Hydrogeochemical Characteristics of Geothermal Water in Ancient Deeply Buried Hills in the Northern Jizhong Depression, Bohai Bay Basin, China
Abstract
:1. Introduction
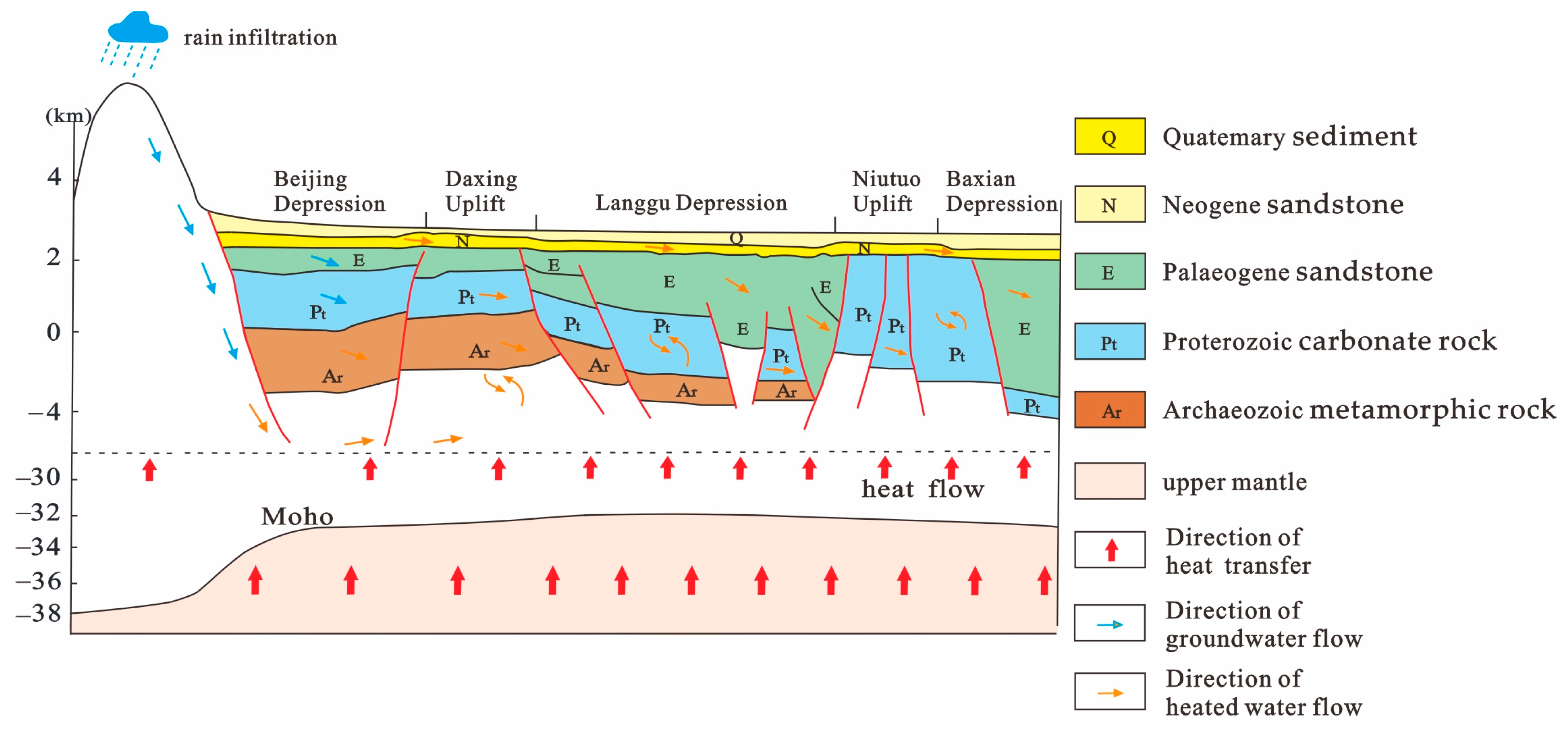
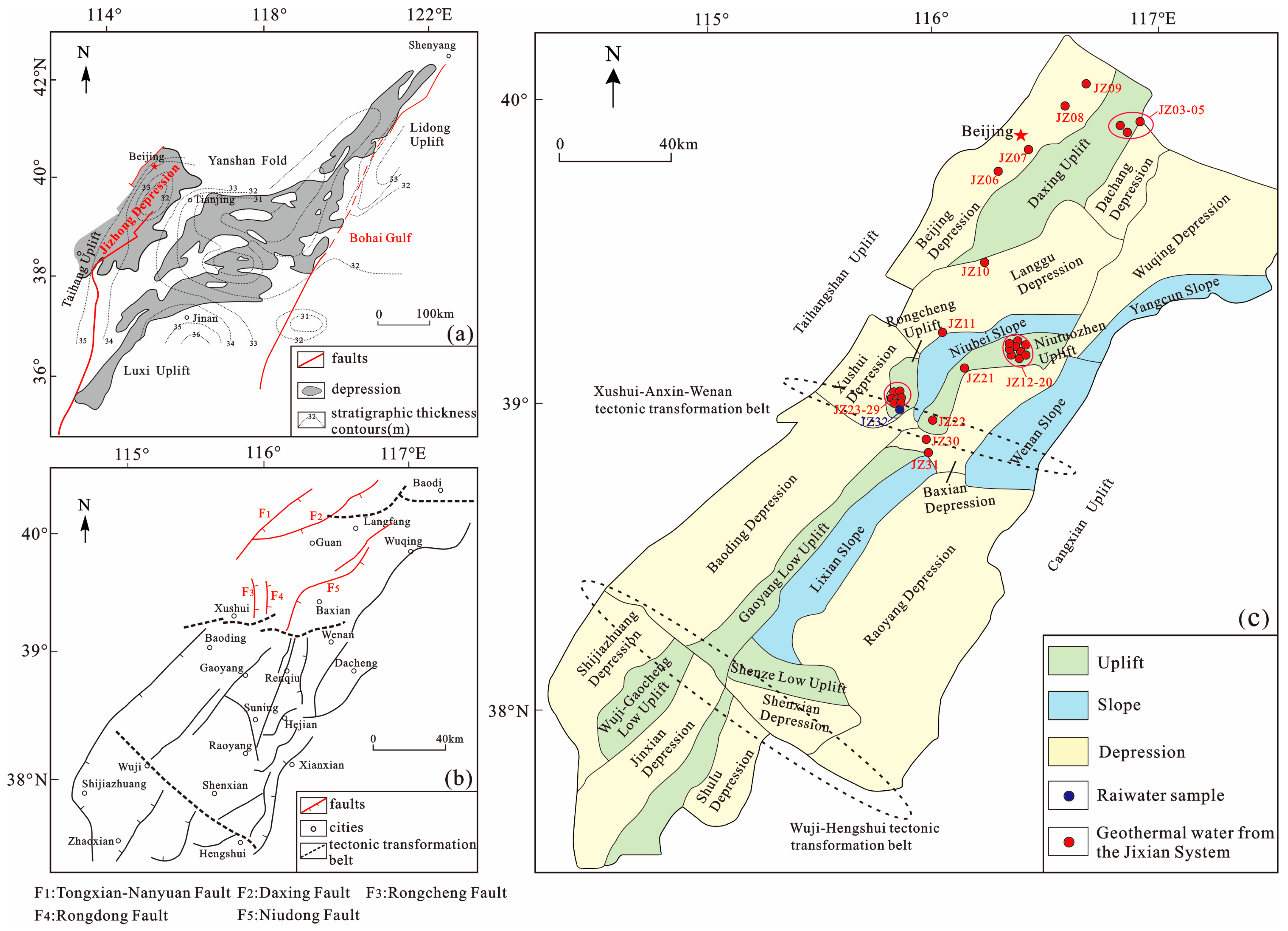
2. Geological Setting
3. Material and Methods
4. Results and Discussion
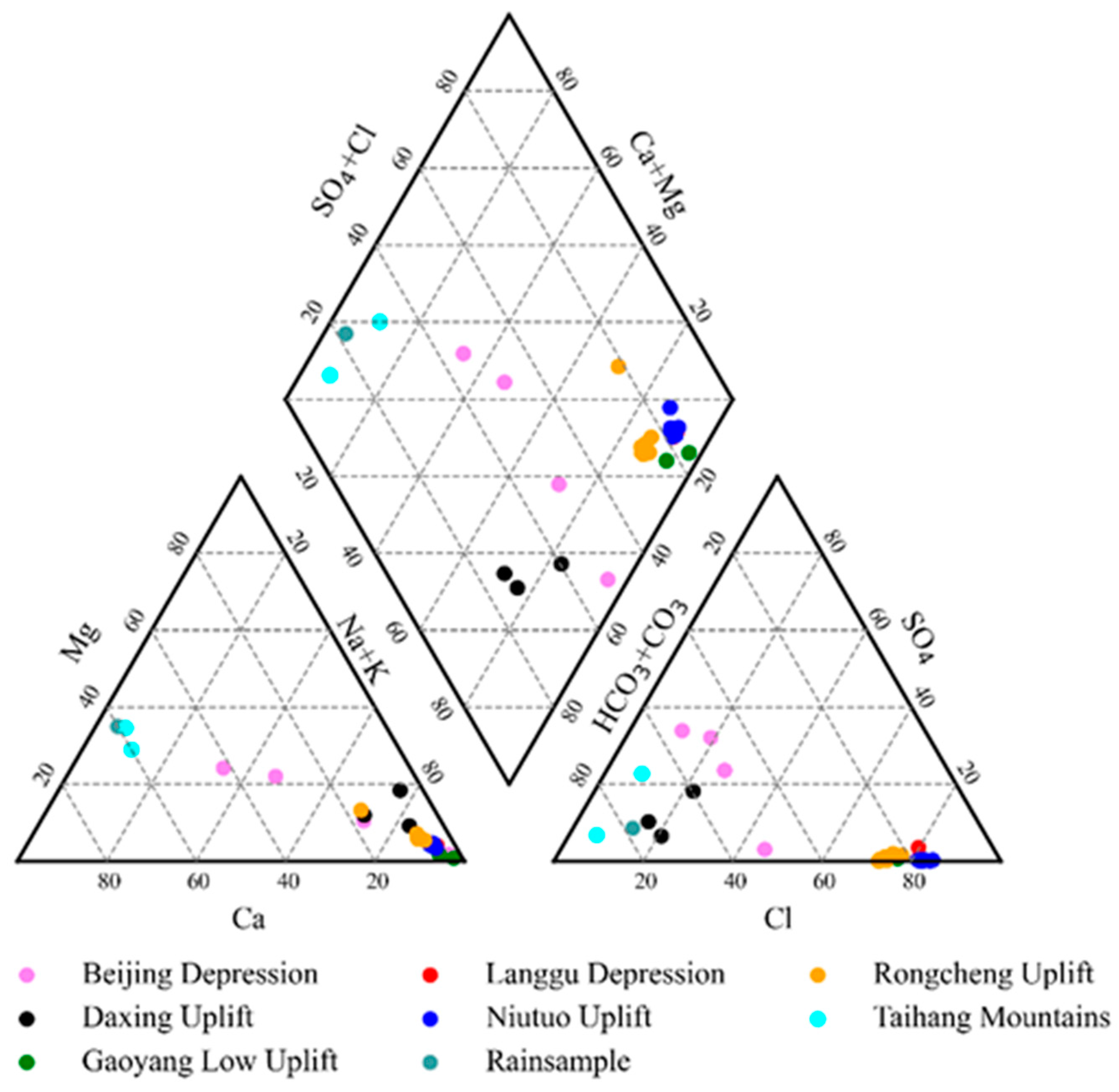
4.1. Hydrochemistry
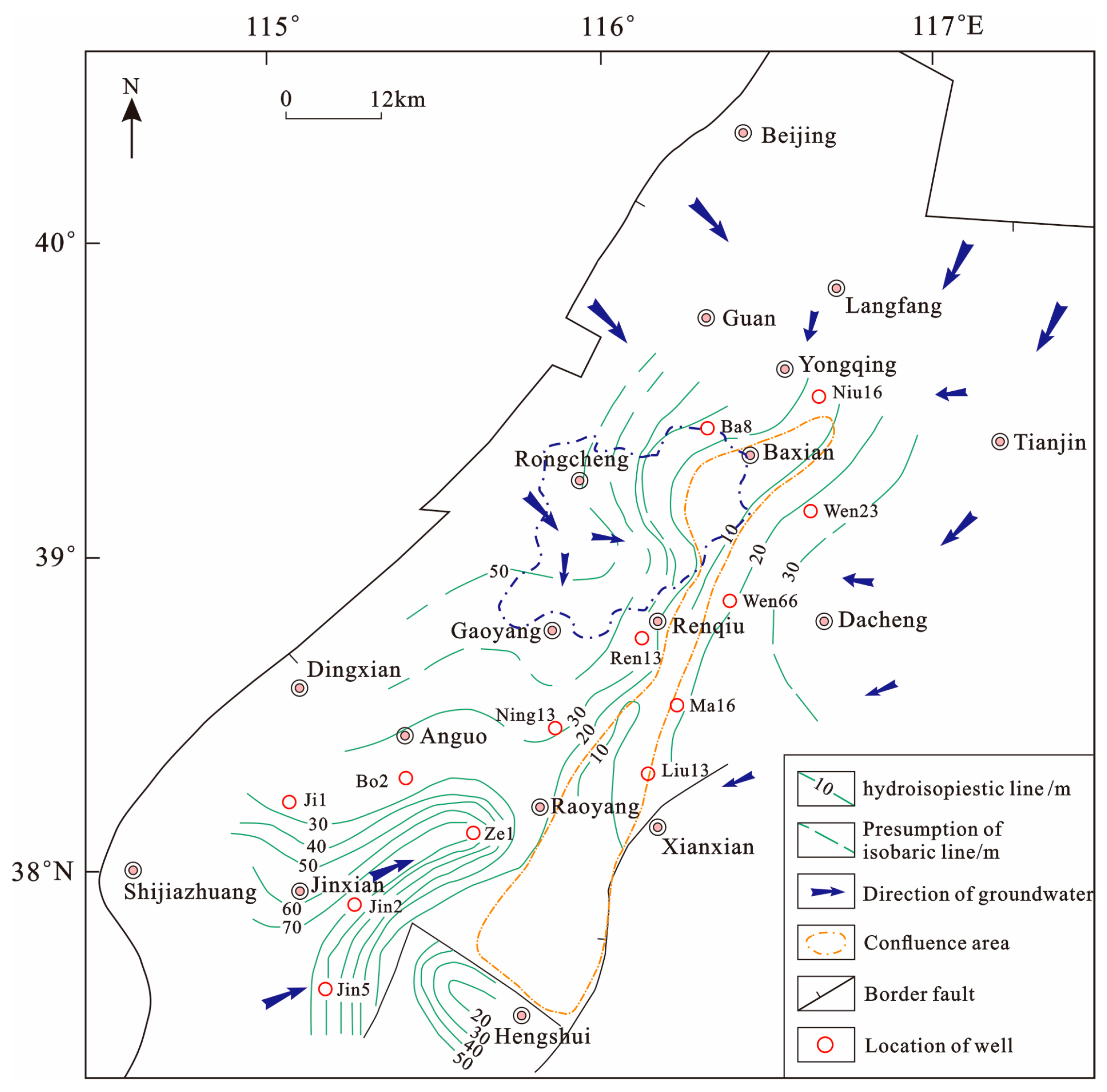
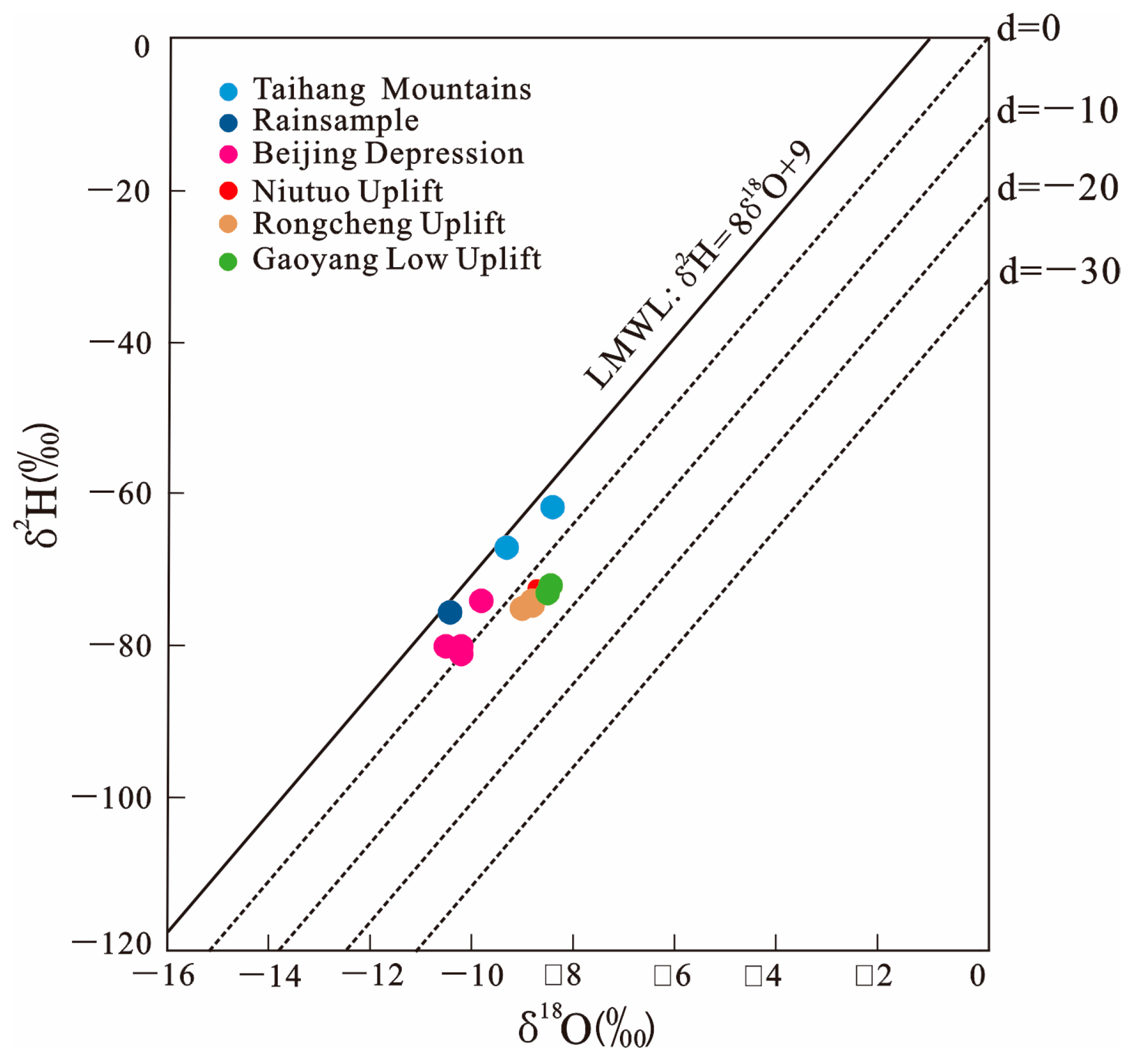
4.2. Isotopic Composition of Water
4.3. Geothermal Reservoir Temperature Estimation
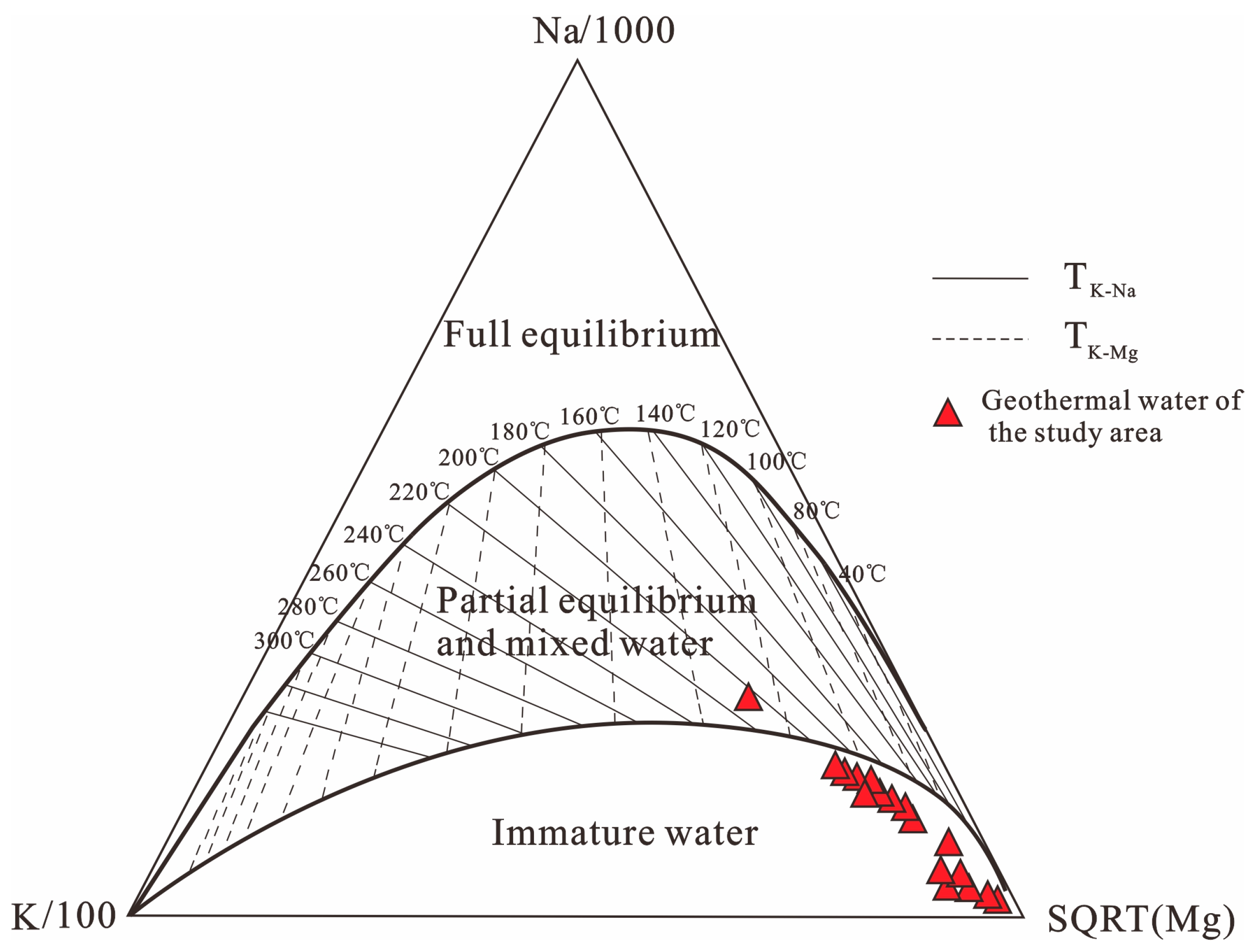
4.3.1. Combination of Empirical Chemical Geothermometers
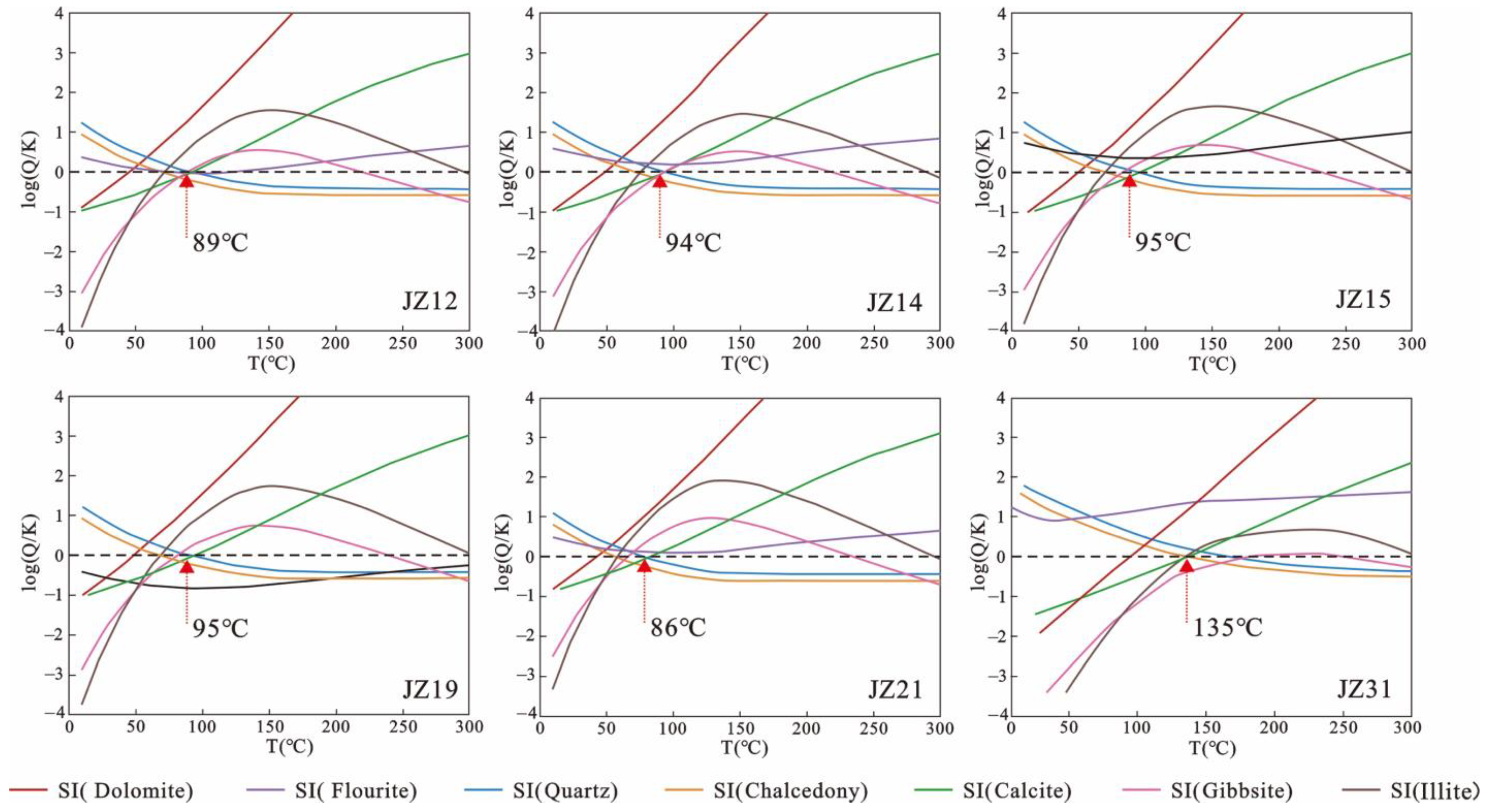
4.3.2. Modeling of Multi-Mineral Saturation States
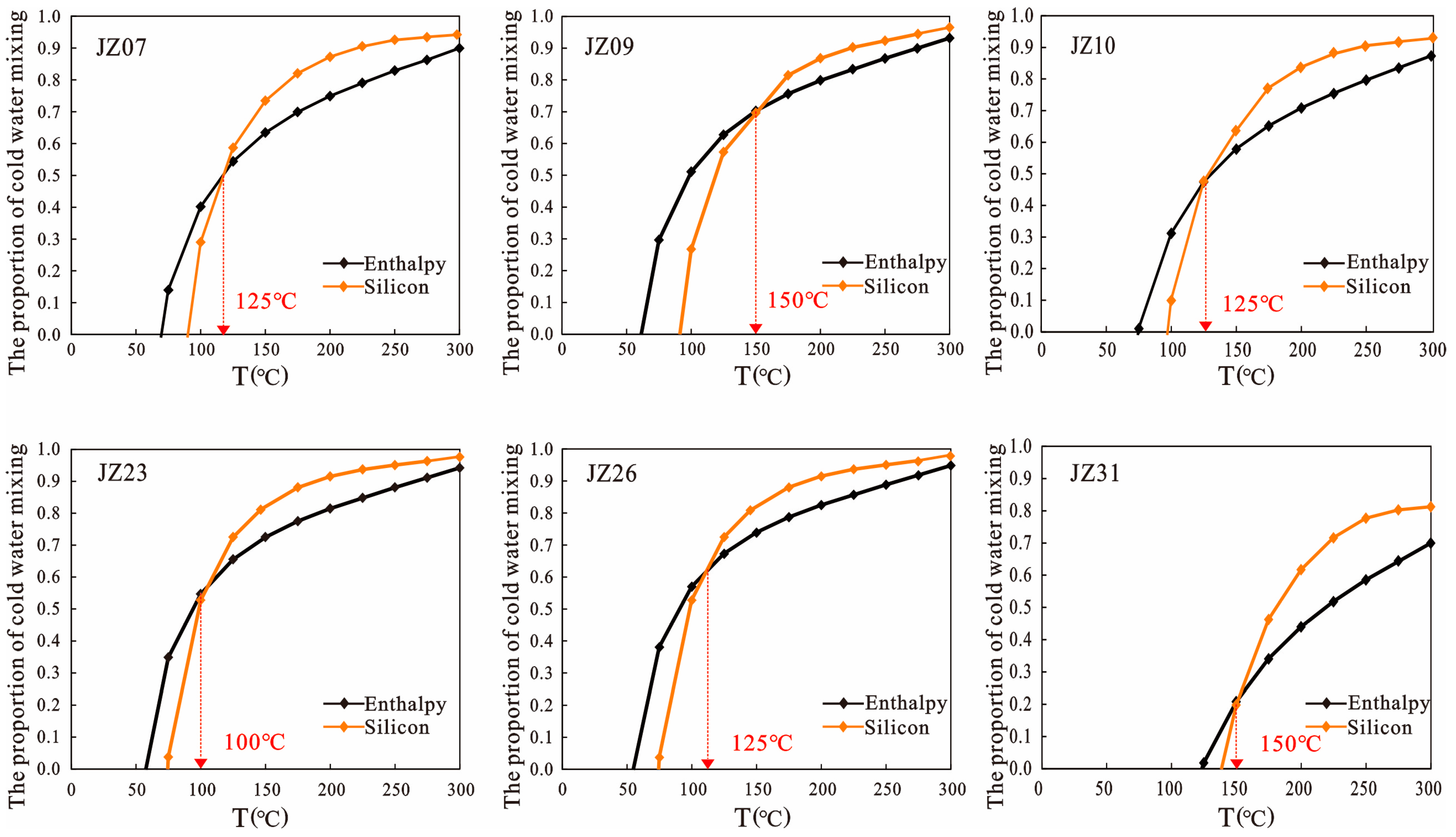
4.3.3. Silica–Enthalpy Mixing Models
4.4. Circulation Depth
5. Conceptual Genetic Model
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tester, J.W.; Anderson, B.J.; Batchelor, A.S.; Blackwell, D.D.; Dipippo, R.; Drake, E.M. The Future of Geothermal Energy. Mass. Inst. Technol. 2006, 358, 1–3. [Google Scholar]
- Hillis, R.R.; Hand, M.; Mildren, S.D.; Reid, P.D.; Reynolds, S.D.; Nelson, E.J. Hot Dry Rock Geothermal Exploration in Australia. ASEG Ext. Abstr. 2004, 1, 1–4. [Google Scholar] [CrossRef]
- Wang, G.L.; Lin, W.J.; Zhang, W.; Lu, C.; Ma, F.; Gan, H.N. Research on Formation Mechanisms of Hot Dry Rock Resources in China. Acta Geol. Sin. 2016, 90, 1418–1433. [Google Scholar]
- Liu, F.; Zhang, W.; Wang, G.L.; Wei, S.C.; Yue, C.; Jiang, G.Z.; Liao, Y.Z. Geothermal anomalies in the Xianshuihe area: Implications for tunnel construction along the Sichuan-Tibet Railway, China. J. Groundw. Sci. Eng. 2023, 11, 237–248. [Google Scholar] [CrossRef]
- Shi, H.L.; Wang, G.L.; Lu, C. Numerical investigation on delaying thermal breakthrough by regulating reinjection fluid path in multi-aquifer geothermal system. Appl. Therm. Eng. 2022, 221, 119692. [Google Scholar] [CrossRef]
- Lin, W.J.; Wang, G.; Gan, H.N.; Zhang, S.S.; Zhao, Z.; Yue, G.F.; Ting, L.X. Heat source model for Enhanced Geothermal Systems (EGS) under different geological conditions in China. Gondwana Res. 2023, 122, 243–259. [Google Scholar] [CrossRef]
- Wang, G.L.; Lin, W.J. Main hydro-geothermal systems and their genetic models in China. Acta Geol. 2020, 94, 1923–1937. (In Chinese) [Google Scholar]
- Ma, F.; Wang, G.L.; Zhang, W.; Zhu, X.; Zhang, H.X.; Yue, G.F. Structure of geothermal reservoirs and resource potential in the Rongcheng geothermal field in Xiong’an New Area. Acta Geol. Sin. 2020, 94, 1981–1990. (In Chinese) [Google Scholar]
- Wang, G.L.; Zhang, W.; Liang, J.Y.; Lin, W.J.; Liu, Z.M.; Wang, W.L. Evaluation of Geothermal Resources Potential in China. Acta Geosci. Sin. 2017, 38, 12. (In Chinese) [Google Scholar]
- Li, M.; Xing, L.X.; Wang, G.L.; Zhang, W.; Zhao, J.Y.; Jin, M.G. Distribution characteristics of fluorine in deep geothermal water in Jizhong Depression and its risk assessment and suggestions for utilization. Geol. China 2023. (In Chinese) [Google Scholar]
- Eberhard, L.; Pettke, T. Antigorite dehydration fluids boost carbonate mobilisation and crustal CO2 outgassing in collisional orogens. Geochim. Cosmochim. Acta 2021, 300, 192–214. [Google Scholar] [CrossRef]
- Weydt, L.M.; Heldmann, C.D.J.; Machel, H.G.; Sass, I. From oil field to geothermal reservoir: Assessment for geothermal utilization of two regionally extensive Devonian carbonate aquifers in Alberta, Canada. Solid Earth 2018, 9, 953–983. [Google Scholar] [CrossRef]
- Hoefs, J. Stable Isotope Geochemistry/Jochen Hoefs; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Chimeddorj, B.; Munkhbat, D.; Altanbaatar, B.; Dolgorjav, O.; Oyuntsetseg, B. Hydrogeochemical characteristics and geothermometry of hot springs in the Altai region, Mongolia. Geochem. Explor. Environ. Anal. 2021, 21, 4. [Google Scholar] [CrossRef]
- Stober, I.; Bucher, K. Geothermal Energy: From Theoretical Models to Exploration and Development; Springer Nature: Basel, Switzerland, 2021. [Google Scholar]
- Yurteri, C.; Simsek, S. Hydrogeological and hydrochemical studies of the Kaman-Savcili-Büyükoba (Kirsehir) geothermal area, Turkey. Geothermics 2017, 65, 99–112. [Google Scholar] [CrossRef]
- Soldatova, E.A.; Guseva, N.V.; Sun, Z.; Mazurova, I.S. Size fractionation of trace elements in the surface water and groundwater of the Ganjiang River and Xiushui River basins, China. IOP Conf. Ser. Earth Environ. Sci. 2015, 27, 012037. [Google Scholar] [CrossRef]
- Han, D.M.; Currell, M.J. Delineating multiple salinization processes in a coastal plain aquifer, northern China: Hydrochemical and isotopic evidence. Hydrol. Earth Syst. Sci. 2018, 22, 3473–3491. [Google Scholar] [CrossRef]
- Pang, J.M.; Pang, Z.H.; Lv, M.; Tian, J.; Kong, Y.L. Geochemical and isotopic characteristics of fluids in the Niutuozhen geothermal field, North China. Environ. Earth Sci. 2018, 77, 12. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, G.L.; Wang, X.Y.; Qi, S.H.; Ma, F.; Zhang, W.; Zhang, H.X. Hydrogeochemical and Isotopic Analyses of Deep Geothermal Fluids in the Wumishan Formation in Xiong’an New Area, China. Lithosphere 2022, 2022, 2576752. [Google Scholar] [CrossRef]
- Yu, M.X.; Wang, G.L.; Ma, F.; Zhang, W.; Lin, W.J.; Zhu, X.; Zhang, H.X. Geochemical characteristics of geothermal fluids of a deep ancient buried hill in the Xiong’an New Area of China. Water 2022, 14, 3182. [Google Scholar] [CrossRef]
- Wang, Y.J.; Ma, F.; Xie, H.P.; Wang, G.L.; Wang, Z.H. Fracture Characteristics and Heat Accumulation of Jixianian Carbonate Reservoirs in the Rongcheng Geothermal Field, Xiong’an New Area. Acta Geol. Sin. Engl. Ed. 2021, 95, 1902–1914. [Google Scholar] [CrossRef]
- He, D.F.; Cui, Y.Q.; Shan, S.Q.; Xiao, Y.; Zhang, Y.Y.; Zhang, C.B. Characteristics of geologic framework of buried-hill in Jizhong depression, Bohai Bay Basin. Chin. J. Geol. 2018, 53, 1–24. (In Chinese) [Google Scholar]
- Chang, J.; Qiu, N.S.; Zhao, X.Z.; Xu, W.; Xu, Q.C.; Jin, F.M.; Han, C.Y.; Ma, X.F.; Dong, X.Y. Present-day geothermal regime of the Jizhong depression in Bohai Bay basin, East China. Chin. J. Geophys. 2016, 59, 1003–1016. (In Chinese) [Google Scholar]
- Zhao, H.G.; Liu, C.Y. Research on the Segmentation of Daxing Fault. Oil Cas Geol. 2002, 23, 368–371. (In Chinese) [Google Scholar]
- Zhao, J.Y.; Zhang, W.; Ma, F.; Zhu, X.; Zhang, H.X.; Wang, G.L. Geothemical characteristics of the geothermal fluid in the Rongcheng geothermal field, Xiong’an New Area. Acta Geol. Sin. 2020, 94, 1992–2001. (In Chinese) [Google Scholar]
- Zhu, X.; Wang, G.L.; Ma, F.; Lin, W.J.; Zhang, W.; Zhang, B.J.; Jia, X.F.; Zhang, H.X. Evaluation of Geothermal Resources of the Xiong’an New Area. Earth Sci. 2023, 48, 1093–1106. (In Chinese) [Google Scholar]
- Song, J.J.; Wang, G.L.; Xing, L.X.; Qian, J.Z.; Dai, L.; Di, H. Influencing factors of rock thermal conductivity and applicability ecaluation of its mixing law predicitive models. Geothermics 2023, 110, 102680. [Google Scholar] [CrossRef]
- Yang, M.H.; Liu, C.Y.; Sun, D.S.; Cui, Y.Q. Extensional tectonic system and its deep-seated setting of Jizhong Basin, China. Geotectinic Metallog. 2002, 26, 113–120. (In Chinese) [Google Scholar]
- DZ/T 0184.6-1997; Determination of Samarium-Neodymium Isotope Geological Age and Neodymium Isotope Ratio. Institute of Geology, Ministry of Geology and Mineral Resources: Nanjing, China, 1997. (In Chinese)
- Deng, J.Z. Chemical Characteristics and Genetic Analysis If Geothermal Water in Carbonate Reservoir in the Northern Jizhong Depression. Master’s Thesis, East China University of Technology, Shanghai, China, 2022. (In Chinese). [Google Scholar]
- Mazela, W.; Krasodomski, W.; Pajda, M. Assessment of the Main Threats to Injection Well Damage Caused by Reservoir Waters using AquaChem Software as well as Laboratory Tests Application. J. China Hydrol. 2012, 29, 50–53. [Google Scholar]
- Dinka, M.O.; Loiskandl, W.; Ndambuki, J.M. Hydrochemical characterization of various surface water and groundwater resources available in Matahara areas, Fantalle Woreda of Oromiya region. J. Hydrol. Reg. Stud. 2015, 3, 444–456. [Google Scholar] [CrossRef]
- Arnórsson, S. Isotopic and Chemical Techniques in Geothermal Exploration, Development and Use; International Atomic Energy Agency: Vienna, Austria, 2000; p. 351. [Google Scholar]
- Liu, M.L.; He, T.; Wu, Q.F.; Guo, Q.H. Hydrogeochemistry of geothermal waters from Xiong’an New Area and its Indicating significance. Earth Sci. 2020, 45, 2221–2231. (In Chinese) [Google Scholar]
- GB8537-2018; National Standard for Food Safety Drinking Natural Mineral Water. National Health Commission of the People's Republic of China State Administration for Market Regulation: Beijing, China, 2018. (In Chinese)
- Wang, S.Q.; Zhang, B.J.; Yan, L.Y.; Xing, Y.F.; Yuan, W.Z.; Li, J.; Gao, J.; Zhao, T. Heat accumulation mechanism of deep ancient buried hill in the northeast of Gaoyang geothermal field, Xiong’an New Area. Bull. Geol. Sci. Technol. 2021, 40, 12–21. (In Chinese) [Google Scholar]
- Can, I. A new improved Na/K geothermometer by artificial neural networks. Geothermics 2002, 31, 751–760. [Google Scholar] [CrossRef]
- Henley, R.W.; Truesdell, A.H.; Barton, P.B., Jr.; Whitney, J.A. Fluid-Mineral Equilibria in Hydrothermal Systems. Soc. Econ. Geol. 1984, 1, 66. [Google Scholar]
- Deng, J.Z.; Lin, W.J.; Xing, L.X.; Chen, L. The Estimation of Geothermal Reservoir Temperature Based on Integrated Multicomponent Geothermometry: A Case Study in the Jizhong Depression, North China Plain. Water 2022, 14, 2489. [Google Scholar] [CrossRef]
- Pang, Z.H.; Kong, Y.L.; Pang, J.M.; Hu, S.B.; Wang, J.Y. Geothermal resources and development in Xiong’an New Area. Bull. Chin. Acad. Sci. 2017, 32, 1224–1230. (In Chinese) [Google Scholar]
- Jiang, Y.; Li, J.; Xing, Y.F.; Liu, Y.L.; Wang, H.Q.; Teng, Y.G.; Wang, G.L. Evaluation of Geochemical Geothermometers with Borehole Geothermal Measurements: A Case study of the Xiong’an New Area. Earth Sci. 2023, 48, 958–972. (In Chinese) [Google Scholar]
- Fournier, R.O. Chemical geothermometers and mixing models for geothermal systems. Geothermics 1977, 5, 41–50. [Google Scholar] [CrossRef]
- Fournier, R.O.; Rowe, J.J. Estimation of underground temperatures from the silica content of water from hot springs and wet-steam wells. Am. J. Sci. 1966, 264, 685–697. [Google Scholar] [CrossRef]
- Fournier, R.O.; Truesdell, A.H. An empirical Na-K-Ca geothermometer for natural waters. Geochim. Cosmochim. Acta 1973, 37, 1255–1275. [Google Scholar] [CrossRef]
- Fournier, R.O.; Truesdell, A.H. Geochemical indicators of subsurface temperature: Part 2, estimation of temperature and fraction of hot water mixed with cold water. J. Res. US Geol. Surv. 1974, 2, 263–270. [Google Scholar]
- Giggenbach, W.F. Geothermal solute equilibria. Derivation of Na-K-Mg-Ca geoindicators. Geochim. Cosmochim. Acta 1988, 52, 2749–2765. [Google Scholar] [CrossRef]
- Li, J.X. Study on the Key Factors of Geothermometers and Their Application: A Case Study of Crystalline Basement Reservoirs. Ph.D. Thesis, China University of Geosciences, Wuhan, China, 2019. (In Chinese). [Google Scholar]
- Wei, Z.A.; Huang, S.P.; Wang, C.S.; Zhang, M. Geochemistry and Its Geothermal Reservoir Implications of Geothermal Water in the Guangdong-Hong Kong-Macao Greater Bay Area, South China. Acta Geosci. Sin. 2023, 44, 117–132. (In Chinese) [Google Scholar]
- Benjamin, T.; Charles, R.; Vidale, R. Thermodynamic parameters and experimental data for the Na-K-Ca geothermometer. J. Volcanol. Geotherm. Res. 1983, 15, 167–186. [Google Scholar] [CrossRef]
- Chiodini, G.; Cioni, R.; Guidi, M.; Marini, L. Chemical geothermometry and geobarometry in hydrothermal aqueous solutions: A theoretical investigation based on a mineral-solution equilibrium model. Geochim. Cosmochim. Acta 1991, 55, 2709–2727. [Google Scholar] [CrossRef]
- Alsemgeest, J.; Auqué, L.F.; Gimeno, M.J. Verification and comparison of two thermodynamic databases through conversion to PHREEQC and multicomponent geothermometrical calculations. Geothermics 2021, 91, 102036. [Google Scholar] [CrossRef]
- Reed, M.; Spycher, N. Calculation of pH and mineral equilibria in hydrothermal water with application to geothermometry and studies of boiling and Dilution. Geochim. Cosmochim. Acta 1984, 48, 1479–1492. [Google Scholar] [CrossRef]
- Spycher, N.; Peiffer, L.; Sonnenthal, E.L.; Saldi, G.; Reed, M.H.; Kennedy, B.M. Integrated multicomponent solute geothermometry. Geothermics 2014, 51, 113–123. [Google Scholar] [CrossRef]
- Tole, M.P.; Ármannsson, H.; Zhong-He, P.; Arnórsson, S. Fluid/mineral equilibrium calculations for geothermal fluids and chemical geothermometry. Geothermics 1993, 22, 17–37. [Google Scholar] [CrossRef]
- Parkhurst, D.L.; Appelo, C.A.J. User’s Guide to PHREEQC (Version 2)—A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations. Water Resour. Investig. Rep. 1999, 99, 1–317. [Google Scholar]
- Xu, W.C. Application of the saturation index method to the study of geothermal geochemistry. J. Xi'an Coll. Geol. 1992, 14, 66–70. (In Chinese) [Google Scholar]
- Truesdell, A.H.; Fournier, R.O. Procedure for estimating the temperature of a hot-water component in a mixed water by using a plot of dissolved silica versus enthalpy. J. Res. U.S. Geol. Surv 1977, 5, 49–52. [Google Scholar]
- Keenan, J.H.; Keyes, F.G.; Hill, P.G.; Moore, J.G. Steam Tables-Thermodynamic Properties of Water Including Vapor, Liquid, and Solid Phases (International Edition—Metric Units); Wiley: Hoboken, NJ, USA, 1969. [Google Scholar]
- Zhao, Z.R.; Zhang, W.; Wang, G.L.; Xing, L.X.; Zhang, H.X.; Zhao, J.Y. Hydrogeochemical characteristics of Gaoyang geothermal field in central Hebei Depression and its constraint on geothermal genesis. Geol. China 2023. (In Chinese) [Google Scholar]
- Zhang, W.; Wang, G.L.; Zhao, J.Y.; Liu, F. Geochemical characteristics of medium-high temperature geothermal fluids in West Sichuan and their geological implications. Geoscience 2021, 35, 188–198. (In Chinese) [Google Scholar]
- Yu, M.X.; Ma, F.; Wang, G.L.; Zhang, W.; Zhu, X.; Zhang, H.X.; Wang, Y.X. Characteristics and Evaluation of Geothermal Resources in the Rongdong Area of Xiong’an New Area. Acta Geosci. Sin. 2023, 44, 180–190. (In Chinese) [Google Scholar]
- Shahbaz, A.M.; Nakashima, Y.; Nishigaki, M. Clogging mechanisms and preventive measures in artificial recharge systems. J. Groundw. Sci. Eng. 2021, 9, 181–201. [Google Scholar]
- Mohammad, A.T.; Jalut, Q.H.; Abbas, N.L. Predicting groundwater level of wells in the Diyala River Basin in eastern Iraq using artificial neural network. J. Groundw. Sci. Eng. 2020, 8, 87–96. [Google Scholar]
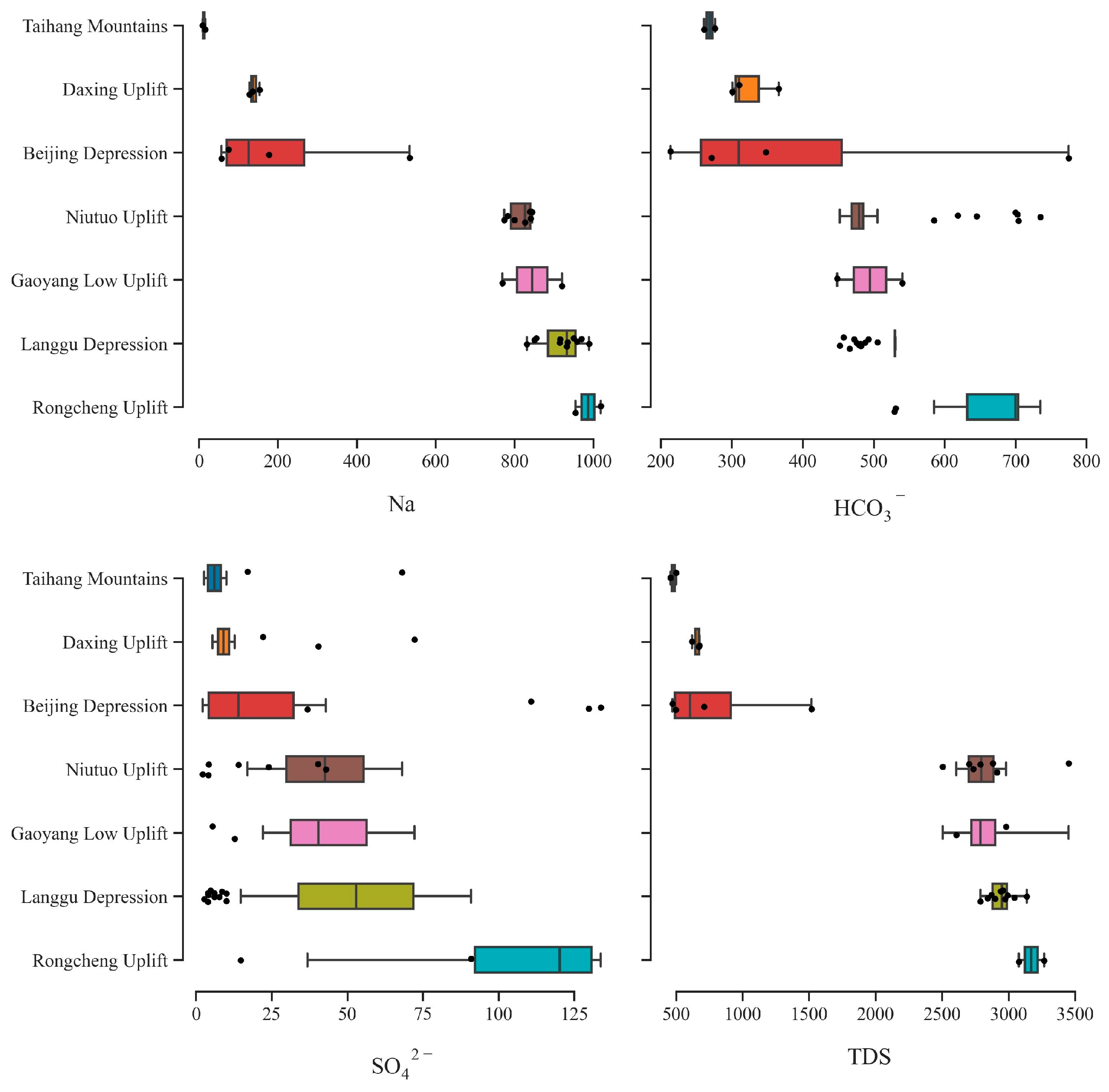
| Sample No. | Station | Longitude | Latitude | T (°C) | TDS | pH | K+ | Na+ | Ca2+ | Mg2+ |
|---|---|---|---|---|---|---|---|---|---|---|
| JZ01 * | Taihang Mountains | 115°12′35.04″ | 39°04′13.37″ | 14.0 | 458 | 7.69 | 0.7 | 9.02 | 70.6 | 25.2 |
| JZ02 * | Taihang Mountains | 115°47′36.9″ | 39°19′51.1″ | 14.0 | 501.9 | 7.92 | 2.3 | 15.6 | 80.8 | 23.5 |
| JZ03 | Daxing Uplift | 116°46′55″ | 39°55′02″ | 33.9 | 619.6 | 8.37 | 19 | 136.5 | 8.7 | 18.7 |
| JZ04 | Daxing Uplift | 116°57′40″ | 40°00′02″ | 59.2 | 675.8 | 7.93 | 24.7 | 128 | 28.6 | 12.5 |
| JZ05 | Daxing Uplift | 116°47′57″ | 39°53′21″ | 37.9 | 670.2 | 7.85 | 19.3 | 153.5 | 13.4 | 9.6 |
| JZ06 * | Beijing Depression | / | / | 36.4 | 498.4 | 8.2 | 9.5 | 57.1 | 67.4 | 23.5 |
| JZ07 * | Beijing Depression | / | / | 67.0 | 473.9 | 7.9 | 9.2 | 74.9 | 46.7 | 19.9 |
| JZ08 * | Beijing Depression | / | / | 45 | 711 | 8.2 | 18.6 | 177.5 | 39.3 | 14.4 |
| JZ09 * | Beijing Depression | / | / | 58 | 1520 | 8.3 | 36.3 | 533.8 | 13.2 | 5.7 |
| JZ10 | Langgu Depression | 116°12′44″ | 39°29′05″ | 74.4 | 3076 | 7.24 | 54.6 | 954.4 | 41.9 | 22.6 |
| JZ11 | Langgu Depression | 116°03′53″ | 39°07′03″ | 82 | 3267.4 | 7.64 | 55.1 | 1019 | 40.8 | 24.8 |
| JZ12 | Niutuozhen Uplift | 116°21′47″ | 39°14′51″ | 86 | 2940 | 7.13 | 55.6 | 970 | 43.7 | 26.5 |
| JZ13 | Niutuozhen Uplift | 116°19′59″ | 39°12′34″ | 86 | 2842 | 7.57 | 50.6 | 915.9 | 41.6 | 21.7 |
| JZ14 | Niutuozhen Uplift | 116°16′35″ | 39°13′42″ | 80 | 2971.9 | 7.61 | 53.6 | 958.7 | 47.3 | 24.8 |
| JZ15 | Niutuozhen Uplift | 116°17′12″ | 39°13′47″ | 80 | 2899.3 | 7.15 | 51.3 | 950.1 | 50 | 22.6 |
| JZ16 | Niutuozhen Uplift | 116°20′15″ | 39°13′12″ | 88 | 2958 | 7.42 | 56.1 | 934.4 | 41.9 | 19.3 |
| JZ17 | Niutuozhen Uplift | 116°19′47″ | 39°13′14″ | 91 | 2871 | 7.5 | 53.8 | 851.1 | 47.5 | 22.2 |
| JZ18 | Niutuozhen Uplift | 116°17′41″ | 39°14′08″ | 81 | 3137.2 | 7.6 | 52.5 | 989.5 | 71.4 | 36.1 |
| JZ19 | Niutuozhen Uplift | 116°20′38″ | 39°13′39″ | 87 | 3044 | 7.31 | 51 | 933.2 | 42.4 | 21 |
| JZ20 | Niutuozhen Uplift | 116°21′54″ | 39°15′08″ | 85 | 2787.6 | 8.2 | 51.6 | 855 | 49 | 23 |
| JZ21 | Niutuozhen Uplift | 116°11′41″ | 39°08′40″ | 83 | 2991 | 7.3 | 54.8 | 914.3 | 48.1 | 23.3 |
| JZ22 | Niutuozhen Uplift | 116°01′12″ | 38°56′15″ | 84 | / | 7.34 | 47.3 | 831.9 | 39.6 | 23.7 |
| JZ23 | Rongcheng Uplift | 115°50′55″ | 39°03′08″ | 55 | 2881 | 7.61 | 44.6 | 841.5 | 65.6 | 30.4 |
| JZ24 | Rongcheng Uplift | 115°52′53″ | 39°02′56″ | 50 | 2505 | 7.33 | 50.5 | 844.5 | 63 | 38.8 |
| JZ25 | Rongcheng Uplift | 115°51′22″ | 39°02′42″ | 52 | 2734 | 8.87 | 41.1 | 800.3 | 62 | 31.5 |
| JZ26 | Rongcheng Uplift | 116°55′03″ | 39°03′19″ | 53.1 | 3452 | 6.61 | 43 | 839.5 | 176.9 | 85.4 |
| JZ27 | Rongcheng Uplift | 115°51′45.00″ | 38°59′07.59″ | 72 | 2787 | 7.4 | 43.2 | 782.6 | 55.4 | 29 |
| JZ28 | Rongcheng Uplift | 115°52′59.11″ | 39°00′55.24″ | 62 | 2914 | 7.1 | 47 | 827.1 | 62.6 | 32 |
| JZ29 | Rongcheng Uplift | 115°52′40.63″ | 39°05′29.03″ | 70.8 | 2704 | 7.32 | 41.8 | 774.3 | 48.9 | 26 |
| JZ30 | Gaoyang Low Uplift | 115°56′37.91″ | 38°52′22.24″ | 109.2 | 2606 | 7.94 | 47.8 | 769.2 | 35.5 | 9.1 |
| JZ31 | Gaoyang Low Uplift | 115°57′24″ | 38°47′09″ | 123.4 | 2980 | 8.48 | 64 | 920.6 | 17 | 4.3 |
| JZ32 * | Rain Sample | 115°55′42.36″ | 38°58′39.22″ | 20 | 26.88 | 7.85 | 1.2 | 4.4 | 54.3 | 19 |
| Sample No. | Station | Li+ | Sr2+ | HCO3− | Cl− | SO42− | SiO2 | F− | Hydrochemical Type | |
| JZ01 * | Taihang Mountains | / | 0.1 | 276.5 | 11.32 | 17 | / | / | HCO3-Ca·Mg | |
| JZ02 * | Taihang Mountains | / | 0.38 | 261.1 | 17.95 | 68.1 | / | / | HCO3-Ca·Mg | |
| JZ03 | Daxing Uplift | 0.36 | 0.17 | 310.6 | 50.9 | 22.1 | 21.6 | 19.2 | HCO3-Na·Mg | |
| JZ04 | Daxing Uplift | 0.34 | 0.23 | 366 | 45.3 | 40.5 | 22.4 | 7.9 | HCO3-Na | |
| JZ05 | Daxing Uplift | 0.43 | 0.3 | 300.8 | 63.7 | 72.2 | 20.1 | 14.3 | HCO3·Cl-Na | |
| JZ06 * | Beijing Depression | 0.15 | 1.89 | 271.5 | 33.5 | 133.8 | 73.4 | 4.7 | HCO3·SO4-Ca·Na·Mg | |
| JZ07 * | Beijing Depression | 0.19 | 0.96 | 213.6 | 47.5 | 110.7 | 61.8 | 4.8 | HCO3·SO4-Na·Ca·Mg | |
| JZ08 * | Beijing Depression | 0.3 | 1.14 | 348.4 | 105.5 | 129.8 | 58.9 | 7.2 | HCO3·Cl·SO4-Na | |
| JZ09 * | Beijing Depression | 0.76 | 0.88 | 774.9 | 394.7 | 36.8 | 63.2 | 13.4 | HCO3·Cl-Na | |
| JZ10 | Langgu Depression | 1.97 | 1.89 | 529.4 | 1345 | 14.83 | 72.9 | 9.8 | Cl-Na | |
| JZ11 | Langgu Depression | / | / | 530.9 | 1453.4 | 90.9 | 68.8 | 11.6 | Cl-Na | |
| JZ12 | Niutuozhen Uplift | 1.51 | 1.65 | 476 | 1350 | 7.7 | 73.6 | 10 | Cl-Na | |
| JZ13 | Niutuozhen Uplift | 1.31 | 1.48 | 479.2 | 1215 | 8.58 | 57.7 | 17 | Cl-Na | |
| JZ14 | Niutuozhen Uplift | 1.85 | 1.66 | 488.1 | 1318.9 | 6 | 68.5 | 8.1 | Cl-Na | |
| JZ15 | Niutuozhen Uplift | 1.91 | 1.62 | 457.4 | 1289.3 | 4.8 | 60.5 | 9.7 | Cl-Na | |
| JZ16 | Niutuozhen Uplift | 1.72 | 1.69 | 481.5 | 1296 | 10 | 63.7 | 9.6 | Cl-Na | |
| JZ17 | Niutuozhen Uplift | 1.64 | 1.73 | 492.5 | 1288 | 6 | 57.9 | 10 | Cl-Na | |
| JZ18 | Niutuozhen Uplift | 1.87 | 1.6 | 452.3 | 1457 | 10.1 | 60.3 | 10.1 | Cl-Na | |
| JZ19 | Niutuozhen Uplift | 1.91 | 1.86 | 466 | 1418 | 3.89 | 60.2 | 9.5 | Cl-Na | |
| JZ20 | Niutuozhen Uplift | 1.61 | 1.47 | 472.4 | 1259.3 | 4 | 52.5 | 10 | Cl-Na | |
| JZ21 | Niutuozhen Uplift | 1.24 | 1.94 | 505.2 | 1375.6 | 3.9 | 46.9 | 2.1 | Cl-Na | |
| JZ22 | Niutuozhen Uplift | / | / | 482.1 | 1240.8 | 2.7 | 43 | 9.6 | Cl-Na | |
| JZ23 | Rongcheng Uplift | 1.25 | 2.17 | 585 | 1084 | 40.4 | 107.4 | 8.2 | Cl·HCO3-Na | |
| JZ24 | Rongcheng Uplift | 1.32 | 1.3 | 702.43 | 1095.4 | 14.1 | 150.3 | 7.5 | Cl·HCO3-Na | |
| JZ25 | Rongcheng Uplift | 1.21 | 1.62 | 618.8 | 1033 | 24 | 28.4 | 8 | Cl·HCO3-Na | |
| JZ26 | Rongcheng Uplift | 1.35 | 2.3 | 699.8 | 1450 | 43 | 50.2 | 7.3 | Cl·HCO3-Na | |
| JZ27 | Rongcheng Uplift | 1.56 | / | 704.3 | 1085 | 2.2 | 34.4 | 7.4 | Cl·HCO3-Na | |
| JZ28 | Rongcheng Uplift | 1.41 | 2.65 | 735 | 1118 | 4.2 | 41.6 | 6.5 | Cl·HCO3-Na | |
| JZ29 | Rongcheng Uplift | 1.36 | 2.51 | 645.2 | 1076 | 4.1 | 40.9 | 7.6 | Cl·HCO3-Na | |
| JZ30 | Gaoyang Low Uplift | 1.33 | 2.57 | 540.7 | 1041 | 12.8 | 46.3 | 7.3 | Cl·HCO3-Na | |
| JZ31 | Gaoyang Low Uplift | 1.75 | 2.32 | 448.7 | 1271 | 5.4 | 46.4 | 11.1 | Cl-Na | |
| JZ32 * | Rain Sample | / | 0.15 | 17.9 | 1.74 | 1.6 | 21.6 | / | HCO3-Ca·Mg | |
| Sample No. | Station | δ2H | δ18O |
|---|---|---|---|
| JZ01 | Taihang Mountains | −61.64 | −8.4 |
| JZ02 | Taihang Mountains | −67 | −9.3 |
| JZ06 * | Beijing Depression | −74 | −9.8 |
| JZ07 * | Beijing Depression | −81 | −10.2 |
| JZ08 * | Beijing Depression | −80 | −10.5 |
| JZ09 * | Beijing Depression | −80 | −10.2 |
| JZ17 | Niutuo Uplift | −73 | −8.5 |
| JZ20 | Niutuo Uplift | −73 | −8.6 |
| JZ25 | Rongcheng Uplift | −74.62 | −8.79 |
| JZ27 | Rongcheng Uplift | −74 | −8.8 |
| JZ28 | Rongcheng Uplift | −75 | −9 |
| JZ29 | Rongcheng Uplift | −75 | −9 |
| JZ30 | Gaoyang Low Uplift | −71.99 | −8.44 |
| JZ31 | Gaoyang Low Uplift | −73 | −8.5 |
| JZ32 | Rain Sample | −75 | −10.5 |
| Geothermometers | Formulas | Numbers |
|---|---|---|
| Na-K | (1) | |
| K-Mg | (2) | |
| Na-K-Ca | = − 273.15 | (3) |
| Quartz | (4) | |
| Quartz (no steam loss) | (5) | |
| Quartz (maximum steam loss) | (6) | |
| Chalcedony | (7) |
| Sample No. | Measured Water Temperatures (°C) | Cationic Geothermometers | SiO2 Geothermometers | |||||
|---|---|---|---|---|---|---|---|---|
| (1) | (2) | (3) | (4) | (5) | (6) | (7) | ||
| JZ07 | 67 | 238.0 | 56.7 | 29.9 | 112.3 | 112.0 | 111.3 | 82.9 |
| JZ08 | 45 | 223.6 | 76.3 | 41.8 | 109.9 | 109.6 | 109.3 | 80.3 |
| JZ09 | 58 | 188.3 | 105.8 | 60.3 | 113.4 | 113.1 | 112.3 | 84.1 |
| JZ10 | 74.4 | 175.5 | 98.1 | 55.3 | 121.0 | 120.8 | 118.9 | 92.5 |
| JZ12 | 86 | 175.6 | 96.4 | 55.3 | 120.8 | 120.6 | 118.7 | 92.3 |
| JZ14 | 80 | 173.9 | 96.3 | 53.6 | 109.9 | 109.6 | 109.3 | 80.3 |
| JZ15 | 80 | 171.4 | 96.4 | 52.0 | 113.4 | 113.1 | 112.3 | 84.1 |
| JZ16 | 88 | 179.0 | 100.9 | 56.3 | 120.5 | 120.3 | 118.4 | 91.9 |
| JZ19 | 87 | 172.2 | 97.1 | 53.5 | 117.4 | 117.1 | 115.7 | 88.5 |
| JZ21 | 83 | 178.9 | 97.8 | 54.5 | 113.7 | 113.4 | 112.6 | 84.5 |
| JZ23 | 55 | 170.1 | 88.8 | 46.8 | 99.2 | 98.8 | 100.0 | 68.7 |
| JZ26 | 53.1 | 167.6 | 75.0 | 37.1 | 102.4 | 102.0 | 102.8 | 72.1 |
| JZ28 | 62 | 175.1 | 89.5 | 48.8 | 93.9 | 93.4 | 95.3 | 63.0 |
| JZ30 | 109.2 | 181.6 | 107.0 | 55.2 | 141.4 | 141.2 | 136.1 | 115.0 |
| JZ31 | 123.4 | 189.9 | 127.0 | 69.3 | 161.6 | 161.3 | 152.9 | 137.6 |
| T (°C) | Enthalpy (×4.1868 J/g) | SiO2 (mg/L) | T (°C) | Enthalpy (×4.1868 J/g) | SiO2 (mg/L) |
|---|---|---|---|---|---|
| 50 | 50 | 13.5 | 200 | 203.6 | 265 |
| 75 | 75 | 26.6 | 225 | 230.9 | 365 |
| 100 | 100.1 | 48 | 250 | 259.2 | 486 |
| 125 | 125.4 | 80 | 275 | 289 | 614 |
| 150 | 151 | 125 | 300 | 321 | 692 |
| 175 | 177 | 185 |
| T (°C) | 50 | 75 | 100 | 125 | 150 | 175 | 200 | 225 | 250 | 275 | 300 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| X1 | JZ07 | −0.52 | 0.14 | 0.40 | 0.54 | 0.63 | 0.70 | 0.75 | 0.79 | 0.83 | 0.86 | 0.90 |
| JZ09 | −0.25 | 0.30 | 0.51 | 0.63 | 0.70 | 0.76 | 0.80 | 0.83 | 0.87 | 0.90 | 0.93 | |
| JZ10 | −0.75 | 0.01 | 0.31 | 0.47 | 0.58 | 0.65 | 0.71 | 0.75 | 0.80 | 0.84 | 0.87 | |
| JZ23 | −0.15 | 0.35 | 0.55 | 0.65 | 0.72 | 0.77 | 0.81 | 0.85 | 0.88 | 0.91 | 0.94 | |
| JZ26 | −0.10 | 0.38 | 0.57 | 0.67 | 0.74 | 0.79 | 0.82 | 0.86 | 0.89 | 0.92 | 0.95 | |
| JZ31 | −2.26 | −0.84 | −0.28 | 0.02 | 0.21 | 0.34 | 0.44 | 0.52 | 0.59 | 0.64 | 0.70 | |
| X2 | JZ07 | 13.23 | −3.72 | −0.45 | 0.29 | 0.59 | 0.73 | 0.82 | 0.87 | 0.90 | 0.93 | 0.93 |
| JZ09 | 13.62 | −3.88 | −0.49 | 0.27 | 0.57 | 0.70 | 0.81 | 0.87 | 0.90 | 0.92 | 0.93 | |
| JZ10 | 16.48 | −4.98 | −0.83 | 0.10 | 0.48 | 0.64 | 0.77 | 0.84 | 0.88 | 0.91 | 0.92 | |
| JZ23 | 9.14 | −2.14 | 0.04 | 0.53 | 0.72 | 0.79 | 0.88 | 0.91 | 0.94 | 0.95 | 0.96 | |
| JZ26 | 10.05 | −2.50 | −0.07 | 0.47 | 0.69 | 0.77 | 0.87 | 0.90 | 0.93 | 0.94 | 0.95 | |
| JZ31 | 37.48 | −13.09 | −3.32 | −1.12 | −0.23 | 0.20 | 0.46 | 0.62 | 0.72 | 0.78 | 0.80 | |
| Sample No. | I/(°C/100 m) | t1/°C | t2/°C | h/m | H/m |
|---|---|---|---|---|---|
| JZ07 | 3.5 | 82.9 | 17.5 | 25 | 1979 |
| JZ08 | 3.5 | 80.3 | 17.5 | 25 | 1820 |
| JZ09 | 3.5 | 84.1 | 17.5 | 25 | 1928 |
| JZ10 | 3.5 | 92.5 | 17.5 | 25 | 2167 |
| JZ12 | 3.5 | 92.3 | 17.5 | 25 | 2162 |
| JZ14 | 3.5 | 80.3 | 17.5 | 25 | 1820 |
| JZ15 | 3.5 | 84.1 | 17.5 | 25 | 1928 |
| JZ16 | 3.5 | 91.9 | 17.5 | 25 | 2151 |
| JZ19 | 3.5 | 88.5 | 17.5 | 25 | 2053 |
| JZ21 | 3.5 | 84.5 | 17.5 | 25 | 1939 |
| JZ23 | 3.5 | 68.7 | 17.5 | 25 | 1488 |
| JZ26 | 3.5 | 72.1 | 17.5 | 25 | 1586 |
| JZ28 | 3.5 | 63.0 | 17.5 | 25 | 1324 |
| JZ30 | 3.5 | 115.0 | 17.5 | 25 | 2811 |
| JZ31 | 3.5 | 137.6 | 17.5 | 25 | 3455 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, M.; Tian, X.; Zhang, H.; Li, J.; Wang, L.; Zhang, Z.; Lin, H.; Yang, X. Hydrogeochemical Characteristics of Geothermal Water in Ancient Deeply Buried Hills in the Northern Jizhong Depression, Bohai Bay Basin, China. Water 2023, 15, 3881. https://doi.org/10.3390/w15223881
Yu M, Tian X, Zhang H, Li J, Wang L, Zhang Z, Lin H, Yang X. Hydrogeochemical Characteristics of Geothermal Water in Ancient Deeply Buried Hills in the Northern Jizhong Depression, Bohai Bay Basin, China. Water. 2023; 15(22):3881. https://doi.org/10.3390/w15223881
Chicago/Turabian StyleYu, Mingxiao, Xia Tian, Hanxiong Zhang, Jun Li, Laibin Wang, Zhigang Zhang, Hailiang Lin, and Xinlong Yang. 2023. "Hydrogeochemical Characteristics of Geothermal Water in Ancient Deeply Buried Hills in the Northern Jizhong Depression, Bohai Bay Basin, China" Water 15, no. 22: 3881. https://doi.org/10.3390/w15223881
APA StyleYu, M., Tian, X., Zhang, H., Li, J., Wang, L., Zhang, Z., Lin, H., & Yang, X. (2023). Hydrogeochemical Characteristics of Geothermal Water in Ancient Deeply Buried Hills in the Northern Jizhong Depression, Bohai Bay Basin, China. Water, 15(22), 3881. https://doi.org/10.3390/w15223881