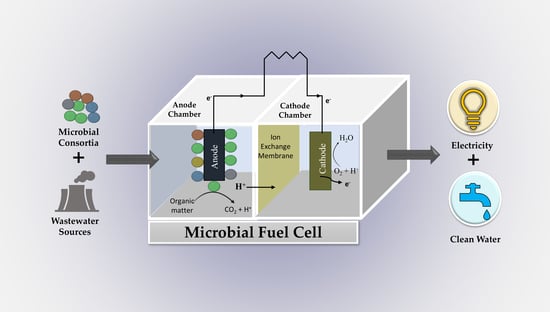
A Perspective Review on Microbial Fuel Cells in Treatment and Product Recovery from Wastewater
Abstract
:1. Introduction
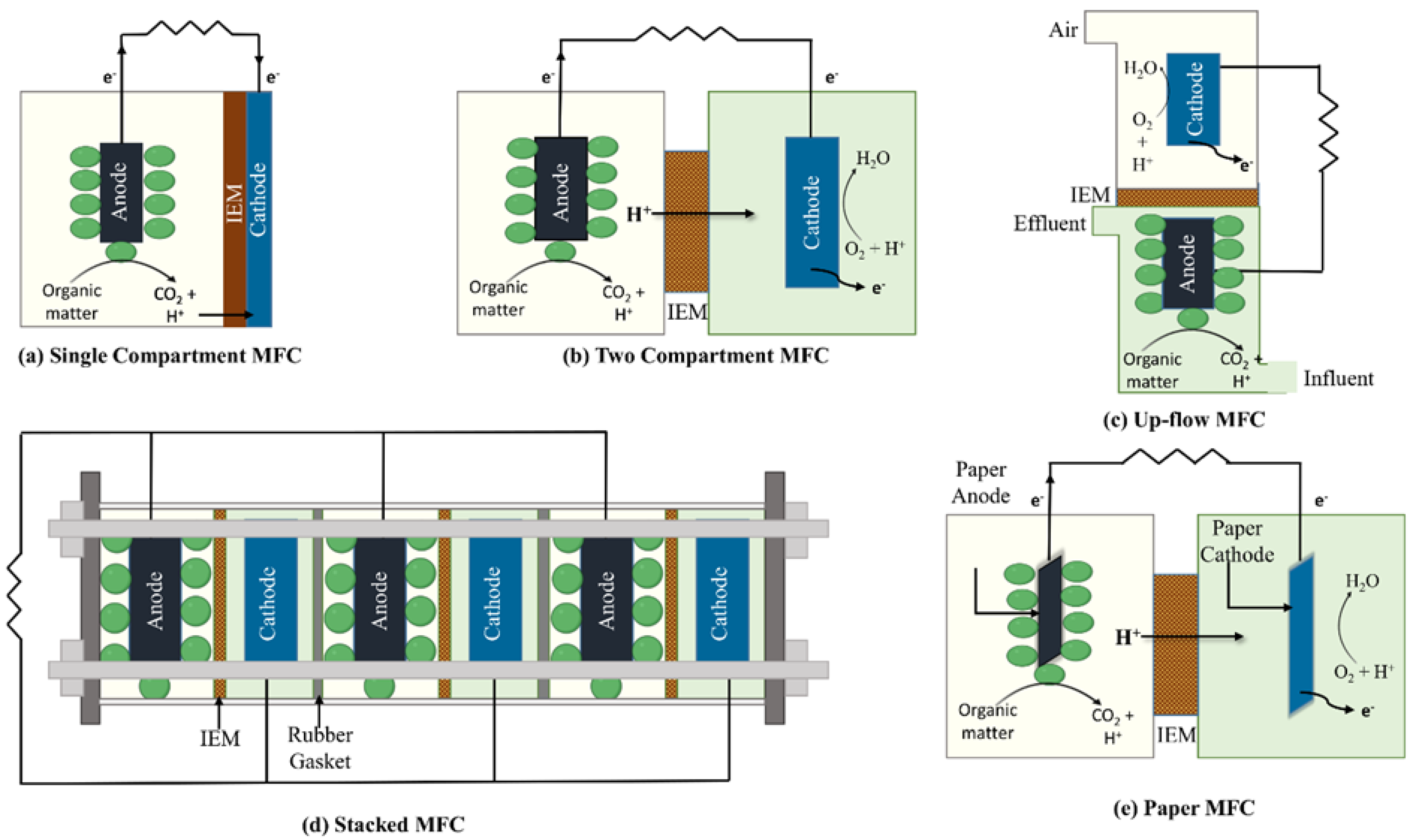
2. General Features, Types, and Designs of MFCs
2.1. Types of MFCs
2.1.1. Single-Compartment MFCs
2.1.2. Two-Compartment MFCs
2.1.3. Up-Flow MFCs
2.1.4. Stacked MFCs
2.1.5. Paper MFCs
2.2. Substrate and Microorganisms That Are Used in MFCs
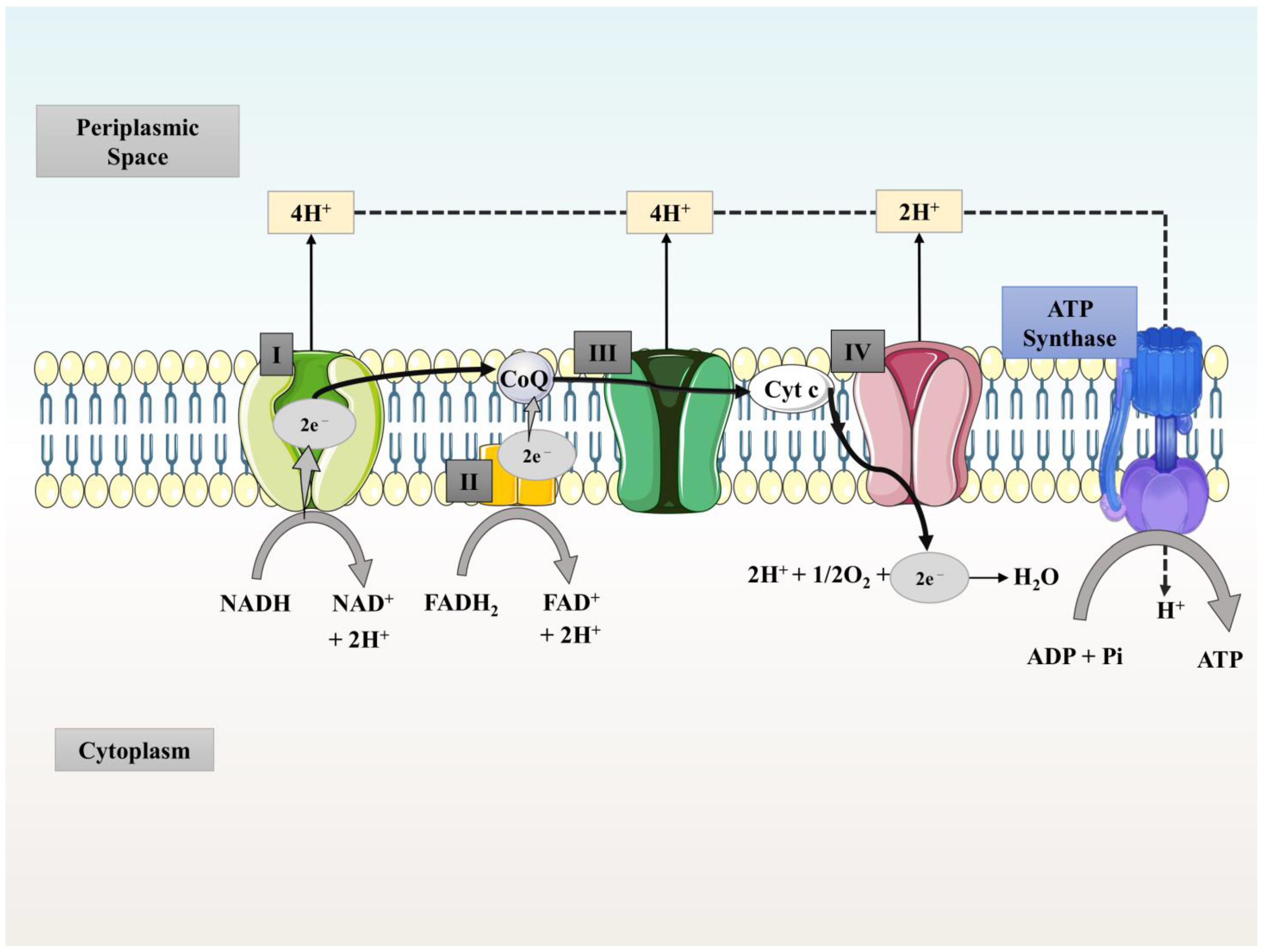
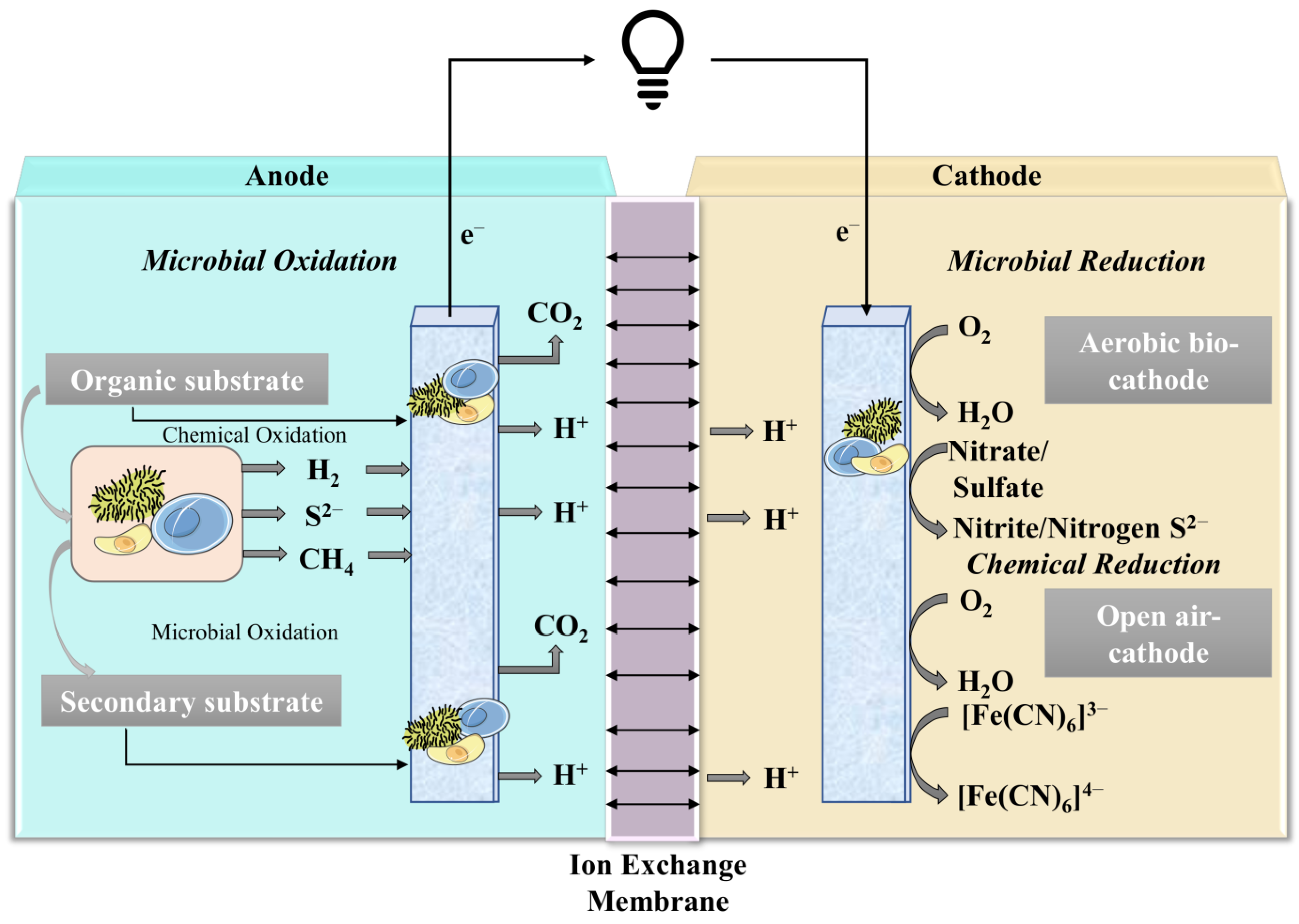
3. Working Principle/Treatability of MFCs and Generation of Energy
4. Application/Performance of MFCs in Wastewater Treatment
4.1. Factors Affecting Performances of MFCs during Wastewater Treatment
4.1.1. Electrode Properties
4.1.2. pH
4.1.3. Temperature
4.1.4. Aeration
5. Different Products’ Recovery from Wastewater Using MFCs
6. Recent Advancements in MFC Technology
7. Challenges and Future Prospects
8. Concluding Remarks
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anand, U.; Adelodun, B.; Cabreros, C.; Kumar, P.; Suresh, S.; Dey, A.; Ballesteros, F.; Bontempi, E. Occurrence, transformation, bioaccumulation, risk and analysis of pharmaceutical and personal care products from wastewater: A review. Environ. Chem. Lett. 2022, 20, 3883–3904. [Google Scholar] [CrossRef]
- Anand, U.; Adelodun, B.; Pivato, A.; Suresh, S.; Indari, O.; Jakhmola, S.; Jha, H.C.; Jha, P.K.; Tripathi, V.; Di Maria, F. A review of the presence of SARS-CoV-2 RNA in wastewater and airborne particulates and its use for virus spreading surveillance. Environ. Res. 2021, 196, 110929. [Google Scholar] [CrossRef]
- Anand, U.; Li, X.; Sunita, K.; Lokhandwala, S.; Gautam, P.; Suresh, S.; Sarma, H.; Vellingiri, B.; Dey, A.; Bontempi, E.; et al. SARS-CoV-2 and other pathogens in municipal wastewater, landfill leachate, and solid waste: A review about virus surveillance, infectivity, and inactivation. Environ. Res. 2022, 203, 111839. [Google Scholar] [CrossRef]
- Anand, U.; Reddy, B.; Singh, V.K.; Singh, A.K.; Kesari, K.K.; Tripathi, P.; Kumar, P.; Tripathi, V.; Simal-Gandara, J. Potential Environmental and Human Health Risks Caused by Antibiotic-Resistant Bacteria (ARB), Antibiotic Resistance Genes (ARGs) and Emerging Contaminants (ECs) from Municipal Solid Waste (MSW) Landfill. Antibiotics 2021, 10, 374. [Google Scholar] [CrossRef]
- Malik, S.; Kishore, S.; Prasad, S.; Shah, M.P. A comprehensive review on emerging trends in industrial wastewater research. J. Basic Microbiol. 2022, 62, 296–309. [Google Scholar] [CrossRef]
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Mariñas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nature 2008, 452, 301–310. [Google Scholar] [CrossRef]
- Ge, Z.; He, Z. Long-term performance of a 200 L modularized microbial fuel cell system treating municipal wastewater: Treatment, energy, and cost. Environ. Sci. Water Res. Technol. 2016, 2, 274–281. [Google Scholar] [CrossRef] [Green Version]
- Lu, S.; Li, H.; Tan, G.; Wen, F.; Flynn, M.T.; Zhu, X. Resource recovery microbial fuel cells for urine-containing wastewater treatment without external energy consumption. Chem. Eng. J. 2019, 373, 1072–1080. [Google Scholar] [CrossRef]
- Yan, T.; Ye, Y.; Ma, H.; Zhang, Y.; Guo, W.; Du, B.; Wei, Q.; Wei, D.; Ngo, H.H. A critical review on membrane hybrid system for nutrient recovery from wastewater. Chem. Eng. J. 2018, 348, 143–156. [Google Scholar] [CrossRef]
- Freguia, S.; Logrieco, M.E.; Monetti, J.; Ledezma, P.; Virdis, B.; Tsujimura, S. Self-powered bioelectrochemical nutrient recovery for fertilizer generation from human urine. Sustainability 2019, 11, 5490. [Google Scholar] [CrossRef]
- Ye, Y.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Zhang, X.; Zhang, S.; Luo, G.; Liu, Y. Impacts of hydraulic retention time on a continuous flow mode dual-chamber microbial fuel cell for recovering nutrients from municipal wastewater. Sci. Total Environ. 2020, 734, 139220. [Google Scholar] [CrossRef] [PubMed]
- Athar, T.; Pandey, A.; Khan, M.; Saqib, Z.A.; Jabeen, M.; Shahid, S.; Hamurcu, M.; Gezgin, S.; Rajput, V.D.; Elinson, M.A. Potential Role of Beneficial Microbes for Sustainable Treatment of Sewage Sludge and Wastewater. In Sustainable Management and Utilization of Sewage Sludge; Springer: Berlin/Heidelberg, Germany, 2022; pp. 71–96. [Google Scholar]
- Tsekouras, G.J.; Deligianni, P.M.; Kanellos, F.D.; Kontargyri, V.T.; Kontaxis, P.A.; Manousakis, N.; Elias, C.N. Microbial fuel cell for wastewater treatment as power plant in smart grids: Utopia or Reality? Front. Energy Res. 2022, 10, 843768. [Google Scholar] [CrossRef]
- Elhenawy, S.; Khraisheh, M.; AlMomani, F.; Al-Ghouti, M.; Hassan, M.K. From Waste to Watts: Updates on Key Applications of Microbial Fuel Cells in Wastewater Treatment and Energy Production. Sustainability 2022, 14, 955. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, J.; Shinde, S.; Wang, X.; Li, Y.; Dai, Y.; Ren, J.; Zhang, P.; Liu, X. Simultaneous wastewater treatment and energy harvesting in microbial fuel cells: An update on the biocatalysts. RSC Adv. 2020, 10, 25874–25887. [Google Scholar] [CrossRef] [PubMed]
- Aelterman, P.; Rabaey, K.; Clauwaert, P.; Verstraete, W. Microbial fuel cells for wastewater treatment. Water Sci. Technol. 2006, 54, 9–15. [Google Scholar] [CrossRef] [Green Version]
- Rabaey, K.; Verstraete, W. Microbial fuel cells: Novel biotechnology for energy generation. TRENDS Biotechnol. 2005, 23, 291–298. [Google Scholar] [CrossRef]
- Kumari, S.; Rajput, V.D.; Sushkova, S.; Minkina, T. Microbial electrochemical system: An emerging technology for remediation of polycyclic aromatic hydrocarbons from soil and sediments. Environ. Geochem. Health 2022, 1–17. [Google Scholar] [CrossRef]
- Tilman, D.; Balzer, C.; Hill, J.; Befort, B.L. Global food demand and the sustainable intensification of agriculture. Proc. Natl. Acad. Sci. USA 2011, 108, 20260–20264. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Sun, D.; Zhang, X.; Liang, P.; Huang, X. Novel self-driven microbial nutrient recovery cell with simultaneous wastewater purification. Sci. Rep. 2015, 5, 15744. [Google Scholar] [CrossRef] [Green Version]
- Gardner-Dale, D.; Bradley, I.; Guest, J. Influence of solids residence time and carbon storage on nitrogen and phosphorus recovery by microalgae across diel cycles. Water Res. 2017, 121, 231–239. [Google Scholar] [CrossRef]
- Yang, Z.; Pei, H.; Hou, Q.; Jiang, L.; Zhang, L.; Nie, C. Algal biofilm-assisted microbial fuel cell to enhance domestic wastewater treatment: Nutrient, organics removal and bioenergy production. Chem. Eng. J. 2018, 332, 277–285. [Google Scholar] [CrossRef]
- Callegari, A.; Cecconet, D.; Molognoni, D.; Capodaglio, A.G. Sustainable processing of dairy wastewater: Long-term pilot application of a bio-electrochemical system. J. Clean. Prod. 2018, 189, 563–569. [Google Scholar] [CrossRef]
- Jain, H.; Kumar, A.; Verma, A.K.; Wadhwa, S.; Rajput, V.D.; Minkina, T.; Garg, M.C. Treatment of textile industry wastewater by using high-performance forward osmosis membrane tailored with alpha-manganese dioxide nanoparticles for fertigation. Environ. Sci. Pollut. Res. 2022, 29, 80032–80043. [Google Scholar] [CrossRef] [PubMed]
- Paucar, N.E.; Sato, C. Microbial fuel cell for energy production, nutrient removal and recovery from wastewater: A review. Processes 2021, 9, 1318. [Google Scholar] [CrossRef]
- Ye, Y.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Liu, Y.; Nghiem, L.D.; Zhang, X.; Wang, J. Effect of organic loading rate on the recovery of nutrients and energy in a dual-chamber microbial fuel cell. Bioresour. Technol. 2019, 281, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Aelterman, P.; Versichele, M.; Marzorati, M.; Boon, N.; Verstraete, W. Loading rate and external resistance control the electricity generation of microbial fuel cells with different three-dimensional anodes. Bioresour. Technol. 2008, 99, 8895–8902. [Google Scholar] [CrossRef] [PubMed]
- Ieropoulos, I.; Winfield, J.; Greenman, J. Effects of flow-rate, inoculum and time on the internal resistance of microbial fuel cells. Bioresour. Technol. 2010, 101, 3520–3525. [Google Scholar] [CrossRef] [PubMed]
- Molognoni, D.; Puig, S.; Balaguer, M.D.; Capodaglio, A.G.; Callegari, A.; Colprim, J. Multiparametric control for enhanced biofilm selection in microbial fuel cells. J. Chem. Technol. Biotechnol. 2016, 91, 1720–1727. [Google Scholar] [CrossRef]
- Sleutels, T.H.; Darus, L.; Hamelers, H.V.; Buisman, C.J. Effect of operational parameters on Coulombic efficiency in bioelectrochemical systems. Bioresour. Technol. 2011, 102, 11172–11176. [Google Scholar] [CrossRef]
- Ortiz-Martínez, V.; Salar-García, M.; De Los Ríos, A.; Hernández-Fernández, F.; Egea, J.; Lozano, L. Developments in microbial fuel cell modeling. Chem. Eng. J. 2015, 271, 50–60. [Google Scholar] [CrossRef]
- Kumar, P.; Dixit, J.; Singh, A.K.; Rajput, V.D.; Verma, P.; Tiwari, K.N.; Mishra, S.K.; Minkina, T.; Mandzhieva, S. Efficient Catalytic Degradation of Selected Toxic Dyes by Green Biosynthesized Silver Nanoparticles Using Aqueous Leaf Extract of Cestrum nocturnum L. Nanomaterials 2022, 12, 3851. [Google Scholar] [CrossRef]
- Capodaglio, A.G.; Molognoni, D.; Pons, A.V. A multi-perspective review of microbial fuel-cells for wastewater treatment: Bio-electro-chemical, microbiologic and modeling aspects. In AIP Conference Proceedings; AIP Publishing LLC: Baltimore, MD, USA, 2016; p. 030032. [Google Scholar]
- Mohan, S.V.; Velvizhi, G.; Modestra, J.A.; Srikanth, S. Microbial fuel cell: Critical factors regulating bio-catalyzed electrochemical process and recent advancements. Renew. Sustain. Energy Rev. 2014, 40, 779–797. [Google Scholar] [CrossRef]
- Khandaker, S.; Das, S.; Hossain, M.T.; Islam, A.; Miah, M.R.; Awual, M.R. Sustainable approach for wastewater treatment using microbial fuel cells and green energy generation—A comprehensive review. J. Mol. Liq. 2021, 344, 117795. [Google Scholar] [CrossRef]
- Kumar, S.S.; Kumar, V.; Malyan, S.K.; Sharma, J.; Mathimani, T.; Maskarenj, M.S.; Ghosh, P.C.; Pugazhendhi, A. Microbial fuel cells (MFCs) for bioelectrochemical treatment of different wastewater streams. Fuel 2019, 254, 115526. [Google Scholar] [CrossRef]
- Kaur, R.; Marwaha, A.; Chhabra, V.A.; Kim, K.-H.; Tripathi, S. Recent developments on functional nanomaterial-based electrodes for microbial fuel cells. Renew. Sustain. Energy Rev. 2020, 119, 109551. [Google Scholar] [CrossRef]
- Cai, T.; Meng, L.; Chen, G.; Xi, Y.; Jiang, N.; Song, J.; Zheng, S.; Liu, Y.; Zhen, G.; Huang, M. Application of advanced anodes in microbial fuel cells for power generation: A review. Chemosphere 2020, 248, 125985. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, D.A.; Das, I.; Ghangrekar, M.M.; Pant, D. Moving towards practical applications of microbial fuel cells for sanitation and resource recovery. J. Water Process Eng. 2020, 38, 101566. [Google Scholar] [CrossRef]
- Obileke, K.; Onyeaka, H.; Meyer, E.L.; Nwokolo, N. Microbial fuel cells, a renewable energy technology for bio-electricity generation: A mini-review. Electrochem. Commun. 2021, 125, 107003. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, L.; Zularisam, A.; Hai, F.I. Microbial fuel cell is emerging as a versatile technology: A review on its possible applications, challenges and strategies to improve the performances. Int. J. Energy Res. 2018, 42, 369–394. [Google Scholar] [CrossRef] [Green Version]
- ElMekawy, A.; Hegab, H.M.; Vanbroekhoven, K.; Pant, D. Techno-productive potential of photosynthetic microbial fuel cells through different configurations. Renew. Sustain. Energy Rev. 2014, 39, 617–627. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, L.; Zularisam, A.W. Exoelectrogens: Recent advances in molecular drivers involved in extracellular electron transfer and strategies used to improve it for microbial fuel cell applications. Renew. Sustain. Energy Rev. 2016, 56, 1322–1336. [Google Scholar] [CrossRef] [Green Version]
- Mercuri, E.G.F.; Kumata, A.Y.J.; Amaral, E.B.; Vitule, J.R.S. Energy by Microbial Fuel Cells: Scientometric global synthesis and challenges. Renew. Sustain. Energy Rev. 2016, 65, 832–840. [Google Scholar] [CrossRef]
- He, L.; Du, P.; Chen, Y.; Lu, H.; Cheng, X.; Chang, B.; Wang, Z. Advances in microbial fuel cells for wastewater treatment. Renew. Sustain. Energy Rev. 2017, 71, 388–403. [Google Scholar] [CrossRef]
- GajendraPrasad, J.; Panda, S. Microbial Fuel Cells: Types of MFC and Different Source of Substrate. IntJ Latest Technol Eng Mgt App Sc 2018, 7, 158–165. [Google Scholar]
- Chaudhuri, S.K.; Lovley, D.R. Electricity generation by direct oxidation of glucose in mediatorless microbial fuel cells. Nat. Biotechnol. 2003, 21, 1229–1232. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, H.S.; Hyun, M.S.; Chang, I.S.; Kim, M.; Kim, B.H. A mediator-less microbial fuel cell using a metal reducing bacterium, Shewanella putrefaciens. Enzym. Microb. Technol. 2002, 30, 145–152. [Google Scholar] [CrossRef]
- Min, B.; Kim, J.; Oh, S.; Regan, J.M.; Logan, B.E. Electricity generation from swine wastewater using microbial fuel cells. Water Res. 2005, 39, 4961–4968. [Google Scholar] [CrossRef]
- Bond, D.R.; Lovley, D.R. Electricity production by Geobacter sulfurreducens attached to electrodes. Appl. Environ. Microbiol. 2003, 69, 1548–1555. [Google Scholar] [CrossRef] [Green Version]
- Pham, C.A.; Jung, S.J.; Phung, N.T.; Lee, J.; Chang, I.S.; Kim, B.H.; Yi, H.; Chun, J. A novel electrochemically active and Fe (III)-reducing bacterium phylogenetically related to Aeromonas hydrophila, isolated from a microbial fuel cell. FEMS Microbiol. Lett. 2003, 223, 129–134. [Google Scholar] [CrossRef] [Green Version]
- Min, B.; Logan, B.E. Continuous electricity generation from domestic wastewater and organic substrates in a flat plate microbial fuel cell. Environ. Sci. Technol. 2004, 38, 5809–5814. [Google Scholar] [CrossRef]
- Tremouli, A.; Antonopoulou, G.; Bebelis, S.; Lyberatos, G. Operation and characterization of a microbial fuel cell fed with pretreated cheese whey at different organic loads. Bioresour. Technol. 2013, 131, 380–389. [Google Scholar] [CrossRef]
- Park, D.H.; Zeikus, J.G. Improved fuel cell and electrode designs for producing electricity from microbial degradation. Biotechnol. Bioeng. 2003, 81, 348–355. [Google Scholar] [CrossRef]
- Logan, B.E.; Rabaey, K. Conversion of wastes into bioelectricity and chemicals by using microbial electrochemical technologies. Science 2012, 337, 686–690. [Google Scholar] [CrossRef] [Green Version]
- Winfield, J.; Gajda, I.; Greenman, J.; Ieropoulos, I. A review into the use of ceramics in microbial fuel cells. Bioresour. Technol. 2016, 215, 296–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aelterman, P.; Rabaey, K.; Pham, H.T.; Boon, N.; Verstraete, W. Continuous Electricity Generation at High Voltages and Currents Using Stacked Microbial Fuel Cells. Environ. Sci. Technol. 2006, 40, 3388–3394. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Ban, J.Y.; Oh, C.-H.; Park, H.-K.; Choi, S. A solvent-free microbial-activated air cathode battery paper platform made with pencil-traced graphite electrodes. Sci. Rep. 2016, 6, 28588. [Google Scholar] [CrossRef]
- Ieropoulos, I.; Greenman, J.; Melhuish, C. Microbial fuel cells based on carbon veil electrodes: Stack configuration and scalability. Int. J. Energy Res. 2008, 32, 1228–1240. [Google Scholar] [CrossRef]
- Das, S.; Kungwani, N. Recent developments in microbial fuel cells: A review. J. Sci. Ind. Res. 2010, 69, 727–731. [Google Scholar]
- Sa’adu, L.; Garba, N.A.; Balarabe, M.D. A Review on Electrode Materials in Microbial Fuel Cell Fabrication. Int. J. Sci. Glob. Sustain. 2019, 5, 5. [Google Scholar]
- Pant, D.; Van Bogaert, G.; Diels, L.; Vanbroekhoven, K. A review of the substrates used in microbial fuel cells (MFCs) for sustainable energy production. Bioresour. Technol. 2010, 101, 1533–1543. [Google Scholar] [CrossRef]
- Walter, X.A.; Greenman, J.; Ieropoulos, I.A. Microbial fuel cells directly powering a microcomputer. J. Power Sources 2020, 446, 227328. [Google Scholar] [CrossRef]
- Ramírez-Vargas, C.A.; Arias, C.A.; Zhang, L.; Brix, H. Microbial Community Function in Electroactive Biofilm-based Constructed Wetlands. Biogeosci. Discuss. 2018, 2018, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Lovley, D.R. The microbe electric: Conversion of organic matter to electricity. Curr. Opin. Biotechnol. 2008, 19, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Heijne, A.T.; Liu, F.; Weijden, R.V.D.; Weijma, J.; Buisman, C.J.N.; Hamelers, H.V.M. Copper Recovery Combined with Electricity Production in a Microbial Fuel Cell. Environ. Sci. Technol. 2010, 44, 4376–4381. [Google Scholar] [CrossRef] [PubMed]
- Sharma, Y.; Li, B. Optimizing energy harvest in wastewater treatment by combining anaerobic hydrogen producing biofermentor (HPB) and microbial fuel cell (MFC). Int. J. Hydrog. Energy 2010, 35, 3789–3797. [Google Scholar] [CrossRef]
- Puig, S.; Coma, M.; Desloover, J.; Boon, N.; Colprim, J.S.; Balaguer, M.D. Autotrophic denitrification in microbial fuel cells treating low ionic strength waters. Environ. Sci. Technol. 2012, 46, 2309–2315. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.; Zhou, S.-G.; Zhuang, L.; Zhang, J.-T.; Ni, J.-R. Electricity generation from starch processing wastewater using microbial fuel cell technology. Biochem. Eng. J. 2009, 43, 246–251. [Google Scholar] [CrossRef]
- Jatoi, A.S.; Akhter, F.; Mazari, S.A.; Sabzoi, N.; Aziz, S.; Soomro, S.A.; Mubarak, N.M.; Baloch, H.; Memon, A.Q.; Ahmed, S. Advanced microbial fuel cell for waste water treatment—A review. Environ. Sci. Pollut. Res. 2021, 28, 5005–5019. [Google Scholar] [CrossRef] [PubMed]
- Mardanpour, M.M.; Yaghmaei, S.; Kalantar, M. Modeling of microfluidic microbial fuel cells using quantitative bacterial transport parameters. J. Power Sources 2017, 342, 1017–1031. [Google Scholar] [CrossRef]
- Corbella, C.; Puigagut, J. Improving domestic wastewater treatment efficiency with constructed wetland microbial fuel cells: Influence of anode material and external resistance. Sci. Total Environ. 2018, 631, 1406–1414. [Google Scholar] [CrossRef]
- Tamta, P.; Rani, N.; Yadav, A.K. Enhanced wastewater treatment and electricity generation using stacked constructed wetland–microbial fuel cells. Environ. Chem. Lett. 2020, 18, 871–879. [Google Scholar] [CrossRef]
- ElMekawy, A.; Hegab, H.M.; Dominguez-Benetton, X.; Pant, D. Internal resistance of microfluidic microbial fuel cell: Challenges and potential opportunities. Bioresour. Technol. 2013, 142, 672–682. [Google Scholar] [CrossRef] [PubMed]
- Hiegemann, H.; Herzer, D.; Nettmann, E.; Lübken, M.; Schulte, P.; Schmelz, K.-G.; Gredigk-Hoffmann, S.; Wichern, M. An integrated 45L pilot microbial fuel cell system at a full-scale wastewater treatment plant. Bioresour. Technol. 2016, 218, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Chi, M.; Wang, H.; He, H.; Zhou, M. Electrochemical surface modification of carbon mesh anode to improve the performance of air-cathode microbial fuel cells. Bioprocess Biosyst. Eng. 2013, 36, 1889–1896. [Google Scholar] [CrossRef]
- Choudhury, P.; Uday, U.S.P.; Mahata, N.; Nath Tiwari, O.; Narayan Ray, R.; Kanti Bandyopadhyay, T.; Bhunia, B. Performance improvement of microbial fuel cells for waste water treatment along with value addition: A review on past achievements and recent perspectives. Renew. Sustain. Energy Rev. 2017, 79, 372–389. [Google Scholar] [CrossRef]
- Nosek, D.; Jachimowicz, P.; Cydzik-Kwiatkowska, A. Anode Modification as an Alternative Approach to Improve Electricity Generation in Microbial Fuel Cells. Energies 2020, 13, 6596. [Google Scholar] [CrossRef]
- Mahdi Mardanpour, M.; Nasr Esfahany, M.; Behzad, T.; Sedaqatvand, R. Single chamber microbial fuel cell with spiral anode for dairy wastewater treatment. Biosens. Bioelectron. 2012, 38, 264–269. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, H.; Hassett, D.J.; Gu, T. Recent advances in microbial fuel cells (MFCs) and microbial electrolysis cells (MECs) for wastewater treatment, bioenergy and bioproducts. J. Chem. Technol. Biotechnol. 2013, 88, 508–518. [Google Scholar] [CrossRef]
- Ma, D.; Jiang, Z.-H.; Lay, C.-H.; Zhou, D. Electricity generation from swine wastewater in microbial fuel cell: Hydraulic reaction time effect. Int. J. Hydrog. Energy 2016, 41, 21820–21826. [Google Scholar] [CrossRef]
- Fangzhou, D.; Zhenglong, L.; Shaoqiang, Y.; Beizhen, X.; Hong, L. Electricity generation directly using human feces wastewater for life support system. Acta Astronaut. 2011, 68, 1537–1547. [Google Scholar] [CrossRef]
- Sciarria, T.P.; Tenca, A.; D’Epifanio, A.; Mecheri, B.; Merlino, G.; Barbato, M.; Borin, S.; Licoccia, S.; Garavaglia, V.; Adani, F. Using olive mill wastewater to improve performance in producing electricity from domestic wastewater by using single-chamber microbial fuel cell. Bioresour. Technol. 2013, 147, 246–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yakar, A.; Türe, C.; Türker, O.C.; Vymazal, J.; Saz, Ç. Impacts of various filtration media on wastewater treatment and bioelectric production in up-flow constructed wetland combined with microbial fuel cell (UCW-MFC). Ecol. Eng. 2018, 117, 120–132. [Google Scholar] [CrossRef] [Green Version]
- Karuppiah, T.; Uthirakrishnan, U.; Sivakumar, S.V.; Authilingam, S.; Arun, J.; Sivaramakrishnan, R.; Pugazhendhi, A. Processing of electroplating industry wastewater through dual chambered microbial fuel cells (MFC) for simultaneous treatment of wastewater and green fuel production. Int. J. Hydrog. Energy 2022, 47, 37569–37576. [Google Scholar] [CrossRef]
- Kong, X.; Sun, Y.; Yuan, Z.; Li, D.; Li, L.; Li, Y. Effect of cathode electron-receiver on the performance of microbial fuel cells. Int. J. Hydrog. Energy 2010, 35, 7224–7227. [Google Scholar] [CrossRef]
- Fadzli, F.S.; Bhawani, S.A.; Adam Mohammad, R.E. Microbial Fuel Cell: Recent Developments in Organic Substrate Use and Bacterial Electrode Interaction. J. Chem. 2021, 2021, 4570388. [Google Scholar] [CrossRef]
- Mukherjee, A.; Patel, V.; Shah, M.T.; Munshi, N.S. Enzymatic and microbial biofuel cells: Current developments and future directions. In Handbook of Biofuels; Elsevier: Amsterdam, The Netherlands, 2022; pp. 551–576. [Google Scholar]
- Rabaey, K.; Rozendal, R.A. Microbial electrosynthesis—Revisiting the electrical route for microbial production. Nat. Rev. Microbiol. 2010, 8, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Catal, T.; Xu, S.; Li, K.; Bermek, H.; Liu, H. Electricity generation from polyalcohols in single-chamber microbial fuel cells. Biosens. Bioelectron. 2008, 24, 849–854. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Cheng, S.; Logan, B.E. Power generation in fed-batch microbial fuel cells as a function of ionic strength, temperature, and reactor configuration. Environ. Sci. Technol. 2005, 39, 5488–5493. [Google Scholar] [CrossRef]
- Ahn, Y.; Logan, B.E. Effectiveness of domestic wastewater treatment using microbial fuel cells at ambient and mesophilic temperatures. Bioresour. Technol. 2010, 101, 469–475. [Google Scholar] [CrossRef]
- Ishii, S.I.; Watanabe, K.; Yabuki, S.; Logan, B.E.; Sekiguchi, Y. Comparison of electrode reduction activities of Geobacter sulfurreducens and an enriched consortium in an air-cathode microbial fuel cell. Appl. Environ. Microbiol. 2008, 74, 7348–7355. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.R.; Jung, S.H.; Regan, J.M.; Logan, B.E. Electricity generation and microbial community analysis of alcohol powered microbial fuel cells. Bioresour. Technol. 2007, 98, 2568–2577. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Minteer, S.D.; Angenent, L.T. Electricity generation from artificial wastewater using an upflow microbial fuel cell. Environ. Sci. Technol. 2005, 39, 5262–5267. [Google Scholar] [CrossRef] [PubMed]
- Dumas, C.; Mollica, A.D. Fe ron, R. Basse guy, L. Etcheverry and A. Bergel. Electrochim. Acta 2007, 53, 468–473. [Google Scholar] [CrossRef]
- Watanabe, K. Recent developments in microbial fuel cell technologies for sustainable bioenergy. J. Biosci. Bioeng. 2008, 106, 528–536. [Google Scholar] [CrossRef]
- Mustakeem, M. Electrode materials for microbial fuel cells: Nanomaterial approach. Mater. Renew. Sustain. Energy 2015, 4, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Logan, B.E. Microbial Fuel Cells; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Liu, X.-W.; Wang, Y.-P.; Huang, Y.-X.; Sun, X.-F.; Sheng, G.-P.; Zeng, R.J.; Li, F.; Dong, F.; Wang, S.-G.; Tong, Z.-H.; et al. Integration of a microbial fuel cell with activated sludge process for energy-saving wastewater treatment: Taking a sequencing batch reactor as an example. Biotechnol. Bioeng. 2011, 108, 1260–1267. [Google Scholar] [CrossRef]
- Jatoi, A.S.; Tunio, M.; Riaz, S.; Abro, R.; Wajahat, M.H.; Qureshi, K.; Shah, A.; Nizamuddin, S.; Mubarak, N. Utilization of distillery effluent as substrate for power generation with optimized parametric conditions using microbial fuel cell. Eurasian J. Anal. Chem. 2018, 13, em49. [Google Scholar] [CrossRef]
- Puig, S.; Serra, M.; Coma, M.; Cabré, M.; Balaguer, M.D.; Colprim, J. Effect of pH on nutrient dynamics and electricity production using microbial fuel cells. Bioresour. Technol. 2010, 101, 9594–9599. [Google Scholar] [CrossRef]
- Quan, X.-C.; Quan, Y.-P.; Tao, K. Effect of anode aeration on the performance and microbial community of an air–cathode microbial fuel cell. Chem. Eng. J. 2012, 210, 150–156. [Google Scholar] [CrossRef]
- Khan, A.; Chen, Z.; Zhao, S.; Ni, H.; Pei, Y.; Xu, R.; Ling, Z.; Salama, E.-S.; Liu, P.; Li, X. Micro-aeration in anode chamber promotes p-nitrophenol degradation and electricity generation in microbial fuel cell. Bioresour. Technol. 2019, 285, 121291. [Google Scholar] [CrossRef]
- Oon, Y.-L.; Ong, S.-A.; Ho, L.-N.; Wong, Y.-S.; Dahalan, F.A.; Oon, Y.-S.; Lehl, H.K.; Thung, W.-E.; Nordin, N. Role of macrophyte and effect of supplementary aeration in up-flow constructed wetland-microbial fuel cell for simultaneous wastewater treatment and energy recovery. Bioresour. Technol. 2017, 224, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhou, M.; Tian, X.; Tan, C.; McDaniel, C.T.; Hassett, D.J.; Gu, T. Microbial fuel cell (MFC) power performance improvement through enhanced microbial electrogenicity. Biotechnol. Adv. 2018, 36, 1316–1327. [Google Scholar] [CrossRef] [PubMed]
- Kishore, S.; Malik, S.; Shah, M.P.; Bora, J.; Chaudhary, V.; Kumar, L.; Sayyed, R.Z.; Ranjan, A. A comprehensive review on removal of pollutants from wastewater through microbial nanobiotechnology-based solutions. Biotechnol. Genet. Eng. Rev. 2022, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Kishore, S.; Bora, J.; Chaudhary, V.; Kumari, A.; Kumari, P.; Kumar, L.; Bhardwaj, A. A Comprehensive Review on Microalgae-Based Biorefinery as Two-Way Source of Wastewater Treatment and Bioresource Recovery. CLEAN—Soil Air Water N/A 2022, 2200044. [Google Scholar] [CrossRef]
- Malik, S.; Kishore, S.; Shah, M.P.; Kumar, S.A. A comprehensive review on nanobiotechnology for bioremediation of heavy metals from wastewater. J. Basic Microbiol. 2022, 62, 361–375. [Google Scholar] [CrossRef]
- Zhang, Y.; He, Q.; Xia, L.; Li, Y.; Song, S. Algae cathode microbial fuel cells for cadmium removal with simultaneous electricity production using nickel foam/graphene electrode. Biochem. Eng. J. 2018, 138, 179–187. [Google Scholar] [CrossRef]
- Hasan, M.N.; Salman, M.S.; Islam, A.; Znad, H.; Hasan, M.M. Sustainable composite sensor material for optical cadmium(II) monitoring and capturing from wastewater. Microchem. J. 2021, 161, 105800. [Google Scholar] [CrossRef]
- Shahat, A.; Kubra, K.T.; Salman, M.S.; Hasan, M.N.; Hasan, M.M. Novel solid-state sensor material for efficient cadmium(II) detection and capturing from wastewater. Microchem. J. 2021, 164, 105967. [Google Scholar] [CrossRef]
- Awual, M.R. A novel facial composite adsorbent for enhanced copper(II) detection and removal from wastewater. Chem. Eng. J. 2015, 266, 368–375. [Google Scholar] [CrossRef]
- Hasan, M.N.; Shenashen, M.A.; Hasan, M.M.; Znad, H.; Awual, M.R. Assessing of cesium removal from wastewater using functionalized wood cellulosic adsorbent. Chemosphere 2021, 270, 128668. [Google Scholar] [CrossRef]
- Salman, M.S.; Znad, H.; Hasan, M.N.; Hasan, M.M. Optimization of innovative composite sensor for Pb(II) detection and capturing from water samples. Microchem. J. 2021, 160, 105765. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, X.; Jin, M.; Li, Y.; Li, S.; Kong, F.; Nan, J.; Wang, A. Copper removal and microbial community analysis in single-chamber microbial fuel cell. Bioresour. Technol. 2018, 253, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, R.; Dhakshina Moorthy, G.P.; Krishnan, H.; Anappara, S. A Study on Polythiophene Modified Carbon Cloth as Anode in Microbial Fuel Cell for Lead Removal. Arab. J. Sci. Eng. 2021, 46, 6695–6701. [Google Scholar] [CrossRef]
- Tao, Q.; Zhang, X.; Prabaharan, K.; Dai, Y. Separation of cesium from wastewater with copper hexacyanoferrate film in an electrochemical system driven by microbial fuel cells. Bioresour. Technol. 2019, 278, 456–459. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.; Hu, N. The modeling of gold recovery from tetrachloroaurate wastewater using a microbial fuel cell. Bioresour. Technol. 2013, 133, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Hirooka, K.; Ichihashi, O. Phosphorus recovery from artificial wastewater by microbial fuel cell and its effect on power generation. Bioresour. Technol. 2013, 137, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Mutnuri, S. Nutrient recovery and microbial diversity in human urine fed microbial fuel cell. Water Sci. Technol. 2019, 79, 718–730. [Google Scholar] [CrossRef]
- Xiao, Y.; Zheng, Y.; Wu, S.; Yang, Z.-H.; Zhao, F. Nitrogen recovery from wastewater using microbial fuel cells. Front. Environ. Sci. Eng. 2016, 10, 185–191. [Google Scholar] [CrossRef]
- Ye, Y.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Liu, Y.; Ni, B.-J.; Zhang, X. Microbial fuel cell for nutrient recovery and electricity generation from municipal wastewater under different ammonium concentrations. Bioresour. Technol. 2019, 292, 121992. [Google Scholar] [CrossRef]
- Santoro, C.; Arbizzani, C.; Erable, B.; Ieropoulos, I. Microbial fuel cells: From fundamentals to applications. A review. J. Power Sources 2017, 356, 225–244. [Google Scholar] [CrossRef]
- Angelucci, D.M.; Donati, E.; Tomei, M.C. Extractive membrane bioreactor to detoxify industrial/hazardous landfill leachate and facilitate resource recovery. Sci. Total Environ. 2022, 806, 150892. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Bapodra, S.L.; Madamwar, D.; Desai, C. Electroactive bacterial community augmentation enhances the performance of a pilot scale constructed wetland microbial fuel cell for treatment of textile dye wastewater. Bioresour. Technol. 2021, 332, 125088. [Google Scholar] [CrossRef] [PubMed]
- Rahman, W.; Yusup, S.; Mohammad, S.N.A.A. Screening of fruit waste as substrate for microbial fuel cell (MFC). AIP Conf. Proc. 2021, 2332, 020003. [Google Scholar] [CrossRef]
- Chen, W.; Liu, Z.; Li, Y.; Xing, X.; Liao, Q.; Zhu, X. Improved electricity generation, coulombic efficiency and microbial community structure of microbial fuel cells using sodium citrate as an effective additive. J. Power Sour. 2021, 482, 228947. [Google Scholar] [CrossRef]
- Arun, S.; Sinharoy, A.; Pakshirajan, K.; Lens, P.N. Algae based microbial fuel cells for wastewater treatment and recovery of value-added products. Renew. Sustain. Energy Rev. 2020, 132, 110041. [Google Scholar] [CrossRef]
- Marzorati, S.; Schievano, A.; Colombo, A.; Lucchini, G.; Cristiani, P. Ligno-cellulosic materials as air-water separators in low-tech microbial fuel cells for nutrients recovery. J. Clean. Prod. 2018, 170, 1167–1176. [Google Scholar] [CrossRef]
- Seo, Y.; Kang, H.; Chang, S.; Lee, Y.-Y.; Cho, K.-S. Effects of nitrate and sulfate on the performance and bacterial community structure of membrane-less single-chamber air-cathode microbial fuel cells. J. Environ. Sci. Health 2018, 53, 13–24. [Google Scholar] [CrossRef]
- Lu, M.; Chen, S.; Babanova, S.; Phadke, S.; Salvacion, M.; Mirhosseini, A.; Chan, S.; Carpenter, K.; Cortese, R.; Bretschger, O. Long-term performance of a 20-L continuous flow microbial fuel cell for treatment of brewery wastewater. J. Power Sour. 2017, 356, 274–287. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Kakarla, R.; Min, B. Algae cathode microbial fuel cells for electricity generation and nutrient removal from landfill leachate wastewater. Int. J. Hydrog. Energy 2017, 42, 29433–29442. [Google Scholar] [CrossRef]
- Prathiba, S.; Kumar, P.S.; Vo, D.-V.N. Recent advancements in microbial fuel cells: A review on its electron transfer mechanisms, microbial community, types of substrates and design for bio-electrochemical treatment. Chemosphere 2022, 286, 131856. [Google Scholar] [CrossRef]
- Dannys, E.; Green, T.; Wettlaufer, A.; Madhurnathakam, C.; Elkamel, A. Wastewater treatment with microbial fuel cells: A design and feasibility study for scale-up in microbreweries. J. Bioprocess Biotech 2016, 6, 2. [Google Scholar]
- Yang, Q.; Wu, Z.; Liu, L.; Zhang, F.; Liang, S. Treatment of oil wastewater and electricity generation by integrating constructed wetland with microbial fuel cell. Materials 2016, 9, 885. [Google Scholar] [CrossRef] [PubMed]
- Bosire, E.M.; Blank, L.M.; Rosenbaum, M.A. Strain- and Substrate-Dependent Redox Mediator and Electricity Production by Pseudomonas aeruginosa. Appl. Environ. Microbiol. 2016, 82, 5026–5038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, Z. Energy-Efficient Wastewater Treatment by Microbial Fuel Cells: Scaling up and Optimization. Doctoral Dissertation, Virginia Polytechnic Institute and State University, Blacksburg, VA, USA, 2015. [Google Scholar]
- Mahendra, B.; Mahavarkar, S. Treatment of wastewater and electricity generation using microbial fuel cell technology. Int. J. Res. Eng. Technol. 2013, 1, 277–282. [Google Scholar]
- Salar-García, M.; Ortiz-Martínez, V. Nanotechnology for Wastewater Treatment and Bioenergy Generation in Microbial Fuel Cells. In Advanced Research in Nanosciences for Water Technology; Springer: Berlin/Heidelberg, Germany, 2019; pp. 341–362. [Google Scholar]
- Khoo, K.S.; Chia, W.Y.; Tang, D.Y.Y.; Show, P.L.; Chew, K.W.; Chen, W.-H. Nanomaterials utilization in biomass for biofuel and bioenergy production. Energies 2020, 13, 892. [Google Scholar] [CrossRef]
- Zhao, C.; Gai, P.; Liu, C.; Wang, X.; Xu, H.; Zhang, J.; Zhu, J.-J. Polyaniline networks grown on graphene nanoribbons-coated carbon paper with a synergistic effect for high-performance microbial fuel cells. J. Mater. Chem. 2013, 1, 12587–12594. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Zhang, Q.; Li, C. Microbial fuel cells: Nanomaterials based on anode and their application. Energy Technol. 2020, 8, 2000206. [Google Scholar] [CrossRef]
- Roh, S.H. Layer-by-layer self-assembled carbon nanotube electrode for microbial fuel cells application. J. Nanosci. Nanotechnol. 2013, 13, 4158–4161. [Google Scholar] [CrossRef]
- Yuan, H.; He, Z. Graphene-modified electrodes for enhancing the performance of microbial fuel cells. Nanoscale 2015, 7, 7022–7029. [Google Scholar] [CrossRef] [Green Version]
- Fan, M.; Zhang, W.; Sun, J.; Chen, L.; Li, P.; Chen, Y.; Zhu, S.; Shen, S. Different modified multi-walled carbon nanotube-based anodes to improve the performance of microbial fuel cells. Int. J. Hydrog. Energy 2017, 42, 22786–22795. [Google Scholar] [CrossRef]
- Premier, G.; Michie, I.; Boghani, H.; Fradler, K.; Kim, J. Reactor design and scale-up. In Microbial Electrochemical and Fuel Cells; Elsevier: Amsterdam, The Netherlands, 2016; pp. 215–244. [Google Scholar]
- Rahimnejad, M.; Bakeri, G.; Najafpour, G.; Ghasemi, M.; Oh, S.-E. A review on the effect of proton exchange membranes in microbial fuel cells. Biofuel Res. J. 2014, 1, 7–15. [Google Scholar] [CrossRef]
- Chin, M.Y.; Phuang, Z.X.; Woon, K.S.; Hanafiah, M.M.; Zhang, Z.; Liu, X. Life cycle assessment of bioelectrochemical and integrated microbial fuel cell systems for sustainable wastewater treatment and resource recovery. J. Environ. Manag. 2022, 320, 115778. [Google Scholar] [CrossRef] [PubMed]
- Ayol, A.; Peixoto, L.; Keskin, T.; Abubackar, H.N. Reactor designs and configurations for biological and bioelectrochemical C1 gas conversion: A review. Int. J. Environ. Res. Public Health 2021, 18, 11683. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, D.A.; Park, S.-G.; Eisa, T.; Mungray, A.K.; Madenli, E.C.; Olabi, A.-G.; Abdelkareem, M.A.; Chae, K.-J. Current outlook towards feasibility and sustainability of ceramic membranes for practical scalable applications of microbial fuel cells. Renew. Sustain. Energy Rev. 2022, 167, 112769. [Google Scholar] [CrossRef]
- Janicek, A.; Fan, Y.; Liu, H. Design of microbial fuel cells for practical application: A review and analysis of scale-up studies. Biofuels 2014, 5, 79–92. [Google Scholar] [CrossRef]
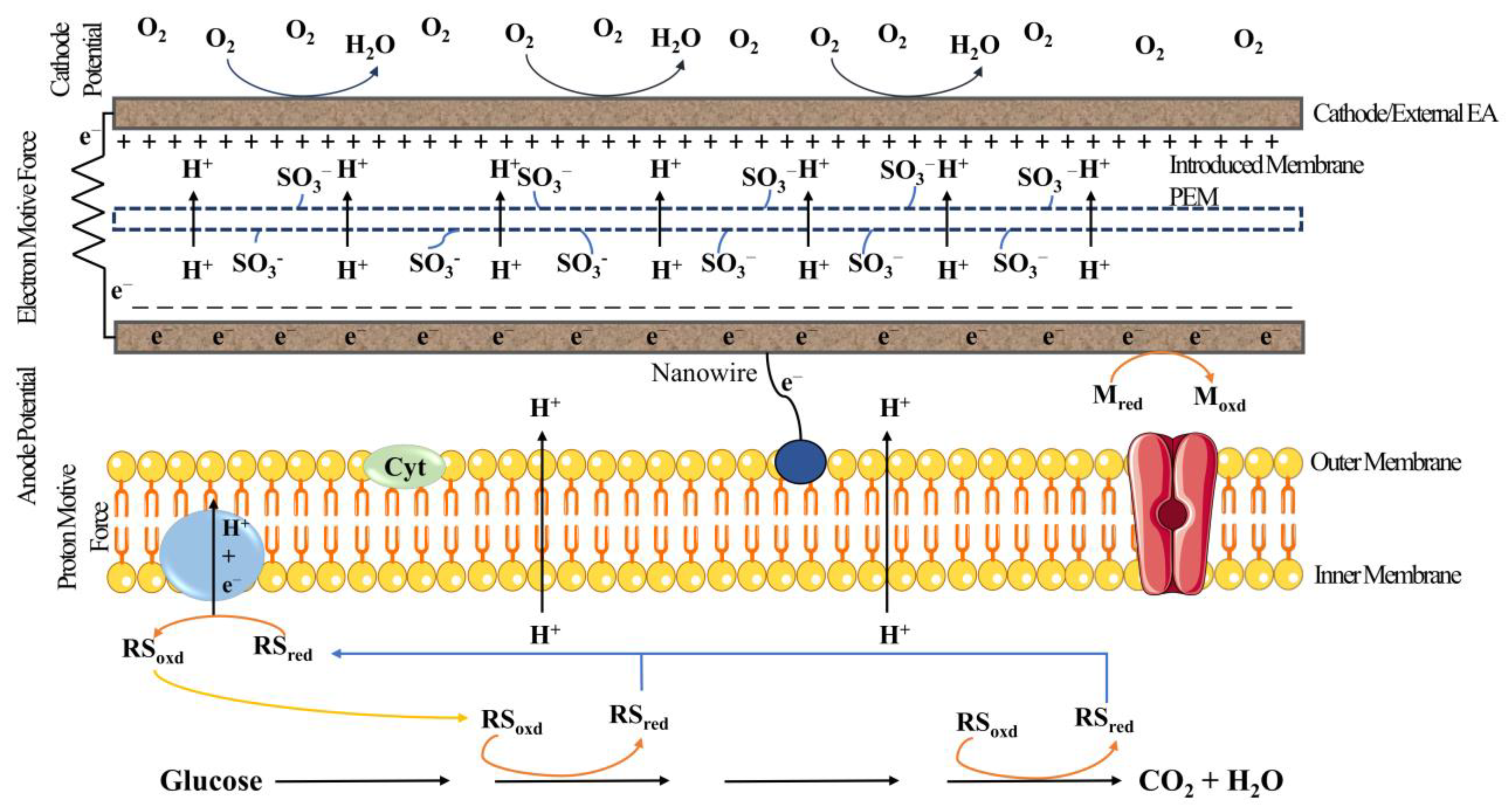
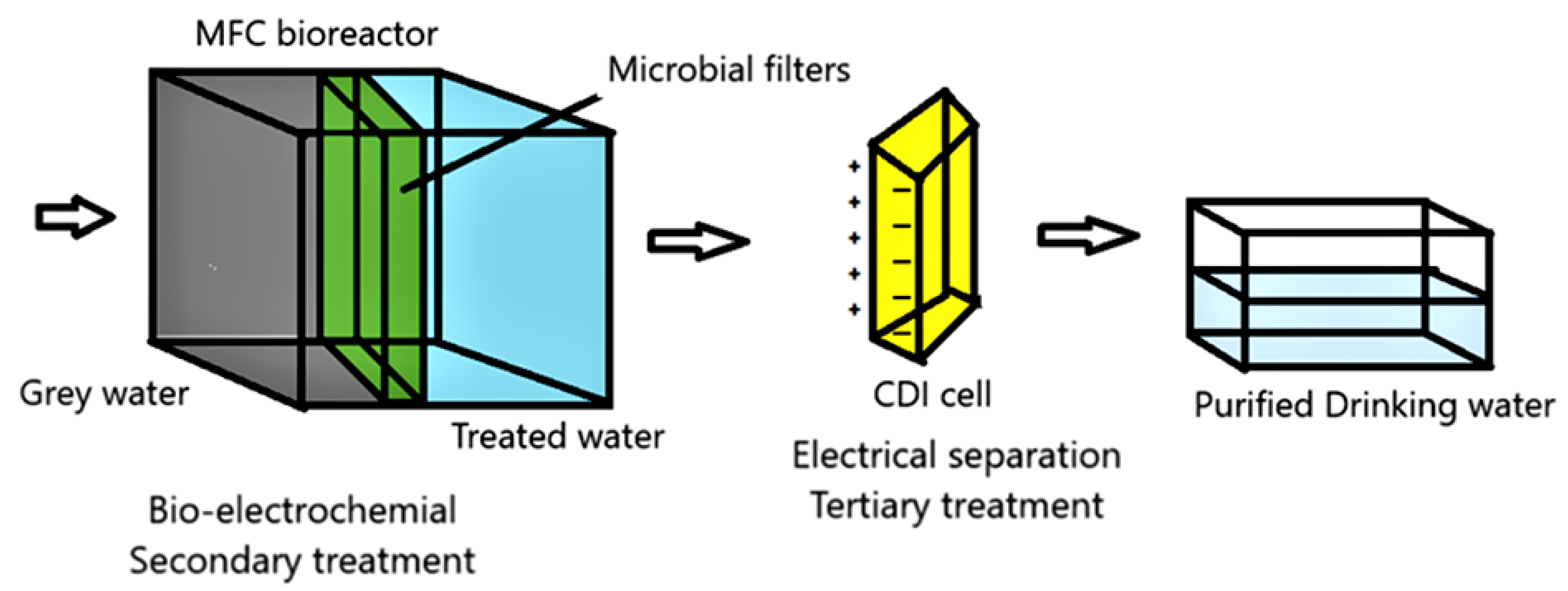
| S. No. | Inoculum and Substrate | Type of MFC | Electrode Material | Power Density/Current Density/Voltage | Treatment Efficiency | Reference |
|---|---|---|---|---|---|---|
| 1 | Swine wastewater manure | Two-chambered | Carbon cloth | 13 mW/m2 | TCOD: 83%, CE: 0.3% | [81] |
| 2 | Agriculture wastewater (Human feces wastewater) | Two-chambered | -Anode: carbon paper -Cathode: carbon paper with 40% platinum | 70.8 mW/m2 | TCOD: 71.0%, SCOD: 88.0%, NH4+: 44.0% | [82] |
| 3 | Domestic and olive mill wastewater | Single-chambered air cathode | -Anode: graphite fiber brush. -Cathodes 7 cm2 (total exposed surface area) | 124.6 mW m−2 | TCOD: 65.0% BOD: 50.0%, CE: 29% | [83] |
| 4 | Dairy wastewater (COD of 1000 mg/L) inoculated by activated sludge from the dairy WWTP | Annular single chambered | -Graphite-coated stainless-steel mesh anode -Cathode: carbon cloth type B | 20.2 W/m3 | COD: 91%, CE: 26.87% | [79] |
| 5 | Synthetic wastewater | Up-flow constructed wetland (UCW-MFC) | -anode: graphite -cathode: magnesium | 15.1 mW/m2 | COD: 92.1%, NH4+: 93.2%, NO3−: 81.1%, CE: 1.64% | [84] |
| 6 | Industrial wastewater | Dual chambered anaerobic MFCs | Anode and cathode | 260 mW/m2 | TCOD: 87%, SCOD: 79%, TSS: 72% | [85] |
| 7 | Acetate | Single-chambered MFC | Substrate as a source of carbon to stimulate electroactive bacteria | 506 mW/m2 | CE (72.3%), butyrate (43.0%), propionate (36.0%), and glucose (15%) | [86] |
| 8 | Arabitol | Single-chambered MFC | Co substrate in a single chamber | 0.68 mA/cm2 | COD: >91% | [87] |
| 9 | Cysteine | MFC with carbon paper electrodes (11.25 cm2) dual chamber | Co-substrate | 36 mW/m2 | - | [86] |
| 10 | Common effluent treatment plant (CETP) wastewater | H-type, dual chamber, mediator-less MFC | graphite plates | 0.6 V | COD: 50% | [88] |
| 11 | Sodium benzoate (0.721 g/L) | H-type, dual chamber, mediator-less MFC | graphite plates | 0.8 V | COD: 89% | [88] |
| S. No. | Material Used | Anode/ Cathode | Advantages | Disadvantages | Reference |
|---|---|---|---|---|---|
| 1 | Graphite rods | Anode | High conductivity, chemical stability, low cost, and easy to handle | Surface area is difficult to increase | [90] |
| 2 | Graphite brushes | Anode | Easy to construct and more specific area | Clogging issues | [91] |
| 3 | Carbon cloth | Anode | Large porosity relatively | Not cost efficient | [92] |
| 4 | Carbon paper | Anode | Easy to construct wire connection | Brittle | [93] |
| 5 | Carbon felt | Anode | Enormous surface area | Elevated resistance | [94] |
| 6 | Reticulated vitreous carbon | Anode | High electrical conductivity | Delicate and large resistance | [48] |
| 7 | Stainless steel | Anode | High conductivity, cost efficient, and easily accessible | Low surface area, compatibility issues, can get corroded | [95] |
| 8 | Pt-based catalyst | Cathode | High surface area and low potential for the oxygen reduction reaction | pH sensitivity, sulfide poisoning, and non-sustainability | [96] |
| 9 | Non-Pt-based catalyst | Cathode | pH control, no sulfide poisoning, and non-sustainability | Compromised electron transfer | [97] |
| 10 | Carbon Nano tubes | Cathode | High surface area and power density | Voltage losses | [98] |
| 11 | Palladium | Cathode | Excellent catalytic properties and low cost | Very low oxygen reduction reaction overpotential for catalytic hydrogen production | [99] |
| 12 | Aerobic biocathode | Cathode | Production of methane, ethanol, and formic acid via microbes and application as a biosensor for BOD detection | Loss of electrons through oxygen | [100] |
| 13 | Anaerobic biocathode | Cathode | Prevention of loss of electrons via anodic end | Biofilms catalyze the reduction of chemically active species | [54] |
| 14 | Cathode with metal-free catalyst | Cathode | Cheap materials, catalytic activity, stability | Superior electrocatalytic activity, with lower overpotential and prolonged stability for ORR | [97] |
| S. No. | MFC System | Outcome | Ref |
|---|---|---|---|
| 1 | Synthetic polymeric tubular MFC having affinity binding group for removal of volatile fatty acids and inorganic compounds | Effective removal (≥98%) of pollutants, up to 95% biodegradation of the toxic compounds | [125] |
| 2 | CW-MFC system developed for electroactive and textile dye wastewater treatment through microbial community | Bioaugmentation and dynamic removal of pollutants through electroactive bacterial community | [126] |
| 3 | Screening of fruit waste for MFCs | The energy production rate of orange waste was maximally up to 357 mV voltage output, followed by banana and mango fruit waste | [127] |
| 4 | Estimated the power generation capacity of sodium citrate-treated MFCs | Significantly improved biocatalytic activity of anode with maximum electrical energy output | [128] |
| 5 | Study the MFC coupled effect with stacked 12 vertically-arranged constructed wetlands | Reduced COD level, uptake of free N and P, electricity generation | [73] |
| 6 | Microalgae can be used in MFCs | Efficient for CO2 uptake, effective removal of N and P, symbiotic microalgae–bacterial interactions for power generation | [129] |
| 7 | Anode–cathode catalysts immersed in biomolecule solutions (monosaccharides, nitrogen and amino acid) | 51% COD, 20 mL methane gas was achieved at 20.5 °C temperature | [10] |
| 8 | MFC operated through bioelectrochemical nutrient from human urine as a self-power generating system | Endured power and electrical current generation at a rate of 3 A/m for over two months and simultaneously increased concentration of N and K by a factor of 1.5–1.7 | [123] |
| 9 | Evaluated the effect of ammonia concentration on MFC power generation and efficiency | A high concentration of ammonia in the influent negatively affected the ammonium recovery and poor uptake of phosphorus by MFCs | [72] |
| 10 | Estimated the treatment efficiency of membrane-less MFCs by simulating core of a shallow un-planted horizontal subsurface flow-constructed wetland system | Effective for domestic wastewater treatment with 25% efficiency | [72] |
| 11 | Applicability of lingo-cellulosic low-cost material for MFCs | Maximum power generation through the high electro-osmotic force and high pH at the cathode with significant recovery of elements | [130] |
| 12 | Evaluated the effect of nitrate and sulphate components on MFC microbial component activity | Nitrate does not show any effect on cathode and anode microbial flora. However, the bacterial community of Desulfovibrio showed dominant growth on the cathode (32.9%) after the addition of sulfate | [131] |
| 13 | Studied the long-term processing of multi-layered MFC for brewery effluent | Maximum removal efficiency for COD up to 94.6 ± 1.0% but system failure due to long-term processing | [132] |
| 14 | Algae cathode MFCs for landfill leaching at different concentrations of pollutants of 5–40% | Enhanced removal of nitrogen and phosphorus with power generation | [133] |
| 15 | 200 L modularized MFC system consisting of 96 MFC modules | The cost-effective system generates ∼200 mW power, 75% of the total COD, and 90% of the suspended solids removal | [7] |
| 16 | Electro-chemicals disruption of pollutants | Non-toxic metabolites | [134] |
| 17 | Two-chamber MFC for wastewater treatment at a rate of 84 L/hr and COD of 3000 mg/L | COD conversion of 91.9%, electricity generation of 26.4 kWh for the feed of 84 L/hr | [135] |
| 18 | Constructed wetland reactor and a microbial fuel cell reactor(CW-MFC) for digestion | MFC digestion rate for 98–100 L/hr and 74% electricity generation | [136] |
| 19 | microbial bioelectrochemical systems (BES) co-culture Pseudomonas aeruginosa and other strain | Highest electrochemical activity | [137] |
| 20 | MFC system with passive aeration method for waste treatment | Cost-effective approaches for electricity generation by the 80% organic compound removal | [138] |
| 21 | Comparative study of a single-chamber (MFC-1) and double-chamber (MFC-2) MFC for wastewater treatment | Effective removal of solutes, maintenance of COD level and electricity production | [139] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malik, S.; Kishore, S.; Dhasmana, A.; Kumari, P.; Mitra, T.; Chaudhary, V.; Kumari, R.; Bora, J.; Ranjan, A.; Minkina, T.; et al. A Perspective Review on Microbial Fuel Cells in Treatment and Product Recovery from Wastewater. Water 2023, 15, 316. https://doi.org/10.3390/w15020316
Malik S, Kishore S, Dhasmana A, Kumari P, Mitra T, Chaudhary V, Kumari R, Bora J, Ranjan A, Minkina T, et al. A Perspective Review on Microbial Fuel Cells in Treatment and Product Recovery from Wastewater. Water. 2023; 15(2):316. https://doi.org/10.3390/w15020316
Chicago/Turabian StyleMalik, Sumira, Shristi Kishore, Archna Dhasmana, Preeti Kumari, Tamoghni Mitra, Vishal Chaudhary, Ritu Kumari, Jutishna Bora, Anuj Ranjan, Tatiana Minkina, and et al. 2023. "A Perspective Review on Microbial Fuel Cells in Treatment and Product Recovery from Wastewater" Water 15, no. 2: 316. https://doi.org/10.3390/w15020316