Degradation of Methylene Blue in the Photo-Fenton-Like Process with WO3-Loaded Porous Carbon Nitride Nanosheet Catalyst
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
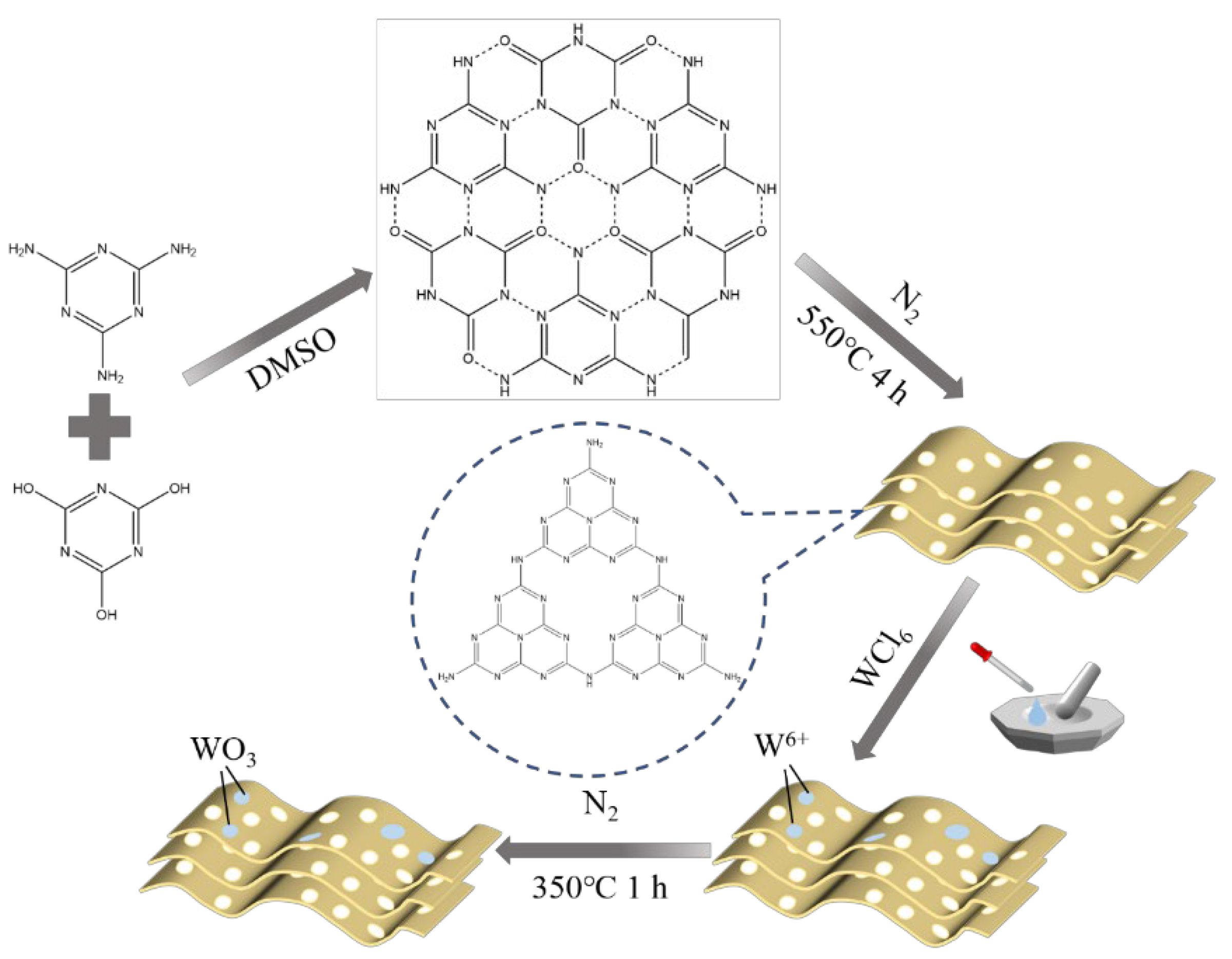
2.2. Synthesis of MCA-CN/WO3
2.3. Characterizations
2.4. Photocatalytic Test
2.5. Photoelectrochemical Measurement
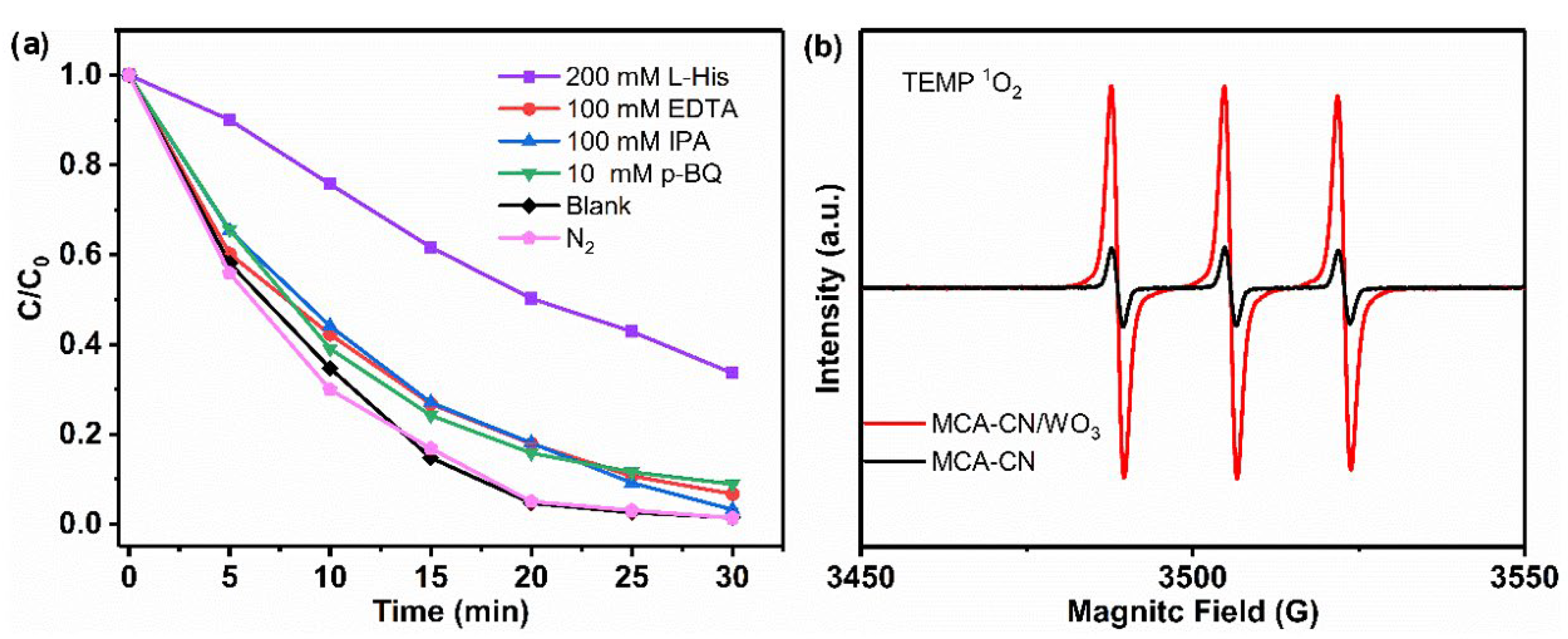
2.6. Radical Quenching Experiments
3. Results
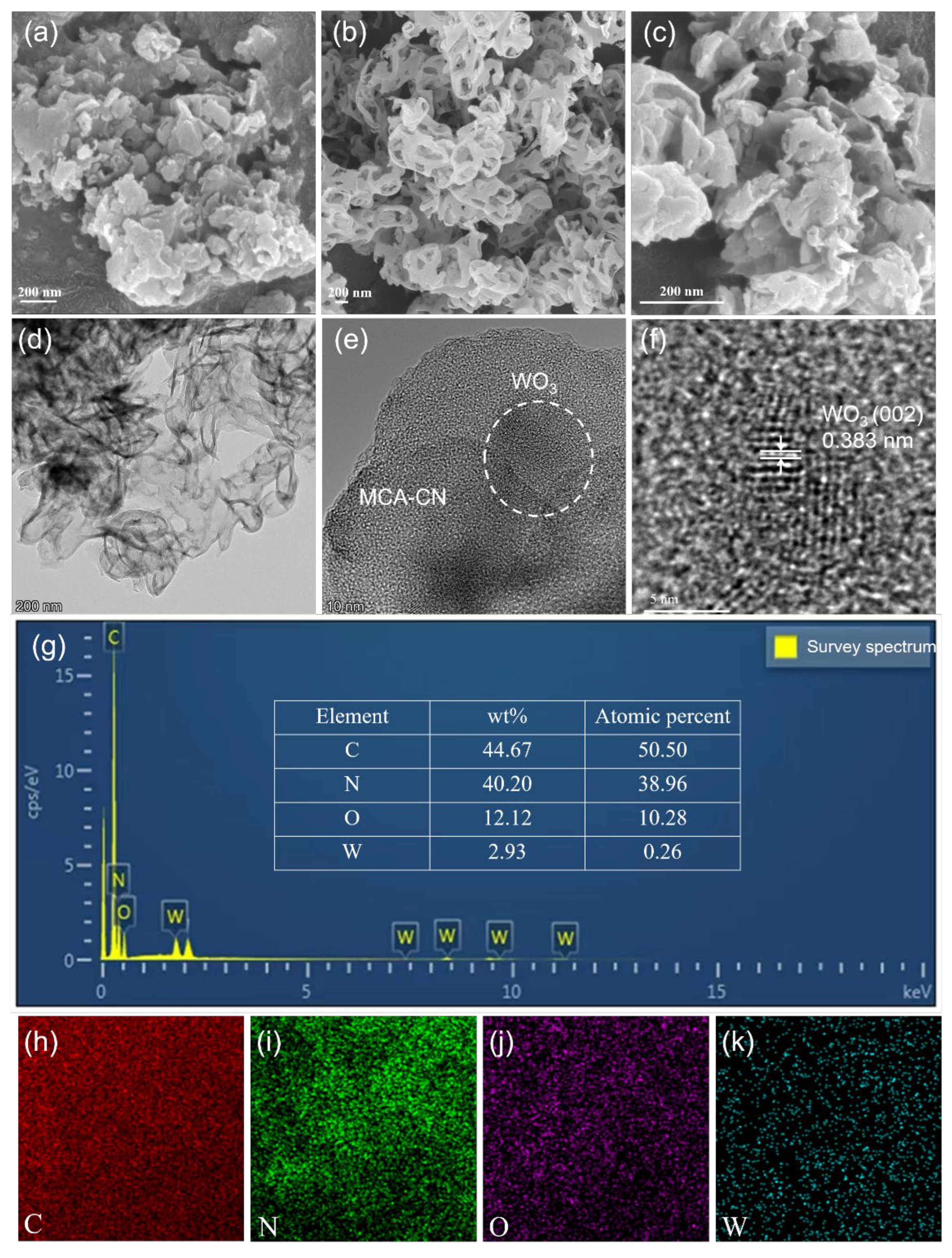
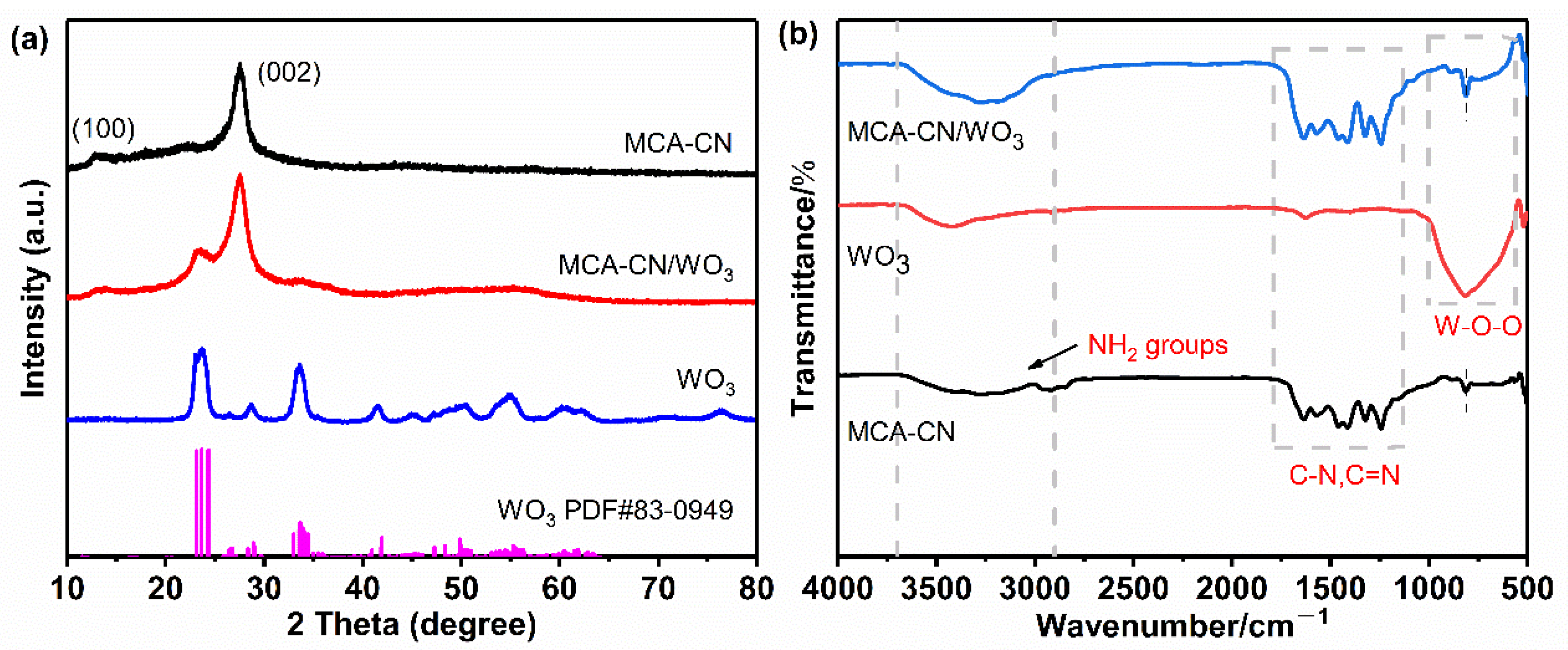
3.1. Characterization
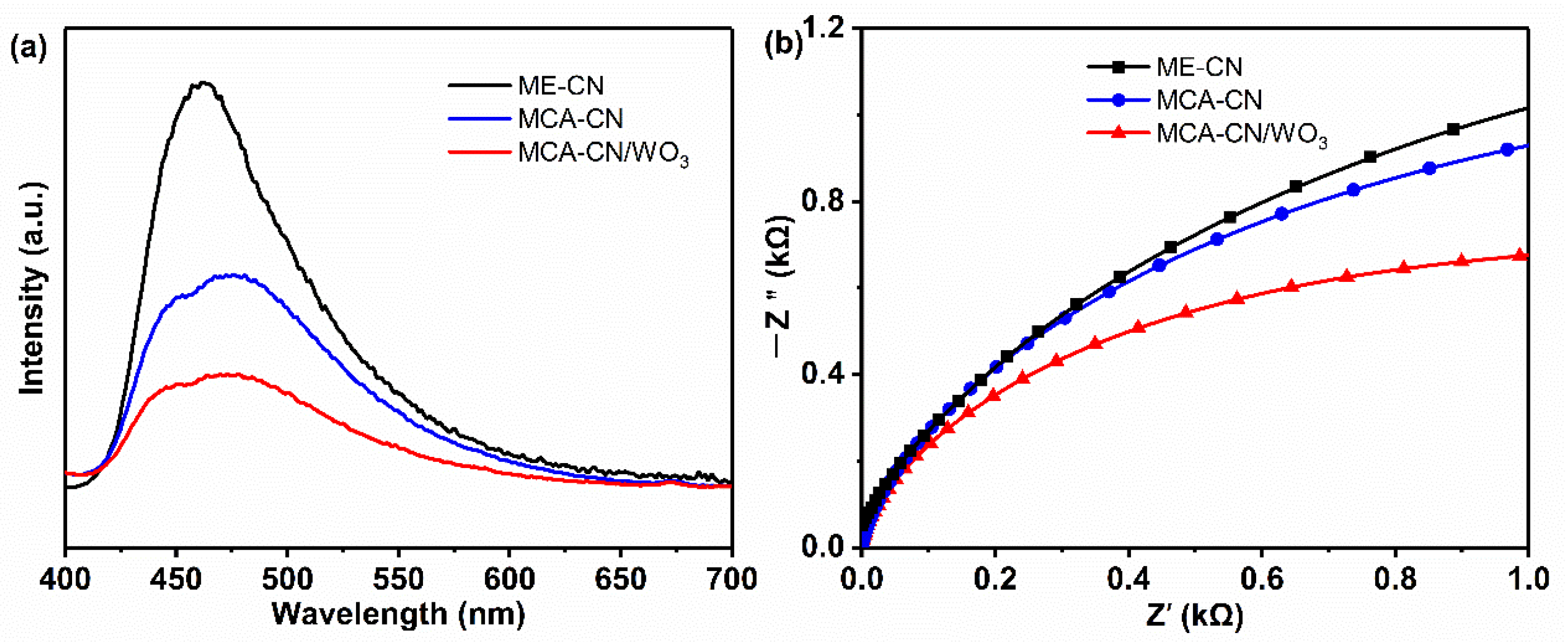
3.2. Photochemical Characterization
3.3. Catalytic Performance
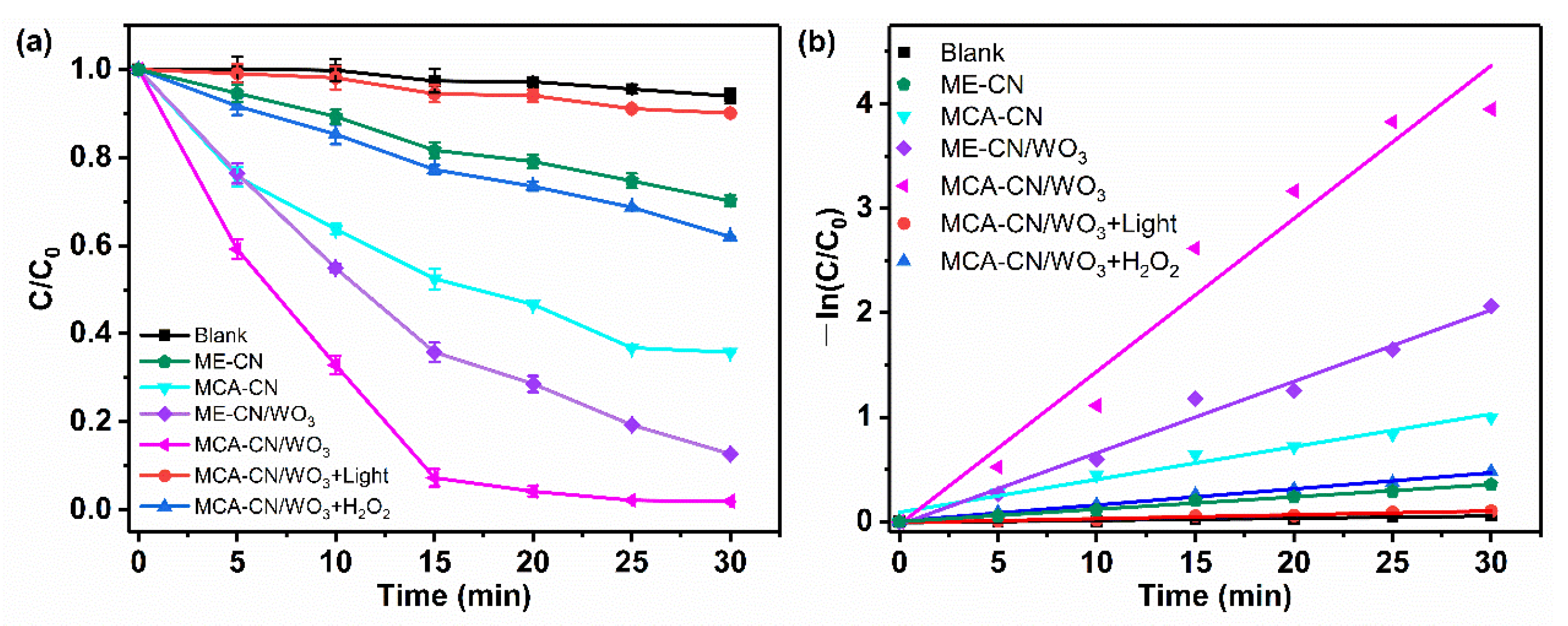
3.3.1. Photo-Fenton Performance of Catalysts
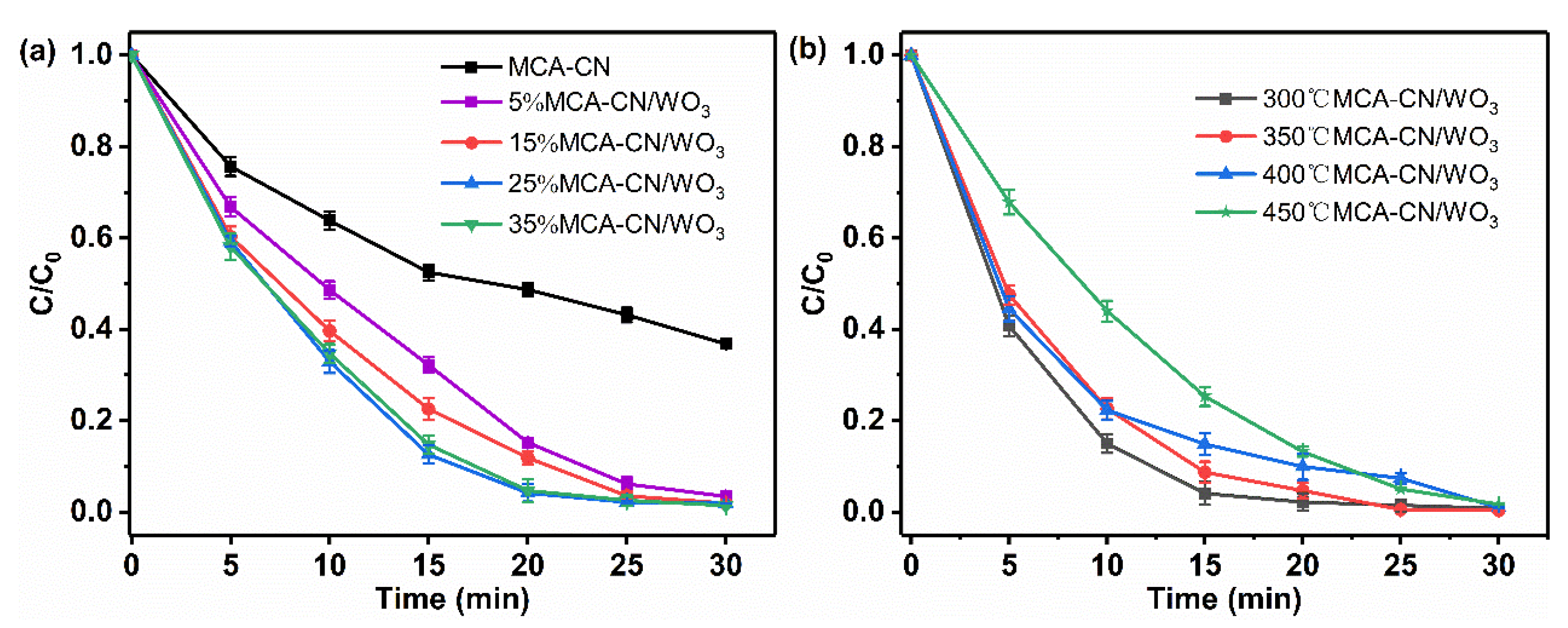
3.3.2. Performance Optimization of MCA-CN/WO3
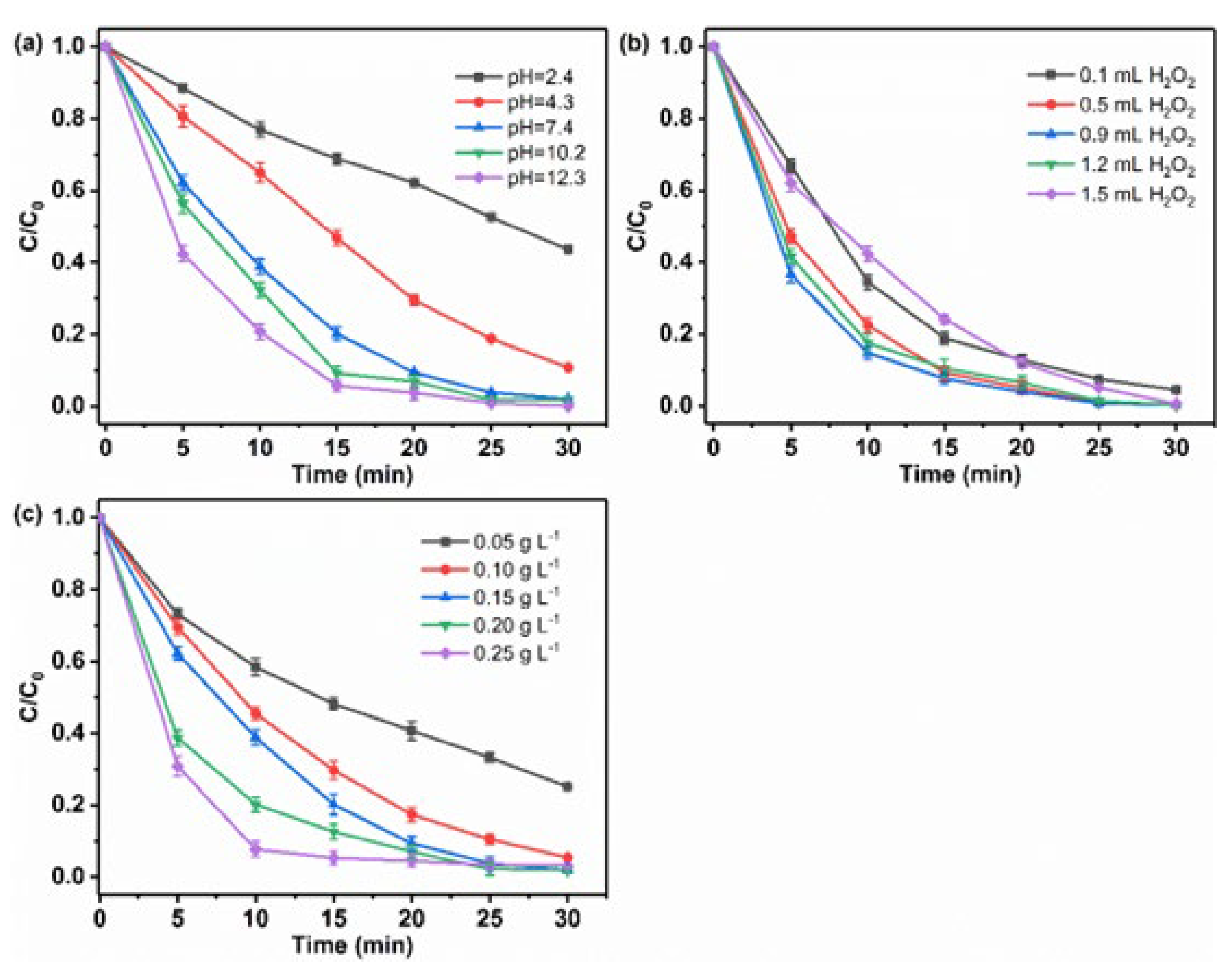
3.3.3. The Effect of Experimental Parameters
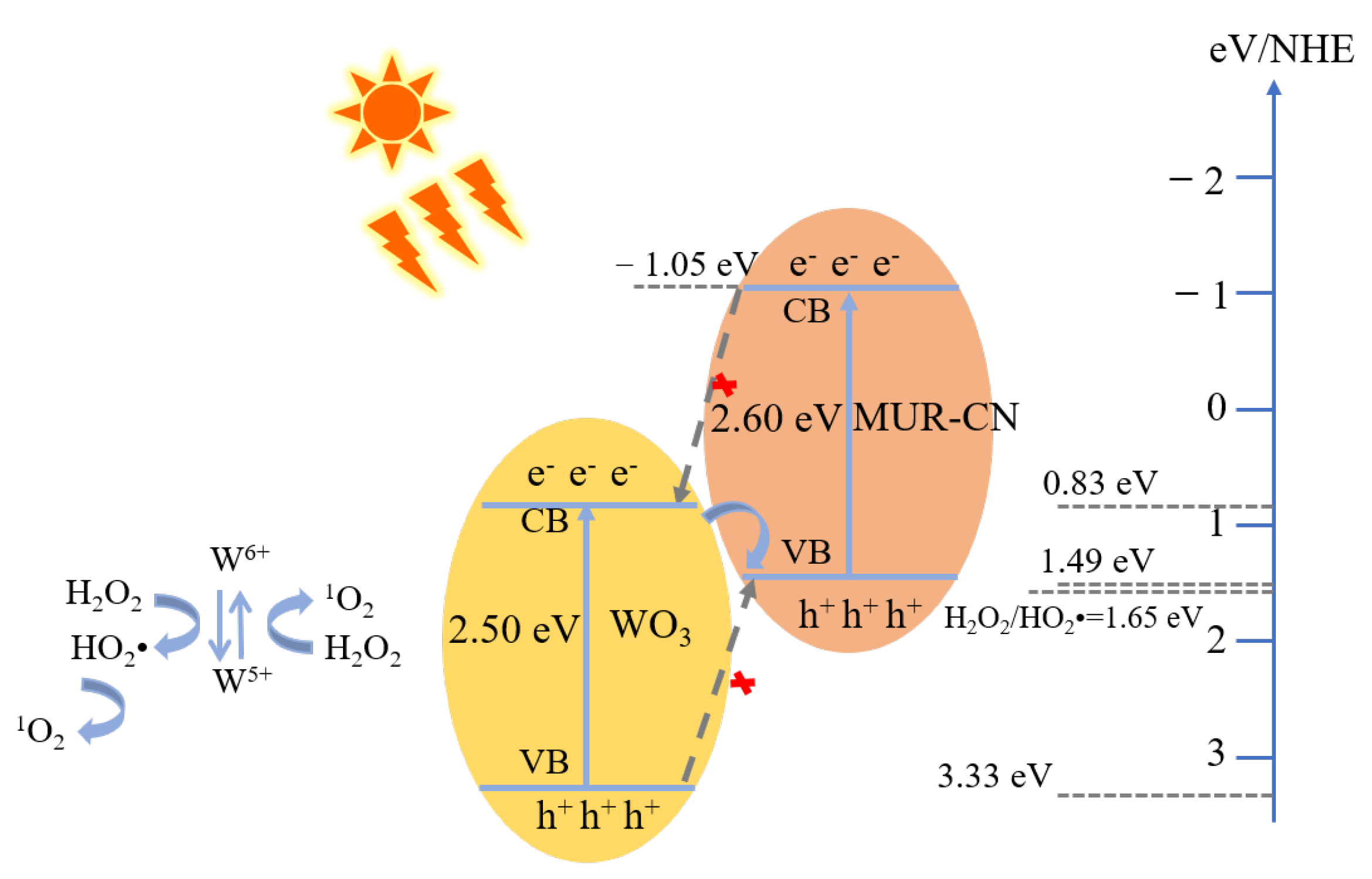
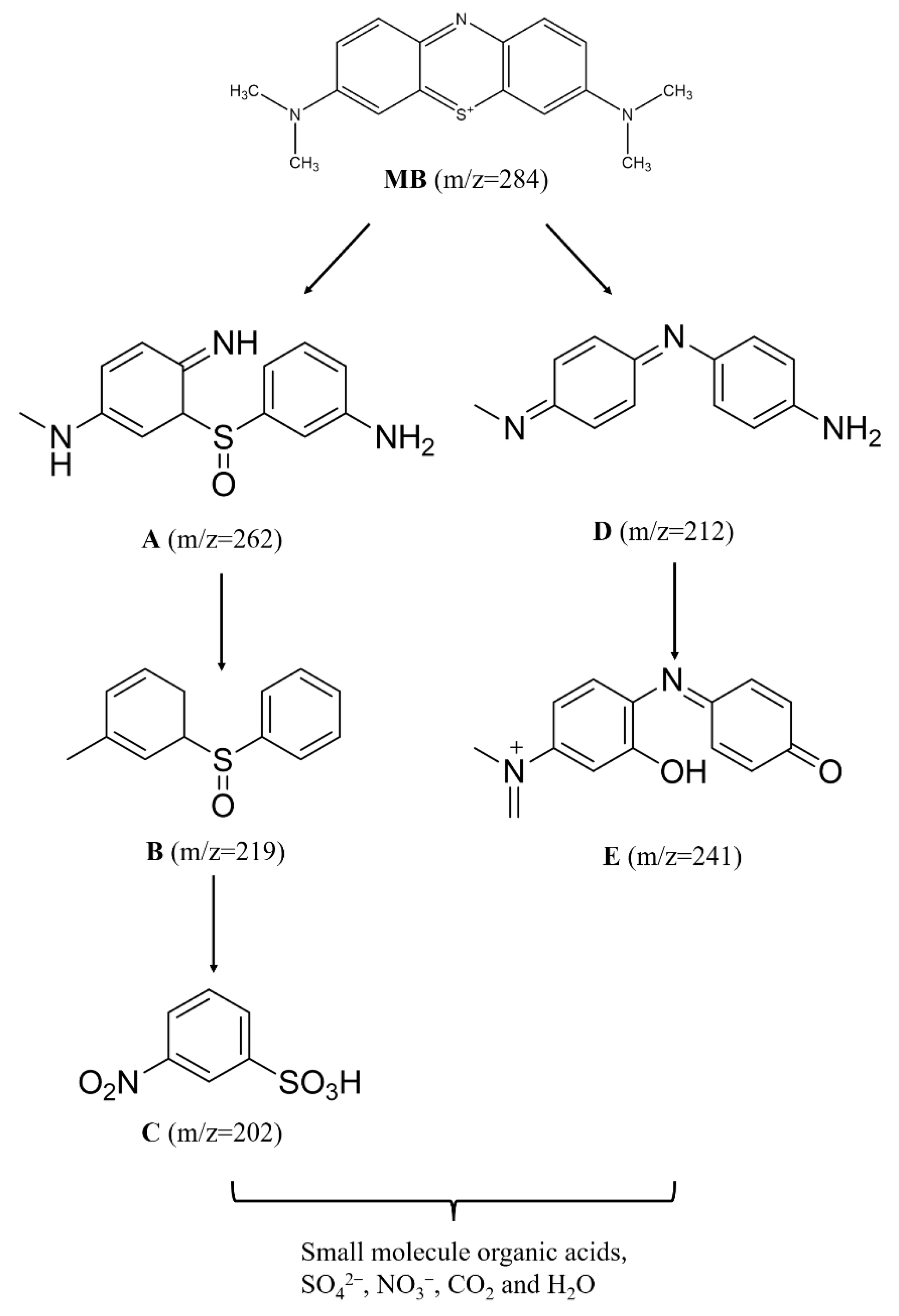
3.4. Degradation Mechanism
3.5. Stability of MCA-CN/WO3 Catalyst
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, X.N.; Ao, Z.M.; Liu, J.Y.; Sun, H.Q.; Rykov, A.I.; Wang, J.H. Topotactic transformation of metal–organic frameworks to graphene-encapsulated transition-metal nitrides as efficient Fenton-like catalysts. ACS Nano 2016, 10, 11532–11540. [Google Scholar] [CrossRef]
- Wols, B.A.; Hofman-Caris, C.H. Review of photochemical reaction constants of organic micropollutants required for UV advanced oxidation processes in water. Water Res. 2012, 46, 2815–2827. [Google Scholar] [CrossRef]
- Zhu, J.N.; Zhu, X.Q.; Cheng, F.F.; Li, P.; Wang, F.; Xiao, Y.W.; Xiong, W.W. Preparing copper doped carbon nitride from melamine templated crystalline copper chloride for Fenton-like catalysis. Appl. Catal. B Environ. 2019, 256, 117830. [Google Scholar] [CrossRef]
- Lim, H.; Lee, J.; Jin, S.; Kim, J.; Yoon, J.; Hyeon, T. Highly active heterogeneous Fenton catalyst using iron oxide nanoparticles immobilized in alumina coated mesoporous silica. Chem. Commun. 2006, 4, 463–465. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.L.; Zhang, J.; Wang, Y.; Chen, Q.; Feng, Z.M.; Sun, T. Concerted catalytic and photocatalytic degradation of organic pollutants over CuS/g-C3N4 catalysts under light and dark conditions. J. Adv. Res. 2019, 16, 135–143. [Google Scholar] [CrossRef]
- Hu, P.D.; Long, M. Cobalt-catalyzed sulfate radical-based advanced oxidation: A review on heterogeneous catalysts and applications. Appl. Catal. B Environ. 2016, 181, 103–117. [Google Scholar] [CrossRef]
- Barrio, J.; Lin, L.; Amo-Ochoa, P.; Tzadikov, J.; Peng, G.; Sun, J.; Shalom, M. Unprecedented centimeter-long carbon nitride needles: Synthesis, characterization and applications. Small 2018, 14, e1800633. [Google Scholar] [CrossRef]
- Sun, S.D.; Liang, S.H. Recent advances in functional mesoporous graphitic carbon nitride (mpg-C3N4) polymers. Nanoscale 2017, 9, 10544–10578. [Google Scholar] [CrossRef]
- Xu, R.P.; Li, J.; Sui, G.Z.; Zhuang, Y.; Guo, D.X.; Luo, Z.; Chen, S. Constructing supramolecular self-assembled porous g-C3N4 nanosheets containing thiophene-groups for excellent photocatalytic performance under visible light. Appl. Surf. Sci. 2022, 578, 152064. [Google Scholar] [CrossRef]
- Fattahimoghaddam, H.; Mahvelati-Shamsabadi, T.; Lee, B.K. Efficient photodegradation of Rhodamine B and tetracycline over robust and green g-C3N4 nanostructures supramolecular design. J. Hazard. Mater. 2021, 403, 123703. [Google Scholar] [CrossRef]
- Zhang, K.; Jin, Y.R.; Guo, Y.X.; Wang, H.W.; Liu, K.F.; Fu, W.J.; Wang, B. Study on Microstructure and Photocatalytic Mechanism of g−C3N4/WO3 Heterojunctions Prepared by Ice Template. Chem. Sel. 2021, 6, 5719–5728. [Google Scholar] [CrossRef]
- Meng, J.Q.; Wang, X.Y.; Liu, Y.Q.; Ren, M.; Zhang, X.Y.; Ding, X.H.; Yang, Y. Acid-induced molecule self-assembly synthesis of Z-scheme WO3/g-C3N4 heterojunctions for robust photocatalysis against phenolic pollutants. Chem. Eng. J. 2021, 403, 126354. [Google Scholar] [CrossRef]
- Li, X.; Song, X.H.; Ma, C.C.; Cheng, Y.M.; Shen, D.; Zhang, S.M.; Wang, H. Direct Z-Scheme WO3/graphitic carbon nitride nanocomposites for the photoreduction of CO2. ACS Appl. Nano Mater. 2020, 3, 1298–1306. [Google Scholar]
- Jun, Y.S.; Lee, E.Z.; Wang, X.; Hong, W.H.; Stucky, G.D.; Thomas, A. From Melamine-Cyanuric acid supramolecular aggregates to carbon nitride hollow spheres. Adv. Funct. Mater. 2013, 23, 3661–3667. [Google Scholar] [CrossRef]
- Song, T.; Xie, C.; Matras-Postolek, K.; Yang, P. 2D layered g-C3N4/WO3/WS2 S-Scheme heterojunctions with enhanced photochemical performance. J. Chem. A 2021, 125, 19382–19393. [Google Scholar] [CrossRef]
- Bai, X.Y.; Li, Y.; Xie, L.B.; Liu, X.H.; Zhan, S.H.; Hu, W.P. A novel Fe-free photo-electro-Fenton-like system for enhanced ciprofloxacin degradation: Bifunctional Z-scheme WO3/g-C3N4. Environ. Sci. Nano 2019, 6, 2850–2862. [Google Scholar] [CrossRef]
- Singh, J.; Arora, A.; Basu, S. Synthesis of coral like WO3/g-C3N4 nanocomposites for the removal of hazardous dyes under visible light. J. Alloys Compd. 2019, 808, 151734. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef]
- Yang, S.B.; Gong, Y.J.; Zhang, J.L.; Zhan, L.; Ma, L.S.; Fang, Z.Y.; Ajayan, P.M. Exfoliated graphitic carbon nitride nanosheets as efficient catalysts for hydrogen evolution under visible light. Adv. Mater. 2013, 25, 2452–2456. [Google Scholar] [CrossRef]
- Xi, J.H.; Xia, H.; Ning, X.M.; Zhang, Z.; Liu, J.; Mu, Z.J.; Lu, X. Carbon-Intercalated 0D/2D hybrid of hematite quantum dots/graphitic carbon nitride nanosheets as superior catalyst for advanced oxidation. Small 2019, 15, e1902744. [Google Scholar] [CrossRef]
- Liang, Q.H.; Li, Z.; Yu, X.L.; Huang, Z.H.; Kang, F.Y.; Yang, Q.H. Macroscopic 3D porous graphitic carbon nitride monolith for enhanced photocatalytic hydrogen evolution. Adv. Mater. 2015, 27, 4634–4639. [Google Scholar] [CrossRef]
- Zhu, W.Y.; Sun, F.Q.; Goei, R.; Zhou, Y. Construction of WO3–g-C3N4 composites as efficient photocatalysts for pharmaceutical degradation under visible light. Catal. Sci. Technol. 2017, 7, 2591–2600. [Google Scholar] [CrossRef]
- Ding, J.; Liu, Q.Q.; Zhang, Z.Y.; Liu, X.; Zhao, J.Q.; Cheng, S.B.; Dai, W.L. Carbon nitride nanosheets decorated with WO3 nanorods: Ultrasonic-assisted facile synthesis and catalytic application in the green manufacture of dialdehydes. Appl. Catal. B Environ. 2015, 165, 511–518. [Google Scholar] [CrossRef]
- Song, T.; Zhang, X.; Yang, P. Bifunctional nitrogen-doped carbon dots in g-C3N4/WOx heterojunction for enhanced photocatalytic water-splitting performance. Langmuir 2021, 37, 4236–4247. [Google Scholar] [CrossRef]
- Zeng, Y.X.; Liu, C.B.; Wang, L.L.; Zhang, S.Q.; Ding, Y.B.; Xu, Y.Z.; Luo, S. A three-dimensional graphitic carbon nitride belt network for enhanced visible light photocatalytic hydrogen evolution. J. Mater. Chem. 2016, 4, 19003–19010. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, S.A.; Wang, J.M.; Peng, T.; Li, R.J. Direct Z-Scheme 2D/2D photocatalyst based on ultrathin g-C3N4 and WO3 nanosheets for efficient visible-light-driven H2 generation. ACS Appl. Mater. Interfaces 2019, 11, 27913–27923. [Google Scholar] [CrossRef]
- Li, X.; Kang, B.B.; Dong, F.; Zhang, Z.Q.; Luo, X.D.; Han, L.; Wang, Z.L. Enhanced photocatalytic degradation and H2/H2O2 production performance of S-pCN/WO2.72 S-scheme heterojunction with appropriate surface oxygen vacancies. Nano Energy 2021, 81, 105671. [Google Scholar] [CrossRef]
- Wang, J.J.; Wang, Z.Y.; Liu, C.J.J. Enhanced activity for CO oxidation over WO3 nanolamella supported Pt catalyst. ACS Appl. Mater. Interfaces 2014, 6, 12860–12867. [Google Scholar] [CrossRef]
- Chen, X.K.; Li, H.; Wu, Y.S.; Wu, H.; Wu, L.F.; Tan, P.; Xiong, X. Facile fabrication of novel porous graphitic carbon nitride/copper sulfide nanocomposites with enhanced visible light driven photocatalytic performance. J. Colloid. Interf. Sci. 2016, 476, 132–143. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, Y.; Yaakob, Z.; Akhtar, P. Degradation and mineralization of methylene blue using a heterogeneous photo-Fenton catalyst under visible and solar light irradiation. Catal Sci. Technol. 2016, 6, 1222–1232. [Google Scholar] [CrossRef]
- Muruganandham, M.; Swaminathan, M. Photocatalytic decolourisation and degradation of Reactive Orange 4 by TiO-UV process. Dye. Pigment. 2006, 68, 133–142. [Google Scholar] [CrossRef]
- Yi, Q.Y.; Ji, J.; Shen, B.; Dong, C.C.; Liu, J.; Zhang, J.L.; Xing, M. Singlet oxygen triggered by superoxide radicals in a molybdenum cocatalytic Fenton reaction with enhanced redox activity in the environment. Environ. Sci Technol. 2019, 53, 9725–9733. [Google Scholar] [CrossRef]
- Zhao, Y.M.; Sun, M.; Wang, X.X.; Wang, C.; Lu, D.W.; Ma, W.; Elimelech, M. Janus electrocatalytic flow-through membrane enables highly selective singlet oxygen production. Nat. Commun. 2020, 11, 6228. [Google Scholar] [CrossRef]
- Yang, Z.C.; Qian, J.S.; Yu, A.Q.; Pan, B.C. Singlet oxygen mediated iron-based Fenton-like catalysis under nanoconfinement. Proc. Natl. Acad. Sci. USA 2019, 116, 6659–6664. [Google Scholar] [CrossRef] [Green Version]
- Pignatello, J.J.; Oliveros, E.; MacKay, A. Advanced oxidation processes for organic contaminant destruction based on the fenton reaction and related chemistry. Crit. Rev. Environ. Sci. Technol. 2006, 36, 1–84. [Google Scholar] [CrossRef]
- Laura, A.; MacManus-Spencer, K.M. Quantification of singlet oxygen production in the reaction of superoxide with hydrogen peroxide using a selective chemiluminescent. Probe 2005, 127, 8954–8955. [Google Scholar] [CrossRef]
- Zheng, N.C.; He, X.; Hu, R.T.; Wang, R.L.; Zhou, Q.; Lian, Y.K.; Hu, Z. In-situ production of singlet oxygen by dioxygen activation on iron phosphide for advanced oxidation processes. Appl. Catal. B Environ. 2022, 307, 121157. [Google Scholar] [CrossRef]
- Jia, J.K.; Jiang, C.Y.; Zhang, X.R.; Li, P.J.; Xiong, J.X.; Zhang, Z.Z.; Wang, Y. Urea-modified carbon quantum dots as electron mediator decorated g-C3N4/WO3 with enhanced visible-light photocatalytic activity and mechanism insight. Appl. Surf. Sci. 2019, 495, 143524. [Google Scholar] [CrossRef]
- Jain, B.; Singh, A.K.; Kim, H.; Lichtfouse, E.; Sharma, V.K. Treatment of organic pollutants by homogeneous and heterogeneous Fenton reaction processes. Environ. Chem. Lett. 2018, 16, 947–967. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.Y.; Chen, Y.; Peng, Q.S.; Wang, Q.N.; Zhao, G.H. Catalytic activity of MOF(2Fe/Co)/carbon aerogel for improving H2O2 and OH generation in solar photo–electro–Fenton process. Appl. Catal. B Environ. 2017, 203, 127–137. [Google Scholar] [CrossRef]
- Yin, Y.; Ren, Y.; Lu, J.H.; Zhang, W.M.; Shan, C.; Hua, M.; Pan, B. The nature and catalytic reactivity of UiO-66 supported Fe3O4 nanoparticles provide new insights into Fe-Zr dual active centers in Fenton-like reactions. Appl. Catal. B Environ. 2021, 286, 119943. [Google Scholar] [CrossRef]
- Zhou, S.Q.; Wang, Y.; Zhou, K.; Ba, D.Y.; Ao, Y.H.; Wang, P.F. In-situ construction of Z-scheme g-C3N4/WO3 composite with enhanced visible-light responsive performance for nitenpyram degradation. Chin. Chem. Lett. 2021, 32, 2179–2182. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, Y.; Yang, D.; Zhao, L.; Ding, H.; Wang, Z. The mixed marriage of copper and carbon ring-g-C3N4 nanosheet: A visible-light-driven heterogeneous Fenton-like catalyst. Appl. Surf. Sci. 2019, 488, 728–738. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, G.; Ji, Q.H.; Lan, H.C.; Qu, J.H.; Liu, H.J. Carbon nanodot-modified FeOCl for photo-assisted Fenton reaction featuring synergistic in-situ H2O2 production and activation. Appl. Catal. B 2020, 266, 118665. [Google Scholar] [CrossRef]
- An, S.F.; Zhang, G.H.; Wang, T.W.; Zhang, W.N.; Li, K.; Song, C.S. nHigh-Density Ultra-small Clusters and Single-Atom Fe Sites Embedded in Graphitic Carbon Nitride (g-C3N4) for Highly Efficient Catalytic Advanced Oxidation Processes. ACS Nano 2018, 12, 9441–9450. [Google Scholar] [CrossRef]
- Li, X.; Pi, Y.H.; Wu, L.Q.; Xia, Q.B.; Wu, J.L.; Li, Z. Facilitation of the visible light-induced Fenton-like excitation of H2O2 via heterojunction of g-C3N4/NH2-Iron terephthalate metal-organic framework for MB degradation. Appl Catal. B 2017, 202, 653–663. [Google Scholar] [CrossRef]

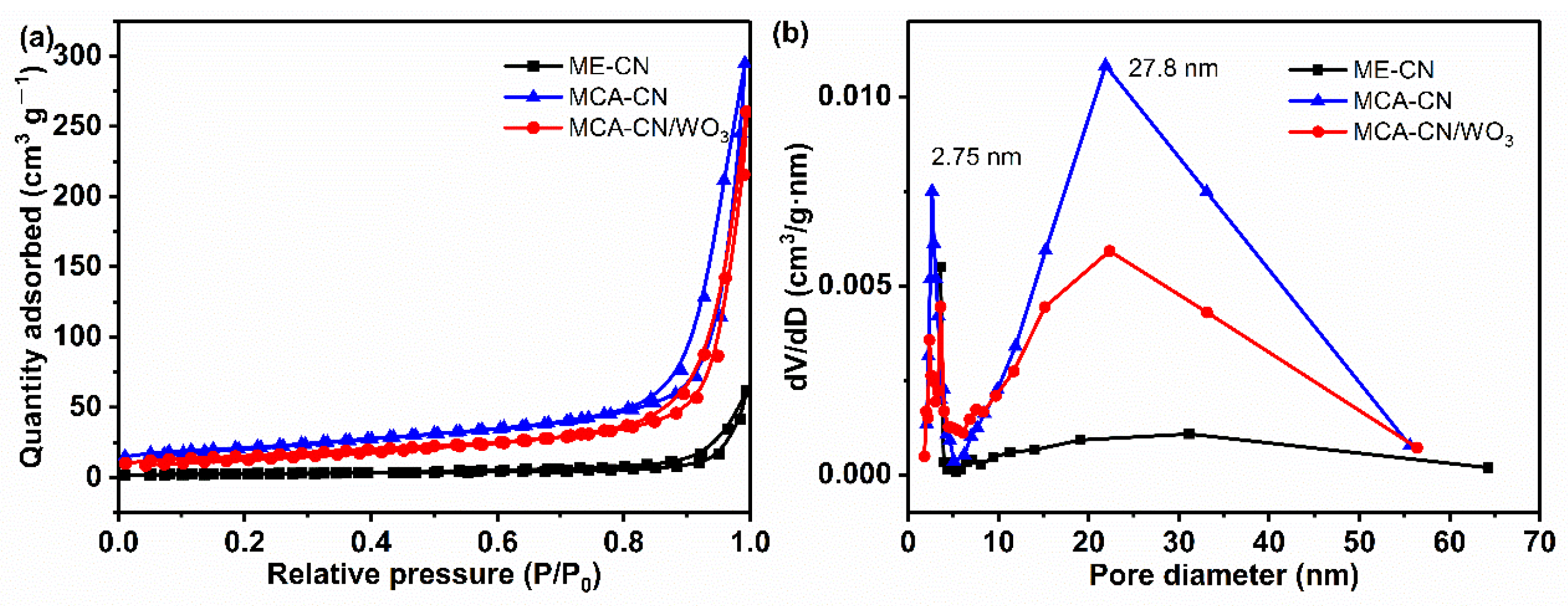


| Samples | Surface Area (m2 g−1) | Pore Volume (cm3 g−1) | Pore Size (nm) |
|---|---|---|---|
| ME-CN | 10.43 | 0.025 | 28.66 |
| MCA-CN | 74.44 | 0.168 | 24.36 |
| MCA-CN/WO3 | 53.84 | 0.135 | 27.50 |
| Samples | Eg (eV) | EVB (eV) | ECB (eV) |
|---|---|---|---|
| ME-CN | 2.62 | 1.53 | −1.09 |
| MCA-CN | 2.54 | 1.49 | −1.05 |
| WO3 | 2.50 | 3.33 | 0.83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, W.; Zhang, G.; Zhang, X.; Zhou, S.; Wang, Z. Degradation of Methylene Blue in the Photo-Fenton-Like Process with WO3-Loaded Porous Carbon Nitride Nanosheet Catalyst. Water 2022, 14, 2569. https://doi.org/10.3390/w14162569
Gao W, Zhang G, Zhang X, Zhou S, Wang Z. Degradation of Methylene Blue in the Photo-Fenton-Like Process with WO3-Loaded Porous Carbon Nitride Nanosheet Catalyst. Water. 2022; 14(16):2569. https://doi.org/10.3390/w14162569
Chicago/Turabian StyleGao, Weifan, Guichang Zhang, Xiaoping Zhang, Shaoqi Zhou, and Zihao Wang. 2022. "Degradation of Methylene Blue in the Photo-Fenton-Like Process with WO3-Loaded Porous Carbon Nitride Nanosheet Catalyst" Water 14, no. 16: 2569. https://doi.org/10.3390/w14162569
APA StyleGao, W., Zhang, G., Zhang, X., Zhou, S., & Wang, Z. (2022). Degradation of Methylene Blue in the Photo-Fenton-Like Process with WO3-Loaded Porous Carbon Nitride Nanosheet Catalyst. Water, 14(16), 2569. https://doi.org/10.3390/w14162569





