Content and Speciation of Phosphorus in Lake Kórnickie
Abstract
1. Introduction
2. Materials and Methods
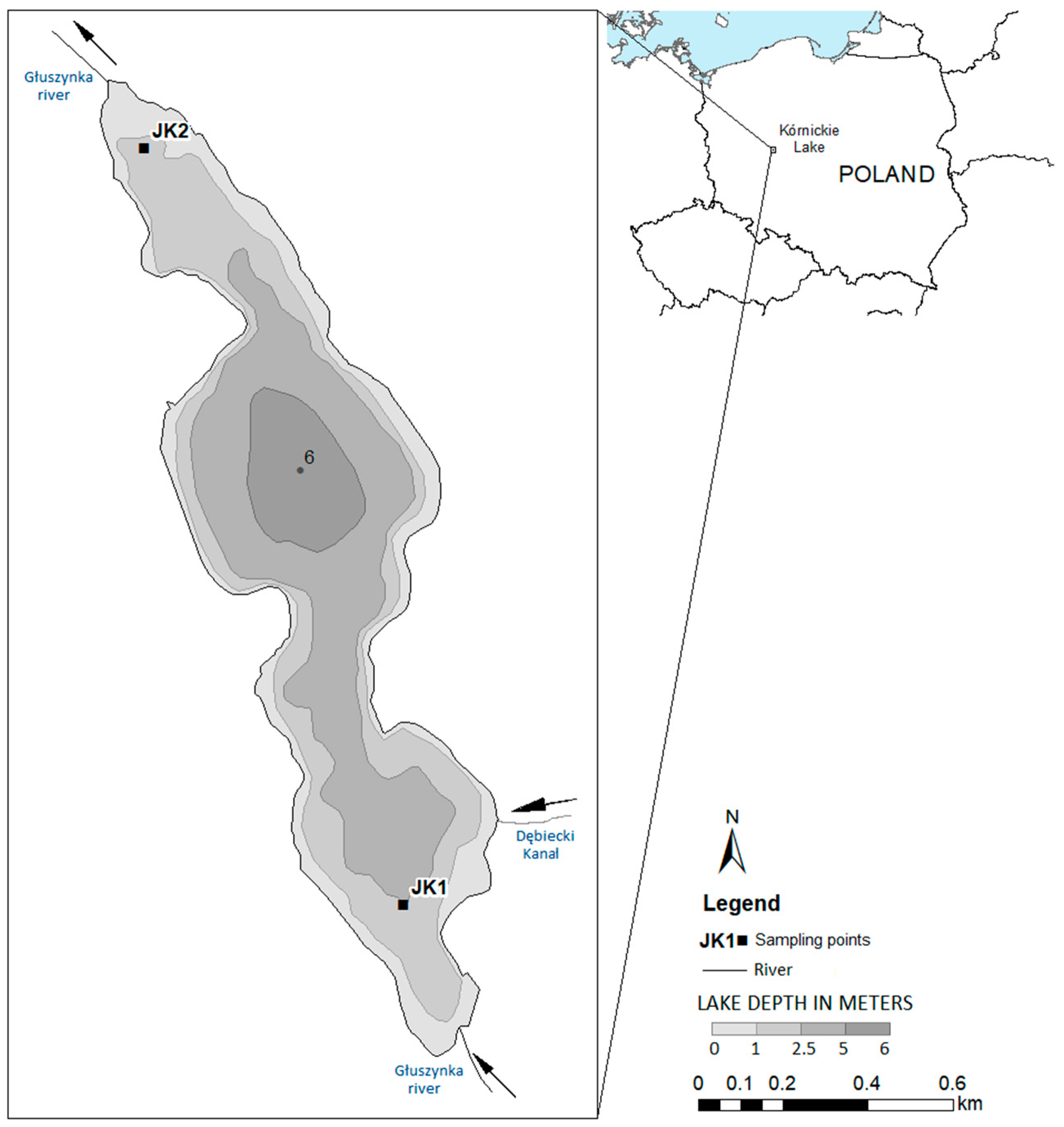
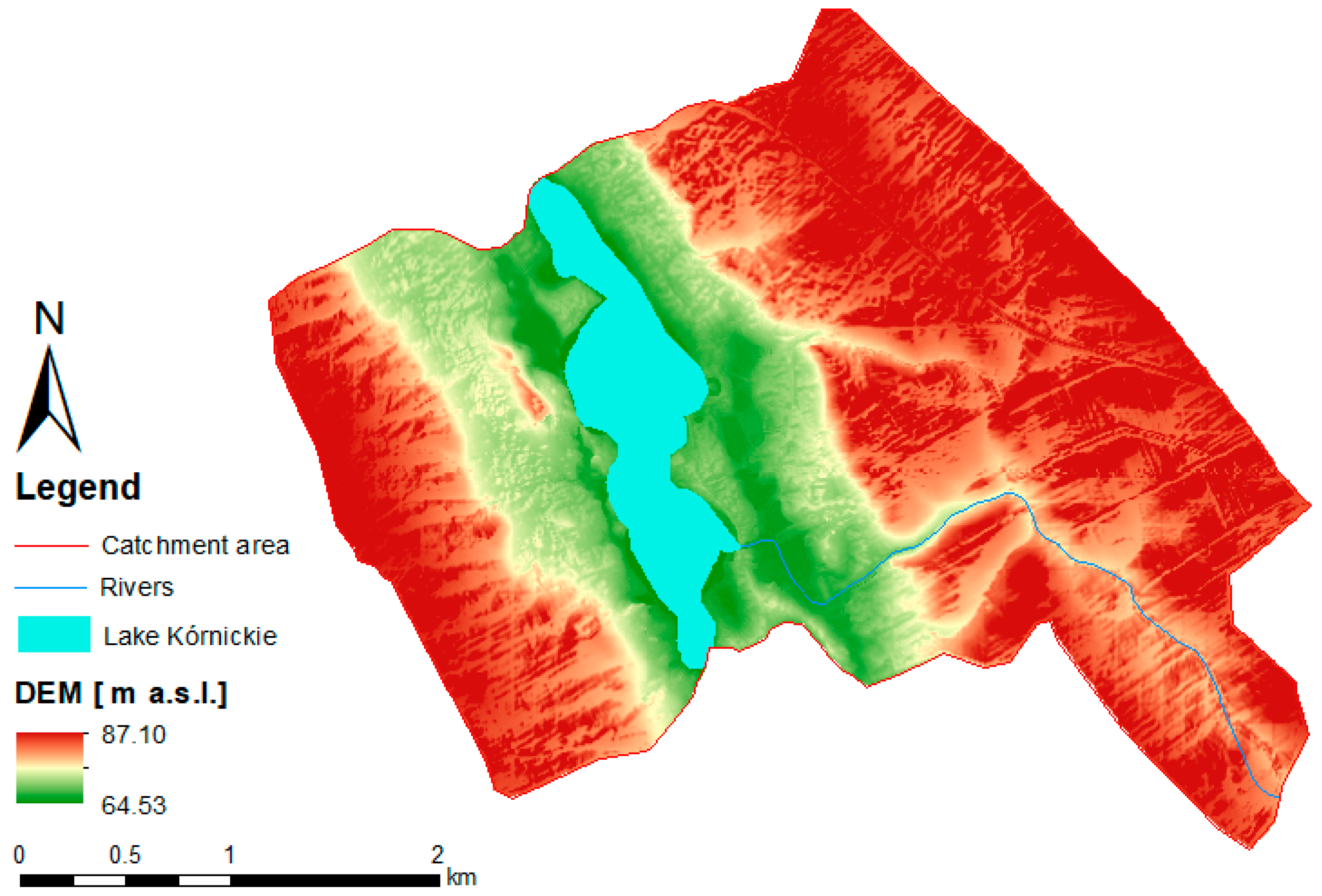
2.1. Study Site Description
2.2. Materials
2.3. Field Works
2.4. Laboratory Works
2.5. Geochemical Model
2.6. Statistical Analyses
3. Results
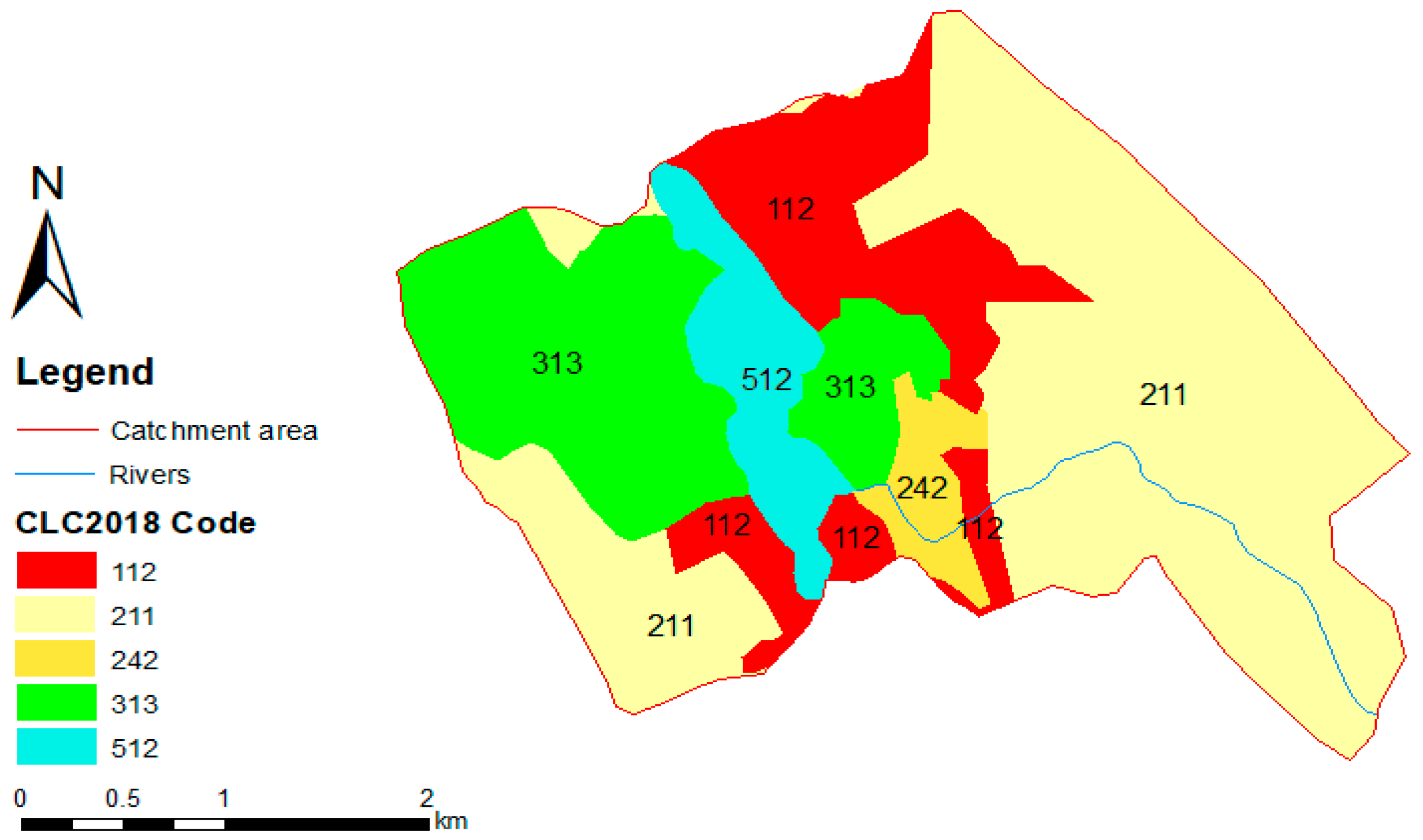
3.1. Characteristics of the Catchment Area
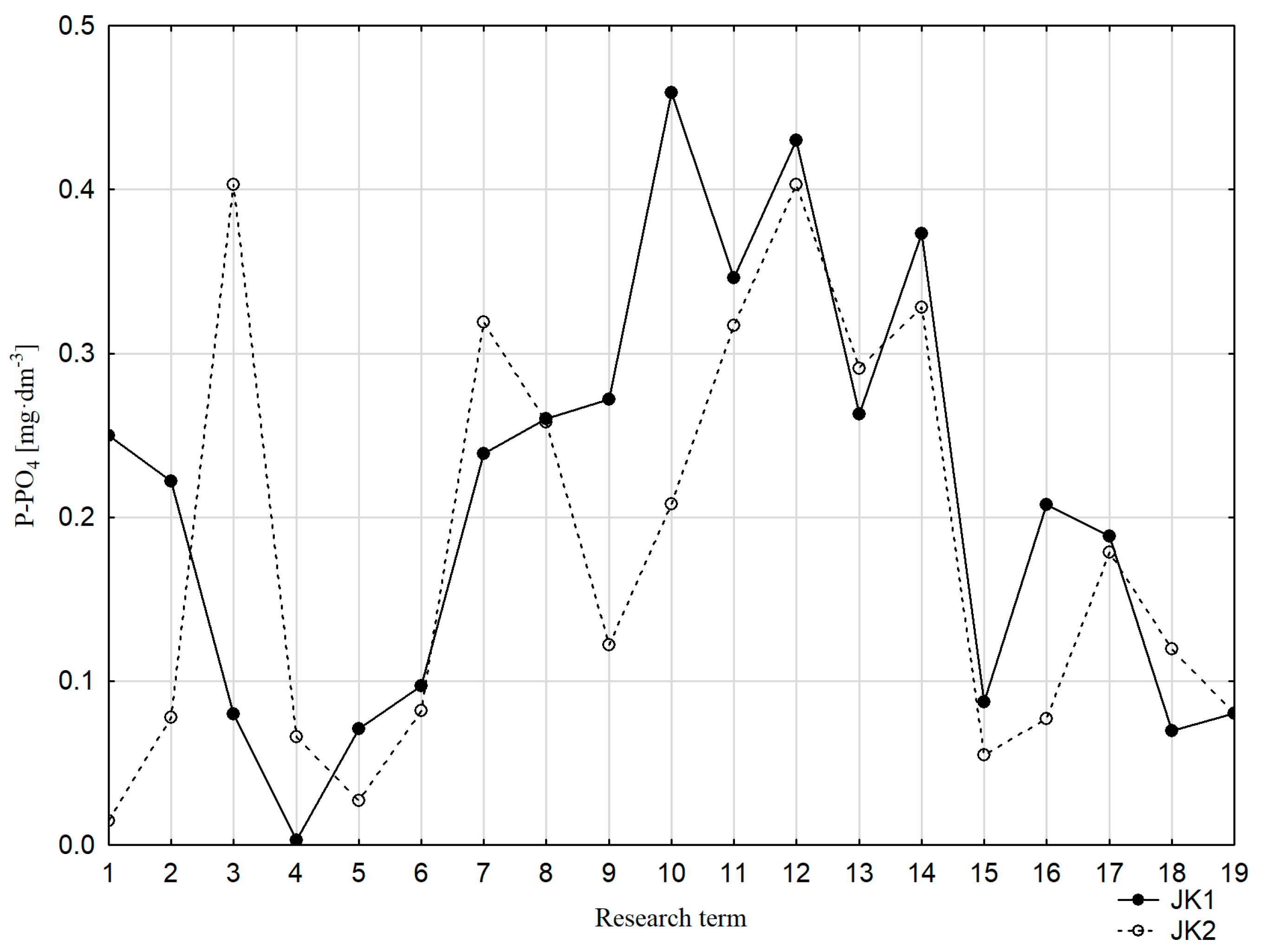
3.2. Water Properties
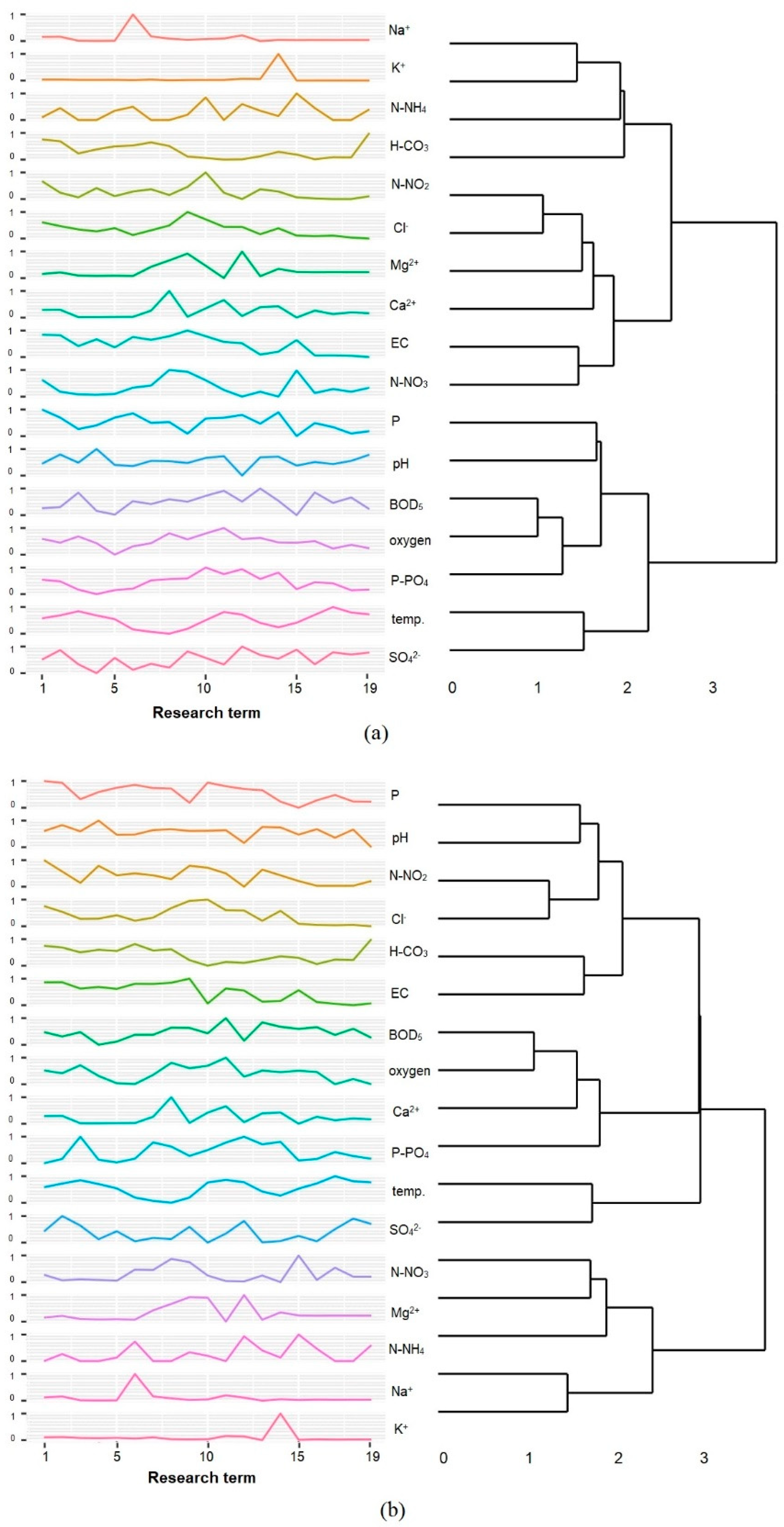
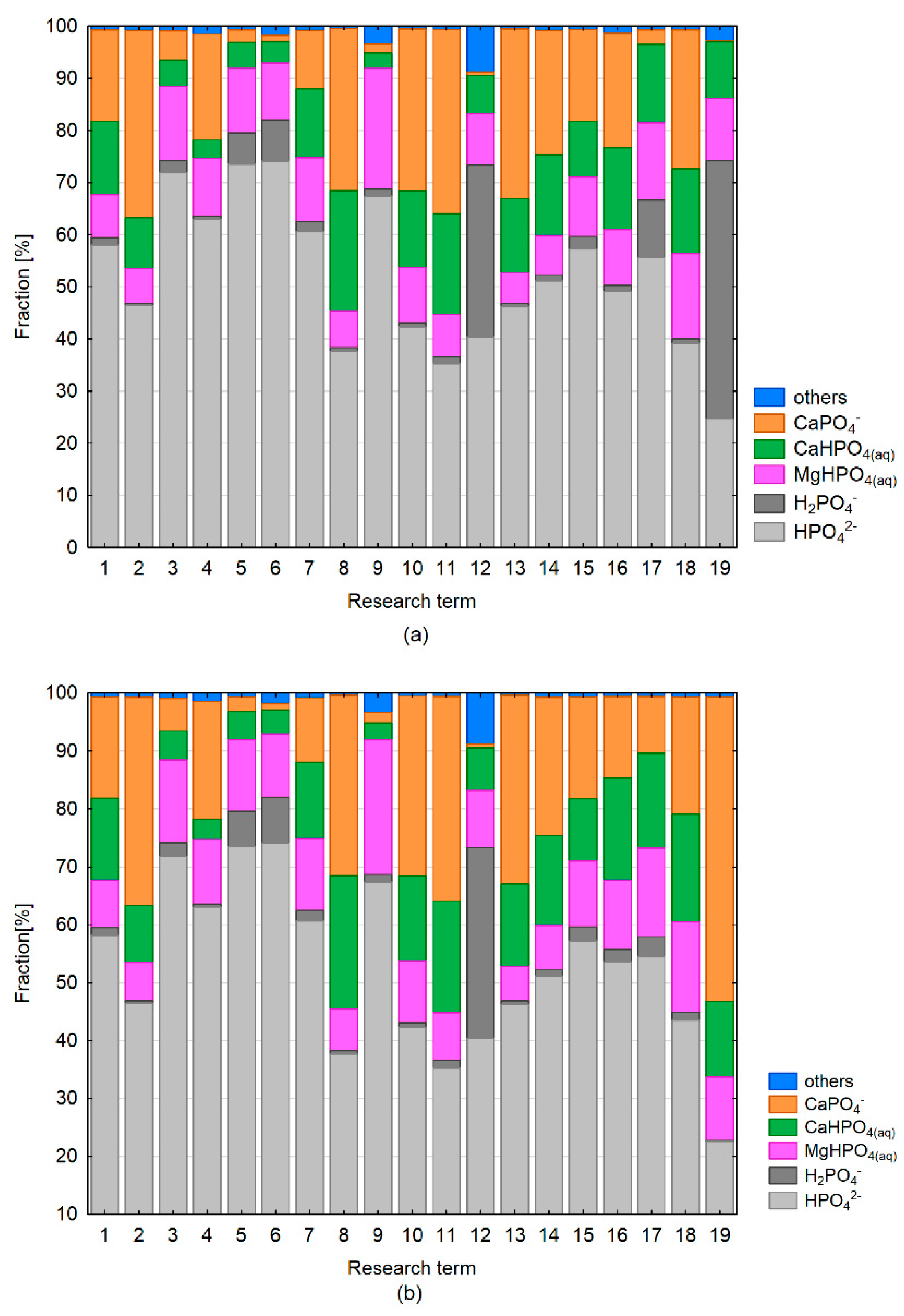
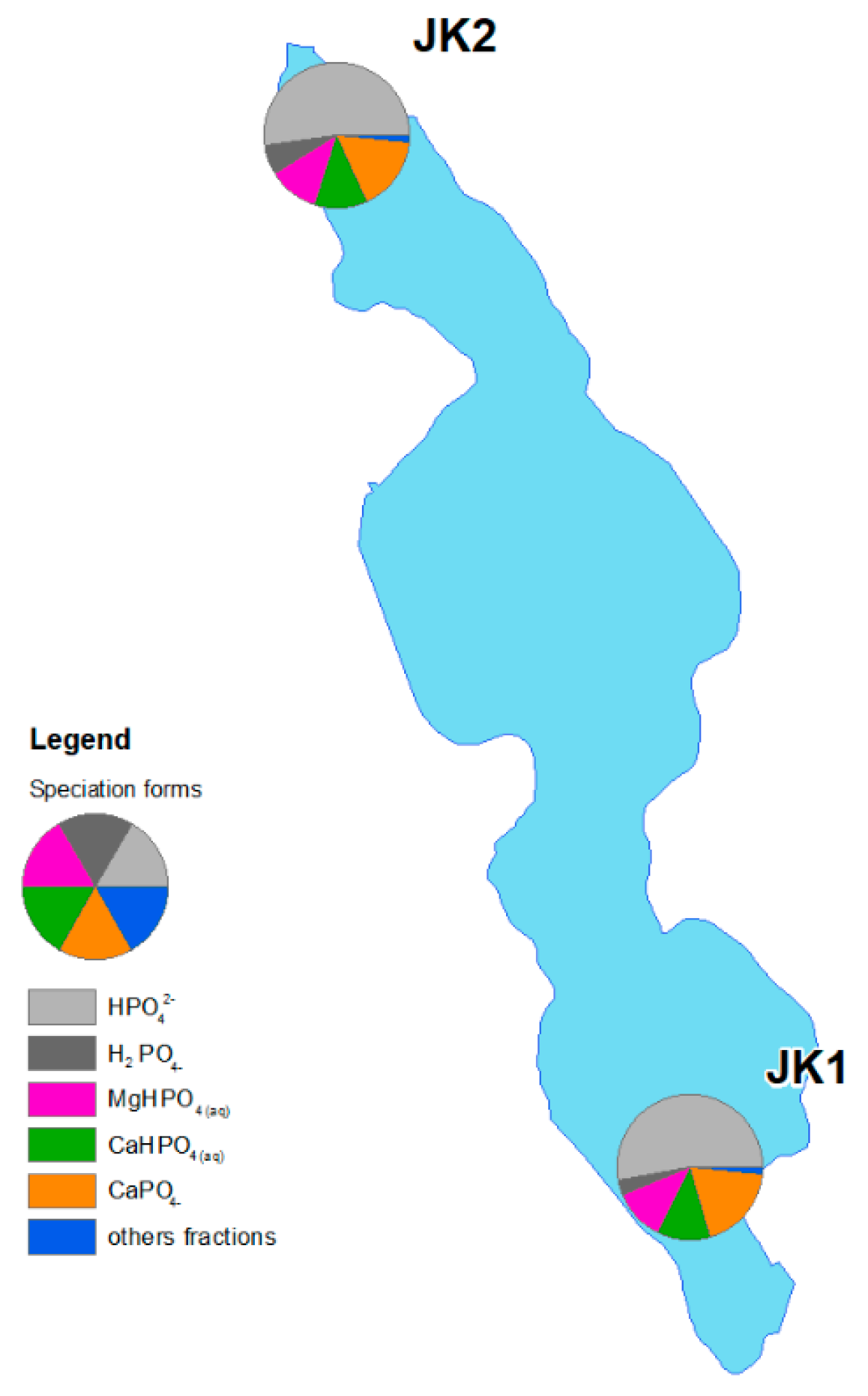
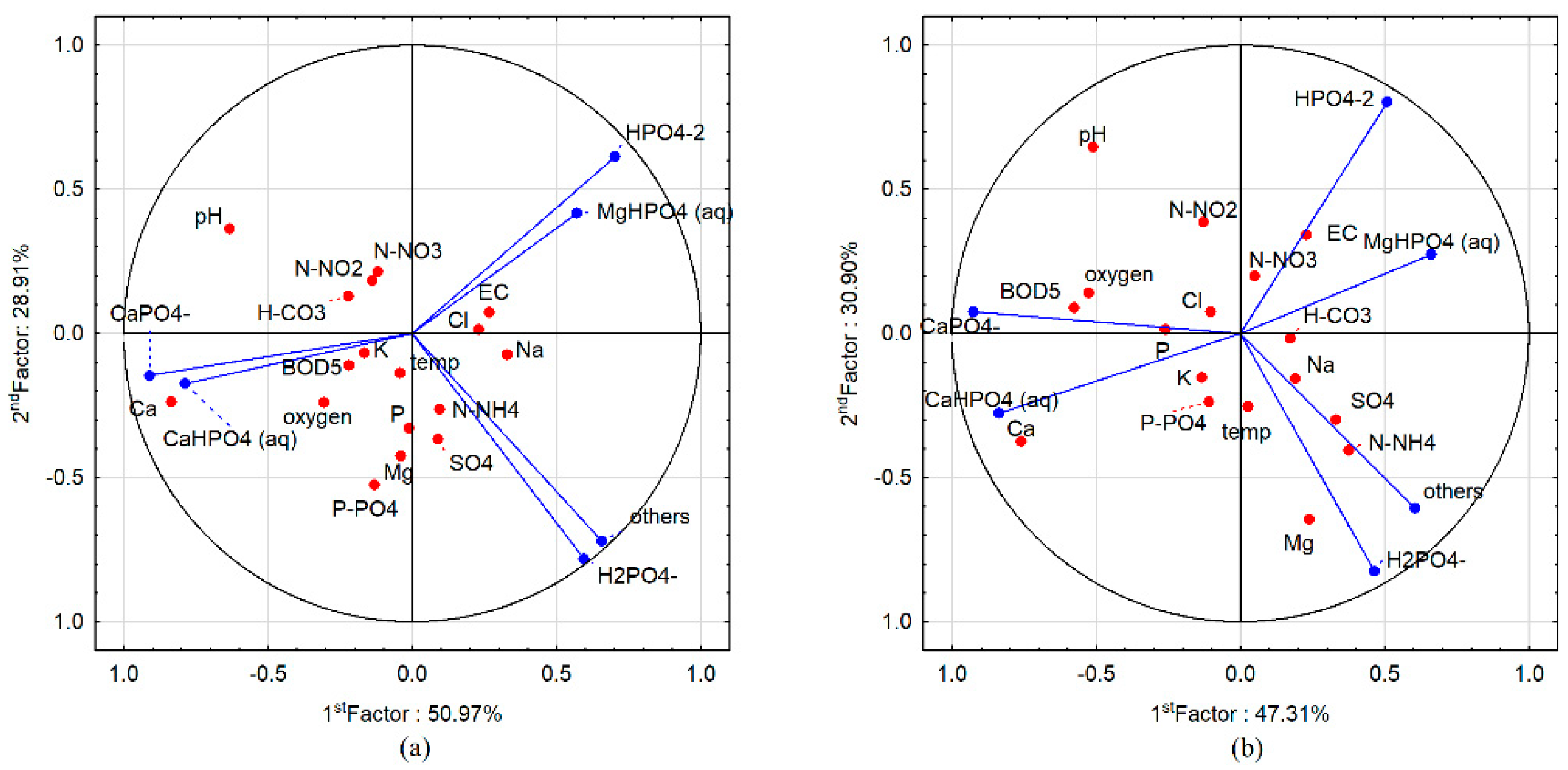
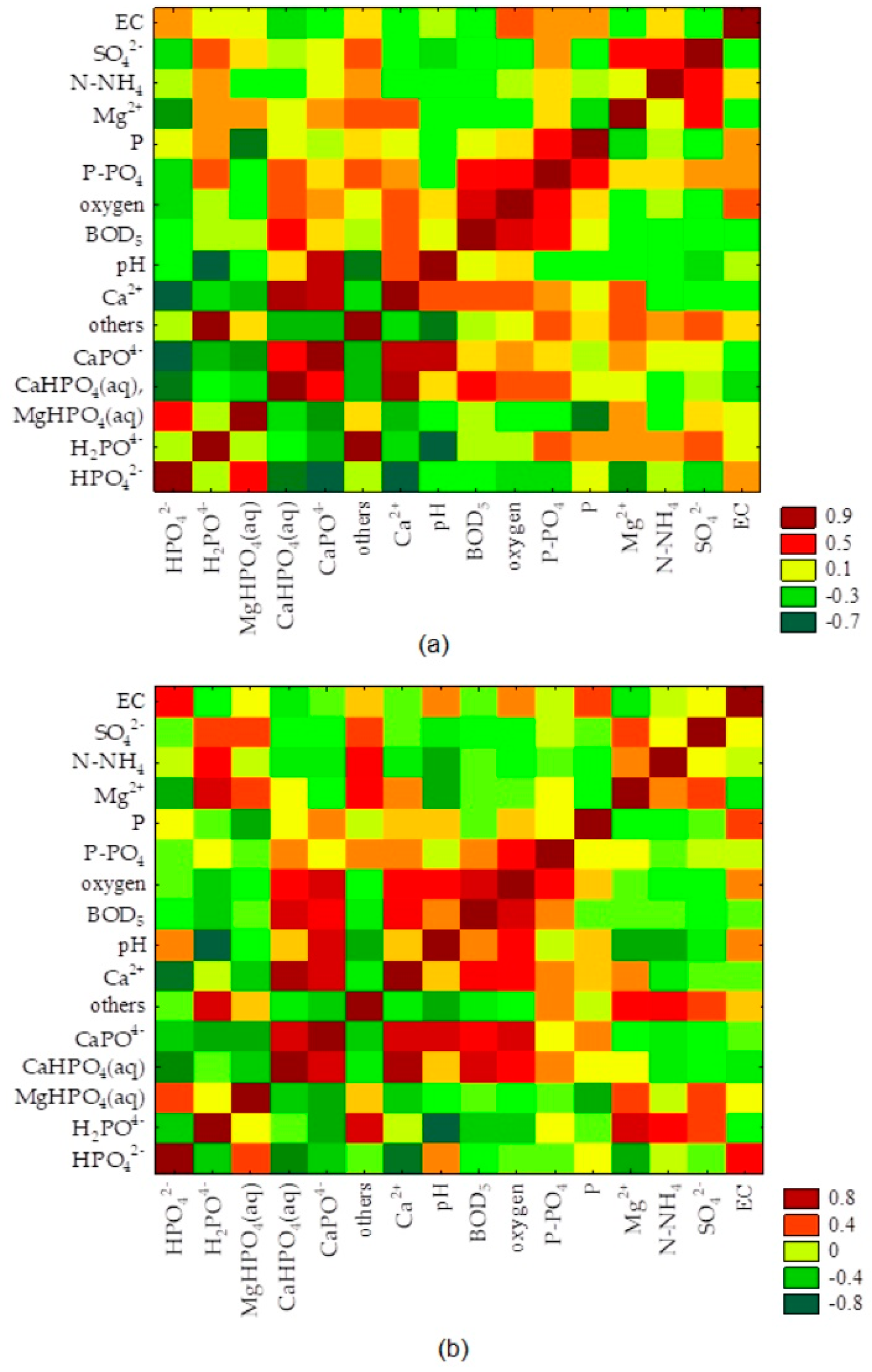
3.3. Speciation Forms and Correlation with Surface Water
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Granlund, K.; Räike, A.; Ekholm, P.; Rankinen, K.; Rekolainen, S. Assessment of water protection targets for agricultural nutrient loading in Finland. J. Hydrol. 2005, 304, 251–260. [Google Scholar] [CrossRef]
- Dokulil, M.T.; Teubner, K. Eutrophication and Climate Change: Present Situation and Future Scenarios. In Eutrophication: Causes, Consequences and Control; Springer: Dordrecht, The Netherlands, 2010; pp. 1–16. [Google Scholar] [CrossRef]
- Schindler, D.W.; Carpenter, S.R.; Chapra, S.C.; Hecky, R.E.; Orihel, D.M. Reducing phosphorus to curb lake eutrophication is a success. Environ. Sci. Technol. 2016, 50, 8923–8929. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Kang, Z.J.; Lu, D.L.; Dan, S.F.; Ning, Z.M.; Lan, W.L.; Zhong, Q.P. Spatial variations in the abundance and chemical speciation of phosphorus across the river–sea interface in the Northern Beibu Gulf. Water 2018, 10, 1103. [Google Scholar] [CrossRef]
- Zhao, G.; Sheng, Y.; Wang, W.; Liu, Q.; Jiang, M.; Li, Z. Effects of suspended particular matters, excess PO43−, and salinity on phosphorus speciation in coastal river sediments. Environ. Sci. Pollut. Res. 2020, 27, 27697–27707. [Google Scholar] [CrossRef]
- Murphy, T.; Lawson, A.; Kumagai, M.; Nalewajko, C. Release of phosphorus from sediments in Lake Biwa. Limnology 2001, 2, 119–128. [Google Scholar] [CrossRef]
- Boers, P.C.M. The Release of Dissolved Phosphorus from Lake Sediments; Wageningen University and Research: Wageningen, The Netherlands, 1991. [Google Scholar]
- Gnauck, A.; Luther, B.; Heinrich, R.; Hofmann, A. Modelling and simulation of phosphorus dynamics in shallow lakes. In Reservoir Limnology and Water Quality; Kluwer: České Budějovice, Czech Republic, 2002; pp. 98–101. [Google Scholar]
- Milenkovic, N.; Damjanovic, M.; Ristic, M. Study of Heavy Metal Pollution in Sediments from the Iron Gate (Danube River), Serbia and Montenegro. Pol. J. Environ. Stud. 2005, 14, 781–787. [Google Scholar]
- Hou, D.; He, J.; Lü, C.; Dong, S.; Wang, J.; Xie, Z.; Zhang, F. Spatial variations and distributions of phosphorus and nitrogen in bottom sediments from a typical north-temperate lake, China. Environ. Earth. Sci. 2014, 71, 3063–3079. [Google Scholar] [CrossRef]
- Dalu, T.; Tshivhase, R.; Cuthbert, R.N.; Murungweni, F.M.; Wasserman, R.J. Metal distribution and sediment quality variation across sediment depths of a subtropical Ramsar declared wetland. Water 2020, 12, 2779. [Google Scholar] [CrossRef]
- Prepas, E.E.; Charette, T. Worldwide eutrophication of water bodies: Causes, concerns, controls. Treatise Geochem. 2003, 9, 313–331. [Google Scholar] [CrossRef]
- Wang, H.; Appan, A.; Gulliver, J.S. Modeling of phosphorus dynamics in aquatic sediments: I—Model development. Water Res. 2003, 37, 3928–3938. [Google Scholar] [CrossRef]
- Huang, Q.; Wang, Z.; Wang, C.; Wang, S.; Jin, X. Phosphorus release in response to pH variation in the lake sedimentswith different ratios of iron-bound P to calcium-bound P. Chem. Speciat. Bioavailab. 2005, 17, 55–61. [Google Scholar] [CrossRef]
- Moore, P.A., Jr.; Reddy, K.R.; Fisher, M.M. Phosphorus flux between sediment and overlying water in Lake Okeechobee, Florida: Spatial and temporal variations. J. Environ. Qual. 1998, 27, 1428–1439. [Google Scholar] [CrossRef]
- Palmer-Felgate, E.J.; Mortimer, R.J.; Krom, M.D.; Jarvie, H.P.; Williams, R.J.; Spraggs, R.E.; Stratford, C.J. Internal loading of phosphorus in a sedimentation pond of a treatment wetland: Effect of a phytoplankton crash. Sci. Total Environ. 2011, 409, 2222–2232. [Google Scholar] [CrossRef] [PubMed]
- Kowalczewska-Madura, K.; Gołdyn, R.; Bogucka, J.; Strzelczyk, K. Impact of environmental variables on spatial and seasonal internal phosphorus loading in a mesoeutrophic lake. Int. J. Sediment Res. 2019, 34, 14–26. [Google Scholar] [CrossRef]
- Lukkari, K.; Hartikainen, H.; Leivuori, M. Fractionation of sediment phosphorus revisited. Fractionation steps and their biogeochemical basis. Limnol. Oceanogr. Methods 2007, 5, 433–444. [Google Scholar] [CrossRef]
- Markovic, S.; Liang, A.; Watson, S.B.; Guo, J.; Mugalingam, S.; Arhonditsis, G.; Morley, A.; Dittrich, M. Biogeochemical mechanisms controlling phosphorus diagenesis and internal loading in a remediated hard water eutrophic embayment. Chem. Geol. 2019, 514, 122–137. [Google Scholar] [CrossRef]
- Yang, C.; Yang, P.; Geng, J.; Yin, H.; Chen, K. Sediment internal nutrient loading in the most polluted area of a shallow eutrophic lake (Lake Chaohu, China) and its contribution to lake eutrophication. Environ. Pollut. 2020, 262, 114292. [Google Scholar] [CrossRef]
- Cavalcante, H.; Araújo, F.; Becker, V.; Barbosa, J.E.D.L. Internal phoshorus loading potential of a semiarid reservoir: An experimental study. Acta Limnol. Bras. 2021, 33, e6. [Google Scholar] [CrossRef]
- Wang, L.; Ruiz-Agudo, E.; Putnis, C.V.; Menneken, M.; Putnis, A. Kinetics of calcium phosphate nucleation andgrowth on calcite: Implications for predicting the fate of dissolved phosphate species in alkaline soils. Environ. Sci. Technol. 2011, 46, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Kondracki, J. Geografia Regionalna Polski (Regional Geography of Poland); Wydawnictwo Naukowe PWN: Warszawa, Poland, 2002. (In Polish) [Google Scholar]
- Studium Uwarunkowań i Kierunków Zagospodarowania Przestrzennego. Gmina Kórnik. Załącznik nr 1 do Uchwały Nr XL/529/2017 Rady Miasta i Gminy Kórnik z Dnia 25 Października 2017 r. Kórnik 2017. Available online: https://www2.kornik.pl/Image/files/2018/tekst_studium_projekt.pdf (accessed on 28 September 2022).
- Michałkiewicz, M.; Osses, A. Wpływ Antropopresji na Stopień Zanieczyszczenia Jeziora Kórnickiego. The Influence of the Anthropopression Process on the Contamination Level of Kórnickie Lake. Water Supply and water Quality. 2008. Available online: https://water.put.poznan.pl/images/fullpapers/2008/OCHRONA_WOD/337_WODA2008_T2.pdf (accessed on 28 September 2022). (In Polish).
- Zwiększenie Atrakcyjności Turystycznej Kórnika Poprzez Budowę Promenady nad Jeziorem Kórnickim—Etap II. Available online: https://fundusze.kornik.pl/zwiekszenie-atrakcyjnosci-turystycznej-kornika-poprzez-budowe-promenady-nad-jeziorem-kornickim-etap (accessed on 28 September 2022).
- Kaczorowska, Z. Opady w Polsce w Przekroju Wieloletnim. Prace Geograficzne. Precipitation in Poland in a Long-Term Perspective: Trends, Periodicity and Probability of Occurrence of 430 Deficiency and Excess of Precipitation; Polska Akademia Nauk: Warsaw, Poland, 1962. (In Polish) [Google Scholar]
- Lorenc, H. Studia nad 220-letnią (1779–1998) Serią Temperatury Powietrza w Warszawie Oraz Ocena jej Wiekowych Tendencji. Studies on the 220-Year (1779–1998); IMGW: Warsaw, Poland, 2000. (In Polish) [Google Scholar]
- ISO 10523:2008; Water Quality—Determination of pH. Polski Komitet Normalizacyjny: Warsaw, Poland, 1997.
- European Communities Environmental Objectives (Surface Water) Regulations 2009. Available online: https://www.irishstatutebook.ie/eli/2009/si/272/made/en/print (accessed on 28 September 2022).
- EPA Method 354.1; Nitrite by Spectrophotometry, Ohio. 1993. Available online: https://www.uvm.edu/bwrl/lab_docs/protocols/354.1_Nitrite_by_spectrophoometry_(EPA_1971) (accessed on 28 September 2022).
- EPA method 350.1; Determination of Ammonia Nitrogen by Semi-Automated. Ohio, 1993. Available online: https://www.epa.gov/sites/default/files/2015-06/documents/epa-350.1.pdf (accessed on 28 September 2022).
- ISO 9963-1:1994; Water Quality—Determination of Alkalinity—Part 1: Determination of Total and Composite Alkalinity. Polski Komitet Normalizacyjny: Warsaw, Poland, 1997.
- ISO 9280:1990; Water Quality—Determination of Chloride—Silver Nitrate Titration with Chromate Indicator, Mohr’s Method. Polski Komitet Normalizacyjny: Warsaw, Poland, 1997.
- ISO 6878:2004; Water Quality—Determination of Phosphorus—Ammonium Molybdate Spectrometric Method. Polski Komitet Normalizacyjny: Warsaw, Poland, 1997.
- ISO 5815:1989; Water Quality—Determination of Biochemical Oxygen Demand after n Days (BOD). Polski Komitet Normalizacyjny: Warsaw, Poland, 1997.
- ISO 8288:1986; Water Quality—Determination of Cobalt, Nickel, Copper, Zinc, Cadmium and Lead—Flame Atomic Absorption Spectrometric Methods. Polski Komitet Normalizacyjny: Warsaw, Poland, 1997.
- Gustafsson, J.P. Visual MINTEQ, ver 2.32. Royal Institute of Technology, Sztokholm, Szwecja, 2005. Available online: https://vminteq.lwr.kth.se/visual-minteq-ver-3-1/ (accessed on 28 September 2022).
- Tibco, T.I.B. InConcert Process Designer User’s Guide; Tibco Software Inc.: Palo Alto, CA, USA, 2000. [Google Scholar]
- Choiński, A. Katalog Jezior Polski (Catalog of Polish Lakes); Wydawnictwo Naukowe UAM: Poznań, Poland, 2006. (In Polish) [Google Scholar]
- Ośrodek Badań i Kontroli Środowiska (OBiKŚ) w Poznaniu. Komunikat nr 59. Available online: https://portalkomunalny.pl/plus/artykul/antropopresja-jeziora-kornickiego/ (accessed on 25 May 2022).
- WIOŚ w Poznaniu. Stan czystości jeziora Kórnickiego w roku 2001. Komunikat nr 215. Poznań. 2002. Available online: http://ekoportal.poznan.wios.gov.pl/imap/ (accessed on 25 May 2022).
- Piechowiak, K. Charakterystyka Regionu Wodnego Warty i Identyfikacja Istotnych Problemów Gospodarki Wodnej. Characteristics of the Warta Water Region and Identification of Significant Water Management Problems; RZGW: Poznań, Poland, 2007. (In Polish) [Google Scholar]
- Potasznik, A.; Szymczyk, S. Magnesium and calcium concentrations in the surface water and bottom deposits of a river-lake system. J. Elem. 2015, 20, 677–692. [Google Scholar] [CrossRef]
- Kanclerz, J.; Wiatrowska, K.; Adamska, A. Formy specjacyjne fosforu w wodach powierzchniowych w zlewni Jeziora Gorzuchowskiego. Phosphorus concentration in Surface water of Gorzuchowskie lake catchment. Pol. J. Agron. 2015, 22, 10–17. (In Polish) [Google Scholar]
- Zhang, Y.; Zhang, H.; Zhang, Z.; Liu, C.; Sun, C.; Zhang, W.; Marhaba, T. pH effect on heavy metal release from a polluted sediment. J. Chem. 2018, 2018, 7597640. [Google Scholar] [CrossRef]
- Spivakov, B.Y.; Maryutina, T.A.; Muntau, H. Phosphorus speciation in water and sediments. Pure Appl. Chem. 1999, 71, 2161–2176. [Google Scholar] [CrossRef]
- Golterman, H.L. The distribution of phosphate over iron-bound and calcium-bound phosphate in stratified sediments. Hydrobiologia 1988, 364, 75–81. [Google Scholar] [CrossRef]
- Serrano, L.; Calzada-Bujak, I.; Toja, J. Variability of the sediment phosphate composition of a temporary pond (Doana National Park, SW Spain). Hydrobiologia 2003, 429, 159–169. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janicka, E.; Kanclerz, J.; Wiatrowska, K. Content and Speciation of Phosphorus in Lake Kórnickie. Water 2022, 14, 3234. https://doi.org/10.3390/w14203234
Janicka E, Kanclerz J, Wiatrowska K. Content and Speciation of Phosphorus in Lake Kórnickie. Water. 2022; 14(20):3234. https://doi.org/10.3390/w14203234
Chicago/Turabian StyleJanicka, Ewelina, Jolanta Kanclerz, and Katarzyna Wiatrowska. 2022. "Content and Speciation of Phosphorus in Lake Kórnickie" Water 14, no. 20: 3234. https://doi.org/10.3390/w14203234
APA StyleJanicka, E., Kanclerz, J., & Wiatrowska, K. (2022). Content and Speciation of Phosphorus in Lake Kórnickie. Water, 14(20), 3234. https://doi.org/10.3390/w14203234






