Results of the First Improvement Step Regarding Removal Efficiency of Kanchan Arsenic Filters in the Lowlands of Nepal—A Case Study
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
- CS = Concrete square filter;
- PR = Plastic round filter;
- Res. = Residence time in minutes.
4. Future Improvements
- (i)
- Installation of the upper sand bed for all filters in order to prevent the nails from drying and moving while pouring ground water as well as diminishing the flow rate;
- (ii)
- Regulation of the outflow by placing a tap at the outlet or by raising the outlet from the plastic bucket to above the level of the nail bed;
- (iii)
- Replacing nails and sand on a regular basis;
- (iv)
- Monitoring of the water quality and filter conditions by trained local inhabitants;
- (v)
- Proper and regularly repeated instructions for the users.
5. Conclusions
- (i)
- Geological background, e.g., Fe and As concentrations of the ground water itself [9];
- (ii)
- Condition and aging of the material intended to remove As efficiently: nails and sand. Both nails and sand must be changed regularly. Sand, especially, has to be replaced on a yearly base (see filter SN51) in order to maintain its absorbing capacity;
- (iii)
- The nails of the nail bed have to be wet constantly but not immersed in water in order to promote oxidation and formation of Fe(III)hydr(oxides);
- (iv)
- Prevention of the formation of holes and dents within the nail bed, as the ground water has to be precluded to flow through the nail bed in nail-free channels. The ground water has to be poured slowly and carefully in order to prevent moving of the nails;
- (v)
- Sufficient contact time between ground water and nails. A high concentration of As and a low concentration of Fe requires an increased contact time.
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, R.M. Research Study on Possible Contamination of Ground water with Arsenic in Jhapa, Morang, and Sunsari Districts of Eastern Terai of Nepal; Report of WHO Project; DWSS Government of Nepal: Kathmandu, Nepal, 1999. [Google Scholar]
- NASC; NRCS. The State of Arsenic in Nepal—2011; Nepal Arsenic Steering Committee; Nepal Red Cross Society: Kathmandu, Nepal, 2011. [Google Scholar]
- Gurung, J.K.; Ishiga, H.; Khadka, M.S. Geological and geochemical examination of arsenic contamination in ground water in the Holocene Terai Basin, Nepal. Environ. Geol. 2005, 49, 98–113. [Google Scholar] [CrossRef]
- Shah, B.A. Role of Quaternary stratigraphy on arsenic-contaminated ground water from parts of Middle Ganga Plain, UP–Bihar, India. Environ. Geol. 2008, 53, 1553–1561. [Google Scholar] [CrossRef]
- Guillot, S.; Garçon, M.; Weinman, B.; Gajurel, A.; Tisserand, D.; France-Lanord, C.; van Geen, A.; Chakraborty, S.; Huyghe, P.; Upreti, B.N.; et al. Origin of arsenic in Late Pleistocene to Holocene sediments in the Nawalparasi district (Terai, Nepal). Environ. Earth Sci. 2015, 74, 2571–2593. [Google Scholar] [CrossRef]
- Brikowski, T.H.; Smith, L.S.; Shei, T.C.; Shrestha, S.D. Correlation of electrical resistivity and ground water arsenic concentration, Nawalparasi, Nepal. J. Nepal Geol. Soc. 2004, 30, 99–106. [Google Scholar]
- Brikowski, T.H.; Neku, A.; Shrestha, S.D.; Smith, L.S. Hydrologic control of temporal variability in ground water arsenic on the Ganges floodplain of Nepal. J. Hydrol. 2014, 518, 342–353. [Google Scholar] [CrossRef]
- Diwakar, J.; Johnston, S.G.; Burton, E.D.; Shrestha, S.D. Arsenic mobilization in an alluvial aquifer of the Terai region, Nepal. J. Hydrol. Reg. Stud. 2015, 4, 59–79. [Google Scholar] [CrossRef] [Green Version]
- Mueller, B.; Hug, S.J. Climatic variations and de-coupling between arsenic and iron in arsenic contaminated ground water in the lowlands of Nepal. Chemosphere 2018, 210, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Mueller, B. Arsenic in ground water in the southern lowlands of Nepal and its mitigation options: A review. Environ. Rev. 2017, 25, 296–305. [Google Scholar] [CrossRef] [Green Version]
- Mueller, B.; Dangol, B.; Ngai, T.K.K.; Hug, S.J. Nepal—Arsenic load, mode of operation, arsenic removal and future improvements. Environ. Geochem. Health 2020, 43, 375. [Google Scholar] [CrossRef] [PubMed]
- Nickson, R.T.; McArthur, J.M.; Ravenscroft, P.; Burgess, W.G.; Ahmed, K.M. Mechanism of arsenic release to ground water, Bangladesh and West Bengal. Appl. Geochem. 2000, 15, 403–413. [Google Scholar] [CrossRef]
- Ngai, T.K.K.; Dangol, B.; Murcott, S.; Shrestha, R.R. Kanchan Arsenic Filter; Massachusetts Institute of Technology (MIT), Environment and Public Health Organization (ENPHO): Katmandu, Nepal, 2005. [Google Scholar]
- Ngai, T.K.K.; Murcott, S.E.; Shrestha, R.R.; Dangol, B.; Maharjan, M. Development and dissemination of KanchanTM arsenic filter in rural Nepal. Water Sci. Technol. 2006, 6, 137–146. [Google Scholar]
- Ngai, T.K.K.; Shrestha, R.R.; Dangol, B.; Maharjan, M.; Murcott, S.E. Design for sustainable development—Household drinking water filter for arsenic and pathogen treatment in Nepal. J. Environ. Sci. Health Part A Toxicol. Hazard 2007, 42, 1879–1888. [Google Scholar] [CrossRef] [PubMed]
- Chiew, H.; Sampson, M.L.; Huch, S.; Ken, S.; Bostick, B.C. Effect of ground water iron and phosphate on the efficacy of arsenic removal by iron-amended bio sand filters. Environ. Sci. Technol. 2009, 43, 6295–6300. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Smith, L.S.; Shrestha, S.; Maden, N. Efficacy of arsenic filtration by Kanchan Arsenic Filter in Nepal. J. Water Health 2014, 12, 596–599. [Google Scholar] [CrossRef] [PubMed]
- Wenk, C.; Kaegi, R.; Hug, S.J. Factors affecting arsenic and uranium removal with zero-valent iron: Laboratory tests with Kanchan-type iron nail filter columns with different ground waters. Environ. Chem. 2014, 11, 547–557. [Google Scholar] [CrossRef] [Green Version]
- Zweifel, E.R. Arsenic Contamination in Nepal: Water Treatment Issues and Geologic Origin of the Pollution. Master’s Thesis, Institute of Geography, University of Bern, Bern, Switzerland, 2018; p. 67. [Google Scholar]
- Guo, H.; Stüben, D.; Berner, Z. Adsorption of arsenic(III) and arsenic(V) from ground water using natural siderite as the adsorbent. J. Colloid Interface Sci. 2007, 315, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.; Li, Z.; Chen, B.; Liang, H.; Zhang, X.; Xu, R.; Li, Z.; Dai, H.; Wei, C.; Liu, S. Comparison of sand-based water filters for point-of-use arsenic removal in China. Chemosphere 2017, 168, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Smiech, K.M.; Tolsma, A.; Kovacs, T.; Dalbosco, V.; Yasadi, K.; Groendijk, L.; Agostinho, L.F. Comparing mixed-media and conventional slow-sand filters for arsenic removal from ground water. Water 2018, 10, 119. [Google Scholar] [CrossRef] [Green Version]
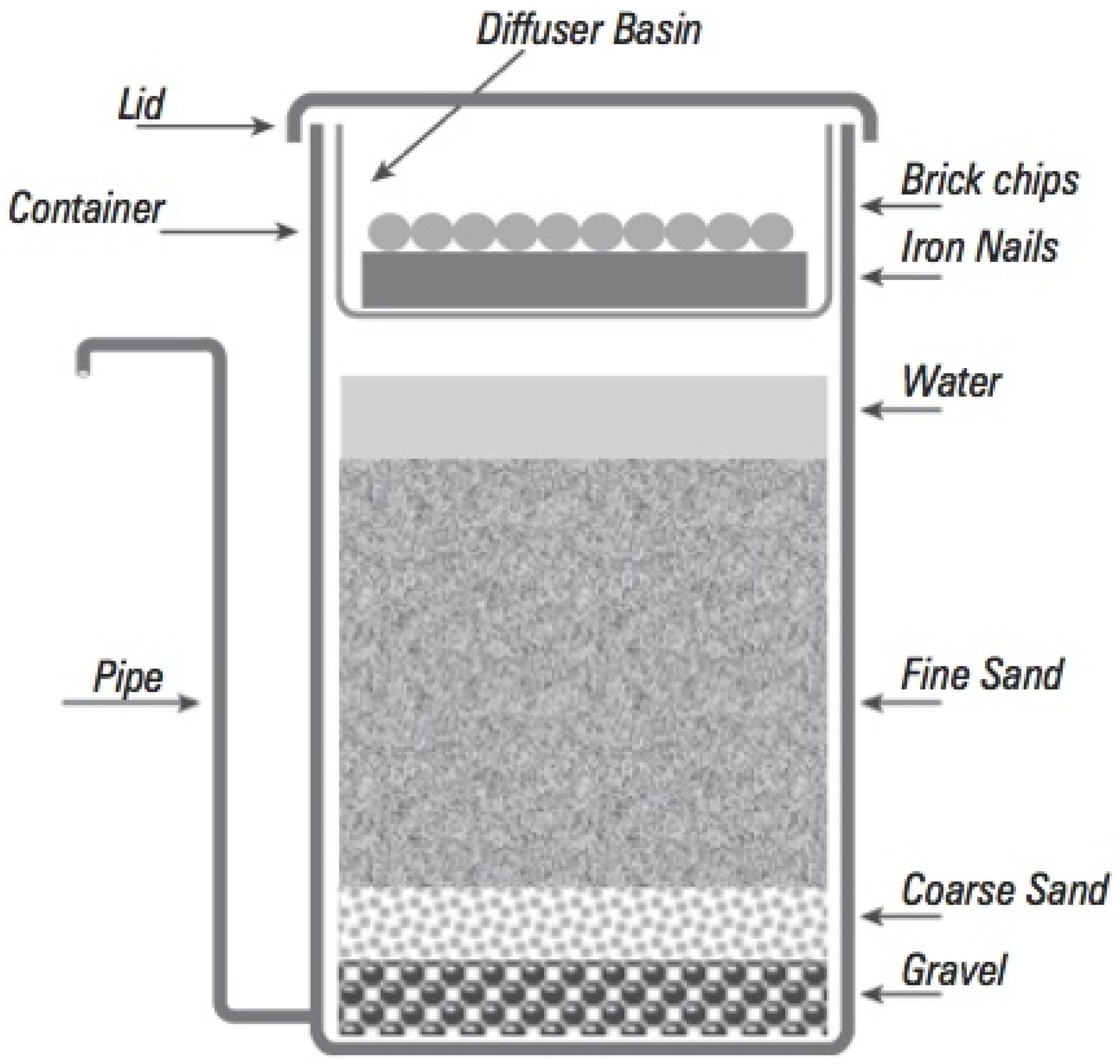
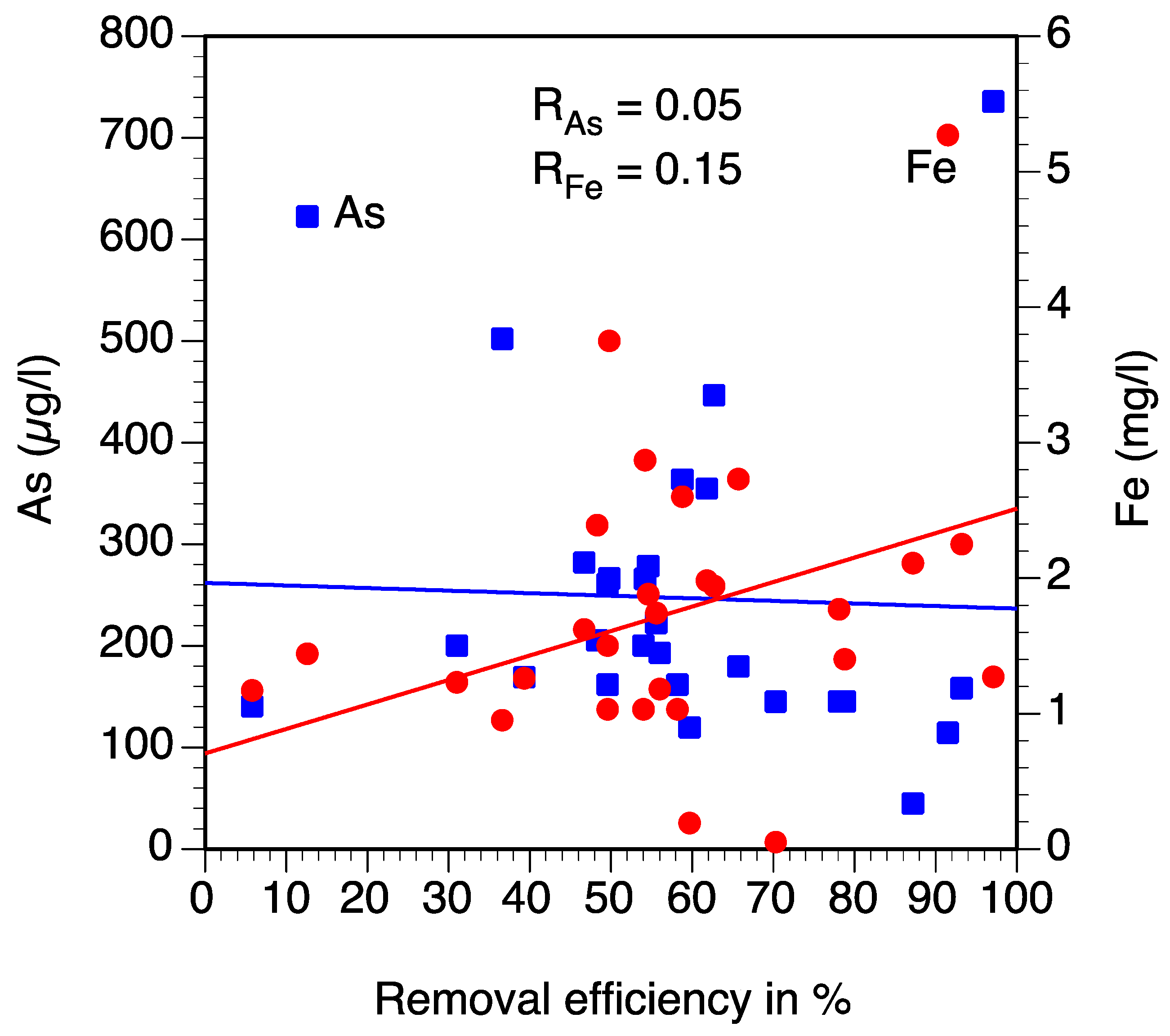
| Tube Well | Fe (mg/L) | As (µg/L) | Res. Time Original | R1 Original | R2 Original | OR Original | Fe (mg/L) | As (µg/L) | Res. Time New 2018 | R1 New 2018 | R2 New 2018 | OR New 2018 | Fe (mg/L) | As (µg/L) | Respective Time New 2 2019 | R1 New 2 2019 | R2 New 2 2019 | OR New 2 2019 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SN14 CS | 1.44 | 622.6 | 15.1 | 30.1 | −25.0 | 12.6 | 1.15 | 644.4 | 7.8 | 18.0 | 89.6 | 91.5 | 1.30 | 640.6 | 15.5 | 14.3 | 31.3 | 41.2 |
| SN26 PR | 3.75 | 266.3 | 8.1 | 54.5 | −10.4 | 49.8 | 1.70 | 326.3 | 7.8 | 10.3 | 70.7 | 73.7 | 1.74 | 328.7 | 3.9 | 22.4 | 52.5 | 63.1 |
| SN33 PR | 2.60 | 363.6 | 12.9 | 15.6 | 51.2 | 58.8 | 1.86 | 363.4 | 16.1 | 17.7 | 37.0 | 48.1 | 1.60 | 401.6 | 17.0 | 27.7 | 27.9 | 47.9 |
| SN35 PR | 1.98 | 354.7 | 6.8 | 43.7 | 32.1 | 61.8 | 1.54 | 333.3 | 14.2 | 12.1 | 50.1 | 56.1 | 1.68 | 332.3 | 13.6 | 48.7 | 20.0 | 58.9 |
| SN51 PR | 1.23 | 200.1 | 12.0 | 8.4 | 24.6 | 31.0 | 0.30 | 239.8 | 5.4 | 7.5 | 92.7 | 93.2 | 1.07 | 259.8 | 4.7 | 17.8 | 46.7 | 53.7 |
| SN53 PR | 1.50 | 260.8 | 11.0 | 30.1 | 27.7 | 49.6 | 0.78 | 220.6 | 12.1 | 24.7 | 21.8 | 36.6 | 1.19 | 229.8 | 10.9 | 13.4 | 26.8 | 36.6 |
| SN54 PR | 1.62 | 281.7 | 9.8 | 17.3 | 35.6 | 46.7 | 1.53 | 277.8 | 9.7 | 22.9 | 40.2 | 54.0 | 1.21 | 265.2 | 21.3 | 39.3 | 3.8 | 41.6 |
| SN55 CS | 1.88 | 278.4 | 6.6 | 15.7 | 46.1 | 54.6 | 0.64 | 215.5 | 38.8 | 52.0 | 23.2 | 63.1 | 1.63 | 232.5 | 22.0 | 5.0 | 62.3 | 64.2 |
| SN57 PR | 2.73 | 179.6 | 26.4 | 37.9 | 44.8 | 65.7 | 2.31 | 188.1 | 29.4 | 28.2 | 49.3 | 63.6 | 2.12 | 214.4 | 29.4 | 35.4 | 47.3 | 66.0 |
| SN62 PR | 1.74 | 222.2 | 12.0 | 139 | 48.4 | 55.6 | 1.57 | 208.9 | 12.6 | 44.5 | 43.1 | 74.0 | 1.71 | 208.6 | 9.0 | 22.7 | 73.5 | 79.5 |
| SN63 PR | 2.25 | 158.3 | 5.7 | 28.0 | 90.5 | 93.2 | 2.60 | 96.54 | 14.9 | 18.8 | 67.9 | 73.9 | 2.29 | 98.58 | 12.3 | 28.5 | 59.4 | 70.9 |
| SN64 PR | −0.27 | 100.66 | 9.7 | −71.2 | 39.5 | −3.5 | 2.21 | 164.8 | 13.6 | 23.0 | 59.6 | 68.9 | −0.01 | 14.54 | 10.3 | −17.1 | −70.5 | −99.6 |
| SN66 PR | 2.87 | 265.6 | 10.0 | 55.3 | −2.4 | 54.2 | 2.65 | 257.3 | 13.6 | 25.4 | 48.9 | 61.9 | 2.15 | 242.8 | 14.2 | 34.3 | 37.0 | 58.6 |
| SN67 CS | 1.26 | 169.0 | 6.1 | 31.7 | 11.1 | 39.3 | 1.77 | 145.1 | 24.3 | 74.5 | 80.7 | 1.16 | 155.4 | 5.7 | 21.5 | 72.6 | 78.5 | |
| SN68 PR | 1.18 | 192.9 | 6.6 | 15.3 | 48.0 | 56.0 | 1.08 | 169.0 | 18.1 | 6.6 | 40.7 | 44.7 | 0.002 | 192.9 | 25.8 | 17.5 | 11.8 | 27.2 |
| SN69 PR | −0.27 | 280.3 | 19.8 | −80.8 | 13.5 | −56.5 | 0.85 | 398.6 | 23.9 | −7.0 | 22.0 | 16.6 | 0.79 | 471.8 | 12.9 | 18.7 | 54.0 | 62.6 |
| SN70 CS | 0.95 | 502.1 | 5.8 | 10.7 | 29.0 | 36.6 | 0.40 | 470.7 | 6.5 | 1.6 | −9.9 | −8.1 | 0.53 | 480.1 | 5.2 | 2.0 | −6.0 | −3.9 |
| SN72 PR | 2.39 | 205.4 | 29.8 | 10.0 | 42.6 | 48.3 | 1.74 | 204.8 | 24.5 | 17.5 | 68.2 | 73.8 | 1.63 | 197.1 | 26.5 | 20.0 | 60.3 | 68.3 |
| SN76 PR | 1.17 | 140.5 | 8.7 | 7.3 | −1.6 | 5.8 | 0.34 | 20.0 | 8.4 | −24.8 | −61.4 | −101.4 | 0.88 | 139.1 | 9.0 | 16.3 | 66.6 | 72.0 |
| SN56 PR | 1.40 | 145.1 | 5.2 | 9.9 | 76.5 | 78.8 | 1.40 | 145.1 | 5.8 | 11.2 | 78.1 | 80.6 | 1.36 | 165.1 | 10.3 | 8.3 | 32.7 | 38.3 |
| SN73 PR | 1.94 | 446.6 | 10.3 | 9.7 | 58.8 | 62.7 | 1.94 | 446.6 | 11.0 | 45.3 | 33.0 | 63.3 | 4.22 | 556.9 | 8.4 | 92.0 | −133.0 | 81.3 |
| SN1A PR | 2.11 | 44.57 | 9.7 | 48.8 | 75.1 | 87.2 | 2.11 | 44.57 | 10.3 | 26.4 | 82.0 | 86.7 | 4.59 | 96.23 | 9.7 | 77.2 | 41.4 | 86.6 |
| SN1B PR | 1.03 | 200.1 | 24.8 | 7.3 | 50.3 | 54.0 | 1.03 | 200.1 | 25.8 | 11.8 | 45.1 | 51.6 | 0.80 | 208.0 | 21.7 | 8.3 | 20.8 | 27.4 |
| SN1C CS | 5.27 | 114.3 | 29.1 | −4.6 | 91.8 | 91.5 | 5.27 | 114.3 | 20.2 | 23.4 | 89.9 | 92.3 | 6.40 | 136.2 | 35.5 | 34.9 | 92.4 | 95.0 |
| SN2A CS | 1.03 | 161.7 | 11.6 | 22.0 | 35.5 | 49.6 | 1.03 | 161.7 | 12.4 | 12.6 | 46.1 | 52.9 | 1.17 | 166.9 | 10.8 | 6.4 | −52.3 | −42.5 |
| SN2B CS | 1.03 | 161.7 | 7.0 | 14.6 | 51.1 | 58.2 | 1.03 | 161.7 | 6.0 | 18.7 | 55.3 | 63.7 | 1.17 | 166.9 | 3.9 | 50.8 | −73.9 | 14.4 |
| SN3A CS | 1.77 | 145.1 | 6.8 | 6.3 | 76.6 | 78.1 | 1.77 | 145.1 | 7.1 | 21.6 | 73.9 | 79.6 | 1.24 | 152.0 | 6.8 | 15.9 | −60.8 | −35.2 |
| SN4A CS | 1.27 | 735.6 | 32.6 | 17.3 | 96.5 | 97.1 | 1.27 | 735.6 | 31.7 | 39.4 | 95.1 | 97.1 | 0.38 | 634.3 | 32.6 | 12.5 | 93.4 | 94.3 |
| SN4B CS | 0.05 | 144.8 | 7.0 | 18.2 | 63.7 | 70.3 | 0.05 | 144.8 | 7.0 | 22.2 | 47.3 | 59.1 | 0.05 | 142.5 | 7.0 | 7.0 | 12.3 | 18.5 |
| SN4C PR | 0.19 | 119.5 | 3.5 | 8.1 | 56.1 | 59.7 | 0.19 | 119.5 | 3.9 | 22.6 | 48.1 | 59.8 | 0.20 | 108.6 | 3.9 | 33.8 | 37.2 | 58.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mueller, B. Results of the First Improvement Step Regarding Removal Efficiency of Kanchan Arsenic Filters in the Lowlands of Nepal—A Case Study. Water 2021, 13, 1765. https://doi.org/10.3390/w13131765
Mueller B. Results of the First Improvement Step Regarding Removal Efficiency of Kanchan Arsenic Filters in the Lowlands of Nepal—A Case Study. Water. 2021; 13(13):1765. https://doi.org/10.3390/w13131765
Chicago/Turabian StyleMueller, Barbara. 2021. "Results of the First Improvement Step Regarding Removal Efficiency of Kanchan Arsenic Filters in the Lowlands of Nepal—A Case Study" Water 13, no. 13: 1765. https://doi.org/10.3390/w13131765
APA StyleMueller, B. (2021). Results of the First Improvement Step Regarding Removal Efficiency of Kanchan Arsenic Filters in the Lowlands of Nepal—A Case Study. Water, 13(13), 1765. https://doi.org/10.3390/w13131765





