Selective Speciation of Inorganic Selenium [Se(IV)] in Water Samples by Inductively Coupled Plasma Mass Spectrometry after Solid Phase Extraction: Blood and Mokolo Rivers, South Africa
Abstract
:1. Introduction
2. Experimental
2.1. Reagents, Standard Solutions and Standard Reference Materials
2.2. Apparatus and Instrumentation
- pH measurement
- Separation and preconcentration of inorganic selenium
- Analysis of total selenium concentration and speciation of inorganic selenium
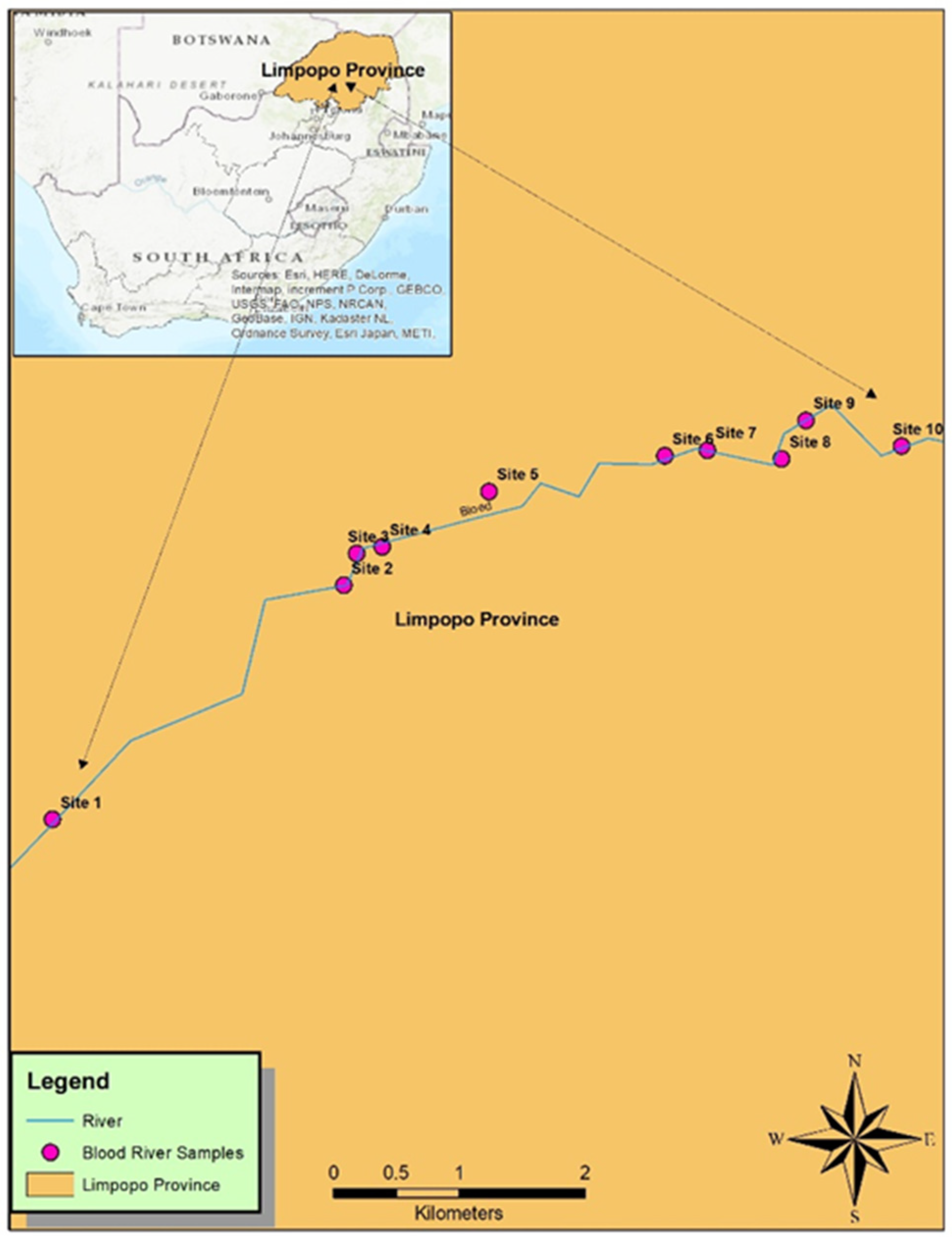
2.3. Sample Collection and Preservation
2.4. Determination of Limit of Detection and Limit of Quantification
2.5. Validation of Methods for the Determination of Total Selenium and Se(IV)
2.6. Total Concentration of Selenium in Water Samples
2.7. Speciation of Inorganic Selenium
3. Results and Discussion
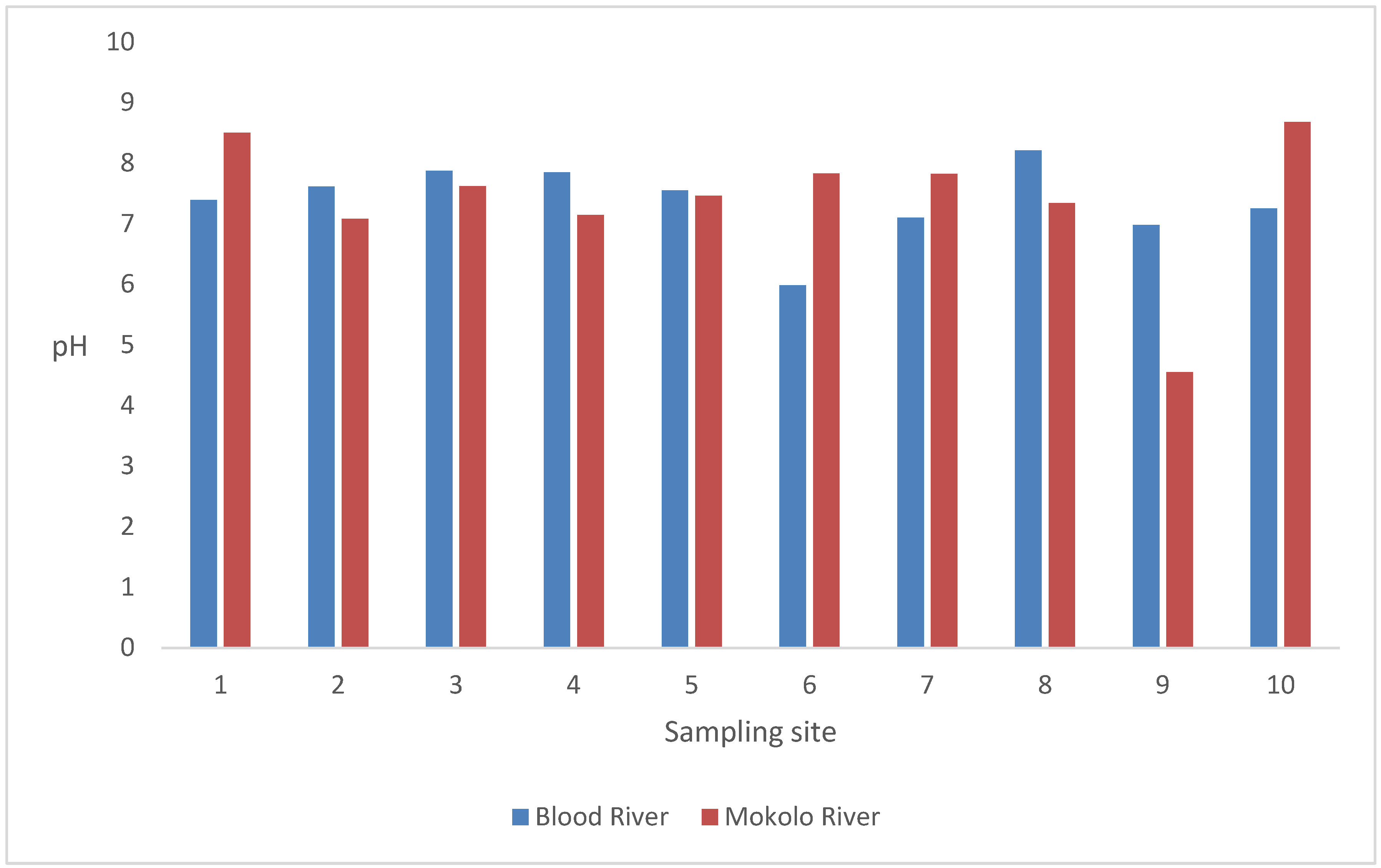
3.1. The pH of Water Samples
3.2. Temperature of Water Samples
3.3. Analytical Performance of the Method
3.3.1. The LODs and LOQs for the Total Determination of Selenium and Speciation of Inorganic Selenium
3.3.2. Linearity of Calibration Curves for the Total Concentration of Selenium
3.3.3. Accuracy and Precision of the Analytical Method
3.4. Influence of pH on the SPE Procedure for the Determination of Se(IV)
3.5. Determination of Total Selenium in River Water Samples
3.5.1. Comparison with Maximum Permissible Levels in Drinking and Irrigation Water
3.5.2. Comparison of Selenium Levels in Water from Different Rivers
3.6. Determination of Se(IV) in Water Samples from Blood and Mokolo River
3.7. Comparison of Se(IV) in Water Samples with Maximum Permissible Levels in Drinking and Irrigation Water
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simonovic, S.P.; Breach, P.A. The role of water supply development in the earth system. Water 2020, 12, 3349. [Google Scholar] [CrossRef]
- Chigor, V.N.; Sibanda, T.; Okoh, A.I. Studies on the bacteriological qualities of the Buffalo River and three source water dams along its course in the Eastern Cape Province of South Africa. Environ. Sci. Pollut. Res. 2013, 20, 4125–4136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gemmell, M.E.; Schmidt, S. Microbiological assessment of river water used for the irrigation of fresh produce in a sub-urban community in Sobantu, South Africa. Food Res. Int. 2012, 47, 300–305. [Google Scholar] [CrossRef]
- Momba, M.N.; Kaleni, P. Regrowth and survival of indicator microorganisms on the surfaces of household containers used for the storage of drinking water in rural communities of South Africa. Water Res. 2002, 36, 3023–3028. [Google Scholar] [CrossRef] [PubMed]
- UN Water. Water a Shared Responsibility. 2006. Available online: http://unesdoc.unesco.org/images/0014/001454/145405e.pdf#page=519 (accessed on 5 November 2022).
- Musingafi, M.C.C. Fresh water sources pollution: A human related threat to fresh water security in South Africa. J. Public Policy Gov. 2014, 1, 72–81. [Google Scholar]
- He, Y.; Xiang, Y.; Zhou, Y.; Yang, Y.; Zhang, J.; Huang, H.; Shang, C.; Luo, L.; Gao, J.; Tang, L. Selenium contamination, consequences and remediation techniques in water and soils: A review. Environ. Res. 2018, 164, 288–301. [Google Scholar] [CrossRef]
- Camara, C.; Cobo, M.G.; Palacios, M.A.; Mu, R.; Donard, O.F.X. 10. Selenium speciation analyses in water and sediment matrices. In Techniques and Instrumentation in Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 1995; Volume 17, pp. 235–262. [Google Scholar]
- Pyrzyńska, K. Speciation of selenium compounds. Anal. Sci. 1998, 14, 479–483. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Chen, B.; Wu, S.; He, M.; Hu, B. Graphene oxide—TiO2 composite solid phase extraction combined with graphite furnace atomic absorption spectrometry for the speciation of inorganic selenium in water samples. Talanta 2016, 154, 474–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Feng, X.; Larssen, T. Selenium speciation, distribution, and transport in a river catchment affected by mercury mining and smelting in Wanshan, China. Appl. Geochem. 2014, 40, 1–10. [Google Scholar] [CrossRef]
- Latorre, C.H.; García, J.B.; Martín, S.G.; Crecente, R.P. Solid phase extraction for the speciation and preconcentration of inorganic selenium in water samples: A review. Anal. Chim. Acta 2013, 804, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Najafi, N.M.; Seidi, S.; Alizadeh, R.; Tavakoli, H. Inorganic selenium speciation in environmental samples using selective electrodeposition coupled with electrothermal atomic absorption spectrometry. Spectrochim. Acta Part B At. Spectrosc. 2010, 65, 334–339. [Google Scholar] [CrossRef]
- World Health Organization. Selenium in Drinking-Water; WHO Guidelines for Drinking-Water Quality, WHO/HSE/WSH/10.01/14; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Torres, J.; Pintos, V.; Gonzatto, L.; Domínguez, S.; Kremer, C.; Kremer, E. Selenium chemical speciation in natural waters: Protonation and complexation behavior of selenite and selenate in the presence of environmentally relevant cations. Chem. Geol. 2011, 288, 32–38. [Google Scholar] [CrossRef]
- Nyaba, L.; Matong, J.M.; Dimpe, K.M.; Nomngongo, P.N. Speciation of inorganic selenium in environmental samples after suspended dispersive solid phase microextraction combined with inductively coupled plasma spectrometric determination. Talanta 2016, 159, 174–180. [Google Scholar] [CrossRef]
- Valencia, M.C.; Nicolas, E.A.; Capitan-Vallvey, L.F. Speciation of selenium (IV) in natural waters by solid phase spectrophotometry. Talanta 1999, 49, 915–921. [Google Scholar] [CrossRef] [Green Version]
- Tadayon, F.; Mehrandoost, S. Taguchi method approach on preconcentration and speciation of trace amounts of inorganic and organic selenium in mushroom samples with cloud point extraction and UV-Vis spectrophotomety. Acad. Res. Int. 2014, 5, 15. [Google Scholar]
- B’hymer, C.; Caruso, J.A. Selenium speciation analysis using inductively coupled plasma-mass spectrometry. J. Chromatogr. A 2006, 1114, 1–20. [Google Scholar] [CrossRef]
- Radulescu, C.; Dulama, I.D.; Stihi, C.; Ionita, I.; Chilian, A.; Necula, C.; Chelarescu, E.D. Determination of heavy metal levels in water and therapeutic mud by atomic absorption spectrometry. J. Phys. 2014, 59, 1057. [Google Scholar]
- Saalidong, B.M.; Aram, S.A.; Out, S.; Lartey, P.O. Examining the dynamics of the relationship between water pH and water quality parameters in ground and surface water systems. PLoS ONE 2022, 17, e0262117. [Google Scholar] [CrossRef]
- Water Research Center. 2015–2021. Available online: https://www.knowyourh2o.com/indoor-4/the-ph-of-water (accessed on 5 November 2022).
- Pansu, M.; Gautheyrou, J. pH measurement; Springer: Berlin/Heidelberg, Germany, 2006; pp. 551–579. [Google Scholar]
- Department of Water Affairs and Forestry (DWAF). Agricultural Water Use, Irrigation. In South African Water Quality Guidelines, 2nd ed.; CSIR Environmental Services: Pretoria, South Africa, 1996; Volume 4. [Google Scholar]
- Chen, A.; Lin, C.; Lu, W.; Wu, Y.; Ma, Y.; Li, J.; Zhu, L. Well water contaminated by acidic mine water from Dabaoshan Mine, South China: Chemistry and toxicity. Chemosphere 2007, 70, 248–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Department of Water Affairs and Forestry (DWAF). Aquatic Ecosystems. In South African Water Quality Guidelines, 2nd ed.; CSIR Environmental Services: Pretoria, South Africa, 1996; Volume 7. [Google Scholar]
- Jitmanee, K.; Teshima, N.; Sakai, T.; Grudpan, K. DRC™ ICP-MS coupled with automated flow injection system with anion exchange minicolumns for determination of selenium compounds in water samples. Talanta 2007, 73, 352–357. [Google Scholar] [CrossRef]
- Lin, T.S. Inorganic selenium speciation in groundwaters by solid phase extraction on Dowex 1X2. J. Hazard. Mater. 2007, 149, 80–85. [Google Scholar] [CrossRef]
- Moosavi, S.M.; Ghassabian, S. Linearity of calibration curves for analytical methods: A review of criteria for assessment of method reliability. In Calibration and Validation of Analytical Methods: A Sampling of Current Approaches; IntechOpen: London, UK, 2018; pp. 109–127. [Google Scholar]
- Chandran, S.; Singh, R.S.P. Comparison of various international guidelines for analytical method validation. Die Pharm.-Int. J. Pharm. Sci. 2007, 62, 4–14. [Google Scholar]
- US EPA. Contract Laboratory Program for National Functional Guidelines for Inorganic Data Review; National Service Center for Environmental Publications (NSCEP): Washington, DC, USA, 2010. [Google Scholar]
- Mathebula, B. Assessment of the Surface Water Quality of the Main Rivers Feeding at Katse Dam Lesotho. Ph.D. Thesis, University of Pretoria, Pretoria, South Africa, 2016. [Google Scholar]
- Gupta, M.; Gupta, S. An overview of selenium uptake, metabolism, and toxicity in plants. Front. Plant Sci. 2017, 7, 2074. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, B.M.; Avenant-Oldewage, A. Arsenic, chromium, copper, iron, manganese, lead, selenium and zinc in the tissues of the largemouth yellowish, Labeobarbuskimberleyensis (Gilchrist and Thompson, 1913), from the Vaal Dam, South Africa, and associated consumption risks. Water SA 2014, 40, 739–748. [Google Scholar] [CrossRef] [Green Version]
- Greenfield, R.; Van Vuren, J.H.J.; Wepener, V. Heavy metal concentrations in the water of the Nyl River system, South Africa. Afr. J. Aquat. Sci. 2012, 37, 219–224. [Google Scholar] [CrossRef]
- Parkman, H.; Hultberg, H. Occurrence and Effects of Selenium in the Environment-A Literature Review; IVL Report B 1486; Swedish Environmental Research Institute: Stockholm, Sweden, 2002. [Google Scholar]
- Lemly, A.D. Guidelines for evaluating selenium data from aquatic monitoring and assessment studies. Environ. Monit. Assess. 1993, 28, 83–100. [Google Scholar] [CrossRef]




| Parameters | Settings |
|---|---|
| Nebulizer gas flow | 1.0 L/min |
| Auxiliary gas flow | 1.2 L/min |
| Plasma gas flow | 14 L/min |
| ICP RF Power | 1400 V |
| Lens voltage | 10 V |
| Analogue Stage Voltage | −2550 V |
| Pulse Stage Voltage | 1050 V |
| Torch box temperature | 30 °C |
| Cooling system | |
| 1 Main water temperature | 18.0 °C |
| 2 Interface water temperature | 32.6 °C |
| Sites | Blood River | Mokolo River | ||
|---|---|---|---|---|
| Latitudes | Longitudes | Latitudes | Longitudes | |
| 1 | −23.851384 | 29.357036 | −23.648206 | 27.762074 |
| 2 | −23.834667 | 29.377833 | −23.650119 | 27.760373 |
| 3 | −23.832393 | 29.37875 | −23.652380 | 27.759355 |
| 4 | −23.831928 | 29.380566 | −23.656405 | 27.757581 |
| 5 | −23.827958 | 29.388218 | −23.684576 | 27.748081 |
| 6 | −23.825434 | 29.400748 | −23.688417 | 27.745652 |
| 7 | −23.825000 | 29.403803 | −23.691378 | 27.744676 |
| 8 | −23.82563 | 29.409094 | 23.697074 | 27.742127 |
| 9 | −23.822885 | 29.41083 | 23.701907 | 27.742141 |
| 10 | −23.824724 | 29.417663 | 23.707893 | 27.743858 |
| Temperature (°C) | Site1 | Site 2 | Site 3 | Site 4 | Site 5 | Site 6 | Site 7 | Site 8 | Site9 | Site 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Blood River | 21.9 | 21.5 | 23.9 | 23.9 | 23.9 | 24.4 | 24.9 | 27.2 | 27.6 | 28.4 |
| Mokolo River | 23.9 | 21.9 | 21.3 | 22.6 | 22.8 | 19.3 | 24.7 | 25.8 | 23.1 | 22.9 |
| Using SRM 1643f | Using a Solution of Known Concentration | ||
|---|---|---|---|
| Analyte | Se | Se(IV) | Se(IV) |
| Measured value | 10.3 | 9.04 | 5.18 |
| Certified value | 11.7 | 11.7 | 5.00 |
| Percentage recovery | 88.1 | 77 | 104 |
| Mean Se Concentration (µg/L) | |||
|---|---|---|---|
| Sampling Site | Blood River | Mokolo River | Standard Guidelines Se (µg/L) |
| 1 | 1.14 ± 0.070 | 0.309 ± 0.012 | |
| 2 | 1.03 ± 0.140 | 0.159 ± 0.0090 | |
| 3 | 0.629 ± 0.061 | 0.297 ± 0.0030 | |
| 4 | 0.678 ± 0.031 | 1.02 ± 0.031 | |
| 5 | 0.563 ± 0.030 | 0.115 ± 0.0030 | |
| 6 | 0.0682 ± 0.020 | 0.109 ± 0.0070 | |
| 7 | 1.77 ± 0.050 | 0.085 ± 0.0030 | 40 (World Health Organization) [32] |
| 8 | 2.72 ± 0.020 | 0.113 ± 0.012 | 50 (Environmental Protection Agency) [32] |
| 9 | 1.48 ± 0.050 | 25.4 ± 0.31 | 2 (South African Target Water Quality |
| 10 | 0.135 ± 0.050 | 0.275 ± 0.021 | Range) [32] |
| River | Se (µg/L) | References |
|---|---|---|
| Vaal Dam | <0.253 | [34] |
| Nyl River | (2.00–6.17) | [35] |
| Blood River | (0.068–2.724) | (Current study) |
| Mokolo River | (0.085–25.405) | (Current study) |
| Blood River | Mokolo River | ||||||
|---|---|---|---|---|---|---|---|
| Site | Se(IV) (µg/L) | Total Se (µg/L) | %Se(IV) | Se(IV) (µg/L) | Total Se (µg/L) | %Se(IV) | Standard Guideline Se(IV), µg/L |
| 1 | 0.323 ± 0.0091 | 1.29 ± 0.070 | 25 | 0.157 ± 0.018 | 1.02 ± 0.012 | 15 | |
| 2 | 0.434 ± 0.0071 | 1.39 ± 0.014 | 31 | 0.280 ± 0.010 | 0.974 ± 0.0090 | 29 | |
| 3 | 0.193 ± 0.017 | 0.717 ± 0.061 | 27 | 0.619 ± 0.015 | 1.72 ± 0.0050 | 36 | |
| 4 | 0.0512 ± 0.010 | 0.526 ± 0.031 | 10 | 0.590 ± 0.027 | 2.93 ± 0.031 | 20 | |
| 5 | 0.163 ± 0.012 | 0.664 ± 0.030 | 24 | 0.174 ± 0.013 | 0.523 ± 0.0030 | 33 | |
| 6 | 0.0411 ± 0.0012 | 0.122 ± 0.020 | 34 | 0.216 ± 0.0041 | 0.488 ± 0.0070 | 44 | |
| 7 | 0.385 ± 0.010 | 1.73 ± 0.050 | 22 | 0.135 ± 0.012 | 0.302 ± 0.0030 | 45 | |
| 8 | 0.333 ± 0.0071 | 2.09 ± 0.020 | 15 | 0.178 ± 0.0071 | 0.699 ± 0.012 | 25 | 2 (South African Target Water Quality Range) [32] |
| 9 | 0.358 ± 0.020 | 1.57 ± 0.020 | 23 | 2.79 ± 0.013 | 17.6 ± 0.31 | 16 | |
| 10 | 0.820 ± 0.050 | 2.11 ± 0.050 | 39 | 0.196 ± 0.0012 | 0.292 ± 0.021 | 67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matjena, M.M.; Mampa, R.M.; Ambushe, A.A. Selective Speciation of Inorganic Selenium [Se(IV)] in Water Samples by Inductively Coupled Plasma Mass Spectrometry after Solid Phase Extraction: Blood and Mokolo Rivers, South Africa. Water 2023, 15, 2679. https://doi.org/10.3390/w15152679
Matjena MM, Mampa RM, Ambushe AA. Selective Speciation of Inorganic Selenium [Se(IV)] in Water Samples by Inductively Coupled Plasma Mass Spectrometry after Solid Phase Extraction: Blood and Mokolo Rivers, South Africa. Water. 2023; 15(15):2679. https://doi.org/10.3390/w15152679
Chicago/Turabian StyleMatjena, Mmakoena Meldred, Richard M. Mampa, and Abayneh Ataro Ambushe. 2023. "Selective Speciation of Inorganic Selenium [Se(IV)] in Water Samples by Inductively Coupled Plasma Mass Spectrometry after Solid Phase Extraction: Blood and Mokolo Rivers, South Africa" Water 15, no. 15: 2679. https://doi.org/10.3390/w15152679
APA StyleMatjena, M. M., Mampa, R. M., & Ambushe, A. A. (2023). Selective Speciation of Inorganic Selenium [Se(IV)] in Water Samples by Inductively Coupled Plasma Mass Spectrometry after Solid Phase Extraction: Blood and Mokolo Rivers, South Africa. Water, 15(15), 2679. https://doi.org/10.3390/w15152679







