Abstract
In the last years, nanoparticles such as TiO2, ZnO, NiO, CuO and Fe2O3 were mainly used in wastewater applications. In addition to the positive aspects concerning using nanoparticles in the advanced oxidation process of wastewater containing pollutants, the impact of these nanoparticles on the environment must also be investigated. The toxicity of nanoparticles is generally investigated by the nanomaterials’ effect on green algae, especially on Chlorella vulgaris. In this review, several aspects are reviewed: the Chlorella vulgaris culture monitoring and growth parameters, the effect of different nanoparticles on Chlorella vulgaris, the toxicity of photocatalyst nanoparticles, and the mechanism of photocatalyst during oxidative stress on the photosynthetic mechanism of Chlorella vulgaris. The Bold basal medium (BBM) is generally recognized as an excellent standard cultivation medium for Chlorella vulgaris in the known environmental conditions such as temperature in the range 20–30 °C and light intensity of around 150 μE·m2·s−1 under a 16/8 h light/dark cycle. The nanoparticles synthesis methods influence the particle size, morphology, density, surface area to generate growth inhibition and further algal deaths at the nanoparticle-dependent concentration. Moreover, the results revealed that nanoparticles caused a more potent inhibitory effect on microalgal growth and severely disrupted algal cells’ membranes.
1. Introduction
Conventional wastewater treatment technologies such as coagulation, precipitation, flocculation, adsorption, and natural aerobic treatment [1] have been sufficient for years. However, the complexity of pollutants discharged into the water has constrained new technologies to removing various emerging pollutants. Technologies based on nanoparticles, such as advanced oxidation processes, are efficient for emerging pollutants in low concentrations, such as pesticides, pharmaceuticals, endocrine disruptors, and personal care products [2]. Nanoparticles are particles obtained by natural or synthetic methods with less than 100 nm [3]. Nanoparticles have applications in various industrial [4,5] and medicine [6,7] fields, including wastewater treatment [8]. The most significant exposures from engineering material applications avenues for assessing environmental risks are water and soil [4,9]. The activity of nanoparticles in the environment depends on several characteristics, such as morphology, porosity, grain size, composition and crystallinity, groups attached on the surface, and charge of the surface. An area of interest for research is the decontamination of wastewater through efficient and inexpensive methods to maintain the environment in normal parameters.The use of nanoparticles is causing a substantial environmental impact due to the aquatic environment’s toxic effect. This work provides an overview of the key parameters of growing Chlorella vulgaris green algae. The current status of photocatalytic nanomaterials and their toxic effect on Chlorella vulgaris’s growth provide a direction for future research in this area and scaling-up and industrial-scale applications.
2. Chlorella vulgaris—Culture, Monitoring and Growth Parameters
Microalgae respond rapidly to environmental changes, and they are essential because of their role as primary producers in aquatic systems due to their short cell-doubling time. In aquatic systems, microalgae Chlorella vulgaris have been used for experiments to determine their crucial role in oxygen production and as the producers of food chains/food webs [10]. Destruction of activity would lead to an imbalance in the aquatic environment [11]. Because of their unicellular structure, microalgae suffer abnormal damage that may affect the whole aquatic system after exposure to pollutants. Simultaneously, in multicellular organisms, there are barriers skin and respiration that protect them [10,12].
Chlorella vulgaris, an essential green eukaryotic photosynthetic microorganism capable of rapid adaptation to new environments, with a structure similar to plants in an aquatic system, is a unicellular organism [13] with a spherical shape 2.5–10 μm in diameter [13,14], with a lipid production capacity range 14–56 dw% (dw—dry weight), protein content 10–58 dw%, and carbohydrate content 10–17 dw% [15]. Chlorella vulgaris reproduces asexually most commonly by auto-sporulation (i.e., four daughter cells with their own and dependent cell wall) [16]. In optimum conditions, Chlorella vulgaris can rapidly multiply asexually, and due to this, it is widely used as a model aquatic organism for toxic studies [17].
2.1. Chlorella vulgarisMaintenance Medium and Culturing Parameters
According to the Organisation for Economic Cooperation and Development (OECD) criteria, the concentrations of the Chlorella vulgaris growth nutrients, OECD Test no. 201: freshwater alga and cyanobacteria, growth inhibition test [18], are presented in Table 1.

Table 1.
Composition of the Organisation for Economic Cooperation and Development (OECD) Test no. 201 medium [18].
Chlorella vulgaris has a rapid growth rate, and it is ideal for production because it is remarkably resistant to conditions and invaders. For growth of this algae, there are three types of method: autotrophic, mixotrophic, and heterotrophic. Microalgae growth depends on pH, temperature, intensity and duration of illumination, and mineral nutrients’ concentration [19]. Chlorella vulgaris grows better at a temperature between 22 and 30 °C [20]; a temperature higher than 30 °C leads to slow growth and cell death [21]. The pH is essential for optimal growth, and optimum pH is neutral, but microalgae can survive at pH 3.0 [22,23]. A high concentration of iron, an essential micronutrient, induced a lipid accumulation while increasing Chlorella vulgaris’s growth [24].
Intensity and wavelength of light are significant for the growth of Chlorella vulgaris. For algae, light helps cell proliferation and photosynthesis. In order to keep the cell growth rate high and the costs low, it is recommended to alternate light with darkness under 8 h:16 h [25], 12 h:12 h [24], 14 h:10 h [26], or 16 h:8 h [27] light/dark cycle, but continuous illumination has also been reported [28,29]. Chlorella vulgaris has registered more cell division after stopping the light [30]. Light intensity is also important from the point of view of economic and operating efficiency and varied from 2800 to 8000 lux m−2 s−1 [28,31,32].
In terms of wavelength, microalgae usually use wavelengths between 400 nm and 700 nm (red light λ = 630–665 nm [33] and blue light λ = 430–465 nm [34]) for photosynthesis. Chlorella vulgaris cell size measurement demonstrated that microalgae cells’ growth had an approximate increase of 60–70% in diameter under blue light, compared to red light [30].
Aeration is ensured through aeration pumps because in the absence of any aeration or mixing, the unicellular form of algae dies, and the growth slowly decreases [35]. After five days of aeration Chlorella vulgaris growth is two times than without aeration [36].
2.2. Chlorella vulgaris Growth Monitoring
Algae growth modeling has been attracting attention over the past century regarding the effect of process parameters such as temperature, pH, light, nutrients, and growth rate. A few essential parameters are usually monitored (Table 2).

Table 2.
Parameters and methods for determining the development of Chlorella vulgaris.
2.2.1. The cell Concentration
The cell concentration is needed to estimate the algae growth; for cell density quantification, a few techniques are presented:
- (1)
- UV-VIS spectrophotometric optical density (OD), estimating chromophore formation in liquid culture and quantitative evaluation using Beer–Lambert low [46] at different wavelengths of 480, 649, and 665 nm is a parameter that measured the cell concentration [47]. This parameter depends on the type of culture (autotrophic, heterotrophic, or mixotrophic) and CO2 concentration, light penetration, and the presence or absence of inorganic or organic compounds can affect it [48]. If the algae concentration is too low, they will not be established in an anaerobic condition of cultures because the dissolved oxygen quantity is too low for the overall cell respiration [49]. For this mechanism, when concentration hits 1.9 × 107 cell mL−1, it is important to keep an active growth phase with density around 1–2 × 104 cell mL−1.
- (2)
- Neubauer haemocytometry using Neubauer improved chamber is currently used as a standard in laboratories for manual cell counting under a light microscope. The literature mentions that the Neubauer chamber showed the best overall performance [50].
- (3)
- Automatic cell counting is based on image analysis to count the particles present on the image.
- (4)
- Particles are counted by flow cytometry for the fast quantification of fluorescent particles excited with a fluorescent light source.
2.2.2. Biomass Quantification
Spectrophotometric analysis of the microalgae containing pigment such as Chlorophyll A has been developed to quantitative the biomass culture of Chlorella vulgaris by measuring the optical density (OD) as absorbance [42]. Chlorophyll A’s wavelengths in a range of 400–460 nm and 650–680 nm have been reported frequently. [37]. The OD of microalgae culture at 680 nm (OD680) is measured to determine the dry biomass concentration of microalgae, according to Gao et al. [41].
2.2.3. Pigments
Microalgae have pigment contents, for example, chlorophylls and carotenoids. Of the total amount of wet biomass, the pigments can be a percentage of 0.1–9.7% [37]. The viability of cells is an essential parameter for determining the growth rate of algae. This index is known as chlorophylls content (sum of Chlorophyll A and Chlorophyll B) of the algal cells [51]. Several methods are available to determine Chlorophyll A, for example, fluorometry, high-performance liquid chromatography (HPLC) using a fluorescence detector, and spectrophotometry.
The chlorophyll content is measured after centrifugation (mechanical breaking of the collected cells), followed by extracting the chlorophyll from the broken cells into alcohol or acetone. The extract is analyzed spectrophotometrically measuring absorbance at 665 nm and 750 nm [52]. Ethanol extraction is less harmful than the acetone method. The Chlorophyll A content (using ethanol extraction method) was calculated using Equation (1) [53].
where Chla is Chlorophyll A concentration (mg·m–3), Vethanol is the constant volume of the extract (mL), Vwater is the volume of filtered water (L), A665 is the absorbance of the samples in 665 nm wave, similar to A750. E665 and E750 indicate the absorbance of the samples acidified with 1 mol/L HCl at 665 nm and 750 nm, respectively [53].
Another method of determining algae pigments described by Wellburn [54] uses dimethylsulphoxide (DMSO). The supernatant extract was diluted with DMSO, and spectrophotometric tests were done at 649, 665, and 480 nm. The pigment content was calculated using the formulae [37]:
where Chla and Chlb are Chlorophyll A and B, respectively, concentration (mg·m−3); and OD649, OD665, and OD480 are the optical densities measured spectrophotometrically at 649, 665, and 480 nm, respectively.
Chlorophyll A (mg/L) = 12.47(OD665) − 3.62(OD649)
Chlorophyll B (mg/L) = 25.06(OD649) − 6.5(OD665)
Total carotenoids (mg/L) = [1000(OD480) − 1.29(Chla)-53.78(Chla)]/220
Total pigment (mg/L) = Chla + Chlb + Total carotenoids
2.2.4. Chemistry and Composition of Chlorella vulgaris
Chlorellavulgaris biomass contained 25–30% protein, 6–10% carbohydrate, and 30–40% lipid. While growing, Chlorella vulgaris can reach 5–40% [55] lipids per dry weight of biomass mainly composed of glycolipids, hydrocarbons, phospholipids, and free fatty acids [13]. To determine the lipids, Blight and Dyer described the extraction process of total lipids from Chlorella vulgaris using a mixture of chloroform and methanol. Quantification is conducted gravimetrically after evaporating the extracting solvent [56]. Bradford [44] and Dubois [45] methods are used for proteins and sugars, respectively.
3. Toxicity of Photocatalyst Nanoparticles to Chlorella vulgaris
The main source of nanoparticles in the environment is engineering nanoparticles for water and air pollution, contaminated soil remediation with hazardous substances, sensors for environmental application, biomedical imaging, and drug production [57].
Heterogeneous photodegradation based on nanoparticles (NPs) as photocatalytic materials is also a well-known practice, and they are utilized for this purpose. Rogozea et al. used NiO/ZnO NPs modified silica for photodegradation purposes. A high surface area of nanoparticles facilitates the efficient photodegradation reaction because of their small size (<10 nm) [58]. In addition to photocatalytic activity of nanoparticles, these have been described by the same group as having optic, fluorescence proprieties, and applications for compound degradation [59,60].
Heterogeneous photocatalysis is an advanced oxidation process for pollutant degradation by generating hydroxyl radicals , electron-hole pairs (, and superoxide radicals () [61]. The hydroxyl radicals react with organic pollutants and transform them into mineral constituents or in less toxic compounds [1]. The mechanism of photocatalysis are explained in Section 4. A fair number of metal oxides have been used as photocatalytic materials for water treatment since the year 1972, e.g., titanium dioxide (TiO2), zinc oxide (ZnO), iron (III) oxide (Fe2O3), vanadium oxide (V2O5), tungsten trioxide (WO3), etc. [62,63]. The photocatalytic materials have unique properties such as chemical stability, non-toxicity, photosensitivity, and low cost. For example, ZnO nanoparticles have a ∼3.22eV bandgap, and they have a small size, increasing the specific surface area. Higher numbers of active surface sites can facilitate absorption and degradation of pollutants, making photogenerated charge carriers able to react with pollutants [64].
Between photocatalysts, TiO2 and ZnO semiconductors present a higher efficiency for pollutant degradation [65], they are UV-light active materials and absorb a small portion of the solar spectrum (4–5%) [66]. In the past twenty years, numerous bodies of research were also developed to efficiently use solar energy and design new photocatalyst activity under visible light irradiation [67].
The continuous exposure of TiO2 nanoparticles increases the probability of contaminating the environment. For example, because of Ag nanoparticles’ use in consumer products, some of them are released into the aquatic environment, thus becoming a source of dissolved Ag that exerts toxic effects on organisms like algae, bacteria, daphnia, and fish [68]. The interactions between the particles and algal cells will lead to aggregation and sedimentation reducing their availability in the system. All these previously mentioned factors contribute to diminishing the toxicity of the nanoparticles during the algae exposure. The oxidative stress indicators with decreased levels across the cycles manifest the decrease in toxic effects. Thiagarajan et al. demonstrate that the nanoparticles’ continuous exposure to algal cells (e.g., Chlorella vulgaris cells) would significantly reduce their toxic impact [69].
Chlorella vulgaris has a high growth rate, a simple cell cycle, and can respond quickly to different nanoparticles [53,70]. When algae are incubated with a different type of nanoparticles, they have specific interactions like nanoparticles-nanoparticles homo-aggregation [71], algal cell-cell interaction [72], and nanoparticles-algae hetero-aggregation [73]. For TiO2 nanoparticles, homo-aggregation interaction is caused by the electrical double layer that is compressed around the nanoparticles [74]. The medium pH influences the interaction between the nanoparticles and algae [75]. pH and ionic strength are parameters that play an essential role in aggregation states of nanoparticles in water. Nanoparticles have toxic effects on the microalgae which is important to determine their impact on water. Moreover, decreasing the nanoparticles concentration will result in lower toxicity across the microalgae cycles from aggregation and sedimentation that plays a vital role in the growth rate of Chlorella vulgaris [76].
Although research papers have addressed the toxic effects of nanoparticles on plant cells, the toxicological studies are still limited.
The ecotoxicity of nanoparticles to algae has attracted the attention of scientists. For different types of nanoparticles, they have different toxicity behaviors. They are identified as causing growth inhibition and further algal deaths at the nanoparticle-dependent concentration and their properties (Table 3) [77]. Depending on the type of nanoparticles and the algae’s experimental conditions, the toxic effects may vary. Some studies demonstrate the toxicity of nanoparticles on algae growth and development (EC50).

Table 3.
Toxic effect of nanoparticles (NPs) to Chlorella vulgaris algae.
The most probable toxic effects of nanoparticles can be determined using a mathematic model (pattern) of the probability theory or calculating the EC50 value. EC50 is the concentration of test substance, resulting in a 50% reduction in either growth (EbC50) or growth rate (ErC50) relative to the control within 72–96 h exposure. Ecotoxicity tests are quantified by determining the EC50 values in several replicates, defined as the concentration that produces a toxic effect in 50% of the tested population [87]. Toxicity tests on the algae must be consistent with the OECD TG 2011 guide [18]. ThI is a test that aims to determine the effects of a substance on the growth of freshwater microalgae.
Most toxicity tests are done using a single type of nanoparticle. Ko et al. tested the toxicity of a mixture of ZnO, NiO, CuO, TiO2 nanoparticles, and Fe2O3 on microalgae Chlorella vulgaris growth. The parameter that was taken into consideration was the chlorophyll content. The tests highlighted the high toxicity of the probes that contained ZnO nanoparticles [79].
Table 3 reviews some types of nanoparticles used in photocatalytic processes; the conclusions resulting from their toxicity studies are the following.
Titanium oxide significantly decreased the growth and biomass (dry weight, Chlorophyll A, and total chlorophyll) of Chlorella vulgaris. TiO2 nanoparticles can adsorb Zn and P from the algal growth medium owing to surface adsorption of the NPs on the algal cell promoting growth inhibition. The acidity significantly increased the oxidative damage of TiO2 NPs on the algal cells. Metzler et al. [88] disclose that the “young” (3–5 days) and the “old” (15–26 days) algal cultures were less resistant to the stress imposed by TiO2 nanoparticles than the “mid-age” (8–14 days) culture.
The interaction between the ZnO NPs and algae altered the morphological characteristics after 72 h treatment. The toxicity of ZnO nanoparticles was attributed to dissolved Zn2+. The oxidative stress induced by ZnO was ten-fold higher than that by TiO2 NPs.
Nickel oxide nanoparticles induced inevitably influenced the growth of aquatic photoautotroph, cellular toxicity, and morphological alteration, cell deterioration, and oxidative stress, which were related to NPs concentration and reactive oxygen species (ROS) production, resulting in biotoxicity on Chlorella vulgaris.
Ag nanoparticles changed Chlorella vulgaris growth kinetics and cell metabolism shown in photosynthetic pigments and chemical composition because it can affect carbon acquisition, photosynthesis, and respiration processes. AgNPs could affect their toxic effects to Chlorella vulgaris by increasing cells’ aggregation, especially at higher concentrations.
4. Mechanism of Photocatalyst Nanoparticles during Oxidative Stress of Algae
The application of photocatalysis in wastewater remediation aimed to evaluate the risk of nanoparticles used during the photodegradation process. Despite the merits of environmental remediation based on photocatalytic materials like TiO2 and ZnO, there are a few drawbacks. Bioaccumulation and toxicity are significant shortcomings that affect the aquatic environment when wastewater is treated by photocatalysis, especially if contaminants’ complete mineralization is not ensured.
During photocatalysis, the reactive oxygen species (ROS), including superoxide (O2•−), hydroxyl radicals (), and hydrogen peroxide (H2O2) are generated on the surface of nanoparticles, such as TiO2, ZnO, WO3, SnO2, and so on.
Heterogeneous photocatalysis relies on the generation of electron-hole pairs when a photon of energy higher or equal to the bandgap energy of the semiconducting photocatalyst (Eg) is absorbed (Equation (6)). The electron-hole recombination on the surface (or in bulk) of the nanoparticles represents the main mechanism responsible for the photocatalyst’s deactivation. The electron-hole pair trapping in surface states leads to reactions with chemisorbed O2 and HO-/H2O to generate reactive species such as superoxide and hydroxyl radicals (Equations (7)–(10), Figure 1).
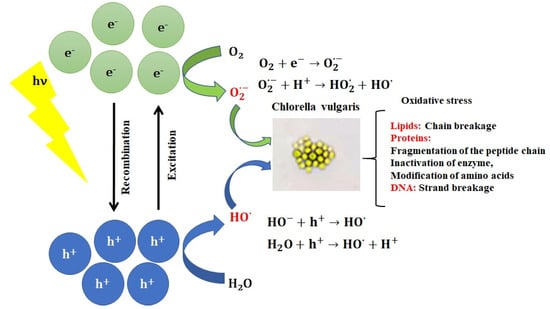
Figure 1.
Reactive oxygen species produced during photocatalysis induce oxidative damage to algae cells.
Numerous studies confirm that dissolved Ti4+, Zn2+, W3+, Sn4+, and ROS contribute to the nanoparticles’ toxicity on green algae.
ROS interaction with cellular components may lead to toxic effects during oxidative stress and act as secondary reactions in numerous cellular processes. ROS disrupt cell function by lipid peroxidation, oxidizing proteins and damaging nucleic acid. Oxidative stress in algal cells is monitored using common markers including lipid peroxidation monitoring by quantifying malondialdehyde content and antioxidant enzyme (glutathione S transferase and peroxidase) activities [78]. Chlorella vulgaris has been reported by Dauda et al. [78] to produce polyunsaturated fatty acids sensitive to reactive oxygen species.
5. Conclusion and Perspectives
This review reflects an image of scientific progress in Chlorella vulgaris toxicity studies and its impact in the aquatic ecosystem. This microalga is a simple plant because of its unicellular structure, but it has a complex role in oxygen production and as a food chains producer. The most common parameters for Chlorella vulgaris algae growth are Bold’s basal medium (BBM); the temperature of 20–30 °C; and the light intensity of around 150 μE·m2·s−1 under a 16/8 h light/dark cycle. Considering that the nanoparticles are used in a wide variety of fields (engineering, construction, medical, electric etc.), toxicity tests became very important when we discussed their involvement in algae or other microorganisms growth. Ecotoxicity tests were conducted to measure the impact of each powder on aquatic and terrestrial ecosystems. The most tested nanoparticles for algae toxicity are the oxides, for example, ZnO, TiO2, Ag, NiO, etc. Analysis of the algae showed that the application of more than 20 ppm of nanoparticles could negatively impact aquatic biology’s growth and physiology.
The nanoparticle exposure concentrations to Chlorella vulgaris is challenging to define, especially in low concentration. The toxicity is estimated based on the effects on the growth rate of algae. Chlorella vulgaris is a valuable bioindicator of nanoparticle suspensions, permitting a better understanding of nanoparticles’ toxicity risks to the aquatic environment.
Author Contributions
L.A. designed and carried out this research, supervised, provided advice, helped in this paper’s revision and editing, and funding acquisition. C.A. prepared and analyzed the data, and conducted theoriginal draft preparation. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a grant of the Romanian National Authority for Scientific Research and Innovation, CCCDI-UEFISCDI, Project number 114/2019 ERANET-M.-TESTIMONIES, within PNCDI III.
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Raizada, P.; Sudhaik, A.; Singh, P. Photocatalytic water decontamination using graphene and ZnO coupled photocatalysts: A review. Mater. Sci. Energy Technol. 2019, 2, 509–525. [Google Scholar] [CrossRef]
- Murgolo, S.; Franz, S.; Arab, H.; Bestetti, M.; Falletta, E.; Mascolo, G. Degradation of emerging organic pollutants in wastewater effluents by electrochemical photocatalysis on nanostructured TiO2 meshes. Water Res. 2019, 164, 114920. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, Applications and Toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Masciangioli, T.; Zhang, W.-X. Peer Reviewed: Environmental Technologies at the Nanoscale. Environ. Sci. Technol. 2003, 37, 102A–108A. [Google Scholar] [CrossRef] [PubMed]
- Corma, A.; Atienzar, P.; García, H.; Chane-Ching, J.-Y. Hierarchically mesostructured doped CeO2 with potential for solar-cell use. Nat. Mater. 2004, 3, 394–397. [Google Scholar] [CrossRef]
- Sangchay, W. The Self-cleaning and Photocatalytic Properties of TiO2 Doped with SnO2 Thin Films Preparation by Sol-gel Method. Energy Procedia 2016, 89, 170–176. [Google Scholar] [CrossRef]
- Guzmán, K.A.D.; Taylor, M.R.; Banfield, J.F. Environmental Risks of Nanotechnology: National Nanotechnology Initiative Funding, 2000−2004. Environ. Sci. Technol. 2006, 40, 1401–1407. [Google Scholar] [CrossRef]
- Ali, I.; Alharbi, O.M.L.; Tkachev, A.; Galunin, E.; Burakov, A.; Grachev, V.A. Water treatment by new-generation graphene materials: Hope for bright future. Environ. Sci. Pollut. Res. 2018, 25, 7315–7329. [Google Scholar] [CrossRef]
- Golobič, M.; Jemec, A.; Drobne, D.; Romih, T.; Kasemets, K.; Kahru, A. Upon Exposure to Cu Nanoparticles, Accumulation of Copper in the IsopodPorcellio scaberIs Due to the Dissolved Cu Ions Inside the Digestive Tract. Environ. Sci. Technol. 2012, 46, 12112–12119. [Google Scholar] [CrossRef]
- Khoshnamvand, M.; Ashtiani, S.; Chen, Y.; Liu, J. Impacts of organic matter on the toxicity of biosynthesized silver nanoparticles to green microalgae Chlorella vulgaris. Environ. Res. 2020, 185, 109433. [Google Scholar] [CrossRef]
- Baker, T.J.; Tyler, C.R.; Galloway, T.S. Impacts of metal and metal oxide nanoparticles on marine organisms. Environ. Pollut. 2014, 186, 257–271. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Garrido, I.; Pérez, S.; Blasco, J. Toxicity of silver and gold nanoparticles on marine microalgae. Mar. Environ. Res. 2015, 111, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Safi, C.; Zebib, B.; Merah, O.; Pontalier, P.-Y.; Vaca-Garcia, C. Morphology, composition, production, processing and applications of Chlorella vulgaris: A review. Renew. Sustain. Energy Rev. 2014, 35, 265–278. [Google Scholar] [CrossRef]
- Yamamoto, M.; Fujishita, M.; Hirata, A.; Kawano, S. Regeneration and maturation of daughter cell walls in the autospore-forming green alga Chlorella vulgaris (Chlorophyta, Trebouxiophyceae). J. Plant Res. 2004, 117, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Júnior, W.G.M.; Gorgich, M.; Corrêa, P.S.; Martins, A.A.; Mata, T.M.; Caetano, N.S. Microalgae for biotechnological applications: Cultivation, harvesting and biomass processing. Aquaculture 2020, 528, 735562. [Google Scholar] [CrossRef]
- Yamamoto, M.; Kurihara, I.; Kawano, S. Late type of daughter cell wall synthesis in one of the Chlorellaceae, Parachlorella kessleri (Chlorophyta, Trebouxiophyceae). Planta 2005, 221, 766–775. [Google Scholar] [CrossRef]
- Oukarroum, A.; Bras, S.; Perreault, F.; Popovic, R. Inhibitory effects of silver nanoparticles in two green algae, Chlorella vulgaris and Dunaliella tertiolecta. Ecotoxicol. Environ. Saf. 2012, 78, 80–85. [Google Scholar] [CrossRef]
- OECD. Test No. 201: Freshwater Alga and Cyanobacteria, Growth Inhibition Test, OECD Guidelines for the Testing of Chemicals; Section 2; OECD Publishing: Paris, France, 2011. [Google Scholar]
- Wehr, J.D. Algae: Anatomy, Biochemistry, and Biotechnology by Barsanti, L. & Gualtieri, P.J. Phycology 2007, 43, 412–414. [Google Scholar]
- Serra-Maia, R.; Bernard, O.; Gonçalves, A.; Bensalem, S.; Lopes, F. Influence of temperature on Chlorella vulgaris growth and mortality rates in a photobioreactor. Algal Res. 2016, 18, 352–359. [Google Scholar] [CrossRef]
- Žitnik, M.; Šunta, U.; Torkar, K.G.; Klemenčič, A.K.; Atanasova, N.; Bulc, T.G. The study of interactions and removal efficiency of Escherichia coli in raw blackwater treated by microalgae Chlorella vulgaris. J. Clean. Prod. 2019, 238, 117865. [Google Scholar] [CrossRef]
- Mayo, A.W. Effects of temperature and pH on the kinetic growth of unialga Chlorella vulgaris cultures containing bacteria. Water Environ. Res. 1997, 69, 64–72. [Google Scholar] [CrossRef]
- Filali, R.; Tebbani, S.; Dumur, D.; Isambert, A.; Pareau, D.; Lopes, F. Identification of the growth model parameters for a culture of Chlorella vulgaris in a photobioreactor. IFAC Proc. Vol. 2010, 43, 431–436. [Google Scholar] [CrossRef]
- Bélanger-Lépine, F.; Tremblay, A.; Huot, Y.; Barnabé, S. Cultivation of an algae-bacteria consortium in wastewater from an industrial park: Effect of environmental stress and nutrient deficiency on lipid production. Bioresour. Technol. 2018, 267, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Khoeyi, Z.A.; Seyfabadi, J.; Ramezanpour, Z. Effect of light intensity and photoperiod on biomass and fatty acid composition of the microalgae, Chlorella vulgaris. Aquac. Int. 2011, 20, 41–49. [Google Scholar] [CrossRef]
- Salgueiro, J.L.; Pérez, L.; Maceiras, R.; Sánchez, A.; Cancela, A. Bioremediation of Wastewater Using Chlorella vulgaris Microalgae: Phosphorus and Organic Matter. Int. J. Environ. Res. 2016, 10, 465–470. [Google Scholar]
- Kube, M.; Mohseni, A.; Fan, L.; Roddick, F. Impact of alginate selection for wastewater treatment by immobilised Chlorella vulgaris. Chem. Eng. J. 2019, 358, 1601–1609. [Google Scholar] [CrossRef]
- González, L.E.; Cañizares, R.O.; Baena, S. Efficiency of ammonia and phosphorus removal from a colombian agroindustrial wastewater by the microalgae Chlorella vulgaris and Scenedesmus dimorphus. Bioresour. Technol. 1997, 60, 259–262. [Google Scholar] [CrossRef]
- Lam, M.K.; Yusoff, M.I.; Uemura, Y.; Lim, J.W.; Khoo, C.G.; Lee, K.T.; Ong, H.C. Cultivation of Chlorella vulgaris using nutrients source from domestic wastewater for biodiesel production: Growth condition and kinetic studies. Renew. Energy 2017, 103, 197–207. [Google Scholar] [CrossRef]
- Daliry, S.; Hallajisani, A.; Mohammadi Roshandeh, J.; Nouri, H.; Golzary, A. Investigation of Optimal Condition for Chlorella vulgaris Microalgae Growth. Glob. J. Environ. Sci. Manag. 2017, 3, 217–230. [Google Scholar]
- Gong, N.; Shao, K.; Che, C.; Sun, Y. Stability of nickel oxide nanoparticles and its influence on toxicity to marine algae Chlorella vulgaris. Mar. Pollut. Bull. 2019, 149, 110532. [Google Scholar] [CrossRef]
- Sreesai, S.; Pakpain, P. Nutrient Recycling by Chlorella vulgaris from Septage Effluent of the Bangkok City, Thailand. ScienceAsia 2007, 33, 293–299. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Fu, C.-C.; Liu, Y.-C. Effects of using light-emitting diodes on the cultivation of Spirulina platensis. Biochem. Eng. J. 2007, 37, 21–25. [Google Scholar] [CrossRef]
- Yeh, K.-L.; Chang, J.-S. Nitrogen starvation strategies and photobioreactor design for enhancing lipid content and lipid production of a newly isolated microalga Chlorella vulgaris ESP-31: Implications for biofuels. Biotechnol. J. 2011, 6, 1358–1366. [Google Scholar] [CrossRef]
- Kim, D.G.; Lee, C.; Park, S.-M.; Choi, Y.-E. Manipulation of light wavelength at appropriate growth stage to enhance biomass productivity and fatty acid methyl ester yield using Chlorella vulgaris. Bioresour. Technol. 2014, 159, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Munir, N.; Imtiaz, A.; Sharif, N.; Naz, S. Optimization of Growth Conditions of Different Algal Strains and Determination of Their Lipid Contents. J. Anim. Plant. Sci. 2015, 25, 546–553. [Google Scholar]
- Griffiths, M.J.; Garcin, C.; Van Hille, R.; Harrison, S.T. Interference by pigment in the estimation of microalgal biomass concentration by optical density. J. Microbiol. Methods 2011, 85, 119–123. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Y.; Liu, J.; Shen, Z.; Li, A.; Ma, T.; Feng, Q.; Sun, Y. Treatment of high-nitrate wastewater mixtures from MnO2 industry by Chlorella vulgaris. Bioresour. Technol. 2019, 291, 121836. [Google Scholar] [CrossRef]
- Takahashi, T. Applicability of Automated Cell Counter with a Chlorophyll Detector in Routine Management of Microalgae. Sci. Rep. 2018, 8, 4967. [Google Scholar] [CrossRef]
- Yu, Q.; Wang, H.; Li, X.; Yin, Y.; Qin, S.; Ge, B. Enhanced biomass and CO2 sequestration of Chlorella vulgaris using a new mixotrophic cultivation method. Process. Biochem. 2020, 90, 168–176. [Google Scholar] [CrossRef]
- Gao, F.; Yang, Z.-H.; Li, C.; Chen, B.-A.; Jin, W.-H.; Deng, Y.-B. Concentrated microalgae cultivation in treated sewage by membrane photobioreactor operated in batch flow mode. Bioresour. Technol. 2014, 167, 441–446. [Google Scholar] [CrossRef]
- Fernández-Linares, L.; Barajas, C.G.; Páramo, E.D.; Corona, J.A.B. Assessment of Chlorella vulgaris and indigenous microalgae biomass with treated wastewater as growth culture medium. Bioresour. Technol. 2017, 244, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Li, C.; Zhang, D. Lipid production of Chlorella vulgaris cultured in artificial wastewater medium. Bioresour. Technol. 2011, 102, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilising the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Etermination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Myers, J.A.; Curtis, B.S.; Curtis, W.R. Improving accuracy of cell and chromophore concentration measurements using optical density. BMC Biophys. 2013, 6, 4. [Google Scholar] [CrossRef]
- Fan, G.; Hong, L.; Luo, J.; You, Y.; Zhang, J.; Hua, P.; Du, B.; Zhan, J.; Ning, R.; Bao, M. Photocatalytic inactivation of harmful algae and degradation of cyanotoxins microcystin-LR using GO-based Z-scheme nanocatalysts under visible light. Chem. Eng. J. 2020, 392, 123767. [Google Scholar] [CrossRef]
- Heredia-Arroyo, T.; Wei, W.; Hu, B. Oil Accumulation via Heterotrophic/Mixotrophic Chlorella protothecoides. Appl. Biochem. Biotechnol. 2010, 162, 1978–1995. [Google Scholar] [CrossRef]
- Hemschemeier, A.; Melis, A.; Happe, T. Analytical approaches to photobiological hydrogen production in unicellular green algae. Photosynth. Res. 2009, 102, 523–540. [Google Scholar] [CrossRef]
- Camacho-Fernández, C.; Hervás, D.; Rivas-Sendra, A.; Marín, M.P.; Seguí-Simarro, J.M. Comparison of six different methods to calculate cell densities. Plant Methods 2018, 14, 1–15. [Google Scholar] [CrossRef]
- Baniamerian, H.; Tsapekos, P.; Alvarado-Morales, M.; Shokrollahzadeh, S.; Safavi, M.; Angelidaki, I.; Safavi, M. Anti-algal activity of Fe2O3–TiO2 photocatalyst on Chlorella vulgaris species under visible light irradiation. Chemosphere 2020, 242, 125119. [Google Scholar] [CrossRef]
- Miao, M.-S.; Yao, X.-D.; Shu, L.; Yan, Y.-J.; Wang, Z.; Li, N.; Cui, X.-T.; Lin, Y.-M.; Kong, Q. Mixotrophic growth and biochemical analysis of Chlorella vulgaris cultivated with synthetic domestic wastewater. Int. Biodeterior. Biodegradation 2016, 113, 120–125. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, X.; Li, R.; Yao, H.; Lu, Z.; Yang, X. Photosynthetic Toxicity and Oxidative Damage Induced by nano-Fe3O4 on Chlorella vulgaris in Aquatic Environment. Open J. Ecol. 2012, 2, 21–28. [Google Scholar] [CrossRef]
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Deviram, G.; Mathimani, T.; Anto, S.; Ahamed, T.S.; Ananth, D.A.; Pugazhendhi, A. Applications of microalgal and cyanobacterial biomass on a way to safe, cleaner and a sustainable environment. J. Clean. Prod. 2020, 253, 119770. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A Rapid Method of Total Lipid Extraction and Purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Tratnyek, P.G.; Johnson, R.L. Nanotechnologies for environmental cleanup. Nano Today 2006, 1, 44–48. [Google Scholar] [CrossRef]
- Rogozea, E.A.; Petcu, A.R.; Olteanu, N.L.; Lazar, C.A.; Cadar, D.; Mihaly, M. Tandem Adsorption-Photodegradation Activity Induced by Light on NiO-ZnO p–n Couple Modified Silica Nanomaterials. Mater. Sci. Semicond. Process. 2017, 57, 1–11. [Google Scholar] [CrossRef]
- Olteanu, N.L.; Lazăr, C.A.; Petcu, A.R.; Meghea, A.; Rogozea, E.A.; Mihaly, M. “One-Pot” Synthesis of Fluorescent Au@SiO2 and SiO2@Au Nanoparticles. Arab. J. Chem. 2016, 9, 854–864. [Google Scholar] [CrossRef]
- Rogozea, E.A.; Olteanu, N.L.; Petcu, A.R.; Lazar, C.A.; Meghea, A.; Mihaly, M. Extension of optical properties of ZnO/SiO2 materials induced by incorporation of Au or NiO nanoparticles. Opt. Mater. 2016, 56, 45–48. [Google Scholar] [CrossRef]
- Barakat, M.A.; Kumar, R. Photocatalytic Activity Enhancement of Titanium Dioxide Nanoparticles; Springer Briefs in Molecular Science; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1–29. [Google Scholar]
- Raizada, P.; Shandilya, P.; Singh, P.; Thakur, P. Solar Light-Facilitated Oxytetracycline Removal from the Aqueous Phase Utilising a H2O2/ZnWO4/CaO Catalytic System. J. Taibah Univ. Sci. 2017, 11, 689–699. [Google Scholar] [CrossRef]
- Raizada, P.; Kumari, J.; Shandilya, P.; Dhiman, R.; Singh, P.V.; Singh, P. Magnetically Retrievable Bi2WO6/Fe3O4 Immobilised on Graphene Sand Composite for Investigation of Photocatalytic Mineralization of Oxytetracycline and Ampicillin. Process Saf. Environ. Prot. 2017, 106, 104–116. [Google Scholar] [CrossRef]
- Raizad, P.; Kumari, J.; Shandilya, P.; Singh, P. Kinetics of photocatalytic mineralization of oxytetracycline and ampicillin using activated carbon supported ZnO/ZnWO4 nanocomposite in simulated wastewater. Desalin. Water Treat. 2017, 79, 204–213. [Google Scholar] [CrossRef]
- Adegoke, K.A.; Iqbal, M.; Louis, H.; Bello, O.S. Synthesis, Characterisation and Application of CdS/ZnO Nanorod Heterostructure for the Photodegradation of Rhodamine B Dye. Mater. Sci. Energy Technol. 2019, 2, 329–336. [Google Scholar]
- Fatin, S.O.; Lim, H.N.; Tan, W.T.; Huang, N.M. Comparison of Photocatalytic Activity and Cyclic Voltammetry of Zinc Oxide and Titanium Dioxide Nanoparticles toward Degradation of Methylene Blue. Int. J. Electrochem. Sci. 2012, 7, 9074–9084. [Google Scholar]
- Maeda, K. Photocatalytic water splitting using semiconductor particles: History and recent developments. J. Photochem. Photobiol. C Photochem. Rev. 2011, 12, 237–268. [Google Scholar] [CrossRef]
- Navarro, E.; Piccapietra, F.; Wagner, B.; Marconi, F.; Kaegi, R.; Odzak, N.; Sigg, L.; Behra, R. Toxicity of Silver Nanoparticles to Chlamydomonas reinhardtii. Environ. Sci. Technol. 2008, 42, 8959–8964. [Google Scholar] [CrossRef]
- Thiagarajan, V.; Pavani, M.; Raghavan, A.; Ramasubbu, S. Diminishing bioavailability and toxicity of P25 TiO2 NPs during continuous exposure to marine algae Chlorella sp. Chemosphere 2019, 233, 363–372. [Google Scholar] [CrossRef]
- Ma, J.; Lin, F.; Zhang, R.; Yu, W.; Lu, N. Differential sensitivity of two green algae, Scenedesmus quadricauda and Chlorella vulgaris, to 14 pesticide adjuvants. Ecotoxicol. Environ. Saf. 2004, 58, 61–67. [Google Scholar] [CrossRef]
- Morelli, E.; Gabellieri, E.; Bonomini, A.; Tognotti, D.; Grassi, G.; Corsi, I. TiO2 nanoparticles in seawater: Aggregation and interactions with the green alga Dunaliella tertiolecta. Ecotoxicol. Environ. Saf. 2018, 148, 184–193. [Google Scholar] [CrossRef]
- Lin, M.; Tseng, Y.H.; Huang, C.P. Interactions between Nano-TiO2 Particles and Algal Cells at Moderate Particle Concentration. Front. Chem. Sci. Eng. 2015, 9, 242–257. [Google Scholar] [CrossRef]
- Ma, S.; Zhou, K.; Yang, K.; Lin, D. Heteroagglomeration of Oxide Nanoparticles with Algal Cells: Effects of Particle Type, Ionic Strength and pH. Environ. Sci. Technol. 2015, 49, 932–939. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.-H.; Zhuang, C.-M.; Peng, Y.-H.; Lin, C.-H.; Tseng, Y.-M. The effect of inorganic ions on the aggregation kinetics of lab-made TiO2 nanoparticles in water. Sci. Total. Environ. 2012, 446–452. [Google Scholar] [CrossRef]
- Khan, S.S.; Srivatsan, P.; Vaishnavi, N.; Mukherjee, A.; Chandrasekaran, N. Interaction of silver nanoparticles (SNPs) with bacterial extracellular proteins (ECPs) and its adsorption isotherms and kinetics. J. Hazard. Mater. 2011, 192, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Dalai, S.; Pakrashi, S.; Nirmala, M.J.; Chaudhri, A.; Chandrasekaran, N.; Mandal, A.; Mukherjee, A. Cytotoxicity of TiO2 nanoparticles and their detoxification in a freshwater system. Aquat. Toxicol. 2013, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.K.; Moon, J.-Y.; Lee, Y.-C. Microalgal ecotoxicity of nanoparticles: An updated review. Ecotoxicol. Environ. Saf. 2020, 201, 110781. [Google Scholar] [CrossRef] [PubMed]
- Dauda, S.; Chia, M.A.; Bako, S.P. Toxicity of titanium dioxide nanoparticles to Chlorella vulgaris Beyerinck (Beijerinck) 1890 (Trebouxiophyceae, Chlorophyta) under changing nitrogen conditions. Aquat. Toxicol. 2017, 187, 108–114. [Google Scholar] [CrossRef]
- Ko, K.-S.; Koh, D.-C.; Kong, I.C. Toxicity Evaluation of Individual and Mixtures of Nanoparticles Based on Algal Chlorophyll Content and Cell Count. Materials 2018, 11, 121. [Google Scholar] [CrossRef]
- Sadiq, I.M.; Dalai, S.; Chandrasekaran, N.; Mukherjee, A. Ecotoxicity study of titania (TiO2) NPs on two microalgae species: Scenedesmus sp. and Chlorella sp. Ecotoxicol. Environ. Saf. 2011, 74, 1180–1187. [Google Scholar] [CrossRef]
- Xia, B.; Sui, Q.; Sun, X.; Han, Q.; Chen, B.; Zhu, L.; Qu, K. Ocean acidification increases the toxic effects of TiO2 nanoparticles on the marine microalga Chlorella vulgaris. J. Hazard. Mater. 2018, 346, 1–9. [Google Scholar] [CrossRef]
- Suman, T.Y.; Radhika, S.R.R.; Kirubagaran, R. Evaluation of zinc oxide nanoparticles toxicity on marine algae Chlorella vulgaris through flow cytometric, cytotoxicity and oxidative stress analysis. Ecotoxicol. Environ. Saf. 2015, 113, 23–30. [Google Scholar] [CrossRef]
- Oukarroum, A.; Zaidi, W.; Samadani, M.; Dewez, D. Toxicity of Nickel Oxide Nanoparticles on a Freshwater Green Algal Strain of Chlorella vulgaris. BioMed Res. Int. 2017, 2017, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gong, N.; Shao, K.; Feng, W.; Lin, Z.; Liang, C.; Sun, Y. Biotoxicity of nickel oxide nanoparticles and bio-remediation by microalgae Chlorella vulgaris. Chemosphere 2011, 83, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Huang, X.; Sun, W.; Too, H.Z.; Laserna, A.K.C.; Li, S.F.Y. A global metabolomic insight into the oxidative stress and membrane damage of copper oxide nanoparticles and microparticles on microalga Chlorella vulgaris. Environ. Pollut. 2020, 258, 113647. [Google Scholar] [CrossRef]
- Romero, N.; Visentini, F.F.; Márquez, V.E.; Santiago, L.G.; Castro, G.R.; Gagneten, A.M. Physiological and morphological responses of green microalgae Chlorella vulgaris to silver nanoparticles. Environ. Res. 2020, 189, 109857. [Google Scholar] [CrossRef] [PubMed]
- Clément, L.; Hurel, C.; Marmier, N. Toxicity of TiO2 nanoparticles to cladocerans, algae, rotifers and plants—Effects of size and crystalline structure. Chemosphere 2013, 90, 1083–1090. [Google Scholar] [CrossRef]
- Metzler, D.M.; Li, M.; Erdem, A.; Huang, C.P. Responses of Algae to Photocatalytic Nano-TiO2 Particles with an Emphasis on the Effect of Particle Size. Chem. Eng. J. 2011, 170, 538–546. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).