Abstract
In many experiments, a partial nitrification device is initiated with the use of highly active nitrating sludge because of the large number of nitrifying bacteria. Ammonia-oxidizing bacteria (AOB) are more adaptable to low-dissolved oxygen environments than nitrite-oxidizing bacteria (NOB). NOB activity was inhibited when the dissolved oxygen (DO) levels were decreased, causing the nitrate-nitrogen concentration to gradually decrease in the effluent and the nitrite-nitrogen concentration to gradually increase, achieving the accumulation of nitrous nitrogen. In this experiment, a sequencing batch reactor (SBR) was used to suppress NOB activity at a given pH while maintaining DO at a very low level so that the ammonia–water reaction mainly occurred in the device, and then the mud and water separated. Compared with other experiments, this approach can occur in 25 days, and it runs stably for more than two months until the device closes when the ammonia-nitrogen concentration is about 170 mg/L. This experiment also compared the difference between the pH change at the beginning of the device operation and after the device was stable. In order to increase the efficiency of bacterial appreciation, supplementing NaHCO3 increased the HCO3− concentration by 300 mg/L on the 25th day. It was found that some nitrification reactions still occurred, but they were not enough to destabilize the device. The nitrosate accumulation efficiency still gradually increased, and the average nitrite accumulation efficiency was 87.25% after NaHCO3 supplementation.
1. Introduction
The question of how to remove nitrogen in sewage economically and efficiently has always been a hot topic in international research. Anammox (anaerobic ammonium oxidation) is widely studied because of its economic advantages, but it is difficult to proliferate anammox. Through the study of anammox many great research results have been achieved. For instance, Du et al. realized the treatment of wastewater containing high-strength nitrate through partial denitrification (PD)-anammox. [1]. Li et al. studied the beneficial effects of influent organics on anammox [2]. In this research, we hope to realize the treatment of ammonia-nitrogen wastewater through partial nitrification–anammox.
It is generally believed that nitrate-nitrogen is generated by ammonia-nitrogen through the ammonia oxidation reaction and the nitrification reaction, and then nitrate-nitrogen is removed from water through the common process for nitrogen removal from wastewater; however, this process requires a large amount of energy, especially if both reactions require a carbon source [3]. In theory, the conversion of 1 g of nitrate-nitrogen to nitrogen removal requires a biochemical oxygen demand (BOD) of 2.86 g, while the conversion of 1 g of nitrite-nitrogen to nitrogen removal only requires a BOD of 1.71 g [4,5]. If one can inhibit the further conversion of nitrite-nitrogen to nitrate-nitrogen, converting it directly to nitrogen removal, this can save a lot of energy [6]. The ammonia oxidation reaction and nitrification reaction are carried out by ammonia-oxidizing bacteria (AOB) and nitrite-oxidizing bacteria (NOB), respectively. When the temperature, pH, dissolved oxygen, and other factors change, the activity of the NOB is inhibited and the AOB become more abundant than the NOB [7]. This allows nitrite-nitrogen to accumulate in the water. In this way, the process of nitrification ends at the nitrite-nitrogen stage. The process of denitrification to achieve the removal of the total nitrogen is called the partial nitrification and denitrification process. Compared with the full nitrification and denitrification process, partial nitrification and denitrification could preserve the carbon source and reduce aeration. Although partial nitrification and denitrification processes have economic advantages, there were many problems to be solved in terms of starting and maintaining the stability of the process.
At a normal temperature, NOB have an advantage over AOB in terms of growth efficiency. In addition, AOB have a greater proliferation efficiency under conditions of relatively high pH and a low dissolved oxygen concentration. There have been many studies on controlling the short-range nitrification process by controlling dissolved oxygen (DO) [8,9]. In this experiment, the effect on the partial nitrification process was observed by controlling DO under extremely low conditions, and then through higher pH and alkalinity. The initiation of the partial nitrification process by a sequencing batch reactor (SBR) can control DO and pH more effectively than initiation with a continuous-flow reactor [10,11]. Sequential batch processing can avoid the accumulation of salts in the reactor. Accumulating too much salt may result in the decreased activity of the ammonia-oxidizing bacteria [12]. The main purpose of this study is to observe the operating characteristics of the partial nitrification device initiated by supplementing the inorganic carbon source and pH control.
2. Materials and Methods
2.1. Partial Nitrification Seed Sludge and the Composition of Influent
The sludge used to start the partial nitrification unit was taken from the aeration section of the sewage treatment station at the Changping campus of Beijing University of Chemical Technology. The sludge already had good nitration activity. The sludge was operated under a DO content of 4.0–6.0 mg/L and a pH of 7.3. It is mainly used for the treatment of wastewater for domestic sewage generated by students in the campus and canteens. The mixed liquid suspended solids (MLSS) in the aeration basin are usually 7000 mg/L. During the operation of this process, no significant accumulation of nitrite-nitrogen occurred.
The composition of the influent is shown in Table 1, below [13].

Table 1.
Characteristics of the short-range nitrification influent.
2.2. Reactor System and the Operation Strategy
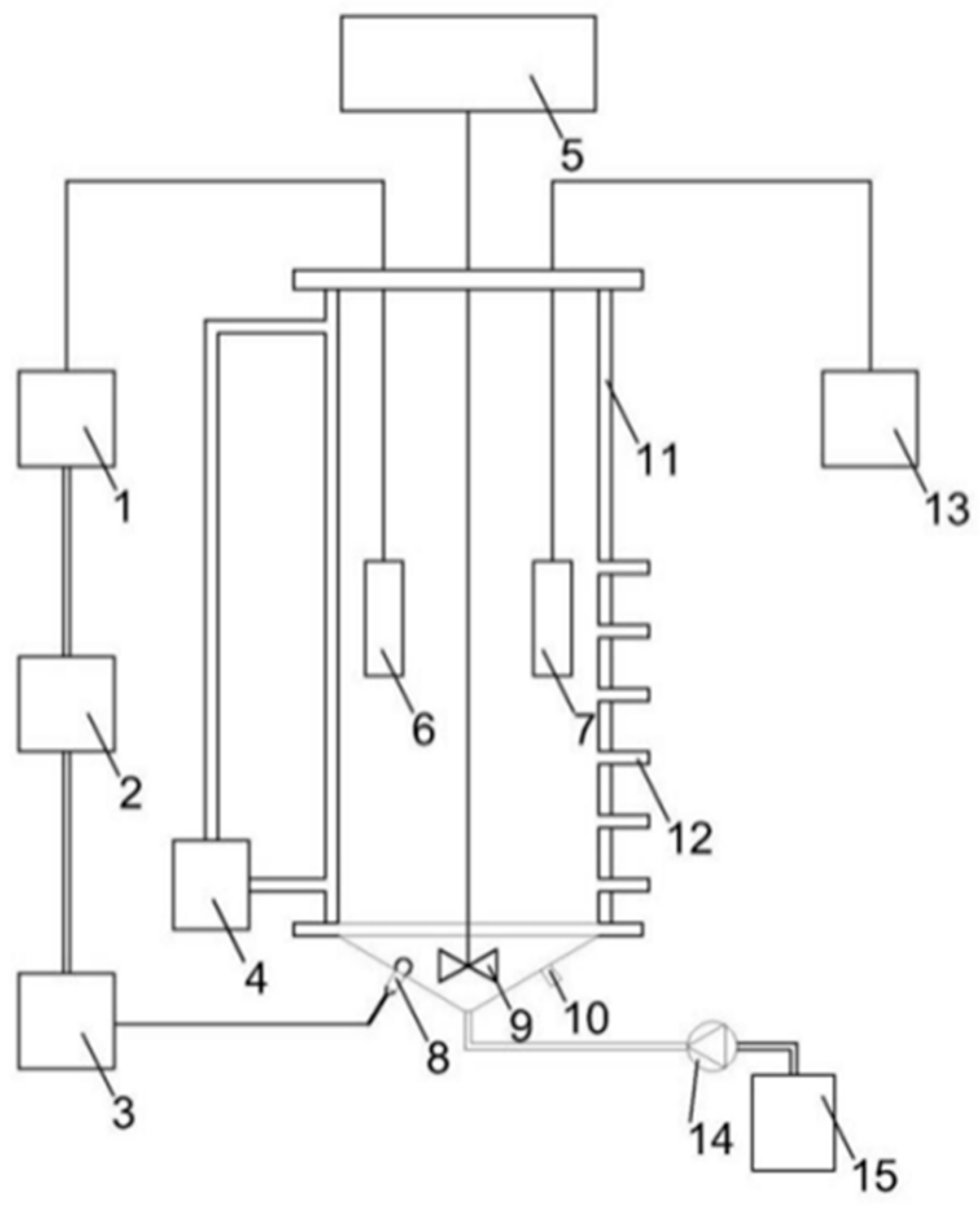
A SBR with a working volume of 10 L was used for the establishment of the partial nitrification process. A schematic diagram of the SBR is shown in Figure 1. As a precursor, the acclimation process was started using 4 L of the partial nitrification seed sludge. The synthetic wastewater was used as a feed; the composition of the feed wastewater is shown in Table 1. The acclimation of the partial nitrification sludge started immediately after the inoculation of the seed sludge. The temperature of the SBR was always controlled at 34 °C through the use of a thermostatic water jacket [14], and the pH was maintained in the range of 8.2–8.5 [15]. An aeration pump was used to provide oxygen to the SBR, a DO meter with a relay was used to detect the concentration of DO, the aeration pump was controlled by the relay, and the concentration of DO was controlled at 0.2–0.7 mg/L through feedback adjustment [16]. The aeration efficiency of the aeration pump was adjusted to make the length of non-aeration periods in the SBR longer [17]. The pH was adjusted to 8.2–8.5 using NaOH and HCl. A part of the NH4+–N in the device was oxidized to NO2−–N, and the NO3−–N concentration in the effluent was low. Triangular blades were used for stirring at 60 rpm to ensure that the SBR was evenly mixed inside. The SBR takes 11 h per cycle, and two cycles occur per day. Each cycle includes water inlet (0.5 h), reaction (9 h), precipitation (0.5 h), and decanting of the supernatant (1.0 h). Eight liters of influent was used per cycle and the volumetric exchange ratio was 0.8. The sludge retention time (SRT) was 30 days.
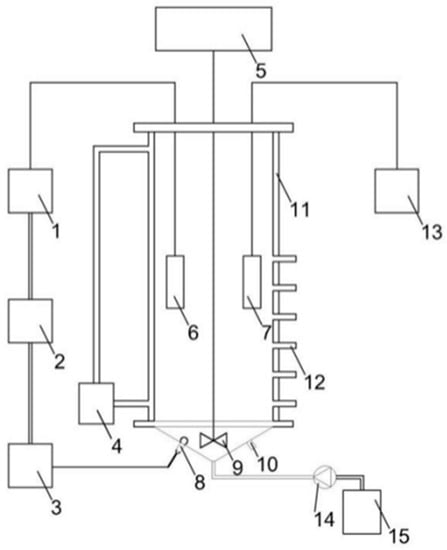
Figure 1.
Partial nitrification device diagram. 1: Online dissolved oxygen (DO) meter; 2: relay; 3: aeration pump; 4: thermostatic circulator; 5: stirrer; 6: DO electrode; 7: pH electrode; 8: aeration port; 9: stirring paddle; 10: mud discharge port; 11: thermal insulation interlayer; 12: sampling/drainage port; 13: online pH meter; 14: peristaltic pump; and 15: storage bucket.
2.3. Analytical Methods
The detection methods used in this experiment are shown in Table 2, below.

Table 2.
Detection method and instrument.
3. Results
3.1. Overview of the Partial Nitrification Operation Started by Controlling pH
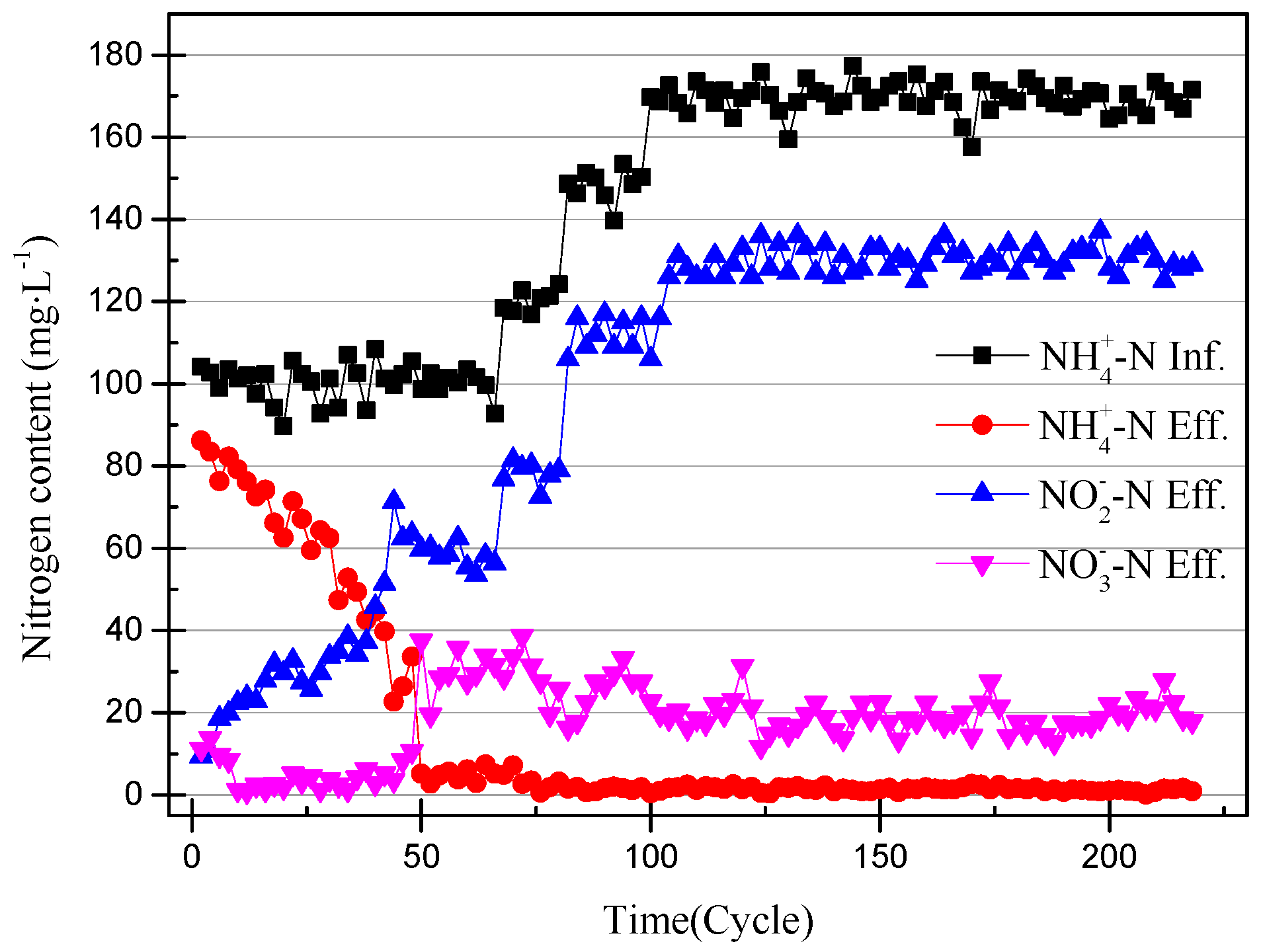
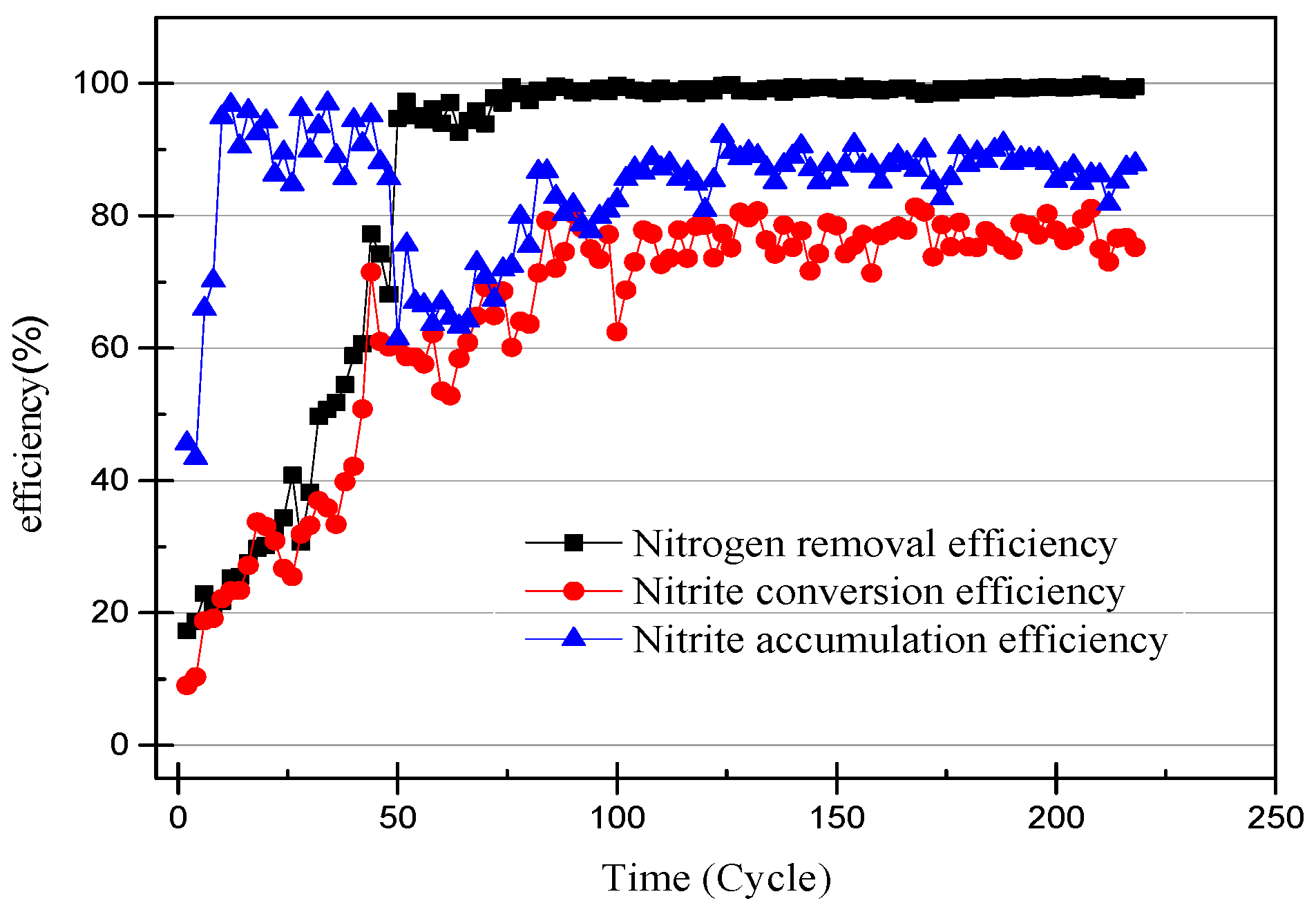
The nitrogen concentration of the influent and effluent was recorded every two cycles (24 h), and the data are shown in Figure 2. The efficiency of the ammonia removal and nitrite accumulation is shown in Figure 3. The nitrite accumulation was not sufficient to explain the early performance of the SBR with high NH4+–N effluent in this experiment. The ratio of effluent NO2−–N to influent NH4+–N was defined as the nitrite conversion efficiency, used to illustrate the performance of the SBR. The closeness of the ammonia-nitrogen removal efficiency and the nitrite conversion efficiency indicates that AOB activity improved and NOB was gradually eliminated. In the first few days of the SBR operation, the higher nitrate concentration of the effluent was likely due to the controlled DO range of 0.3–1.0 mg/L (0.65 mg/L on average) [18]. After reducing the DO to 0.2–0.7 mg/L (0.45 mg/L on average) the nitrate concentration in the effluent was reduced to below 5 mg/L. At the same time, under the condition that the ammonia-nitrogen concentration in the influent was constant, with the operation of the SBR, the ammonia concentration in the effluent gradually decreased and the nitrite concentration gradually increased. The results prove that the concentration of AOB was enriched and that of NOB reduced. Unlike the usual partial nitrification start-up method for controlling DO, controlling the pH at 8.2–8.5 inhibited the activity of both AOB and NOB. The high ammonia-nitrogen content in the early plant effluent illustrates this, but it is clear that AOB is more adaptable to the environment. As time passed, it was found that there was no increase in nitrite and no significant change in nitrate. This phenomenon proves that AOB are more active than NOB at high pH levels.
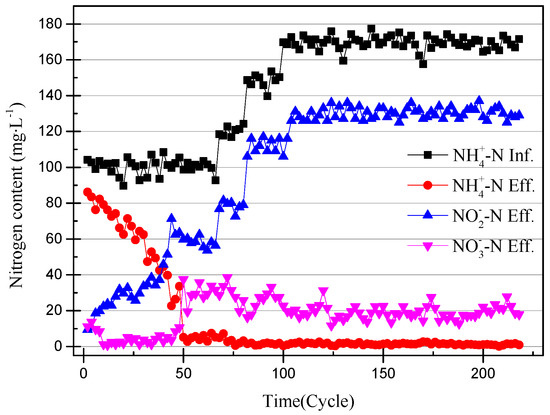
Figure 2.
The change in the nitrogen content of the partial nitrification device.
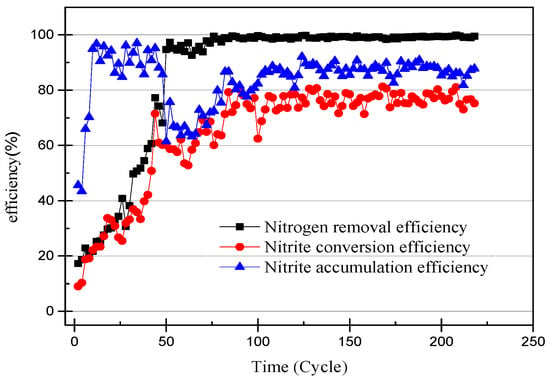
Figure 3.
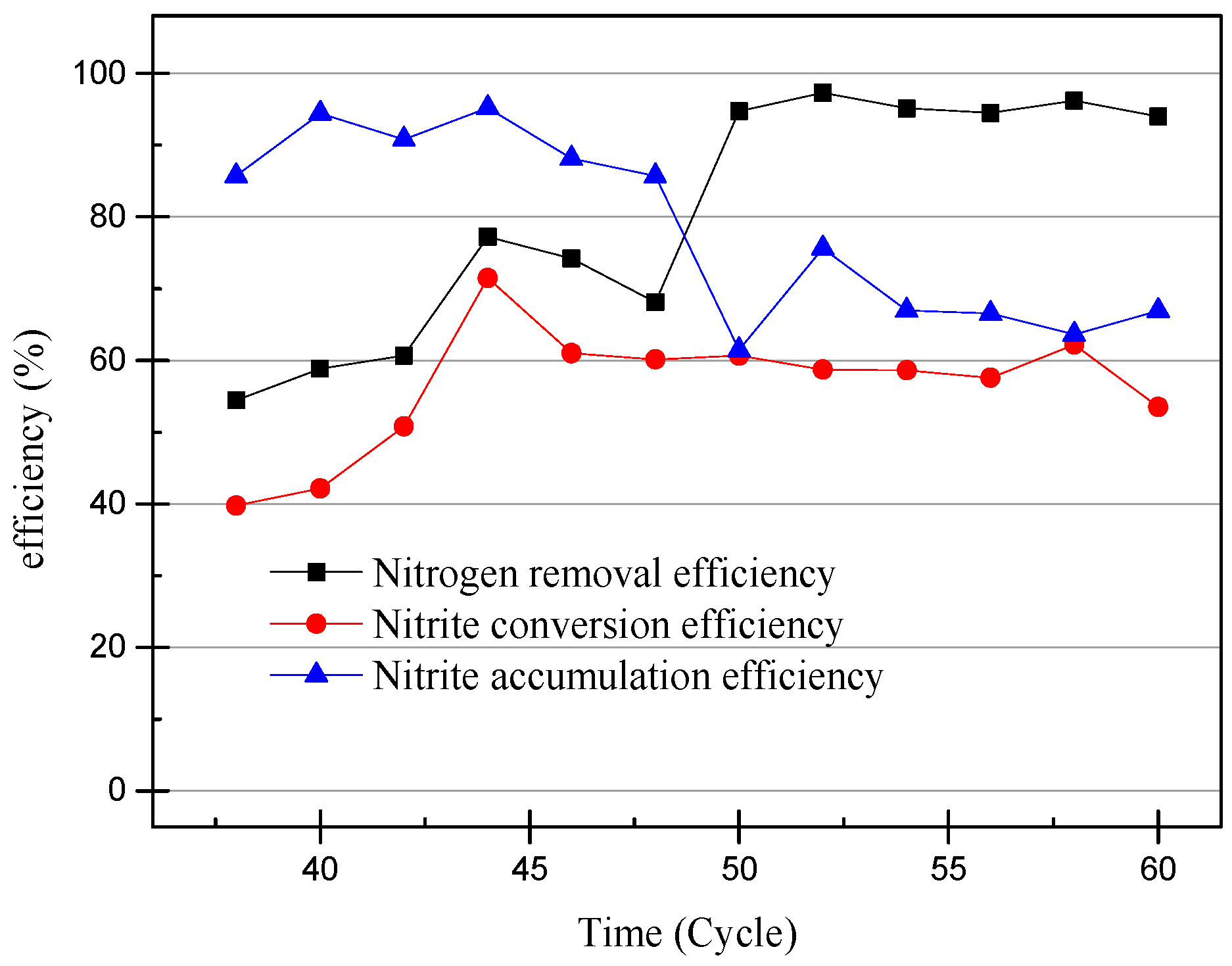
The efficiency of ammonia-nitrogen removal, nitrite conversion, and nitrite accumulation.
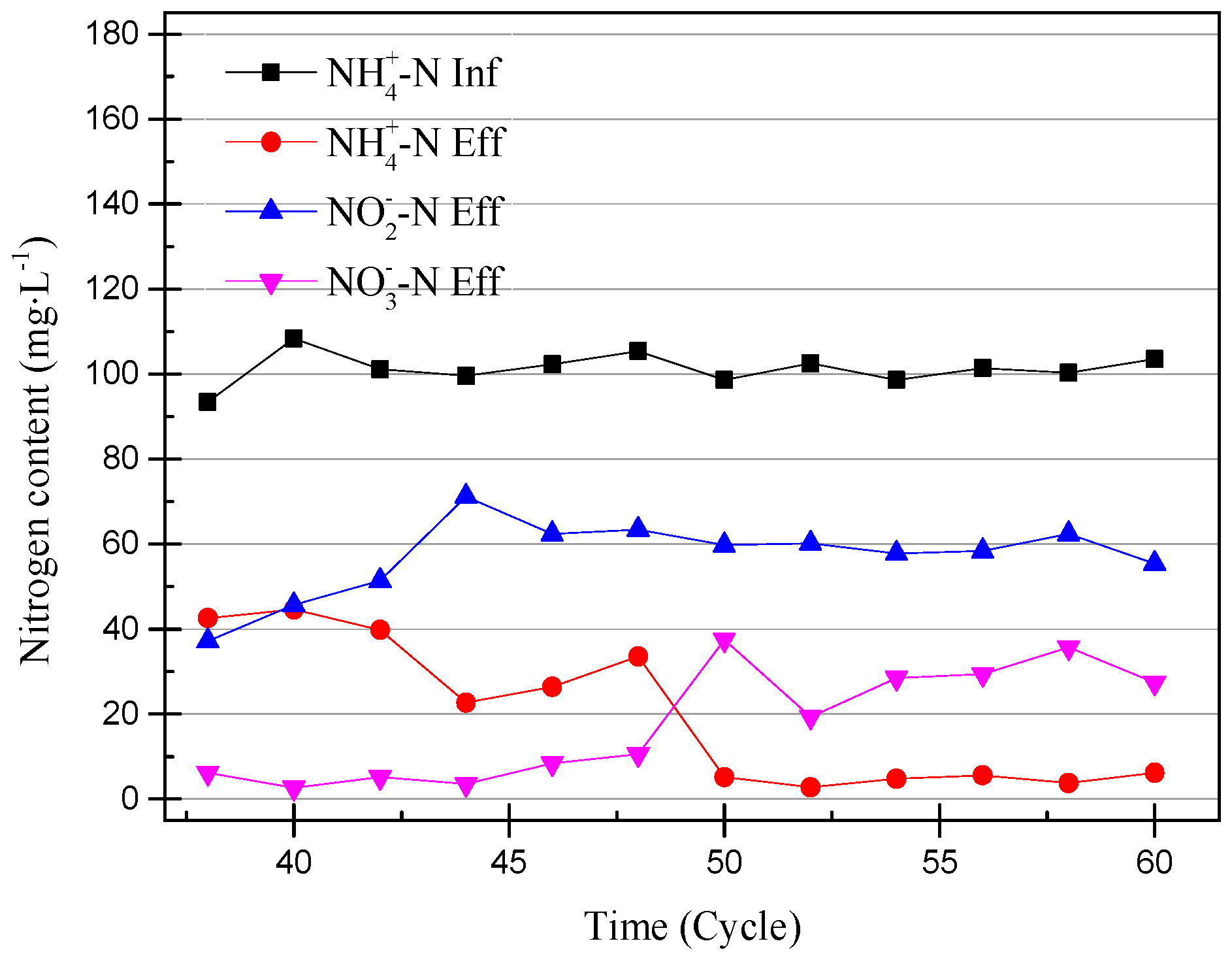
At the 40th cycle, the concentration of nitrite in the effluent was already higher than that of ammonia. Therefore, it was considered that the partial nitrification has successfully started. In the 49th cycle, the concentration of NaHCO3 had increased to 300 mg/L in the influent and the pH remained unchanged. Increasing the concentration of the inorganic carbon source can make the partial nitrification activity greater and the cycle shorter [19]. However, the concentration of nitrate in the effluent exceeded the experimental expectations. The partial data of the partial nitrification device operation are shown in Figure 4 and Figure 5, and changes in the process after increasing the alkalinity can be observed. After increasing the alkalinity, the nitrite accumulation efficiency decreased from 85.86% to 61.46%, and there was a significant difference between the nitrite conversion efficiency and the ammonia removal efficiency. The average NO2−–N concentration increased from 55.22 to 59.00 mg/L without a significant increase, as shown in Figure 4, but the NH4+–N concentration decreased to 4.73 mg/L and the nitrate concentration increased to 29.65 mg/L. This result was not as expected, because the start-up period was not long enough to increase the alkalinity in the influent enough to improve the activity of the nitrifying bacteria. There was still a certain amount of NOB in the SBR, and the activity was expressed under the stimulation of NaHCO3. At the same time, although the NO2−–N concentration in the effluent did not increase, the concentration of ammonia in the effluent was very low, which proved that the AOB activity also increased greatly. In the case of little dissolved oxygen, AOB still expressed ammonia oxidation activity.
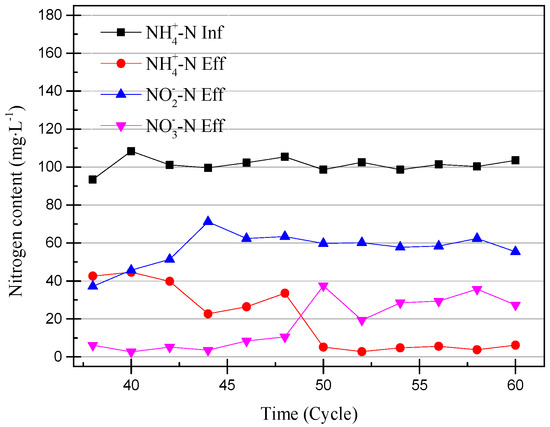
Figure 4.
Ammonia, nitrite, and nitrate concentrations from the 38th cycle to 60th cycle.
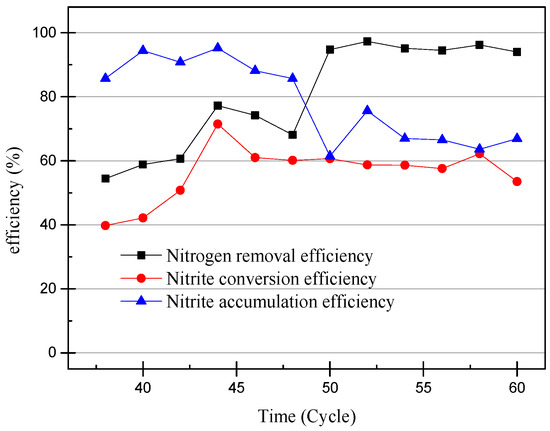
Figure 5.
The efficiency of ammonia-nitrogen removal and nitrite conversion and nitrite accumulation from 38th cycle to 60th cycle.
NOB will be further eliminated from the device with the operation of the SBR, because both DO and pH are not suitable for the proliferation of NOB. After increasing the alkalinity in the influent, the nitrite accumulation efficiency increases with the SBR operation, so the influent ammonia concentration was increased to 170 mg/L to obtain higher ammonia oxidation activity [20]. The NO3−–N concentration of the effluent decreases with an increase in the NH4+–N concentration of the influent, and because when there is little dissolved oxygen, AOB preferentially obtains O2 over NOB. In addition, an elevated concentration of substrate will inhibit NOB activity [21]. The data in Figure 2 and Figure 3 illustrate that the nitrite accumulation efficiency rises with an increase in the NH4+–N concentration in the influent. The average nitrite accumulation efficiency was found to be 87.22% when the NH4+-N influent was 170 mg/L.
3.2. Analysis of Partial Nitrification Process
After 200 operation cycles, partial nitrification stabilized. Since it was tested every two cycles, we selected the cycle in which we changed the working conditions to see what impact this had on the partial nitrification process. After the SBR water inlet stage, the online pH meter was started, and NH4+–N, NO2−–N, NO3−–N, and total nitrogen (TN) data were measured every 30 min until the end of the reaction stage. The DO was increased at the 213th cycle from 0.2–0.7 mg/L to 0.4–0.8 mg/L (0.45 mg/L to 0.6 mg/L on average). The data from the 213th cycle are shown in Figure 6.
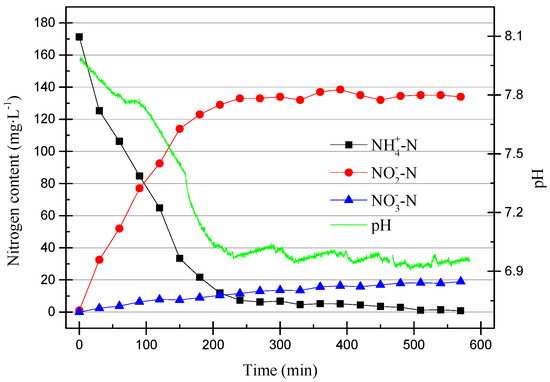
Figure 6.
The operating data for the 213th cycle.
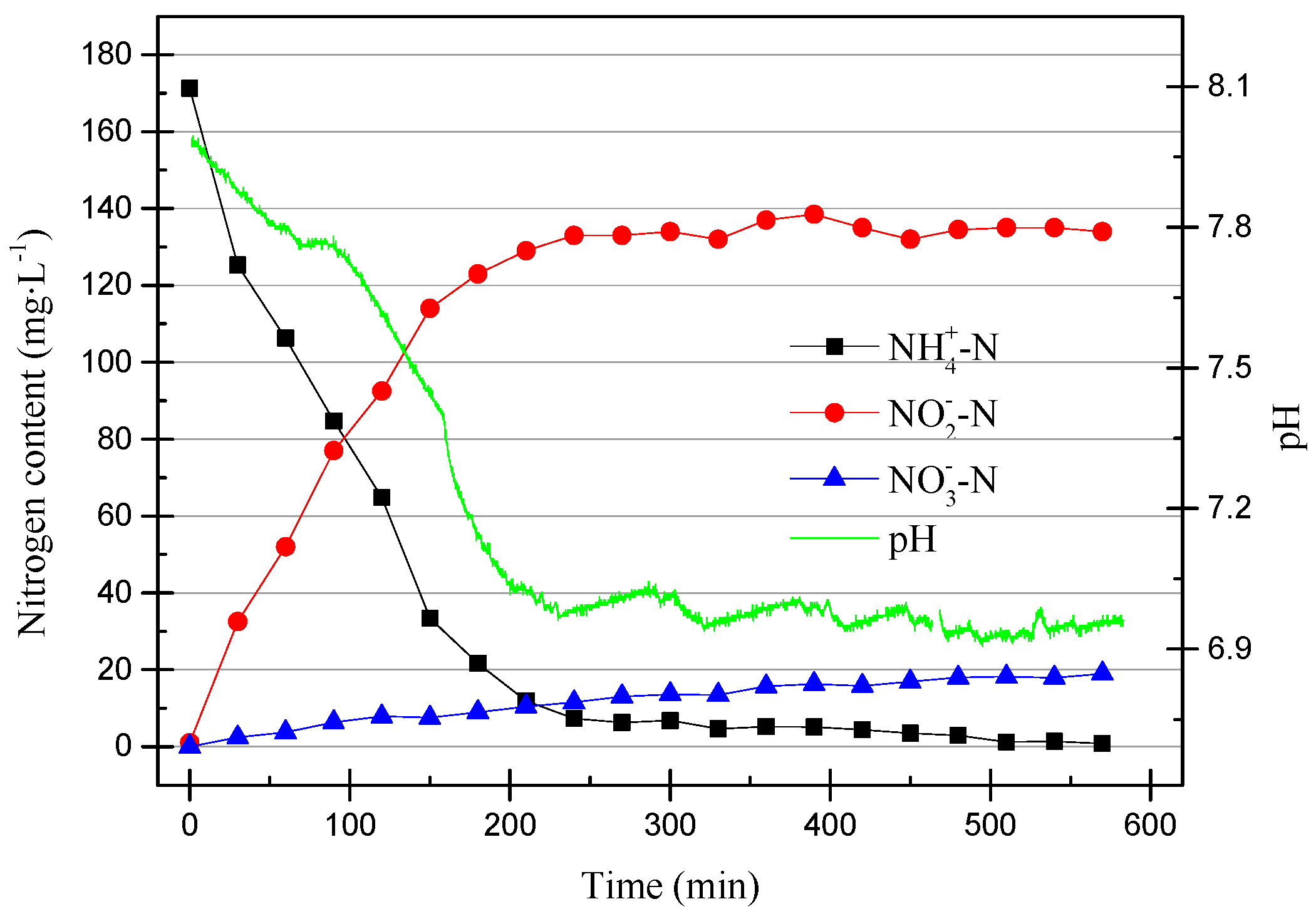
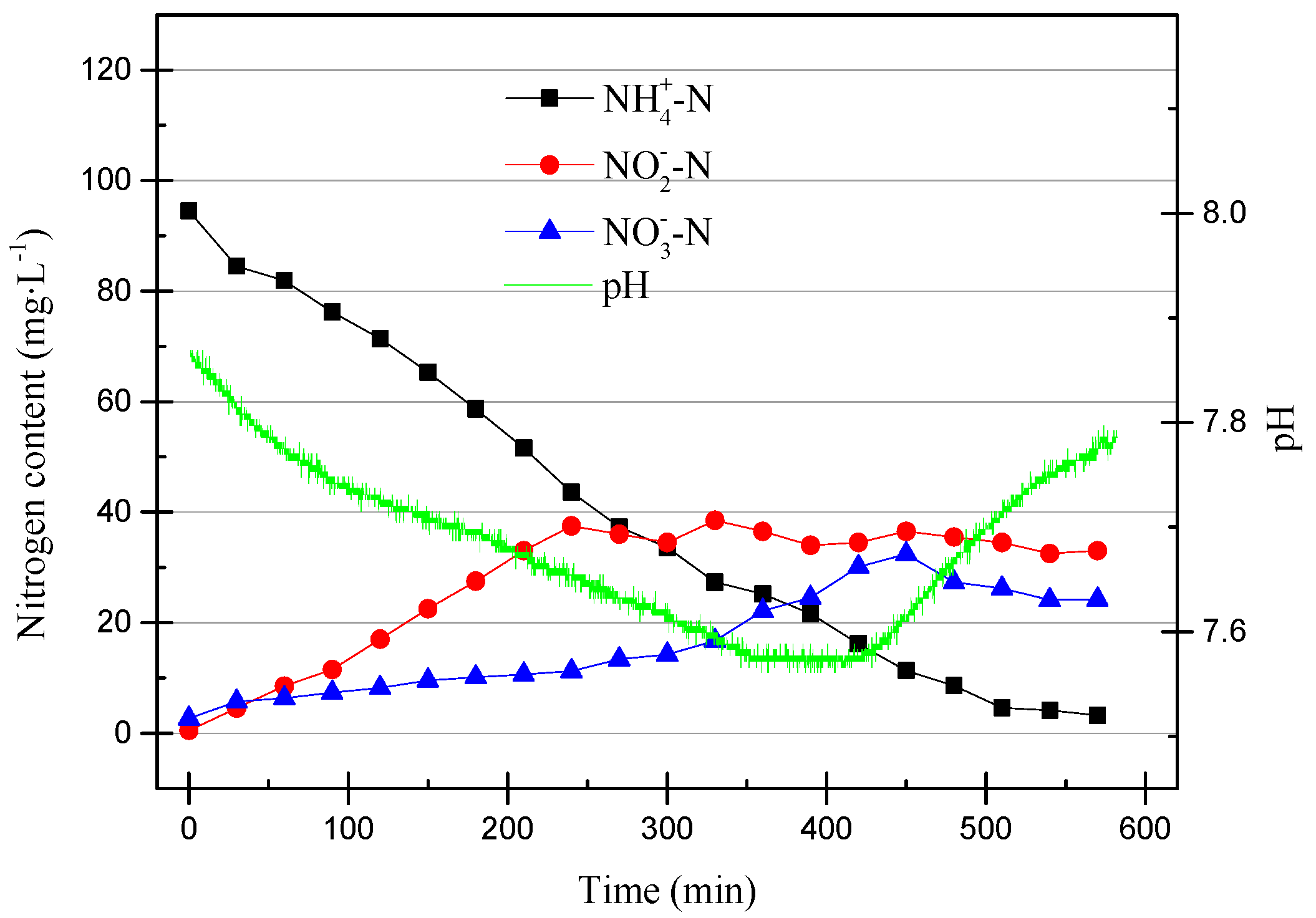
As can be seen from Figure 6, the pH dropped rapidly within the first 200 min and then stabilized at around pH of 6.9. At the same time, NO2−–N increased as the pH decreased. This was due to the ammonia oxidation reaction, which AOB participates in. The reaction is shown in Equation (1).
AOB consumes HCO3− to synthesize cells, NH4+–N is oxidized by O2 to NO2−–N, and the consumption of HCO3− leads to a decrease in pH. In the data shown in Figure 6, it can be seen that NO3−–N underwent a slight increase, while the TN was basically unchanged. The increase of NO3−–N may have been due to the effect of a small amount of active NOB or NO2−–N being oxidized by O2. In addition, it can be found that after the pH was reduced to about 6.9, the nitrification reaction basically stopped, and NH4+–N was also consumed. After the partial nitrification activity improves, the reaction time can be shortened appropriately to improve efficiency.
In the 215th cycle, the DO range was reduced to 0.2–0.6 mg/L (0.4 mg/L on average). The data from the 215th cycle are shown in Figure 7.
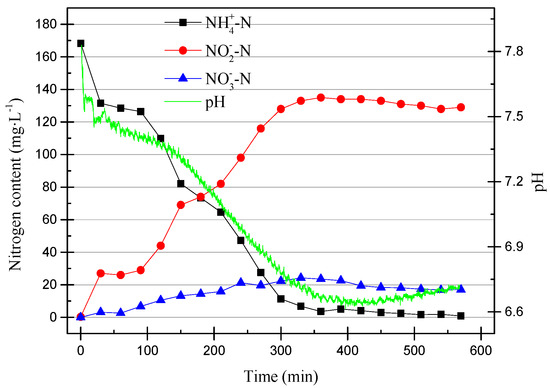
Figure 7.
The operating data for the 215th cycle.
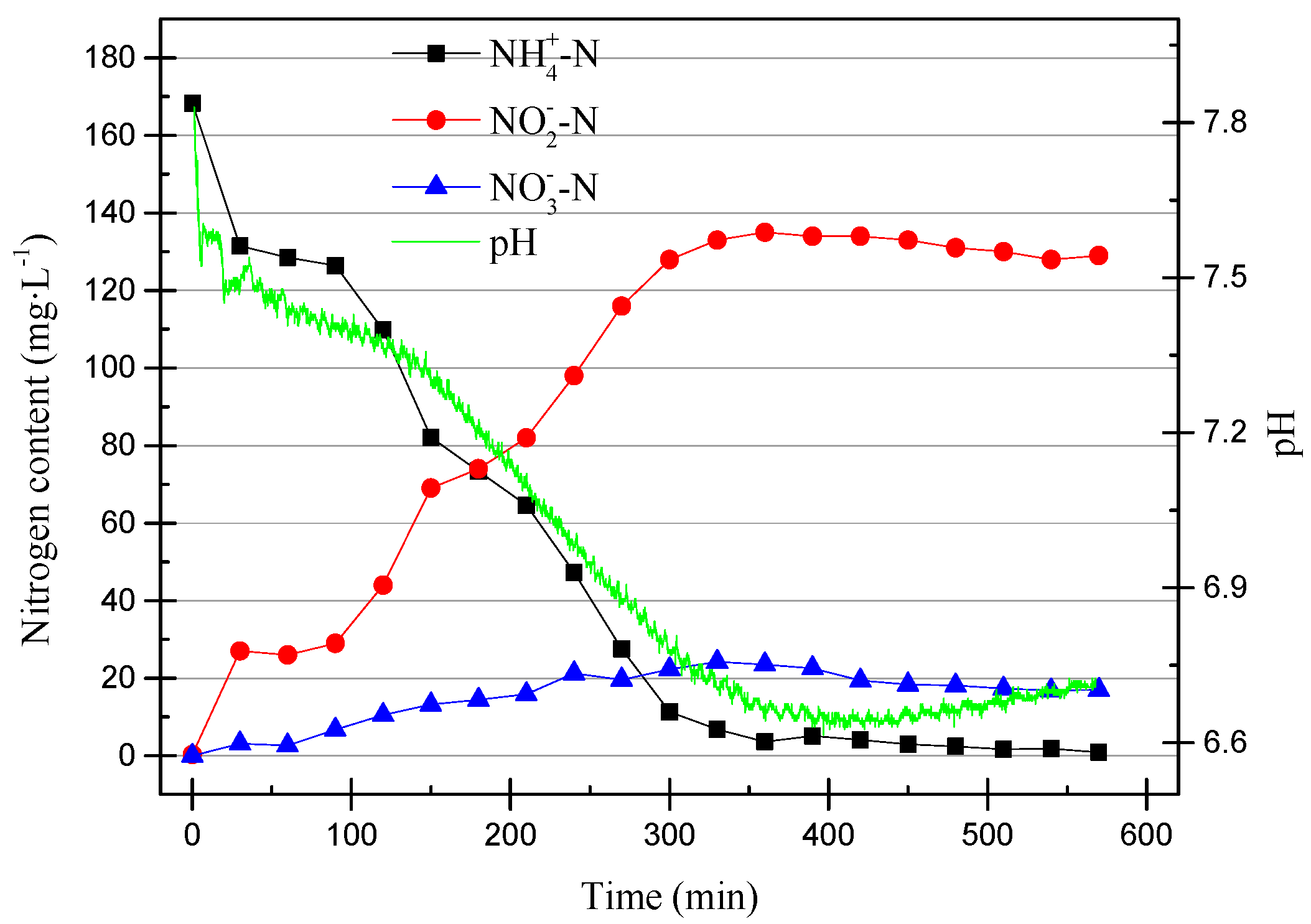
It can be clearly seen in Figure 7 that the removal efficiency of NH4+–N decreased after the dissolved oxygen content decreased. The ammonia oxidation efficiency reduced because the DO is also one of the factors that affects the activity of AOB. The denitrification reaction occurred before the end of the reaction stage because the dissolved oxygen was reduced, which provided suitable conditions for the denitrification reaction. The data were already stable and so the partial nitrification operation was stopped after 218 cycles. In order to compare the difference between pH control and DO control, another set of partial nitrification was started using the SBR. The working conditions were the same as in the previous group, except that the pH range was controlled at 7.6–7.8. When running to the 11th cycle, the DO range was adjusted to 0.4–0.8 mg/L (0.6 mg/L on average). The data from the 11th cycle are shown in Figure 8.
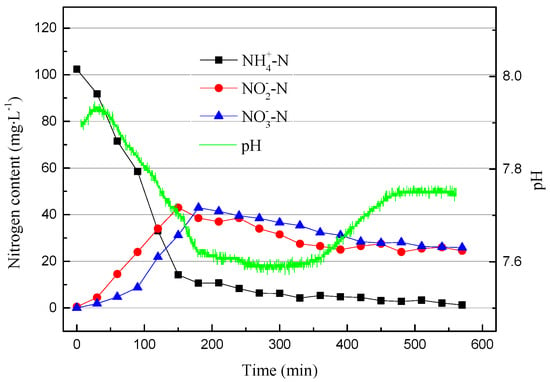
Figure 8.
The operating data for the 11th cycle.
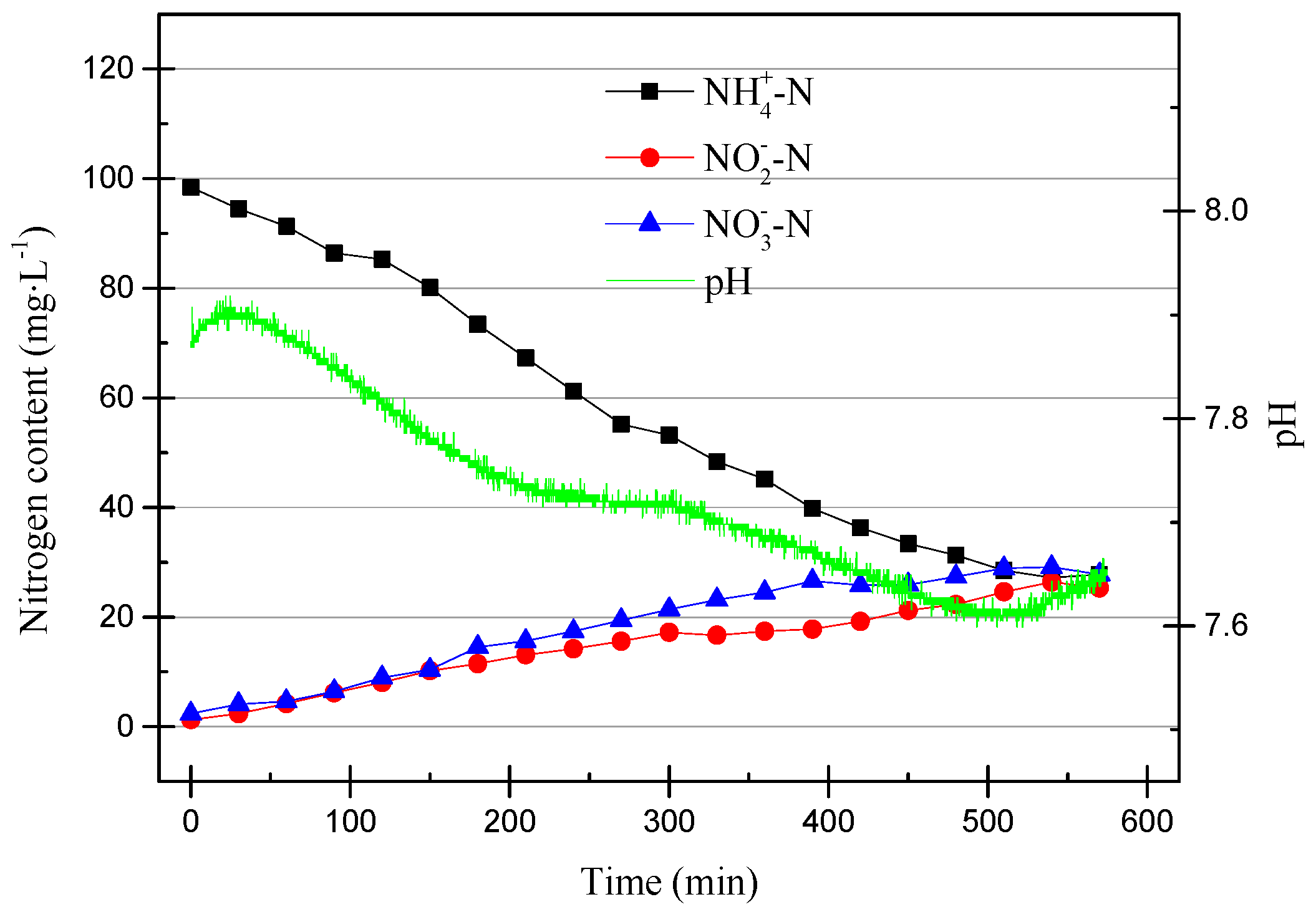
Compared with the partial nitrification process that ran stably, there were significant differences in the early operation of the SBR. First, the online pH meter detected that the initial pH increase was due to autolysis in the early stages of the operation [22]. The pH dropped first and then stabilized, and the final increase was the result of the combined actions of nitrification and denitrification. In the early stage of the SBR operation, there was a large amount of denitrifying bacteria in the SBR. Their activity and cell lysis caused this pH change.
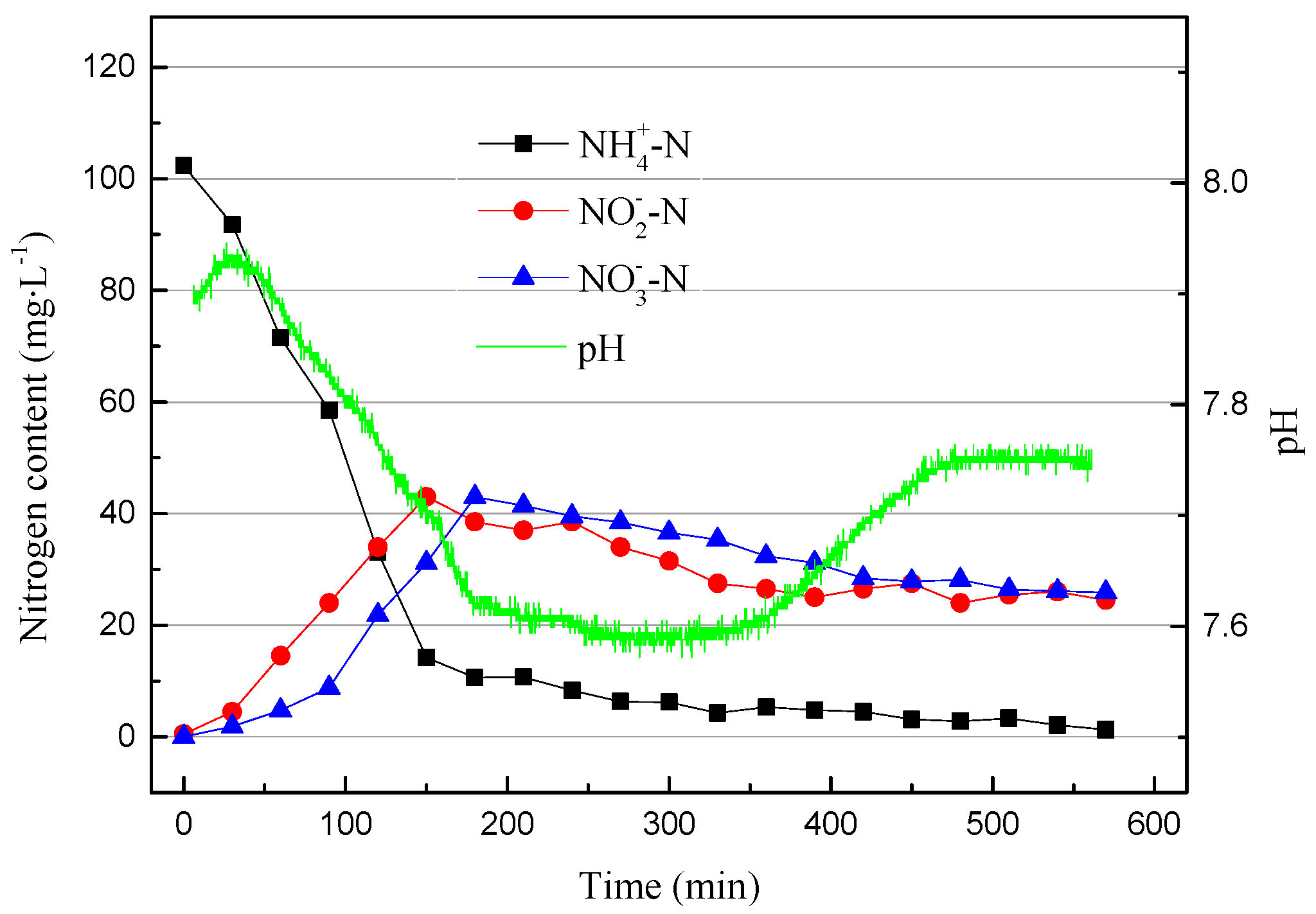
The DO range was adjusted to 0.2–0.6 mg/L (0.4 mg/L on average) in the 13th cycle, and the operating data are shown in Figure 9.
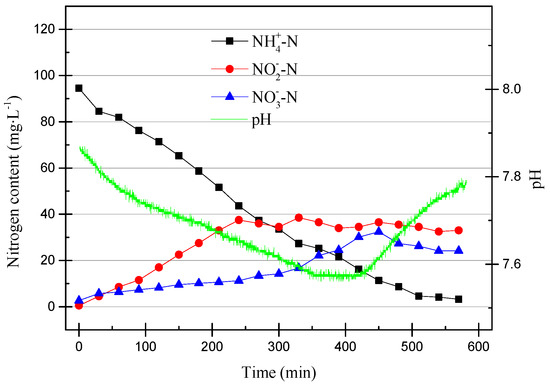
Figure 9.
The operating data for the 13th cycle.
In addition to the significant nitrification activity and denitrification reaction shown in Figure 9, the nitrite accumulation efficiency was higher than that in the 11th cycle, because it is difficult for NOB to obtain O2 and oxidize NO2−–N to NO3−–N when DO is low. In addition, shortening the reaction time to 6 h is beneficial to the SBR in starting partial nitrification.
The DO range was adjusted to 0–0.4 mg/L (0.2 mg/L on average) in the 15th cycle, and the operating data are shown in Figure 10.
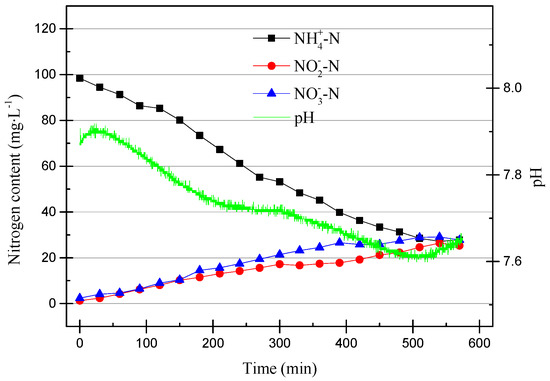
Figure 10.
The operating data for the 15th cycle.
The ammonia oxidation efficiency was low because the dissolved oxygen was further reduced. Denitrification activity did not occur in this cycle.
4. Conclusions
First, the inorganic carbon source content of the SBR-fed water was controlled at a low concentration, and the pH was controlled at 8.2–8.5, so the activity of nitrifying bacteria was suppressed. Because AOB are more suitable in a high pH and a low dissolved oxygen environment than NOB, the amount of NOB decreased faster than that of AOB. The alkalinity was increased to provide AOB with an inorganic carbon source. The results prove that increasing the HCO3− concentration in the influent to 300 mg/L can stimulate the nitrifying bacteria, improving their activity. Through first inhibiting the activity of the nitrifying bacteria at a high pH and then stimulating them by increasing the influent inorganic carbon source, partial nitrification can be initiated faster than by controlling the DO. The partial nitrification initiated by the pH in this inhibition–stimulation mode is also more stable than in the controlled DO mode [10]. Increasing the concentration of ammonia in the influent is conducive to increasing the efficiency of nitrite accumulation. An increased ammonia concentration stimulates AOB to obtain more O2 under low levels of dissolved oxygen, further reducing the possible O2 content obtained by NOB. Therefore, properly increasing the concentration of NH4+–N in the influent water is conducive to improving the efficiency of nitrite accumulation.
DO and pH are decisive factors for the partial nitrification process. During the partial nitrification start-up phase, the DO range should be limited to 0.2–0.8 mg/L, and the pH should be controlled at 8.2–8.5. A low dissolved oxygen content can inhibit NOB activity, and a high pH is beneficial for inhibiting the activity of denitrifying bacteria. In order to improve the overall nitrite accumulation efficiency during the SBR operation, a low DO content is beneficial for suppressing NOB and reducing denitrification activity in the early stage of operation. After partial nitrification is stabilized, increasing the dissolved oxygen content is beneficial for shortening the reaction time, and partial nitrification initiated by controlling the pH is more difficult to disturb than partial nitrification initiated by controlling DO. This work is of significance in making it easier to start partial nitrification and ensure the stability of this operation.
Author Contributions
Conceptualization, Y.S., Y.Y. and C.C.; methodology, Y.S., Y.Y. and C.C.; investigation, C.C.; writing—original draft preparation, C.C.; writing—review and editing, Y.S. and C.C.; supervision, Y.S.; funding acquisition, Y.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a Major Project of the High-tech Research Institute of Beijing University of Chemical Technology.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Du, R.; Cao, S.B.; Peng, Y.Z.; Zhang, H.Y.; Wang, S.Y. Combined Partial Denitrification (PD)-Anammox: A method for high nitrate wastewater treatment. Environ. Int. 2019, 126, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Li, J.L.; Li, J.W.; Peng, Y.Z.; Wang, S.Y.; Zhang, L.; Yang, S.H.; Li, S. Insight into the impacts of organics on anammox and their potential linking to system performance of sewage partial nitrification-anammox (PN/A): A critical review. Bioresour. Technol. 2020, 300. [Google Scholar] [CrossRef] [PubMed]
- Wiesmann, U. Biological nitrogen removal from wastewater. In Biotechnics/Wastewater; Springer: Berlin/Heidelberg, Germany, 1994; Volume 51, pp. 113–154. [Google Scholar] [CrossRef]
- Hallingsørensen, B. Process chemistry and biochemistry of denitrification. In Removal of Nitrogen Compounds from Wastewater; Elsevier Science: Amsterdam, The Netherlands, 1993. [Google Scholar]
- Ahn, J.H.; Kwan, T.; Chandran, K. Comparison of Partial and Full Nitrification Processes Applied for Treating High-Strength Nitrogen Wastewaters: Microbial Ecology through Nitrous Oxide Production. Environ. Sci. Technol. 2011, 45, 2734–3740. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Tang, J.H. Nitrogen Removal with Nitrification and Denitrification via Nitrite. In Advanced Materials Research; Trans Tech Publications Ltd.: Stafa-Zurich, Switzerland, 2014; Volume 908, pp. 175–178. [Google Scholar] [CrossRef]
- Peng, Y.; Zhu, G. Biological nitrogen removal with nitrification and denitrification via nitrite pathway. Appl. Microbiol. Biotechnol. 2006, 73, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Blackburne, R.; Yuan, Z.; Keller, J. Partial nitrification to nitrite using low dissolved oxygen concentration as the main selection factor. Biodegradation 2008, 19, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Jianlong, W.; Ning, Y. Partial nitrification under limited dissolved oxygen conditions. Process Biochem. 2004, 39, 1223–1229. [Google Scholar] [CrossRef]
- Guo, J.; Peng, Y.; Wang, S.; Zheng, Y.; Huang, H.; Ge, S. Effective and robust partial nitrification to nitrite by real-time aeration duration control in an SBR treating domestic wastewater. Process Biochem. 2009, 44, 979–985. [Google Scholar] [CrossRef]
- Wan, C.; Sun, S.; Lee, D.; Liu, X.; Wang, L.; Yang, X.; Pan, X. Partial nitrification using aerobic granules in continuous-flow reactor: Rapid startup. Biores. Technol. 2013, 142, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Mosqueracorral, A.; Gonzalez, F.L.V.; Campos, J.L.; Mendez, R. Partial nitrification in a SHARON reactor in the presence of salts and organic carbon compounds. Process Biochem. 2005, 40, 3109–3118. [Google Scholar] [CrossRef]
- Zhang, X.; Jian, Z.; Zhen, H.; Xie, H.; Li, W. Effect of influent COD/N ratio on performance and N2O emission of partial nitrification treating high-strength nitrogen wastewater. Rsc Adv. 2015, 5, 61345–61353. [Google Scholar] [CrossRef]
- Guo, J.; Peng, Y.; Huang, H.; Wang, S.; Ge, S.; Zhang, J.; Wang, Z. Short- and long-term effects of temperature on partial nitrification in a sequencing batch reactor treating domestic wastewater. J. Hazard. Mater. 2010, 179, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Teichgräber, B. Alkalinity and pH-Control in Activated Sludge Plants with Nitrification. In Instrumentation, Control and Automation of Water and Wastewater Treatment and Transport Systems, Proceedings of the 5th IAWPRC Workshop Held in Yokohama and Kyoto, Japan, 26 July–3 August 1990; Elsevier Science: Amsterdam, The Netherlands, 1990. [Google Scholar] [CrossRef]
- Sinha, B.; Annachhatre, A.P. Partial nitrification—Operational parameters and microorganisms involved. Rev. Environ. Sci. Bio/Technol. 2007, 6, 285–313. [Google Scholar] [CrossRef]
- Mota, C.; Head, M.A.; Ridenoure, J.A.; Cheng, J.J.; Francis, L. Effects of Aeration Cycles on Nitrifying Bacterial Populations and Nitrogen Removal in Intermittently Aerated Reactors. Appl. Environ. Microbiol. 2006, 71, 8565–8572. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Kim, J.H.; Behera, S.K.; Park, H.S. Influence of dissolved oxygen concentration and aeration time on nitrite accumulation in partial nitrification process. Int. J. Environ. Sci. Technol. 2008, 5, 527–534. [Google Scholar] [CrossRef]
- Mousavi, S.A.; Ibrahim, S.; Aroua, M.K. Effect of carbon source on acclimatization of nitrifying bacteria to achieve high-rate partial nitrification of wastewater with high ammonium concentration. Appl. Water Sci. 2017, 7, 165–173. [Google Scholar] [CrossRef]
- Zhang, X.J.; Li, D.; Zhang, Y.L.; He, Y.P.; Zhang, J. Influence of Alkalinity on Partial Nitrification Treating Domestic Sewage and the Microbial Community in MBR. Adv. Mater. Res. 2013, 807, 1564–1569. [Google Scholar] [CrossRef]
- Wang, X.; Gao, D. In-situ restoration of one-stage partial nitritation-anammox process deteriorated by nitrate build-up via elevated substrate levels. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Wang, Y.Y.; Tang, C.J.; Chai, L.Y.; Xu, K.Q.; Song, Y.X.; Ali, M.; Zheng, P. Start-Up Characteristics of a Granule-Based Anammox UASB Reactor Seeded with Anaerobic Granular Sludge. Biomed Res. Int. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).