Variation in Diet Patterns of the Invasive Top Predator Sander lucioperca (Linnaeus, 1758) across Portuguese Basins
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Sampling and Laboratory Procedures
2.2. Data Analyses
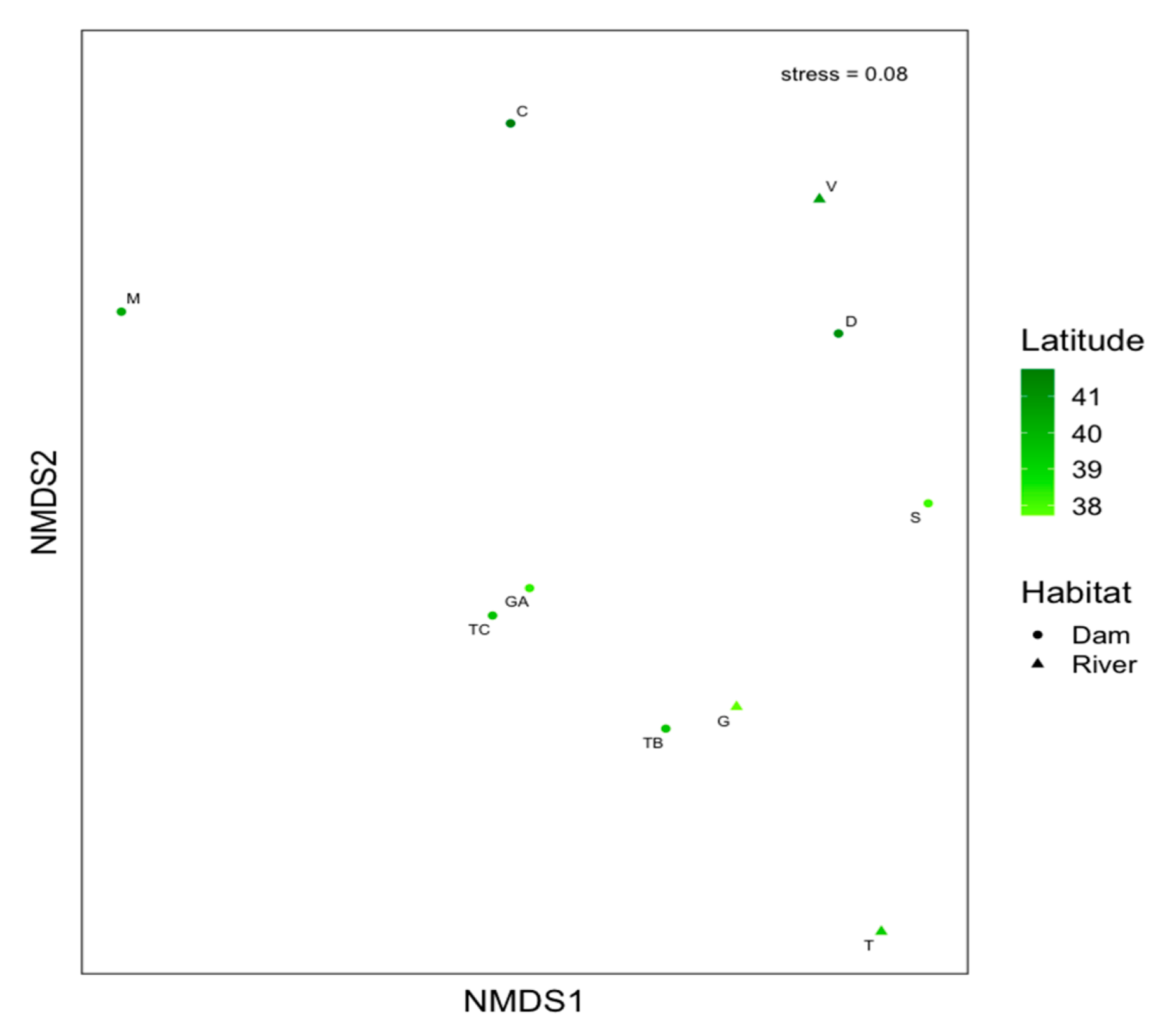
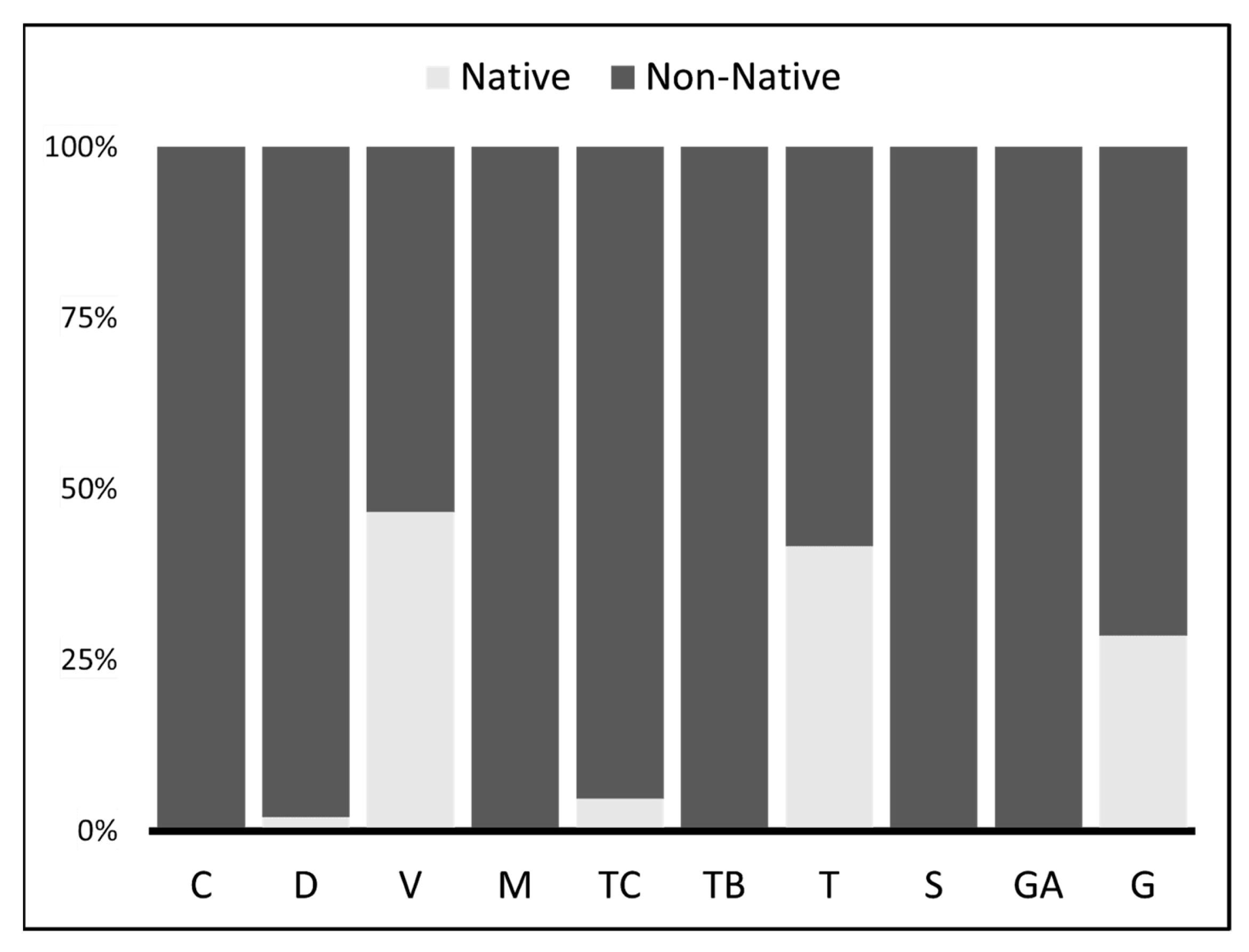
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Revenga, C.; Mock, G. Freshwater Biodiversity in Crisis; Earth Trends World Resources Institute: Washington, DC, USA, 2000; pp. 1–4. [Google Scholar]
- van Rees, C.B.; Waylen, K.A.; Schmidt-Kloiber, A.; Thackeray, S.J.; Kalinkat, G.; Martens, K.; Jähnig, S.C. Safeguarding freshwater life beyond 2020: Recommendations for the new global biodiversity framework from the European experience. Conserv. Lett. 2021, 14, e12771. [Google Scholar] [CrossRef]
- Mazor, T.; Doropoulos, C.; Schwarzmueller, F.; Gladish, D.W.; Kumaran, N.; Merkel, K.; Di Marco, M.; Gagic, V. Global mismatch of policy and research on drivers of biodiversity loss. Nat. Ecol. Evol. 2018, 2, 1071–1074. [Google Scholar] [CrossRef] [PubMed]
- World Wildlife Fund. Living Planet. Report 2018: Aiming Higher; Grooten, M., Almond, R.E.A., Eds.; WWF International: Gland, Switzerland, 2018. [Google Scholar]
- Welcomme, R.L. A history of international introductions of inland aquatic species. ICES Mar. Sci. Symp. 1992, 194, 3–14. [Google Scholar]
- Prenda, J.; Clavero, M.; Blanco-Garrido, F.; Menor, A.; Hermoso, V. Threats to the conservation of biotic integrity in Iberian fluvial ecosystems. Limnetica 2006, 25, 377–388. [Google Scholar]
- Almeida, D.; Almodóvar, A.; Nicola, G.G.; Elvira, B. Feeding tactics and body condition of two introduced populations of pumpkinseed Lepomis gibbosus: Taking advantages of human disturbances? Ecol. Freshw. Fish. 2009, 18, 15–23. [Google Scholar] [CrossRef]
- Reid, A.J.; Carlson, A.K.; Creed, I.F.; Eliason, E.J.; Gell, P.A.; Johnson, P.T.; Cooke, S.J. Emerging threats and persistent conservation challenges for freshwater biodiversity. Biol. Rev. 2019, 94, 849–873. [Google Scholar] [CrossRef]
- Strayer, D.L.; Dudgeon, D. Freshwater biodiversity conservation: Recent progress and future challenges. J. N. Am. Benthol. Soc. 2010, 29, 344–358. [Google Scholar] [CrossRef]
- Crivelli, A.J. Are fish introductions a threat to endemic freshwater fishes in the northern Mediterranean region? Biol. Conserv. 1995, 72, 311–319. [Google Scholar] [CrossRef]
- Elvira, B.; Almodóvar, A. Freshwater fish introductions in Spain: Facts and figures at the beginning of the 21st century. J. Fish. Biol. 2001, 59, 323–331. [Google Scholar] [CrossRef]
- Copp, G.H.; Bianco, P.G.; Bogutskaya, N.G.; Erős, T.; Falka, I.; Ferreira, M.T.; Kováč, V. To be, or not to be, a non-native freshwater fish? J. Appl. Ichthyol. 2005, 21, 242–262. [Google Scholar] [CrossRef]
- García-Berthou, E.; Alcaraz, C.; Pou-Rovira, Q.; Zamora, L.; Coenders, G.; Feo, C. Introduction pathways and establishment rates of invasive aquatic species in Europe. Can. J. Fish. Aquat. Sci. 2005, 62, 453–463. [Google Scholar] [CrossRef]
- Leunda, P.M. Impacts of non-native fishes on Iberian freshwater ichthyofauna: Current knowledge and gaps. Aquat. Invasions 2010, 5, 239–262. [Google Scholar] [CrossRef]
- Merciai, R.; Almeida, D.; Aparicio, E.; Cruset, E.; Fuentes, M.Á.; Pou i Rovira, Q.; García-Berthou, E. First record of the asp Leuciscus aspius introduced into the Iberian Peninsula. Limnetica 2018, 37, 341–344. [Google Scholar]
- Carpio, A.J.; De Miguel, R.J.; Oteros, J.; Hillström, L.; Tortosa, F.S. Angling as a source of non-native freshwater fish: A European review. Biol. Invasions 2019, 21, 3233–3248. [Google Scholar] [CrossRef]
- Ribeiro, F.; Collares-Pereira, M.J.; Moyle, P.B. Non-native fish in the fresh waters of Portugal, Azores and Madeira Islands: A growing threat to aquatic biodiversity. Fish. Manag. Ecol. 2009, 16, 255–264. [Google Scholar] [CrossRef]
- Anastácio, P.M.; Ribeiro, F.; Capinha, C.; Banha, F.; Gama, M.; Filipe, A.F.; Rebelo, R.; Sousa, R. Non-native freshwater fauna in Portugal: A review. Sci. Total Environ. 2019, 6, 1923–1934. [Google Scholar] [CrossRef]
- Ribeiro, F.; Leunda, P.M. Non-native fish impacts on Mediterranean freshwater ecosystems: Current knowledge and research needs. Fish. Manag. Ecol. 2012, 19, 142–156. [Google Scholar] [CrossRef]
- Collares-Pereira, M.J.; Alves, M.J.; Ribeiro, F.; Domingos, I.M.; Raposo de Almeida, P.; Moreira da Costa, L.D.; Gante, H.F.; Filipe, A.F.; Aboim, M.A.; Rodrigues, P.; et al. Guia dos Peixes de Água Doce e Migradores de Portugal Continental; Edições Afrontamento: Porto, Portugal, 2021; p. 292. [Google Scholar]
- Cucherousset, J.; Olden, J.D. Ecological impacts of nonnative freshwater fishes. Fisheries 2011, 36, 215–230. [Google Scholar] [CrossRef]
- Milardi, M.; Aschonitis, V.; Gavioli, A.; Lanzoni, M.; Fano, E.A.; Castaldelli, G. Run to the hills: Exotic fish invasions and water quality degradation drive native fish to higher altitudes. Sci. Total Environ. 2018, 624, 1325–1335. [Google Scholar] [CrossRef]
- Baxter, C.V.; Fausch, K.D.; Murakami, M.; Chapman, P.L. Fish invasion restructures stream and forest food webs by interrupting reciprocal prey subsidies. Ecology 2004, 85, 2656–2663. [Google Scholar] [CrossRef]
- Clavero, M.; Hermoso, V.; Aparicio, E.; Godinho, F.N. Biodiversity in heavily modified waterbodies: Native and introduced fish in Iberian reservoirs. Freshw. Biol. 2013, 58, 1190–1201. [Google Scholar] [CrossRef]
- Kopp, D.; Cucherousset, J.; Syväranta, J.; Martino, A.; Céréghino, R.; Santoul, F. Trophic ecology of the pikeperch (Sander lucioperca) in its introduced areas: A stable isotope approach in southwestern France. Comptes Rendus Biol. 2009, 332, 741–746. [Google Scholar] [CrossRef]
- Miñano, P.A.; Oliva-Paterna, F.J.; Torralva, M. Primera cita de Sander lucioperca (L.) (Actinopterygii, Percidae) en la cuenca del río Segura, SE de España. Anales de Biología 2002, 24, 77–79. [Google Scholar]
- Barros, J.S.; Cunha, M.J.; Lino, M.; Vieira, N.; Valente, A.C.N. Evaluation of the water quality and biotic communities of two Portuguese reservoirs (Alto Lindoso and Ermal) and their relationship with recreational fishing. Ver. Theor. Angew. Limnol. 2000, 27, 2693–2698. [Google Scholar] [CrossRef]
- Ribeiro, F.; Gante, H.F.; Sousa, G.; Filipe, A.F.; Alves, M.J.; Magalhaes, M.F. New records, distribution and dispersal pathways of Sander lucioperca in Iberian freshwaters. Cybium 2009, 33, 255–256. [Google Scholar]
- Gago, J.; Neves, A.; Gkenas, C.; Ribeiro, D.; Ribeiro, F. Condition and size of the non-native pikeperch Sander lucioperca (Linnaeus, 1758) in Portuguese river basins. Ecol. Evol. 2021, 11, 5065–5074. [Google Scholar] [CrossRef]
- Martelo, J.; da Costa, L.M.; Ribeiro, D.; Gago, J.; Magalhães, M.F.; Gante, H.F.; Ribeiro, F. Evaluating the range expansion of recreational non-native fishes in Portuguese freshwaters using scientific and citizen science data. BioInvasions Rec. 2021, 10, 378–389. [Google Scholar] [CrossRef]
- Dörner, H.; Berg, S.; Jacobsen, L.; Hülsmann, S.; Brojerg, M.; Wagner, A. The feeding behaviour of large perch Perca fluviatilis (L.) in relation to food availability: A comparative study. Hydrobiologia 2003, 506, 427–434. [Google Scholar] [CrossRef]
- Mittelbach, G.G.; Persson, L. The ontogeny of piscivory and its ecological consequences. Can. J. Fish. Aquat. Sci. 1998, 55, 1454–1465. [Google Scholar] [CrossRef]
- Ginter, K.; Kangur, K.; Kangur, A.; Kangur, P.; Haldna, M. Diet patterns and ontogenetic diet shift of pikeperch, Sander lucioperca (L.) fry in lakes Peipsi and Võrtsjärv (Estonia). Hydrobiologia 2011, 660, 79–91. [Google Scholar] [CrossRef]
- Campbell, R.N.B. Food of an introduced population of pikeperch, Stizostedion lucioperca L., in lake Egirdir, Turkey. Aquac. Res. 1992, 23, 71–85. [Google Scholar] [CrossRef]
- Keskinen, T.; Marjomäki, T.J. Diet and prey size spectrum of pikeperch in lakes in central Finland. J. Fish. Biol. 2004, 65, 1147–1153. [Google Scholar] [CrossRef]
- Pérez-Bote, J.L.; Roso, R. Diet of the introduced pikeperch Sander lucioperca (L.)(Osteichthyes, Percidae) in a recent colonised reservoir in south-western Iberian Peninsula. Ital. J. Zool. 2012, 79, 617–626. [Google Scholar] [CrossRef]
- Pérez-Bote, J.L.; Roso, R. Growth and length–weight relationships of Sander lucioperca (Linnaeus, 1758) in the Alcántara Reservoir, south-western Spain: Comparison with other water bodies in Eurasia. J. Appl. Ichthyol. 2012, 28, 264–268. [Google Scholar] [CrossRef]
- Didenko, A.V.; Gurbyk, A.B. Spring diet and trophic relationships between piscivorous fishes in Kaniv Reservoir (Ukraine). J. Vertebr. Biol. 2016, 65, 15–26. [Google Scholar] [CrossRef]
- Libois, R.; Hallet, C.; Rosoux, R. Éléments pour l’identification des restes crâniens des poissons dulçaquicoles de Belgique et du Nord de la France. 1. Anguilliformes, Gastérostéiformes, Cyprinodontiformes et Perciformes. Fiches d’Ostéologie Animale pour l’Archéologie Série A Poissons 1987, 3, 1–15. [Google Scholar]
- Libois, R.; Hallet, C. Eléments pour l’identification des restes crâniens des poissons dulçaquicoles de Belgique et du Nord de la France. 2. Cypriniformes. Fiches d’Ostéologie Anim. Pour l’Archéologie Série A Poissons 1988, 4, 1–24. [Google Scholar]
- Prenda, J.; Freitas, D.; Santos-Reis, M.; Collares-Pereira, M.J. Guía para la identificación de restos óseos pertenecientes a algunos peces comunes en las aguas continentales de la Península Ibérica para el estudio de la dieta de depredadores ictiófagos. Doñana Acta Vertebr. 1997, 24, 155–180. [Google Scholar]
- Miranda, R.; Escala, M.C. Guía de Identificación de Restos Óseos de los Ciprínidos Presentes en España. Escamas, Opérculos, Cleitros y Arcos Faríngeos; Publicaciones de Biología de la Universidad de Navarra, Serie Zoológica: Navarra, Spain, 2002; pp. 1–239. [Google Scholar]
- Tachet, H.; Richoux, P.; Bournaud, M.; Usseglio-Polatera, P. Invertébrés d’Eau Douce (2nd Corrected Impression); CNRS Éditions: Paris, France, 2002. [Google Scholar]
- Miranda, R.; Escala, M.C. Morphological and biometric revision of the cleithra, opercular and pharyngeal bones of Iberian teleosts belonging to the genus Barbus (Pisces, Cyprinidae). Eur. J. Morphol. 2003, 41, 175–183. [Google Scholar] [PubMed]
- Nunes, T.P. ATLAS de Otólitos de Peixes do Rio Minho. Master’s Thesis, Porto University, Porto, Portugal, 2012. Available online: https://repositorio-aberto.up.pt/bitstream/10216/67126/2/24245.pdf (accessed on 20 March 2017).
- Bowen, S.H. Quantitative description of the diet. In Fisheries Techniques, Murphy, B.R., Willis, D.W., Eds.; American Fisheries Society: Bethesda, MD, USA, 1996; pp. 513–532. [Google Scholar]
- Hyslop, E.J. Stomach contents Analysis—A review of methods and their application. J. Fish. Biol. 1980, 17, 411–429. [Google Scholar] [CrossRef]
- Clarke, K.R.; Gorley, R.N.; Somerfield, P.J.; Warwick, R.M. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation, 3rd ed.; Primer-E Ltd.: Plymouth, UK, 2014; 256p. [Google Scholar]
- Clarke, K.R.; Warwick, R.M. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation; Primer-E Ltd.: Plymouth, UK, 2001. [Google Scholar]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral. Ecol. 2001, 26, 32–46. [Google Scholar]
- Dufrêne, M.; Legendre, P. Species assemblages and indicator species: The need for a flexible asymmetrical approach. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar] [CrossRef]
- Cardoso, P.; Rigal, F.; Fattorini, S.; Terzopoulou, S.; Borges, P.A.V. Disturbance and Indicator Species in Conservation Studies. PLoS ONE 2013, 8, e63294. [Google Scholar] [CrossRef]
- Muotka, T.; Paavola, R.; Haapala, A.; Novikmecb, M.; Laasonen, P. Long-term recovery of stream habitat structure and benthic invertebrate communities from in-stream restoration. Biol. Conserv. 2002, 105, 243–253. [Google Scholar] [CrossRef]
- Levins, R. Evolution in Changing Environments; Princeton University Press: Princeton, NJ, USA, 1968; 120p. [Google Scholar]
- Krebs, C.J. Ecological Methodology; Harper & Row: New York, NY, USA, 1989. [Google Scholar]
- Bolnick, D.I.; Yang, L.H.; Fordyce, J.A.; Davis, J.M.; Svanbäck, R. Measuring individual-level resource specialization. Ecology 2002, 83, 2936–2941. [Google Scholar] [CrossRef]
- The R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 1 February 2021).
- Kangur, P.; Kangur, A.; Kangur, K. Dietary importance of various prey fishes for pikeperch Sander lucioperca (L.) in large shallow lake Võrtsjärv (Estonia). Proc. Estonian Acad. Sci. Biol. Ecol. 2007, 56, 154–167. [Google Scholar]
- Schulze, T.; Dörner, H.; Baade, U.; Hölker, F. Dietary niche partitioning in a piscivorous fish guild in response to stocking of an additional competitor–The role of diet specialisation. Limnologica 2012, 42, 56–64. [Google Scholar] [CrossRef]
- Argillier, C.; Barral, M.; Irz, P. Growth and diet of the pikeperch Sander lucioperca (L.) in two French reservoirs. Fish. Aquat. Life 2012, 20, 191–200. [Google Scholar] [CrossRef]
- Guillerault, N.; Bouletreau, S.; Iribar, A.; Valentini, A.; Santoul, F. Application of DNA metabarcoding on faeces to identify European catfish Silurus glanis diet. J. Fish. Biol. 2017, 90, 2214–2219. [Google Scholar] [CrossRef]
- Nolan, E.T.; Britton, J.R. Diet of invasive pikeperch Sander lucioperca: Developing non-destructive tissue sampling for stable isotope analysis with comparisons to stomach contents analysis. Knowl. Manag. Aquat. Ecosyst. 2018, 419, 49. [Google Scholar] [CrossRef]
- Ribeiro, F.; Veríssimo, A. Full westward expansion of Rutilus rutilus (Linnaeus, 1758) in the Iberian Peninsula. J. Appl. Ichthyol. 2014, 30, 540–542. [Google Scholar] [CrossRef]
- Veríssimo, A.; Gante, H.F.; Santos, C.D.; Cheoo, G.; Oliveira, J.M.; Cereja, R.; Ribeiro, F. Distribution and demography of the critically endangered Lisbon arched-mouth nase, Iberochondrostoma olisiponense. Fishes Mediterr. Environ. 2018. [Google Scholar] [CrossRef]
- Ferreira, M.; Gago, J.; Ribeiro, F. Diet of European catfish in a newly invaded region. Fishes 2019, 4, 58. [Google Scholar] [CrossRef]
- Specziár, A. First year ontogenetic diet patterns in two coexisting Sander species, S. lucioperca and S. volgensis in Lake Balaton. Hydrobiologia 2005, 549, 115–130. [Google Scholar] [CrossRef]
- Lappalainen, J.; Dörner, H.; Wysujack, K. Reproduction biology of pikeperch (Sander lucioperca (L.))—A review. Ecol. Freshw. Fish. 2003, 12, 95–106. [Google Scholar] [CrossRef]
- Lappalainen, J.; Olin, M.; Vinni, M. Pikeperch cannibalism: Effects of abundance, size and condition. In Annales Zoologici Fennici; Finnish Zoological and Botanical Publishing Board: Helsinki, Finland, 2006; pp. 35–44. [Google Scholar]
- Gkenas, C.; Magalhães, M.F.; Cucherousset, J.; Domingos, I.; Ribeiro, F. Long term patterns in the late summer trophic niche of the invasive pumpkinseed sunfish Lepomis gibbosus. Knowl. Manag. Aquat. Ecosyst. 2016, 417, 19. [Google Scholar] [CrossRef][Green Version]


| Overall | C | D | V | M | TC | TB | T | S | GA | G | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prey Categories | Class I | Class II | Class I | Class II | Class I | Class II | Class I | Class II | Class II | Class I | Class II | Class I | Class II | Class I | Class II | Class II | Class II | |
| A. alburnus | 10.7 (28.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 21.3 (31.8) | 0.3 (5.3) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 20.6 (25.0) | 0.0 (0.0) | 76.9 (91.7) | 0.0 (0.0) | 18.2 (33.3) | 0.0 (0.0) | 6.7 (8.3) | 52.4 (72.7) | 25.0 (40.0) |
| S. lucioperca | 8.9 (25.6) | 0.0 (0.0) | 0.0 (0.0) | 30.8 (66.7) | 36.2 (54.5) | 0.9 (15.8) | 14.3 (28.6) | 0.0 (0.0) | 0.0 (0.0) | 5.9 (12.5) | 0.0 (0.0) | 0.0 (0.0) | 5.0 (9.1) | 9.1 (16.7) | 100.0 (100.0) | 73.3 (75.0) | 0.0 (0.0) | 8.3 (20.0) |
| R. rutilus | 3.4 (6.5) | 33.3 (50.0) | 50.0 (55.6) | 0.0 (0.0) | 27.7 (27.3) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) |
| Mugilidae | 1.1 (3.6) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 42.9 (71.4) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 8.3 (20.0) |
| M. salmoides | 2.1 (5.4) | 0.0 (0.0) | 7.1 (11.1) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 100.0 (100.0) | 0.0 (0.0) | 20.6 (18.8) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 4.8 (9.1) | 0.0 (0.0) |
| L. gibbosus | 1.3 (4.8) | 0.0 (0.0) | 21.4 (22.2) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 11.8 (25.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 8.3 (20.0) |
| O. Fish | 2.6 (9.5) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0)) | 2.1 (4.5) | 0.6 (10.5) | 7.1 (14.3) | 0.0 (0.0) | 0.0 (0.0) | 2.9 (6.3) | 0.0 (0.0) | 2.6 (4.2) | 30.0 (54.5) | 18.2 (33.3) | 0.0 (0.0) | 6.7 (8.3) | 0.0 (0.0) | 8.3 (20.0) |
| Diptera | 54.2 (10.7) | 66.7 (100.0) | 21.4 (33.3) | 26.9 (33.3) | 0.0 (0.0) | 93.5 (52.6) | 0.0 (0.0) | 0.0 (0.0) | 84.6 (33.3) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 14.3 (9.1) | 0.0 (0.0) |
| Ephemeroptera | 1.8 (3.0) | 0.0 (0.0) | 0.0 (0.0) | 19.2 (22.2) | 10.6 (9.1) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 2.9 (6.3) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) |
| Odonata | 1.3 (2.4) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 2.5 (21.1) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) |
| Atyidae | 7.4 (12.5) | 0.0 (0.0) | 0.0 (0.0) | 19.2 (22.2) | 2.1 (4.5) | 0.3 (5.3) | 35.7 (14.3) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 100.0 (100.0) | 15.4 (25.0) | 65.0 (54.5) | 54.5 (16.7) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 41.7 (20.0) |
| P. clarkii | 3.1 (5.4) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 15.4 (66.7) | 29.4 (31.3) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 6.7 (8.3) | 28.6 (9.1) | 0.0 (0.0) |
| O. insects | 2.0 (6.0) | 0.0 (0.0) | 0.0 (0.0) | 3.8 (11.1) | 0.0 (0.0) | 1.8 (21.1) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 5.9 (12.5) | 0.0 (0.0) | 5.1 (8.3) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 6.7 (8.3) | 0.0 (0.0) | 0.0 (0.0) |
| Total prey | 609 | 3 | 14 | 26 | 47 | 325 | 14 | 4 | 3 | 34 | 3 | 39 | 20 | 11 | 8 | 15 | 21 | 12 |
| Total fish (n) | 168 (88) | 2 (3) | 9 (16) | 9 (7) | 22 (3) | 19 (9) | 7 (1) | 4 (1) | 3 (5) | 16 (7) | 2 (0) | 24 (8) | 11 (3) | 6 (4) | 6 (3) | 12 (8) | 11 (9) | 5 (1) |
| SL cm | 29.5 | 23.6 | 34.6 | 19.8 | 32.0 | 12.2 | 38.0 | 21.5 | 42.7 | 40.5 | 19.6 | 29.6 | 15.8 | 29.2 | 22.2 | 39.3 | 40.2 | 41.3 |
| min–max | 9.4–60.3 | 22.9–24.2 | 25.4–53.7 | 12.4–24.1 | 28.1–39.2 | 9.4–19.0 | 31.8–60.3 | 20.2–22.9 | 31.6–48.5 | 25.7–52.2 | 15.9–23.2 | 25.1–38.2 | 9.5–24.2 | 27.4–30.8 | 19.6–23.9 | 25.2–56.6 | 32.4–43.2 | 31.0–51.0 |
| Parameter | df | Pseudo F-Ratio | p-Value |
|---|---|---|---|
| Sites * Size | 6 | 2.38 | <0.0001 |
| Sites | 9 | 6.56 | <0.0001 |
| Size | 1 | 3.14 | 0.004 |
| Residual | 151 |
| Prey Items | Site/Size Class | IndVal | p-Value | Frequency |
|---|---|---|---|---|
| Diptera | C (I) | 0.381 | 0.016 | 18 |
| Mugilidae | V (II) | 0.545 | 0.002 | 6 |
| M. salmoides | M (I) | 0.754 | 0.001 | 9 |
| P. clarkii | M (II) | 0.432 | 0.009 | 9 |
| Atyidae | TB (I) | 0.466 | 0.002 | 21 |
| A. alburnus | TB (II) | 0.266 | 0.001 | 47 |
| O. fish | T (I) | 0.195 | 0.054 | 16 |
| S. lucioperca | S (I) | 0.309 | 0.001 | 43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, D.; Gkenas, C.; Gago, J.; Ribeiro, F. Variation in Diet Patterns of the Invasive Top Predator Sander lucioperca (Linnaeus, 1758) across Portuguese Basins. Water 2021, 13, 2053. https://doi.org/10.3390/w13152053
Ribeiro D, Gkenas C, Gago J, Ribeiro F. Variation in Diet Patterns of the Invasive Top Predator Sander lucioperca (Linnaeus, 1758) across Portuguese Basins. Water. 2021; 13(15):2053. https://doi.org/10.3390/w13152053
Chicago/Turabian StyleRibeiro, Diogo, Christos Gkenas, João Gago, and Filipe Ribeiro. 2021. "Variation in Diet Patterns of the Invasive Top Predator Sander lucioperca (Linnaeus, 1758) across Portuguese Basins" Water 13, no. 15: 2053. https://doi.org/10.3390/w13152053
APA StyleRibeiro, D., Gkenas, C., Gago, J., & Ribeiro, F. (2021). Variation in Diet Patterns of the Invasive Top Predator Sander lucioperca (Linnaeus, 1758) across Portuguese Basins. Water, 13(15), 2053. https://doi.org/10.3390/w13152053






