of adenoviruses were analyzed with our sequences using the ClustalW tool on a default setting with an opening penalty of 15 and extension penalty of 6.66 (MEGA). Using a minimal Bayesian Information Criterion, a phylogenetic tree was created in accordance with the best-suited model for nucleotide substitution. Using the Kimura 3-parameter model, genetic distances were estimated (Supplementary Table S3). The name of all isolates was abbreviated on the phylogenetic according to the following formula: Species/Country/Isolate/Year/Type. Our six isolates were denoted by a red characterization.
2.6. Prevalence
The prevalence of HAdVs in water samples was determined with PCR and calculated according to the following formula: (P = Np/Nt × 100), where P refers to the prevalence of HAdVs, Np denotes the number of positive samples, and Nt is the number of total samples. The following formula was calculated regarding HAdV prevalence with different meteorological factors: (MP = Np/NL × 100), where MP refers to the prevalence of HAdVs according to the meteorological factor, Np denotes the number of positive samples, and NL is the number of total samples in the investigated location. We categorized daily temperature to two groups: Low Temp (9–18 °C, 19–18 °C, 29–38 °C) and High Temp (20–29 °C, 30–39 °C, 40–49 °C). Wind speed (5–10 km/h, 11–16 km/h, 17–22 km/h, 23–28 km/h) and relative humidity (15–25%, 26–36%, 37–47%, 48–58%, 59, 69%) were also categorized.
2.7. Statistical Analysis
The XL-STAT statistical package software program (Ver. 2019, Excel Add-ins soft SARL, New York, NY, USA) was used to conduct all statistical analyses. A one-way analysis of variance was carried out to determine the significance of the correlation between temperature, relative humidity, wind speed, and the prevalence of HAdV.
3. Results
3.1. The Prevalence of Human Adenoviruses
Out of 48 water samples tested, 47 showed a positive PCR. HAdVs were present overall, with (97.9%) prevalence indicated by the detection of the 261-bp Hexon gene amplicon as shown in (Figure 2).
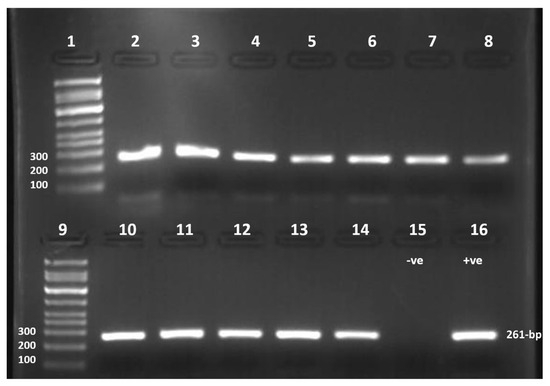
Figure 2.
PCR Product gel image. Lane 1 and 9, DNA Ladder GelPilot 100-bp Plues Ladder (100–10,000 bp), Lanes 2 to 5 for January (H-IW, AH-IW, AW-DB, DW-DB, respectively), lanes 6 to 8 and 10 for February (H-IW, AH-IW, AW-DB, DW-DB, respectively), lanes 11 to 14 for March (H-IW, AH-IW, AW-DB, DW-DB, respectively), 261-bp HAdV amplicons. Lane 15, negative control (Nuclease-free water). Lane 16, HAdV positive control (provided by King Khalid University Hospital).
The prevalence of HAdVs in the four sites was as follows: Al Harriq (100%), Al Hayer (100%), Aluraiji (91.6%) and Diriyah (100%), respectively. The presence of HAdV type F-41 in the positive samples was confirmed by Sanger sequencing.
3.2. The Predominance of Human Adenovirus F Serotype 41
All samples positive for an HAdV were sequenced and compared to sequences available in the GenBank database with the BLAST tool. Hexon gene (261-bp) amplicon sequences showed a typical relationship with serotype 41 (Type F) (Figure 3). Six separate HAdV isolates were found by pairwise distance analysis, and they all displayed a strong relationship to type 41, since they belonged to the same branch. The red italicized sequences on the phylogenetic tree (Figure 3) indicate this study’s accession numbers (OP784580, OP784581, OP784582, OP784583, OP784584, and OP784585). Furthermore, in all sites, the isolate (HAdV/SA/00-2B64/2021/IW) was the most predominant (86.4%) compared to other isolates. The genetic tree analysis showed the isolate clustering to other isolates from Brazil and Saudi Arabia. Interestingly, this isolate showed zero-distance (d = 0.00) in evolutionary divergence with the following isolates from Brazil: MH201101, MH152686, MW088515, MW088507, MT341422, MT341352, MH223626, MW088519, MH251892, and MH152673, as well as the Saudi Arabian isolate MW936375. On the other hand, the isolate (HAdV/SA/08-2B64/2021/41) showed a close relationship to an MW936373 isolate recovered in 2018 from an AnNazim landfill (d = 0.00) in Riyadh, Saudi Arabia. Moreover, (HAdV/SA/01-2B64/2021/41) and (HAdV/SA/27-2B64/2021/41) showed a close relationship to the Brazilian isolate with the accession number (MH201101) (d = 0.0038) rather than (HAdV/SA/45-2B64/2021/41) (d = 0.0078) (Supplementary Table S2).
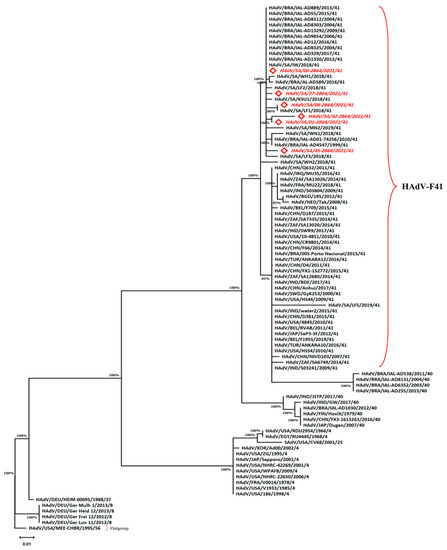
Figure 3.
Phylogenetic tree for the HAdV Hexon sequences constructed by the maximum likelihood method and Tamura 3-parameter model. The red italicized sequences indicate this study’s sequences. The outgroup refers to the highest divergent sequence. The taxa are abbreviated to the formulation: Species/Country/Isolate/Year/Type (Supplementary Table S1).
3.3. The Distribution Isolates of Human Adenovirus F Serotype 41
Remarkably, the isolate HAdV/SA/00-2B64/2021/41 with the accession numbers (OP784585) was the most observed in all different locations with various temperatures, wind speeds, and humidity, at a prevalence of (86.4%) in comparison with the other five isolates (Table 1). Otherwise, the isolates HAdV/SA/01-2B64/2021/41, HAdV/SA/08-2B64/2021/41, HAdV/SA/22-2B64/2021/41, HAdV/SA/42-2B64/2021/41, and HAdV/SA/45-2B64/2021/41 were observed once during the study period. Notably, 3 out of 6 isolates were detected in (AH-IW) (Table 2).

Table 1.
The detection of adenoviruses during a 12-month period by PCR according to location.

Table 2.
The distribution current isolates of HAdVs in 2021 with different meteorological factors.
3.4. The Influence of Metrological Variation on HAdV Prevalence
Generally, HAdVs were detected in all 12 months of the year 2021 (except for May 2021) in the (AW-DB) site. However, statistical analysis showed that meteorological factor variations were observed to have no significant effect on the prevalence of HAdV in all four sites (Table 3). The lowest temperature ranges (19–28 °C) saw the highest HAdV prevalence in the four sampling locations. Noticeably, the (H-WI) site had the highest prevalence detected at 58.3% in the temperature range 19–28 °C, with the lowest prevalence detected at 16.6% in a high temperature range of 30–39 °C. In (DW-DB), different temperature ranges showed no effect on the prevalence of HAdVs (Figure 4).

Table 3.
A statistical analysis of meteorological factors on the prevalence of HAdVs.
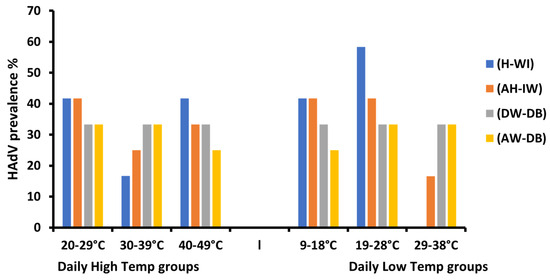
Figure 4.
The effect of the temperature (Daily High and Low) variations of HAdV prevalence on different sampling locations.
3.5. Humidity Variations’ Impact on Adenoviruses’ Prevalence
HAdVs were detected with the highest prevalence (66.66%) at the lowest relative humidity range of 15–25% in the (DW-DB) and (AW-DB) sites, whereas lowest HAdV prevalence (8.33%) was recorded at high humidity range of 59–69% in all samples’ locations. Moreover, according to the sampling schedule, no sample was taken in the range (37–47% and 48–58%) (Figure 5).
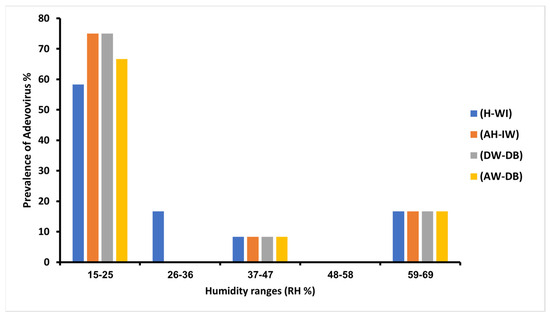
Figure 5.
The prevalence of adenoviruses in a different relative humidity range in all sample sites.
3.6. Wind Speed Impact on the Prevalence of Adenoviruses
The impact of wind speed shows no significance in our study (Table 2). However, the ranged wind speed 11–16 km/h showed the highest prevalence of HAdVs in all four sites: 50%, 50%, 50%, and 41.66% in AW-DB, DW-DB, AH-IW, and H-WI, respectively. Unlike the range 23–28 km/h, where it had the lowest prevalence of HAdVs in all sites (Figure 6).
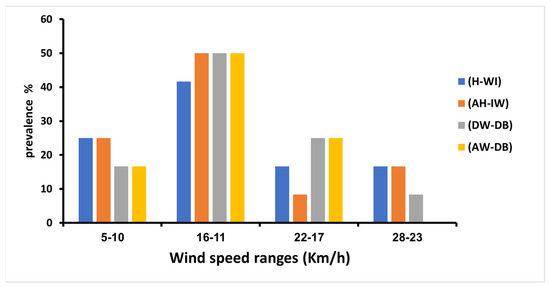
Figure 6.
Adenoviruses’ prevalence at different wind speed ranges during the period of study.
4. Discussion
Human enteric viruses may result in serious public health concerns because of their significant stability in different water matrices and low infectious doses [29,30,31]. The higher HAdV occurrence prevalence (97.9%) in our study could be due to the dewatered sludge-receiving landfills in Saudi Arabia that contaminate shallow groundwater aquifers and disseminate enteric viruses [32,33]. The detection of these viruses in irrigation water, even with small amounts, poses a serious hazard to the general populace, especially young children under the age of ten. Apart from agricultural areas, irrigation water is used occasionally for recreational purposes, such as swimming in pools, especially by this age group. In addition, the high prevalence of HAdVs is linked to the survival of double-stranded DNA and the capacity of HAdVs to persist under natural environmental settings for a long time. The frequent incidence of HAdVs were reported in several water environments involving surface water, groundwater, raw water, and even in treatment plants of wastewater. Likewise, we found HAdVs in all inspected water sources, including irrigation and groundwater sources. HAdV prevalence was previously reported in irrigation water used for restricted irrigation in the northern area of Riyadh [34]. However, the recorded HAdV prevalence of 52.78% was much lower than that detected in our study (97.9%). This mismatch may be due to spatial and temporal factors. The highest prevalence of HAdVs were found in irrigation water in comparison with groundwater. Pang et al. have reported HAdVs as the most frequently detected virus (48.9%) in private well groundwater in rural Alberta, Canada [35], whereas Sedji found a (100%) prevalence of HAdV-F41 over a six-month period in a river located in Northeastern France [36]. In another one-year study in the United Kingdom, Farkas found a prevalence of HAdVs in surface water at (88%), raw sewage water at (90%), and treated effluent at (87%) [14]. In the same manner, the frequency of HAdV detection was significantly higher in surface water (93–100%) in Nepal [37]. Additionally, HAdVs were detected in a river downstream (92%) by Pang in six rivers in Canada [31]. HAdV prevalence in raw sewage water and in Nile water was reported at 100%, whereas treated effluent water exhibited 85% HAdV prevalence [38]. The results of all the studies above are in line with our results (HAdV prevalence 97.9%) obtained during a one-year period. The higher HAdV occurrence in well-dependent discharge basins (91.6–100%) in our study could be due to the dewatered sludge-receiving landfills in Saudi Arabia that contaminate shallow groundwater aquifers and disseminate enteric viruses [32,39].
This study explored circulating HAdV serotypes in both ground and irrigation water and found the entirely recovered HAdV sequences belonging to serotype 41 of species F, which is the principal cause of infantile acute gastroenteritis [30]. Recently, Nour et al. detected HAdV type 41 in all water samples including irrigation water, wastewater treatment plant effluents, wastewater landfills, and lakes in Riyadh, Saudi Arabia, which is consistent with our findings [34]. Moreover, in the past, HAdV type 41 was documented in wastewater treatment plants in the Kingdom of Saudi Arabia and even in diarrheal patients from Mecca, Riyadh, and Jeddah, supporting this study’s observations [32,40].
Moreover, this study revealed the predominance of an HAdV sequence (90%) that had the closest relationship to a Brazilian HAdV, which could suggest it being an imported strain. A recent study conducted in Barra da Tijuca at the west coast of Rio de Janeiro in Brazil, revealed the highest prevalence of HAdV-F species (44%) in hospital sewage among the other detected species (B and D) [41]. Moreover, the growing requirements of technology and innovation in Saudi Arabia has led expatriates to represent about a quarter of the total population, which is associated with imported viral strains from foreign countries [42]. The AdV08-2B64 sequence recovered from AH-IW was found to be highly related to a previous HAdV sequence obtained from an AnNazim landfill at the east of Riyadh, Saudi Arabia [34].
Adenoviruses are characterized by their significant environmental stability and higher persistence in different water environments. Therefore, several studies have reported the insignificant seasonal variability of HAdVs [16,43].
Our findings suggest that temporal variation has no effect on the prevalence of HAdVs, and the virus’s preferred temperature ranges are between 19–28 °C. Similarly, a prior study found the maximum prevalence of HAdVs to be in a range of 22–25 °C in five sample regions in Riyadh, Saudi Arabia [34]. However, due to regional variances, HAdVs was found twice as frequently in irrigation waters in Gyeonggi Province, Korea, during the spring and summer than during the autumn. There was no discernible relationship between the seasons and the prevalence of HAdVs, and there was no discernible relationship between the prevalence of HAdVs on surveyed sites and weather factors like temperature, humidity, and wind speed [44].
Contaminated irrigation water by HAdVs is considered as a potential hazard to agricultural products during the growing and pre-harvest periods [44]. Mainly, virus contamination of irrigation water should be prevented during the pre-harvest period for the production of safe, fresh produce and products. It is important to detect adenoviruses in irrigation water because they were found to be linked with foodborne diseases at higher rates due to the use of higher quantities of contaminated irrigation water for crops and other products [45]. Our study detected HAdVs in irrigation groundwater, and previous studies indicated that irrigation water was susceptible to contamination by human fecal material [46,47]. As a result of the fact that human waste may have access to agricultural land and water, the adenovirus F41-40 is commonly detected in water used for irrigation and agricultural lands [1,3,45,48]. Foodborne outbreaks associated with raw vegetable and fruit consumption were linked to the pre-harvest contamination that occurred in the field due to contaminated irrigation water [49].
This study provides knowledge regarding HAdV prevalence, molecular serotyping, and meteorological influences in different water environments in Riyadh. The dominance of the HAdV species F type 41 was reported for the first time in groundwater in Saudi Arabia. The existence of an unexpectedly higher prevalence of HAdV sequences in irrigation water than was previously reported in Saudi Arabia demands the extensive monitoring of existing HAdV types in the future for the control of associated public health concerns and the establishment of convenient preventive measures. The current findings presented in this paper highlight the urgent need for intensified monitoring efforts to track and understand the dynamics of HAdV transmission in irrigation systems.
5. Conclusions
This study identified human adenoviruses F-41 as the predominant HAdV serotype in Riyadh, Saudi Arabia. Moreover, we discovered six different isolates in the year 2021, where the isolate HAdV/SA/00-2B64/2021/41 with accession number (OP784585) was the most dominant out of the other five isolates. In addition, genetic analysis showed a close relationship with Brazilian and Saudi isolates. However, there was no evidence of the influence of HAdVs in relation to seasonal change. Since the water in the detected regions is used for irrigation purposes, the existence of HAdVs should be considered a potential source of contamination. Increased awareness and surveillance of measurements of HAdVs would be necessary to determine public health threats and should, therefore, be considered. Future studies should be expanded by the addition of multiple locations, because HAdVs have a significance influence on public health and are directly tied to agriculture.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/w15183318/s1. Table S1: Sequences used for phylogenetic analysis of HAdV. Abbreviated name to the formulation: Species/Country/Isolate/Year/Type. Table S2: Estimates of Evolutionary Divergence between Sequences. The red borders refer to closest distance with current studied sequences. Table S3: Best fitting model selection using Maximum Likelihood fits of 24 different nucleotide substitution models.
Author Contributions
Conceptualization, I.N., A.A. and S.E.; methodology, A.A., A.E.A.-A. and I.N.; software, A.A. and A.H.; validation, I.N. and A.H.; formal analysis, A.A., A.E.A.-A. and R.A.; investigation, A.A., K.M. and Y.A.; resources, S.E.; data curation, I.N. and A.A.; writing—original draft preparation, A.A., Y.A. and R.A.; writing—review and editing, A.H., I.N. and S.E.; visualization, A.A. and A.E.A.-A.; supervision, A.H. and S.E; project administration, I.N. and S.E; funding acquisition, S.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Deputyship for research and innovation Project, number (IFKSUOR3-589-2).
Data Availability Statement
The sequences used in this study for phylogenetic analysis are openly available in the NCBI GenBank repository with accession numbers mentioned.
Acknowledgments
The authors extend their appreciation to the Deputyship for research and innovation and to the “Ministry of Education” in Saudi Arabia for funding this research (IFKSUOR3-589-2).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kokkinos, P.; Kozyra, I.; Lazic, S.; Söderberg, K.; Vasickova, P.; Bouwknegt, M.; Rutjes, S.; Willems, K.; Moloney, R.; de Roda Husman, A.M.; et al. Virological Quality of Irrigation Water in Leafy Green Vegetables and Berry Fruits Production Chains. Food Environ. Virol. 2017, 9, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Aw, T.G.; Howe, A.; Rose, J.B. Metagenomic Approaches for Direct and Cell Culture Evaluation of the Virological Quality of Wastewater. J. Virol. Methods 2014, 210, 15–21. [Google Scholar] [CrossRef]
- Ebo Yahans Amuah, E.; Amanin-Ennin, P.; Antwi, K. Irrigation Water Quality in Ghana and Associated Implications on Vegetables and Public Health. A Systematic Review. J. Hydrol. 2022, 604, 127211. [Google Scholar] [CrossRef]
- Dhingra, A.; Hage, E.; Ganzenmueller, T.; Böttcher, S.; Hofmann, J.; Hamprecht, K.; Obermeier, P.; Rath, B.; Hausmann, F.; Dobner, T.; et al. Molecular Evolution of Human Adenovirus (HAdV) Species C. Sci. Rep. 2019, 9, 1039. [Google Scholar] [CrossRef]
- Hassou, N.; Bouseettine, R.; Abouchoaib, N.; Ennaji, M.M. Enteric Adenoviruses: Emerging of a Public Health Threat. In Emerging and Reemerging Viral Pathogens: Volume 1: Fundamental and Basic Virology Aspects of Human, Animal and Plant Pathogens; Elsevier: Amsterdam, The Netherlands, 2019; pp. 879–905. ISBN 9780128194003. [Google Scholar]
- Yoshitomi, H.; Sera, N.; Gonzalez, G.; Hanaoka, N.; Fujimoto, T. First Isolation of a New Type of Human Adenovirus (Genotype 79), Species Human Mastadenovirus B (B2) from Sewage Water in Japan. J. Med. Virol. 2017, 89, 1192–1200. [Google Scholar] [CrossRef]
- Yang, Z.; Zhu, Z.; Tang, L.; Wang, L.; Tan, X.; Yu, P.; Zhang, Y.; Tian, X.; Wang, J.; Zhang, Y.; et al. Genomic Analyses of Recombinant Adenovirus Type 11a in China. J. Clin. Microbiol. 2009, 47, 3082–3090. [Google Scholar] [CrossRef]
- Rabaan, A.A.; Bakhrebah, M.A.; Nassar, M.S.; Natto, Z.S.; Al Mutair, A.; Alhumaid, S.; Aljeldah, M.; Garout, M.; Alfouzan, W.A.; Alshahrani, F.S.; et al. Suspected Adenovirus Causing an Emerging HEPATITIS among Children below 10 Years: A Review. Pathogens 2022, 11, 712. [Google Scholar] [CrossRef]
- Meqdam, M.M.; Thwiny, I.R. Prevalence of group a rotavirus, enteric adenovirus, norovirus and astrovirus infections among children with acute gastroenteritis in Al-Qassim, Saudi Arabia. Pak. J. Med. Sci. 2007, 23, 551–555. [Google Scholar]
- Saeed, M.; Al-Ayed, Z.; Asaad, A.M.; Qureshi, M.A.; Saeed, M.; Alayed, Z.; Morad Asaad, A.; Mahdi, A.A. Aetiology of Acute Gastroenteritis in Children in Najran Region, Saudi Arabia. J. Health Spec. 2013, 1, 84. [Google Scholar]
- Khalaf, R.J.; Fadhil, H.Y.; Auf, I.M.; Namdar, S.A. Molecular Diagnosis of Human Adenovirus in Children with Upper Respiratory Tract Infections. IOSR J. Pharm. Biol. Sci. 2017, 12, 9–13. [Google Scholar] [CrossRef]
- Rames, E.; Roiko, A.; Stratton, H.; Macdonald, J. Technical Aspects of Using Human Adenovirus as a Viral Water Quality Indicator. Water Res. 2016, 96, 308–326. [Google Scholar] [CrossRef]
- Nour, I.; Hanif, A.; Ryan, M.; Eifan, S. Insights into Gastrointestinal Virome: Etiology and Public Exposure. Water 2021, 13, 2794. [Google Scholar] [CrossRef]
- Farkas, K.; Cooper, D.M.; McDonald, J.E.; Malham, S.K.; de Rougemont, A.; Jones, D.L. Seasonal and Spatial Dynamics of Enteric Viruses in Wastewater and in Riverine and Estuarine Receiving Waters. Sci. Total Environ. 2018, 634, 1174–1183. [Google Scholar] [CrossRef]
- Umuhoza, T.; Bulimo, W.D.; Oyugi, J.; Musabyimana, J.P.; Kinengyere, A.A.; Mancuso, J.D. Prevalence of Human Respiratory Syncytial Virus, Parainfluenza and Adenoviruses in East Africa Community Partner States of Kenya, Tanzania, and Uganda: A Systematic Review and Meta-Analysis (2007–2020). PLoS ONE 2021, 16, e0249992. [Google Scholar] [CrossRef]
- Elmahdy, E.M.; Shaheen, M.N.F.; Rizk, N.M.; Saad-Hussein, A. Quantitative Detection of Human Adenovirus and Human Rotavirus Group A in Wastewater and El-Rahawy Drainage Canal Influencing River Nile in the North of Giza, Egypt. Food Environ. Virol. 2020, 12, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Saleh, S.; Almalki, R. Molecular Detection of Hepatitis A Virus and Rotavirus in Water Samples Collected from Albaha, Saudi Arabia. Egypt. Acad. J. Biol. Sci. C Physiol. Mol. Biol. 2018, 10, 59–68. [Google Scholar]
- Waso, M.; Khan, S.; Khan, W. Microbial Source Tracking Markers Associated with Domestic Rainwater Harvesting Systems: Correlation to Indicator Organisms. Environ. Res. 2018, 161, 446–455. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking-Water Quality: First Addendum to the Third Edition, Volume 1: Reccommendations; World Health Organization: Geneva, Switzerland, 2006; ISBN 9241546964.
- US EPA. Drinking Water Contaminant List 5—Final; Federal Register Notice: Drinking Water Contaminant Candidate List 5 2022, EPA 815-F-22-005; EPA: Washington, DC, USA, 2022.
- Silva, H.D.; García-Zapata, M.T.A.; Anunciação, C.E. Why the Use of Adenoviruses as Water Quality Virologic Marker? Food Environ. Virol. 2011, 3, 138–140. [Google Scholar] [CrossRef]
- MEWA. Statistics Book 2021; Ministry of Environment Water and Agriculture: Riyadh, Saudi Arabia, 2021.
- Mena, K.D.; Gerba, C.P. Waterborne Adenovirus. Rev. Environ. Contam. Toxicol. 2009, 198, 133–167. [Google Scholar]
- Beaudeau, P.; de Valk, H.; Vaillant, V.; Mannschott, C.; Tillier, C.; Mouly, D.; Ledrans, M. Lessons Learned from Ten Investigations of Waterborne Gastroenteritis Outbreaks, France, 1998–2006. J. Water Health 2008, 6, 491–503. [Google Scholar] [CrossRef]
- Hjelmsø, M.H.; Hellmér, M.; Fernandez-Cassi, X.; Timoneda, N.; Lukjancenko, O.; Seidel, M.; Elsässer, D.; Aarestrup, F.M.; Löfström, C.; Bofill-Mas, S.; et al. Evaluation of Methods for the Concentration and Extraction of Viruses from Sewage in the Context of Metagenomic Sequencing. PLoS ONE 2017, 12, e0170199. [Google Scholar] [CrossRef]
- Maniah, K.; Nour, I.; Hanif, A.; Yassin, M.T.; Alkathiri, A.; Al-Ashkar, I.; Eifan, S. Molecular Identification of Human Adenovirus Isolated from Different Wastewater Treatment Plants in Riyadh, Saudi Arabia: Surveillance and Meteorological Impacts. Water 2023, 15, 1367. [Google Scholar] [CrossRef]
- Dey, R.S.; Ghosh, S.; Chawla-Sarkar, M.; Panchalingam, S.; Nataro, J.P.; Sur, D.; Manna, B.; Ramamurthy, T. Circulation of a Novel Pattern of Infections by Enteric Adenovirus Serotype 41 among Children below 5 Years of Age in Kolkata, India. J. Clin. Microbiol. 2011, 49, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Bertrand, I.; Schijven, J.F.; Sánchez, G.; Wyn-Jones, P.; Ottoson, J.; Morin, T.; Muscillo, M.; Verani, M.; Nasser, A.; de Roda Husman, A.M.; et al. The Impact of Temperature on the Inactivation of Enteric Viruses in Food and Water: A Review. J. Appl. Microbiol. 2012, 112, 1059–1074. [Google Scholar] [CrossRef]
- Iaconelli, M.; Muscillo, M.; Della Libera, S.; Fratini, M.; Meucci, L.; De Ceglia, M.; Giacosa, D.; La Rosa, G. One-Year Surveillance of Human Enteric Viruses in Raw and Treated Wastewaters, Downstream River Waters, and Drinking Waters. Food Environ. Virol. 2017, 9, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Qiu, Y.; Pang, X.L.; Ashbolt, N.J. Spiked Virus Level Needed to Correctly Assess Enteric Virus Recovery in Water Matrices. Appl. Environ. Microbiol. 2019, 85, e00111-19. [Google Scholar] [CrossRef]
- Jumat, M.R.; Hasan, N.A.; Subramanian, P.; Heberling, C.; Colwell, R.R.; Hong, P.Y. Membrane Bioreactor-Basedwastewater Treatment Plant in Saudi Arabia: Reduction of Viral Diversity, Load, and Infectious Capacity. Water 2017, 9, 534. [Google Scholar] [CrossRef]
- Silva, D.; Luz, D.; der Sand, V. First Description of Adenovirus, Enterovirus, Rotavirus and Torque Teno Virus in Water Samples Collected from the Arroio Dilúvio, Porto Alegre, Brazil. Braz. J. Biol. 2012, 72, 323–329. [Google Scholar]
- Nour, I.; Hanif, A.; Zakri, A.M.; Al-Ashkar, I.; Alhetheel, A.; Eifan, S. Human Adenovirus Molecular Characterization in Various Water Environments and Seasonal Impacts in Riyadh, Saudi Arabia. Int. J. Environ. Res. Public Health 2021, 18, 4773. [Google Scholar] [CrossRef]
- Pang, X.; Gao, T.; Qiu, Y.; Caffrey, N.; Popadynetz, J.; Younger, J.; Lee, B.E.; Neumann, N.; Checkley, S. The Prevalence and Levels of Enteric Viruses in Groundwater of Private Wells in Rural Alberta, Canada. Water Res. 2021, 202, 117425. [Google Scholar] [CrossRef] [PubMed]
- Sedji, M.I.; Varbanov, M.; Meo, M.; Colin, M.; Mathieu, L.; Bertrand, I. Quantification of Human Adenovirus and Norovirus in River Water in the North-East of France. Environ. Sci. Pollut. Res. 2018, 25, 30497–30507. [Google Scholar] [CrossRef] [PubMed]
- Haramoto, E.; Kitajima, M.; Hata, A.; Torrey, J.R.; Masago, Y.; Sano, D.; Katayama, H. A Review on Recent Progress in the Detection Methods and Prevalence of Human Enteric Viruses in Water. Water Res. 2018, 135, 168–186. [Google Scholar] [CrossRef] [PubMed]
- Rashed, M.K.; El-Senousy, W.M.; Sayed, E.S.T.A.E.S.; AlKhazindar, M. Infectious Pepper Mild Mottle Virus and Human Adenoviruses as Viral Indices in Sewage and Water Samples. Food Environ. Virol. 2022, 14, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Mallick, J.; Singh, C.K.; Almesfer, M.K.; Singh, V.P.; Alsubih, M. Groundwater Quality Studies in the Kingdom of Saudi Arabia: Prevalent Research and Management Dimensions. Water 2021, 13, 1266. [Google Scholar] [CrossRef]
- Tayeb, H.T.; Dela Cruz, D.M.; Al-Qahtani, A.; Al-Ahdal, M.N.; Carter, M.J. Enteric Viruses in Pediatric Diarrhea in Saudi Arabia. J. Med. Virol. 2008, 80, 1919–1929. [Google Scholar] [CrossRef]
- da Graça Pedrosa de Macena, L.; Baur Vieira, C.; Gonçalves Maranhão, A.; César Ferreira, F.; Sampaio Lemos, E.R.; Miagostovich, M.P. Genetic Diversity and Quantification of Human Adenoviruses and JC Polyomaviruses in Wastewater Samples. Int. J. Plant Anim. Environ. Sci. 2021, 11, 614–626. [Google Scholar] [CrossRef]
- Khraif, R.M.; Salam, A.A.; Nair, P.S.; Elsegaey, I. Migration in Saudi Arabia: Present and Prospects. In India’s Low-Skilled Migration to the Middle East: Policies, Politics and Challenges; Palgrave Macmillan: Singapore, 2019. [Google Scholar]
- Fong, T.T.; Phanikumar, M.S.; Xagoraraki, I.; Rose, J.B. Quantitative Detection of Human Adenoviruses in Wastewater and Combined Sewer Overflows Influencing a Michigan River. Appl. Environ. Microbiol. 2010, 76, 715–723. [Google Scholar] [CrossRef]
- Wang, Z.; Shin, H.; Jung, S.; Yeo, D.; Park, H.; Shin, S.; Seo, D.J.; Park, K.H.; Choi, C. Effects of Weather and Environmental Factors on the Seasonal Prevalence of Foodborne Viruses in Irrigation Waters in Gyeonggi Province, Korea. Microorganisms 2020, 8, 1224. [Google Scholar] [CrossRef]
- Shaheen, M.N.F.; Elmahdy, E.M.; Chawla-Sarkar, M. Quantitative PCR-Based Identification of Enteric Viruses Contaminating Fresh Produce and Surface Water Used for Irrigation in Egypt. Environ. Sci. Pollut. Res. 2019, 26, 21619–21628. [Google Scholar] [CrossRef]
- Wei, J.; Kniel, K.E. Pre-Harvest Viral Contamination of Crops Originating from Fecal Matter. Food Environ. Virol. 2010, 2, 195–206. [Google Scholar] [CrossRef]
- Jung, J.H.; Yoo, C.H.; Koo, E.S.; Kim, H.M.; Na, Y.; Jheong, W.H.; Jeong, Y.S. Occurrence of Norovirus and Other Enteric Viruses in Untreated Groundwaters of Korea. J. Water Health 2011, 9, 544–555. [Google Scholar] [CrossRef] [PubMed]
- Cheong, S.; Lee, C.; Song, S.W.; Choi, W.C.; Lee, C.H.; Kim, S.J. Enteric Viruses in Raw Vegetables and Groundwater Used for Irrigation in South Korea. Appl. Environ. Microbiol. 2009, 75, 7745–7751. [Google Scholar] [CrossRef] [PubMed]
- Bintsis, T. Microbial Pollution and Food Safety. AIMS Microbiol. 2018, 4, 377–396. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).