Silver Doped Zinc Stannate (Ag-ZnSnO3) for the Photocatalytic Degradation of Caffeine under UV Irradiation
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Photocatalyst Sample Preparation
2.3. Instruments
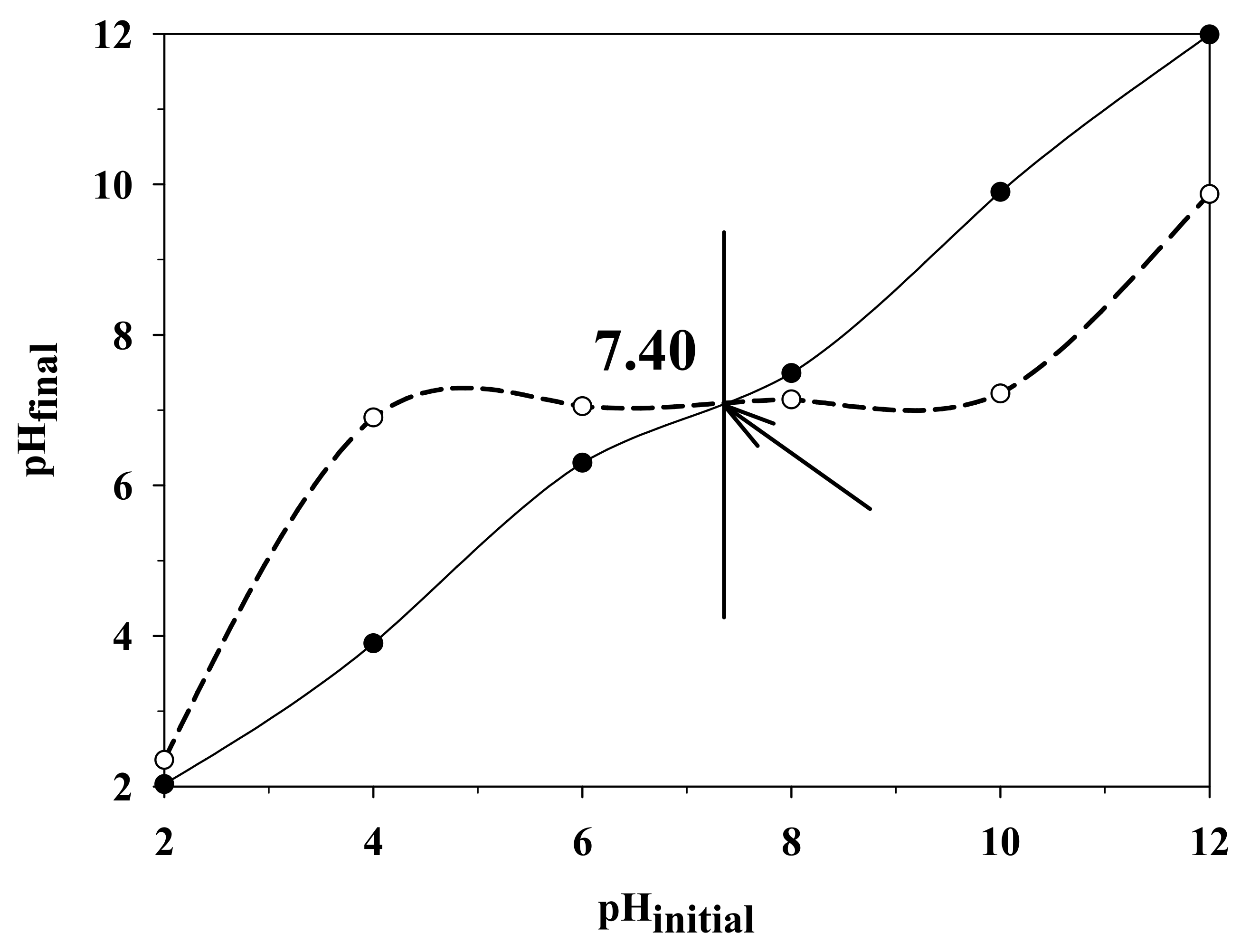
2.4. Determination of pHpzc of Ag-ZnSnO3 Catalyst Material
2.5. Photocatalytic Measurement
3. Results and Discussion
3.1. Material Sample Charcaterizations
3.2. Photocatalytic Activity
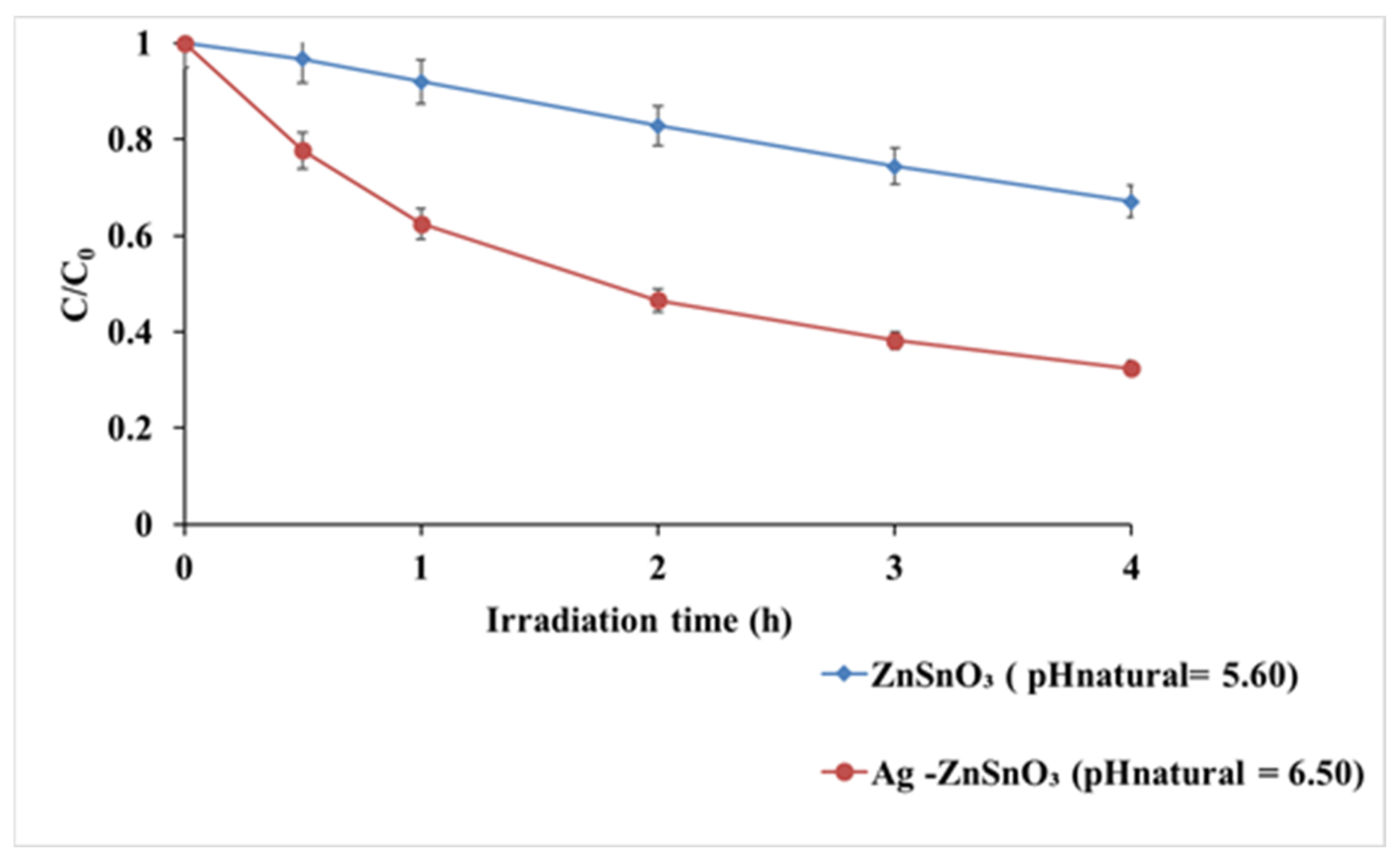
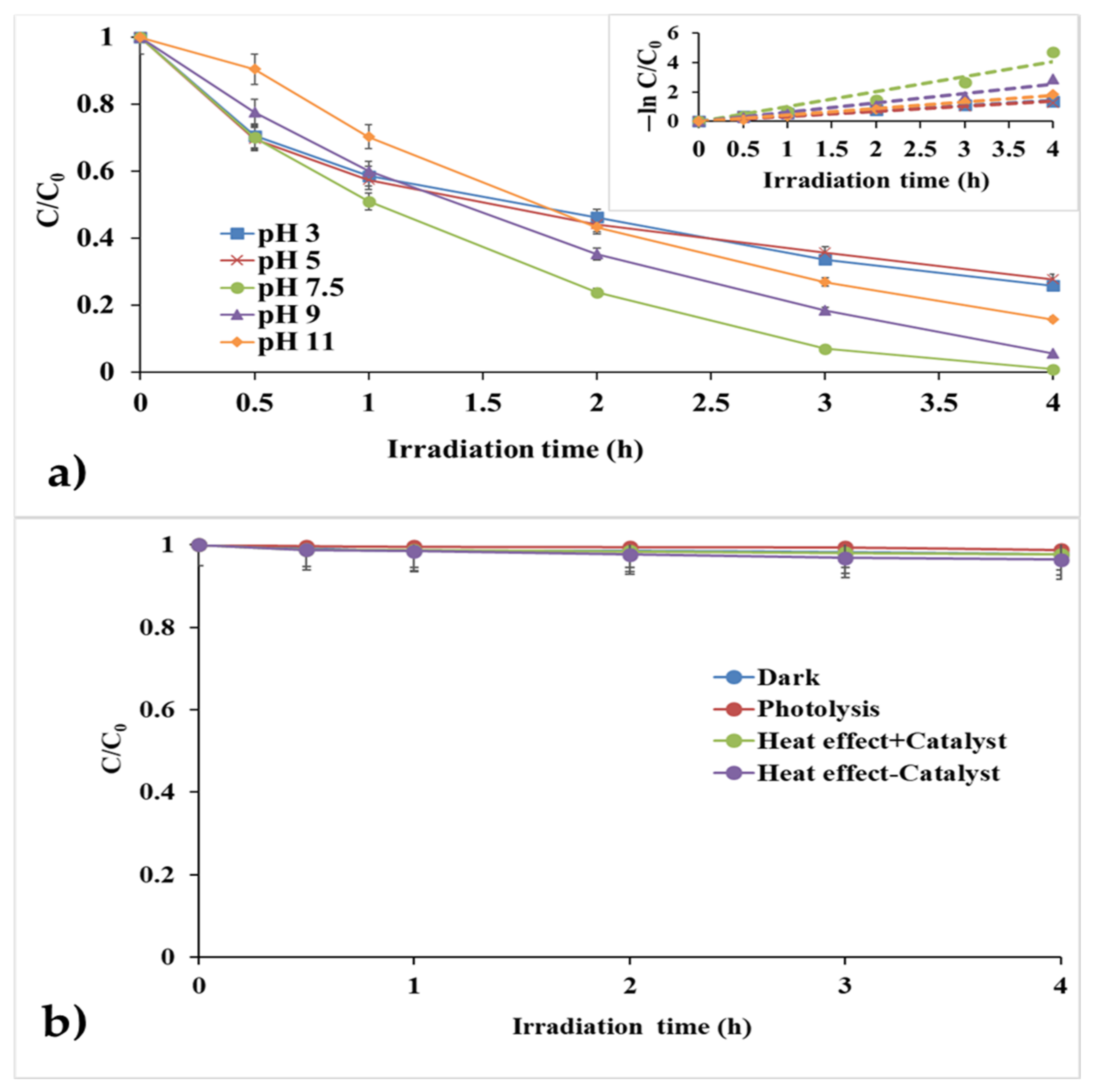
3.2.1. Effect of pH
- pH Point of Zero Charge (pHpzc).
3.2.2. Effect of Catalyst Dosage and Initial Caffeine Concentration
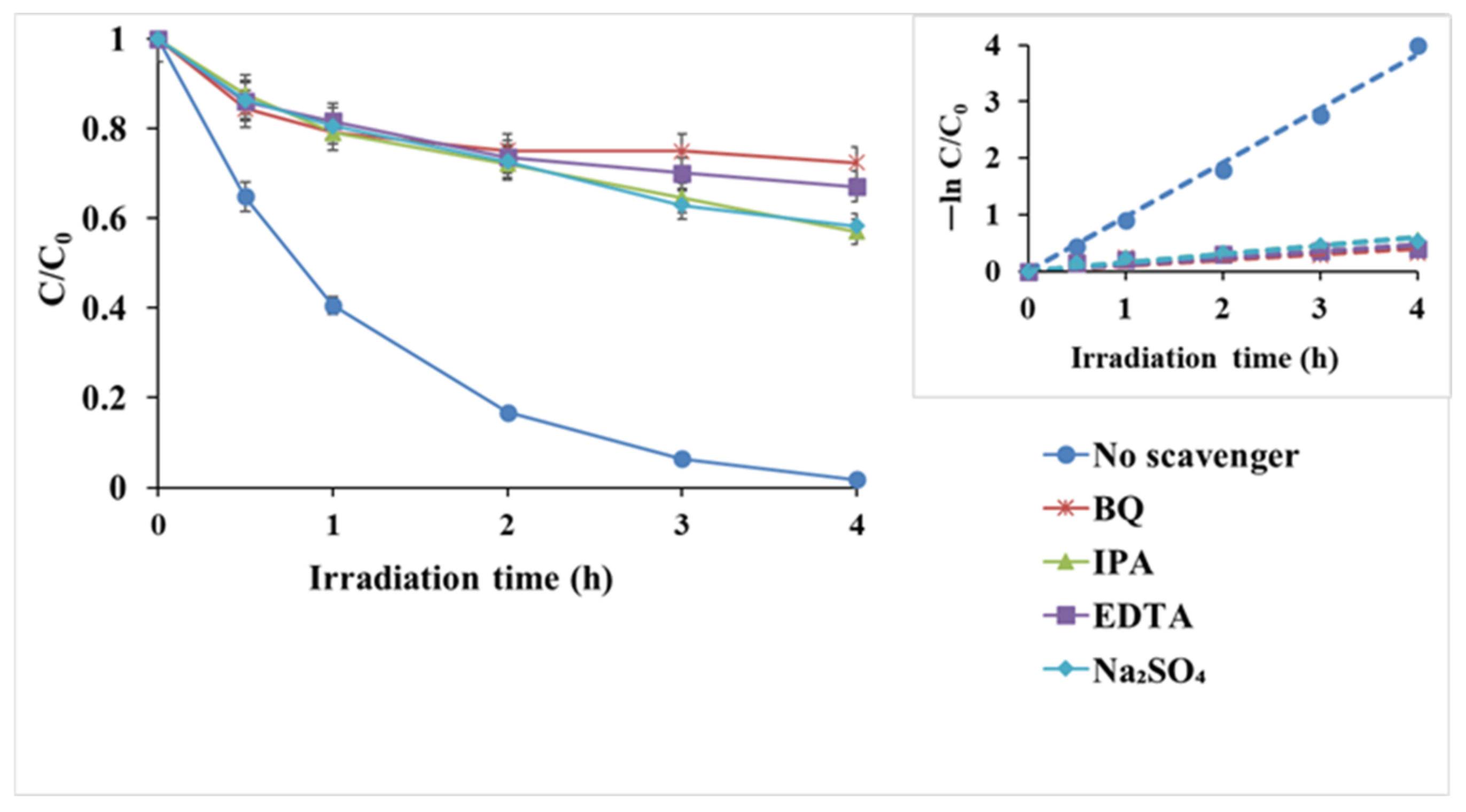
3.2.3. Reactive Oxygen Species (ROS) Probe
3.2.4. Hydrogen Peroxide (H2O2) Effect
3.2.5. Effects of Contaminant Ions (Anions) and Humic Acid (HA)
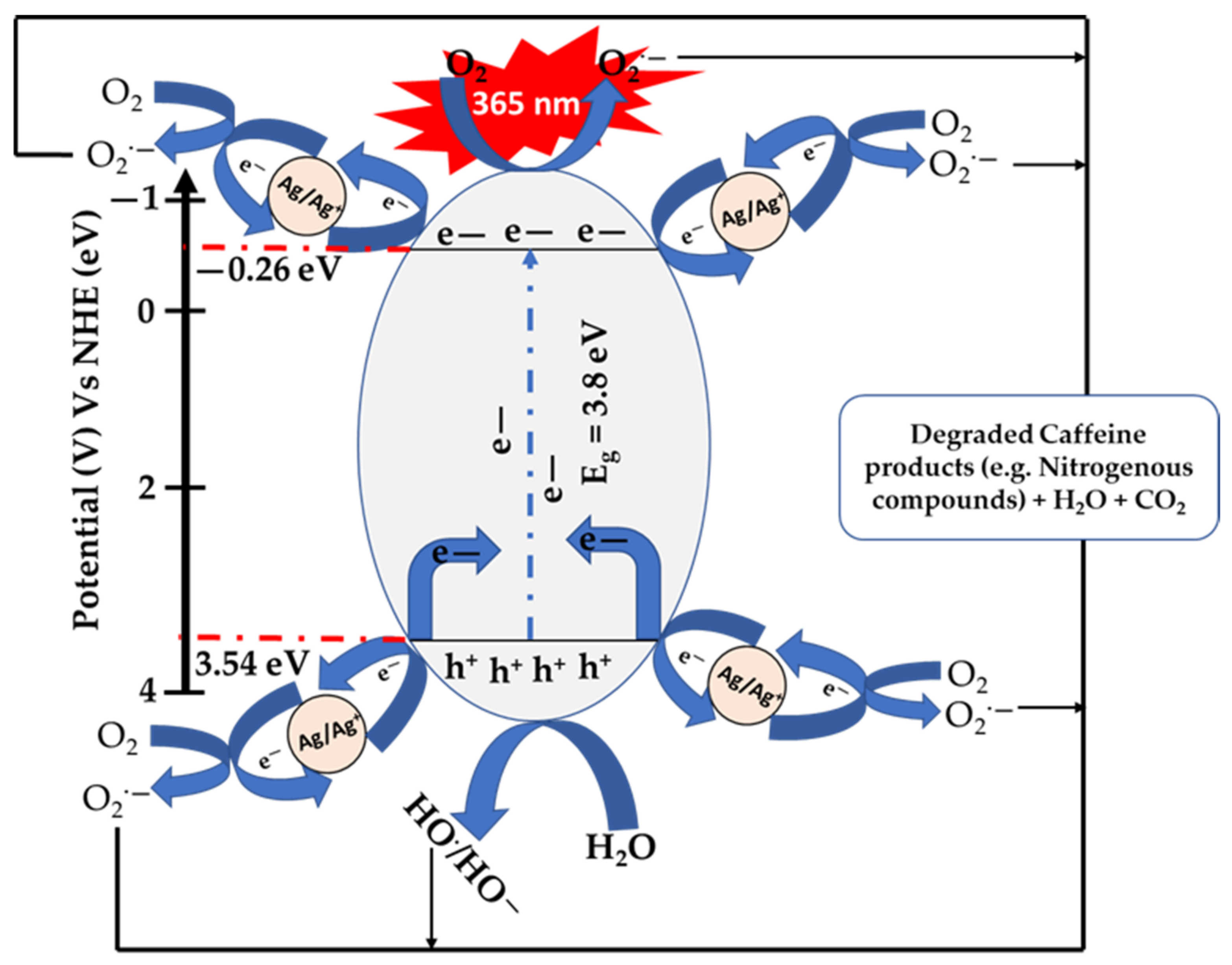
3.3. Proposed Reaction Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lawrence, J.R.; Swerhone, G.D.W.; Wassenaar, L.I.; Neu, T.R. Effects of Selected Pharmaceuticals on Riverine Biofilm Communities. Can. J. Microbiol. 2005, 51, 655–669. [Google Scholar] [CrossRef] [PubMed]
- Kummerer, K. The Presence of Pharmaceuticals in The Environment Due to Human Use—Present Knowledge and Future Challenges. J. Environ. Manag. 2009, 90, 2354–2366. [Google Scholar] [CrossRef] [PubMed]
- Himenez-Holgado, C.; Chrimatopoulos, C.; Stathopoulos, V.; Sakkas, V. Extraction and HPLC-UV-Vis/DAD to Determine Antidepressant Drugs in Environmental Aqueous Samples. Separations 2020, 7, 39. [Google Scholar]
- Anucha, C.B.; Altin, I.; Biyiklioglu, Z.; Bacaksiz, E.; Polat, I.; Stathopoulos, V.N. Synthesis, Characterization, and Photocatalytic Evaluation of Manganese (III) Phythalocyanine Sensitized ZnWO4 (ZnWO4MnPc) for Bisphenol A Degradation Under UV Irradiation. Nanomaterials 2020, 10, 2139. [Google Scholar] [CrossRef]
- Anucha, C.B.; Altin, I.; Fabbri, D.; Degirmencioglu, I.; Calza, P.; Magnacca, G.; Stathopoulos, V.N.; Bacaksiz, E. Synthesis and Characterization of B/NaF and Silicon Phthalocyanine-Modified TiO2 and Evaluation of Their Photocatalytic Removal of Carbamazepine. Separations 2020, 7, 71. [Google Scholar] [CrossRef]
- Anucha, C.B.; Altin, I.; Bacaksiz, E.; Degirmencioglu, I.; Kucukomeroglu, T.; Yilmaz, S.; Stathopoulos, V.N. Immobilized TiO2/ZnO Sensitized Copper (II) Phthalocyanine Heterostructure for The Degradation of Ibuprofen Under UV Irradiation. Separations 2021, 8, 24. [Google Scholar]
- Anucha, C.B.; Altin, I.; Bacaksiz, E.; Kucukomeroglu, T.; Belay, M.H.; Stathopoulos, V.N. Enhanced Photocatalytic Activity of CuWO4 Doped TiO2 Photocatalyst Towards Carbamazepine Under UV Irradiation. Separations 2021, 8, 25. [Google Scholar] [CrossRef]
- Seiler, R.L.; Zaugg, S.D.; Thomas, J.M.; Howcroft, D.L. Caffeine and Pharmaceuticals as Indicators of Wastewater Contamination in Wells. Groundwater 1999, 37, 405–410. [Google Scholar] [CrossRef]
- Marques, R.R.N.; Sampaio, M.J.; Carrapico, P.M.; Silva, C.G.; Morales-Torres, S.; Drazic, G.; Faria, J.L.; Silva, A.M.T. Photocatalytic Degradation of Caffeine: Developing Solutions for Emerging Pollutants. Catal. Today 2013, 209, 108–115. [Google Scholar] [CrossRef]
- Yamal-Turbay, E.; Graells, M.; Perez-Moya, M. Systematic Assessment of The Influence of Hydrogen peroxide Dosage on Caffeine Degradation by The FotoPhenton Process. Ind. Eng. Chem. Res. 2012, 51, 4770–4778. [Google Scholar] [CrossRef]
- Aly, A.A. Determination of Caffeine in Roasted and Irradiated Coffee Beans with Gamma Rays by High Performance Liquid Chromatography. Food Sci. Qual. Manag. 2013, 22, 28–34. [Google Scholar]
- Guo, S.; Zhu, Q.; Yang, B.; Wang, J.; Ye, B. Determination of Caffeine Content in Tea Based on Poly (Safranine T) Electroactive Film Modified Electrode. Food Chem. 2011, 129, 1311–1314. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.P.; Guo, W.; Peng, X.D.; Li, M.; Yuan, Z.B. Sensitive Differential Pulse Sripping Voltammetry of Caffeine in Medicines and Cola Using a Sensor Based on Multi-walled Carbon Nanotubes and Nafion. Int. J. Electrochem. Sci. 2011, 6, 997–1006. [Google Scholar]
- Torres, A.C.; Barsan, M.M.; Brett, C.M.A. Simple Electrochemical Sensor for Caffeine Based on Carbon and Nanofion-Modified Carbon Electrodes. Food Chem. 2014, 149, 215–220. [Google Scholar] [CrossRef]
- Ali, M.M.; Eisa, M.; Taha, M.I.; Abdallah, B.Z.; Elbashir, A. Determination of Caffeine in Some Sudanese Beverages by High Performance Liquid Chromatography. Pak. J. Nutr. 2012, 11, 336–342. [Google Scholar]
- Barcelo, D.; Petrovic, M. Emerging Contaminants from Industrial and Municipal Wastes: Occurrence, Analysis and Effects. The Handbook of Environmental Chemistry; Springer: Berlin, Germany, 2008; Volume 5/S1. [Google Scholar]
- Maroga Mboula, V.; Hequeta, V.; Andres, Y.; Gru, Y.; Colin, R.; Dona-Rodriguez, J.M.; Pastrana-Martinez, L.M.; Silva, A.M.T.; Leleu, M.; Tindall, A.J.; et al. Photocatalytic Degradation of Estradiol Under Simulated Solar Light and Assessment of Estrogenic Activity. Appl. Catal. B 2015, 162, 437–444. [Google Scholar] [CrossRef]
- Moustakas, N.G.; Katsaros, F.K.; Kontos, A.G.; Em, G.; Romanos, D.D.; Falaras, P.; Dionysiou, D.D. Visible Light Active TiO2 Photocatalytic Filtration Membranes with Improved Permeability and Low Energy Consumption. Catal. Today 2014, 224, 56–69. [Google Scholar] [CrossRef]
- Prieto-Rodriguez, L.; Miralles-Cuevas, S.; Oller, I.; Aguera, A.; Li Puma, G.; Malato, S. Treatment of Emerging Contaminants in Wastewater Treatment Plants (WWTP) Effluents by Solar Photocatalysis Using Low TiO2 Concentrations. J. Hazard. Mater. 2012, 211–212, 131–137. [Google Scholar] [CrossRef]
- Souza, B.S.; Dantas, R.F.; Cruz, A.; Sans, C.; Esplugas, S.; Dezzotti, M. Photochemical Oxidation of Municipal Secondary Effluents at Low H2O2 Dosage: Study of Hydroxyl Radical Scavenging and Process Performance. Biochem. Eng. J. 2014, 237, 268–276. [Google Scholar] [CrossRef]
- Stefa, S.; Lykaki, M.; Fragkoulis, D.; Binas, V.; Pandis, P.K.; Stathopoulos, V.N.; Konsolakis, M. Effect of the Preparation Method on the Physicochemical Properties and the CO Oxidation Performance of Nanostructured CeO2/TiO2 Oxides. Processes 2020, 8, 847. [Google Scholar] [CrossRef]
- Stathopoulos, V.N.; Costa, C.N.; Pomonis, J.P.; Efstathiou, A.M. CH4/NO/O2 “Lean-deNOx” Reaction on Mesoporous Mn-Based Mixed Oxides. Top. Catal. 2001, 16, 231–235. [Google Scholar] [CrossRef]
- Lykaki, M.; Stefa, S.; Carabiniero, S.A.S.; Pandis, P.K.; Stathopoulos, V.N.; Konsolakis, M. Facet Dependent Reactivity of Fe2O3/CeO2 Nanocomposites. Catalysts 2019, 9, 371. [Google Scholar] [CrossRef]
- Corberan, V.C.; Rives, V.; Sthatopoulos, V. Recent Applications of Nanometal Oxide Catalyst in Oxidation Reactions. In Advanced Nanomaterials for Catalysis and Energy Synthesis, Characterization and Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 227–293. [Google Scholar]
- Sanchez, C.; Bellevile, P.; Popall, M.; Nicole, L. Applications of Advanced Hybrid Organic-Inorganic Nanomaterials: From Laboratory to Market. Chem. Soc. Rev. 2011, 40, 696–753. [Google Scholar] [CrossRef]
- Yuan, C.; Wu, H.B.; Xie, Y.; Lou, X.W. Mixed Transition-Metal Oxides: Design, Synthesis, and Energy- Related Applications. Angew. Chem. Int. Ed. 2014, 53, 1488–1504. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.L.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’Shea, K. A Review on The Visible Light Active Titanium Dioxide Photocatalysis for Environmental Applications. Appl. Catal. B Eviron. 2012, 125, 331–349. [Google Scholar] [CrossRef]
- Mills, A.; LeHunte, S. An Overview of Semiconductor Photocatalysis. J. Phtochem. Photobiol. A Chem. 1997, 108, 1–35. [Google Scholar] [CrossRef]
- Tong, H.; Ouyang, S.X.; Bi, Y.P.; Umezawa, N.; Oshikiri, M.; Ye, J.H. Nano-Photocatalytic Materials: Possibilities and Challenges. Adv. Mater. 2012, 24, 229–251. [Google Scholar] [CrossRef]
- Ge, J.; Zhang, Y.; Heo, Y.-J.; Park, S.-J. Advanced Design and Synthesis of Composite Photocatalysts for the Remediation of Wastewater: A Review. Catalyst 2019, 9, 122. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, M.; Meng, Y.; Huang, B.; Pu, X.; Shao, X. A Novel Method for the Synthesis Ag3VO4/AgVO2PO4 Photocatalyst with Improved Visible-Light Photocatalytic Properties. Sep. Purif. Technol. 2018, 206, 149–157. [Google Scholar] [CrossRef]
- Liang, S.; He, M.; Guo, J.; Yue, J.; Pu, X.; Ge, B.; Li, W. Fabrication and Characterization of BiOBr:Yb3+, Er3+/g-C3N4 P-N Junction Photocatalyst with Enhanced Visible-NIR-Light Driven Photoactivities. Sep. Purif. Technol. 2018, 206, 69–79. [Google Scholar] [CrossRef]
- Shao, Z.; Zeng, T.; He, Y.; Zhang, D.; Pu, X. A Novel Magnetically Separable CoFe2O4/Cd0.9Zn0.1S Photocatalyst with Remarkably Enhanced H2 Evolution Activity Under Visible Light Irradiation. Chem. Eng. J. 2019, 359, 485–495. [Google Scholar] [CrossRef]
- Ma, L.; Ma, S.Y.; Shen, X.F.; Wang, T.T.; Jiang, X.H.; Chen, Q.; Qiang, Z.; Yang, H.M.; Chen, H. PrFeO3 Hollow Nanofibers as a Highly Efficient Gas Sensor for Acetone Detection. Sens. Actuators B Chem. 2018, 253, 2546–2554. [Google Scholar] [CrossRef]
- Shi, W.L.; Li, M.Y.; Ren, H.J.; Guo, F.; Huang, X.L.; Shi, Y.; Tang, Y.B. Construction of a 0D/1D Composite Based on Au Nanoparticles/CuBi2O4 Microrods for Efficient -Light- Driven Photocatalytic Activity. Beilstein J. Nanotechnol. 2019, 10, 1360–1367. [Google Scholar] [CrossRef]
- Guo, F.; Li, M.; Ren, H.; Huang, X.; Hou, W.; Wang, C.; Shi, W.; Lu, C. Fabrication of P-N CuBi2O4/MoS2 Heterojunction with Nanosheets-on- Microrods Structure for Enhanced Photocatalytic Activity Towards Tetracycline Degradation. Appl. Surf. Sci. 2019, 491, 88–94. [Google Scholar] [CrossRef]
- Tian, Z.; Liang, C.; Liu, J.; Zhang, H.; Zhang, L. Zinc Stannate Nanocubes and Nanourchins with High Photocatalytic Activity for Methyl Orange and 2,5-DCP Degradation. J. Mater. Chem. 2012, 22, 17210. [Google Scholar] [CrossRef]
- Xue, X.Y.; Guo, T.L.; Lin, X.L.; Wang, T.H. Individual Core-Shell Structured ZnSnO3 Nanowires as Photoconductors. Mater. Lett. 2008, 62, 1356–1358. [Google Scholar] [CrossRef]
- Luo, P.; Zhang, H.J.; Liu, L.; Fang, L.; Wang, Y. Sandwich-Like Nanostructure of Amorphous ZnSnO3 Encapsulated in Carbon Nanosheets for Enhanced Lithium Storage. Electrochim. Acta 2016, 219, 734–741. [Google Scholar] [CrossRef]
- Wang, L.; Xiao, L.; Qingqing, L.; Zhao, Y.; Che, R. Enhanced Polarization from Hollow Cube-Like ZnSnO3 Wrapped by Multiwalled Carbon Nanotubes: As a Lightweight and High-Performance Microwave Absorber. ACS Appl. Mater. Int. 2018, 10, 22602–22610. [Google Scholar] [CrossRef]
- Zhang, L.S.; Wang, W.Z.; Zhou, L.; Xu, H.L. Bi2WO6 Nano- and microstructures: Shape control and Associated Visible-Light-Driven Photocatalytic Activities. Small 2007, 3, 1618–1625. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, Y.F. Synthesis of Square Bi2WO6 Nanoplates as High -Activity Visible-Light Driven Photocatalysis. Chem. Mater. 2005, 17, 3537–3545. [Google Scholar] [CrossRef]
- Li, X.Y.; Hou, Y.; Zhao, Q.D.; Teng, W.; Hu, X.J.; Chen, G.H. Capability of Novel ZnFe2O4 Nanotube Arrays for Simulated-Sunlight Induced Degradation of 4-Chlorophenol. Chemosphere 2011, 82, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Xu, X.; Gu, C.; Wang, W.; Geng, B.; Sun, Y.; Liu, J. Size-Controlled Synthesis of Porous ZnSnO3 Cubes and Their Gas-Sensing and Photocatalysis Properties. Sens. Actuators B Chem. 2012, 171–172, 572–579. [Google Scholar] [CrossRef]
- Liang, D.; Wu, S.; Wang, P.; Cai, Y.; Tian, Z.; Liu, J.; Liang, C. Recyclable Chestnut-Like Fe3O4@C@ZnSnO3 Core-Shell Particles for The Photocatalytic Degradation of 2,5-diclorophenol. RSC Adv. 2014, 4, 26201–26206. [Google Scholar] [CrossRef]
- Dong, S.; Sun, J.; Li, Y.; Yu, C.; Li, Y.; Sun, J. ZnSnO3 Hollow Nanospheres/Reduced Graphene Oxide Nanocomposites as High Performance Photocatalyst for Degradation of Metronidazole. Appl. Catal. B Environ. 2014, 144, 386–393. [Google Scholar] [CrossRef]
- Lui, Y.; Yang, Z.-H.; Song, P.-P.; Xu, R.; Wang, H. Facile Synthesis of Bi2MoO6/ZnSnO3 Heterojunction with Enhanced Visible Light Photocatalytic Degradation of Methylene Blue. Appl. Surf. Sci. 2018, 430, 561–570. [Google Scholar]
- Beshkar, F.; Amiri, O.; Salehi, Z. Synthesis of ZnSnO3 Nanostructures by Using Novel Gelling Agents and Their Application in Degradation of Textile Dye. Sep. Purif. Technol. 2017, 184, 66–71. [Google Scholar] [CrossRef]
- Khan, M.N.; Dutta, J. Comparison of Photocatalytic Activity of Zinc Stannate Particles and Zinc Stannate/Zinc Oxide Composites for The Removal of Phenol from Water and a Study on The Effect of pH on Photocatalytic Efficiency. Mater. Sci. Semicon. Proc. 2015, 36, 124–133. [Google Scholar] [CrossRef]
- Fang, C.H.; Geng, B.Y.; Liu, J.; Zhan, F.M. D-Fructose Molecule Template Route to Ultra-Thin ZnSnO3 Nanowire Architectures and Their Application as Efficient Photocatalyst. Chem. Commun. 2009, 2350–2352. [Google Scholar] [CrossRef]
- Liu, C.H.; Roder, R.; Zhang, L.C.; Ren, Z.; Chen, H.Y.; Zhang, Z.H.; Ronning, C.; Gao, P.X. Highly Efficient Visible-Light Driven Photocatalyst: A Case of Zinc Stannate Based Nanocrystal Assemblies. J. Mater. Chem. A 2014, 2, 4157–4167. [Google Scholar] [CrossRef]
- Dong, S.; Cui, L.; Zhang, W.; Xia, L.; Zhou, S.; Russel, C.K.; Fan, M.; Feng, J.; Sun, J. Double-Shelled ZnSnO3 Hollow Cubes for Efficient Photocatalytic Degradation of Antibiotic Wastewater. Chem. Eng. J. 2020, 384, 123279. [Google Scholar] [CrossRef]
- Yin, Y.; Li, F.; Zhang, N.; Ruan, S.; Zhang, H.; Chen, Y. Improved Gas Sensing Properties of Ag-Functionalized ZnSnO3 Hollow Nanocubes. Inorg. Chem. Front. 2018, 5, 2123–2131. [Google Scholar] [CrossRef]
- Wang, H.-L.; Li, Y.; Pang, L.; Zhang, W.-Z.; Jiang, W.F. Preparation and Application of Thermosensitive Poly (NIPAM-co-MAH-β-CD)/(TiO2-MWCNTs) Composites for Photocatalytic Degradation of Dinitro Butyl Phenol (DNBP) under Visible Light Irradiation. Appl. Catal. B Environ. 2013, 130, 132–142. [Google Scholar] [CrossRef]
- Geng, B.; Fang, C.; Zhan, F.; Yu, N. Synthesis of Polyhedral ZnSnO3 Microcrystals with Controlled Exposed Facets and Their Selective Gas-Sensing Properties. Small 2008, 4, 1337–1343. [Google Scholar] [CrossRef]
- Aziz, S.; Bum, K.G.; Yang, Y.J.; Yang, B.S.; Kang, C.U.; Doh, Y.H.; Choi, K.H.; Kim, H.C. Fabrication of ZnSnO3 Based Humidity Sensor onto Arbitrary Substrates by Micro-Nano Scale Transfer Printing. Sens. Actuators A Phys. 2016, 246, 1–8. [Google Scholar] [CrossRef]
- Wang, X.Y.; Ding, B.N.; Liu, Y.P.; Zhu, X.T.; Li, H.; Xia, M.Z.; Fu, X.; Li, M.X. Synthesis of 3D Flower-Like ZnSnO3 and Improvement of Ethanol-Sensing Properties at Room Temperature Based on Nano-TiO2 Decoration and UV Irradiation. Sens. Actuators B Chem. 2018, 246, 119–127. [Google Scholar] [CrossRef]
- Bespalko, Y.; Kuznetkova, T.; Kriger, T.; Chesalov, Y.; Lapina, O.; Ischenko, A.; Larina, T.; Sadykov, V.; Stathopoulos, V. La2Zr2O7/LaAlO3 Composite Prepared by Mixing Precipitated Precursors: Evolution of Its Structure Under Sintering. Mater. Chem. Phys. 2020, 251, 123093. [Google Scholar] [CrossRef]
- Huang, S.; Liu, L.; Zheng, Y.; Wang, Y.; Kong, D.; Zhang, Y.; She, Y.; Zhang, L.; Schimdt, O.G.; Yang, H.Y. Efficient Sodium Storage in Rolled-Up Amorphous Si Nanomembranes. Adv. Mater. 2018, 30, 1706637. [Google Scholar] [CrossRef]
- Huang, X.; Guo, F.; Li, M.; Ren, H.; Shi, Y.; Chen, L. Hydrothermal Synthesis of ZnSnO3 Nanoparticles Decorated on g-C3N4 Nanosheets for Accelerated Photocatalytic Degradation of Tetracycline Under the Visible Light Irradiation. Sep. Purif. Technol. 2020, 230, 115854. [Google Scholar] [CrossRef]
- Zhou, X.; Li, X.; Sun, H.; Sun, P.; Liang, X.; Liu, F.; Hu, X.; Lu, G. Nanosheet- Assembled ZnFe2O4 Hollow Microspheres for High- Sensitive Acetone Sensor. ACS Appl. Mater. Interfaces 2015, 7, 15414–15421. [Google Scholar] [CrossRef]
- Li, L.; Cheah, Y.; Ko, Y.; The, P.; Wee, G.; Wong, C.; Peng, S.; Srinivasan, M. The Facile Synthesis of Hierarchical Porous Flower-Like NiCo2O4 with Superior Lithium Storage Properties. J. Mater. Chem. A 2013, 1, 10935–10941. [Google Scholar] [CrossRef]
- Hailili, R.; Wang, C.Y.; Lichtfouse, E. Perovskite Nanostructures Assembled in Molten Salt Based on Halogen Anions KX (X = F, Cl and Br): Regulated Morphology and Defect-Mediated Photocatalytic Activity. Appl. Catal. B Environ. 2018, 232, 531–543. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, P.; Bao, D.; Wang, L.Q.; Chen, Y.J.; Zhou, X.M.; Yang, P.P.; Sun, S.C.; Zhang, M.L. One Pot, Two Phases: Individual Orthorhombic and Faced-Centred Cubic ZnSnO3 Obtained Synchronously in One Solution. Inorg. Chem. 2014, 53, 12289–12296. [Google Scholar] [CrossRef]
- Rovisco, A.; dos Santos, A.; Cramer, T.; Martins, J.; Branquinho, R.; Aquas, H. Piezoelectricity Enhancement of Nanogenerators Based on PDMS and ZnSnO3 Nanowires Through Microstructuration. ACS Appl. Mater. Interfaces 2020, 12, 18421–18430. [Google Scholar] [CrossRef]
- Elhalil, A.; Elmoubarki, R.; Farnane, M.; Machrouhi, A.; Sadiq, M.; Mahjoubi, F.Z.; Qouzarl, S. Photocatalytic Degradation of Caffeine as a Model Pharmaceutical Pollutant on Mg Doped ZnO-Al2O3 Heterostructure. Environ. Nanotechnol. Monitor. Manag. 2018, 10, 63–72. [Google Scholar] [CrossRef]
- Elhalil, A.; Elmoubarki, A.; Machrouhi, A.; Sadiq, M.; Abdennouri, M.; Quozarl, S.; Barka, N. Photodegradation of Caffeine by ZnO-ZnAl2O4 Nanoparticles Derived from LDH Structure. J. Environ. Chem. Eng. 2017, 5, 3719–3726. [Google Scholar] [CrossRef]
- Jagannatha, R.B.; Ramu, S.R.; Padaki, M.; Balakrishna, R.G. An Efficient Method for The Synthesis of Photocatalytically Active ZnO Nanoparticles by a Gel-Combustion Method for The Photodegradation of Caffeine. Nanochem. Res. 2017, 2, 86–95. [Google Scholar]
- Arfanis, M.K.; Adamou, P.; Moustakas, N.G.; Triantis, T.M.; Kontos, A.G.; Falaras, P. Photocatalytic Degradation of Salicylic Acid and Caffeine Emerging Contaminants Using Titania Nanotubes. J. Chem. Eng. 2017, 310, 525–536. [Google Scholar] [CrossRef]
- Tatarchuk, T.; Peter, A.; Al-Najar, B.; Vijaya, J.; Bououdina, M. Photocatalysis: Activity of Nanomaterials. Nanotech. Environ. Sci. 2018, 209–292. [Google Scholar] [CrossRef]
- Haroun, L.; Salaun, M.; Menard, A.; Legault, C.-Y.; Bellenger, J.-P. Photodegradation of Carbamazepine and Three Derivatives Using TiO2 and ZnO: Effect of pH, Ionic Strength, and Natural Organic Matter. Sci. Total Environ. 2014, 475, 16–22. [Google Scholar] [CrossRef]
- Ma, H.-Y.; Zhao, L.; Guo, L.H.; Zhang, H.; Chen, F.-J.; Yu, W.C. Roles of Reactive Oxygen Species (ROS) in The Photocatalytic Degradation of Pentachlorophenol and Its Main Toxic Intermediates by TiO2/UV. J. Hazard. Mater. 2019, 369, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Kudlek, E.; Dudziak, M.; Bohdziewicz, J. Influence of Inorganic Ions and Organic Substances on The Degradation of Pharmaceutical Compound in Water Matrix. Water 2016, 8, 532. [Google Scholar] [CrossRef]
- Murali, A.; Sarswat, P.K.; Free, M.L. Minimizing Electron-Hole Recombination Through Bang-gap Engineering in Novel ZnO-CeO2-rGO Ternary Composite Nanocomposite for Photoelectrochemical and and Photocatalytic Applications. Environ. Sci. Pollut. Res. 2020, 27, 25042–25056. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zeng, W. Room-Temperature Gas Sensing of ZnO Based Gas Sensor: A Review. Sens. Actuators A Phys. 2017, 267, 242–261. [Google Scholar] [CrossRef]

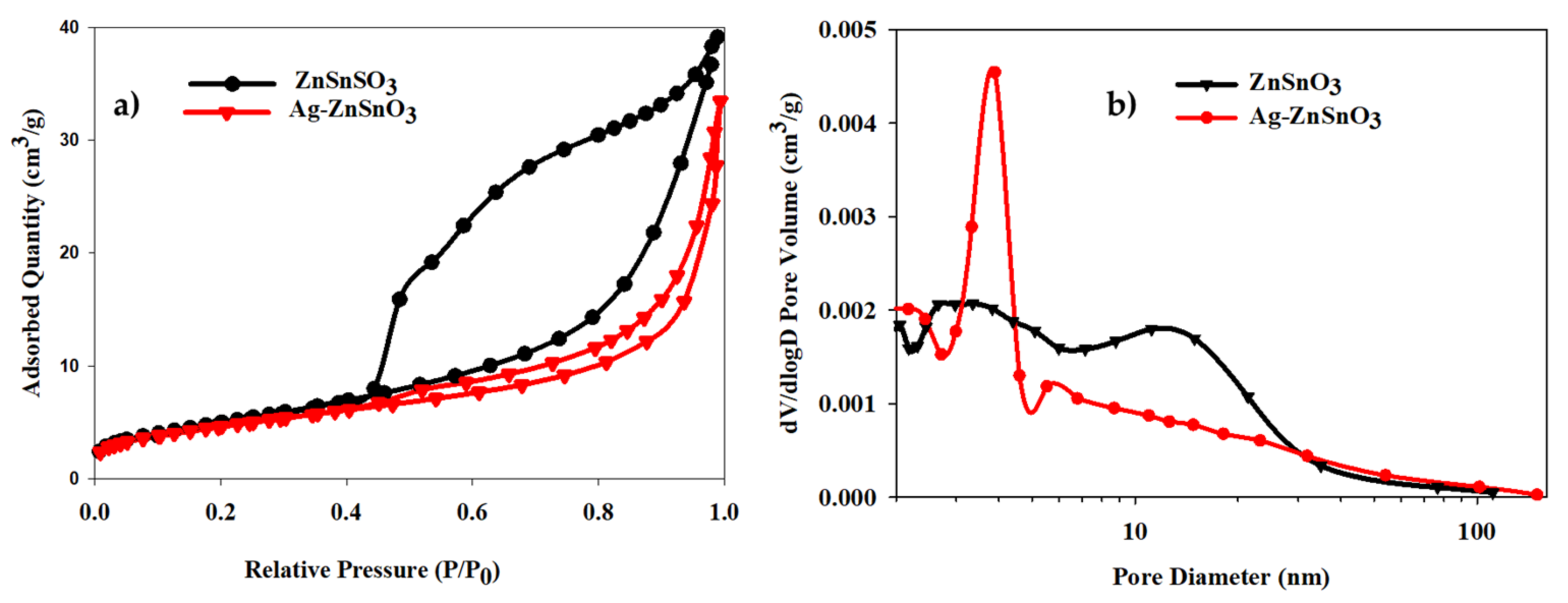



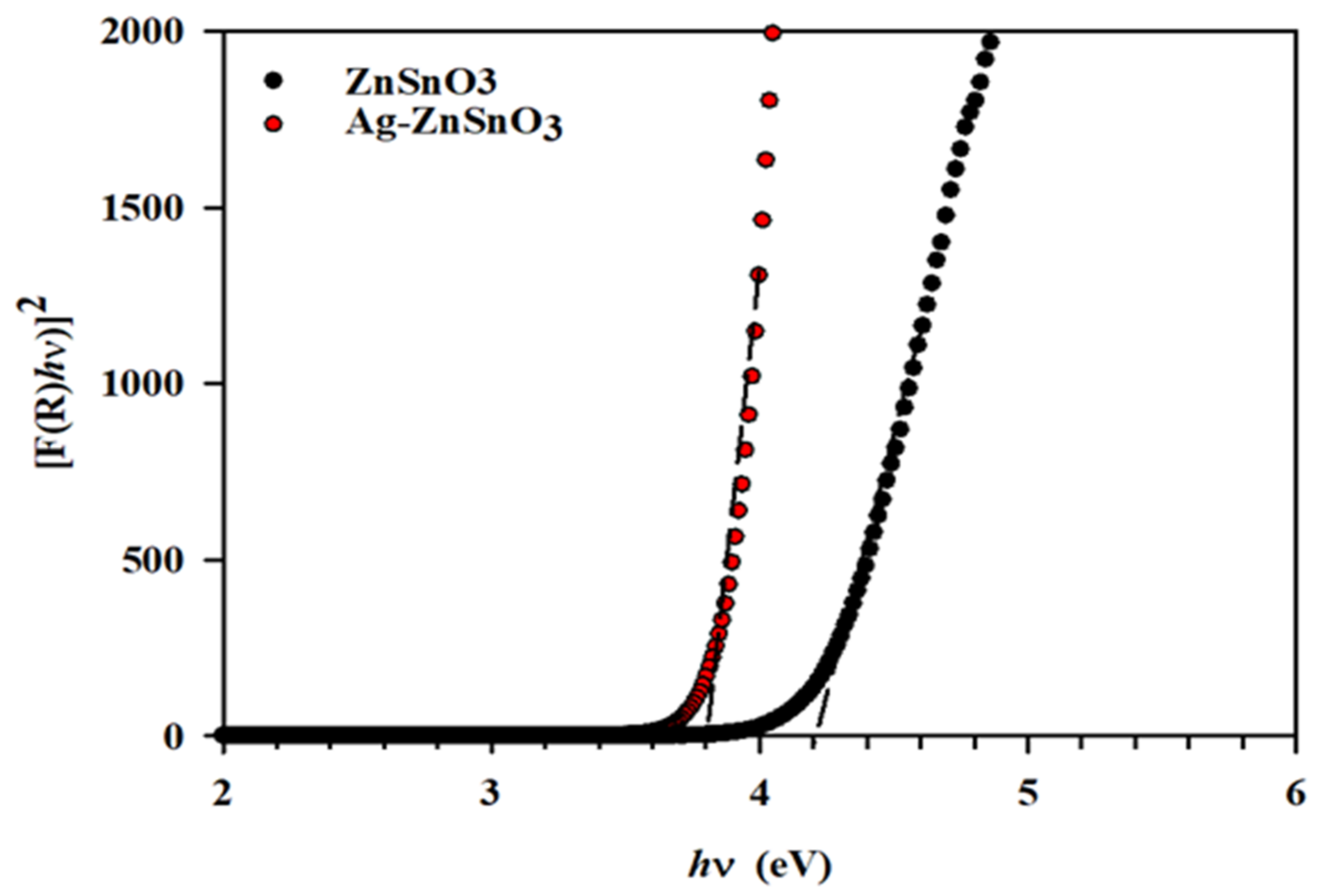



| Materials | dXRD (nm) | dTEM (nm) | SBET (m2/g) | Vp (cm3/g) | dp (nm) | Eg (eV) |
|---|---|---|---|---|---|---|
| ZnSnO3 | 29.3 | 98.2 | 18.8 | 0.06 | 11.5 | 4.2 |
| 1 mol.% Ag-ZnSnO3 | 19.4 | 79.0 | 17.2 | 0.05 | 12.8 | 3.8 |
| ZnSnO3 | 1 mol.% Ag-ZnSnO3 | ||||
|---|---|---|---|---|---|
| Chemical Phase | wt.% | at.% | Chemical Phase | wt.% | at.% |
| Sn 3d | 41.59 | 9.31 | Sn 3d | 38.00 | 8.83 |
| Zn 2p | 19.10 | 6.86 | Zn 2p | 25.55 | 9.85 |
| O 1s | 24.92 | 43.95 | O 1s | 23.73 | 55.26 |
| C 1s | 14.39 | 39.88 | C 1s | 9.59 | 25.34 |
| Ag 3d | 3.12 | 0.71 | |||
| Chemical Property/Name | Caffeine |
|---|---|
| Chemical structure |  |
| Molecular formula | C8H10N4O2 |
| CAS No. | 58-08-2 |
| Molecular weight, g/mol | 194.19 |
| Solubility in water, g/L | 18.7 |
| pKa | 10.4 |
| Test | Degradation Rate Constant k (h−1) | R2 |
|---|---|---|
| pH effects on Ag-ZnSnO3 #,* | ||
| pH = 3 | 0.36 | 0.98 |
| pH = 5 | 0.35 | 0.97 |
| pH = 7.5 | 1.01 | 0.97 |
| pH = 9 | 0.64 | 0.98 |
| pH = 11 | 0.44 | 0.99 |
| Catalyst dosage effects on Ag-ZnSnO3 #,* | ||
| 0.5 g/L | 0.54 | 0.97 |
| 0.75 g/L | 0.75 | 0.96 |
| 1 g/L | 1.01 | 0.96 |
| 1.25 g/L | 0.80 | 0.99 |
| 2 g/L | 1.10 | 0.98 |
| Pollutant concentration effects on Ag-ZnSnO3 #,* | ||
| 5 mg/L | 2.20 | 0.99 |
| 10 mg/L | 0.87 | 0.99 |
| 15 mg/L | 0.62 | 0.99 |
| 20 mg/L | 0.42 | 0.99 |
| 25 mg/L | 0.42 | 0.99 |
| Reactive oxygen species (ROS) effects on Ag-ZnSnO3 #,* | ||
| No scavenger | 0.96 | 0.99 |
| 1 mM Benzoquinone (BQ) | 0.10 | 0.87 |
| 1 mM Isopropyl alcohol (IPA) | 0.15 | 0.98 |
| 1 mM Ethylenediamine acetic acid (EDTA) | 0.12 | 0.94 |
| 1 mM Sodium sulphate (Na2SO4) | 0.15 | 0.98 |
| Hydrogen peroxide (H2O2) effects on Ag-ZnSnO3 #,* | ||
| 0 mM H2O2 | 0.96 | 0.99 |
| 1 mM H2O2 | 0.20 | 0.99 |
| 1 mM H2O2 | 0.20 | 0.99 |
| Ion effects (anions) on Ag-ZnSnO3 #,* | ||
| No anion | 1.10 | 0.99 |
| 1 mM HCO3− | 0.44 | 0.99 |
| 1 mM CO32− | 0.41 | 0.99 |
| 1 mM SO42− | 0.35 | 0.99 |
| 1 mM NO3− | 0.33 | 0.99 |
| Humic acid (HA) effects on Ag-ZnSnO3 #,* | ||
| 0 mg/L HA | 0.96 | 0.99 |
| 5 mg/L HA | 0.23 | 0.99 |
| 10 mg/L HA | 0.20 | 0.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anucha, C.B.; Altin, I.; Bacaksiz, E.; Stathopoulos, V.N.; Polat, I.; Yasar, A.; Yüksel, Ö.F. Silver Doped Zinc Stannate (Ag-ZnSnO3) for the Photocatalytic Degradation of Caffeine under UV Irradiation. Water 2021, 13, 1290. https://doi.org/10.3390/w13091290
Anucha CB, Altin I, Bacaksiz E, Stathopoulos VN, Polat I, Yasar A, Yüksel ÖF. Silver Doped Zinc Stannate (Ag-ZnSnO3) for the Photocatalytic Degradation of Caffeine under UV Irradiation. Water. 2021; 13(9):1290. https://doi.org/10.3390/w13091290
Chicago/Turabian StyleAnucha, Chukwuka Bethel, IIknur Altin, Emin Bacaksiz, Vassilis N. Stathopoulos, Ismail Polat, Ahmet Yasar, and Ömer Faruk Yüksel. 2021. "Silver Doped Zinc Stannate (Ag-ZnSnO3) for the Photocatalytic Degradation of Caffeine under UV Irradiation" Water 13, no. 9: 1290. https://doi.org/10.3390/w13091290
APA StyleAnucha, C. B., Altin, I., Bacaksiz, E., Stathopoulos, V. N., Polat, I., Yasar, A., & Yüksel, Ö. F. (2021). Silver Doped Zinc Stannate (Ag-ZnSnO3) for the Photocatalytic Degradation of Caffeine under UV Irradiation. Water, 13(9), 1290. https://doi.org/10.3390/w13091290







