Abstract
Groundwater pollution is a threat to the environment and human health because it is an important source of drinking water. Groundwater is used to supply water to communities and pollution occurs when harmful substances and contaminants infiltrate into the groundwater. Through excessive use of fertilizers, agriculture is a major contributor to groundwater pollution. This study tests the impact of organic and mineral fertilization on the groundwater physiochemical parameters and explores the potential consequences of using manure as fertilizer on groundwater nitrate pollution. The experiment was carried out in Satu Mare County, Romania, where both organic (manure) and mineral fertilizers were applied to potato cultures to test their impact on 18 physiochemical parameters of groundwater quality. Basic Statistics, Nitrate Pollution Index (NPI), and Principal Components Analysis were used for emphasizing the impact of mineral and organic fertilization on groundwater quality and relationships between analyzed groundwater parameters. The results show that groundwater corresponding to the site where the higher dose of organic fertilization was applied is characterized by nitrate concentrations (64.92 mg/L) and pH values (6.3 pH units) beyond the allowed limits. Based on the calculated NPI (2.21), it falls within the significant pollution category. Two principal factors were identified as having an impact on groundwater quality: fertilizer type and administered dose, respectively.
1. Introduction
Agriculture is an important factor in the development of the economy, with the aim of providing food for the population [1]. Food requirements have not only increased over the last century, but interest in food quality has also developed; consumers not only want to benefit from food safety but also want to know in detail the activities that contribute to obtaining high-quality food [2,3]. In order to meet the need for food, farmers resorted to the expansion of irrigation and the increasingly intensive use of fertilizers and pesticides. The effect of these measures led to an increase in the degree of water pollution from agriculture, with a high potential impact on human health [4,5]. One of the challenges of the 21st century is the development of sustainable agriculture. According to the FAO definition in the Water Strategy for the Development of a Sustainable Agriculture from 1990, sustainable development involves the responsible handling of natural resources and the adaptation of technologies and institutions to guarantee the fulfilment of present and future generations’ needs while safeguarding the environment. Soil–water symbiosis is a principle on which agriculture is based, thus water is both a “victim” and a “participant” in agricultural pollution [1]. Groundwater represents 0.5% of the total water resources on earth, serving as the main source of drinking water worldwide and meeting the water needs of 2.5 billion people. As a result, managing and monitoring groundwater, as well as its quantity and quality, is essential. In addition, treatment of contaminated groundwater is difficult and expensive [6,7,8].
Due to population growth, the safe use of groundwater has become more difficult [9]. Groundwater pollution from agricultural activities occurs due to increased concentrations of nutrients (nitrogen N and phosphorus P compounds) resulting from the excessive use of fertilizers, animal husbandry, and incorrect storage of agricultural inputs [10,11]. Nitrate (NO3−) is one of the most common inorganic pollutants [6], because in the last decades, the overconsumption of both synthetic and organic nitrogen fertilizers has resulted in soil and groundwater contamination. Due to this reality, nitrogen groundwater pollution became one of the most challenging environmental concerns [12,13,14]. Infiltration from landfills, mining activities, and wastewater discharges lead to pollution with other inorganic substances, toxic substances, and compounds that can be associated with water salinity due to high concentrations of Ca2+, Mg2+, Na+, Cl−, and F− [7,15,16]. Thanks to the soil–water symbiosis, both nutrients and toxic substances are absorbed at the plant level.
For obtaining general information concerning groundwater contamination with nitrate, the calculation of the nitrate pollution index became a common tool [17]. To analyze the potential pollution of groundwater resulting from organic and inorganic pollutants, a statistical approach is used. Research concerning the analysis of anions and cations content, whether through an integrated approach or the assessment of the sewerage network construction impact on groundwater quality, uses statistical tools, such as Principal Components Analysis, Discriminant Analysis, or Cluster Analysis [18,19,20,21].
Despite a significant reduction in the cultivated area of potatoes (Solanum tuberosum L.) in recent years, due to the multidirectional use of tubers, the potato is still one of the most important crops [22], providing major contributions to human nutrition, animal feed, places of work, and income [7]. In addition to minerals, potato tubers contain unwanted substances called antinutritive or toxic. Potassium (K) is the basic mineral in potatoes. Phosphorus (P) and magnesium (Mg) are present in potato tubers in moderate amounts, while calcium (Ca) is present in small amounts [23]. The main anti-nutrients contained in potato tubers are nitrates. Tubers also contain small amounts of toxic nitrites. The presence of nitrates in excessive amounts is dangerous because they are precursors to the highly toxic nitrites that cause methemoglobinemia or vitamin A deficiency [24]. According to Commission Regulation (EC) No. 1822/2005 of 8 November 2005, the maximum limit of nitrates in potatoes must not exceed 200 mg kg−1 fresh weight of the tubers (food). Nitrogen absorbed from the soil in the form of nitrate and ammonium is used by plants to synthesize amino acids, proteins, chlorophyll, and other substances. However, when there is an excessive amount of nitrogen, mainly from nitrogen fertilization, plants are not able to metabolize it all and may accumulate it as nitrates [21,23]. In order to reduce nitrate pollution due to mineral and organic nitrogen supply, a series of sustainable fertilization strategies are tested [25,26,27].
The specific agricultural practices in North West Romania involve the extensive use of organic fertilizers, but research concerning their impact on groundwater quality has not been conducted. For this reason, this study aims to identify the impact of using organic fertilizers compared to mineral fertilization on the physiochemical parameters of groundwater quality and test the potential groundwater pollution by nitrates resulting from the use of manure as a fertilizer. This research is of great importance, being the first complex study conducted in the area.
The use of organic fertilizers, such as manure, in agricultural practices is presumed to have a significant impact on the physiochemical parameters of groundwater quality compared to mineral fertilization. Specifically, in this study, we adopt the hypothesis that the use of organic fertilizers will lead to higher concentrations of nitrates in groundwater, indicating a potential risk of groundwater pollution.
2. Materials and Methods
2.1. Experimental Area and Treatments Description
The experiment was carried out in Apa commune in Satu Mare county 47°45′48.6″ N 23°11′53.2″ E, Romania. Satu Mare county is located in the extreme North–West of Romania, bordering Hungary to the west and Ukraine to the north, and in the interior, it borders the counties of Maramures, Salaj, and Bihor. The Apa commune is located in the eastern part of Satu Mare county in the Someș Plain (Figure 1).
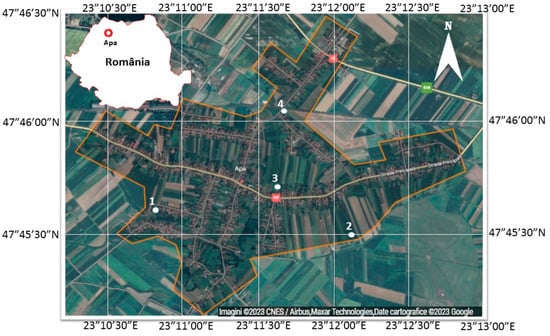
Figure 1.
Study area (location map and distribution of sampling points). Site 1-Sheep Manure, 39.52 t/ha; Site 2-Sheep Manure, 79.04 t/ha; Site 3-Sheep Manure, 118.56 t/ha; Site 4-Mineral Fertilization N14:P7:K21.
The experimental sites were located in four private vegetal farms. Four plots (one by each farm) of 100 m2 were organized. Cambisol was the type of soil identified in all experimental sites. Potato culture was selected for this study because it is one of the most spread cultures in the area. It was installed on the same day at each of the four experimental sites, 15 March 2022, respectively, and harvested during 5-7.06.6.2022.
Nitrogen mineral and organic fertilizations were applied to potato cultures (Table 1). NPK fertilizer was applied for mineral fertilization. In the early phase of potato growing, after plantation, only nitrogen was applied (N34:P0:K0) as ammonium nitrate, and afterwards, the doses were adjusted to N14:P7:K21. The organic fertilization was performed using sheep manure in three doses. The sheep manure content in NPK and dry matter (DM) was as follows: 0.85% N, 0.19% P, 0.76% K, and 29.83% DM [28]. It was applied on the field four weeks prior to the installation of the potato culture. The groundwater sampling was performed after potato harvesting (10 June 2022) and laboratory analysis in July 2022.

Table 1.
The fertilization pattern.
The samples were taken from underground waters, located in proximity to the experimental fields from Apa commune, where potato crops were installed (site 1—47°45′42.29″ N 23°11′23.74″ E, site 2—47°45′36.11″ N 23°12′42.56″ E, site 3—47°45′48.81″ N 23°12′13.4″ E, site 4—47°46′9.19″ N 23°12′13.21″ E). Ten water samples were collected during potato cultivation development as follows: once in March 2022, three times in April 2022, four times in May 2022, and twice in June. Before analysis, the water samples were mixed to obtain one sample at each experimental site. From each of the 10 samples collected by the experimental site, 18 groundwater quality parameters were quantified. The collection of samples was carried out following the sampling protocols [29], avoiding sample contamination during sampling and transport. Groundwater samples were taken from a depth of 20 m by drilling.
2.2. Methodology
For a more accurate picture of the groundwater quality, in addition to the nutrient values, the pH and turbidity values were also analyzed, according to standardized methodology (Table 2).

Table 2.
The standardized methodology applied for groundwater quality assessment.
Turbidity was determined using a HACH 2100P Turbidimeter, according to European Standard EU ISO 7027 [30]. The pH measurements were performed using an Inolab 740 multimeter WTW and the determination was performed according to the International Standard ISO 10523/2012 [30]. The determination of ammonium, nitrites and nitrates, was performed using a LAMBDA Spectrophotometer UV-VIS BIO 40 from PERKIN ELMER [35,36,37].
The determination of nitrogen content was carried out in accordance with ISO 7890 for Water Quality, Determination of nitrogen content part 3—Spectrometric method with sulfosalicylic acid. It was performed by the spectrometric measurement of the absorbance of the yellow compound obtained by the reaction of sulfosalicylic acid (formed by adding sodium salicylate and sulfuric acid to the sample) with nitrogen, followed by treatment with an alkaline solution. The disodium salt of ethylenediaminetetraacetic acid (EDTANa2) was added to the alkaline solution to prevent the precipitation of calcium and magnesium salts. Sodium azide is added to remove interference with nitrites. During the analysis, only reagents of recognized analytical quality and distilled water or equivalent purity are used. Reagents and doses: 1. sulfuric acid c(H2SO4) = 18 mol/L, ρ = 1.84 g/mL; 2. glacial acetic acid, c(CH3COOH) = 17 mol/l, ρ = 1.05 g/mL; 3. alkaline solution ρNAOH = 200 g/L, ρ [CH2-N(CH2COOH)CH2-COONa]*2H2O = 50 g/L; 4. sodium azide, ρNaN3 = 0.5 g/L—is a very toxic solution. As an alternative, the sulfamic acid solution can be used with ρNH2-SO3H = 0.75 g/L; 5. sodium salicylate, ρHO-C5H4-COONa = 10 g/L; 6. nitrate, basic standard solution ρ = 1000 mg/L or ρ = 1 mg/L. Cuvettes with an optical path of 40 mm and a sample volume of 25 mL were used. The detection limit is in the range ρN = 0.003 mg/L to 0.013 mg/L. The equipment used is the specific one of an accredited water analysis laboratory and includes common materials and a spectrometer LAMBDA Spectrophotometer UV-VIS BIO 40 from PERKIN ELMER [37].
For the determination of chemical compounds such as chloride, calcium, and magnesium, the volumetric titration method was used [33,38,39].
To identify the potential nitrate groundwater contamination, the nitrate pollution index was calculated [16], according to the formula:
where NPI is the contamination pollution index, HAV is the threshold value for the nitrates of anthropogenic origin (20 mg/L), and Cs is the nitrate concentration of each sample [37]. According to Iqbal et al. (2023) and Ramalingam et al. (2022), based on NPI values, water quality can be classified into five classes as follows: 1—unpolluted (NPI < 0), 2—lightly polluted (NPI = 0–1), 3—moderately polluted (NPI = 1–2), 2—significantly polluted (NPI = 2–3), and 5—very significantly polluted (NPI > 3) [16,26].
2.3. Statistics
The statistical analysis was performed using the XLSTAT program. Basic statistics were implemented for the calculation of the means and dispersion parameters of the analyzed groundwater quality parameters. One-step ANOVA was used to emphasize the significance of differences between the studied parameters based on fertilization type and doses, at a threshold of 95%. The Pearson correlations between groundwater parameters were calculated after testing the normal distributions using Skewness and Kurtosis values (K-test, S-test), which were verified by histograms. The significance of the Pearson correlation coefficient was determined at 95% and 99% confidence intervals. The ”factor” analysis by its component Principal Components Analysis (PCA) was performed to summarize results from the multitude of physiochemical variables characterizing the groundwater quality. According to statistical methodology, at least 5 cases are recommended for performing the basic statistics, while in order to conduct PCA, a minimum sample size of 150 cases (5 to 10 cases/variable) has been recommended. In our case, we have 18 variables; thus, the analysis was conducted on 180 cases, with 10 cases per variable. The validity of implementing PCA was tested using the Kaiser–Maier–Olkin (KMO) test for sampling adequacy and Bartlett probes. If p < 0.05 for the Bartlett test and the KMO value > 0.5, then the conditions for applying PCA are met [46].
3. Results and Discussions
3.1. Physiochemical Characterization of the Groundwater Quality
The results of the statistical study of the evolution of the 18 physiochemical parameters analyzed in 40 water samples collected from the four experimental sites are presented in Table 2. The pH values and nitrate content in groundwater samples collected from experimental site 3, where potato culture was fertilized with sheep manure at doses of 118.56 t/Ha, exceed the allowed limits mentioned by the Romanian standards (Table 1). A lower mean pH is reported in above mentioned site (pH = 6.37) compared to allowed limits (≥6.5 ≤9.5) and higher mean nitrate content (NO3− = 64.29 mg/L) compared to the allowed limit of 50 mg/L. These means differ significantly from those reported for the groundwater samples corresponding to the other experimental sites. Concerning the groundwater of all experimental sites, the nitrate (NO3−) concentrations over 20 mg/L identified in all samples allow their framing within the category of samples with high nitrate (NO3−) content [47]. Also, higher mean concentrations of conductivity, permanganate index, alkalinity, and bicarbonates (HCO3−) are observed in groundwater samples collected from experimental site 3, even though they do not exceed the allowed limits. Concerning most physiochemical parameters, no significant differences are reported between samples corresponding to all experimental sites (Table 3). Li et al. (2022) reported similar limits and content hierarchy in groundwater cations (Ca, Mg), iron, and major anions (HCO3−, SO42−, Cl−, NO3−), but higher contents in F− and NH4+ in studies performed to emphasize the quality parameters of the groundwater collected from different sites [48]. Suthar et al. (2009) reported greater groundwater contamination in rural areas of India due to mineral and organic fertilization practices, compared to our results, concerning nitrates(NO3−), chlorine (Cl−), and sulfates (SO42−) [49]. Studies on groundwaters quality emphasize that often their quality is depreciated mainly by NO3− pollution from anthropogenic sources in rural areas, where issues concerning communal wastewater occur, which are the result of the improper disposal of wastewater (discharged untreated onto agricultural lands) and may contain high quantities of NO2− and NO3−. This is the result of failing septic systems and inadequate sanitation practices. The main reason for the incidence of these issues in rural areas is the lack of adequate infrastructure and proper wastewater treatment facilities [18,50,51,52].

Table 3.
Descriptive statistics for the characterization of groundwater from experimental sites with organic and mineral fertilization.
3.2. Groundwater Pollution with Nitrates
According to NPI values (Table 4), the groundwater analyzed from three locations corresponding to mineral fertilization and organic fertilization with sheep manure falls within the moderate pollution category, while the groundwater collected from experimental site 3, where potato culture was fertilized with sheep manure at doses of 118.56 t/Ha, falls within the significant pollution category.

Table 4.
Descriptive statistics for nitrate pollution index (NPI) of groundwater collected from the experimental sites.
We consider that the reason for the highest concentration of NO3− and the highest NPI value reported at the experimental site fertilized with the highest quantity of organic fertilizer is not only due to the high nitrogen input from the fertilizer but also due to the contribution of other factors. Thus, some factors that could contribute may be the historical accumulations from infiltrations of untreated sewage and from uninsulated septic tanks [16] and also the high water permeability of the Cambisol structure. Another factor may be the higher biodisponibility of NO3− from the organic fertilizer compared to mineral fertilization, or even inappropriate management of fertilization practices [5]. The NPI is widely used to classify water quality for different purposes [53,54,55]. In a study conducted to assess NO3− groundwater pollution in 10 different sites, the mean NPI values ranged from 2.32 to 15.7, indicating a very significant polluted sampling area [56,57].
3.3. The Study of the Simple Correlations
Pearson’s simple correlation matrices between water quality parameters for each type of fertilization and experimental site were calculated to emphasize their relationships, and the opportunity of performing the PCA, which correlates the relationships among multiple parameters of groundwater quality. The simple Pearson correlations have values between −1 and +1. The negative correlation signifies an inverse evolution of the analyzed traits (the increase of one parameter is accompanied by the decrease of the other, in a manner described by the intensity of correlation, which increases in intensity as close as to 1 the correlation coefficient value) while the positive one signifies similar evolutions [51,52]. In all experimental sites, both positive and negative correlations between the same analyzed groundwater parameters are observed. Regardless of the type of fertilizer and doses administered, the most significant correlations are reported for pH (Table 5, Table 6, Table 7 and Table 8). The simple Pearson correlations are considered moderate when values of the correlation coefficients are ranging between r = 0.51–0.69 at a significance threshold of 5% (p < 0.05%) and strong when the correlation coefficients are over r = 0.77 at a significance threshold of 1%. Turbidity is moderately and significantly (p < 0.05%) correlated with conductivity (with values of r = −0.57 corresponding to sites 1 and 3, and r = −0.58 corresponding to sites 2 and 4) and permanganate index (with values ranging between r = 0.51 corresponding to site 1 and r = 0.55 corresponding to site 2). pH is moderately and significantly (p < 0.05%) correlated with conductivity (with values ranging between r = 0.65 corresponding to sites 1 and 3 and r = 0.69 corresponding to site 2), nitrites NO2− (with values ranging between r = −0.63 corresponding to site 4, and r = −0.65 corresponding to sites 2 and 3), nitrates (NO3−) for groundwater located in sites 1 and 4 (r = −0.68), sulfates SO42− (with values ranging between r = 0.62 corresponding to sites 1 and 4, and r = 0.65 corresponding to sites 2, and 3), and phosphates PO43− in case of groundwater located in site 4 that was minerally fertilized (r = 0.69). pH is strongly and significantly (p < 0.01%) correlated with nitrates (NO3−) for groundwater located in site 3 (r = −0.73) and phosphates PO43− (with values ranging between r = 0.77 corresponding to site 1 and r = 0.82 corresponding to site 3). Except for pH, all other correlations between parameters of groundwater are moderate and significant (p < 0.05%). Conductivity is correlated with ammonium NH4+, with correlation coefficients ranging between r = 0.55 corresponding to site 3 and r = 0.59 corresponding to site 4 (Table 5, Table 6, Table 7 and Table 8).

Table 5.
The simple correlations for Site 1-Sheep manure, administered at doses of 39.52 t/Ha.

Table 6.
The simple correlations for Site 2-Sheep manure, administered at doses of 79.04 t/Ha.

Table 7.
The simple correlations for Site 3-Sheep manure, administered at doses of 118.56 t/Ha.

Table 8.
The simple correlations for Site 4-Mineral fertilization N14:P7:K21.
Chlorides (Cl−) are correlated with nitrites, with correlation coefficients ranging between r = 0.68 corresponding to sites 1 and 3 and r = 0.69 corresponding to sites 2 and 4. The permanganate index is correlated with ammonium (NH4+), with values ranging between r = 0.52 corresponding to site 3 and r = 0.58 corresponding to site 2. Ammonium (NH4+) is correlated with phosphates PO43−, with correlation coefficients ranging between r = −0.53 corresponding to sites 1 and 3 and r = −0.56 corresponding to sites 2 and 4. Nitrites (NO2−) are correlated with nitrates NO3− (with correlation coefficients ranging between r = 0.61 corresponding to sites 1 and 2, and r = 0.64 corresponding to site 3) and magnesium Mg (with correlation coefficients ranging between r = 0.60 corresponding to sites 1 and 2 and r = 0.64 corresponding to site 4). Nitrates (NO3−) are also negatively correlated with ammonium NH4+ (with correlation coefficients ranging between r = −0.63 corresponding to site 1 and r = −0.64 corresponding to sites 2–4). Total hardness is positively correlated with bicarbonates (HCO3−) content in groundwater with correlation coefficients ranging between r = 0.61 and r = 0.64 corresponding to sites 1 and 2, respectively. Calcium (Ca) is correlated with iron with correlation coefficients ranging between r = 0.58 corresponding to sites 1 and 4, respectively, and r = −0.61 corresponding to site 3. Magnesium (mg) is correlated with bicarbonate content in groundwater with correlation coefficients ranging between r = 0.53 and r = 0.57 corresponding to sites 4 and 2, respectively. Iron is correlated with alkalinity (with correlation coefficients ranging between r = 0.52 corresponding to sites 3 and r = 0.55 corresponding to sites 2 and 4, respectively) and bicarbonate (HCO3−) contents in groundwater with correlation coefficients ranging between r = −0.52 corresponding to sites 3 and r = −0.59 corresponding to sites 3 and 2, respectively (Table 5, Table 6, Table 7 and Table 8).
The significant correlations identified in groundwater are similar for all analyzed sites, regardless of fertilization type and administered doses. Differences are reported only concerning the intensity of correlations. Between pH and phosphates (PO43−), strong significant positive correlations at the significance threshold of 1% (p< 0.01%) are reported when organic fertilization is applied, while moderate correlation is observed between pH and phosphates (PO43−) in the groundwater when mineral fertilization was administered. This suggests the stronger effect of phosphates (PO43−) on groundwater acidity and/or alkalinity when organic fertilization is used, compared with mineral fertilization. The strong correlation between pH and nitrates (NO3−) recorded in site 3, where exceeding of nitrates (NO3−) allowed limit is reported, emphasizes that the increase in nitrates (NO3−) in groundwater influences to a great extent the pH, which reaches values not considered within allowed limits.
Unlike results reported by Tanwer et al. (2023) where alkalinity is significantly correlated with total hardness and nitrates (NO3−) [20], our study does not demonstrate strong or significant correlations between above-mentioned parameters. Similar to our results, Suthar et al. (2009) also reported strong correlation between pH and NO3− in groundwater samples collected from mineral and organic fertilized areas [49], which indicates the contribution of nitrates (NO3−) to the increase of the groundwater acidity. Alramthi et al. (2022) identified stronger and statistically significant correlations between chloride (Cl−) and nitrites (NO2−), compared to those reported in the present study [6]. Li et al. (2020) reported weaker correlations (r = −0.277 and −0.302) between NO3− and NH4+ compared to those obtained in our study, which indicates the intensified transformation processes (nitrification versus denitrification) in experimental sites analyzed in our study [52].
3.4. Principal Components Analysis (PCA)
For a better understanding of the relationships between the main groundwater quality parameters when different fertilization strategies are applied, a PCA analysis was applied. The suitability of using PCA was demonstrated by KMO values above 0.500 and p < 0.01 for Bartlett test. In all experimental sites, two main factors are identified: F1, the dose of nitrogen administered and F2, the type of fertilization, while F1 accounts for a larger share of variance compared to F1 (Figure 1, Table 9).

Table 9.
The factor loadings of the groundwater parameters.
PC1 and PC2 explain different percentages of variation across the experimental sites: PC1 explains 65.25% of variance, and PC2 explains 34.75% of variance corresponding to Site 1-Sheep manure, 39.52 t/ha; PC1 explains 64.92% of variance, and PC2 explains 35.75% of variance corresponding to Site 2-Sheep manure, 79.04 t/ha; PC1 explains 67.83% of variance, and PC2 explains 32.17% of variance corresponding to Site 3-Sheep manure 118.56 t/ha; PC1 explains 61.22% of variance, and PC2 explains 38.78% of variance corresponding to Site 4-mineral fertilization N14:P7:K21. According to the biplot plan of the PCA (Figure 1), results show that the dose of nitrogen administered influences in a different manner the evolution of the groundwater parameters. The lowest doses of nitrogen administered by organic fertilization and the dose of nitrogen administered by mineral fertilization mainly led to increases in the salts (nitrites NO2−, nitrates NO3−, phosphates PO43−, sulfates SO42−), calcium Ca, ammonium NH4+, and total hardness of the groundwater (Figure 2a,b,d, Table 9), while the biggest dose of nitrogen administered by organic fertilization increases the values of the physical parameters (turbidity, conductivity), alkalinity, sulfate SO42−, nitrates NO3−, and iron Fe concentrations (Figure 2c, Table 9). These results may be attributed not only to the consequence of nitrogen source or administered dose, but also to the physiochemical and biological soil processes influencing soil solution and groundwater quality, and consequently, by nitrogen form release (NO3−, and or NH4+) [16,57,58].
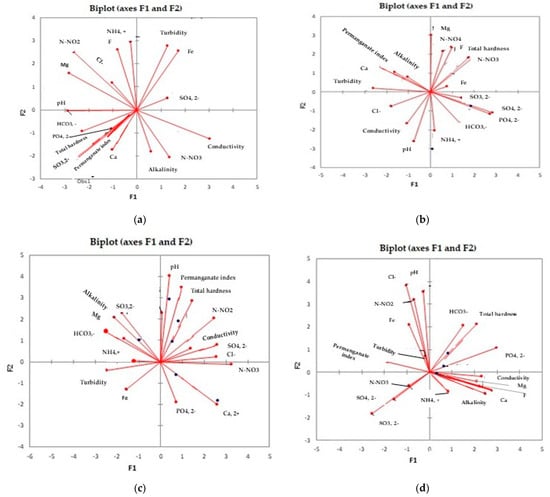
Figure 2.
The biplot of principal factors identified for studied groundwater parameters in experimental sites. (a) Site 1-Sheep manure, administered at doses of 39.52 t/Ha; (b) Site 2-Sheep manure, administered at doses of 79.04 t/hA; (c) Site 3-Sheep manure, administered at doses of 118.56 t/Ha; and (d) Site 4-Mineral fertilization N14:P7:K21.
The common groundwater parameters positively influenced by both mineral and organic fertilization are turbidity, pH, permanganate, index, bicarbonate (HCO3−), chlorine (Cl−), nitrites (NO3−), iron (Fe) content, and total hardness. Differences between the influences of the type of fertilization on groundwater parameters concern the phosphate (PO43−) content increased only with mineral fertilization and fluoride (F), magnesium (Mg), calcium (Ca), ammonium (NH4+), alkalinity, and sulfides (SO32−), increased only with organic fertilization (Figure 2d, Table 9). Unlike the results of our study, where we identified two principal factors, in studies conducted on groundwater quality from a western Saudi Arabia area, Alshehri and Abdelrahman (2023) identified three principal factors, while Li et al. (2020) in southwestern China five principal factors, both accounting for low variances [18,52]. Both principal factors identified in our study strongly weighed nitrites (NO3−), nitrates (NO2−), sulfides (SO32−), or phosphates (PO43−), showing the importance of fertilizers inputs regardless of their origin [58,59,60,61].
4. Conclusions
The impact of organic (manure) fertilization, based on the administered dose, and mineral fertilization on physiochemical parameters of groundwater quality, was assessed. The administration of higher doses of organic fertilizer leads to the exceeding of allowed limits for nitrates (NO3−) occurrence and groundwater acidifying. Few positive and negative significant correlations are identified between the same analyzed groundwater parameters in experimental sites, and this indicates similar interactions between fertilizers inputs and groundwater. The doses of nitrogen administered through organic and mineral fertilization have different influences on the groundwater parameters. The lowest doses of nitrogen administered by organic fertilization and mineral fertilization mainly led to increases in the salts, calcium (Ca), ammonium (NH4+), and total hardness in groundwater and the biggest dose of nitrogen administered through organic fertilization increases the values of the groundwater physical parameters (turbidity, conductivity), alkalinity, sulfates (SO42−), nitrates (NO3−), and iron (Fe) concentrations. The type of fertilization (organic versus mineral) has different influences on groundwater quality parameters. The phosphate content increased only when mineral fertilization is applied, while fluoride (F), magnesium (Mg), calcium (Ca), ammonium (NH4+), alkalinity, and sulfides (SO32−) increased only when organic fertilization was used, regardless of administered dose. The administration of high organic fertilizer (manure) doses, which is a very common practice in rural areas, has a high harmful potential because wells that are in proximity to agricultural fields are still used as sources of drinking water. Further research needs to be conducted to obtain a clear picture of groundwater pollution due to anthropic contributions, in rural areas of Romania, including methodologies involving isotopic measurements.
Author Contributions
Conceptualization, D.C.C. and A.C.M.O.; methodology, S.C.M.; validation, C.V.N.; writing—original draft preparation, A.C.B., D.B. and S.D.; writing—review and editing, D.C.C. and C.V.N.; supervision, A.C.M.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Document provided for peer review.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Roș, V.; Nașcu, H.; Chira, R.; Ghereș, M.I.; Fechete, L.V. Control of Water Pollution in Agriculture; Todesco Publishing House: Cluj-Napoca, Romania, 2003. (In Romanian) [Google Scholar]
- Canhong, G.; El-Sawah, A.M.; Dina, F.I.A.; Yousef, A.H.; Hiba, S.; Mohamed, S.S. The Integration of Bio and Organic Fertilizers Improve Plant Growth, Grain Yield, Quality and Metabolism of Hybrid Maize (Zea mays L.). Agronomy 2020, 10, 319. [Google Scholar]
- Rizzo, A.; Sarti, C.; Nardini, A.; Conte, G.; Masi, F.; Pistocchi, A. Nature-based solutions for nutrient pollution control in European agricultural regions: A literature review. Ecol. Eng. 2023, 186, 106772. [Google Scholar] [CrossRef]
- Liu, L.; Wu, J.; He, S.; Wang, L. Occurrence and distribution of groundwater fluoride and manganese in the Weining Plain (China) and their probabilistic health risk quantifcation. Expo Health 2022, 14, 263–279. [Google Scholar] [CrossRef]
- Rakib, M.A.; Sasaki, J.; Matsuda, H.; Quraishi, S.B.; Mahmud, M.J.; Bodrud Doza, M.; Ullah, A.; Fatema, K.J.; Newaz, M.A.; Bhuiyan, M. Groundwater salinization and associated co-contamination risk increase severe drinking water vulnerabilities in the southwestern coast of Bangladesh. Chemosphere 2020, 246, 125646. [Google Scholar] [CrossRef]
- Alramthi, S.M.; Ali, G.H.; Shaban, A.M.; Abdou, T.A.; Elthagafi, A.M.; Eldosari, S.H.; Zhu, B.-K.; Safaa, H.M. Quality Characterization of Groundwater for Drinking Purposes and Its Network Distribution to Assure Sustainability in Southern Region of Saudi Arabia. Water 2022, 14, 3565. [Google Scholar] [CrossRef]
- Munthali, C.; Kinoshita, R.; Onishi, K.; Rakotondrafara, A.; Mikami, K.; Koike, M.; Tani, M.; Palta, J.; Aiuchi, D. A Model Nutrition Control System in Potato Tissue Culture and Its Influence on Plant Elemental Composition. Plants 2022, 11, 2718. [Google Scholar] [CrossRef]
- Panneerselvam, B.; Muniraj, K.; Duraisamy, K.; Pande, C.; Karuppannan, S.; Thomas, M. An integrated approach to explore the suitability of nitrate-contaminated groundwater for drinking purposes in a semiarid region of India. Environ. Geochem. Health 2023, 45, 647–663. [Google Scholar] [CrossRef]
- Gaikwad, R.W.; Warade, A.R. Removal of Nitrate from Groundwater by Using Natural Zeolite of Nizarneshwar Hills of Western India. J. Water Resour. Hydraul. Eng. 2014, 3, 74–80. [Google Scholar]
- Valin, H.; Sands, R.D.; van der Mensbrugghe, D.; Nelson, G.C.; Ahammad, H.; Blanc, E.; Bodirsky, B.; Fujimori, S.; Hasegawa, T.; Havlik, P.; et al. The future of food demand: Understanding differences in global economic models. Agric. Econ. 2014, 45, 51–67. [Google Scholar] [CrossRef]
- Shukla, S.; Saxena, A. Global Status of Nitrate Contamination in Groundwater: Its Occurrence, Health Impacts, and Mitigation Measures. In Handbook of Environmental Materials Management; Hussain, C.M., Ed.; Springer International Publishing: Cham, Switzerland, 2019. [Google Scholar]
- Malyan, S.K.; Singh, R.; Rawat, M.; Kumar, M.; Pugazhendhi, A.; Kumar, A.; Kumar, V.; Kumar, S.S. An overview of carcinogenic pollutants in groundwater of India. Biocatal. Agric. Biotechnol. 2019, 21, 101288. [Google Scholar] [CrossRef]
- Mohammed, A.M.; Refaee, A.; El-Din, G.K.; Harb, S. Hydrochemical characteristics and quality assessment of shallow groundwater under intensive agriculture practices in arid region, Qena, Egypt. Appl. Water Sci. 2022, 12, 92. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, Y.; Yang, S.; Zhao, X.; Ouyang, L.; Lai, C. Evaluating Surface Water Nitrogen Pollution via Visual Clustering in Megacity Chengdu. Water 2023, 15, 2113. [Google Scholar] [CrossRef]
- Vasilache, N.; Diacu, E.; Modrogan, C.; Chiriac, F.L.; Paun, I.C.; Tenea, A.G.; Pirvu, F.; Vasile, G.G. Groundwater Quality Affected by the Pyrite Ash Waste and Fertilizers in Valea Calugareasca, Romania. Water 2022, 14, 2022. [Google Scholar] [CrossRef]
- Ramalingam, S.; Panneerselvam, B.; Kaliappan, S.P. Effect of high nitrate contamination of groundwater on human health and water quality index in semi-arid region, South India. Arab. J. Geosci. 2022, 15, 242. [Google Scholar] [CrossRef]
- Mester, T.; Szabó, G.; Sajtos, Z.; Baranyai, E.; Szabó, G.; Balla, D. Environmental Hazards of an Uncultivated Liquid Waste Disposal Site on Soil and Groundwater. Water 2022, 14, 226. [Google Scholar] [CrossRef]
- Alshehri, F.; Abdelrahman, K. Integrated approach for the investigation of groundwater quality using hydrochemical and geostatistical analyses in Wadi Fatimah, western Saudi Arabia. Front. Earth Sci. 2023, 11, 1166153. [Google Scholar] [CrossRef]
- Mester, T.; Szabó, G.; Balla, D. Assessment of Shallow Groundwater Purification Processes after the Construction of a Municipal sewerage Network. Water 2021, 13, 1946. [Google Scholar] [CrossRef]
- Tanwer, N.; Deswal, M.; Khyalia, P.; Laura, J.S.; Khosla, B. Fluoride and nitrate in groundwater: A comprehensive analysis of health risk and potability of groundwater of Jhunjhunu district of Rajasthan, India. Environ. Monit. Assess. 2023, 195, 267. [Google Scholar] [CrossRef]
- Wang, Y.; Li, R.; Wu, X.; Yan, Y.; Wei, C.; Luo, M.; Xiao, Y.; Zhang, Y. Evaluation of Groundwater Quality for Drinking and Irrigation Purposes Using GIS-Based IWQI, EWQI and HHR Model. Water 2023, 15, 2233. [Google Scholar] [CrossRef]
- Wszelaczynska, E.; Poberezny, J.; Keutgen, A.J.; Keutgen, N.; Goscinna, K.; Milczarek, D.; Tatarowska, B.; Flis, B. Antinutritional Nitrogen Compounds Content in Potato (Solanum tuberosum L.) Tubers Depending on the Genotype and Production System. Agronomy 2022, 12, 2415. [Google Scholar] [CrossRef]
- Wadas, W. Effect of Foliar Silicon Application on Nutrient Content in Early Crop Potato Tubers. Agronomy 2022, 12, 2706. [Google Scholar] [CrossRef]
- Hansen, B.; Thorling, L.; Schullehner, J.; Termansen, M.; Dalgaard, T. Groundwater nitrate response to sustainable nitrogen management. Sci. Rep. 2017, 7, 8566. [Google Scholar] [CrossRef] [PubMed]
- Nyiraneza, J.; Chen, D.; Fraser, T.; Comeau, L.-P. Improving Soil Quality and Potato Productivity with Manure and High-Residue Cover Crops in Eastern Canada. Plants 2021, 10, 1436. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Su, C.; Wang, M.; Abbas, H.; Baloch, M.Y.J.; Ghani, J.; Ullah, Z.; Huq, M.E. Groundwater fluoride and nitrate contamination and associated human health risk assessment in South Punjab, Pakistan. Environ. Sci. Pollut. Res. 2023, 30, 61606–61625. [Google Scholar] [CrossRef]
- Eslamian, S.; Harooni, Y.; Sabzevari, Y. Simulation of nitrate pollution and vulnerability of groundwater resources using MODFLOW and DRASTIC models. Sci. Rep. 2023, 13, 8211. [Google Scholar] [CrossRef] [PubMed]
- Șara, A.; Odagiu, A. Determination of Fodder Quality; AcademicPres Publishing House: Cluj-Napoca, Romania, 2002. (In Romanian) [Google Scholar]
- ISO 5667-3; Water Quality. Sampling-Part 3: Preservation and Handling of Water Samples. International Standardization Organization: Geneve, Switzerland, 2018.
- EN ISO 7027; Water Quality. Determination of Turbidity. European Committee for Standardization: Brussels, Belgium, 2012.
- Internal Laboratory Procedures for Water Quality Assessment; Somes Water Company, Water Analysis Laboratory of the Water Treatment Plant: Gilău, Romania, 2019. (In Romanian)
- ISO 10523; Water Quality. Determination of pH. International Standardization Organization: Geneve, Switzerland, 2012.
- EN 27888; Water quality. Determination of electrical conductivity. International Standardization Organization: Geneve, Switzerland, 1997.
- ISO 9297; Water Quality. Determination of Cloride. Silver Nitrate Titration with Chromate Indicator (Mohr’s Method). International Standardization Organization: Geneve, Switzerland, 2001.
- EN ISO 8467; Water quality — Determination of permanganate index. European Committee for Standardization: Brussels, Belgium, 2019.
- ISO 7150-1; Water Quality. Determination of Ammonium. Part 1: Manual Spectrometric Method. International Standardization Organization: Geneve, Switzerland, 2001.
- EN ISO 26777; Water Quality. Determination of Nitrite. Molecular Absorption Spectrometric Method. European Committee for Standardization: Brussels, Belgium, 2006.
- ISO 7890; Water Quality. Determination of Nitrate. Part 3: Spectrometric Method Using Sulfosalicylic Acid. International Standardization Organization: Geneve, Switzerland, 2000.
- ISO 6058; Water Quality. Determination of Calcium. EDTA Titrimetric Method. International Standardization Organization: Geneve, Switzerland, 2008.
- ISO 6059; Water Quality. Determining the Sum of Calcium and Magnesium. EDTA Titrimetric Method. International Standardization Organization: Geneve, Switzerland, 2008.
- U.S. EPA. 1997; Method 300.1: Determination of Inorganic Anions in Drinking Water by Ion Chromatography, Revision 1.0. United States Environmental Protection Agency: Cincinnati, OH, USA, 1997.
- U.S. EPA 2018; Methylene Blue Method. Measures total sulfides, H2S, HS–, and some metal sulfides in groundwater, wastewater, brines and seawater. United States Environmental Protection Agency: Colorado, CO, USA, 2018.
- EN ISO 9963-1; Water quality—Determination of alkalinity—Part 1: Determination of total and composite alkalinity, 2002. International Standardization Organization: Geneve, Switzerland, 2002.
- U.S. EPA 1989; EZ1301 Iron, FerroVer® Method, for water, wastewater and seawater; digestion is required for determining total iron. United States Environmental Protection Agency: Colorado, CO, USA, 1989.
- EN ISO 6878; Water quality—Determination of phosphorus—Ammonium molybdate spectrometric method, 2004. International Standardization Organization: Geneve, Switzerland, 2004.
- Merce, E.; Merce, C. Statistică—Paradigme Consacrate şi Paradigme Întregitoare; AcademicPres Publishing House: Cluj-Napoca, Romania, 2009. (In Romanian) [Google Scholar]
- Bahrami, M.; Zarei, A.R.; Rostami, F. Temporal and spatial assessment of groundwater contamination with nitrate by nitrate pollution index (NPI) and GIS (case study: Fasarud plain, southern Iran). Environ. Geochem. Health 2020, 43, 3119–3130. [Google Scholar] [CrossRef]
- Li, Z.; Yang, Q.; Xie, C.; Lu, X. Source identification and health risks of nitrate contamination in shallow groundwater: A case study in Subei Lake basin. Environ. Sci. Pollut. Res. 2023, 30, 13660–13670. [Google Scholar] [CrossRef]
- Suthar, S.; Bishnoi, P.; Singh, S.; Mutiyar, P.K.; Nema, A.K.; Patil, N.S. Nitrate contamination in groundwater of some rural areas of Rajasthan, India. J. Hazard. Mater. 2009, 171, 189–199. [Google Scholar] [CrossRef]
- Cardona, A.; Carrillo-Rivera, J.J.; Huizar-Aolvarez, R.; Graniel-Castro, E. Salinization in coastal aquifers of arid zones: An example from santo domingo, baja California sur, Mexico. Environ. Geol. 2004, 45, 350–366. [Google Scholar] [CrossRef]
- Chae, G.T.; Kim, K.; Yun, S.T.; Kim, K.H.; Kim, S.O.; Choi, B.Y.; Kim, H.S.; Rhee, C.W. Hydro geochemistry of alluvial groundwaters in an agricultural area: An implication for groundwater contamination susceptibility. Chemosphere 2004, 55, 369–378. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, H.; Guo, S.; Fu, K.; Liao, L.; Xu, Y.; Cheng, S. Groundwater pollution source apportionment using principal component analysis in a multiple land-use area in southwestern China. Environ. Sci. Pollut. Res. 2020, 27, 9000–9011. [Google Scholar] [CrossRef] [PubMed]
- Al-Hamdany, N.; Al-Shaker, Y.M.S.; Al-Saffawi, A.Y.T. Application of nitrate pollution index (Npi) to evaluate the health safety of wells water for some quarters of the leftside of Mosul City, Iraq. Biochem. Cell. Arch. 2020, 20, 6063–6068. [Google Scholar]
- Al-Sinjari, W.E.A.; Al-Shanoona, R.A.A.; Al-Saffawi, A.Y.T. Valuation of Drinking Water Human Health Risk Using Nitrate Pollution Index (NPI): A Case Study for The Ground Water of Al-Hamdaniya District. Iraq. HIV Nurs. 2023, 23, 486–490. [Google Scholar]
- El Mountassir, O.; Bahir, M.; Ouazar, D.; Chehbbouni, A.; Careirra, P.M. Temporal and spatial assessment of groundwater contamination with nitrate using nitrate pollution index (NPI), groundwater pollution index (GPI), and GIS (case study: Essaouira basin, Morocco). Environ. Sci. Pollut. Res. 2022, 29, 17132–17149. [Google Scholar] [CrossRef]
- Obeidat, M.M.; Awawdeh, M.; Al-Rub, F.A.; Al-Ajlouni, A. An innovative nitrate pollution index and multivariate statistical investigations of groundwater chemical quality of Umm Rijam Aquifer (B4), North Yarmouk River Basin, Jordan. In Water Quality Monitoring and Assessment; Vouddouris, K., Voutsa, D., Eds.; InTech: Rijeka, Croatia, 2012; pp. 169–188. [Google Scholar]
- Pang, T.; Zhang, H.; Wen, L.; Tang, J.; Zhou, B.; Yang, Q.; Li, Y.; Wang, J.; Chen, A.; Zeng, Z. Quantitative Analysis of a Weak Correlation between Complicated Data on the Basis of Principal Component Analysis. J. Anal. Methods Chem. 2021, 2021, 8874827. [Google Scholar] [CrossRef]
- Rovetta, A. Raiders of the Lost Correlation: A Guide on Using Pearson and Spearman Coefficients to Detect Hidden Correlations in Medical Sciences. Cureus 2020, 12, e11794. [Google Scholar] [CrossRef]
- Brindha, K.; Vaman, K.N.; Srinivasan, K.; Babu, M.S.; Elango, L. Identifcation of surface water-groundwater interaction by hydrogeochemical indicators and assessing its suitability for drinking and irrigational purposes in Chennai. South. India Appl. Water Sci. 2014, 4, 159–174. [Google Scholar] [CrossRef]
- Dartan, G.; Taspinar, F.; Toroz, I. Assessment of heavy metals in agricultural soils and their source apportionment: A Turkish district survey. Environ. Monit. Assess. 2015, 187, 99. [Google Scholar] [CrossRef]
- Bhuiyan, M.A.H.; Bodrud-Doza, M.; Islam, A.R.M.T.; Rakib, M.A.; Rahman, M.S.; Ramanathan, A.L. Assessment of groundwater quality of Lakshimpur district of Bangladesh using water quality indices, geostatistical methods, and multivariate analysis. Environ. Earth Sci. 2016, 75, 1020. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).