Filtration Process and Alternative Filter Media Material in Water Treatment
Abstract
1. Introduction
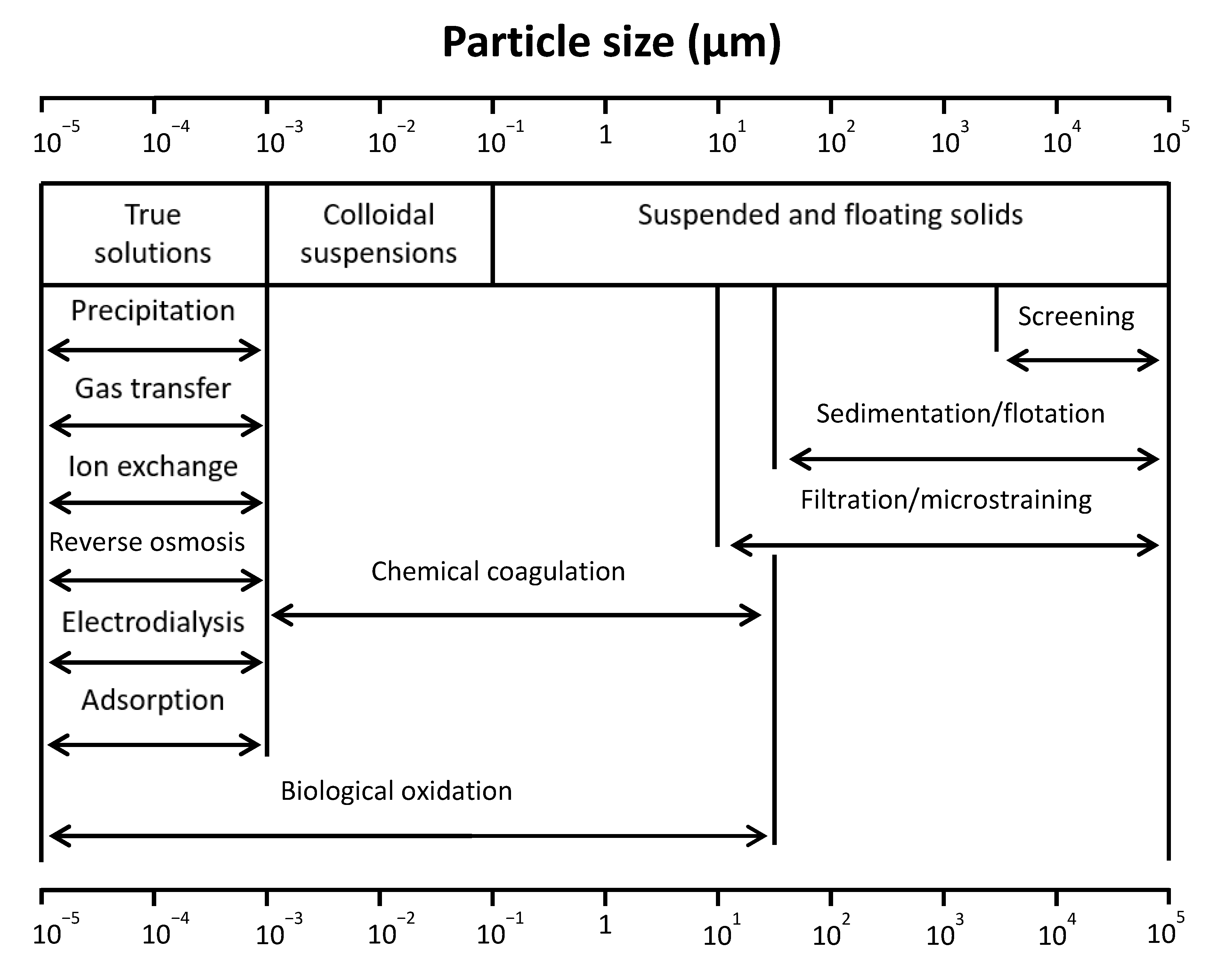
2. Filtration for Water Treatment
2.1. Filtration Process
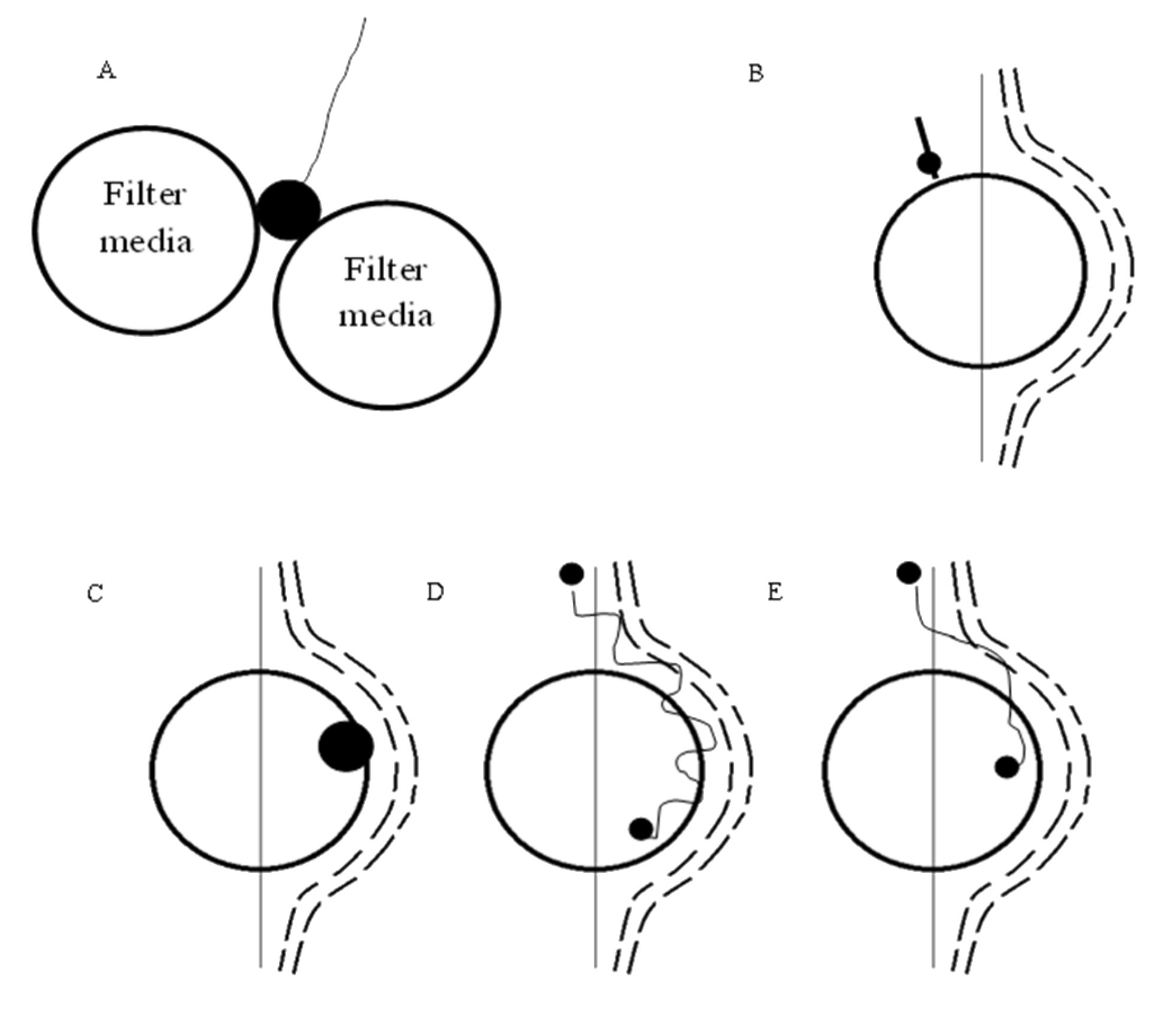
- Straining. It is not desirable as collected particles clog the upper part of the bed (blinding), preventing an efficient use of the filter [12].
- Sedimentation. This is favoured when the density of the suspended material is greater than that of water. The particle will deviate from the streamline because of gravity and it will impact the medium surface [12,20]. This depends upon particle density and temperature [16], the diameter of the particle and more generally on the ratio between the settling velocity of the particle and the velocity of the fluid approaching the media [12]. Larger particles and lower filtration velocities will lead to higher collection efficiency for this mechanism [9].
- Interception. This occurs when a particle is transiting within a distance equal to its radius from the surface of the grain. The contact between the particle and the grain can result in attachment (12). The mechanism is very similar to straining, but smaller particles are involved [6,12,21]; it depends on the ratio of the particle diameter to the media diameter [12]. Its efficiency increases with increasing particle size and decreasing collector size [9].
- Diffusion. This is due to the thermal energy of the fluid, which is transferred to the particles. This causes them to drift from the streamlines to impact the surface of the grain or on other particles [9]. As mentioned previously, diffusion is efficient for sizes below 1 µm because viscous drag is not restricting the particles; the lower the particle size, the more significant the mechanism [12].
- Furthermore, every particle is subjected to hydrodynamic action, caused by the velocity gradients within pore openings. As it experiences higher velocities on one side, the particle tends to rotate and create an additional spherical field, which causes the particle to move across the flow field. Because of deformable non-spherical shapes and non-ideal flow conditions, the results are non-predictable random paths, leading to movement across the streamlines and collision with the grains [12,23]. This is usually negligible; however, it appears to be more effective for lower particle–grain size ratios [11].
2.2. Filtration Operating Setup
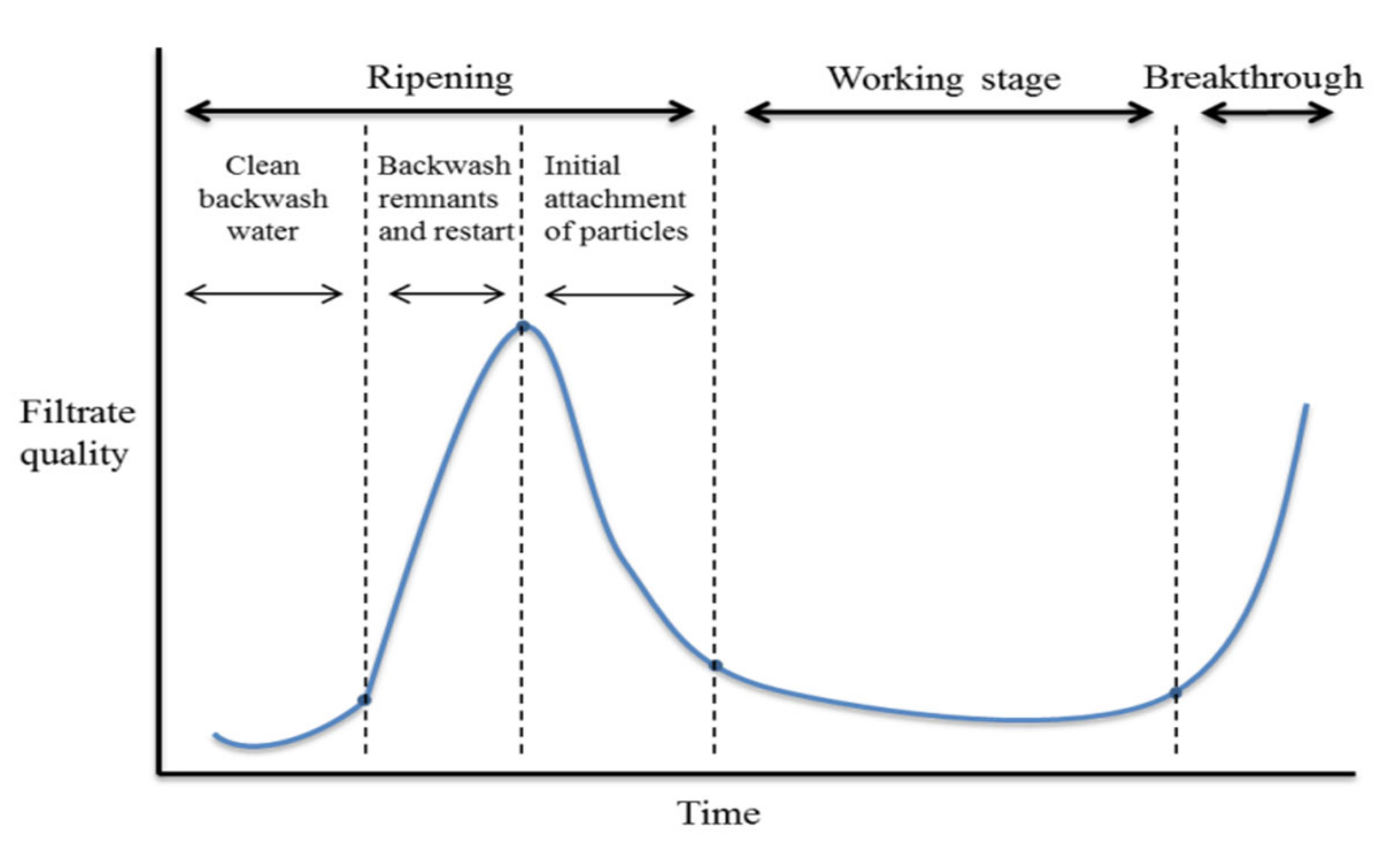
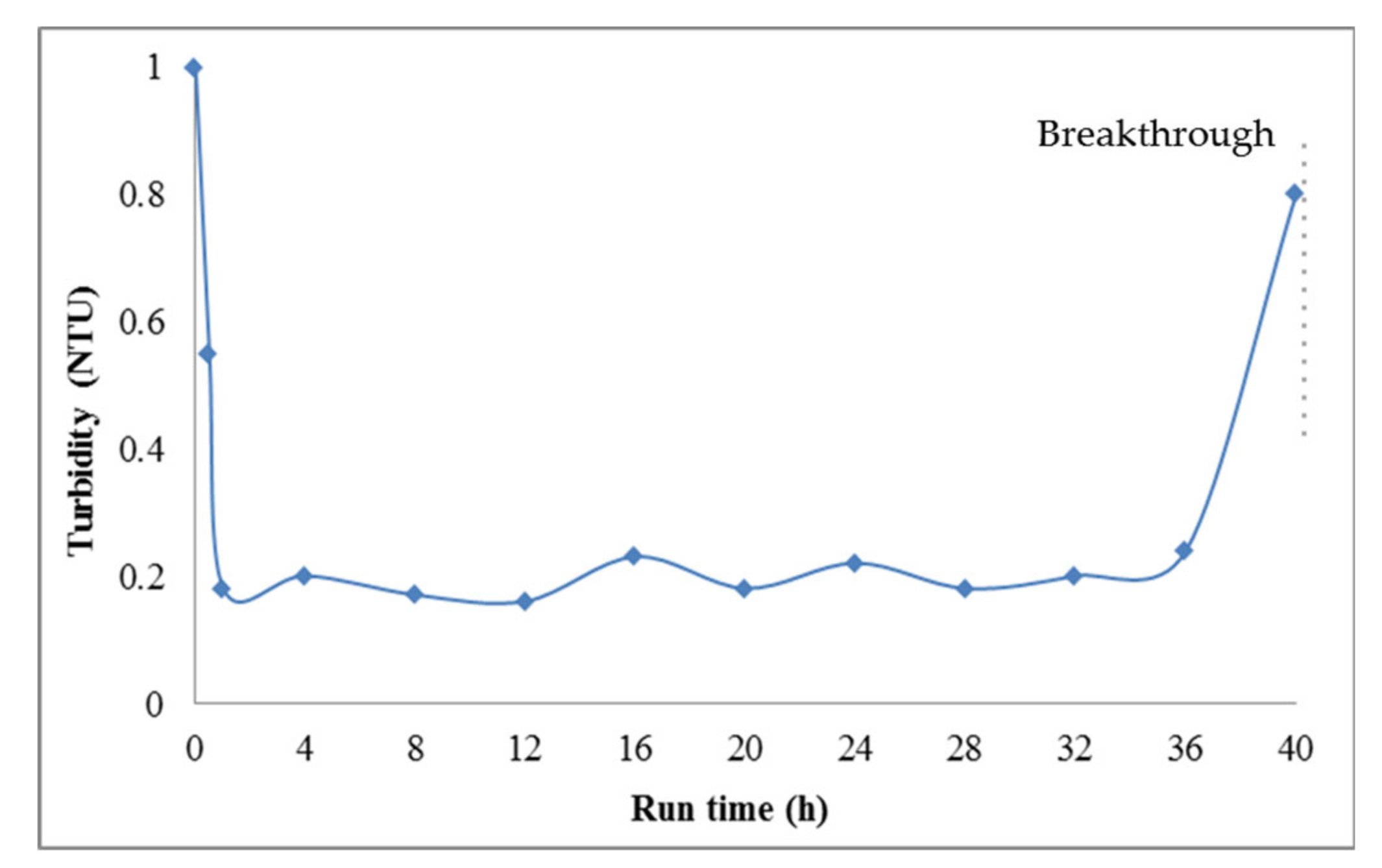
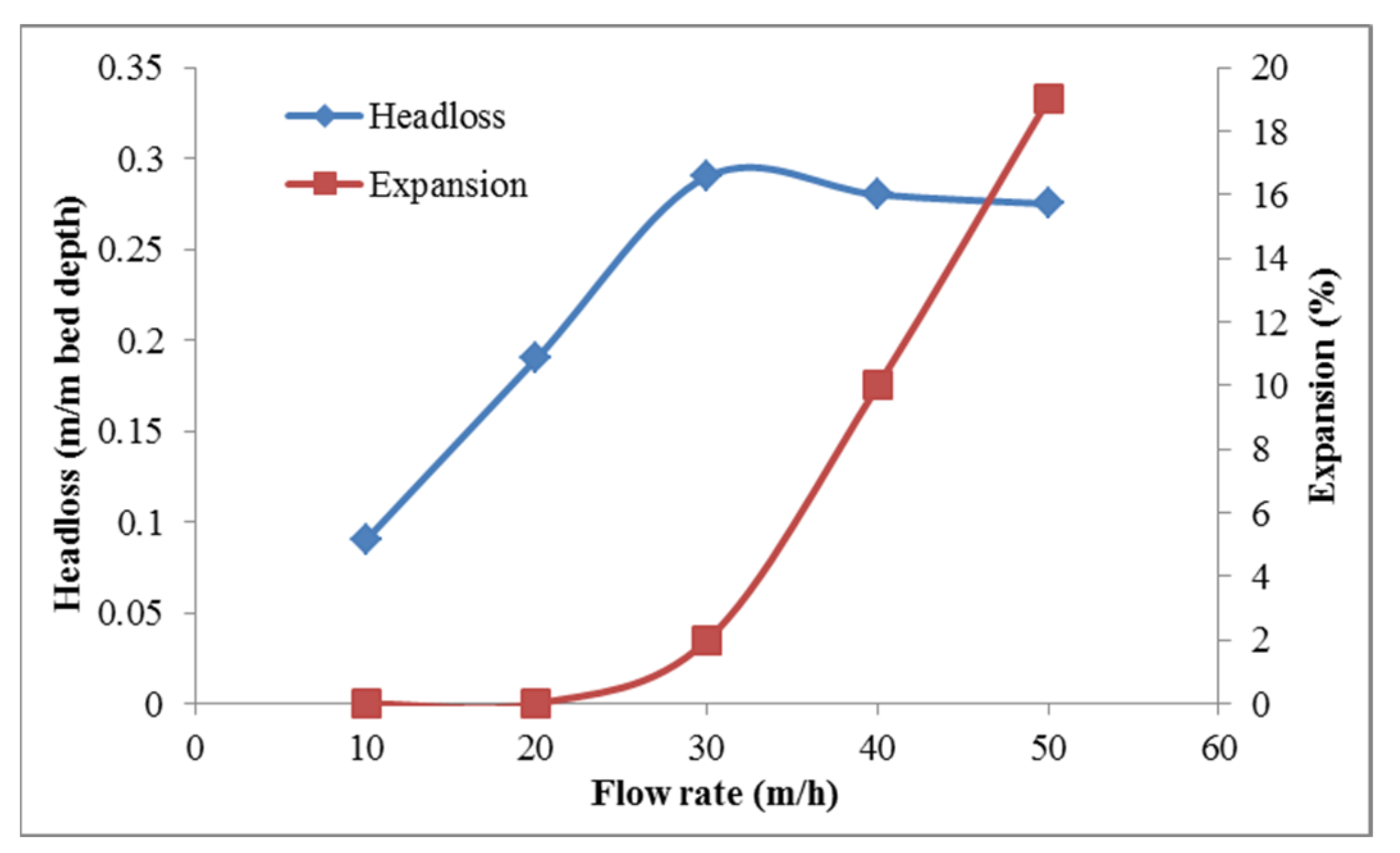
2.3. Process Performance Monitoring and Filter Backwash
3. Development and Testing of New Filtration Media
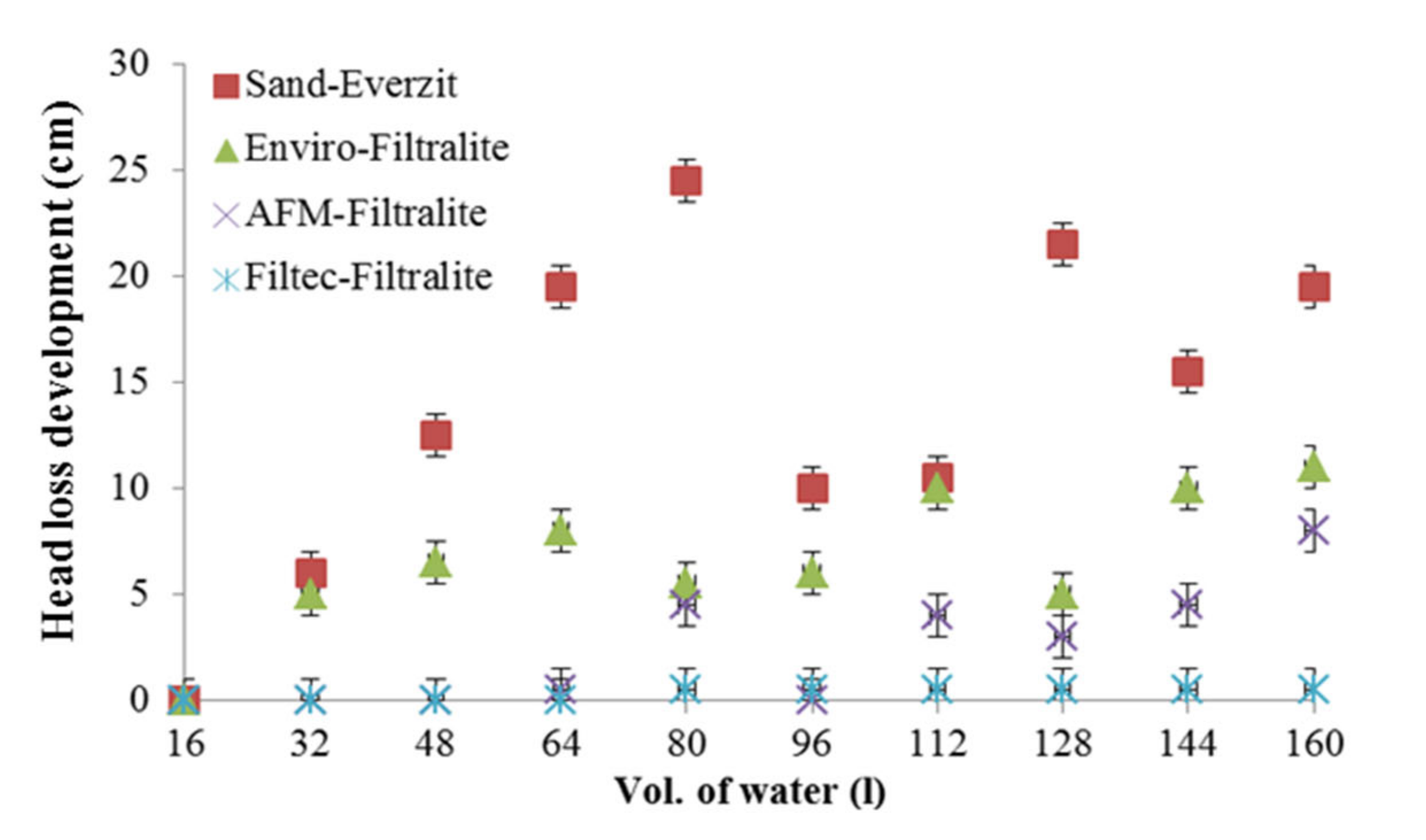
3.1. Expanded Aluminosilicate–Filtralite
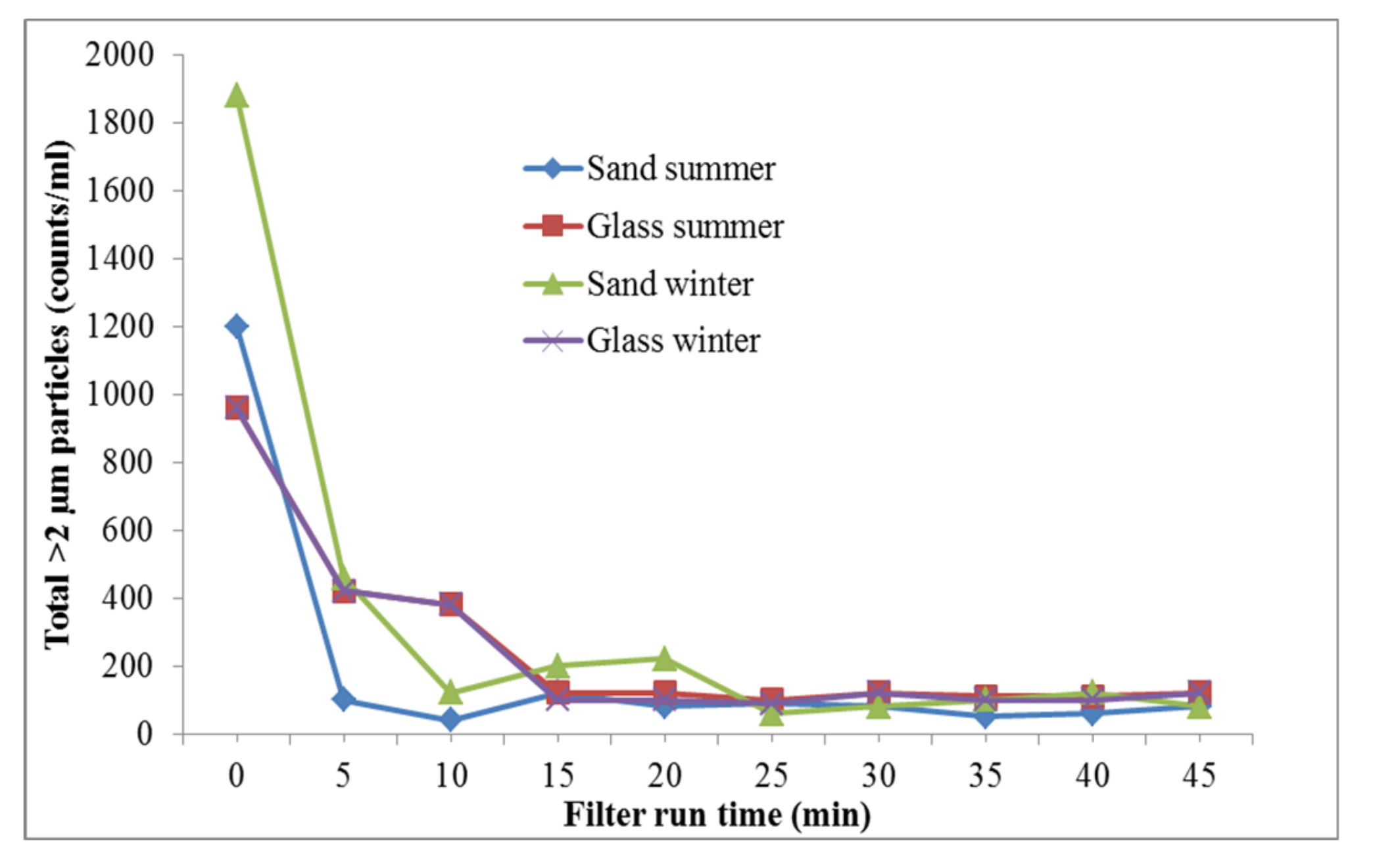
3.2. Glass-Based Media
3.3. Polypropylene Fibre
3.4. Sand with Granular Activated Carbon
4. Conclusions and Future Work Recommendation
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Binnie, C.; Kimber, M. Basic Water Treatment, 5th ed.; ICE Publishing: London, UK, 2013. [Google Scholar]
- LeChevallier, M.W.; Au, K.K. Water Treatment and Pathogen Control—Process Efficiency in Achieving Safe Drinking Water; WHO Drinking Water Quality Series; IWA Publishing: London, UK, 2004. [Google Scholar]
- Ritson, J.P.; Graham, N.J.D.; Templeton, M.R.; Clark, J.M.; Gough, R.; Freeman, C. The impact of climate change on the treatability of dissolved organic matter (DOM) in upland water supplies: A UK perspective. Sci. Total Environ. 2014, 473–474, 714–730. [Google Scholar] [CrossRef] [PubMed]
- Cheremisinoff, N.P. Handbook of Water and Wastewater Treatment Technologies; Butterworth-Heinemann: Oxford, UK, 2002. [Google Scholar]
- Ratnayaka, D.D.; Brandt, M.J.; Johnson, K.M. Twort’s Water Supply, 6th ed.; Butterworth-Heinemann: Oxford, MA, USA, 2009. [Google Scholar]
- Tebbutt, T.H.Y. Principles of Water Quality Control, 5th ed.; Butterworth-Heinemann: Oxford, UK, 1998. [Google Scholar]
- McGivney, W.; Kawamura, S. Cost Estimating Manual for Water Treatment Facilities; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008. [Google Scholar]
- Gray, N.F. Water Technology, 3rd ed.; IWA Publishing: London, UK, 2010. [Google Scholar]
- O’Melia, C.R. Particles, Pretreatment and Performance in water filtration. J. Environ. Eng. 1985, 111, 874–890. [Google Scholar] [CrossRef]
- Jegatheesan, V.; Vigneswaran, S. Deep Bed Filtration: Mathematical Models and Observations. Crit. Rev. Environ. Sci. Technol. 2005, 35, 515–569. [Google Scholar] [CrossRef]
- Ison, C.R.; Ives, K.J. Removal mechanisms in deep bed filtration. Chem. Eng. Sci. 1969, 24, 717–729. [Google Scholar] [CrossRef]
- Ives, K.J. Rapid Filtration. Water Res. 1970, 4, 201–223. [Google Scholar] [CrossRef]
- Zamani, A.; Maini, B. Flow of dispersed particles through porous media—Deep bed filtration. J. Pet. Sci. Eng. 2009, 69, 71–88. [Google Scholar] [CrossRef]
- Cleasby, J.L.; Logsdon, G.S. Granular bed and precoat filtration. In Water Quality and Treatment: A Handbook of Community Water Supplies; Letterman, R.D., Ed.; McGraw-Hill: New York, NY, USA, 1999. [Google Scholar]
- Rajagopolan, R.; Tien, C. Single collector analysis of collection mechanisms in water filtration. Can. J. Chem. Eng. 1977, 55, 246–255. [Google Scholar] [CrossRef]
- Amirtharajah, M. Some theoretical and conceptual views of filtration. J. Am. Water Works Assoc. 1988, 80, 36–46. [Google Scholar] [CrossRef]
- O’Melia, C.R.; Ali, W. The role of retained particles in deep bed filtration. Prog. Water Technol. 1978, 10, 167–182. [Google Scholar]
- Han, S.; Fitzpatrick, C.; Wetherill, A. Mathematical modelling of particle removal and head loss in rapid gravity filtration. Sep. Sci. Technol. 2008, 43, 1798–1812. [Google Scholar] [CrossRef]
- Hess, A.; Logsdon, G.S.; Chipps, M.J.; Rachwal, A.J. Filter Maintenance and Operations Guidance Manual; AWWA Research Foundation and American Water Works Association: Denver, CO, USA, 2002. [Google Scholar]
- Yao, K.-M.; Habibian, M.T.; O’Melia, C.R. Water and waste water filtration. Concepts and applications. Environ. Sci. Technol. 1971, 5, 1105–1112. [Google Scholar] [CrossRef]
- Stevenson, D.G. Flow and filtration through granular media—The effect of grain and particle size dispersion. Water Resour. 1997, 31, 310–322. [Google Scholar] [CrossRef]
- Ives, K.J. Filtration of Clay Suspensions through Sand. Clay Miner. 1987, 22, 49–61. [Google Scholar] [CrossRef]
- Ives, K.J. Capture mechanisms in filtration. In The Scientific Basis of Filtration; Noordhoff: Leyden, The Netherlands, 1975. [Google Scholar]
- Castro, K.; Ahmed, R. Filtration. In Operational Control of Coagulation and Filtration Processes—Manual of Water Supply Practices, M37, 3rd ed.; American Water Works Association (AWWA): Denve, CO, USA, 2011. [Google Scholar]
- Kim, J.; Tobiason, J. Particles in filter effluent: The roles of deposition and detachment. Environ. Sci. Technol. 2004, 38, 6132–6138. [Google Scholar] [CrossRef]
- Bai, R.; Tien, C. Particle detachment in deep bed filtration. J. Colloid Interface Sci. 1997, 186, 307–317. [Google Scholar] [CrossRef]
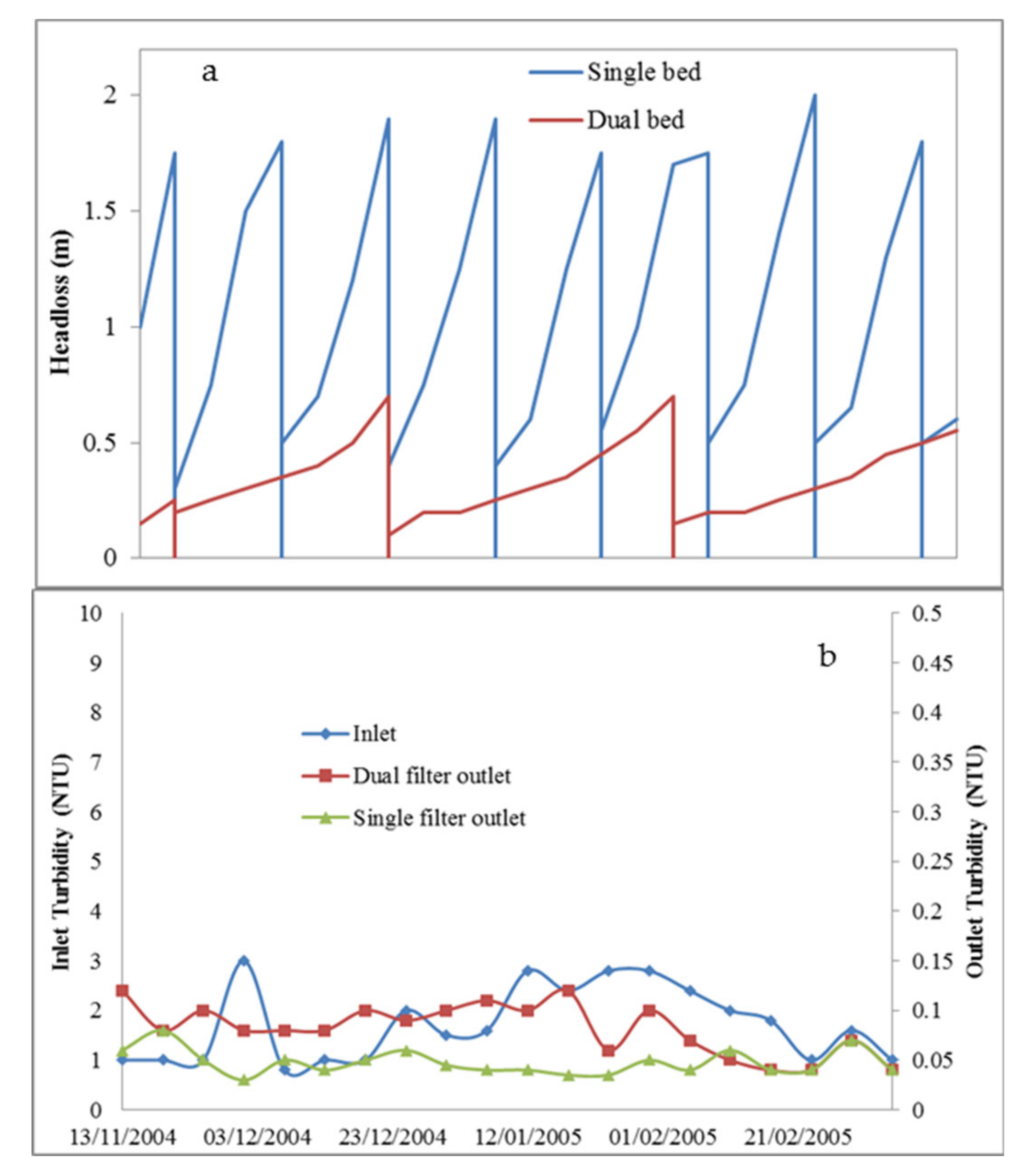
- Zouboulis, A.; Traskas, G.; Samaras, P. Comparison of single and dual media filtration in a full-scale drinking water treatment plant. Desalination 2007, 213, 334–342. [Google Scholar] [CrossRef]
- Asano, T.; Burton, F.; Leverenz, H. Removal of Residual Particulate Matter. In Water Reuse: Issues, Technologies and Application; McGraw-Hill: New York, NY, USA, 2007. [Google Scholar]
- Voutchkov, N. Considerations for selection of seawater filtration pretreatment system. Desalination 2010, 261, 354–364. [Google Scholar] [CrossRef]
- Logsdon, G.S.; Horsley, M.B.; Freeman, S.D.N.; Neemann, J.J.; Budd, G.C. Filtration processes—A distinguished history and a promising future. J. Am. Water Works Assoc. 2006, 98, 150–162. [Google Scholar] [CrossRef]
- Collins, M.R.; Eighmy, T.T.; Fenstermacher, J.M.; Spanos, S.K. Using granular media amendments to enhance NOM removal. J. Am. Water Works Assoc. 1996, 88, 48–61. [Google Scholar] [CrossRef]
- Twort, A.C.; Ratnayaka, D.D.; Brandt, M.J. Water Supply, 3rd ed.; Butterworth-Heinemann: Oxford, MA, USA, 2000. [Google Scholar]
- Fitzpatrick, C. Media properties and their effect on filter performance and backwashing. Water Sci. Technol. 1998, 38, 105–111. [Google Scholar] [CrossRef]
- Stevenson, D.G. The specification of filtering materials for rapid-gravity filtration. J. Inst. Water Environ. Manag. 1994, 8, 527–533. [Google Scholar] [CrossRef]
- Stevenson, D.G. Process conditions for the backwashing of filters with simultaneous air and water. Water Res. 1995, 29, 2594–2597. [Google Scholar] [CrossRef]
- Morgeli, B.; Ives, K.J. New media for effluent filtration. Water Res. 1979, 13, 1001–1007. [Google Scholar] [CrossRef]
- Ives, K.J. Specification for granular filter media. Effl. Water Treat. J. 1975, 15, 296–305. [Google Scholar]
- Humby, M.S.; Fitzpatrick, C.S.B. Attrition of granular filter media during backwashing with combined air and water. Water Res. 1996, 30, 291–294. [Google Scholar] [CrossRef]
- Slavik, I.; Jehmlich, A.; Uhl, W. Impact of backwashing procedures on deep bed filtration productivity in drinking water treatment. Water Res. 2013, 47, 6348–6357. [Google Scholar] [CrossRef]
- Farizoglu, B.; Nuhoglu, A.; Yildiz, E.; Keskinler, B. The performance of pumice as a filter bed material under rapid filtration conditions. Filtr. Sep. 2003, 40, 41–47. [Google Scholar] [CrossRef]
- Suthaker, S.; Smith, D.W.; Stanley, S.J. Evaluation of filter media for upgrading existing filter performance. Environ. Technol. 1995, 16, 625–643. [Google Scholar] [CrossRef]
- Kitis, M.; Kaplan, S.; Karakaya, E. Adsorption of natural organic matter from waters by iron coated pumice. Chemosphere 2007, 66, 130–138. [Google Scholar] [CrossRef]
- Ndi, K.; Dihang, D.; Aimar, P.; Kayem, G.J. Retention of bentonite in granular natural pozzolan: Implications for water filtration. Sep. Sci. Technol. 2008, 43, 1621–1631. [Google Scholar] [CrossRef]
- Drinking Water Inspectorate. List of Approved Products for Use in Public Water Supply in the United Kingdom; Drinking Water Inspectorate: London, UK, 2016.
- Mitrouli, S.T.; Yiantsios, S.G.; Karabelas, A.J. Pretreatment for desalination of seawater from an open intake by dual-media filtration: Pilot testing and comparison of two different media. Desalination 2008, 222, 24–37. [Google Scholar] [CrossRef]
- Mitrouli, S.T.; Karabelas, A.J.; Yiantsios, S.G.; Kjølseth, P.A. New granular materials for dual-media filtration of seawater: Pilot testing. Sep. Purif. Technol. 2009, 65, 147–155. [Google Scholar] [CrossRef]
- Saltnes, T.; Elkebrokk, B.; Odegaard, H. Contact filtration of humic waters: Performance of an expanded clay aggregate filter (Filtralite) compared to a dual anthracite/sand filter. Water Sci. Technol. Water Supply 2002, 2, 17–23. [Google Scholar] [CrossRef]
- Mikol, A.; Fitzpatrick, C.; Chipps, M.J.; Steele, M.E. Novel dual media combination for drinking water treatment. Water Sci. Technol. Water Supply 2007, 7, 131–139. [Google Scholar] [CrossRef]
- Davies, P. Alternative Filter Media in Rapid Gravity Filtration of Potable Water. Ph.D. Thesis, Loughborough University, Loughborough, UK, 2011. [Google Scholar]
- Davies, P.; Wheatley, A.D. Pilot plant study of alternative filter media for rapid gravity filtration. Water Sci. Technol. 2012, 66, 2779–2784. [Google Scholar] [CrossRef]
- Eikebrokk, B.; Saltnes, T. Removal of natural organic matter (NOM) using different coagulants and lightweight expanded clay aggregate filters. Water Sci. Technol. Water Supply 2001, 1, 131–140. [Google Scholar] [CrossRef]
- Lavender, P. Filter media: Treating chemical wastewaters. Filtr. Sep. 2008, 45, 16–18. [Google Scholar] [CrossRef]
- Gill, L.W.; Veale, P.L.; Murray, M. Recycled glass compared to sand as a media in polishing filters for on-site wastewater treatment. Water Pract. Technol. 2011, 6, wpt2011058. [Google Scholar] [CrossRef]
- Korkosz, A.; Malakowska, A.; Hänel, A.; Niewiadomski, M.; Jan, H. Cullet as filter medium for swimming pool water treatment. Physicochem. Probl. Miner. Process. 2012, 48, 295–301. [Google Scholar]
- Jonsson, J.; Watts, M. Glass Media Report; Water Res. Centre: Swindon, UK, 2011. [Google Scholar]
- Water Development Services. Full Scale Operational Trials Involving the Use of Recycled Glass in Selected Markets (WRAP); The Waste & Resources Action Programme: Banbury, UK, 2005. [Google Scholar]
- Soyer, E.; Akgiray, Ö.; Eldem, N.Ö.; Saatçi, A.M. On the use of crushed recycled glass instead of silica sand in dual-media filters. Clean Soil Air Water 2013, 41, 325–332. [Google Scholar] [CrossRef]
- Soyer, E.; Akgiray, Ö.; Eldem, N.Ö.; Saatçi, A.M. Crushed recycled glass as a filter medium and comparison with silica sand. Clean Soil Air Water 2010, 38, 927–935. [Google Scholar] [CrossRef]
- Rutledge, S.O.; Gagnon, G.A. Comparing crushed recycled glass to silica sand for dual media filtration. J. Environ. Eng. Sci. 2002, 1, 349–358. [Google Scholar] [CrossRef]
- Evans, G.; Dennis, P.; Cousins, M.; Campbell, R. Use of recycled crushed glass as a filtration medium in municipal potable water treatment plants. Water Sci. Technol. Water Supply 2002, 2, 9–16. [Google Scholar] [CrossRef]
- Cescon, A.; Jiang, J.-Q.; Haffey, M.; Moore, G.; Callaghan, K. Assessment of recycled glass and expanded clay in a dual media configuration for drinking water treatment. Sep. Sci. Technol. 2016, 51, 2455–2464. [Google Scholar] [CrossRef]
- Coffey, B.M.; Krasner, S.W.; Sclimenti, M.J.; Hacker, P.A.; Gramith, J.T. A comparison of biologically active filters for the removal of ozone by-products, turbidity, and particles. In Proceedings of the Water Quality Technology Conference, New Orleans, LA, USA, 12–16 November 1995. [Google Scholar]
- Droste, R.L. Theory and Practice of Water and Wastewater Treatment; John Wiley and Sons, Inc.: New York, NY, USA, 1997. [Google Scholar]
- Piccirillo, J.B.; Letterman, R.D. Examination of Pulverized Waste Recycled Glass as Filter Media in Slow Sand Filtration; Final Report, PB-98-158959/XAB; TRN: 82432439; U.S. Department of Energy Office of Scientific and Technical Information: Washington, DC, USA, 1997.
- Morita, A.K.M.; Reali, M.A.P. Fiber filter built with polypropylene fibers applied to water clarification. Water Supply 2019, 19, 1036–1043. [Google Scholar] [CrossRef]
- Kim, J.; Kang, B. DBPs removal in GAC filter-adsorber. Water Res. 2008, 42, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Wang, T.; Wang, Y.; Sun, S.; Yu, C. Experimental study on GAC-sand filter for advanced treatment in drinking water. Adv. Mater. Res. 2013, 726–731, 3044–3047. [Google Scholar] [CrossRef]
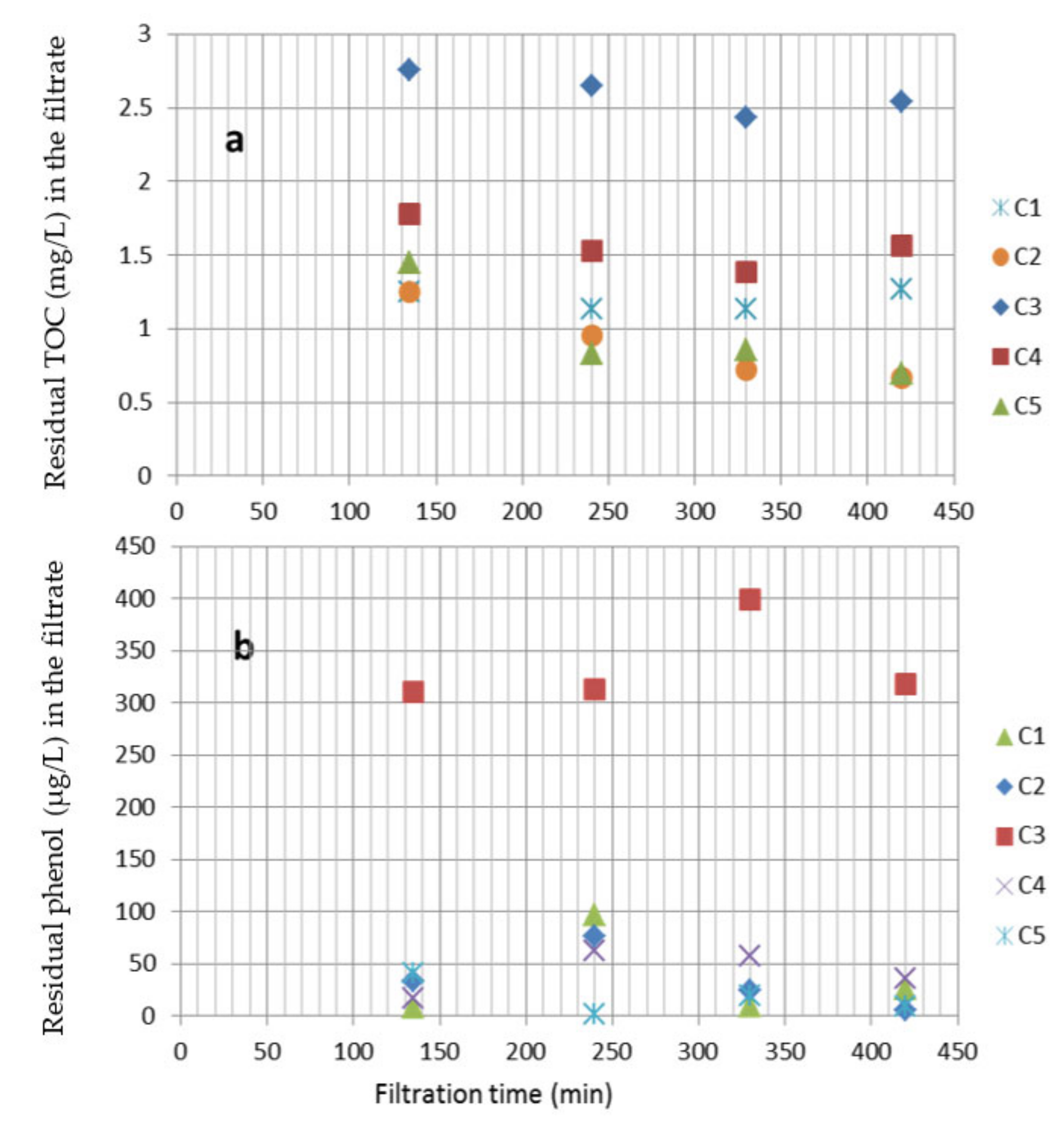
- Fuentes-López, L.; Amézquita-Marroquín, C.; Barba-Ho, L.E.; Cruz-Vélez, C.H.; Torres-Lozada, P.J. Application of double filtration with activated carbon for the removal of phenols in drinking water treatment processes. Water Supply Res. Technol. AQUA 2018, 67, 227–235. [Google Scholar] [CrossRef]

| Flow Rate (m/h)/Type of Test Water | Bed Depth (cm) Filter 1 | Size Ranges (mm) Filter 1 | Bed Depth (cm) Filter 2 | Size Ranges (mm) Filter 2 | Reference |
|---|---|---|---|---|---|
| 5, 10, 15/Raw seawater | Anthracite: 70 | 1.2–2.5 | Filtralite MC: 70 | 1.5–2.5 | [45] |
| Sand: 50 | 0.8–1.25 | Sand: 50 | 0.8–1.25 | ||
| 5, 10, 15/Raw seawater | Anthracite: 70 | 1.2–2.5 | Filtralite NC: 70 | 1.5–2.5 | [46] |
| Sand: 50 | 0.8–1.25 | Filtralite HC: 50 | 0.8–1.6 | ||
| /Tap water with added humic concentrate and/or bentonite clay | Anthracite: 60 | 0.8–1.6 | Filtralite NC: 48 | 1.5–2.5 | [47] |
| Sand: 35 | 0.4–0.8 | Filtralite HC: 47 | 0.8–1.6 | ||
| 10/Raw water | Anthracite: 50 | 1.7–2.5 | Filtralite NC: 50 | 1.5–2.5 | [48] |
| Sand: 50 | 0.6–1.18 | Filtralite HC: 50 | 0.8–1.6 | ||
| 8.6, 11.1, 13.6/Clarified water | Sand: 60 | 0.59 (d10) | Filtralite: 60 | 0.77 (d10) | [49,50] |
| 5–12/Tap water with added humic concentrate | Anthracite: 60 | 0.8–1.6 | Filtralite NC: 60 | 0.8–1.6 | [51] |
| Sand: 35 | 0.4–0.8 | Sand: 35 | 0.4–0.8 |
| Type of Configuration/Type of Test Water | Coagulant | Bed Depth (cm) | Flow Rate (m/h) | Effective Size Glass (d10, mm) | Effective Size Sand (d10, mm) | Uniformity Coefficient Glass (UC) | Uniformity Coefficient Sand (UC) | Ref |
|---|---|---|---|---|---|---|---|---|
| Dual media/Raw water | PACl | Anthracite: 60 Sand or Glass: 40 Garnet: 6 | 5 | 0.59 | 0.33 | 1.58 | 1.82 | [59] |
| Single media/Raw water | Alum (plus additional filter aid) | Sand or Glass: 90 Gravel: 10 | 7.5, 10, 12.5 | 0.98 | 0.97 | 1.31 | 1.27 | [60] |
| Single media/Raw water | Alum or Ferric Chloride | Sand or Glass: 104 | 11.5 | 0.77 | 0.79 | 1.41 | 1.33 | [58] |
| Dual media/Raw water | Alum or Ferric Chloride | Anthracite: 41.5 Sand or Glass: 62.5 | 11.5 | 0.77 | 0.79 | 1.41 | 1.33 | [57] |
| Single media/Raw water | Ferric sulphate | Sand or Glass: 60 | 0–9 | 0.76 | 0.59 | 1.21 | 1.27 | [50] |
| Single media/Tap water with added kaolin clay | No coagulation | Sand or Glass: 60 Gravel: 41 | 8.6, 11.1, 13.5 | 0.76 | 0.59 | 1.21 | 1.27 | [49] |
| Single media/Raw water | PACl | Glass: 80 | 6 | 0.56 | 0.58 | 1.28 | / | [55] |
| Configuration | Vegetable Activated Carbon (VAC) (%) | Mineral Activated Carbon (MAC) (%) | Sand (%) |
|---|---|---|---|
| C1 | 100 | - | - |
| C2 | - | 100 | - |
| C3 | - | - | 100 |
| C4 | 50 | - | 50 |
| C5 | - | 50 | 50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cescon, A.; Jiang, J.-Q. Filtration Process and Alternative Filter Media Material in Water Treatment. Water 2020, 12, 3377. https://doi.org/10.3390/w12123377
Cescon A, Jiang J-Q. Filtration Process and Alternative Filter Media Material in Water Treatment. Water. 2020; 12(12):3377. https://doi.org/10.3390/w12123377
Chicago/Turabian StyleCescon, Anna, and Jia-Qian Jiang. 2020. "Filtration Process and Alternative Filter Media Material in Water Treatment" Water 12, no. 12: 3377. https://doi.org/10.3390/w12123377
APA StyleCescon, A., & Jiang, J.-Q. (2020). Filtration Process and Alternative Filter Media Material in Water Treatment. Water, 12(12), 3377. https://doi.org/10.3390/w12123377






