1. Introduction
Water caltrop (
Trapa natans L.) is an annual plant with floating leaves. It roots on the bottom, preferring eutrophic sites in stagnant and slowly flowing waters that are well insolated. Water caltrop also occurs in oxbow lakes and ponds where the bottom consists of bottom sediments that are rich in organic matter [
1,
2,
3]. This species is monitored at six sites in Poland that are not representative of the number of sites throughout the country. Additional in-depth studies on ponds located in nature reserves are nationally important for the conservation of both species and natural small water bodies.
On a global scale, according to the IUCN, the status of
T. natans is "least concern" (LC). Its distribution includes Eurasia and Africa, but it has also been introduced to Australia and the United States [
4]. In Europe,
T. natans is considered a species in need of protection and is therefore listed in Appendix 1 to the Bern Convention. It mainly occurs in the central part of the continent. In North America, it is considered invasive [
4]. The species is highly variable, as evidenced by the distinction of up to 20 species of the genus
Trapa [
5]. However, in the Integrated Taxonomic Information System, the two main subspecies of
T. natans are var.
natans and var.
bispinosa [
6]. Previously, in Poland,
T. natans was common; however, in the period from 1870 to 1980, the species lost approximately 82% of its known sites [
1]. In the last two decades of the 20th century, it lost another 60 sites, and in the last decade of the 20th century, its occurrence was confirmed at only 39 sites [
7].
T. natans has been under strict species protection since 1946. Currently considered to be threatened with extinction in recent years, new sites of the species have been found, which resulted in the reduction in the risk category from critically endangered (CR) in 2001 [
8] and 2006 [
9] to endangered (EN) in 2014 [
10], and then to vulnerable (VU) in 2016 [
11].
T. natans sites are currently monitored by the Institute of Nature Conservation, Polish Academy of Sciences in Cracow [
12]. The approved locations are available online in the form of a map with an Open Street Maps base (
https://www.iop.krakow.pl/kotewka, accessed on 12 May 2022).
In Poland,
Trapa natans has an endangered species status, but in some parts of the world, it is an invasive plant, and its growing population is a problem for native species and the economy [
13]. The Potomac, Hudson Rivers, and Lake Champlain, USA, are examples of the rapid growth of the water caltrop population, where it has been known since the mid-19th century. The extensive and costly work undertaken to remove it has not been successful. The water caltrop colonizes water, being introduced from aquariums or horticulture, thereby qualitatively and quantitatively changing the primary production, as well as the characteristics of bottom sediment [
14,
15]. In such situations, water caltrop, like any invasive species appearing in new areas, may reduce local biodiversity [
16]. Water caltrop, with fruit having two horns instead of four, has also been appearing in the USA since 2014, including
Trapa bispinosa Roxb. var.
iinumai [
16,
17], which may further adversely affect native species through competition for light and nutrients.
The distribution of water caltrop in contemporary Poland also decreased during periods of climate cooling. The findings of paleobotanic studies conducted by Lewandowska [
18] in the areas of the Kusowo and Mechacz Wielki swamps indicated that water caltrop retreated from the very high calcium contents in the sediments, but it persisted with high levels of iron and magnesium. In the warmer periods of the Holocene, the
T. natans distribution extended much further north to the area of Pomerania (Kashubia). This finding was confirmed during stratigraphic studies, during which soil profiles were collected from peatlands where fossilized remains of water caltrop were found [
19,
20].
The forms of protecting the species include introduction, reintroduction, and water level regulation [
1,
7,
21,
22,
23,
24,
25,
26]. For water caltrop, the procedure is not always 100% effective. An attempt to rebuild the population of
T. natans in the Oświęcim Valley in 2002 by introducing its fruits was successful only in 8 out of 15 reservoirs [
3]. Of the many reasons for the loss of water caltrop, those mainly mentioned are the progressive eutrophication of aquatic ecosystems (sewage inflow, changes in water chemical mechanism, and overgrowing and shallowing of lakes and oxbow lakes), as well as all amelioration treatments (renovation of ponds, mowing macrophytes, soil drainage, river regulation, and lowering the water level), and the introduction of
Ctenopharyngodon idella. The water caltrop habitat is also affected by average annual temperature, the speed of water flow, water deposition during the flood season, and too low water and air temperatures; under such conditions, it is replaced by other macrophytes. The rate at which the water caltrop population dies out does not depend on their number.
Trapetum natantis plant associations can regenerate in a relatively short time, from 3–10 years, even from single plants [
1,
23,
27]. Studies on water caltrop have recently been conducted in many regions and subspecies. For instance, the possibility of using
Trapa bispinosa in constructed wetlands was studied, and it performed well under P and N limitations [
28]. Based on such results,
T. bispinosa was recommended as an f floating-leaved plant for constructed wetlands [
29].
Trapa natans (T. longicarpa ssp.
scutarensis) was examined by Krivokapić [
30] according to its usefulness in managing pollution in Skadar Lake (Montenegro).
Our aim in this study was to assess the habitat and conservation status of the water caltrop (
T. natans) in the Szumirad reservoir and the Nowokuźnicki pond reserve during 1977–2020 and to identify potential threats to the analyzed habitats. We compared our results with the historical data describing the condition of these habitats in previous years [
1,
23,
31,
32,
33,
34].
2. Materials, Methods, and Study Area
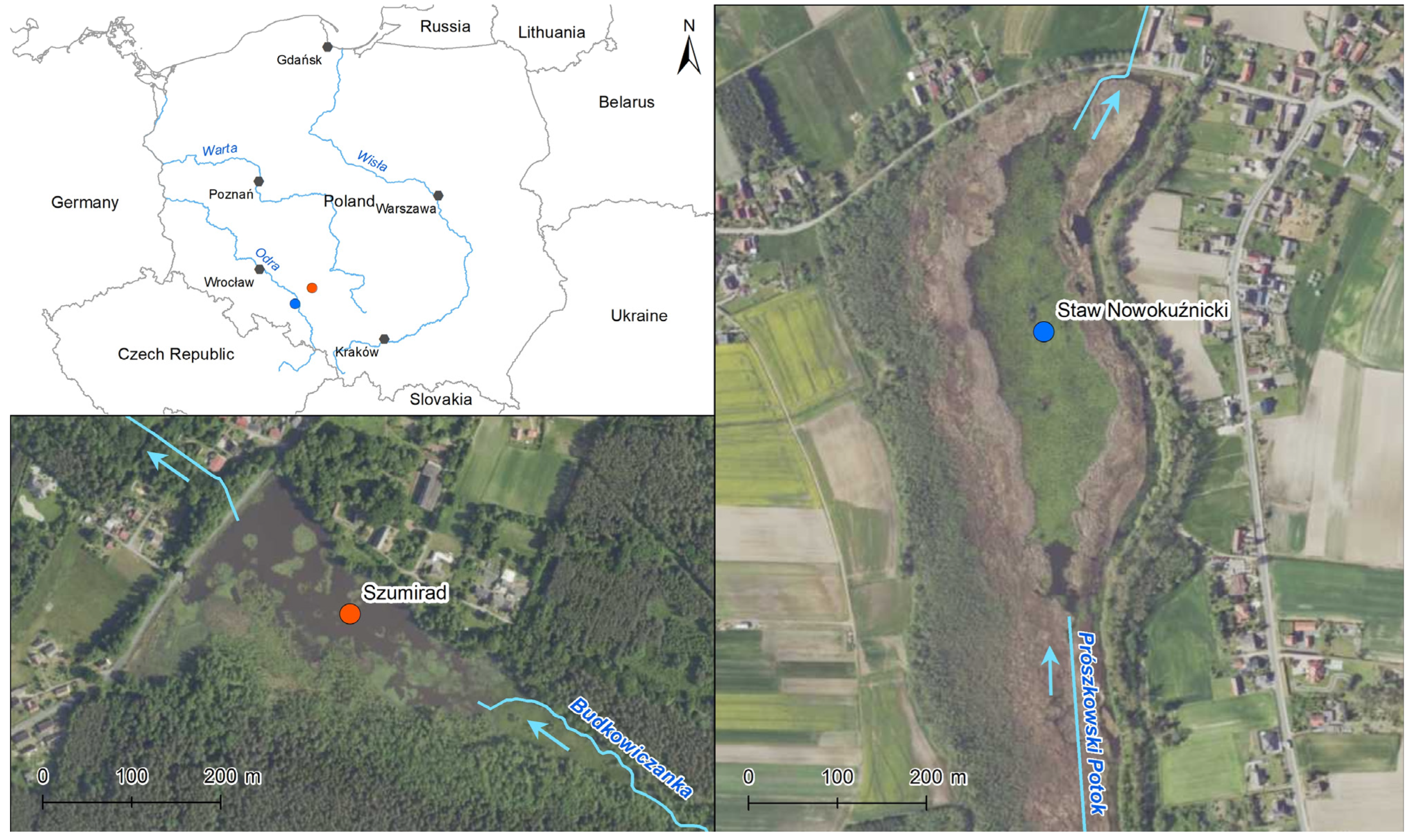
The study objects included two small dam reservoirs located in the southern part of Poland in the Opolskie Voivodship (
Figure 1). The Szumirad water reservoir is a part of the Smolnik nature reserve, which was established in 1958. It is located in the village of Szumirad in the Opolskie Voivodship in the Lasy Stobrawsko-Turawskie Protected Landscape Area and in the Natura 2000 Szumirad area (PLH160020). The reservoir has an area of 7.60 ha, including an open water surface area of 5.44 ha. Its average depth was 1.5 m, and its maximum depth was 2.1 m during the study in 2014. The thickness of the bottom sediments ranged between 0.1 and 0.9 m. The Budkowiczanka River (inflow of the Brynica River) flowed through the reservoir. In addition to sedimentation of the suspension carried by the river, the succession of rush vegetation was also responsible for the process of changing the water into a land area. In 2014, phytolittoral covered 56.6% of the water table, including communities of plants with floating leaves (nymphaeids) with an area cover of 22.2%. The area extending from the south to the east of the reservoir was a complex of wetlands, including rush communities (mainly
Typhetum latifoliae and
Caricetum acutiformis), transitional peatlands, and forest communities, e.g., alder and ash–alder riparian forests [
35].
The second studied object was the Nowokuźnicki pond nature reserve, established in 1957 and located about 45 km from the Szumirad reservoir. It is located in the town of Nowa Kuźnia in the Opolskie Voivodeship within the Bory Niemodlińskie Protected Landscape Area. The reservoir has an area of 17.16 ha, including an open water surface area of 5.78 ha. During the study carried out in 2014, its average depth ranged from 1.2–1.4 m, while the thickness of bottom sediments, rich in organic matter, ranged from 0.1–0.9 m. The Prószkowski Stream (an inflow of the Oder River) flowed through the eastern part of the reservoir. Additionally, it was supplied by a small stream carrying water from the nearby forests and a drainage ditch carrying water from the agricultural areas adjacent to the reserve. The reservoir was overgrown with rushes, especially on the south and west sides. From 1978 to 2020, the rush area increased threefold. In 2014, phytolittoral covered over 95% of the water surface, including a
Nuphar lutea community covering 43.3%. The direct vicinity of the reservoir consisted of arable land, meadows, alder and willow woodlands, and reed stands, mainly
Phragmitetum australis and
Typhetum angustifoliae [
35].
The study included the following:
Spatial analysis of the changes over time of the total area of reservoirs, rushes, and open water surfaces in the 1977–2020 period;
Detailed field studies in 2014, including assessment of the conservation status of the T. natans population and habitat;
Analysis of changes in the population of T. natans and the performed active protection actions;
Analysis of physico-chemical parameters of water in the years 1975, 1998, and 2014;
Analysis of climatic data (temperature and precipitation) in the period 1950–2020.
Our spatial analyses of the changes over time in the total area of reservoirs, rush area, open water surface area, and population of
T. natans were based on our own research, materials provided by the Regional Directorate for Environmental Protection in Opole, archival maps, and orthophotomaps available from WMS service. We scanned and recorded data from 1977–1978 [
1] and 1997–1998 [
22,
27], available in the form of paper maps at a scale of 1:3000 (Nowokuźnicki pond) and 1:5000 (Shumirad reservoir) in the EPSG:2180 coordinate system. Then, we vectorized the coastline, range of rushes, and the presence of
T. natans. In 2014, we performed field mapping of the vegetation in both reservoirs based on GPX tracks recorded in the field using a GPS device. Based on obtained results, we developed vector maps of vegetation [
26]. In 2019–2021, the Regional Directorate for Environmental Protection in Opole monitored the state of the water caltrop population with the use of a drone, which we used to create detailed orthophotomaps with a resolution of 10 cm/pixel, from which the size of the population and reed range was determined. In addition, we used orthophotomaps with a resolution of 25 cm/pixel to verify the course of the coastline and the range of the reed in 2010–2020, made available in the form of a WMS service by the Head Office of Geodesy and Cartography [
36]. We used these orthophotomaps to verify and correct the generated vector layers. We assessed the rate of overgrowth by rushes of the analyzed reservoirs and the predicted time of their disappearance on the basis of nonlinear regression.
We performed detailed field studies during the full growing season in 2014 with the use of a pneumatic boat in accordance with the official methodology for assessing the conservation status of the
Trapa natans population and habitat [
37]. In the course of the study, we completed observation cards of the species at the site and its conservation status; we also prepared lists of the most important impacts on the species and its habitat, which may pose a serious threat in the long term. We valorized the values of the indicators of the population and habitat status of the species, specified numerically or descriptively, on a three-level scale: FV, proper condition; U1, improper, unsatisfactory; U2, improper, bad. We assumed that the worst-rated indicator determined the final assessment of a given parameter (population, habitat). During the field study, we also performed a full mapping of the vegetation within the entire reservoir.
We collected water samples for the physico-chemical analysis during the vegetation season. We filtered all samples using Sartorius Cellulose filters with a nominal pore size of 0.45 μm, except those used for the determination of total phosphorous. The physico-chemical tests of water in 1975 and 1998 included 8 parameters. In 2014, they were extended with an additional 5 parameters (
Table 1). Moreover, in 2014, we collected water samples for analyses at three sites: above, middle, and below the ponds. We assessed the differences between the analyzed reservoirs and the inflow–reservoir–outflow system on the basis of the nonparametric Kruskal–Wallis analysis of variance.
We used public data provided by the Institute of Meteorology and Water Management: monthly average temperature, average annual temperature, and monthly rainfall total [
38]. We obtained Walter diagrams for the Opole station in 3 periods: 1951–1986, 1999–2010, and 2011–2020. The Opole weather station is located approximately 10 km from the Nowokuźnicki pond and approximately 30 km from the Szumirad reservoir. We assessed the rate of annual average air temperature increase at the Opole station on the basis of linear regression.
3. Results
In the Szumirad reservoir during the analyzed period (1977–2019), the pond surface area decreased by 22.3% and the water table area by 37.5%; the average rate of disappearance was 519 and 778 m
2/year, respectively. The reduction in the open water table resulted from the growth of rush at an average rate of 259 m
2/year. In the Nowokuźnicki pond, these processes occurred much faster. The reservoir area decreased by 32.8% and the water table area by as much as 68.0% (during 1978–2020). The average rate of disappearance was 1993 m
2/year of the reservoir area and 2919 m
2/year of the water table area. The rush quickly grew, amounting to 926 m
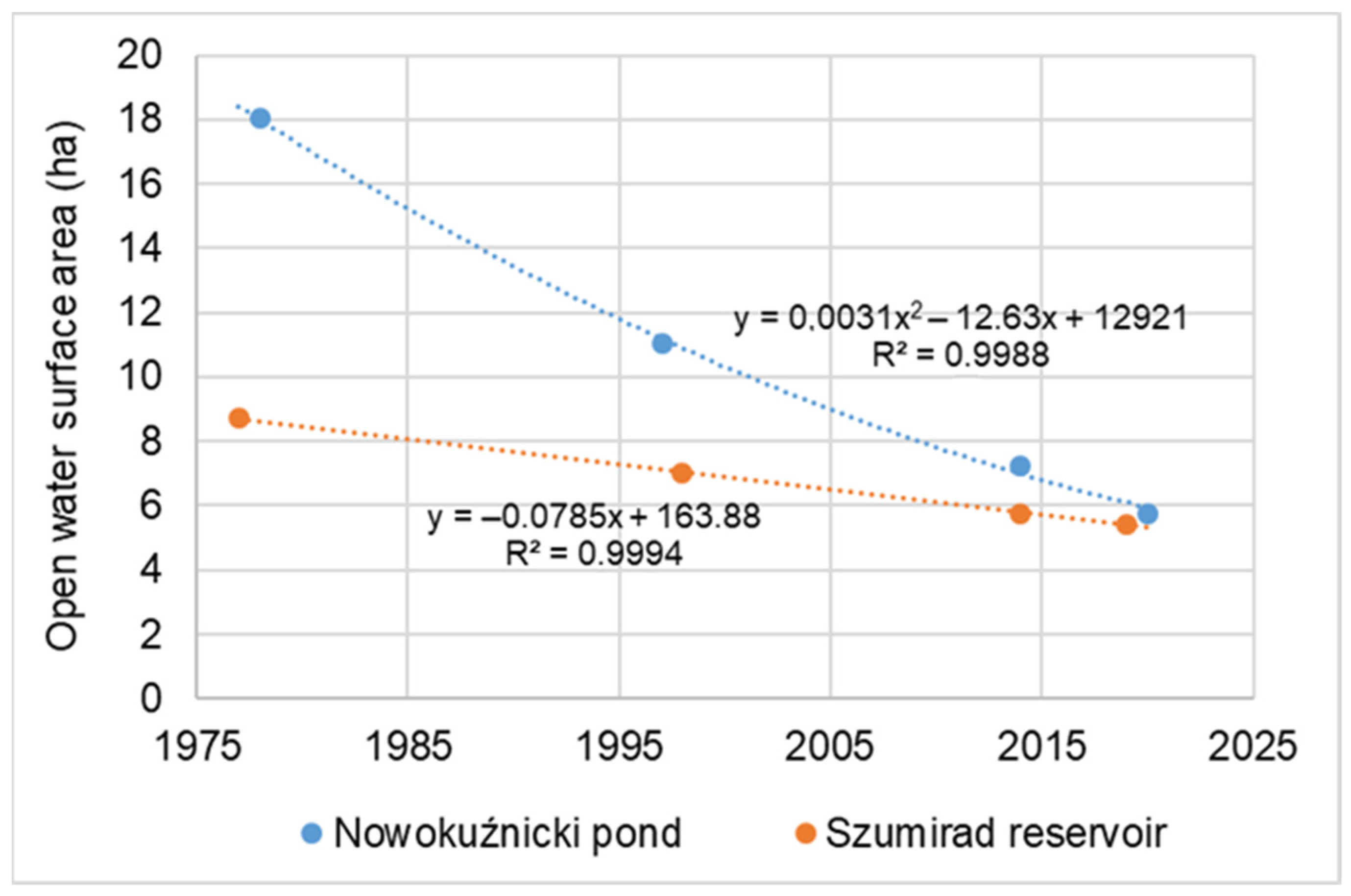
2/year. The disappearance of the reservoir, the reduction in the open water table area, and the growth of the rush were approximately 3.8 times faster than in Szumirad. For example, the open water table area at the end of the 1970s was 18.03 ha in the Nowokuźnicki pond and 8.71 ha in Szumirad; they have decreased to a comparable level of 5.48 and 5.44 ha, respectively (
Figure 2,
Figure 3 and
Figure 4). Moreover, in the Nowokuźnicki pond, more than 90% of the water surface area of the main part of the reservoir is covered by a dense community of
Nuphar lutea; in Szumirad, it covers only approximately 30% of the water surface. By extrapolating the current rate of the disappearance of the reservoirs, we predicted when they would be completely covered by land if no countermeasures were taken. The disappearance of the Nowokuźnicki pond is expected to occur around 2050 and 2090 for Szumirad (
Figure 4).
Comparing the results for the population size parameter, the Nowokuźnicki pond has a much larger population of this species (15–20,000) than the hundreds of rosettes (151) in the Szumirad reservoir. This high density of plants in one patch with an area of about 1875 m
2 in the Nowokuźnicki pond most likely had an impact on the smaller (25 cm on average) diameter of the leaf rosettes, the diameter of the leaf blades (3 cm on average), and the number of leaves in the rosette (26 on average), compared with those of the Szumirad reservoir. Here, the water caltrop appeared in 2 patches with a total area of 245 m
2; the other parameters were higher: 35 cm average leaf rosettes diameter, 3.9 cm average leaf blade diameter, and 36 leaves in a rosette of on average (
Table 2). In both cases, the final assessment of the population conservation status was unsatisfactory (U1). However, this was due to different reasons. In the case of Szumirad, it was due to the low density of rosettes (on average 7 pcs/m
2); in the case of Nowokuźnicki pond, the reason was the relatively small diameter of leaf rosettes (25 cm on average). Additionally, in both areas, the total assessments of the conservation status of the habitat were described as unsatisfactory (U1). In the case of the Szumirad reservoir, this was caused by the fragmentation of the habitat and competition from rush and
Nuphar lutea communities, covering a total of 46% of the reservoir surface. Increased competition, especially from the yellow water lily, occurred in the Nowokuźnicki pond, where, together with the rush, it covered as much as 97% of the reservoir’s surface. To summarize, the final assessment of both the Szumirad reservoir and the Nowokuźnicki pond was described as unsatisfactory (U1).
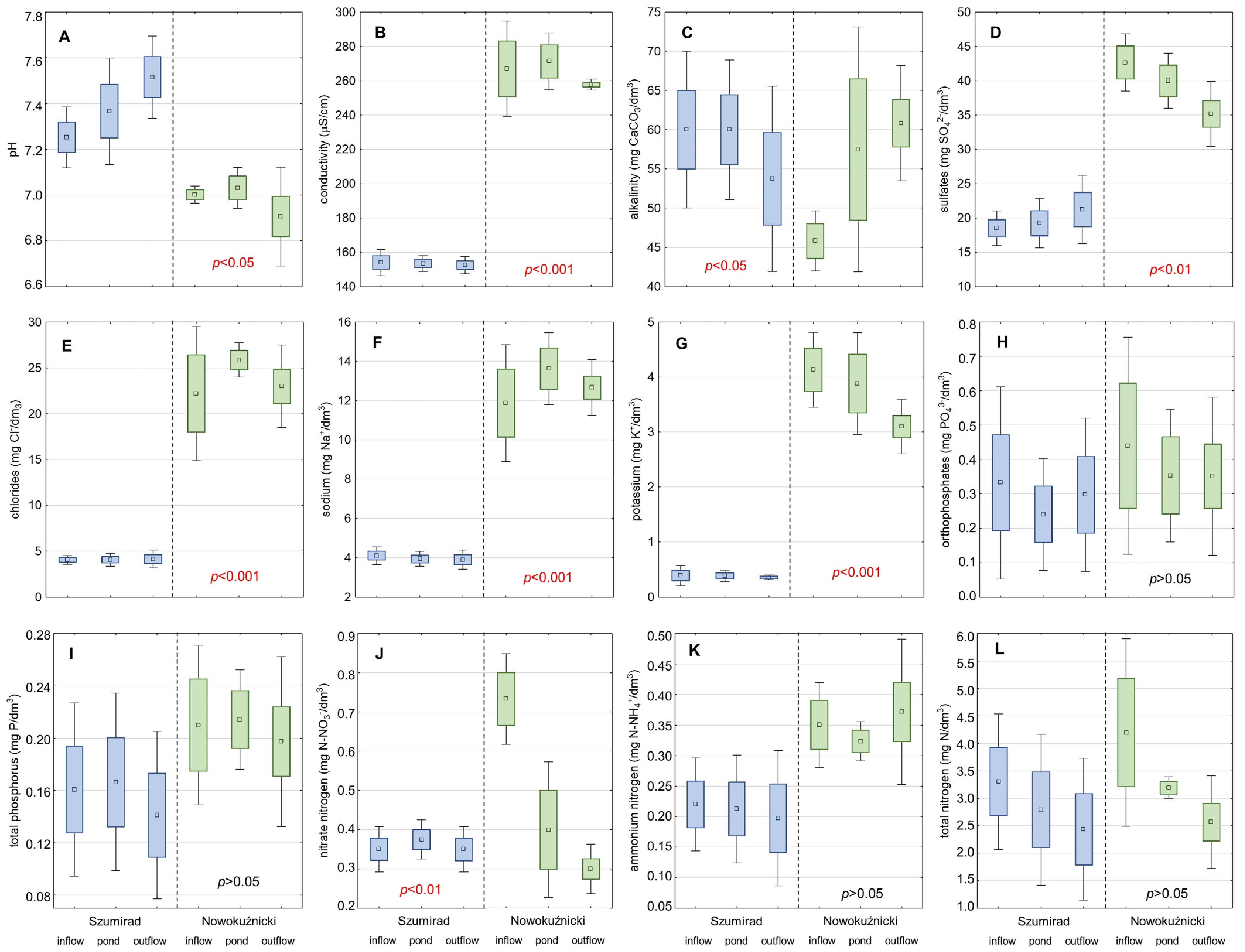
The water in the Szumirad reservoir was characterized by a low concentration of most of the analyzed ions (
Figure 5). The poor mineralization of water was confirmed by the low electrolytic conductivity. However, this parameter was constant during the study in 2014, ranging from 150 to 154 µS/cm. The reaction of the water was close to neutral. The water in the Budkowiczanka River was heated in the reservoir, averaging 13.4–17.8 °C during the growing season. The forest is located basin resulting in a lack of water pollution in the reservoir. The nearest built-up area, Kamieniec, is located a few kilometers from the reservoir. The area in between is covered with forest. When analyzing the long-term trends in the changes in the physico-chemical parameters of the water in the Szumirad reservoir from 1975–2014, we found that most of the parameters were relatively stable (
Table 3). However, we recorded a noticeable increase in trophic water level (concentration of orthophosphates and nitrates). We also noted a decrease in the water potassium concentration.
The water in Nowokuźnicki pond, compared with that in the Szumirad reservoir, was characterized by a higher concentration of almost every analyzed parameter (
Figure 5). It contained many more ions, and the conductivity was 1.7 times higher; the concentrations of sulfates, chloride, sodium, and potassium were 2, 5, 3, and 10 times higher, respectively. This also applied to all the analyzed forms of nutrients, the concentrations of which were higher by 1.2–2 times than those in the Szumirad reservoir. Only pH was lower: it was slightly acidic. The Nowokuźnicki pond water was also slightly warmer than that in Szumirad. When analyzing the long-term trends in the changes in the physico–chemical parameters of Nowokuźnicki pond water over 1975–2014, we observed a more notable increase in water trophic level than in Szumirad (
Table 4). This was indicated by all the analyzed forms of nutrients except for ammonium nitrogen. For example, phosphates, nitrates, and nitrite nitrogen concentrations increased five, almost three, and two times, respectively. As in the Szumirad reservoir, we also observed a decrease in the concentration of potassium in the water. The standards for healthy water status were exceeded in the case of phosphates in the Nowokuźnicki pond in 2014, which can cause excessive phytoplankton development and water blooms. The increase in the trophic water level may have supported the intensive development of
Ceratophyllum demersum in the Nowokuźnicki pond, forming a layer of plants submerged within the
Nuphar lutea patches with a density of up to 60%. This species is an indicator of the progressive eutrophication of water reservoirs.
The results of analyzing the physico-chemical parameters of the water in the hydrographic system showed that both ponds slightly purified the water of the watercourses flowing through them via the retention of nutrients (Budkowiczanka in the case of the Szumirad reservoir and Prószkowski Stream in the case of the Nowokuźnicki pond). However, these differences were, in most cases, statistically insignificant, except for nitrate and total nitrogen in the Nowokuźnicki pond (
Figure 5). In conclusion, from the point of view of
T. natans, suitable habitat conditions prevail in both analyzed reservoirs; however, the physico-chemical water parameters of the water were more favorable in the Szumirad reservoir. This was particularly true in terms of the lower concentrations of nutrients in the water.
4. Discussion and Summary
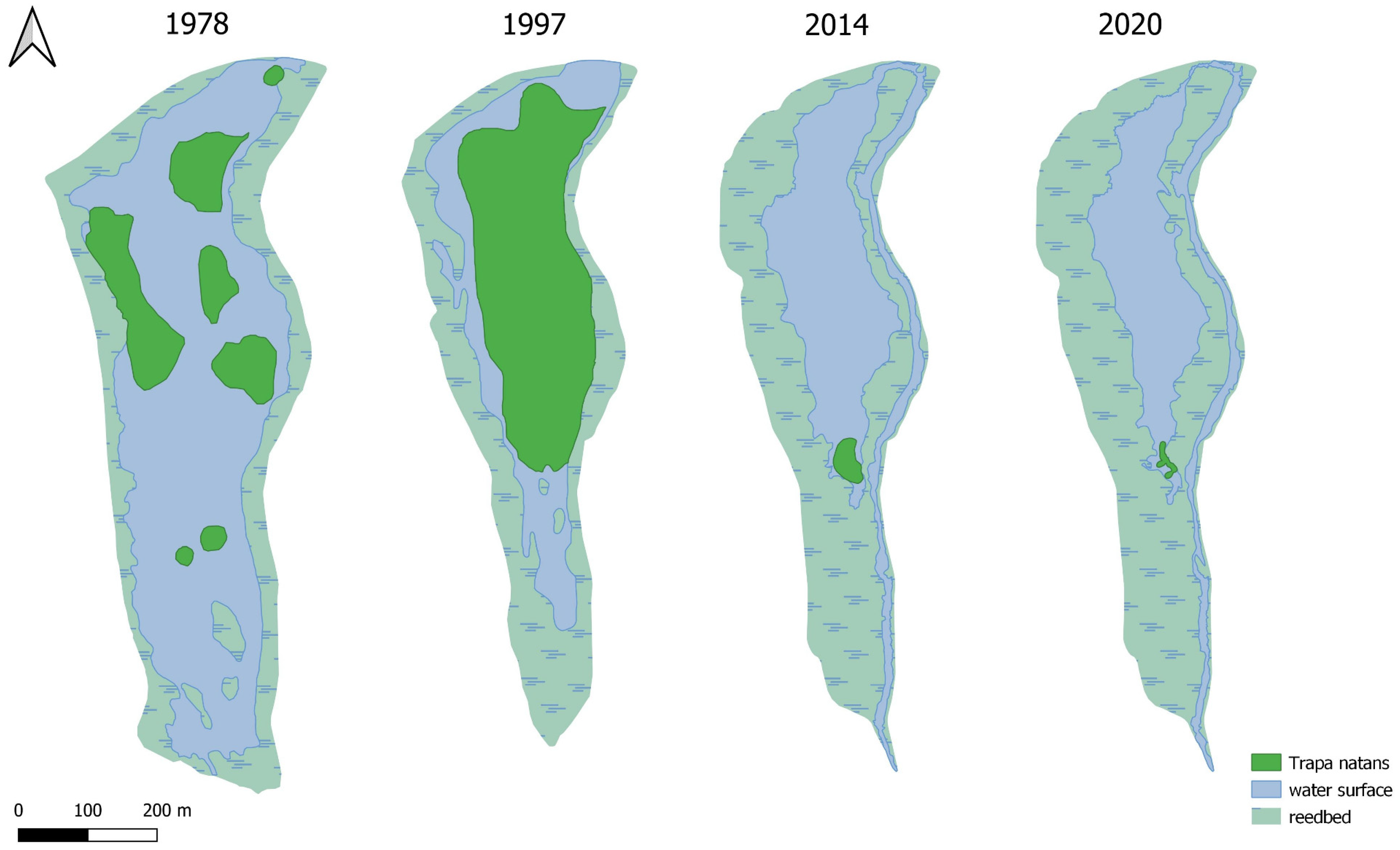
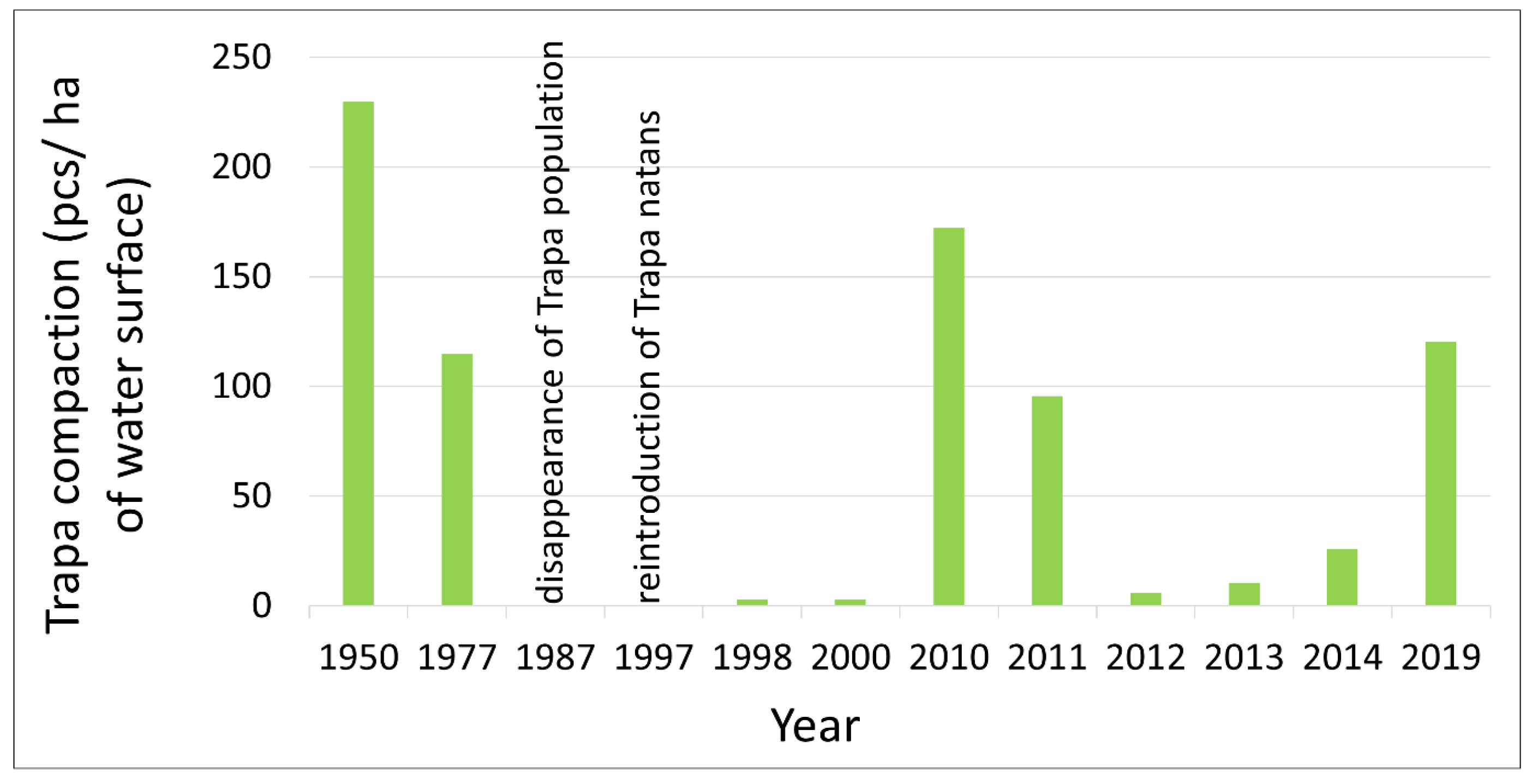
Water caltrop in Szumirad Reservoir was first recorded in 1887 [
31], but its gradual disappearance has been observed since 1950. In the 1950s, the Szumirad reservoir was used for recreational purposes by a resort located on its banks [
1]. In addition to the swimming area, boats, canoes, and pedal boats were used, and the reservoir was also used for fishing [
32]. At that time, the population of
T. natans was large and stable in the pond, covering approximately 0.87 ha, with an average density of 230 rosettes/ha of water surface. However, the recreational use of the reservoir resulted in a gradual reduction in the abundance of water caltrop (1200 m
2 and 115 rosettes/ha of water surface in 1977) and finally its disappearance in 1987 (
Figure 6). In 1997, the species was successfully reintroduced using seeds transferred from fish ponds in Krogulin [
34]. In the following year, rosettes were recorded over an area of approximately 20 m
2 [
27,
33]. By 2010, both the area occupied by
T. natans and the density of its population had almost returned to the state in the 1950s (0.87 ha and 172 rosettes/ha water surface). However, in the following two years, the population started to decline once again dramatically. In 2012,
Trapa was already only found singly in clusters of
Nuphar lutea or forming small patches in the southern margins of the pond. In 2014, an expert report was produced regarding the causes of the disappearance of
T. natans in the Szumirad reservoir [
35]. During the survey, 150 rosettes were counted in two main clusters over a total area of approximately 145 m
2. The expert report confirmed the suspected angling exploitation of the pond despite prohibitions, which can lead to the mechanical uprooting of water caltrop rosettes from the substrate. Since 2019, drone monitoring has been conducted, indicating a renewed recovery of the
T. natans population in the Szumirad reservoir (
Figure 2 and
Figure 6). The order of the Regional Director for Environmental Protection in Opole of 4 August 2014 on the establishment of a protection plan for the Smolnik nature reserve is currently in force in the reserve. The decline in the water caltrop population and the gradual disappearance of the reservoir, to which the natural processes of alluvial accumulation and the accompanying development of rushes and peatland vegetation have contributed, have been identified as the most important threats. Among the most important conservation tasks are the maintenance of a stable water level in the Budkowiczanka River, monitoring the water caltrop population, and prohibiting fishing and swimming in the reservoir. The restrictions implemented in 2014 likely enabled the significant increase in the water caltrop population (
Figure 6), mainly through lowering the intensity of reservoir usage, which meant that any mechanical damage to
Trapa rosettes was substantially reduced by minimizing activities such as fishing and recreational boating.
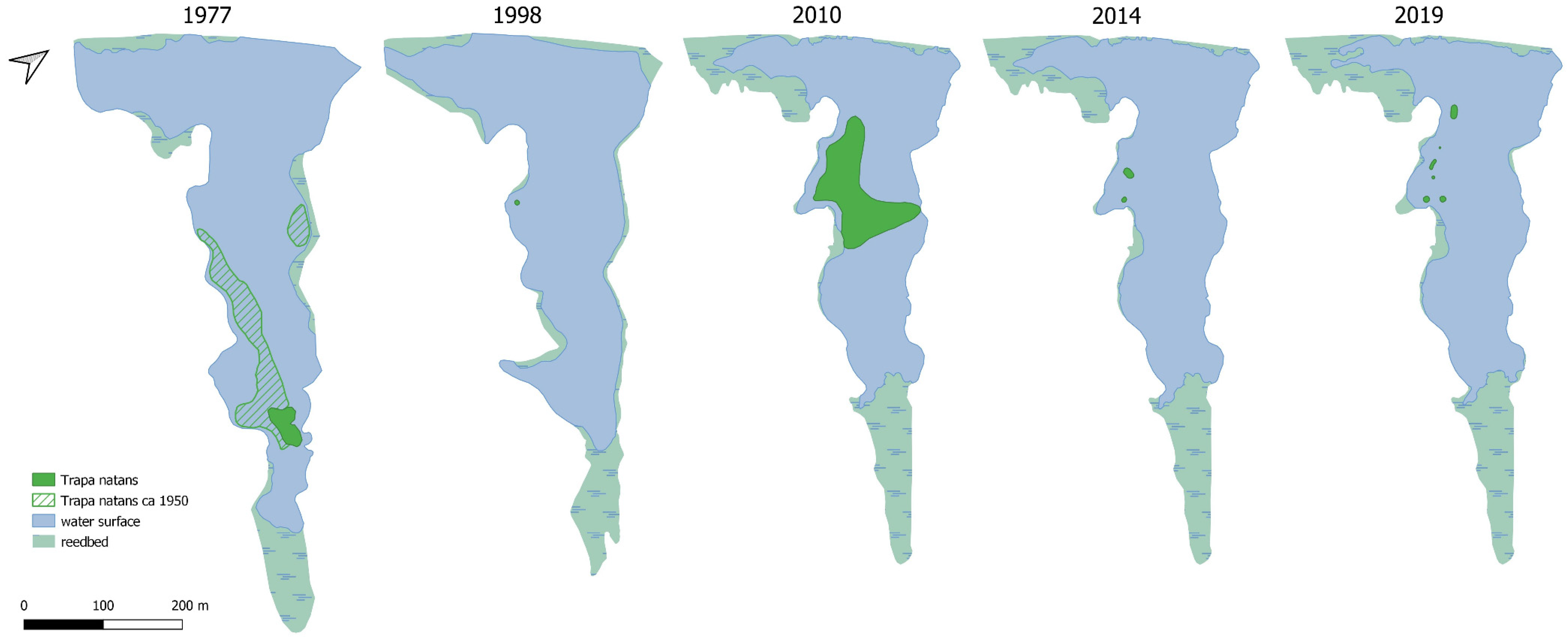
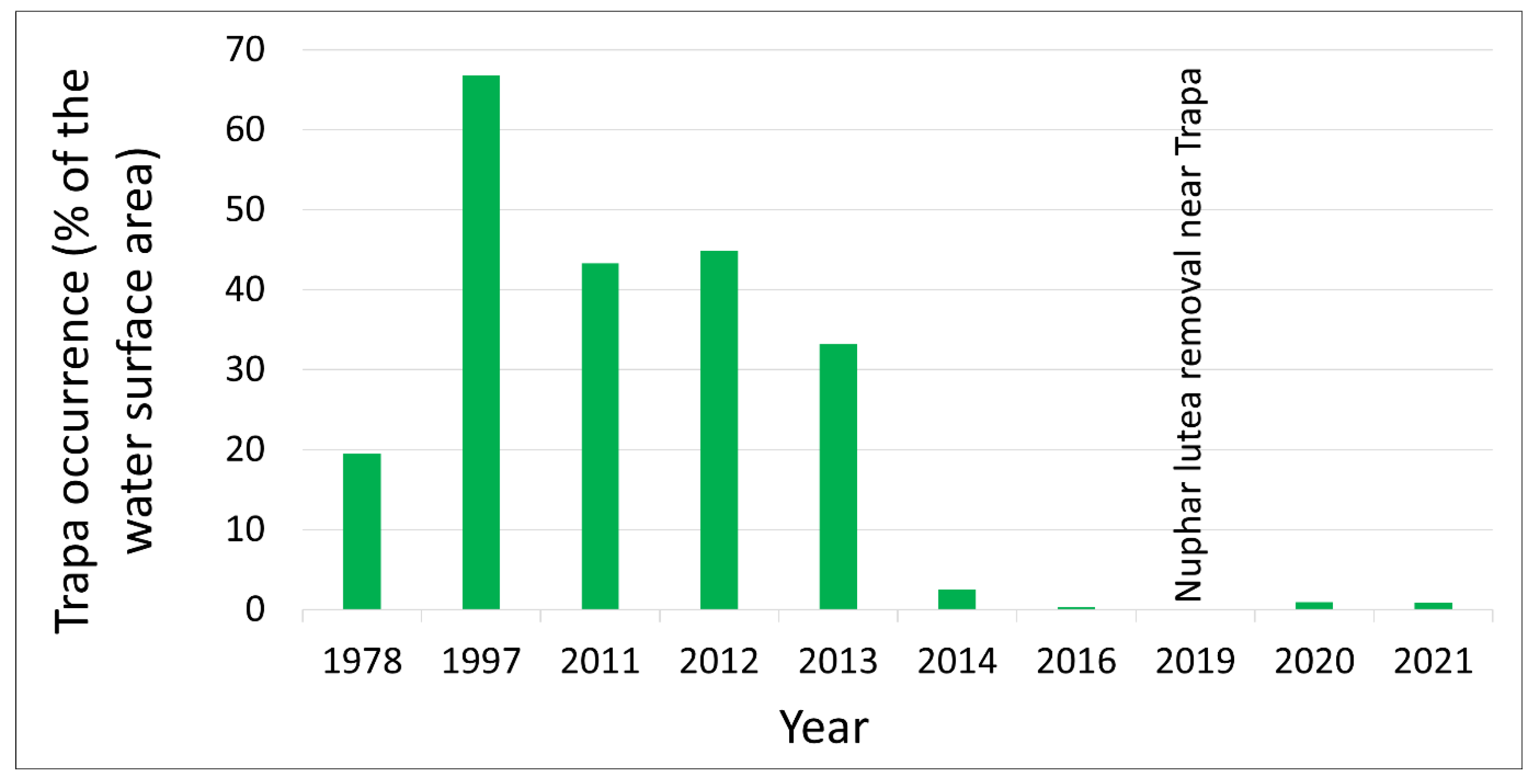
The first studies and floristic notes on Staw Nowokuźnicki were recorded by German [
39,
40,
41] and Polish [
1,
42,
43] researchers. In the late 1920s and early 1930s, a girdling ditch was dug a dozen meters to the east of the Nowokuźnicki pond, which drained approximately one-third of the Prószkowski Stream waters that had previously fed the reservoir. The reduction in the volume of water flowing through the pond considerably accelerated the rate of rush expansion and land formation in the southern part of the reservoir. In the 1970s, the average water depth was 1.2–1.4 m, and the thickness of the bottom sediments was 0.6–0.8 m [
1]. By the late 1990s,
Trapetum natantis occupied almost 70% of the open water surface (
Figure 7), but this had the largest area of water caltrop population in Silesia [
1]. By the beginning of the 21st century, the reservoir had become markedly shallower (0.8–1.0 m). To slow this process, the water flow in the Nowokuźnicki pond was intensified. In 2003, a 10–15 m wide bed of the eastern part of the Prószkowski Stream was reconstructed. In addition, desilting was performed in the southern part of the reservoir, at the boundary between the rushes and the open water table. However, the lateral location of the restored bed meant that the water of the Prószkowski Potok flowed little through the central part of the reservoir, so it did not slow down the expansion of the rushes. In addition, the stagnant water, shallow depth, and deposition of a considerable thickness of organic sediments resulted in the expansion of
Nuphar lutea in the main part of the pond. Its northern and central parts have now become more than 90% overgrown by water lily (
Figure 1). This phenomenon, along with hydrological drought, was also observed during the 2014 survey [
26]. This year was critical for the water caltrop population, as the area covered by
Trapetum natantis decreased from approximately 33% of the water surface area in 2013 to only 2.5% in 2014 (
Figure 3 and
Figure 7). In the southern part in the immediate vicinity of
T. natans, in addition to the desilting in 2003, work was performed in 2019 and 2021 at the end of the growing season (October–November) to mechanically remove
Nuphar lutea biomass together with rhizomes outside the reserve. Owing to these measures, the degree of overgrowth of yellow water lily in the southern part of the pond with has considerably reduced. The results of the monitoring indicated that active protection treatments were performed at the last minute, as the population status was extremely endangered at the time the first treatments were applied (
Figure 7).
Currently, the order of the Regional Director for Environmental Protection in Opole of 2 June 2017 on the establishment of a protection plan for the Nowokuźnicki nature reserve is in force for the Nowokuźnicki pond (Dz. Urz., Official Journal of the Opolskie Voivodship of 2017, item 1584), the protection goal of which is, in particular, the preservation of water caltrop, mainly due to the dynamic disappearance of this species in the reservoir as a result of the notable expansion of yellow water lily in recent years. Consequently, among the active protection measures, which will be implemented only in the southern part of the reservoir, the following are planned: removal of water lilies from the immediate vicinity of water caltrop sites, annual observation of the expansion degree of water lily, assessment of the effectiveness of the work performed, and annual monitoring of the status of the population and habitat of water caltrop and measures related to the unblocking of the Prószkowski stream. These measures include desilting its bed for a distance of 565 m to create a stream bottom slope equal to 1% and increase discharge intensity. This modification aims to return the main current to the center of the pond, thus enhancing water exchange and limiting reservoir shallowing. Depending on the water level of the Prószkowski Stream, the average water velocity will range from 0.37–0.55 m/s. According to the planned protection activities, sediments will be removed in the southern part of the pond at the site where the Prószkowski Stream flows into the pond. The cost of these measures over the life of the plan will be approximately PLN 150,000 and will contribute to stabilizing the existing ecosystem by suppressing the Nuphar lutea population, allowing the water caltrop population to recover.
On the basis of the results of our tests and analyses, we found that the conservation prospects for the T. natans habitat in the analyzed reservoirs are moderate due to wide fluctuations in population abundance in the past. The following reasons should be excluded as their causes: habitat parameters (adequate depth, thickness of bottom sediments, stable water level, and physico-chemical parameters of water being within the optimum of water caltrop), the influence of fish (low abundance and dominance of predatory fish, and no occurrence of Ctenopharyngodon idella and Cyprinus carpio). In the case of the Szumirad reservoir, intensive recreational use (including boating and canoeing on the reservoir) and angling were likely the cause of fluctuations in Trapa numbers in the past. The reason for the disappearance of the water caltrop habitat at this site was not the expansion of Nuphar lutea. We found much less favorable conditions in the Nowokuźnicki pond, where plant succession was much more advanced, the phytolittoral was overgrowing almost 95% of the water surface, rushes were up to 100 m wide, and the open water table was almost completely overgrown with Nuphar lutea and Ceratophyllum demersum. The physico-chemical parameters of the water differed between the analyzed reservoirs (the Nowokuźnicki pond water was characterized by higher concentrations of nutrients). However, they are within the range of ecological range of T. natans. Therefore, they cannot be considered a direct cause of the disappearance of water caltrop. The unfavorable hydrological situation occurring in the summer season after 2010 accelerated eutrophication (especially in Nowokuźnicki pond), leading to rapid overgrowth by rushes, Nuphar lutea, and Ceratophyllum demersum.
Changes in aquatic ecosystems regarding water and sediment chemical mechanisms will increase due to the observed climate warming [
44], which will also affect the populations of some aquatic plants. In contrast, rising temperatures alone will not have a negative effect on
T. natans, which is more affected by low ambient temperatures [
18]. Presumably, the water caltrop population also has a high chance of persisting in fish ponds [
45] if the water is not drained at times critical for species survival. The success of the development of the
T. natans population in Nowokuźnicki and Szumirad ponds can also be achieved using cheap methods based on winter drawdown. For instance, winter drawdown was repeatedly used as the sole management method in the National Nature Reserve of Brehynsky fishpond in Northern Bohemia to remove excessive biomass of the invasive species
Myriophyllum spicatum [
46]. This treatment was based on the idea that winter drawdown slightly reduces the biomass of nymphaeids in water bodies but can enhance the growth of rare annual plant species. Additionally, this method can limit the nutrient load in the sediment because part of the nutrients released from frozen biomass can be washed out of the pond. Another method of maintaining
T. natans’ presence in the studied ponds is the introduction of micropopulations in different parts of water bodies, as was successfully achieved with
Potamogaton praelongus in the Czech Republic [
47].
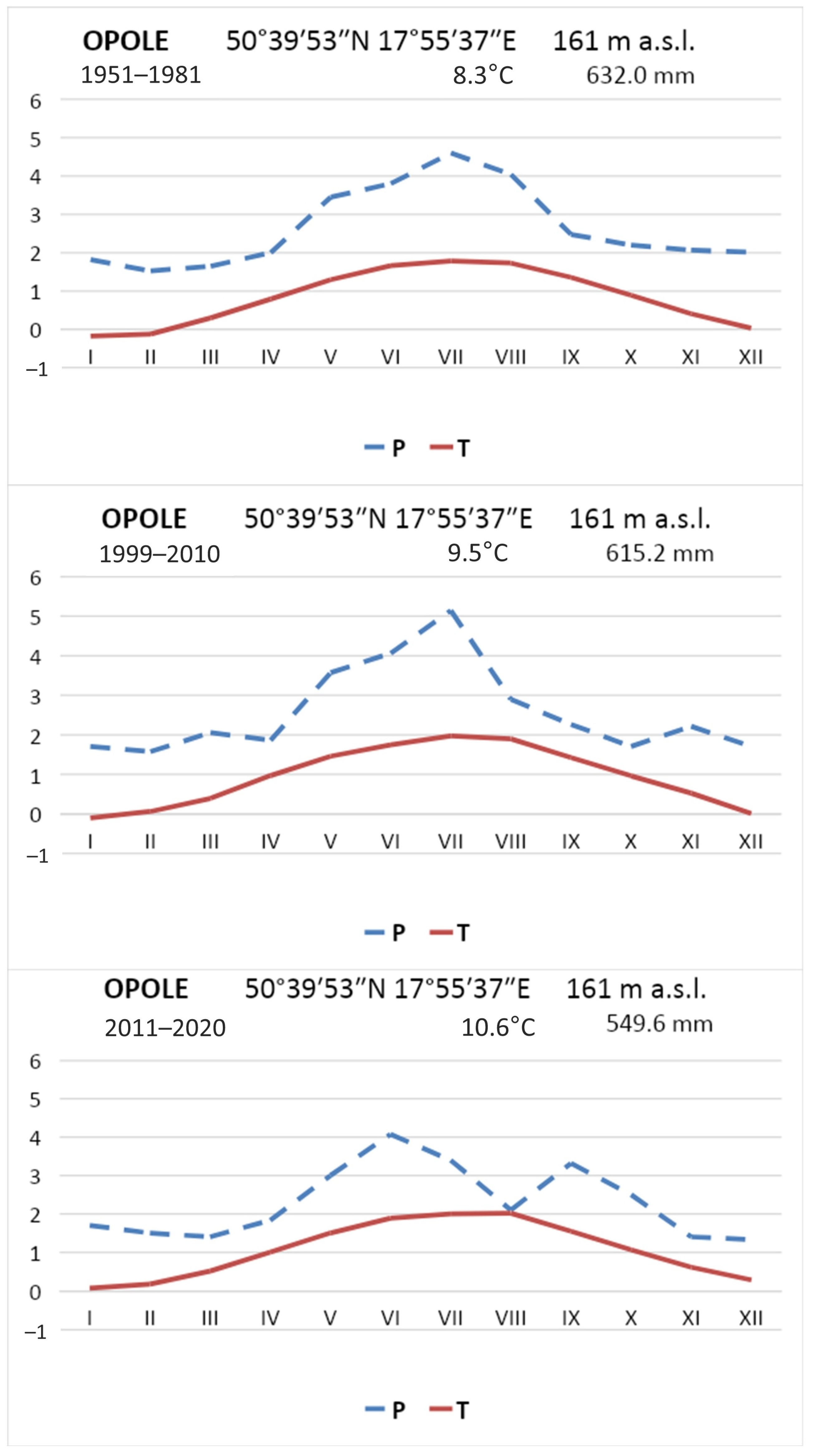
The example of the Szumirad reservoir allowed us to conclude that the reintroduction of water caltrop may have the intended effect, but the final success is not certain. The number of rosettes was low in 1998 and 1999, and only in 2010 was a significant increase as observed (
Figure 6), presumably due, among other things, to suitable weather conditions in 1999–2010. The average precipitation in this period was 615.2 mm, slightly lower than in the 1951–1986 multiyear period when it reached 632.7 mm [
48]. In the period preceding the rapid expansion of the water caltrop population, an increase in the mean annual air temperature was observed from 8.3°C in the 1950–1986 multiyear period to 9.5°C in 1999–2010 (
Figure 8). The observed changes in weather conditions were favorable to the development of
T. natans. During 2011–2020, substantial changes in the distribution of rainfall were observed. In the 1951–2020 multiyear period, an average of 88.9 mm of precipitation was recorded in July, representing 15% of the year’s rainfall, whereas only 67.8 mm, or 12.3% of the average annual total, fell in July in 2011–2020. A continuous increase in air temperatures was recorded between 2011 and 2020, with an average annual temperature of 10.6 °C (
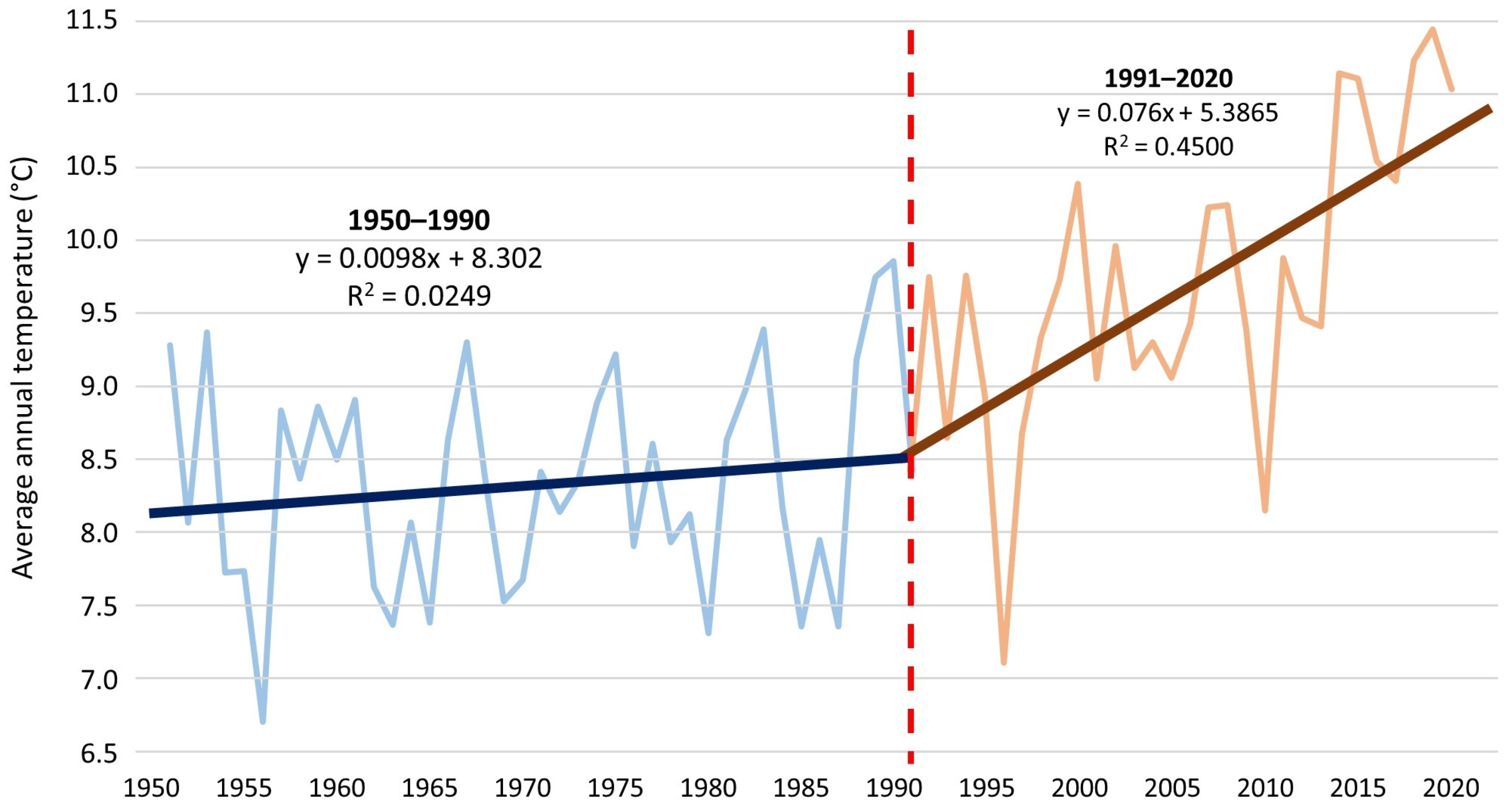
Figure 8). Between 1990 and 2020, the air temperature in Opole increased at an average rate of 1 °C per 13 years (
Figure 9). Thus, the last decade of the study was at least partly favorable for the occurrence of water caltrop, precisely because of the high air temperatures. However, further increases in temperature in the future will result in increased water evaporation, which may consequently accelerate the rate of overgrowth, especially in the Nowokuźnicki pond.
When analyzing the population changes and disappearance of water caltrop from previously occupied habitats, the whole spectrum of factors negatively affecting this plant must be considered. Often, the simultaneous occurrence of several stressors can lead to a sharp reduction in water caltrop populations due to the synergies between two or more factors that often pose little threat when occurring individually. Increased salinity of aquatic environments, oxidative stress, and the occurrence of natural enemies and diseases (insects, mollusks, fungi, and viruses) can markedly reduce the chances of survival of populations of different subspecies [
49,
50,
51], despite the exceptional resistance of seeds to mechanical damage and drying out [
52]. In countries where water caltrop occupies large areas, targeted manual or mechanical removal is performed, which also considerably reduces its biomass in surface waters [
53]. Excessive removal or destruction of plants in areas where water caltrop is an endangered species can lead to its complete disappearance [
2].