Metal Pollution and Mining in the Iberian Pyrite Belt: New Remediation Technologies to Improve the Ecosystem Services of the River Basins
Abstract
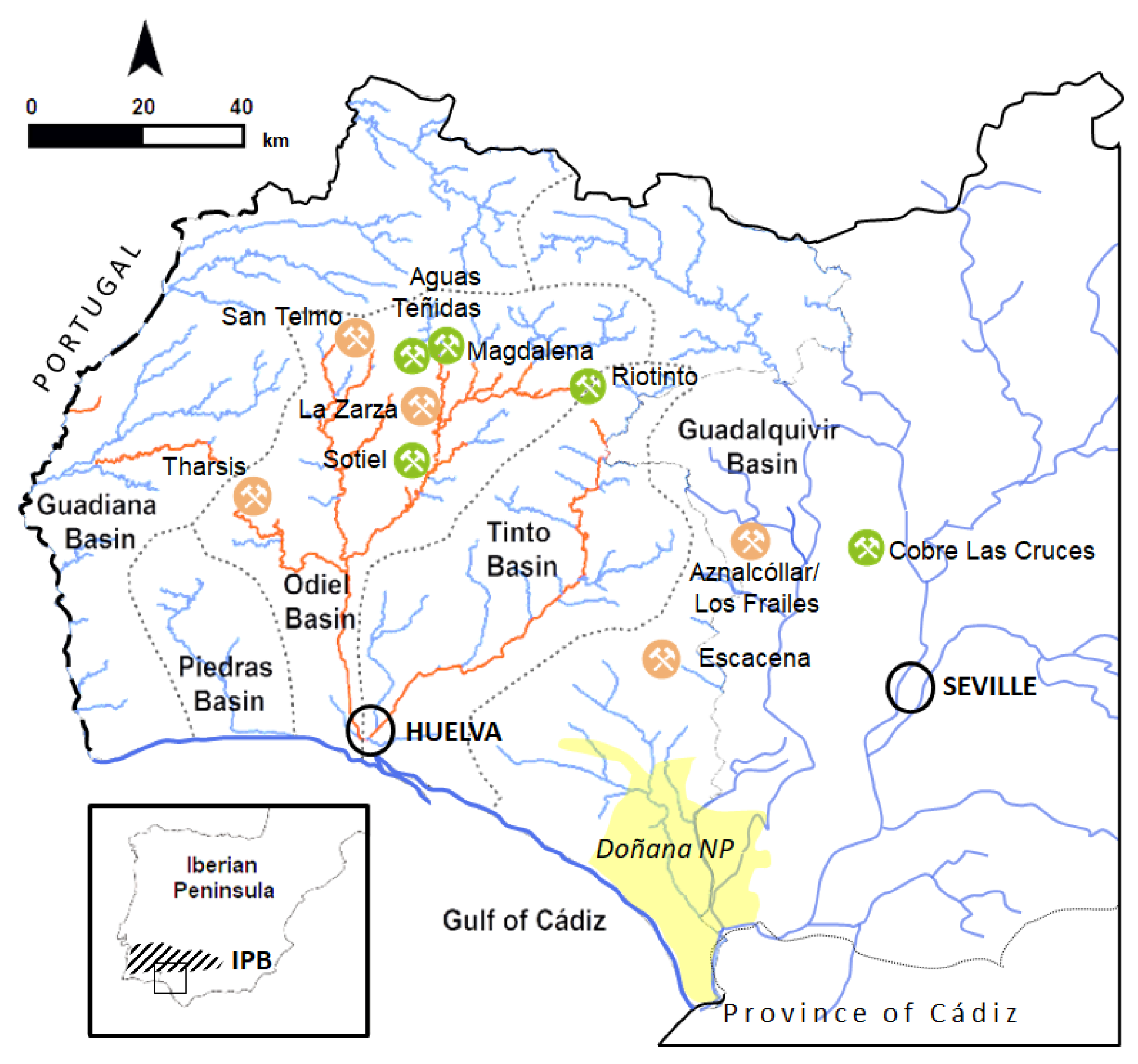
1. Introduction
2. Materials and Methods
2.1. DAS-Passive Treatment
2.2. ASE&C Disruptive Technology
2.3. Sample Analyses
3. Results and Discussion
3.1. Advantages of DAS and ASE&C Technologies
3.2. Some Other Recent Technologies Used for AMD Remediation and Improvement of Ecosystem Services
3.3. Using DAS and ASE&C in an Old Mining Spill (Los Frailes Mine, 1995), an Approach to the Improvement of Ecosystem Services in the River Basin of the Iberian Pyrite Belt
3.4. Other Mining Spillages
4. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sáez, R.; Pascual, E.; Toscano, M.; Almodóvar, G.R. The Iberian type of volcano-sedimentary massive sulphide deposits. Miner. Depos. 1999, 34, 549–570. [Google Scholar] [CrossRef]
- Sánchez-España, J.; Pamo, E.L.; Santofimia, E.; Aduvire, O.; Reyes, J.; Barettino, D. Acid mine drainage in the Iberian Pyrite Belt (Odiel river watershed, Huelva, SW Spain): Geochemistry, mineralogy and environmental implications Appl. Geochem. 2005, 20, 1320–1356. [Google Scholar] [CrossRef]
- Cánovas, C.R.; Olías, M.; Nieto, J.M.; Sarmiento, A.M.; Cerón, J.C. Hydrogeochemical characteristics of the Tinto and Odiel Rivers (SW Spain). Factors controlling metal contents. Sci. Total Environ. 2007, 373, 363–382. [Google Scholar] [CrossRef]
- Riba, I.; Forja, J.M.; Gómez-Parra, A.; DelValls, T.A. Sediment quality in littoral regions of the Gulf of Cádiz: A triad approach to address the influence of mining activities. Environ. Pollut. 2004, 132, 341–353. [Google Scholar] [CrossRef]
- Riba, I.; Conradi, M.; Forja, J.M.; DelValls, T.A. Sediment quality in the Guadalquivir estuary: Lethal effects associated with the Aznalcóllar mining spill. Mar. Pollut. Bullet. 2004, 48, 144–152. [Google Scholar] [CrossRef]
- Riba, I.; De Canales, M.L.G.; Forja, J.M.; DelValls, T.A. Sediment quality in the Guadalquivir estuary: Sublethal effects associated with the Aznalcóllar mining spill. Mar. Pollut. Bullet. 2004, 48, 153–163. [Google Scholar] [CrossRef]
- Riba, I.; DelValls, T.A.; Reynoldson, T.B.; Milani, D. Sediment quality in Rio Guadiamar (SW, Spain) after a tailing dam collapse: Contamination, toxicity and bioavailability. Environ. Int. 2006, 32, 891–900. [Google Scholar] [CrossRef]
- AMINER (Asociación de Empresas Investigadoras, Extractoras, Transformadoras Minero-Metalúrgicas, Auxiliares y de Servicios). Memoria Anual 2021. Available online: https://www.aminer.es/wp-content/uploads/2022/06/AMINER_Memoria-2021_definitiva-V2-comprimido.pdf (accessed on 1 January 2023).
- Agboola, O.; Babatunde, D.E.; Isaac Fayomi, O.S.; Sadiku, E.R.; Popoola, P.; Moropeng, L.; Yahaya, A.; Mamudu, O.A. A review on the impact of mining operation: Monitot. Asses. Manag. 2020, 8, 100181. [Google Scholar] [CrossRef]
- Sonter, L.J.; Saleem, H.A.; Watson, J.E.M. Mining and biodiversity: Key issues and research needs in conservation science. Proc. R. Soc. B. Biol. Sci. 2018, 285, 1892. [Google Scholar] [CrossRef]
- Santofimia, E.; González-Toril, E.; López-Pamo, E.; Gomariz, M.; Amils, R.; Aguilera, Á. Microbial Diversity and Its Relationship to Physicochemical Characteristics of the Water in Two Extreme Acidic Pit Lakes from the Iberian Pyrite Belt (SW Spain). PLoS ONE 2013, 8, e66746. [Google Scholar] [CrossRef]
- Sanz, J.L.; Rodriguez, N.; Escudero, C.; Carrizo, D.; Amils, R. Biological production of H2, CH4 and CO2 in the deep subsurface of the Iberian Pyrite Belt. Environ. Microbiol. 2021, 23, 3913–3922. [Google Scholar] [CrossRef] [PubMed]
- Amils, R.; Fernández-Remolar, D.; Parro, V.; Rodríguez-Manfredi, J.A.; Timmis, K.; Oggerin, M.; Sánchez-Román, M.; López, F.J.; Fernández, J.P.; Puente, F. Iberian Pyrite Belt Subsurface Life (IPBSL), a drilling project of biohydrometallurgical interest. Adv. Mater. Res. 2013, 825, 15–18. [Google Scholar] [CrossRef]
- Escudero, C.; Del Campo, A.; Ares, J.R.; Sánchez, C.; Martinez, J.M.; Gomez, F.; Amils, R. Visualizing Microorganism-Mineral Interaction in the Iberian Pyrite Belt Subsurface: The Acidovorax Case. Front. Microbiol. 2020, 11, 572104. [Google Scholar] [CrossRef]
- FEDER. Proyecto Faja-Junta de Andalucía. Available online: https://www.juntadeandalucia.es/medioambiente/web/ContenidosOrdenacion/red_informacion_ambiental/PDF/Geodiversidad/Faja_Piritica/proyecto_faja.pdf (accessed on 1 December 2022).
- Sánchez-Mata, D.; Chueca, R. Caracterización de la Biodiversidad de la Cuenca del Río Tinto y del Subsuelo de la Faja Pirítica Ibérica que lo Origina, Aplicaciones Biotecnológicas. Available online: https://portalcientifico.uam.es/es/ipublic/item/8899519 (accessed on 1 January 2023).
- Naidu, G.; Ryu, S.; Thiruvenkatachari, R.; Choi, Y.; Jeong, S.; Vigneswaran, S. A critical review on remediation, reuse, and resource recovery from acid mine drainage. Environ. Pollut. 2019, 249, 1110–1124. [Google Scholar] [CrossRef]
- Cánovas, C.R.; Basallote, M.D.; Macías, F.; Olías, M.; Pérez-López, R.; Ayora, C.; Nieto, J.M. Geochemical behaviour and transport of technology critical metals (TCMs) by the Tinto River (SW Spain) to the Atlantic Ocean. Sci. Total Environ. 2021, 764, 143796. [Google Scholar] [CrossRef]
- Nieto, J.M.; Sarmiento, A.M.; Olías, M.; Canovas, C.R.; Riba, I.; Kalman, J.; Delvalls, T.A. Acid mine drainage pollution in the Tinto and Odiel rivers (Iberian Pyrite Belt, SW Spain) and bioavailability of the transported metals to the Huelva Estuary. Environ. Int. 2007, 33, 445–455. [Google Scholar] [CrossRef]
- ICMAN/CSIC. Scientific Report on ASEC Efficiency in the Purification of Contaminated Mining Waters; Technical Report; ICMAN/CSIC: Cádiz, Spain, 2022. [Google Scholar]
- ICMAN/CSIC. Scientific Report on ASEC Efficiency in the Purification of Swine Slurries; Technical Report; ICMAN/CSIC: Cádiz, Spain, 2022. [Google Scholar]
- ICMAN/CSIC. Scientific Report on ASEC Efficiency in the Purification of Contaminated Waters from Textile Industry; Technical Report; ICMAN/CSIC: Cádiz, Spain, 2022. [Google Scholar]
- Macías, F.; Caraballo, M.A.; Nieto, J.M.; Rötting, T.S.; Ayora, C. Natural pretreatment and passive remediation of highly polluted acid mine drainage. J. Environ. Manag. 2012, 104, 93e100. [Google Scholar] [CrossRef]
- Macías, F.; Caraballo, M.A.; Rötting, T.S.; Pérez-López, R.; Nieto, J.M.; Ayora, C. From highly polluted Zn-rich acid mine drainage to nonmetallic waters: Implementation of a multi-step alkaline passive treatment system to remediate metal pollution. Sci. Total Environ. 2012, 433, 323e330. [Google Scholar] [CrossRef]
- Caraballo, M.A.; Macías, F.; Nieto, J.M.; Castillo, J.; Quispe, D.; Ayora, C. Hydrochemical performance and mineralogical evolution of a dispersed alkaline substrate (DAS) remediating the highly polluted acid mine drainage in the full-scale passive treatment of Mina Esperanza (SW Spain). Am. Mineral. 2011, 96, 1270–1277. [Google Scholar] [CrossRef]
- Macías, F.; Pérez-López, R.; Caraballo, M.A.; Sarmiento, A.M.; Canovas, C.R.; Nieto, J.M.; Olias, M.; Ayora, C. A geochemical approach to the restoration plans for the Odiel River basin (SW Spain), a watershed deeply polluted by acid mine drainage. Environ. Sci. Pollut. Res. 2017, 24, 4506–4516. [Google Scholar] [CrossRef]
- Orden, S.; Macías, F.; Cánovas, C.R.; Nieto, J.M.; Pérez-López, R.; Ayora, C. Eco-sustainable passive treatment for mine waters: Full-scale and long-term demonstration. J. Environ. Manag. 2021, 280, 111699. [Google Scholar] [CrossRef] [PubMed]
- Castillo, J.; Vera, S. ASE system from volume reduction to the elimination of radiactive isotopes in the management of Fukushima nuclear residues. In Proceedings of the Spanish National Congress, Granada, Spain, 25–28 October 2020. [Google Scholar]
- Sarmiento, A.M.; Mora-Figueroa, V.; Nieto, J.M.; DelValls, A. Evaluación de la toxicidad de las aguas de una planta de tratamiento pasivo de drenajes ácidos de mina. Bioensayos con Gambusia holbrooki Toxicity assessment with Gambusia holbrooki in a passive treatment system for acid mine drainage in the Iberian Pyrite Belt. Geotemas 2016, 16, 589–592. [Google Scholar]
- Sarmiento, A.M.; Bonnail, E.; Nieto, J.M.; DelValls, A. Preliminary results of ecotoxicological assessment of an Acid Mine Drainage (AMD) Passive Treatment System testing water quality of depurated lixiviates. Procedia Earth Planet. Sci. 2017, 17, 269–272. [Google Scholar] [CrossRef]
- Bonnail, E.; Macías, F.; Osta, V. Ecological improvement assessment of a passive remediation technology for acid mine drainage: Water quality biomonitoring using bivalves. Chemosphere 2019, 219, 695–703. [Google Scholar] [CrossRef]
- Ayers, R.S.; Westcot, D.W. Water Quality for Agriculture; Eurospan Bookstore: London, UK, 1985. [Google Scholar]
- Canadian Environmental Quality Guidelines (CEQGs) for Canadian Environment Council. Available online: https://ccme.ca/en/resources/water-aquatic-life. (accessed on 1 November 2022).
- Bonnail, E.; Cunha Lima, R.; Bautista-Chamizo, E.; Salamanca, M.J.; Cruz-Hernández, P. Biomarker responses of the freshwater clam Corbicula fluminea in acid mine drainage polluted systems. Environ. Pollut. 2018, 212, 1659–1668. [Google Scholar] [CrossRef]
- Martínez, N.M.; Basallote, M.D.; Meyer, A.; Cánovas, C.R.; Macías, F.; Schneider, P. Life cycle assessment of a passive remediation system for acid mine drainage: Towards more sustainable mining activity. J. Clean. Prod. 2019, 211, 1100–1111. [Google Scholar] [CrossRef]
- Ayora, C.; Macías, F.; Torres, E.; Lozano, A.; Carrero, S.; Nieto, J.-M.; Perez-Lopez, R.; Fernandez-Martinez, A.; Castillo-Michel, H. Recovery of rare earth elements and yttrium from passive-remediation systems of acid mine drainage. Environ. Sci. Technol. 2016, 50, 8255–8262. [Google Scholar] [CrossRef]
- León, R.; Macías, F.; Cánovas, C.R.; Pérez-López, R.; Ayora, C.; Nieto, J.M.; Olías, M. Mine waters as secondary source of rare earth elements worldwide: The case of the Iberian Pyrite Belt. J. Geochem. Explor. 2021, 224, 106742. [Google Scholar] [CrossRef]
- European Environment Agency 2000. Directive 2000/60/EC, 2000. Directive 2000/60/EC. Council Decision of 23 October 2000: Establishing a communitarian frame of action in the scope of water policy. Official Journal L 327 (22/12/2000); European Environment Agency: Copenhagen, Denmark, 2000; pp. 1–88.
- Directive 2008/105/EC of the European Parliament and of the Council of 16 December 2008 on Environmental Quality Standards in the Field of Water Policy, Amending and Subsequently Repealing Council Directives 82/176/EEC, 83/513/EEC, 84/156/EEC, 84/491/EEC, 86/280/EEC and Amending Directive 2000/60/EC of the European Parliament and of the Council. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32008L0105 (accessed on 1 November 2022).
- Brown, M.; Barley, B.; Wood, H. Minewater Treatment: Technology, Application and Policy; IWA Publishing: London, UK, 2002. [Google Scholar]
- Johnson, D.B.; Hallberg, K.B. Acid mine drainage remediation options: A review. Sci. Total Environ. 2005, 338, 3–14. [Google Scholar] [CrossRef]
- Asta, M.P.; Ayora, C.; Román-Ross, G.; Cama, J.; Acero, P.; Gault, A.G.; Charnock, J.M.; Bardelli, F. Natural attenuation of arsenic in the Tinto Santa Rosa acid stream (Iberian Pyritic Belt, SW Spain): The role of iron precipitates. Chem. Geol. 2010, 271, 1–12. [Google Scholar] [CrossRef]
- Masindi, V.; Ramakokovhu, M.M.; Osman, M.S.; Tekere, M. Advanced application of BOF and SAF slags for the treatment of acid mine drainage (AMD): A comparative study. Mater. Today Proc. 2021, 38, 934–941. [Google Scholar] [CrossRef]
- Hedin, B.C.; Capoa, R.C.; Stewart, B.W.; Hedin, R.S.; Lopano, C.L.; Stuckmand, M.Y. The evaluation of critical rare earth element (REE) enriched treatment solids from coal mine drainage passive treatment systems. Int. J. Coal Geol. 2019, 208, 54. [Google Scholar] [CrossRef]
- Wadekar, S.S.; Hayes, T.; Lokare, O.R.; Mittal, D.; Vidic, R.D. Laboratory and pilot-scale nanofiltration treatment of abandoned mine drainage for the recovery of products suitable for industrial reuse. Ind. Eng. Chem. Res. 2017, 56, 7355–7364. [Google Scholar] [CrossRef]
- Aguiar, A.; Andrade, L.; Grossi, L.; Pires, W.; Amaral, M. Acid mine drainage treatment by nanofiltration: A study of membrane fouling, chemical cleaning, and membrane ageing. Separ. Purif. Technol. 2018, 192, 185–195. [Google Scholar] [CrossRef]
- Andalaf, J.; Schwarz, A.; Pino, L.; Fuentes, P.; Bórquez, R.; Aybar, M. Assessment and modeling of nanofiltration of acid mine drainage. Ind. Eng. Chem. Res. 2018, 57, 14727–14739. [Google Scholar] [CrossRef]
- Atrei, A.; Fiorani, M.; Bellingeri, A.; Protano, G.; Corsi, I. Remediation of acid mine drainage-affected stream waters by means of eco-friendly magnetic hydrogels crosslinked with functionalized magnetite nanoparticles. Environmental Nanotechnology. Monitor. Manag. 2019, 12, 100263. [Google Scholar] [CrossRef]
- Rambabu, K.; Banat, F.; Pham, Q.M.; Ho, S.-H.; Ren, N.Q.; Show, P.L. Biological remediation of acid mine drainage: Review of past trends and current outlook. Environ. Sci. Ecotechnol. 2020, 2, 100024. [Google Scholar] [CrossRef]
- Schwarz, A.; Suárez, J.I.; Aybar, M.; Nancucheo, I.; Martínez, P.; Rittmann, B.E. A membrane-biofilm system for sulfate conversion to elemental sulfur in mining-influenced waters. Sci Total Environ. 2020, 740, 140088. [Google Scholar] [CrossRef]
- Ramasamy, D.L.; Porada, S.; Sillanpää, M. Marine algae: A promising resource for the selective recovery of scandium and rare earth elements from aqueous systems. Chem. Eng. J. 2019, 371, 759–768. [Google Scholar] [CrossRef]
- Jacinto, J.; Henriques, B.; Duarte, A.C.; Vale, C.; Pereira, E. Removal and recovery of Critical Rare Elements from contaminated waters by living Gracilaria gracilis. J. Hazard. Mater. 2018, 15, 531–538. [Google Scholar] [CrossRef]
- Shi-Tian, H.; Jing-Yi, C.; Ke-Yan, T.; Yang, H.; Hu, X.; Wang, Z.; Yuan, X. A mine drainage treatment system for AMD in remediation of metal sulfide mines. China Geol. 2019, 2, 396–397. [Google Scholar] [CrossRef]
- Royer-Lavallée, A.; Neculita, C.M.; Coudert, L. Removal and potential recovery of rare earth elements from mine water. J. Ind. Eng. Chem. 2020, 89, 47–57. [Google Scholar] [CrossRef]
- DelValls, T.A.; Blasco, J. Integrated coastal area management and assessment of the Aznalcollar mining spill a case study to lind natural and social sciences. In Integrated Assessment and Management of the Ecosystems Affected by Aznalcollar Mining Spill (SW Spain); UNESCO/IOC/ICAM Technical report Sevilla; UNESCO: Paris, France, 2001. [Google Scholar]
- Arenas, J.M.; Carrero, G.; Galache, J.; Mediavilla, C.; Silgado, A.; Vázquez, E.M. Actuaciones realizadas tras el accidente de Aznalcóllar. Boletín Geológico Y Min. 2001, 112, 35–56. Available online: http://www.igme.es/boletin/2001/112_esp_2-2001/2-ACONTECIMIENTO.pdf (accessed on 1 September 2022).
- Blasco, J.; Arias, A.; Saénz, V. Heavy metals in organisms of the River Guadalquivir estuary: Possible incidence of the Aznalcóllar disaster. Sci. Total Environ. 1999, 242, 249–259. [Google Scholar] [CrossRef]
- Riba, I.; DelValls, T.A.; Forja, J.M.; Gómez-Parra, A. Monitoring the impact of the Aznalcóllar Mining Spill on recent sediments from the Guadalquivir estuary, Southwest Spain. Bull. Environ. Contam. Toxicol. 2002, 69, 129–138. [Google Scholar] [CrossRef]
- Riba, I.; Blasco, J.; Jiménez-Tenorio, N.; De Canales, M.L.G.; DelValls, T.A. Heavy metal bioavailability and effects: II. Histopathology–bioaccumulation relationships caused by mining activities in the Gulf of Cádiz (SW, Spain). Chemosphere 2005, 58, 671–682. [Google Scholar] [CrossRef]
- Riba, I.; Blasco, J.; Jiménez-Tenorio, N.; DelValls, T.A. Heavy metal bioavailability and effects: I. Bioaccumulation caused by mining activities in the Gulf of Cádiz (SW, Spain). Chemosphere 2005, 58, 659–669. [Google Scholar] [CrossRef]
- DelValls, T.A.; Riba, I. A weight of evidence approach to assess sediment quality in the Guadalquivir estuary. Aq. Ecosyst. Health Manag. 2007, 10, 101–106. [Google Scholar] [CrossRef]
- Pirrie, D.; Power, M.R.; Rollinson, G.; Camm, G.S.; Hughes, S.H.; Butcher, A.R.; Hughes, P. The spatial distribution and source of arsenic, copper, tin and zinc within the surface sediments of the Fal Estuary, Cornwall, UK. Sedimentology 2003, 50, 579–595. [Google Scholar] [CrossRef]
- Leblanc, M.; Morales, J.A.; Borrego, J.; Elbaz-Poulichet, F. 4500 year-old mining pollution in southwestern Spain: Long-term implications for modern mining pollution. Econ. Geol. 2000, 95, 655–662. [Google Scholar] [CrossRef]
- Gómez-Parra, A.; Forja, J.; DelValls, T.A.; Sáenz, I.; Riba, I. Early contamination by heavy metals of the Guadalquivir Estuary after the Aznalcóllar mining spill (SW Spain). Mar. Pollut. Bull. 2000, 40, 1115–1123. [Google Scholar] [CrossRef]
- Gelencsér, A.; Kováts, N.; Turóczi, B.; Rostási, Á.; Hoffer, A.; Imre, K.; Nyirő-Kósa, I.; Csákberényi-Malasics, D.; Tóth, Á.; Czitrovszky, A.; et al. The red mud accident in Ajka (Hungary): Characterization and potential health effects of fugitive dust. Environ. Sci. Technol. 2011, 45, 1608–1615. [Google Scholar] [CrossRef]
- Rodriguez-Freire, L.; Avasarala, S.; Ali, A.-M.S.; Agnew, D.; Hoover, J.H.; Artyushkova, K.; Latta, D.E.; Peterson, E.J.; Lewis, J.; Crossey, L.J.; et al. Post gold king mine spill investigation of metal stability in water and sediments of the animas river watershed. Environ. Sci. Technol. 2016, 50, 11539–11548. [Google Scholar] [CrossRef]
- Silva, D.C.; Bellato, C.R.; Marqués Neto, J.O.; Fontes, M.P.F. Trace elements in river waters and sediments before and after the mining dam breach (Bento Rodrigues, Brazil). Quim. Nova. 2018, 41, 857–866. [Google Scholar] [CrossRef]
- Queiroz, H.M.; Nóbrega, G.N.; Ferreira, T.O.; Almeida, L.S.; Romero, T.B.; Santaella, S.T.; Bernardino, A.F.; Oterob, X.L. The Samarco mine tailing disaster: A possible time-bomb for heavy metals contamination. Sci. Total Environ. 2018, 637, 498–506. [Google Scholar] [CrossRef]
- Santos, J.L.; Malvar, J.L.; Martín, J.; Aparicio, I.; Alonso, E. Distribution of metals in sediments of the Guadiamar river basin 20 years after the Aznalcóllar mine spill: Bioavailability and risk assessment. J. Environ. Manag. 2020, 260, 110146. [Google Scholar] [CrossRef]
- Segura, F.R.; Nunes, E.A.; Paniz, F.P.; Paulelli, A.C.C.; Rodrigues, G.B.; Braga, G.U.L.; Filho, W.D.R.P.; Barbosa, F., Jr.; Cerchiaro, G.; Silva, F.F.; et al. Potential risks of the residue from Samarco’s mine dam burst (Bento Rodrigues, Brazil). Environ. Pollut. 2016, 218, 813–825. [Google Scholar] [CrossRef]
- Hatje, V.; Pedreira, R.M.; De Rezende, C.E.; Schettini, C.A.F.; De Souza, G.C.; Marin, D.C.; Hackspacher, P.C. The environmental impacts of one of the largest tailing dam failures worldwide. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]

| DAS | ASE&C | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| AMD | AMD1 | AMD2 | AMD3 | ||||||
| Units | input | output | input | output | Input | output | input | output | |
| EC | µS cm−1 | 3293 | 341 | 4450 | 120 | 4340 | 110 | 4730 | 105 |
| T | °C | 14.8 | 11.2 | 25.2 | 25.1 | 25.7 | 25.2 | 24.9 | 24.8 |
| pH | 2.72 | 6.51 | 2.7 | 5.9 | 2.8 | 6.1 | 2.7 | 6.4 | |
| HCO3 | mg L−1 | <25 | n.d. | <25 | n.d. | <25 | n.d. | ||
| CN | µg L−1 | <12 | n.d. | <12 | n.d. | <12 | n.d. | ||
| DO | mg L−1 | 2.9 | 3.1 | 7.7 | 7.9 | 6.1 | 8.1 | 6.9 | 7.9 |
| Al | µg L−1 | 111,790 | 106 | 157,900 | n.d. | 142,400 | n.d. | 129,300 | n.d. |
| Sb | µg L−1 | <5.0 | n.d. | <10 | n.d. | <10 | n.d. | ||
| As | µg L−1 | 350 | 76 | 99 | n.d. | 159 | n.d. | 63 | n.d. |
| Ba | µg L−1 | <50 | n.d. | <100 | n.d. | <100 | n.d. | ||
| Be | µg L−1 | 17 | n.d. | 17 | n.d. | 18 | n.d. | ||
| Cd | µg L−1 | 63.02 | <1 | 120 | n.d. | 221 | n.d. | 230 | n.d. |
| Ca | mg L−1 | 133a | 551 a | 182 | n.d. | 185 | n.d. | 201 | n.d. |
| Cl | mg L−1 | 13 | n.d. | 14 | n.d. | 14 | n.d. | ||
| Co | µg L−1 | 417 | 5.94 | 927 | 1.1 | 907 | n.d. | 1003 | n.d. |
| Cu | µg L−1 | 13,509 | 17.2 | 19,810 | 1.6 | 25,220 | 4.2 | 25,600 | 3.2 |
| Cr | µg L−1 | 17.14 | <1 | 21 | n.d. | 22 | n.d. | 20 | n.d. |
| PO4 | mg L−1 | 0.19 | n.d. | 0.29 | n.d. | 0.14 | n.d. | ||
| Fe | µg L−1 | 348,663 | 220 | 180,200 | 12.5 | 232,600 | 15.1 | 213,000 | 21.2 |
| Mg | mg L−1 | 455 | n.d. | 465 | n.d. | 526 | n.d. | ||
| Mn | µg L−1 | 3171 | 3769 | 37,090 | 1.6 | 38,950 | 2.1 | 41,540 | 2.4 |
| Hg | µg L−1 | 0.023 | n.d. | 0.04 | n.d. | 0.03 | n.d. | ||
| Mo | µg L−1 | 6.3 a | 0.2 a | 2.2 | n.d. | 4.3 | n.d. | 2.0 | n.d. |
| Ni | µg L−1 | 126.7 | 2.81 | 480 | n.d. | 476 | n.d. | 521 | n.d. |
| Ag | µg L−1 | <50 | n.d. | <100 | n.d. | <100 | n.d. | ||
| Pb | µg L−1 | 19.07 | <1 | 62 | n.d. | 61 | n.d. | 60 | n.d. |
| K | mg L−1 | 1.5 | n.d. | 1.4 | n.d. | 1.7 | n.d. | ||
| Se | µg L−1 | 3.319 | <1 | 22 | n.d. | 21 | n.d. | 22 | n.d. |
| Na | mg L−1 | 17 | n.d. | 17 | n.d. | 20 | n.d. | ||
| SO4 | mg L−1 | 2872 a | 2061 a | 5502 | n.d. | 6066 | n.d. | 6648 | n.d. |
| Tl | µg L−1 | 0.06 a | 0.00 | <50 | n.d. | <100 | n.d. | <100 | n.d. |
| U | µg L−1 | 10.21 | <2 | 20 | n.d. | 20 | n.d. | 21 | n.d. |
| V | µg L−1 | 70.8 a | 0.4 a | 1.7 | n.d. | 5.3 | n.d. | 1.8 | n.d. |
| Zn | µg L−1 | 11,650 | 31.91 | 67,320 | n.d. | 70,200 | n.d. | 72,210 | n.d. |
| DAS technology | ASE&C technology | |
|---|---|---|
| Treatment type | Passive | Active |
| Treatment capacity | 0.8 L s−1 a | Unlimited * |
| CAPEX | 196100 € a | Under development |
| OPEX | 8428 € a | Under development |
| Installation | In situ built with significant extension and sloped terrain | Small portable module |
| Energy consumption | No (potential energy) | 5 kWh m−3 (renewable energy) |
| Chemicals | Limestone-DAS, pine wood chips, limestone sand | None |
| Valorization of residues as secondary sources | Water + re-valuable residue | Distilled water + metallic conglomerates |
| CO2 footprint | 1.86 kg CO2 eq m−3 | 0.5 kg CO2 eq m−3. |
| Compliance legislation/improvement ecosystem services | Yes (except SO4−,Mn, Fe) | Yes (all elements) |
| Aznalcóllar Characterization a | DAS Removal | ASE&C Removal | DAS Profit | ASE&C Profit | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| W (mg L−1) | S (g kg−1) | W (t yr−1) | S (t yr−1) | W (t yr−1) | S (t yr−1) | W (k$ yr−1) | S (k$ yr−1) | W (k$ yr−1) | S (k$ yr−1) | |
| As | 0.27 | 2.784 | 0.0059 | 61.0 | 0.063 | 609 | 0.019 | 195 | 0.189 | 1951 |
| Cd | 0.854 | 0.107 | 0.02353 | 2.95 | 0.187 | 23.4 | 0.061 | 7.66 | 0.486 | 60.9 |
| Zn | 462.8 | 38.82 | 12.92 | 1083 | 101 | 8501 | 25.85 | 2168 | 202 | 17,003 |
| Cu | 0.021 | 9.509 | 0.0006 | 265 | 0.004 | 2082 | 0.004 | 1595 | 0.028 | 12,495 |
| CrT | 0.03 | 0.009 | 0.0008 | 0.237 | 0.006 | 1.97 | 0.006 | 1.89 | 0.053 | 15.7 |
| Fe | 138.5 | 234 | 3.875 | 6550 | 30.3 | 51,267 | 0.678 | 1146 | 5.308 | 8972 |
| Mn | 91.7 | 0.27 | n.e. | n.e | 20.0 | 59.1 | n.a. | n.a. | n.a. | n.a. |
| Hg | <0.008 | 0.053 | n.e. | n.e | 0.002 | 11.6 | n.a. | n.a. | n.a. | n.a. |
| Ni | 1.115 | 0.003 | 0.0305 | 0.082 | 0.244 | 0.657 | 0.394 | 1.06 | 3.150 | 8.47 |
| Pb | 3.655 | 39.9 | 0.0969 | 1058 | 0.800 | 8738 | 0.165 | 1800 | 1.361 | 14,855 |
| Wheal Jane a | Aznalcóllar b | Aznalcóllar c | Aznalcóllar d | Ajka e | Colorado f | Mariana g | Mariana h | ||
|---|---|---|---|---|---|---|---|---|---|
| Spill volume | m3 | 50,000 | 7 M | 7 M | 7 M | 50 M | 700,000 | 50 M | 50 M |
| As | ppm | 983–2803 | 6100 | 4.25 ± 0.25 | 89–249 | ||||
| Cd | ppm | 31 | 0.79–3.25 | 0.58–1.38 | 0.66 ± 0.01 | 0.11–0.20 | |||
| Co | ppm | 60 | 15–41.6 | 10.7 ± 4.8 | |||||
| Cr | ppm | 553 | 75–145 | 63.9 ± 15.1 | |||||
| Cu | ppm | 1172–5073 | 2120 | 20.95–78.82 | 13.1–18.5 | 57 | 32.4 ± 0.5 | 17.8–47 | 21.3 ± 4.6 |
| Fe | % | 4.06–5. | 14.6–23.7 | 51 ± 0.3 | 45.2 ± 2.8 | ||||
| Mn | ppm | 1163–1562 | 412–535 | 433 ± 110 | |||||
| Ni | ppm | 248 | 9.1–53.7 | 24.7 ± 10.4 | |||||
| Pb | ppm | 217–570 | 8500 | 7.22–31.07 | 28.7–33.8 | 128 | 108.4 ± 1.8 | 4.7–12.5 | 20.2 ± 4.6 |
| Zn | ppm | 650–6600 | 21,200 | 162–290 | 166–395 | 105 | 729 ± 5.7 | 37.6–78.4 | 62.4 ± 28.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonnail, E.; Vera, S.; Blasco, J.; Conradi, M.; DelValls, T.Á. Metal Pollution and Mining in the Iberian Pyrite Belt: New Remediation Technologies to Improve the Ecosystem Services of the River Basins. Water 2023, 15, 1302. https://doi.org/10.3390/w15071302
Bonnail E, Vera S, Blasco J, Conradi M, DelValls TÁ. Metal Pollution and Mining in the Iberian Pyrite Belt: New Remediation Technologies to Improve the Ecosystem Services of the River Basins. Water. 2023; 15(7):1302. https://doi.org/10.3390/w15071302
Chicago/Turabian StyleBonnail, Estefanía, Sebastián Vera, Julián Blasco, Mercedes Conradi, and T. Ángel DelValls. 2023. "Metal Pollution and Mining in the Iberian Pyrite Belt: New Remediation Technologies to Improve the Ecosystem Services of the River Basins" Water 15, no. 7: 1302. https://doi.org/10.3390/w15071302
APA StyleBonnail, E., Vera, S., Blasco, J., Conradi, M., & DelValls, T. Á. (2023). Metal Pollution and Mining in the Iberian Pyrite Belt: New Remediation Technologies to Improve the Ecosystem Services of the River Basins. Water, 15(7), 1302. https://doi.org/10.3390/w15071302







