Effects of the Antidepressants Citalopram and Venlafaxine on the Big Ramshorn Snail (Planorbarius corneus)
Abstract
:1. Introduction
2. Material and Methods
2.1. Test Organism
2.2. Test Substances
2.3. Experimental Design
2.4. Water Analysis
2.5. Behavioural Analysis
2.6. Histopathology
2.7. B-Esterase Activity
2.8. Superoxide Dismutase Activity
2.9. Stress Protein Level
2.10. Statistics
2.11. CRED
3. Results
3.1. Water Parameters
3.2. Mortality and Weight
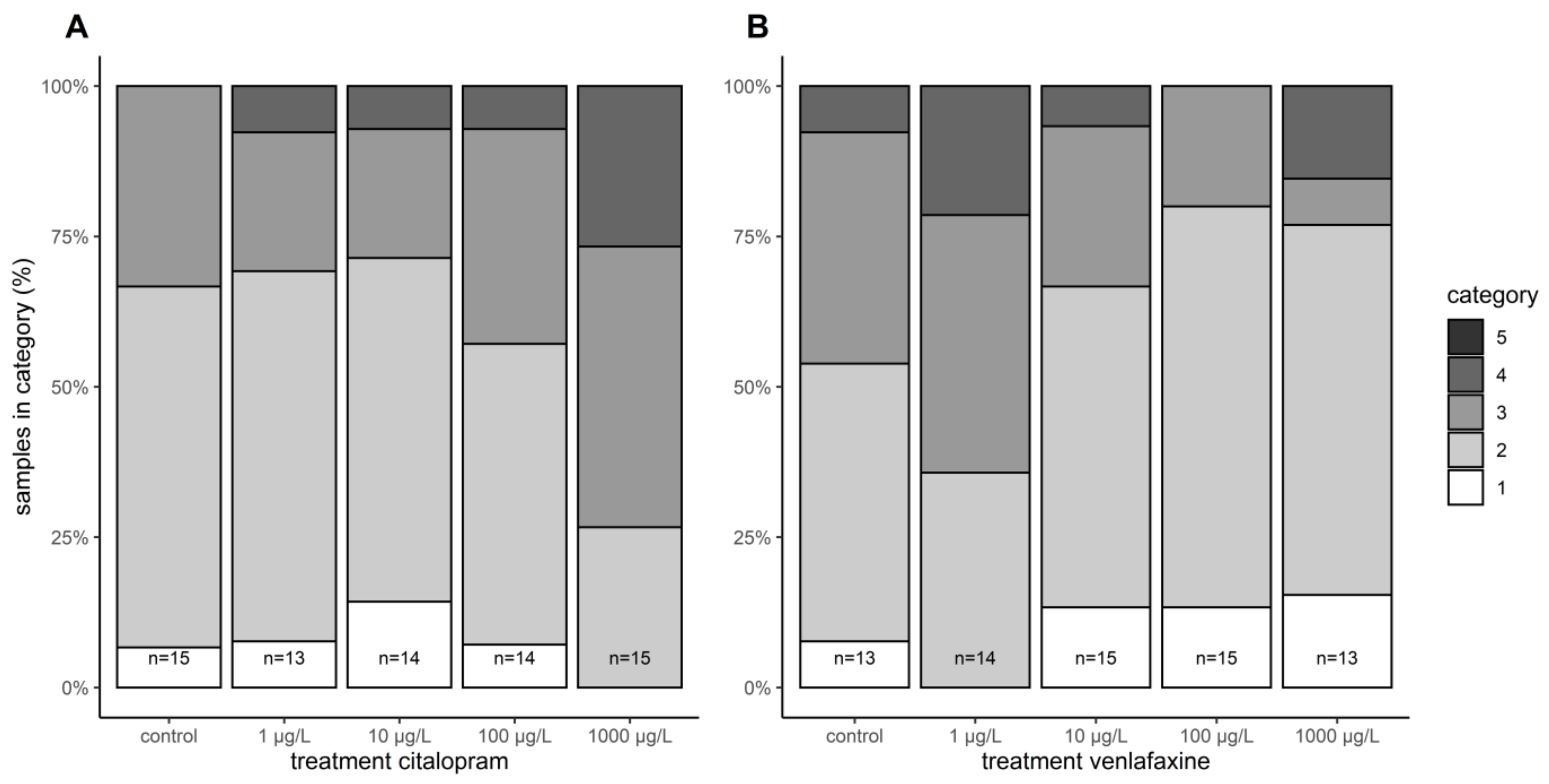
3.3. Behaviour
3.4. Biochemical Biomarkers
3.5. Histopathology
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analy-sis for the Global Burden of Disease Study. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; He, H.; Yang, J.; Feng, X.; Zhao, F.; Lyu, J. Changes in the global burden of depression from 1990 to 2017: Findings from the Global Burden of Disease study. J. Psychiatr. Res. 2020, 126, 134–140. [Google Scholar] [CrossRef]
- Arzneiverordnungs-Report 2019; Springer: Berlin/Heidelberg, Germany, 2019.
- Noble, S.; Benfield, P. Citalopram. CNS Drugs 1997, 8, 410–431. [Google Scholar] [CrossRef]
- Holliday, S.M.; Benfield, P. Venlafaxine. Drugs 1995, 49, 280–294. [Google Scholar] [CrossRef]
- Golovko, O.; Kumar, V.; Fedorova, G.; Randak, T.; Grabic, R. Seasonal changes in antibiotics, antidepressants/psychiatric drugs, antihistamines and lipid regulators in a wastewater treatment plant. Chemosphere 2014, 111, 418–426. [Google Scholar] [CrossRef]
- Lajeunesse, A.; Smyth, S.; Barclay, K.; Sauvé, S.; Gagnon, C. Distribution of antidepressant residues in wastewater and biosolids following different treatment processes by municipal wastewater treatment plants in Canada. Water Res. 2012, 46, 5600–5612. [Google Scholar] [CrossRef]
- Schultz, M.M.; Furlong, E.T.; Kolpin, D.W.; Werner, S.L.; Schoenfuss, H.L.; Barber, L.B.; Blazer, V.S.; Norris, D.O.; Vajda, A.M. Antidepressant Pharmaceuticals in Two, U.S. Effluent-Impacted Streams: Occurrence and Fate in Water and Sediment, and Selective Uptake in Fish Neural Tissue. Environ. Sci. Technol. 2010, 44, 1918–1925. [Google Scholar] [CrossRef]
- Rúa-Gómez, P.C.; Püttmann, W. Impact of wastewater treatment plant discharge of lidocaine, tramadol, venlafaxine and their metabolites on the quality of surface waters and groundwater. J. Environ. Monit. 2012, 14, 1391–1399. [Google Scholar] [CrossRef]
- Himmelsbach, M.; Buchberger, W.; Klampfl, C.W. Determination of antidepressants in surface and wastewater samples by capillary electrophoresis with electrospray ionization mass spectrometric detection after preconcentration using off-line solid-phase extraction. Electrophoresis 2006, 27, 1220–1226. [Google Scholar] [CrossRef]
- Schlüsener, M.P.; Hardenbicker, P.; Nilson, E.; Schulz, M.; Viergutz, C.; Ternes, T.A. Occurrence of venlafaxine, other antidepressants and selected metabolites in the Rhine catchment in the face of climate change. Environ. Pollut. 2015, 196, 247–256. [Google Scholar] [CrossRef] [Green Version]
- Fick, J.; Söderström, H.; Lindberg, R.H.; Phan, C.; Tysklind, M.; Larsson, D.J. Contamination of surface, ground, and drinking water from pharmaceutical production. Environ. Toxicol. Chem. 2009, 28, 2522–2527. [Google Scholar] [CrossRef]
- Acuña, V.; Von Schiller, D.; Galán, M.J.G.; Rodríguez-Mozaz, S.; Corominas, L.; Petrovic, M.; Poch, M.; Barceló, D.; Sabater, S. Occurrence and in-stream attenuation of wastewater-derived pharmaceuticals in Iberian rivers. Sci. Total Environ. 2015, 503–504, 133–141. [Google Scholar] [CrossRef] [Green Version]
- Huber, S.; Remberger, M.; Kaj, L.; Schlabach, M.; Jörundsdóttir, H.Ó.; Vester, J.; Arnórsson, M.; Mortensen, I.; Schwartson, R.; Dam, M. A first screening and risk assessment of pharmaceuticals and additives in personal care products in waste water, sludge, recipient water and sediment from Faroe Islands, Iceland and Greenland. Sci. Total Environ. 2016, 562, 13–25. [Google Scholar] [CrossRef]
- Kellner, M.; Porseryd, T.; Hallgren, S.; Porsch-Hällström, I.; Hansen, S.; Olsén, K. Waterborne citalopram has anxiolytic effects and increases locomotor activity in the three-spine stickleback (Gasterosteus aculeatus). Aquat. Toxicol. 2016, 173, 19–28. [Google Scholar] [CrossRef]
- Kellner, M.; Porseryd, T.; Hällström, I.P.; Borg, B.; Roufidou, C.; Olsén, K.H. Developmental exposure to the SSRI citalopram causes long-lasting behavioural effects in the three-spined stickleback (Gasterosteus aculeatus). Ecotoxicology 2018, 27, 12–22. [Google Scholar] [CrossRef]
- Kellner, M.; Porseryd, T.; Porsch-Hällström, I.; Hansen, S.; Olsen, K. Environmentally relevant concentrations of citalopram partially inhibit feeding in the three-spine stickleback (Gasterosteus aculeatus). Aquat. Toxicol. 2015, 158, 165–170. [Google Scholar] [CrossRef] [Green Version]
- Porseryd, T.; Kellner, M.; Caspillo, N.R.; Volkova, K.; Elabbas, L.; Ullah, S.; Olsén, H.; Dinnétz, P.; Hällström, I.P. Combinatory effects of low concentrations of 17α-etinylestradiol and citalopram on non-reproductive behavior in adult zebrafish (Danio rerio). Aquat. Toxicol. 2017, 193, 9–17. [Google Scholar] [CrossRef]
- Painter, M.M.; Buerkley, M.A.; Julius, M.L.; Vajda, A.M.; Norris, D.O.; Barber, L.B.; Furlong, E.T.; Schultz, M.M.; Schoenfuss, H.L. Antidepressants at environmentally relevant concentrations affect predator avoidance behavior of larval fathead minnows (pimephales promelas). Environ. Toxicol. Chem. 2009, 28, 2677–2684. [Google Scholar] [CrossRef] [Green Version]
- Maulvault, A.L.; Santos, L.H.; Paula, J.R.; Camacho, C.; Pissarra, V.; Fogaça, F.H.D.S.; Barbosa, V.; Alves, R.; Ferreira, P.P.; Barceló, D.; et al. Differential behavioural responses to venlafaxine exposure route, warming and acidification in juvenile fish (Argyrosomus regius). Sci. Total Environ. 2018, 634, 1136–1147. [Google Scholar] [CrossRef]
- Bisesi, J.H.; Bridges, W.; Klaine, S.J. Effects of the antidepressant venlafaxine on fish brain serotonin and predation behavior. Aquat. Toxicol. 2014, 148, 130–138. [Google Scholar] [CrossRef]
- Olsén, K.H.; Ask, K.; Olsén, H.; Porsch-Hällström, I.; Hallgren, S. Reprint of Effects of the SSRI citalopram on behaviours connected to stress and reproduction in Endler guppy, Poecilia wingei. Aquat. Toxicol. 2014, 151, 97–104. [Google Scholar] [CrossRef]
- Thompson, W.A.; Vijayan, M.M. Environmental levels of venlafaxine impact larval behavioural performance in fathead minnows. Chemosphere 2020, 259, 127437. [Google Scholar] [CrossRef]
- Maulvault, A.L.; Camacho, C.; Barbosa, V.; Alves, R.; Anacleto, P.; Pousão-Ferreira, P.; Rosa, R.; Marques, A.; Diniz, M. Living in a multi-stressors environment: An integrated biomarker approach to assess the ecotoxicological response of meagre (Argyrosomus regius) to venlafaxine, warming and acidification. Environ. Res. 2019, 169, 7–25. [Google Scholar] [CrossRef]
- Mehdi, H.; Bragg, L.M.; Servos, M.R.; Craig, P.M. Multiple Stressors in the Environment: The Effects of Exposure to an Antidepressant (Venlafaxine) and Increased Temperature on Zebrafish Metabolism. Front. Physiol. 2019, 10. [Google Scholar] [CrossRef]
- Yang, H.; Lu, G.; Yan, Z.; Liu, J.; Ma, B.; Dong, H. Biological effects of citalopram in a suspended sediment-water system on Daphnia magna. Environ. Sci. Pollut. Res. 2017, 24, 21180–21190. [Google Scholar] [CrossRef]
- Schultz, M.M.; Painter, M.M.; Bartell, S.E.; Logue, A.; Furlong, E.T.; Werner, S.L.; Schoenfuss, H.L. Selective uptake and biological consequences of environmentally relevant antidepressant pharmaceutical exposures on male fathead minnows. Aquat. Toxicol. 2011, 104, 38–47. [Google Scholar] [CrossRef]
- Bidel, F.; Di Poi, C.; Budzinski, H.; Pardon, P.; Callewaert, W.; Arini, A.; Basu, N.; Dickel, L.; Bellanger, C.; Jozet-Alves, C. The antidepressant venlafaxine may act as a neurodevelopmental toxicant in cuttlefish (Sepia officinalis). NeuroToxicology 2016, 55, 142–153. [Google Scholar] [CrossRef]
- Buřič, M.; Grabicova, K.; Kubec, J.; Kouba, A.; Kuklina, I.; Kozák, P.; Grabic, R.; Randák, T. Environmentally relevant concentrations of tramadol and citalopram alter behaviour of an aquatic invertebrate. Aquat. Toxicol. 2018, 200, 226–232. [Google Scholar] [CrossRef]
- Fong, P.P.; Bury, T.B.; Dworkin-Brodsky, A.D.; Jasion, C.M.; Kell, R.C. The antidepressants venlafaxine (“Effexor”) and fluoxetine (“Prozac”) produce different effects on locomotion in two species of marine snail, the oyster drill (Urosalpinx cinerea) and the starsnail (Lithopoma americanum). Mar. Environ. Res. 2015, 103, 89–94. [Google Scholar] [CrossRef]
- Fong, P.P.; Hoy, C.M. Antidepressants (venlafaxine and citalopram) cause foot detachment from the substrate in freshwater snails at environmentally relevant concentrations. Mar. Freshw. Behav. Physiol. 2012, 45, 145–153. [Google Scholar] [CrossRef]
- Fong, P.P.; Molnar, N. Antidepressants cause foot detachment from substrate in five species of marine snail. Mar. Environ. Res. 2013, 84, 24–30. [Google Scholar] [CrossRef]
- Minguez, L.; Farcy, E.; Ballandonne, C.; Lepailleur, A.; Serpentini, A.; Lebel, J.-M.; Bureau, R.; Halm-Lemeille, M.-P. Acute toxicity of 8 antidepressants: What are their modes of action? Chemosphere 2014, 108, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Henry, T.B.; Kwon, J.-W.; Armbrust, K.L.; Black, M.C. Acute and chronic toxicity of five selective serotonin reuptake inhibitors in ceriodaphnia dubia. Environ. Toxicol. Chem. 2004, 23, 2229–2233. [Google Scholar] [CrossRef] [PubMed]
- Christensen, A.M.; Faaborg-Andersen, S.; Ingerslev, F.; Baun, A. Mixture and single-substance toxicity of selective serotonin reuptake inhibitors toward algae and crustaceans. Environ. Toxicol. Chem. 2007, 26, 85–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jopp, F. Comparative studies on the dispersal of the Great Ramshorn (Planorbarius corneus L.): A modelling approach. Limnology 2006, 36, 17–25. [Google Scholar] [CrossRef] [Green Version]
- Glöer, P.; Diercking, R. Atlas der Süßwassermollusken: Rote Liste, Verbreitung, Ökologie, Bestand und Schutz; Freie und Hansestadt Hamburg: Hamburg, Germany, 2010. [Google Scholar]
- Ågerstrand, M.; Arnold, K.; Balshine, S.; Brodin, T.; Brooks, B.W.; Maack, G.; McCallum, E.S.; Pyle, G.G.; Saaristo, M.; Ford, A.T. Emerging investigator series: Use of behavioural endpoints in the regulation of chemicals. Environ. Sci. Process. Impacts 2019, 22, 49–65. [Google Scholar] [CrossRef] [Green Version]
- Ford, A.T.; Ågerstrand, M.; Brooks, B.W.; Allen, J.; Bertram, M.G.; Brodin, T.; Dang, Z.; Duquesne, S.; Sahm, R.; Hoffmann, F.; et al. The Role of Behavioral Ecotoxicology in Environmental Protection. Environ. Sci. Technol. 2021, 55, 5620–5628. [Google Scholar] [CrossRef]
- Rőszer, T. The invertebrate midintestinal gland (“hepatopancreas”) is an evolutionary forerunner in the integration of immunity and metabolism. Cell Tissue Res. 2014, 358, 685–695. [Google Scholar] [CrossRef]
- Van Weel, P.B. The comparative physiology of digestion in molluscs. Am. Zool. 1961, 1, 245–252. [Google Scholar] [CrossRef]
- Jacob, S.; Köhler, H.-R.; Tisler, S.; Zwiener, C.; Triebskorn, R. Impact of the Antidiabetic Drug Metformin and Its Transformation Product Guanylurea on the Health of the Big Ramshorn Snail (Planorbarius corneus). Front. Environ. Sci. 2019, 7. [Google Scholar] [CrossRef] [Green Version]
- Sawasdee, B.; Köhler, H.-R.; Triebskorn, R. Histopathological effects of copper and lithium in the ramshorn snail, Marisa cornuarietis (Gastropoda, Prosobranchia). Chemosphere 2011, 85, 1033–1039. [Google Scholar] [CrossRef]
- Osterauer, R.; Köhler, H.-R.; Triebskorn, R. Histopathological alterations and induction of hsp70 in ramshorn snail (Marisa cornuarietis) and zebrafish (Danio rerio) embryos after exposure to PtCl2. Aquat. Toxicol. 2010, 99, 100–107. [Google Scholar] [CrossRef]
- Otludil, B.; Cengiz, E.I.; Yildirim, M.; Ünver, Ö.; Ünlü, E. The effects of endosulfan on the great ramshorn snail Planorbarius corneus (Gastropoda, Pulmonata): A histopathological study. Chemosphere 2004, 56, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Ünlü, E.; Cengiz, E.I.; Yildirim, M.Z.; Otludil, B.; Ünver, Ö. Histopathological effects in tissues of snailLymnaea stagnalis (Gastropoda, Pulmonata) exposed to sublethal concentration of Thiodan® and recovery after exposure. J. Appl. Toxicol. 2005, 25, 459–463. [Google Scholar] [CrossRef]
- Triebskorn, R. Ultrastructural changes in the digestive tract of Deroceras reticulatum (Müller) induced by a carbamate mol-luscicide and by metaldehyde. Malacolog. Mostra Mond. 1989, 31, 141–156. [Google Scholar]
- Smina, A.H.; Samira, B.; Mohamed, D.; Fatiha, Y.; Houria, B.; Hamlet, S.A.; Bensoltane, S.; Djekoun, M.; Yassi, F.; Berrebbah, H. Histological changes and biochemical parameters in the hepatopancreas of terrestrial gastropod Helix aspersa as biomarkers of neonicotinoid insecticide exposure. Afr. J. Biotechnol. 2012, 11, 16277–16283. [Google Scholar] [CrossRef] [Green Version]
- Schwaiger, J. Histopathological alterations and parasite infection in fish: Indicators of multiple stress factors. J. Aquat. Ecosyst. Stress Recover. 2001, 8, 231–240. [Google Scholar] [CrossRef]
- Bighiu, M.A.; Watermann, B.; Guo, X.; Almroth, B.C.; Eriksson-Wiklund, A.-K. Mortality and histopathological effects in harbour-transplanted snails with different exposure histories. Aquat. Toxicol. 2017, 190, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Stentiford, G.; Feist, S. A histopathological survey of shore crab (Carcinus maenas) and brown shrimp (Crangon crangon) from six estuaries in the United Kingdom. J. Invertebr. Pathol. 2005, 88, 136–146. [Google Scholar] [CrossRef]
- Feder, M.E.; Hofmann, G.E. Heat-shock proteins, molecular chaperones, and the stress response: Evolutionary and Ecological Physiology. Annu. Rev. Physiol. 1999, 61, 243–282. [Google Scholar] [CrossRef] [Green Version]
- Fink, A.L. Chaperone-Mediated Protein Folding. Physiol. Rev. 1999, 79, 425–449. [Google Scholar] [CrossRef]
- Köhler, H.-R.; Bartussek, C.; Eckwert, H.; Farian, K.; Gränzer, S.; Knigge, T.; Kunz, N. The hepatic stress protein (hsp70) response to interacting abiotic parameters in fish exposed to various levels of pollution. J. Aquat. Ecosyst. Stress Recover. 2001, 8, 261–279. [Google Scholar] [CrossRef]
- Xie, Z.; Lu, G.; Li, S.; Nie, Y.; Ma, B.; Liu, J. Behavioral and biochemical responses in freshwater fish Carassius auratus exposed to sertraline. Chemosphere 2015, 135, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Regoli, F.; Giuliani, M.E. Oxidative pathways of chemical toxicity and oxidative stress biomarkers in marine organisms. Mar. Environ. Res. 2014, 93, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Vutukuru, S.S.; Chintada, S.; Madhavi, K.R.; Rao, J.V.; Anjaneyulu, Y. Acute effects of copper on superoxide dismutase, catalase and lipid peroxidation in the freshwater teleost fish, Esomus danricus. Fish. Physiol. Biochem. 2006, 32, 221–229. [Google Scholar] [CrossRef]
- Pedrajas, J.; Peinado, J.; López-Barea, J. Oxidative stress in fish exposed to model xenobiotics. Oxidatively modified forms of Cu,Zn-superoxide dismutase as potential biomarkers. Chem. Interact. 1995, 98, 267–282. [Google Scholar] [CrossRef]
- Yang, M.; Qiu, W.; Chen, J.; Zhan, J.; Pan, C.; Lei, X.; Wu, M. Growth inhibition and coordinated physiological regulation of zebrafish (Danio rerio) embryos upon sublethal exposure to antidepressant amitriptyline. Aquat. Toxicol. 2014, 151, 68–76. [Google Scholar] [CrossRef]
- Ziegler, M.; Eckstein, H.; Ottmann, S.; Reinelt, L.; Stepinski, S.; Köhler, H.-R.; Triebskorn, R. Biochemical and cellular biomarkers in brown trout (Salmo trutta f. fario) in response to the antidepressants citalopram and venlafaxine. Environ. Sci. Eur. 2020, 32, 1–15. [Google Scholar] [CrossRef]
- Aldridge, W.N. Serum esterases. Two types of esterase (A and B) hydrolysing p-nitrophenyl acetate, propionate and butyrate, and a method for their determination. Biochem. J. 1953, 53, 110–117. [Google Scholar] [CrossRef]
- Laguerre, C.; Sanchez-Hernandez, J.C.; Köhler, H.R.; Triebskorn, R.; Capowiez, Y.; Rault, M.; Mazzia, C. B-type esterases in the snail Xeropicta derbentina: An enzymological analysis to evaluate their use as biomarkers of pesticide exposure. Environ. Pollut. 2009, 157, 199–207. [Google Scholar] [CrossRef]
- Rault, M.; Collange, B.; Mazzia, C.; Capowiez, Y. Dynamics of acetylcholinesterase activity recovery in two earthworm species following exposure to ethyl-parathion. Soil Biol. Biochem. 2008, 40, 3086–3091. [Google Scholar] [CrossRef]
- Sugimoto, H.; Yamanish, Y.; Iimura, Y.; Kawakami, Y. Donepezil Hydrochloride (E2020) and Other Acetylcholinesterase Inhibitors. Curr. Med. Chem. 2000, 7, 303–339. [Google Scholar] [CrossRef]
- Carr, R.L.; Chambers, J.E. Acute effects of the organophosphate paraoxon on schedule-controlled behavior and esterase activity in rats: Dose-response relationships. Pharmacol. Biochem. Behav. 1991, 40, 929–936. [Google Scholar] [CrossRef]
- Küster, E. Cholin- and carboxylesterase activities in developing zebrafish embryos (Danio rerio) and their potential use for insecticide hazard assessment. Aquat. Toxicol. 2005, 75, 76–85. [Google Scholar] [CrossRef]
- Sanchez-Hernandez, J.C.; Mazzia, C.; Capowiez, Y.; Rault, M. Carboxylesterase activity in earthworm gut contents: Potential (eco)toxicological implications. Comp. Biochem. Physiol. Part. C: Toxicol. Pharmacol. 2009, 150, 503–511. [Google Scholar] [CrossRef]
- Duarte, I.A.; Pais, M.P.; Reis-Santos, P.; Cabral, H.N.; Fonseca, V.F. Biomarker and behavioural responses of an estuarine fish following acute exposure to fluoxetine. Mar. Environ. Res. 2019, 147, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Munari, M.; Marin, M.G.; Matozzo, V. Effects of the antidepressant fluoxetine on the immune parameters and acetylcholinesterase activity of the clam Venerupis philippinarum. Mar. Environ. Res. 2014, 94, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Walker, C. Biochemical biomarkers in ecotoxicology—some recent developments. Sci. Total. Environ. 1995, 171, 189–195. [Google Scholar] [CrossRef]
- Albrecht, C.; Stelbrink, B.; Clewig, C. Planorbidae Rafinesque. In Freshwater Mollusks od the World: A Distribution Atlas; Cummings, K.S., Lydeard, C., Eds.; Johns Hopkins University Press: Baltimore, MD, USA, 2019. [Google Scholar]
- Costil, K.; Théron, A.; Gérard, C. Influence of temperature on survival and growth of two freshwater planorbid species, Planorbarius corneus (L.) and Planorbis planorbis (L.). J. Molluscan Stud. 1994, 60, 223–235. [Google Scholar] [CrossRef]
- Costil, K. Effect of temperature on embryonic development of two freshwater pulmonates, Planorbarius corneus (L.) and Planorbis planorbis (L.). J. Molluscan Stud. 1997, 63, 293–296. [Google Scholar] [CrossRef] [Green Version]
- Ziegler, M.; Banet, M.; Bauer, R.; Köhler, H.-R.; Stepinski, S.; Tisler, S.; Huhn, C.; Zwiener, C.; Triebskorn, R. Behavioral and Developmental Changes in Brown Trout After Exposure to the Antidepressant Venlafaxine. Front. Environ. Sci. 2021, 8. [Google Scholar] [CrossRef]
- Ziegler, M.; Knoll, S.; Köhler, H.-R.; Tisler, S.; Huhn, C.; Zwiener, C.; Triebskorn, R. Impact of the antidepressant citalopram on the behaviour of two different life stages of brown trout. PeerJ 2020, 8, e8765. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.-W.; Armbrust, K.L. Degradation of citalopram by simulated sunlight. Environ. Toxicol. Chem. 2005, 24, 1618–1623. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Markwell, M.A.K.; Haas, S.M.; Bieber, L.; Tolbert, N. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal. Biochem. 1978, 87, 206–210. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Chanda, S.M.; Mortensen, S.R.; Moser, V.C.; Padilla, S. Tissue-Specific Effects of Chlorpyrifos on Carboxylesterase and Cholinesterase Activity in Adult Rats: An in Vitro and in Vivo Comparison. Fundam. Appl. Toxicol. 1997, 38, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Cayman Chemical. Superoxide Dismutase Assay Kit, Cayman Chemical Company. Ann. Arbor. 2018, 1, 1–23. Available online: https://www.caymanchem.com/pdfs/706002.pdf (accessed on 4 March 2021).
- Dieterich, A.; Troschinski, S.; Schwarz, S.; Di Lellis, M.A.; Henneberg, A.; Fischbach, U.; Ludwig, M.; Gärtner, U.; Triebskorn, R.; Köhler, H.-R. Hsp70 and lipid peroxide levels following heat stress in Xeropicta derbentina (Krynicki 1836) (Gastropoda, Pulmonata) with regard to different colour morphs. Cell Stress Chaperon. 2015, 20, 159–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-Dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Moermond, C.T.; Kase, R.; Korkaric, M.; Ågerstrand, M. CRED: Criteria for reporting and evaluating ecotoxicity data. Environ. Toxicol. Chem. 2016, 35, 1297–1309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, G. The cytology, histochemistry, and ultrastructure of the cell types found in the digestive gland of the slug, Agriolimax reticulatus (Muller). Protoplasma 1970, 71, 91–109. [Google Scholar] [CrossRef]
- Zaldibar, B.; Cancio, I.; Soto, M.; Marigómez, I. Changes in cell-type composition in digestive gland of slugs and its influence in biomarkers following transplantation between a relatively unpolluted and a chronically metal-polluted site. Environ. Pollut. 2008, 156, 367–379. [Google Scholar] [CrossRef]
- Gust, M.; Buronfosse, T.; Giamberini, L.; Ramil, M.; Mons, R.; Garric, J. Effects of fluoxetine on the reproduction of two prosobranch mollusks: Potamopyrgus antipodarum and Valvata piscinalis. Environ. Pollut. 2009, 157, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Péry, A.; Gust, M.; Vollat, B.; Mons, R.; Ramil, M.; Fink, G.; Ternes, T.; Garric, J. Fluoxetine effects assessment on the life cycle of aquatic invertebrates. Chemosphere 2008, 73, 300–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mennigen, J.A.; Sassine, J.; Trudeau, V.; Moon, T.W. Waterborne fluoxetine disrupts feeding and energy metabolism in the goldfish Carassius auratus. Aquat. Toxicol. 2010, 100, 128–137. [Google Scholar] [CrossRef]
- Hexal, A.G. Gebrauchsinformation: Information für Patienten. Citalopram HEXAL® 10 mg Filmtabletten, Hexal AG. 2019. Available online: https://www.hexal.biz/praeparate/dokumente/gi/2019_10_citalopram_10mg_gi-1570717672.pdf (accessed on 17 June 2020).
- Hexal, A.G. Gebrauchsinformation: Information für Patienten. Venlafaxin HEXAL® 37,5 mg Hartkapseln, retardiert. Venlafaxin HEXAL® 75 mg Hartkapseln, retardiert. Venlafaxin HEXAL® 150 mg Hartkapseln, retardiert, Hexal AG, Holzkirchen. 2019. Available online: https://www.hexal.biz/praeparate/dokumente/gi/46259701_165×620_lf_it-1575531248.pdf (accessed on 17 June 2020).
- Fong, P.P.; Bury, T.B.S.; Donovan, E.E.; Lambert, O.J.; Palmucci, J.R.; Adamczak, S.K. Exposure to SSRI-type antidepressants increases righting time in the marine snail Ilyanassa obsoleta. Environ. Sci. Pollut. Res. 2016, 24, 725–731. [Google Scholar] [CrossRef]
- Aonuma, H.; Mezheritskiy, M.; Boldyshev, B.; Totani, Y.; Vorontsov, D.; Zakharov, I.; Ito, E.; Dyakonova, V. The Role of Serotonin in the Influence of Intense Locomotion on the Behavior Under Uncertainty in the Mollusk Lymnaea stagnalis. Front. Physiol. 2020, 11, 221. [Google Scholar] [CrossRef] [Green Version]
- Lewis, S.L.; Lyons, D.E.; Meekins, T.L.; Newcomb, J.M. Serotonin influences locomotion in the nudibranch mollusc Melibe leonina. Biol. Bull. 2011, 220, 155–160. [Google Scholar] [CrossRef] [Green Version]
- Pavlova, G. Effects of serotonin, dopamine and ergometrine on locomotion in the pulmonate mollusc Helix lucorum. J. Exp. Biol. 2001, 204, 1625–1633. [Google Scholar] [CrossRef]
- McKenzie, J.D.; Caunce, M.; Hetherington, M.S.; Winlow, W. Serotonergic innervation of the foot of the pond snail Lymnaea stagnalis (L.). J. Neurocytol. 1998, 27, 459–470. [Google Scholar] [CrossRef]
- Neuparth, T.; Lopes, A.I.; Alves, N.; Oliveira, J.M.; Santos, M.M. Does the antidepressant sertraline show chronic effects on aquatic invertebrates at environmentally relevant concentrations? A case study with the keystone amphipod, Gammarus locusta. Ecotoxicol. Environ. Saf. 2019, 183, 109486. [Google Scholar] [CrossRef] [PubMed]
- Horsmans, Y.; De Clercq, M.; Sempoux, C. Venlaxafine-Associated Hepatitis. Ann. Intern. Med. 1999, 130, 944. [Google Scholar] [CrossRef] [PubMed]
- Stadlmann, S.; Portmann, S.; Tschopp, S.; Terracciano, L.M. Venlafaxine-induced Cholestatic Hepatitis. Am. J. Surg. Pathol. 2012, 36, 1724–1728. [Google Scholar] [CrossRef]
- Dittbrenner, N.; Lazzara, R.; Köhler, H.-R.; Mazzia, C.; Capowiez, Y.; Triebskorn, R. Heat tolerance in Mediterranean land snails: Histopathology after exposure to different temperature regimes. J. Molluscan Stud. 2008, 75, 9–18. [Google Scholar] [CrossRef] [Green Version]
- Hunfeld, N.G.; Berge, R.L.T.; LeBrun, P.P.; Smith, S.J.; Melief, P.H. Hepatotoxicity related to citalopram intake: A case report. Int. J. Risk Saf. Med. 2010, 22, 1–5. [Google Scholar] [CrossRef]
- Neumann, H.; Csepregi, A.; Evert, M.; Malfertheiner, P. Drug-Induced Liver Disease Related to Citalopram. J. Clin. Psychopharmacol. 2008, 28, 254–255. [Google Scholar] [CrossRef]
- Ahmadian, E.; Eftekhari, A.; Fard, J.K.; Babaei, H.; Nayebi, A.M.; Mohammadnejad, D.; Eghbal, M.A. In vitro and in vivo evaluation of the mechanisms of citalopram-induced hepatotoxicity. Arch. Pharmacal. Res. 2017, 40, 1296–1313. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, Z.; Azarnia, M.; Mirabolghasemi, G.; Shiravi, A.; Mohammadi, Z. Histological changes in the liver of fetuses of pregnant rats following citalopram administration. Indian J. Pharmacol. 2013, 45, 517–521. [Google Scholar] [CrossRef] [Green Version]
- Zhou, S.; Di Paolo, C.; Wu, X.; Shao, Y.; Seiler, T.-B.; Hollert, H. Optimization of screening-level risk assessment and priority selection of emerging pollutants—The case of pharmaceuticals in European surface waters. Environ. Int. 2019, 128, 1–10. [Google Scholar] [CrossRef] [PubMed]
- EU Commission Implementing Decision (EU) 2020/1161 of 4 August 2020 Establishing A Watch List of Substances for Union-Wide Monitoring in the Field of Water Policy Pursuant to Directive 2008/105/EC of the European Parliament and of the Council 2020; Official Journal of the European Union: Brussels, Belgium, 2020.
- Cortes, L.G.; Marinov, D.; Sanseverino, I.; Cuenca, A.N.; Niegowska, M.; Rodriguesz, E.P.; Lettieri, T. Selection of Substances for the 3rd Watch List under the Water Framework Directive, EUR 30297 EN.; European Union: Luxembourg, 2020. [Google Scholar] [CrossRef]

| Citalopram Concentration (µg/L) | 0 | 1 | 10 | 100 | 1000 |
|---|---|---|---|---|---|
| Mortality (%) | 0 ± 0 | 6.67 ± 24.49 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Weight (g) | 2.88 ± 0.81 | 2.16 ± 0.74 | 2.19 ± 0.73 | 2.12 ± 0.90 | 1.94 ± 0.86 * |
| AChE activity (mu/mg protein) | 105.31 ± 53.79 | 113.79 ± 42.29 | 100.82 ± 30.15 | 104.33 ± 36.57 | 101.1 ± 42.02 |
| CbE NPA activity (mu/mg protein) | 149.04 ± 32.87 | 155.6 ± 27.53 | 164.09 ± 49.26 | 151.29 ± 46.89 | 156.01 ± 32.34 |
| CbE NPV activity (mu/mg protein) | 93.98 ± 44.21 | 111.73 ± 29.71 | 96.1 ± 41.32 | 79.23 ± 25.14 | 78.31 ± 29.87 |
| SOD activity (U/mL) | 96.67 ± 48.09 | 131.65 ± 50.09 | 128.87 ± 34.03 | 107.85 ± 30.64 | 105.92 ± 43.24 |
| Hsp70-level (relative grey value) | 0.97 ± 0.28 | 1.12 ± 0.27 | 1.18 ± 0.39 | 1.26 ± 0.34 | 1.04 ± 0.42 |
| Aqueous citalopram concentration (µg/L) | <LoD | 1.15 ± 0.04 | 8.47 ± 0.39 | 137 ± 6.48 | 1172 ± 50.41 |
| Venlafaxine Concentration (µg/L) | 0 | 1 | 10 | 100 | 1000 |
|---|---|---|---|---|---|
| Mortality (%) | 4.76 ± 6.73 | 0 ± 0 | 9.52 ± 6.73 | 4.76 ± 6.73 | 0 ± 0 |
| Weight (g) | 1.93 ± 1.00 | 1.80 ± 0.63 | 1.82 ± 0.62 | 1.76 ± 0.62 | 1.52 ± 0.40 |
| Snails detached from surface (%) | 1.97 ± 3.49 | 2.54 ± 3.84 | 5 ± 4.83 | 10.16 ± 6.47 ** | 16.83 ± 6.7 *** |
| AChE activity (mu/mg Protein) | 117.10 ± 47.01 | 100.82 ± 37.95 | 89.71 ± 20.85 | 91.01 ±34.85 | 97.46 ± 21.47 |
| CbE NPA activity (mu/mg Protein) | 133.32 ± 32.92 | 151.45 ± 34.92 | 168.22 ± 23.38 | 157.35 ± 30.32 | 149.31 ± 36.36 |
| CbE NPV activity (mu/mg Protein) | 98.35 ± 52.61 | 108.20 ± 32.24 | 137.43 ± 40.56 | 111.48 ± 44.18 | 101.32 ± 36.36 |
| SOD activity (U/mL) | 114.33 ± 42.57 | 124.73 ± 38.54 | 128.01 ± 37.71 | 138.98 ± 32.21 | 127.93 ± 44.04 |
| Hsp70-level (relative grey value) | 1.39 ± 0.26 | 1.59 ± 0.37 | 1.37 ± 0.36 | 1.35 ± 0.29 | 1.34 ± 0.26 |
| Aqueous venlafaxine concentration (µg/L) | <LoD | 0.73 ± 0.03 | 8.2 ± 0.07 | 79.63 ± 3.25 | 864.15 ± 12.52 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziegler, M.; Eckstein, H.; Köhler, H.-R.; Tisler, S.; Zwiener, C.; Triebskorn, R. Effects of the Antidepressants Citalopram and Venlafaxine on the Big Ramshorn Snail (Planorbarius corneus). Water 2021, 13, 1722. https://doi.org/10.3390/w13131722
Ziegler M, Eckstein H, Köhler H-R, Tisler S, Zwiener C, Triebskorn R. Effects of the Antidepressants Citalopram and Venlafaxine on the Big Ramshorn Snail (Planorbarius corneus). Water. 2021; 13(13):1722. https://doi.org/10.3390/w13131722
Chicago/Turabian StyleZiegler, Michael, Helene Eckstein, Heinz-R. Köhler, Selina Tisler, Christian Zwiener, and Rita Triebskorn. 2021. "Effects of the Antidepressants Citalopram and Venlafaxine on the Big Ramshorn Snail (Planorbarius corneus)" Water 13, no. 13: 1722. https://doi.org/10.3390/w13131722
APA StyleZiegler, M., Eckstein, H., Köhler, H.-R., Tisler, S., Zwiener, C., & Triebskorn, R. (2021). Effects of the Antidepressants Citalopram and Venlafaxine on the Big Ramshorn Snail (Planorbarius corneus). Water, 13(13), 1722. https://doi.org/10.3390/w13131722






