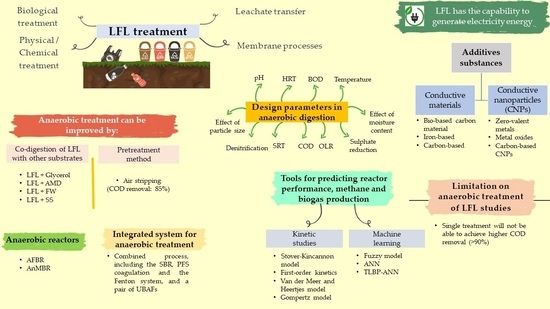
State of the Art in Anaerobic Treatment of Landfill Leachate: A Review on Integrated System, Additive Substances, and Machine Learning Application
Abstract
1. Introduction
2. Anaerobic Co-Digestion of Leachate with Industrial Wastewater and Solid Waste
2.1. Anaerobic Co-Digestion of Leachate with Industrial Wastewater
2.1.1. Anaerobic Co-Digestion of Landfill Leachate and Crude Glycerol
| Types of Anaerobic Digestion | HRT (Hours) | Volume of Leachate | COD Removal Efficiency (%) | BOD/COD | Specific Biogas Production (mL/g VSS) | Specific Biomethane Production (mL CH4/g VSS) | Methane Production | Reference |
|---|---|---|---|---|---|---|---|---|
| Anaerobic Co-Digestion of Leachate with Industrial Wastewater | ||||||||
| Anaerobic co-digestion leachate and crude glycerol (crude glycerol content: 1.50%) | 720 | - | 92.03 (Soluble COD) | 312.37 | 244.59 | 78.3% | [38] | |
| Anaerobic co-digestion of landfill leachate and acid mine drainage | 20 | - | 83 | - | - | 1805 (mL/d) | [45] | |
| Anaerobic co-digestion of leachate with solid waste | ||||||||
| Anaerobic co-digestion of food waste and landfill leachate | 840 | 568 mL | - | 1.48 | 878 | - | 466 mL/g VS | [46] |
| Anaerobic co-digestion of sewage sludge with landfill leachate | 319.2 | 100 (mL/d) | - | 1.07 | - | - | 375 L | [47] |
| Leachate | Crude Glycerol | References |
|---|---|---|
|
| [38] |
|
| [38,42] |
|
| [38,42,43] |
|
| [37,38,44] |
|
| [38,43,44] |
| Leachate | Acid mine drainage | References |
|
| [45] |
|
| |
| Leachate | Food waste | References |
|
| [46] |
|
| |
|
| |
| Leachate | Sewage sludge | References |
| Optimum mixing ratio: 20:80 | [47] | |
2.1.2. Anaerobic Co-Digestion of Landfill Leachate with Acid Mine Drainage
2.2. Anaerobic Co-Digestion of Leachate with Solid Waste
2.2.1. Anaerobic Co-Digestion of Food Waste and Landfill Leachate
2.2.2. Anaerobic Co-Digestion of Sewage Sludge and Landfill Leachate
3. Pre-Treatment of Landfill Leachate
3.1. Coagulation/Fenton/Air Stripping
3.2. Electrochemical Oxidation
3.3. Coagulation-Adsorption
| Type of Pre-Treatment | Coagulation/Fenton/Air Stripping | Remarks/Scale of Study | References | |||
| Parameter | ||||||
| pH | COD removal (%) | Biogas yield (mL/g CODin) | ||||
| Coagulation-flocculation | 7.96 | 75 | 370.90 |
| [6] | |
| Fenton’s oxidation | 8.09 | 77 | 448.00 | |||
| Air stripping | 8.29 | 85 | 588.88 | |||
| Raw LFL | 7.93 | 68 | 163.69 | |||
| Type of pre-treatment | Electrochemical Oxidation (EO) | |||||
| Parameter | ||||||
| Leachate (mL) | Inoculum (mL) | Methane yields (NL/g sCOD removed) | Methane content (%) | |||
| Control: |
| [59] | ||||
| System 1 | 350 | 200 | 0.1712 | 48 | ||
| Assisted with EO pre-treatment: | ||||||
| System 2 | 350 | 200 | 0.2925 | 54 | ||
| Type of pre-treatment | Coagulation and Adsorption | |||||
| Parameter | ||||||
| Coagulation: | ||||||
| Type of leachate | Ferric chloride dosage (g/L) | COD removal (%) | Alum dosage (g/L) | COD removal (%) |
| [62] |
| Old leachate | 0.7 | 59% | 0.6 | 75% | ||
| Young leachate | 0.6 | 35% | 0.8 | 55% | ||
| Adsorption: | ||||||
| Type of leachate | Fly ash dosage (g/L) | COD removal (%) | ||||
| Old leachate | 6 | 28% | ||||
4. Anaerobic Digestion of Landfill Leachate
4.1. Anaerobic Reactor
4.2. Design Parameters in LFL Anaerobic Treatment
5. Integrated System for Anaerobic Treatment of Landfill Leachate
| Type of Integrated System | Type of Leachate | Pollutant Content | Removal Efficiency | Remarks | References |
|---|---|---|---|---|---|
| Integrated Electrocoagulation (EC) a and the Anaerobic Treatment b | Old |
|
|
| [72] |
| Integrated treatment via Active Filtration a and Anaerobic Digestion b | Old |
| ZVI/lapillus:
|
| [73] |
| Anoxic/aerobic granular active carbon assisted MBR a integrated with nanofiltration and reverse osmosis b (A/O-GAC–MBR integrated with NF and RO membranes) | Old |
|
|
| [74] |
| Ozone direct oxidation pre-treatment a and catalytic oxidation post-treatment coupled with anaerobic baffled membrane bioreactor (ABMBR) b | Old |
|
|
| [75] |
| Combined process including sequencing batch reactor (SBR) c, with polyferric sulfate (PFS) coagulation and the Fenton system d, and a pair of up-flow biological aerated filters (UBAFs) e | Old |
|
|
| [76] |
| Anaerobic digestion a combined with coagulation and flocculation (CF) b using ferric chloride as coagulant and cationic polymer as flocculant | Young |
|
|
| [77] |
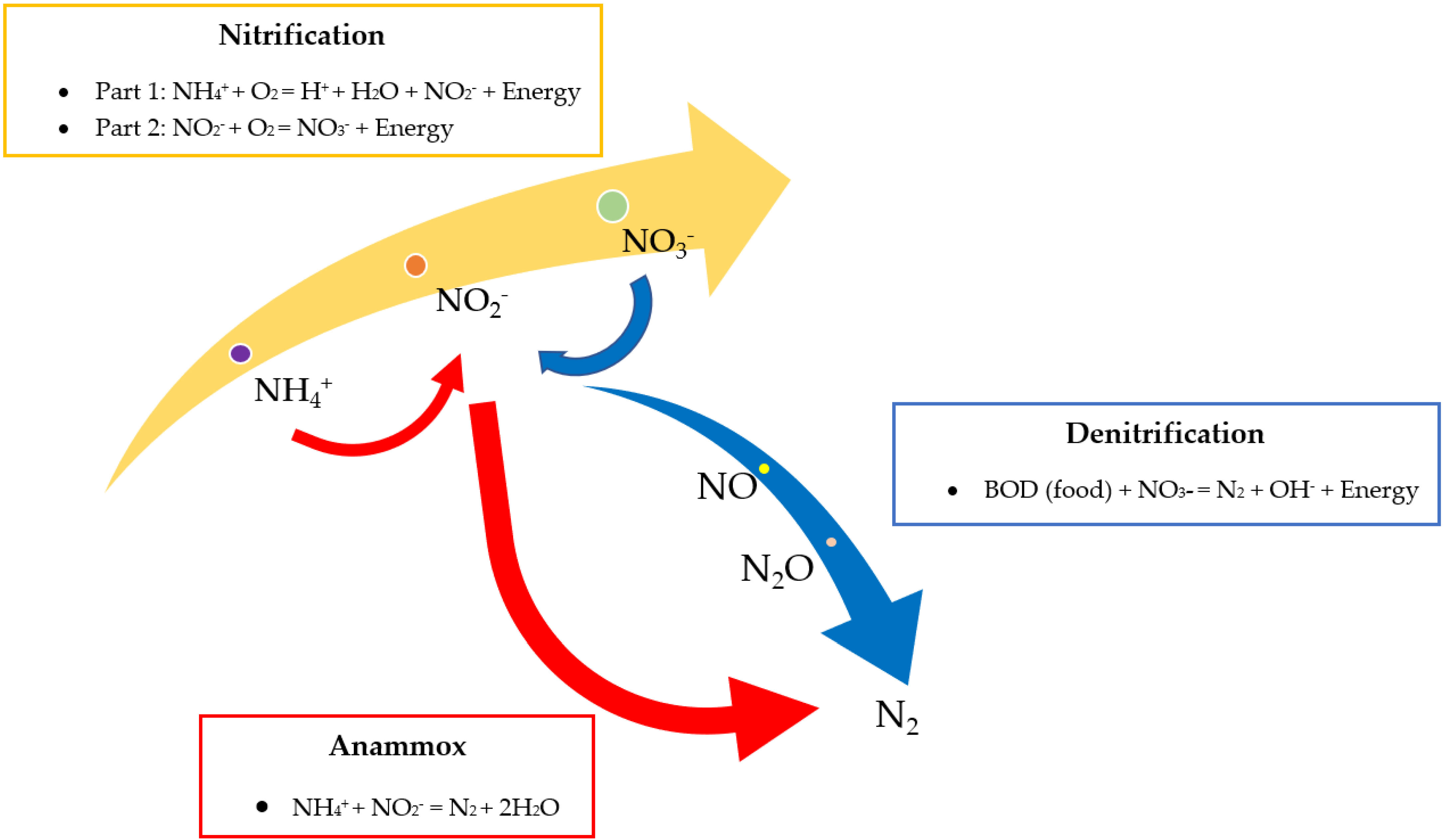
Post Treatment for Ammonia Removal in Landfill Leachate
6. Potential Inhibitors in the Anaerobic Treatment of Landfill Leachate
7. Application of Additive Substances into Anaerobic System
| Conductive Materials | Conductive Nanoparticles (CNPs) | ||||
|---|---|---|---|---|---|
| Type | Concentration | Performance | Type | Concentration | Performance |
| Bio-based carbon material |
|
| Zero-valent metals |
|
|
|
|
|
| ||
| Iron-based |
|
| Metal oxides |
|
|
|
|
|
| ||
| Carbon-based |
|
| Carbon-based conductive nanoparticles |
|
|
|
|
|
| ||
8. Kinetic and Machine Learning Evaluation
| Type of Model | Purpose | Experimental Result | Parameters | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Kinetic Coefficient/(Umax) | R2/Regression R Value | Mean (SD) | RMSE/ MSE | IA | FV | Predicted Results | Remarks | References | |||
| First-order model, Stover-Kincannon, Modified Stover-Kincannon, and Van der Meer and Heertjes | First-order model and Stover-Kincannon were used to investigate the kinetics of COD removal via AMBR biological process | Effluent (observed) COD: 1850 mg/L (OLR = 1.04 g COD/L.d), and 25,000 mg/L (OLR = 19.65 g COD/L.d) Mean (SD) for Effluent COD: 11,188 (8644) mg/L | - | R2 First-order model: 0.926 R2 Stover-Kincannon: 0.999 | First-order model: 9903 (9078) mg/L Stover- Kincannon: 11,025 (8489) mg/L | - | - | - | Predicted COD: First-order model: 1582 mg/L (OLR = 1.04 g COD/L.d), and 27,018 mg/L (OLR = 19.65 g COD/L.d) Stover- Kincannon: 1852 mg/L (OLR = 1.04 g COD/L.d), and 24,038 mg/L (OLR = 19.65 g COD/L.d) |
| [114] |
| Modified Stover-Kincannon and Van der Meer and Heertjes were used to check the kinetic constants of biogas and methane gas production | Biogas: 769 mL/d (OLR = 1.04 g COD/L.d), and 10,470 mL/d (OLR = 18.52 g COD/L.d) Mean (SD) biogas: 4613 (3517) Methane: 423 mL/d (OLR = 1.04 g COD/L.d), and 6177 mL/d (OLR = 18.52 g COD/L.d) Mean (SD) methane: 2705 (2010) | - | R2 Modified Stover-Kincannon; R2 Biogas: 0.947907 Methane: 0.934727 R2 Van der Meer and Heertjes; R2 Methane: 0.9095 | Modified Stover-Kincannon; Biogas: 3845 (3130) Methane: 1928 (1453) Van der Meer and Heertjes; Methane: 2101 (1915) | - | - | - | Predicted biogas and methane: Modified Stover- Kincannon;
|
| - | |
| Gompertz model | To predict methane production | Measured Biochemical methane potential (BMP): 78.39 mL/g vs. removed | Umax: 11.28 mL/g vs. removed.d | R2: 0.994 | - | - | - | - | Predicted BMP: 77.98 mL/g vs. removed |
| [59] |
| Fuzzy-based model and Gompertz model | To predict biogas and methane production | - | - | R1 (with nano-ZnO):
| - | RSME: R1 (with nano-ZnO):
| R1 (with nano-ZnO):
| - | - |
| [111] |
| Three Layer Back Propagation Artificial Neural Network model (TLBP-ANN) | To determine effective substrate concentration and maximum biogas yield |
| - | R2: 0.9703 | - | - | 0.9882 | 0.0014 | Best linear fit function = 0.9779 experimental + 1.1679, R2 0.97045 |
| [112] |
| First-order kinetic (biodegradability) and dynamic activated sludge model (COD removal) | To predict leachate biodegradation and effluent COD in aerobic biological treatment |
|
| - | - | - | - | - | - |
| [115] |
| Artificial neural network (ANN) | To predict biogas production from co-digestion of leachate and pineapple peel | - | - | R Values:
| - | MSE:
| - | - |
|
| [113] |
9. Energy Generation from Landfill Leachate Treatment
10. Limitations and Strategies
11. Conclusions and Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zayen, A.; Mnif, S.; Jlaeïl, L.; Bouaziz, M.; Sayadi, S. Phthalates accumulation inside an anaerobic membrane bioreactor for landfill leachate treatment. Desalination Water Treat. 2015, 53, 1136–1143. [Google Scholar] [CrossRef]
- Bove, D.; Merello, S.; Frumento, D.; Arni, S.; Aliakbarian, B.; Converti, A. A Critical Review of Biological Processes and Technologies for Landfill Leachate Treatment. Chem. Eng. Technol. 2015, 38, 2115–2126. [Google Scholar] [CrossRef]
- Karak, T.; Bhagat, R.M.; Bhattacharyya, P. Municipal solid waste generation, composition, and management: The World Scenario. Crit. Rev. Environ. Sci. Technol. 2012, 42, 1509–1630. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, L.; Xu, Y.; Liang, C.; Kong, H.; Shi, X.; Peng, Y. Advanced nitrogen removal using bio-refractory organics as carbon source for biological treatment of landfill leachate. Sep. Purif. Technol. 2016, 170, 306–313. [Google Scholar] [CrossRef]
- Bashir, M.J.K.; Isa, M.H.; Kutty, S.R.; Awang, Z.B.; Aziz, H.A.; Mohajeri, S.; Farooqi, I.H. Landfill leachate treatment by electrochemical oxidation. Waste Manag. 2009, 29, 2534–2541. [Google Scholar] [CrossRef]
- Smaoui, Y.; Mlaik, N.; Bouzid, J.; Sayadi, S. Improvement of anaerobic digestion of landfill leachate by using coagulation-flocculation, Fenton’s oxidation and air stripping pretreatments. Environ. Prog. Sustain. Energy 2017, 37, 1041–1049. [Google Scholar] [CrossRef]
- Thomas, M.; Kozik, V.; Barbusiński, K.; Sochanik, A.; Jampilek, J.; Bąk, A. Potassium ferrate (VI) as the multifunctional agent in the treatment of landfill leachate. Materials 2020, 13, 5017. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Tiwary, D.; Ohri, A.; Agnihotri, A.K. Impact of municipal solid waste landfill leachate on groundwater quality in Varanasi, India. Groundw. Sustain. Dev. 2019, 9, 100230. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Chan GY, S.; Lo, W.-H.; Babel, S. Physico–chemical treatment techniques for wastewater laden with heavy metals. Chem. Eng. J. 2006, 118, 83–98. [Google Scholar] [CrossRef]
- Guo, J.-S.; Abbas, A.A.; Chen, Y.-P.; Liu, Z.-P.; Fang, F.; Chen, P. Treatment of landfill leachate using a combined stripping, Fenton, SBR, and coagulation process. J. Hazard. Mater. 2010, 178, 699–705. [Google Scholar] [CrossRef]
- Aendo, P.; Netvichian, R.; Thiendedsakul, P.; Khaodhiar, S.; Tulayakul, P. Carcinogenic risk of PB, Cd, Ni, and Cr and critical ecological risk of CD and CU in soil and groundwater around the municipal solid waste open dump in central Thailand. J. Environ. Public Health 2022, 2022, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Abdullah, N.; Chelliapan, S.; Yuzir, A.; Koji, I.; Al-Dailami, A.; Arumugham, T. Strategies of Sustainable Solid Waste Management; IntechOpen: UTM Kuala Lumpur, Malaysia, 2020. [Google Scholar]
- Nawaz, T.; Rahman, A.; Pan, S.; Dixon, K.; Petri, B.; Selvaratnam, T. A review of landfill leachate treatment by microalgae: Current status and Future Directions. Processes 2020, 8, 384. [Google Scholar] [CrossRef]
- Renou, S.; Givaudan, J.G.; Poulain, S.; Dirassouyan, F.; Moulin, P. Landfill leachate treatment: Review and opportunity. J. Hazard. Mater. 2008, 150, 468–493. [Google Scholar] [CrossRef] [PubMed]
- Jagaba, A.H.; Kutty, S.R.M.; Lawal, I.M.; Abubakar, S.; Hassan, I.; Zubairu, I.; Umaru, I.; Abdurrasheed, A.S.; Adam, A.A.; Ghaleb, A.A.S.; et al. Sequencing batch reactor technology for landfill leachate treatment: A state-of-the-art review. J. Environ. Manag. 2021, 282, 111946. [Google Scholar] [CrossRef]
- Spagni, A.; Marsili-Libelli, S.; Lavagnolo, M.C. Optimisation of sanitary landfill leachate treatment in a sequencing batch reactor. Water Sci. Technol. 2008, 58, 337–343. [Google Scholar] [CrossRef]
- Abbas, A.A.; Jingsong, G.; Ping, L.Z.; Ya, P.Y.; Al-Rekabi, W.S. Review on landfill leachate treatments. J. Appl. Sci. Res. 2009, 5, 534–545. [Google Scholar] [CrossRef]
- Aziz, S.Q.; Aziz, H.A.; Mojiri, A.; Bashir MJ, K.; Amr, S.S. Landfill leachate Treatment Using Sequencing Batch Reactor (SBR) Process: Limitation of Operational Parameters and Performance. Int. J. Sci. Res. Knowl. 2013, 1, 34–43. [Google Scholar] [CrossRef]
- Loukidou, M.X.; Zouboulis, A.I. Comparison of two biological treatment processes using attached-growth biomass for sanitary landfill leachate treatment. Environ. Pollut. 2001, 111, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Li, L.; Tan, F.; Wu, D. Treatment of Landfill Leachate Using Activated Sludge Technology: A Review. Archaea 2018, 2018, 1039453. [Google Scholar] [CrossRef]
- Mehmood, M.K.; Adetutu, E.; Nedwell, D.B.; Ball, A.S. In situ microbial treatment of landfill leachate using aerated lagoons. Bioresour. Technol. 2009, 100, 2741–2744. [Google Scholar] [CrossRef]
- Castillo, E.; Vergara, M.; Moreno, Y. Landfill leachate treatment using a rotating biological contactor and an upward-flow anaerobic sludge bed reactor. Waste Manag. 2007, 27, 720–726. [Google Scholar] [CrossRef]
- Cortez, S.; Teixeira, P.; Oliveira, R.; Mota, M. Rotating biological contactors: A review on main factors affecting performance. Rev. Environ. Sci. Bio/Technol. 2008, 7, 155–172. [Google Scholar] [CrossRef]
- Heavey, M. Low-cost treatment of landfill leachate using peat. Waste Manag. 2003, 23, 447–454. [Google Scholar] [CrossRef]
- Ozturk, I.; Altinbas, M.; Koyuncu, I.; Arikan, O.; Gomec-Yangin, C. Advanced physico-chemical treatment experiences on young municipal landfill leachates. Waste Manag. 2003, 23, 441–446. [Google Scholar] [CrossRef]
- Silva, A.C.; Dezotti, M.; Sant’Anna, G.L., Jr. Treatment and detoxification of a sanitary landfill leachate. Chemosphere 2004, 55, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Tatsi, A.A.; Zouboulis, A.I.; Matis, K.A.; Samaras, P. Coagulation–Flocculation pretreatment of sanitary landfill leachates. Chemosphere 2003, 53, 737–744. [Google Scholar] [CrossRef]
- Marttinen, S.K.; Kettunen, R.H.; Sormunen, K.M.; Soimasuo, R.M.; Rintala, J. Screening of physical–chemical methods for removal of organic material, nitrogen and toxicity from low strength landfill leachates. Chemosphere 2002, 46, 851–858. [Google Scholar] [CrossRef]
- Lim, C.K.; Seow, T.W.; Neoh, C.H.; Md Nor, M.H.; Ibrahim, Z.; Ware, I.; Mat Sarip, S.H. Treatment of landfill leachate using ASBR combined with zeolite adsorption technology. 3 Biotech 2016, 6, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hilles, A.H.; Abu Amr, S.S.; Hussein, R.A.; El-Sebaie, O.D.; Arafa, A.I. Performance of combined sodium persulfate/H2O2 based advanced oxidation process in stabilized landfill leachate treatment. J. Environ. Manag. 2016, 166, 493–498. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, H.; Zhang, P.; Wu, Y.; Gou, X.; Song, Y.; Tian, Z.; Zeng, G. Two-stage anoxic/oxic combined membrane bioreactor system for landfill leachate treatment: Pollutant removal performances and microbial community. Bioresour. Technol. 2017, 243, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Marzuki, T.N.; Idrus, S.; Musa, M.A.; Wahab, A.M.; Jamali, N.S.; Man, H.C.; Ng, S.N. Enhancement of bioreactor performance using acclimatised seed sludge in anaerobic treatment of chicken slaughterhouse wastewater: Laboratory Achievement, Energy Recovery, and Its Commercial-scale Potential. Animals 2021, 11, 3313. [Google Scholar] [CrossRef] [PubMed]
- Jaman, K.; Amir, N.; Musa, M.A.; Zainal, A.; Yahya, L.; Abdul Wahab, A.M.; Suhartini, S.; Tuan Mohd Marzuki, T.N.; Harun, R.; Idrus, S. Anaerobic Digestion, Codigestion of Food Waste, and Chicken Dung: Correlation of Kinetic Parameters with Digester Performance and On-Farm Electrical Energy Generation Potential. Fermentation 2022, 8, 28. [Google Scholar] [CrossRef]
- Wijetunga, S.; Li, X.-F.; Jian, C. Effect of organic load on decolourization of textile wastewater containing acid dyes in upflow anaerobic sludge blanket reactor. J. Hazard. Mater. 2010, 177, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Nanda, S.; Reddy, S.N.; Mitra, S.K.; Kozinski, J.A. The progressive routes for carbon capture and sequestration. Energy Sci. Eng. 2016, 4, 99–122. [Google Scholar] [CrossRef]
- Bhatt, A.H.; Tao, L. Economic perspectives of Biogas Production via Anaerobic Digestion. Bioengineering 2020, 7, 74. [Google Scholar] [CrossRef]
- Begum, S.; Anupoju, G.R.; Sridhar, S.; Bhargava, S.K.; Jegatheesan, V.; Eshtiaghi, N. Evaluation of single and two stage anaerobic digestion of landfill leachate: Effect of pH and initial organic loading rate on volatile fatty acid (VFA) and biogas production. Bioresour. Technol. 2018, 251, 364–373. [Google Scholar] [CrossRef]
- Takeda, P.Y.; Gotardo, J.T.; Gomes, S.D. Anaerobic co-digestion of leachate and glycerol for renewable energy generation. Environ. Technol. 2020, 43, 1118–1128. [Google Scholar] [CrossRef]
- Miyuranga, K.A.; Arachchige, U.S.; Jayasinghe, R.A.; Samarakoon, G. Purification of residual glycerol from biodiesel production as a value-added raw material for glycerolysis of free fatty acids in waste cooking oil. Energies 2022, 15, 8856. [Google Scholar] [CrossRef]
- Binhayeeding, N.; Klomklao, S.; Sangkharak, K. Utilization of waste glycerol from biodiesel process as a substrate for mono-, di-, and triacylglycerol production. Energy Procedia 2017, 138, 895–900. [Google Scholar] [CrossRef]
- Chilakamarry, C.R.; Sakinah, A.M.; Zularisam, A.W.; Pandey, A. Glycerol waste to value added products and its potential applications. Syst. Microbiol. Biomanuf. 2021, 1, 378–396. [Google Scholar] [CrossRef]
- Guven, H.; Akca, M.S.; Iren, E.; Keles, F.; Ozturk, I.; Altinbas, M. Co-digestion performance of organic fraction of municipal solid waste with leachate: Preliminary studies. Waste Manag. 2018, 71, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Silvestre, G.; Bonmatí, A.; Fernández, B. Optimisation of sewage sludge anaerobic digestion through co-digestion with OFMSW: Effect of collection system and particle size. Waste Manag. 2015, 43, 137–143. [Google Scholar] [CrossRef] [PubMed]
- McNutt, J.; Yang, J. Utilization of the residual glycerol from biodiesel production for renewable energy generation. Renew. Sustain. Energy Rev. 2017, 71, 63–76. [Google Scholar]
- Zhou, S.; Wang, J.; Peng, S.; Chen, T.; Yue, Z. Anaerobic co-digestion of landfill leachate and acid mine drainage using up-flow anaerobic sludge blanket reactor. Environ. Sci. Pollut. Res. 2020, 28, 8498–8506. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Zhu, S.; Zhong, D.; Zhu, J.; Liao, L. Anaerobic co-digestion of food waste and landfill leachate in single-phase batch reactors. Waste Manag. 2014, 34, 2278–2284. [Google Scholar] [CrossRef]
- Hombach, S.T.; Oleszkiewicz, J.A.; Lagasse, P.; Amy, L.B.; Zaleski, A.A.; Smyrski, K. Impact of landfill leachate on anaerobic digestion of sewage sludge. Environ. Technol. 2003, 24, 553–560. [Google Scholar] [CrossRef]
- Zan, F.; Hao, T. Sulfate in anaerobic co-digester accelerates methane production from food waste and waste activated sludge. Bioresour. Technol. 2020, 298, 122536. [Google Scholar] [CrossRef]
- Lakovleva, E.; Mäkilä, E.; Salonen, J.; Sitarz, M.; Wang, S.; Sillanpää, M. Acid mine drainage (AMD) treatment: Neutralization and toxic elements removal with unmodified and modified limestone. Ecol. Eng. 2015, 81, 30–40. [Google Scholar] [CrossRef]
- Sun, W.; Ji, B.; Khoso, S.A.; Tang, H.; Liu, R.; Wang, L.; Hu, Y. An extensive review on restoration technologies for mining tailings. Environ. Sci. Pollut. Res. 2018, 25, 33911–33925. [Google Scholar] [CrossRef]
- Li, Q.; Li, Y.-Y.; Qiao, W.; Wang, X.; Takayanagi, K. Sulfate addition as an effective method to improve methane fermentation performance and propionate degradation in thermophilic anaerobic co-digestion of coffee grounds, milk and waste activated sludge with AnMBR. Bioresour. Technol. 2015, 185, 308–315. [Google Scholar] [CrossRef]
- Cetecioglu, Z.; Dolfing, J.; Taylor, J.; Purdy, K.J.; Eyice, Ö. COD/sulfate ratio does not affect the methane yield and microbial diversity in anaerobic digesters. Water Res. 2019, 155, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Dearman, B.; Bentham, R.H. Anaerobic digestion of food waste: Comparing leachate exchange rates in sequential batch systems digesting food waste and biosolids. Waste Manag. 2007, 27, 1792–1799. [Google Scholar] [CrossRef]
- Shahriari, H.; Warith, M.; Hamoda, M.; Kennedy, K.J. Effect of leachate recirculation on mesophilic anaerobic digestion of food waste. Waste Manag. 2012, 32, 400–403. [Google Scholar] [CrossRef] [PubMed]
- Stabnikova, O.; Liu, X.-Y.; Wang, J.-Y. Anaerobic digestion of food waste in a hybrid anaerobic solid–liquid system with leachate recirculation in an acidogenic reactor. Biochem. Eng. J. 2008, 41, 198–201. [Google Scholar] [CrossRef]
- Berenjkar, P.; Islam, M.; Yuan, Q. Co-treatment of sewage sludge and mature landfill leachate by anaerobic digestion. Int. J. Environ. Sci. Technol. 2018, 16, 2465–2474. [Google Scholar] [CrossRef]
- Montusiewicz, A.; Lebiocka, M. Co-digestion of intermediate landfill leachate and sewage sludge as a method of leachate utilization. Bioresour. Technol. 2011, 102, 2563–2571. [Google Scholar] [CrossRef]
- Ghosh, P.; Thakur, I.S. Developments in Fungal Biology and Applied Mycology; Springer: New Delhi, India, 2017; pp. 341–357. [Google Scholar]
- Pasalari, H.; Esrafili, A.; Rezaee, A.; Gholami, M.; Farzadkia, M. Electrochemical oxidation pretreatment for enhanced methane potential from landfill leachate in anaerobic co-digestion process: Performance, Gompertz model, and energy assessment. Chem. Eng. J. 2021, 422, 130046. [Google Scholar] [CrossRef]
- Fernandes, A.; Pacheco, M.J.; Ciríaco, L.; Lopes, A. Review on the electrochemical processes for the treatment of sanitary landfill leachates: Present and future. Appl. Catal. B Environ. 2015, 176–177, 183–200. [Google Scholar] [CrossRef]
- Ersahin, M.E.; Ozgun, H.; Dereli, R.K.; Ozturk, I. Waste Water Treatment and Reutilization; IntechOpen: Instabul, Turkey, 2011. [Google Scholar]
- Gandhimathi, R.; Durai, N.J.; Nidheesh, P.V.; Ramesh, S.T.; Kanmani, S. Use of combined coagulation-adsorption process as pretreatment of landfill leachate. Iran. J. Environ. Health Sci. Eng. 2013, 10, 24. [Google Scholar] [CrossRef]
- Gulsen, H.; Turan, M.; Armagan, B. Anaerobic Fluidized Bed Reactor for the Treatment of Landfill Leachates. J. Environ. Sci. Health Part A 2007, 39, 2195–2204. [Google Scholar] [CrossRef]
- Zayen, A.; Schories, G.; Sayadi, S. Incorporation of an anaerobic digestion step in a multistage treatment system for sanitary landfill leachate. Waste Manag. 2016, 53, 32–39. [Google Scholar] [CrossRef]
- Ridzuan, M.B.; Daud, Z.; Ahmad, Z.; Abd Latiff, A.A.; Awang, H. Leachate treatment using Up-Flow Anaerobic Sludge Blanket System. Int. J. Integr. Eng. 2018, 10, 62–65. [Google Scholar] [CrossRef]
- Zhang, Y.; Ji, F.; Hu, Q.; Luo, T.; Jin, Z.; Xu, G.; Zhan, Y.; Wang, H. Effect of organic shock loading on anaerobic performance of pumice-reinforced up-flow anaerobic sludge bed for incineration leachate treatment. Braz. J. Chem. Eng. 2022, 1–10. [Google Scholar] [CrossRef]
- Bohdziewicz, J.; Neczaj, E.; Kwarciak, A. Landfill leachate treatment by means of anaerobic membrane bioreactor. Desalination 2008, 221, 559–565. [Google Scholar] [CrossRef]
- Nain, A.; Lohchab, R.K.; Singh, K.; Kumari, M.; Saini, J.K. MSW stabilization in an anaerobic bioreactor landfill and evaluation of in-situ leachate treatment potential with the help of Quadric Model. J. Mater. Cycles Waste Manag. 2021, 23, 2192–2207. [Google Scholar] [CrossRef]
- Zhu, G.; Zou, R.; Jha, A.K.; Huang, X.; Liu, L.; Liu, C. Recent developments and future perspectives of Anaerobic Baffled Bioreactor for Wastewater Treatment and Energy Recovery. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1243–1276. [Google Scholar] [CrossRef]
- Meegoda, J.; Li, B.; Patel, K.; Wang, L. A review of the Processes, Parameters, and Optimization of Anaerobic Digestion. Int. J. Environ. Res. Public Health 2018, 15, 2224. [Google Scholar] [CrossRef] [PubMed]
- Lohani, S.P.; Havukainen, J. Waste Bioremediation; Springer: Singapore, 2017; pp. 343–359. [Google Scholar]
- Tezcan Un, U.; Filik Iscen, C.; Oduncu, E.; Akcal Comoglu, B.; Ilhan, S. Treatment of landfill leachate using integrated continuous electrocoagulation and the anaerobic treatment technique. Environ. Prog. Sustain. Energy 2017, 37, 1668–1676. [Google Scholar] [CrossRef]
- Fazzino, F.; Bilardi, S.; Moraci, N.; Calabrò, P.S. Integrated treatment at laboratory scale of a mature landfill leachate via active filtration and anaerobic digestion: Preliminary results. Water 2021, 13, 2845. [Google Scholar] [CrossRef]
- Wang, G.; Fan, Z.; Wu, D.; Qin, L.; Zhang, G.; Gao, C.; Meng, Q. Anoxic/aerobic granular active carbon assisted MBR integrated with nanofiltration and reverse osmosis for advanced treatment of municipal landfill leachate. Desalination 2014, 349, 136–144. [Google Scholar] [CrossRef]
- Yuan, Y.; Liu, J.; Gao, B.; Hao, J. Ozone direct oxidation pretreatment and catalytic oxidation post-treatment coupled with ABMBR for landfill leachate treatment. Sci. Total Environ. 2021, 794, 148557. [Google Scholar] [CrossRef] [PubMed]
- Li, H.S.; Zhou, S.Q.; Sun, Y.B.; Feng, P. Advanced treatment of landfill leachate by a new combination process in a full-scale plant. J. Hazard. Mater. 2009, 172, 408–415. [Google Scholar] [CrossRef]
- Bakraouy, H.; Souabi, S.; Digua, K.; Dkhissi, O.; Sabar, M.; Fadil, M. Optimization of the treatment of an anaerobic pretreated landfill leachate by a coagulation–flocculation process using experimental design methodology. Process Saf. Environ. Prot. 2017, 109, 621–630. [Google Scholar] [CrossRef]
- Miao, L.; Yang, G.; Tao, T.; Peng, Y. Recent advances in nitrogen removal from landfill leachate using biological treatments—A Review. J. Environ. Manag. 2019, 235, 178–185. [Google Scholar] [CrossRef]
- Gamoń, F.; Tomaszewski, M.; Ziembińska-Buczyńska, A. Ecotoxicological study of landfill leachate treated in the ANAMMOX process. Water Qual. Res. J. 2019, 54, 230–241. [Google Scholar] [CrossRef]
- Ye, J.; Liu, J.; Ye, M.; Ma, X.; Li, Y.-Y. Towards advanced nitrogen removal and optimal energy recovery from leachate: A critical review of anammox-based processes. Crit. Rev. Environ. Sci. Technol. 2019, 50, 612–653. [Google Scholar] [CrossRef]
- Jin, R.-C.; Yang, G.-F.; Yu, J.-J.; Zheng, P. The inhibition of the Anammox process: A Review. Chem. Eng. J. 2012, 197, 67–79. [Google Scholar] [CrossRef]
- Adekunle, K.F.; Okolie, J.A. A review of biochemical process of anaerobic digestion. Adv. Biosci. Biotechnol. 2015, 6, 205–212. [Google Scholar] [CrossRef]
- Piątek, M.; Lisowski, A.; Kasprzycka, A.; Lisowska, B. The dynamics of an anaerobic digestion of crop substrates with an unfavourable carbon to nitrogen ratio. Bioresour. Technol. 2016, 216, 607–612. [Google Scholar] [CrossRef]
- Lin, L.; Xu, F.; Ge, X.; Li, Y. Biological treatment of organic materials for energy and nutrients production—Anaerobic digestion and composting. Adv. Bioenergy 2019, 121–181. [Google Scholar]
- Hillion, M.-L.; Moscoviz, R.; Trably, E.; Leblanc, Y.; Bernet, N.; Torrijos, M.; Escudié, R. Co-ensiling as a new technique for long-term storage of agro-industrial waste with low sugar content prior to anaerobic digestion. Waste Manag. 2018, 71, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; McAdam, E.; Zhang, Y.; Heaven, S.; Banks, C.; Longhurst, P. Ammonia inhibition and toxicity in anaerobic digestion: A critical review. J. Water Process Eng. 2019, 32, 100899. [Google Scholar] [CrossRef]
- Náthia-Neves, G.; Berni, M.; Dragone, G.; Mussatto, S.I.; Forster-Carneiro, T. Anaerobic digestion process: Technological aspects and recent developments. Int. J. Environ. Sci. Technol. 2018, 15, 2033–2046. [Google Scholar] [CrossRef]
- Liu, Y.; Yuan, Y.; Wang, W.; Wachemo, A.C.; Zou, D. Effects of adding osmoprotectant on anaerobic digestion of kitchen waste with high level of salinity. J. Biosci. Bioeng. 2019, 128, 723–732. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, Y.; Wang, D.; Chen, F.; Li, X.; Zeng, G.; Yang, Q. Potential impact of salinity on methane production from food waste anaerobic digestion. Waste Manag. 2017, 67, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Matheri, A.N.; Belaid, M.; Seodigeng, T.; Ngila, J.C. The Role of Trace Elements on Anaerobic Co-Digestion in Biogas Production. In Proceedings of the World Congress on Engineering 2016, London, UK, 29 June–1 July 2016. [Google Scholar]
- Nabi, M.; Liang, H.; Cheng, L.; Yang, W.; Gao, D. A comprehensive review on the use of conductive materials to improve anaerobic digestion: Focusing on landfill leachate treatment. J. Environ. Manag. 2022, 309, 114540. [Google Scholar] [CrossRef]
- Jadhav, P.; Muhammad, N.; Bhuyar, P.; Krishnan, S.; Razak, A.S.; Zularisam, A.W.; Nasrullah, M. A review on the impact of conductive nanoparticles (CNPs) in anaerobic digestion: Applications and limitations. Environ. Technol. Innovation. 2021, 23, 101526. [Google Scholar] [CrossRef]
- Cheng, Q.; Call, D.F. Hardwiring microbes via direct interspecies electron transfer: Mechanisms and applications. Environ. Sci. Process. Impacts 2016, 18, 968–980. [Google Scholar] [CrossRef]
- Kumar, G.; Sivagurunathan, P.; Sen, B.; Kim, S.-H.; Lin, C.-Y. Mesophilic continuous fermentative hydrogen production from acid pretreated de-oiled jatropha waste hydrolysate using immobilized microorganisms. Bioresour. Technol. 2017, 240, 137–143. [Google Scholar] [CrossRef]
- Batstone, D.J.; Virdis, B. The role of anaerobic digestion in the emerging energy economy. Curr. Opin. Biotechnol. 2014, 27, 142–149. [Google Scholar] [CrossRef]
- Purnomo, D.M.J.; Richter, F.; Bonner, M.; Vaidyanathan, R.; Rein, G. Role of optimisation method on kinetic inverse modelling of biomass pyrolysis at the Microscale. Fuel 2020, 262, 116251. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, M.; Wall, J.D.; Hu, Z. Nanosilver impact on methanogenesis and biogas production from municipal solid waste. Waste Manag. 2012, 32, 816–825. [Google Scholar] [CrossRef] [PubMed]
- Demirel, B. The impacts of engineered nanomaterials (ENMs) on anaerobic digestion processes. Process Biochem. 2016, 51, 308–313. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, Y.; Holmes, D.E.; Dang, Y.; Woodard, T.L.; Nevin, K.P.; Lovley, D.R. Potential enhancement of direct interspecies electron transfer for syntrophic metabolism of propionate and butyrate with biochar in up-flow anaerobic sludge blanket reactors. Bioresour. Technol. 2016, 209, 148–156. [Google Scholar] [CrossRef]
- Amen TW, M.; Eljamal, O.; Khalil AM, E.; Matsunaga, N. Biochemical methane potential enhancement of domestic sludge digestion by adding pristine iron nanoparticles and iron nanoparticles coated zeolite compositions. J. Environ. Chem. Eng. 2017, 5, 5002–5013. [Google Scholar] [CrossRef]
- Lei, Y.; Sun, D.; Dang, Y.; Chen, H.; Zhao, Z.; Zhang, Y.; Holmes, D.E. Stimulation of methanogenesis in anaerobic digesters treating leachate from a municipal solid waste incineration plant with carbon cloth. Bioresour. Technol. 2016, 222, 270–276. [Google Scholar] [CrossRef]
- Baniamerian, H.; Isfahani, P.G.; Tsapekos, P.; Alvarado-Morales, M.; Shahrokhi, M.; Vossoughi, M.; Angelidaki, I. Application of nano-structured materials in anaerobic digestion: Current status and Perspectives. Chemosphere 2019, 229, 188–199. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Y.; Quan, X.; Zhang, Y. Towards engineering application: Potential mechanism for enhancing anaerobic digestion of complex organic waste with different types of conductive materials. Water Res. 2017, 115, 266–277. [Google Scholar] [CrossRef]
- Asam, Z.-u.l.-Z.; Poulsen, T.G.; Nizami, A.-S.; Rafique, R.; Kiely, G.; Murphy, J.D. How can we improve biomethane production per unit of feedstock in biogas plants? Appl. Energy 2011, 88, 2013–2018. [Google Scholar] [CrossRef]
- Lei, Y.; Wei, L.; Liu, T.; Xiao, Y.; Dang, Y.; Sun, D.; Holmes, D.E. Magnetite enhances anaerobic digestion and methanogenesis of fresh leachate from a municipal solid waste incineration plant. Chem. Eng. J. 2018, 348, 992–999. [Google Scholar] [CrossRef]
- Mustapha, N.A.; Toya, S.; Maeda, T. Effect of Aso limonite on anaerobic digestion of waste sewage sludge. AMB Express 2020, 10, 74. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Geng, S.; Li, Z.; Song, K. Effect of microplastic on anaerobic digestion of wasted activated sludge. Chemosphere 2020, 247, 125874. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhang, Y.; Woodard, T.L.; Nevin, K.P.; Lovley, D.R. Enhancing syntrophic metabolism in up-flow anaerobic sludge blanket reactors with conductive carbon materials. Bioresour. Technol. 2015, 191, 140–145. [Google Scholar] [CrossRef]
- Gil, A.; Siles, J.A.; Serrano, A.; Chica, A.F.; Martín, M.A. Effect of variation in the C/[N+P] ratio on anaerobic digestion. Environ. Prog. Sustain. Energy 2018, 38, 228–236. [Google Scholar] [CrossRef]
- Jaman, K.; Idrus, S.; Wahab, A.M.; Harun, R.; Daud, N.N.; Ahsan, A.; Shams, S.; Uddin, M.A. Influence of molasses residue on treatment of cow manure in an anaerobic filter with perforated weed membrane and a conventional reactor: Variations of organic loading and a machine learning application. Membranes 2023, 13, 159. [Google Scholar] [CrossRef]
- Di Addario, M.; Temizel, I.; Edes, N.; Onay, T.T.; Demirel, B.; Copty, N.K.; Ruggeri, B. Development of fuzzylogic model to predict the effects of Zno nanoparticles on methane production from simulated landfill. J. Environ. Chem. Eng. 2017, 5, 5944–5953. [Google Scholar] [CrossRef]
- Yukesh Kannah, R.; Bhava Rohini, K.; Gunasekaran, M.; Gokulakrishnan, K.; Kumar, G.; Rajesh Banu, J. Prediction of effective substrate concentration and its impact on biogas production using Artificial Neural Networks in Hybrid Upflow anaerobic Sludge Blanket reactor for treating landfill leachate. Fuel 2022, 313, 122697. [Google Scholar] [CrossRef]
- Jaroenpoj, S.; Jimmy Yu, Q.; Ness, J. Development of artificial neural network models for biogas production from co-digestion of leachate and Pineapple Peel. Glob. Environ. Eng. 2015, 1, 42–47. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Hashemi, H.; Eslami, H.; Fallahzadeh, R.A.; Khosravi, R.; Askari, R.; Ghahramani, E. Kinetics of biogas production and chemical oxygen demand removal from compost leachate in an anaerobic migrating blanket reactor. J. Environ. Manag. 2018, 206, 707–714. [Google Scholar] [CrossRef]
- Tamrat, M.; Costa, C.; Márquez, M.C. Biological treatment of leachate from solid wastes: Kinetic study and simulation. Biochem. Eng. J. 2012, 66, 46–51. [Google Scholar] [CrossRef]
- Gu, N.; Liu, J.; Ye, J.; Chang, N.; Li, Y.-Y. Bioenergy, ammonia and humic substances recovery from municipal solid waste leachate: A review and process integration. Bioresour. Technol. 2019, 293, 122159. [Google Scholar] [CrossRef] [PubMed]
- Sonawane, J.M.; Adeloju, S.B.; Ghosh, P.C. Landfill leachate: A promising substrate for microbial fuel cells. Int. J. Hydrog. Energy 2017, 42, 23794–23798. [Google Scholar] [CrossRef]
- Abdoli, M.A.; Karbassi, A.R.; Samiee, Z.R.; Rashidi, Z.; Gitipour, S.; Pazoki, M. Electricity Generation from Leachate Treatment Plant. Int. J. Environ. Res. 2012, 6, 493–498. [Google Scholar]
- Li, Y.; Tang, F.; Xu, D.; Xie, B. Advances in biological nitrogen removal of landfill leachate. Sustainability 2021, 13, 6236. [Google Scholar] [CrossRef]
- Liu, B.; Li, B. Single chamber microbial fuel cells (SCMFCs) treating wastewater containing methanol. Int. J. Hydrog. Energy 2014, 39, 2340–2344. [Google Scholar] [CrossRef]
- Jayashree, S.; Ramesh, S.T.; Lavanya, A.; Gandhimathi, R.; Nidheesh, P.V. Wastewater treatment by microbial fuel cell coupled with peroxicoagulation process. Clean Technol. Environ. Policy 2019, 21, 2033–2045. [Google Scholar] [CrossRef]
- Yuvendius, H.; Zondra, E.; Zainuri; Sari, V.I. Study of biogas utilization as waste-to-energy plant and transport modelling of iron (Fe), lead (Pb) and copper (Cu) in leachate at Muara Fajar Landfill Pekanbaru. IOP Conf. Ser. Earth Environ. Sci. 2022, 1041, 012056. [Google Scholar] [CrossRef]
- Iskander, S.M.; Brazil, B.; Novak, J.T.; He, Z. Resource recovery from landfill leachate using bioelectrochemical systems: Opportunities, challenges, and perspectives. Bioresour. Technol. 2016, 201, 347–354. [Google Scholar] [CrossRef]
- Nikolausz, M.; Kretzschmar, J. Anaerobic digestion in the 21st century. Bioengineering 2020, 7, 157. [Google Scholar] [CrossRef]
- Bernat, K.; Kulikowska, D.; Zielińska, M.; Zaborowska, M.; Wojnowska-Baryła, I.; Łapińska, M. Post-treatment of the effluent from anaerobic digestion of the leachate in two-stage SBR system using alternative carbon sources. Sustainability 2021, 13, 6297. [Google Scholar] [CrossRef]
- Li, X.; Bao, D.; Zhang, Y.; Xu, W.; Zhang, C.; Yang, H.; Ru, Q.; Wang, Y.F.; Ma, H.; Zhu, E.; et al. Development and application of membrane aerated biofilm reactor (MABR)—A review. Water 2023, 15, 436. [Google Scholar] [CrossRef]
- Świechowski, K.; Matyjewicz, B.; Telega, P.; Białowiec, A. The influence of low-temperature food waste biochars on anaerobic digestion of food waste. Materials 2022, 15, 945. [Google Scholar] [CrossRef] [PubMed]

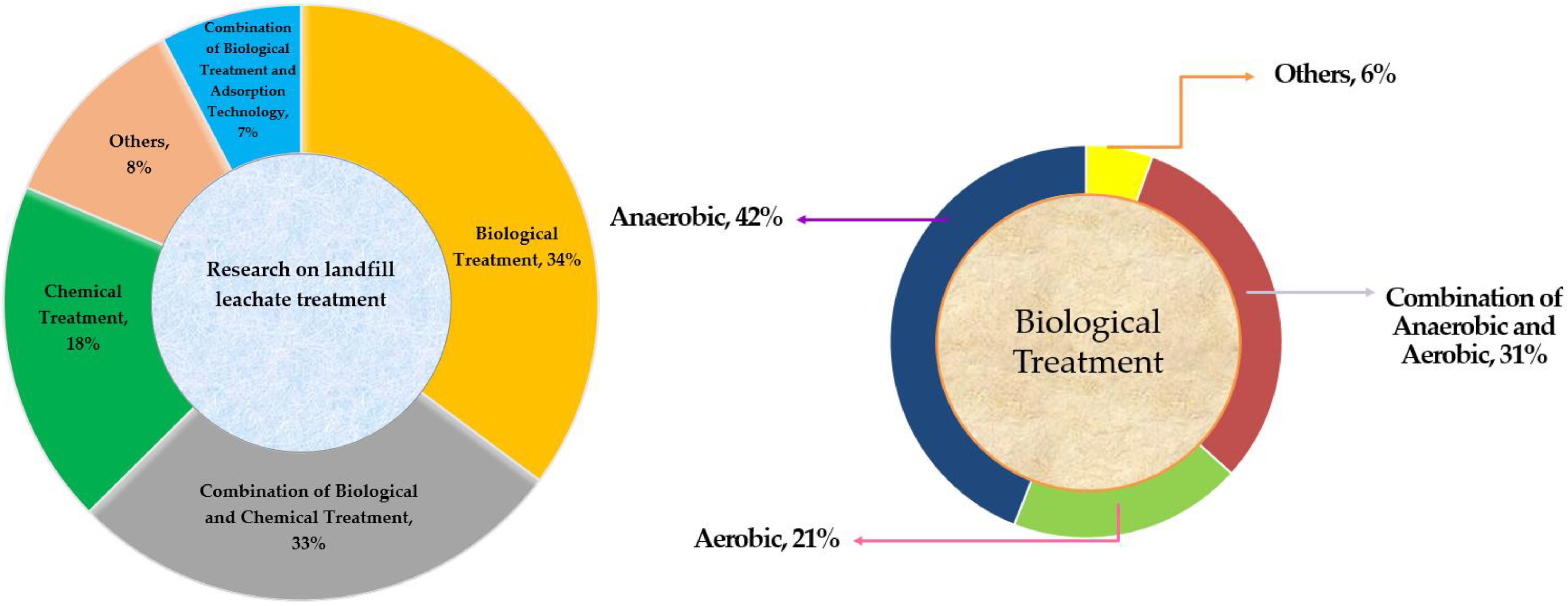
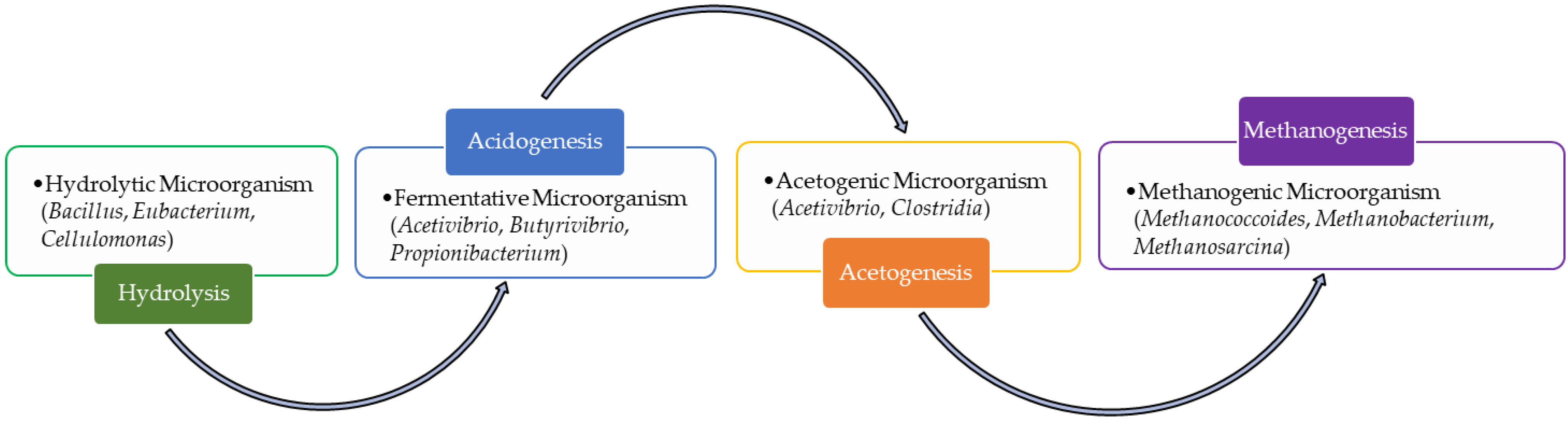
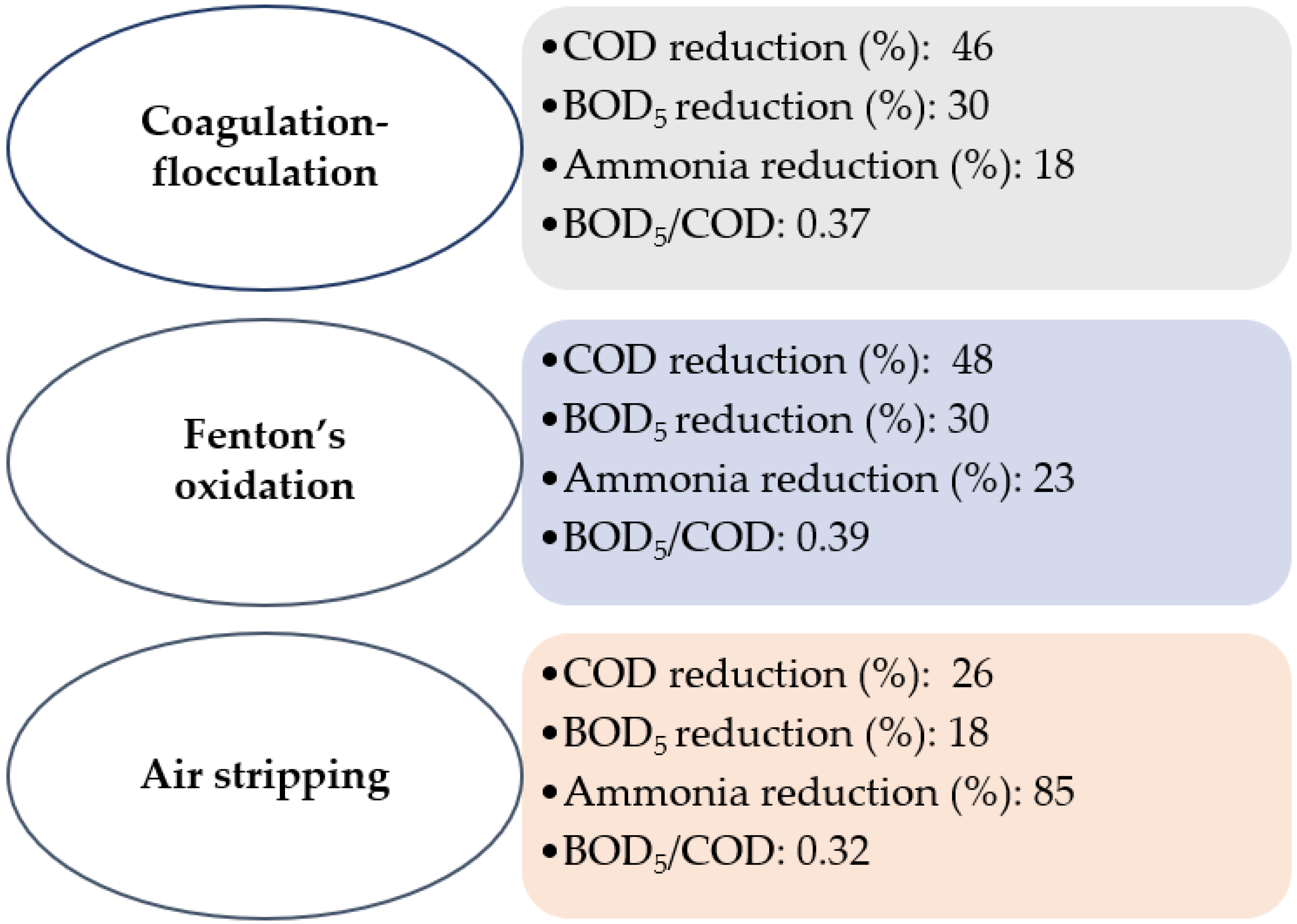

| Heavy Metals (mg/L) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Types of Landfill Leachate | COD (g/L) | BOD5 (g/L) | BOD5/COD | pH | Specific Conductivity (μs/cm) | Alkalinity | Zinc (Zn) | Copper (Cu) | Cadmium (Cd) | Nickel (Ni) | Chromium (Cr) | Age (years) | References |
| Young | 0.41–15 | 0.036–0.984 | 0.5–1.0 | <6.5 | <28,430 | <9682 | <7.64 a | <2.42 b | <0.007 b | <0.66 b | <1.44 b | <1 | [2,12,13] |
| Intermediate | 0.19–15 | 0.006–0.98 | 0.1–0.5 | 6.5–7.5 | 2606–41,500 | 10–2100 | <1.43 b | <0.39 b | <0.03 b | <0.37 b | <0.28 b | 1–5 | |
| Old | 0.70–10.4 | 1.5–3.0 | <0.1 | >7.5 | <15,030 | 1754–5573 | <0.003 b | <0.15 b | <0.04 b | <1.34 b | <0.002 b | >5 | |
| Operational Variables | Removal Rate (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Type of Reactors | Leachate Type a | Temperature (°C) | HRT (days) | Nitrogen | COD | Advantages of Reactor | Scale of Study | References |
| Sequencing batch reactor (SBR) | Old | 40–50 | 20–40 | 99 | 75 | Suitable for nitrification and denitrification, simple construction and low capital cost. | Pilot scale | [14,15,16,17,18] |
| Old | 18–25 | 2–12 | 70–82 | 71.2–76.2 | Laboratory scale | |||
| Intermediate | 20 ± 0.5 | 5.63–5.8 | 90 | 30–40 | Laboratory scale | |||
| Moving-bed biofilm reactor (MBBR) | Old | 21 | 1 | - | 75 | No long settling times for sludge, and less sensitivity to toxic compounds. | Laboratory scale | [14,17,19] |
| Intermediate | - | 20–24 | - | 81 | Laboratory scale | |||
| Activated sludge reactor (ASR) | Old | 21 | 6.25 | - | 46–64 | Low processing costs and can effectively eliminate biodegradable organic matter by converting it to carbon dioxide and water. | Laboratory scale | [14,20] |
| Intermediate | 24 | 0.42 | - | 75 | Laboratory scale | |||
| Aerobic lagoon (AL) | Intermediate | 13.5 | 56 | - | 75 | Low operation and maintenance costs. | Pilot scale | [17,21] |
| Rotating biological contactor (RBC) | Old | - | 1 | - | 52 | Easy to operate, short start-up, minimal land area, low energy consumption, low operating and maintenance costs. | Laboratory scale | [22,23] |
| Adsorption | ||||
|---|---|---|---|---|
| Leachate Type a | Adsorbent | COD Removal (%) | References | |
| Intermediate | Powdered activated carbon | 38 | [14] | |
| Old | Peat soil | 69 | [24] | |
| Chemical precipitation | ||||
| Leachate Type a | Precipitant | COD removal (%) | References | |
| Old | Ca(OH)2 (1 g/L) | 27 | [14] | |
| Young | Struvite (Mg:NH4:PO4 = 1:1:1) | 50 | [25] | |
| Coagulation/Flocculation | ||||
| Leachate Type a | Coagulant | Concentration Range (g/L) | COD Removal (%) | References |
| Old | Al2(SO4)3 | 0.7 | 10–25 | [26] |
| Intermediate | FeCl3 + Al2(SO4)3 | 1.0–5.0 | 75 | [27] |
| Air Stripping | ||||
| Leachate Type a | Time Stripping (days) | Temperature (°C) | Ammonia Removal (%) | References |
| Old | 5 | - | 99.5 | [26] |
| Old | 1 | 20 | 89 | [28] |
| Example of Treatment | Leachate Type a | Operational Variables | Removal Efficiency | Advantages of Reactor | Critical Remarks/Scale of Study | References |
|---|---|---|---|---|---|---|
| Aerobic Sequencing Batch Reactor (ASBR) combined with zeolite technology | Old |
| Ammoniacal nitrogen:
|
|
| [29] |
| Combined sodium persulfate/Hydrogen peroxide based advanced oxidation process | Intermediate |
| Hydrogen peroxide alone:
alone:
|
|
| [30] |
| Two-stage anoxic/oxic (A/O) combined membrane bioreactor (MBR) | Old |
|
|
|
| [31] |
| Type of Anaerobic Reactors | Characteristics | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Leachate Type | Chemical Oxygen Demand, COD Content (mg/L) | Hydraulic Retention Time, HRT (days) | Organic Loading Rate, OLR | Removal Efficiency | Methane Production | Critical Remarks/Scale of Study | COD Concentration in Effluent (mg/L) | References | |
| Upflow anaerobic sludge blanket reactors (UASB) | Old | 14,640 | 30 | - |
| - |
| 3806.4 > 250 a | [65,66] |
| Anaerobic fluidised bed reactors (AFBR) | Young | 35,000 (avg) | 1 | 12 g COD/L/day |
| 75% |
| 3500 > 250 a | [63] |
| Anaerobic Filter (AF) | Young | 15,200 | 4.5 | 3.3 g COD/L/day |
| 19.24 L/d |
| 3842.56 > 250 a | [64] |
| Anaerobic membrane bioreactors (AnMBR) | Old | 39,000 (avg) | 2 | 2.5 kg COD/m3d |
| - |
| 3900 > 250 a | [67] |
| Trace Element | Toxic Threshold Concentration (mg/L) | Composition of Heavy Metals in Landfill Leachate (µg/L) | References |
|---|---|---|---|
| Calcium | 2800 | - | [13,90] |
| Manganese | 50 | - | |
| Copper | 400 | 3–157 | |
| Zinc | 1 | 10–303 | |
| Iron | 10 | - | |
| Cadmium | 0.18 | 0.1–35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zamrisham, N.A.F.; Wahab, A.M.A.; Zainal, A.; Karadag, D.; Bhutada, D.; Suhartini, S.; Musa, M.A.; Idrus, S. State of the Art in Anaerobic Treatment of Landfill Leachate: A Review on Integrated System, Additive Substances, and Machine Learning Application. Water 2023, 15, 1303. https://doi.org/10.3390/w15071303
Zamrisham NAF, Wahab AMA, Zainal A, Karadag D, Bhutada D, Suhartini S, Musa MA, Idrus S. State of the Art in Anaerobic Treatment of Landfill Leachate: A Review on Integrated System, Additive Substances, and Machine Learning Application. Water. 2023; 15(7):1303. https://doi.org/10.3390/w15071303
Chicago/Turabian StyleZamrisham, Nur Ain Fitriah, Abdul Malek Abdul Wahab, Afifi Zainal, Dogan Karadag, Dinesh Bhutada, Sri Suhartini, Mohamed Ali Musa, and Syazwani Idrus. 2023. "State of the Art in Anaerobic Treatment of Landfill Leachate: A Review on Integrated System, Additive Substances, and Machine Learning Application" Water 15, no. 7: 1303. https://doi.org/10.3390/w15071303
APA StyleZamrisham, N. A. F., Wahab, A. M. A., Zainal, A., Karadag, D., Bhutada, D., Suhartini, S., Musa, M. A., & Idrus, S. (2023). State of the Art in Anaerobic Treatment of Landfill Leachate: A Review on Integrated System, Additive Substances, and Machine Learning Application. Water, 15(7), 1303. https://doi.org/10.3390/w15071303