Contrasting the Optical Characterization of Dissolved Organic Matter in Water and Sediment from a Nascent River-Type Lake (Chongqing, China)
Abstract
1. Introduction
2. Materials and Methods
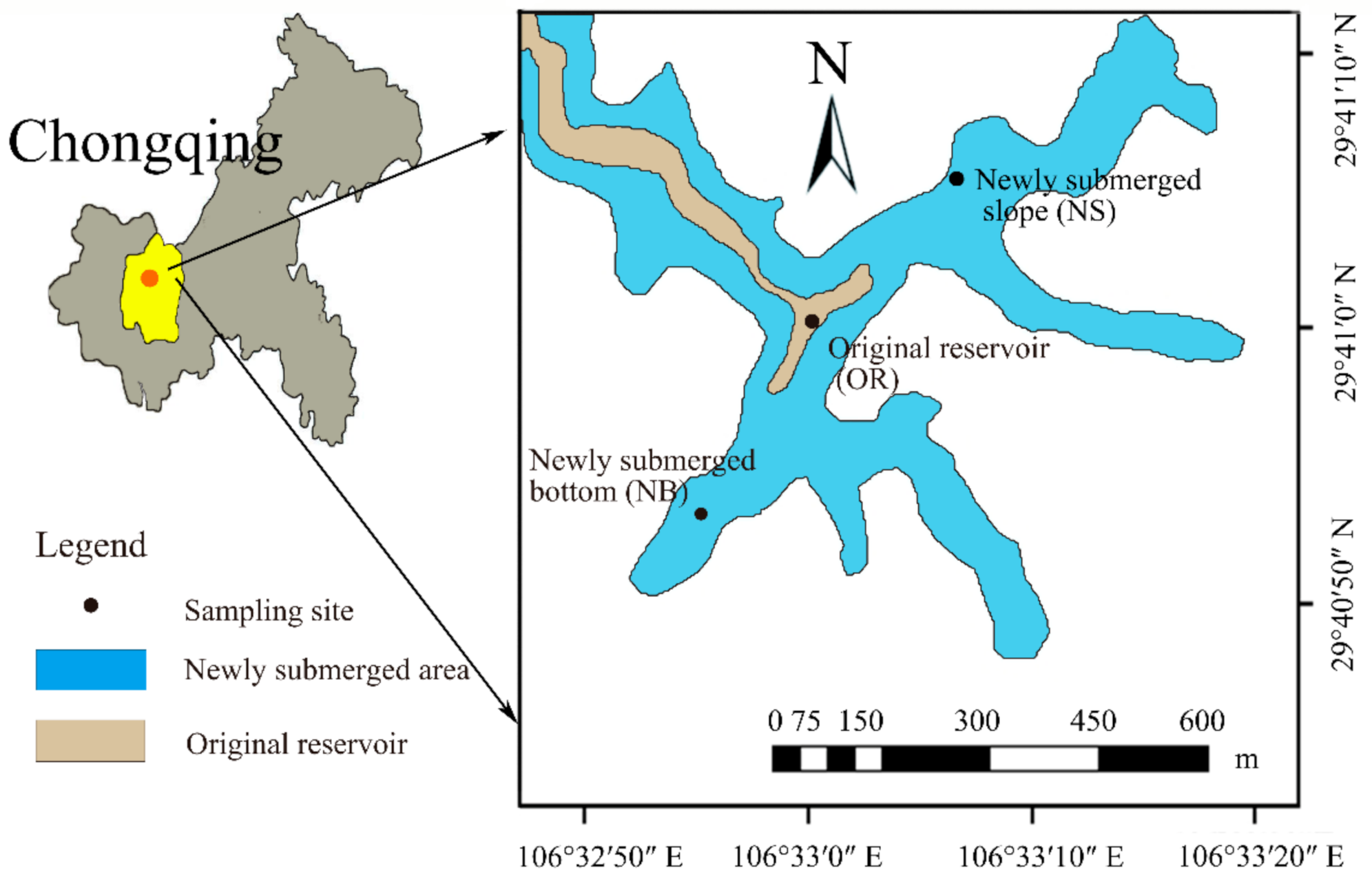
2.1. Site Description
2.2. Sampling and Preprocessing
2.3. Analytical Measurements of PW and DOC Flux Estimation
2.4. Absorbance Measurement
2.5. Fluorescence Measurement
2.6. PARAFAC Modelling
2.7. Statistical Analysis
3. Results and Discussion
3.1. Water Chemistry Properties
3.2. Spatial Distributions of DOC and Optical Indicators
3.2.1. DOC Concentrations and Benthic Flux
3.2.2. Spatial Distributions of Optical Indicators
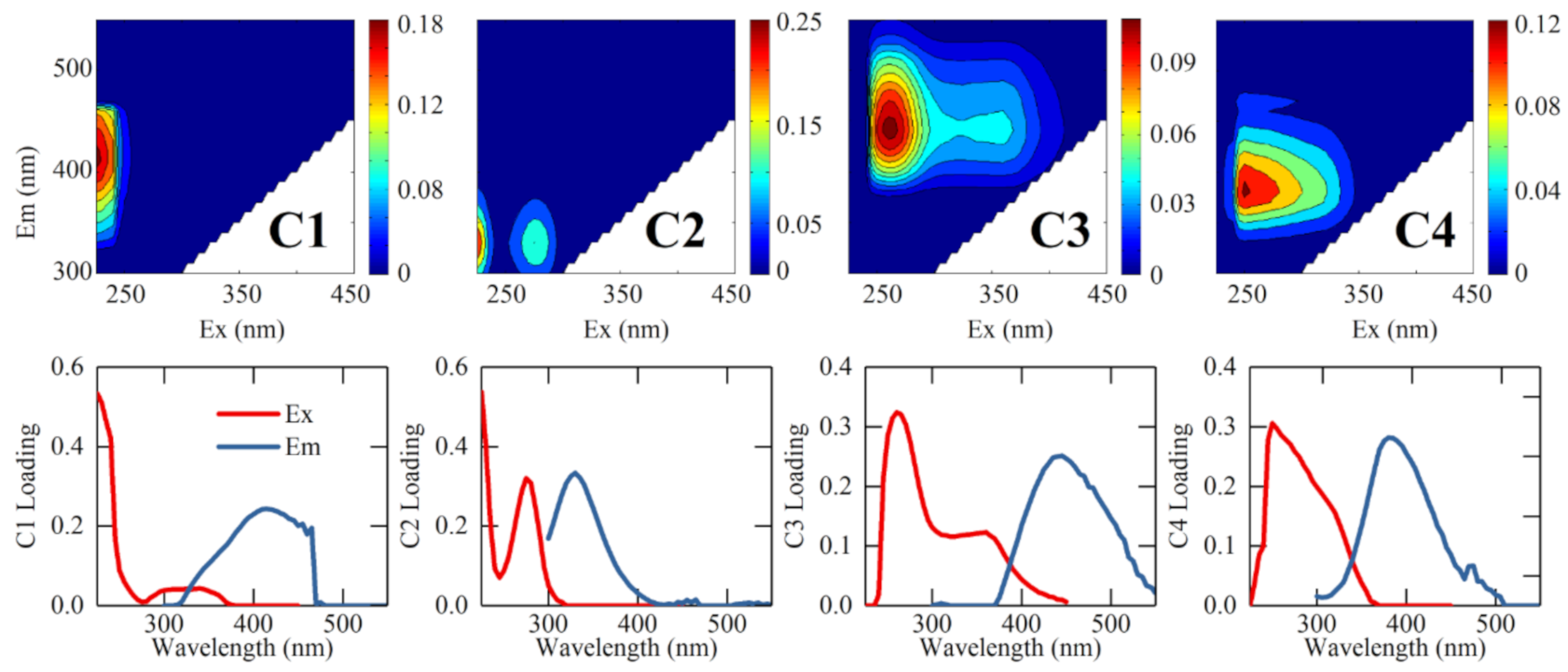
3.3. Composition of Fluorescent PARAFAC Components
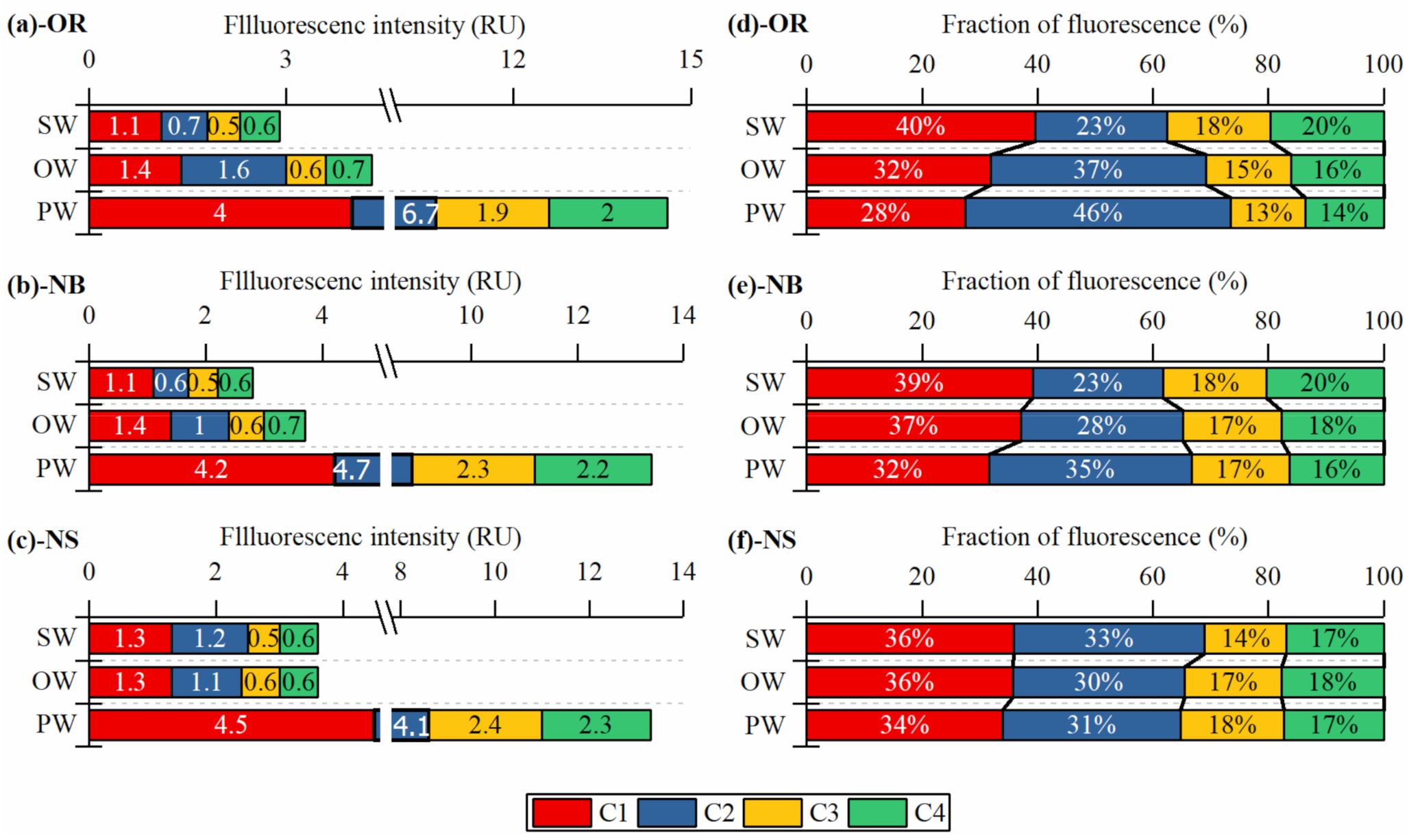
3.4. Spatial Distribution of Fluorescent PARAFAC Components
3.5. Potential Influential Factors to DOM Distribution Behavior
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, M.L.; Kim, S.H.; Jung, H.J.; Hyun, J.H.; Choi, J.H.; Lee, H.J.; Huh, I.A.; Hur, J. Dynamics of dissolved organic matter in riverine sediments affected by weir impoundments: Production, benthic flux, and environmental implications. Water Res. 2017, 121, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Zuijdgeest, A.; Wehrli, B. Carbon and nutrient fluxes from floodplains and reservoirs in the Zambezi basin. Chem. Geol. 2017, 467, 1–11. [Google Scholar] [CrossRef]
- Maavara, T.; Lauerwald, R.; Regnier, P.; Van Cappellen, P. Global perturbation of organic carbon cycling by river damming. Nat. Commun. 2017, 8, 15347. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Pang, Y.; He, C.; Li, P.; Xiao, S.; Sun, Y.; Pan, Q.; Zhang, Y.; Shi, Q.; He, D. Optical and molecular signatures of dissolved organic matter in Xiangxi Bay and mainstream of Three Gorges Reservoir, China: Spatial variations and environmental implications. Sci. Total Environ. 2019, 657, 1274–1284. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Hur, J. Pre-treatments, characteristics, and biogeochemical dynamics of dissolved organic matter in sediments: A review. Water Res. 2015, 79, 10–25. [Google Scholar] [CrossRef] [PubMed]
- Kellerman, A.M.; Kothawala, D.N.; Dittmar, T.; Tranvik, L.J. Persistence of dissolved organic matter in lakes related to its molecular characteristics. Nat. Geosci. 2015, 8, 454–457. [Google Scholar] [CrossRef]
- Meingast, K.M.; Grunert, B.K.; Green, S.A.; Kane, E.S.; Khademimoshgenani, N. Insights on Dissolved Organic Matter Production Revealed by Removal of Charge-Transfer Interactions in Senescent Leaf Leachates. Water 2020, 12, 2356. [Google Scholar] [CrossRef]
- Freixa, A.; Ejarque, E.; Crognale, S.; Amalfitano, S.; Fazi, S.; Butturini, A.; Romani, A.M. Sediment microbial communities rely on different dissolved organic matter sources along a Mediterranean river continuum. Limnol. Oceanogr. 2016, 61, 1389–1405. [Google Scholar] [CrossRef]
- Yang, L.; Choi, J.H.; Hur, J. Benthic flux of dissolved organic matter from lake sediment at different redox conditions and the possible effects of biogeochemical processes. Water Res. 2014, 61, 97–107. [Google Scholar] [CrossRef]
- Withers, P.J.A.; Jarvie, H.P. Delivery and cycling of phosphorus in rivers: A review. Sci. Total Environ. 2008, 400, 379–395. [Google Scholar] [CrossRef]
- Schmidt, F.; Koch, B.P.; Goldhammer, T.; Elvert, M.; Witt, M.; Lin, Y.-S.; Wendt, J.; Zabel, M.; Heuer, V.B.; Hinrichs, K.-U. Unraveling signatures of biogeochemical processes and the depositional setting in the molecular composition of pore water DOM across different marine environments. Geochim. Cosmochim. Acta 2017, 207, 57–80. [Google Scholar] [CrossRef]
- Burdige, D.J.; Kline, S.W.; Chen, W.H. Fluorescent dissolved organic matter in marine sediment pore waters. Mar. Chem. 2004, 89, 289–311. [Google Scholar] [CrossRef]
- Osburn, C.L.; Handsel, L.T.; Mikan, M.P.; Paerl, H.W.; Montgomery, M.T. Fluorescence Tracking of Dissolved and Particulate Organic Matter Quality in a River-Dominated Estuary. Environ. Sci. Technol. 2012, 46, 8628–8636. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, D.; Shen, Z.Y.; Feng, C.H.; Chen, J. Revealing Sources and Distribution Changes of Dissolved Organic Matter (DOM) in Pore Water of Sediment from the Yangtze Estuary. PLoS ONE 2013, 8, e76633. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, D.; Shen, Z.Y.; Chen, J.; Feng, C.H. Characterization and spacial distribution variability of chromophoric dissolved organic matter (CDOM) in the Yangtze Estuary. Chemosphere 2014, 95, 353–362. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Jung, H.; Lee, J.H.; Hur, J. Differences in spectroscopic characteristics between dissolved and particulate organic matters in sediments: Insight into distribution behavior of sediment organic matter. Sci. Total Environ. 2016, 547, 1–8. [Google Scholar] [CrossRef]
- Lee, M.H.; Jung, H.J.; Kim, S.H.; An, S.U.; Choi, J.H.; Lee, H.J.; Huh, I.A.; Hur, J. Potential linkage between sediment oxygen demand and pore water chemistry in weir-impounded rivers. Sci. Total Environ. 2018, 619–620, 1608–1617. [Google Scholar] [CrossRef]
- Ziegelgruber, K.L.; Zeng, T.; Arnold, W.A.; Chin, Y.P. Sources and composition of sediment pore-water dissolved organic matter in prairie pothole lakes. Limnol. Oceanogr. 2013, 58, 1136–1146. [Google Scholar] [CrossRef]
- Mostofa, K.M.G.; Li, W.; Wu, F.C.; Liu, C.Q.; Liao, H.Q.; Zeng, L.; Xiao, M. Environmental characteristics and changes of sediment pore water dissolved organic matter in four Chinese lakes. Environ. Sci. Pollut. Res. 2018, 25, 2783–2804. [Google Scholar] [CrossRef]
- Chen, Y.H.; Yu, K.F.; Zhou, Y.Q.; Ren, L.F.; Kirumba, G.; Zhang, B.; He, Y.L. Characterizing spatiotemporal variations of chromophoric dissolved organic matter in headwater catchment of a key drinking water source in China. Environ. Sci. Pollut. Res. 2017, 24, 27799–27812. [Google Scholar] [CrossRef]
- Pan, Y.; Lei, P.; Zhang, H.; Shan, B.; Li, J. Distribution of nitrogen and phosphorus in the sediments and estimation of the nutrients fluxes in Longjinghu Lake, Chongqing City, during the initial impoundment period. Environ. Sci. 2014, 35, 1727–1734. (In Chinese) [Google Scholar]
- Niu, F.; Ji, F.; Zhao, G.; Zhang, Q.; Shen, Q.; He, Q.; Yan, H. Vertical distribution of bacterial community structure in sediments of Longjing Lake, Chongqing, China. China Environ. Sci. 2017, 37, 2322–2331. (In Chinese) [Google Scholar]
- Tian, T.; Zhang, D.; Li, Y.; He, Q.; Lu, P. Specific features of inorganic sulfur distribution and the effects of the organic contents in the Expo-Garden Longjing Lake, Chongqing. J. Saf. Environ. 2016, 16, 304–308. (In Chinese) [Google Scholar]
- Ji, F.; Yan, H.; Zhao, G.; He, Q.; Niu, F. Distribution of nitrogen speciation at the sediment-water interface in Longjinggou Catchment Area of Longjinghu Lake. China Environ. Sci. 2015, 35, 3101–3107. (In Chinese) [Google Scholar]
- Song, H.; Li, Z.; Du, B.; Wang, G.; Ding, Y. Bacterial communities in sediments of the shallow Lake Dongping in China. J Appl Microbiol 2012, 112, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.; Zou, W.; Jiang, X.; Wan, X.; Hsu, T.-C.; Zheng, Z.; Chen, L.; Xu, M.; Dai, M.; Kao, S. Organic matter decomposition sustains sedimentary nitrogen loss in the Pearl River Estuary, China. Sci. Total Environ. 2019, 648, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Burdige, D.J.; Martens, C.S. Biogeochemical cycling in an organic-rich coastal marine basin: 11. The sedimentary cycling of dissolved, free amino acids. Geochim. Cosmochim. Acta 1990, 54, 3033–3052. [Google Scholar] [CrossRef]
- Helms, J.R.; Stubbins, A.; Ritchie, J.D.; Minor, E.C.; Kieber, D.J.; Mopper, K. Absorption spectral slopes and slope ratios as indicators of molecular weight, source, and photobleaching of chromophoric dissolved organic matter. Limnol. Oceanogr. 2008, 53, 955–969. [Google Scholar] [CrossRef]
- De Haan, H.; De Boer, T. Applicability of light absorbance and fluorescence as measures of concentration and molecular size of dissolved organic carbon in humic Lake Tjeukemeer. Water Res. 1987, 21, 731–734. [Google Scholar] [CrossRef]
- Timko, S.A.; Gonsior, M.; Cooper, W.J. Influence of pH on fluorescent dissolved organic matter photo-degradation. Water Res. 2015, 85, 266–274. [Google Scholar] [CrossRef]
- Zhou, Y.; Jeppesen, E.; Zhang, Y.; Shi, K.; Liu, X.; Zhu, G. Dissolved organic matter fluorescence at wavelength 275/342 nm as a key indicator for detection of point-source contamination in a large Chinese drinking water lake. Chemosphere 2016, 144, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Weishaar, J.L.; Aiken, G.R.; Bergamaschi, B.A.; Fram, M.S.; Fujii, R.; Mopper, K. Evaluation of specific ultraviolet absorbance as an indicator of the chemical composition and reactivity of dissolved organic carbon. Environ. Sci. Technol. 2003, 37, 4702–4708. [Google Scholar] [CrossRef] [PubMed]
- Cory, R.M.; McKnight, D.M. Fluorescence spectroscopy reveals ubiquitous presence of oxidized and reduced quinones in dissolved organic matter. Environ. Sci. Technol. 2005, 39, 8142–8149. [Google Scholar] [CrossRef] [PubMed]
- McKnight, D.M.; Boyer, E.W.; Westerhoff, P.K.; Doran, P.T.; Kulbe, T.; Andersen, D.T. Spectrofluorometric characterization of dissolved organic matter for indication of precursor organic material and aromaticity. Limnol. Oceanogr. 2001, 46, 38–48. [Google Scholar] [CrossRef]
- Huguet, A.; Vacher, L.; Relexans, S.; Saubusse, S.; Froidefond, J.M.; Parlanti, E. Properties of fluorescent dissolved organic matter in the Gironde Estuary. Org. Geochem. 2009, 40, 706–719. [Google Scholar] [CrossRef]
- Lawaetz, A.J.; Stedmon, C.A. Fluorescence Intensity Calibration Using the Raman Scatter Peak of Water. Appl. Spectrosc. 2009, 63, 936–940. [Google Scholar] [CrossRef]
- Kothawala, D.N.; Murphy, K.R.; Stedmon, C.A.; Weyhenmeyer, G.A.; Tranvik, L.J. Inner filter correction of dissolved organic matter fluorescence. Limnol. Oceanogr. Methods 2013, 11, 616–630. [Google Scholar] [CrossRef]
- Stedmon, C.A.; Bro, R. Characterizing dissolved organic matter fluorescence with parallel factor analysis: A tutorial. Limnol. Oceanogr. Methods 2008, 6, 572–579. [Google Scholar] [CrossRef]
- Stedmon, C.A.; Markager, S.; Bro, R. Tracing dissolved organic matter in aquatic environments using a new approach to fluorescence spectroscopy. Mar. Chem. 2003, 82, 239–254. [Google Scholar] [CrossRef]
- Zhou, Y.Q.; Zhang, Y.L.; Jeppesen, E.; Murphy, K.R.; Shi, K.; Liu, M.L.; Liu, X.H.; Zhu, G.W. Inflow rate-driven changes in the composition and dynamics of chromophoric dissolved organic matter in a large drinking water lake. Water Res. 2016, 100, 211–221. [Google Scholar] [CrossRef]
- Chen, M.; Kim, J.H.; Nam, S.I.; Niessen, F.; Hong, W.L.; Kang, M.H.; Hur, J. Production of fluorescent dissolved organic matter in Arctic Ocean sediments. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Burdige, D.J.; Komada, T.; Magen, C.; Chanton, J.P. Modeling studies of dissolved organic matter cycling in Santa Barbara Basin (CA, USA) sediments. Geochim. Cosmochim. Acta 2016, 195, 100–119. [Google Scholar] [CrossRef]
- Burdige, D.J.; Komada, T. Sediment Pore Waters. In Biogeochemistry of Marine Dissolved Organic Matter, 2nd ed.; Hansell, D.A., Carlson, C.A., Eds.; Academic Press: Boston, MA, USA, 2015; pp. 535–577. [Google Scholar] [CrossRef]
- Skoog, A.C.; Arias-Esquivel, V.A. The effect of induced anoxia and reoxygenation on benthic fluxes of organic carbon, phosphate, iron, and manganese. Sci. Total Environ. 2009, 407, 6085–6092. [Google Scholar] [CrossRef] [PubMed]
- Twardowski, M.S.; Boss, E.; Sullivan, J.M.; Donaghay, P.L. Modeling the spectral shape of absorption by chromophoric dissolved organic matter. Mar. Chem. 2004, 89, 69–88. [Google Scholar] [CrossRef]
- Bai, L.; Zhang, Q.; Wang, C.; Yao, X.; Zhang, H.; Jiang, H. Effects of natural dissolved organic matter on the complexation and biodegradation of 17α-ethinylestradiol in freshwater lakes. Environ. Pollut. 2019, 246, 782–789. [Google Scholar] [CrossRef]
- Hansen, A.M.; Kraus, T.E.C.; Pellerin, B.A.; Fleck, J.A.; Downing, B.D.; Bergamaschi, B.A. Optical properties of dissolved organic matter (DOM): Effects of biological and photolytic degradation. Limnol. Oceanogr. 2016, 61, 1015–1032. [Google Scholar] [CrossRef]
- Abdelrady, A.; Sharma, S.; Sefelnasr, A.; Kennedy, M. The Fate of Dissolved Organic Matter (DOM) During Bank Filtration under Different Environmental Conditions: Batch and Column Studies. Water 2018, 10, 1730. [Google Scholar] [CrossRef]
- Stubbins, A.; Spencer, R.G.M.; Chen, H.M.; Hatcher, P.G.; Mopper, K.; Hernes, P.J.; Mwamba, V.L.; Mangangu, A.M.; Wabakanghanzi, J.N.; Six, J. Illuminated darkness: Molecular signatures of Congo River dissolved organic matter and its photochemical alteration as revealed by ultrahigh precision mass spectrometry. Limnol. Oceanogr. 2010, 55, 1467–1477. [Google Scholar] [CrossRef]
- Fellman, J.B.; Hood, E.; Spencer, R.G.M. Fluorescence spectroscopy opens new windows into dissolved organic matter dynamics in freshwater ecosystems: A review. Limnol. Oceanogr. 2010, 55, 2452–2462. [Google Scholar] [CrossRef]
- Lipczynska-Kochany, E. Humic substances, their microbial interactions and effects on biological transformations of organic pollutants in water and soil: A review. Chemosphere 2018, 202, 420–437. [Google Scholar] [CrossRef]
- Coble, P.G. Characterization of marine and terrestrial DOM in seawater using excitation emission matrix spectroscopy. Mar. Chem. 1996, 51, 325–346. [Google Scholar] [CrossRef]
- Coble, P.G.; Del Castillo, C.E.; Avril, B. Distribution and optical properties of CDOM in the Arabian Sea during the 1995 Southwest Monsoon. Deep Sea Res. Part Ii Top. Stud. Oceanogr. 1998, 45, 2195–2223. [Google Scholar] [CrossRef]
- Hudson, N.; Baker, A.; Ward, D.; Reynolds, D.M.; Brunsdon, C.; Carliell-Marquet, C.; Browning, S. Can fluorescence spectrometry be used as a surrogate for the Biochemical Oxygen Demand (BOD) test in water quality assessment? An example from South West England. Sci. Total Environ. 2008, 391, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Coble, P.G. Marine optical biogeochemistry: The chemistry of ocean color. Chem. Rev. 2007, 107, 402–418. [Google Scholar] [CrossRef] [PubMed]
- Maie, N.; Scully, N.M.; Pisani, O.; Jaffé, R. Composition of a protein-like fluorophore of dissolved organic matter in coastal wetland and estuarine ecosystems. Water Res. 2007, 41, 563–570. [Google Scholar] [CrossRef]
- Hernes, P.J.; Bergamaschi, B.A.; Eckard, R.S.; Spencer, R.G.M. Fluorescence-based proxies for lignin in freshwater dissolved organic matter. J. Geophys. Res. Biogeosci. 2009, 114, G00F03. [Google Scholar] [CrossRef]
- Cammack, W.K.L.; Kalff, J.; Prairie, Y.T.; Smith, E.M. Fluorescent dissolved organic matter in lakes: Relationships with heterotrophic metabolism. Limnol. Oceanogr. 2004, 49, 2034–2045. [Google Scholar] [CrossRef]
- Elliott, S.; Lead, J.R.; Baker, A. Characterisation of the fluorescence from freshwater, planktonic bacteria. Water Res. 2006, 40, 2075–2083. [Google Scholar] [CrossRef]
- Tedetti, M.; Cuet, P.; Guigue, C.; Goutx, M. Characterization of dissolved organic matter in a coral reef ecosystem subjected to anthropogenic pressures (La Reunion Island, Indian Ocean) using multi-dimensional fluorescence spectroscopy. Sci. Total Environ. 2011, 409, 2198–2210. [Google Scholar] [CrossRef]
- Hur, J.; Lee, B.-M.; Shin, K.-H. Spectroscopic characterization of dissolved organic matter isolates from sediments and the association with phenanthrene binding affinity. Chemosphere 2014, 111, 450–457. [Google Scholar] [CrossRef]
- Xiao, M.; Wu, F.; Yi, Y.; Han, Z.; Wang, Z. Optical Properties of Dissolved Organic Matter and Controlling Factors in Dianchi Lake Waters. Water 2019, 11, 1967. [Google Scholar] [CrossRef]
- Kellerman, A.M.; Dittmar, T.; Kothawala, D.N.; Tranvik, L.J. Chemodiversity of dissolved organic matter in lakes driven by climate and hydrology. Nat. Commun. 2014, 5, 3804. [Google Scholar] [CrossRef] [PubMed]
- Ishii, S.K.L.; Boyer, T.H. Behavior of Reoccurring PARAFAC Components in Fluorescent Dissolved Organic Matter in Natural and Engineered Systems: A Critical Review. Environ. Sci. Technol. 2012, 46, 2006–2017. [Google Scholar] [CrossRef] [PubMed]
- Vecchio, R.D.; Blough, N.V. On the origin of the optical properties of humic substances. Environ. Sci. Technol. 2004, 38, 3885–3891. [Google Scholar] [CrossRef]
- Kothawala, D.N.; von Wachenfeldt, E.; Koehler, B.; Tranvik, L.J. Selective loss and preservation of lake water dissolved organic matter fluorescence during long-term dark incubations. Sci. Total Environ. 2012, 433, 238–246. [Google Scholar] [CrossRef]
- Hertkorn, N.; Benner, R.; Frommberger, M.; Schmitt-Kopplin, P.; Witt, M.; Kaiser, K.; Kettrup, A.; Hedges, J.I. Characterization of a major refractory component of marine dissolved organic matter. Geochim. Cosmochim. Acta 2006, 70, 2990–3010. [Google Scholar] [CrossRef]
- Smilauer, P.; Lepš, J. Multivariate Analysis of Ecological Data Using Canoco 5; Cambridge University Press: New York, NY, USA, 2014; p. 193. [Google Scholar]


| Formula | Calculation | Purpose |
|---|---|---|
| Spectral slopes from 275 to 295 and 350 to 400 nm (S275–295 and S350–400, respectively) were calculated by performing linear regressions of the natural log transformed absorbance spectra [28]. | A proxy (inversely related) for DOM molecular weight [28] | |
| The ratio of Naperian absorption coefficients at 250 to 365 nm [29,30]. | To estimate relative molecular size of DOM [31] | |
| Calculated by dividing the absorption at wavelength 254 (a254, m−1) by DOC concentration (CDOC, mg/L). | A proxy for aromaticity [32] | |
| The ratio of Em 470 nm and Em 520 nm at Ex at 370 nm for instrument-corrected spectra [33]. | To distinguish the terrestrially- and microbially-derived DOMs [34] | |
| The ratio of Em 380 nm and Em 430 nm at Ex 310 nm [35]. | An indicator of recent autotrophic productivity [35] |
| Sampling Site | Water Layer | Water Depth(m) | pH Values | Water Temperature (°C) | DO (mg/L) | Sediment Area (m2) |
|---|---|---|---|---|---|---|
| OR | SW | 0.5 | 8.07 | 25.2 | 4.40 | - |
| OW | 16.1 | 7.15 | 21.6 | 1.14 | 31,836 | |
| NB | SW | 0.5 | 8.07 | 25.1 | 4.29 | - |
| OW | 10.4 | 6.60 | 24.3 | 2.71 | 138,427 | |
| NS | SW | 0.5 | 8.07 | 25.0 | 4.14 | - |
| OW | 7.0 | 8.02 | 24.6 | 4.09 | 213,168 |
| Exmax/Emmax (nm/nm) | Traditional Classification [52] | Comp. | Previous Findings | Assignment [13,18] | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | V | VI | VII | VIII | ||||
| <225/415 | A | C1 | C2 | C2 | C1 | C1 | C2 | C3 | C1 | C1 | Terrestrial humic-like |
| <225(275)/330 | T | C2 | C5 | C4 | C3 | C2 | C3 | C2 | C3 | C3 | Protein-like |
| 260(360)/445 | C | C3 | C4 | C3 | C2 | C3 | C1 | C4 | C2 | C2 | Terrestrial humic-like |
| 255(295)/385 | M | C4 | - | C1 | -- | -- | -- | -- | -- | -- | Microbial humic-like |
| SR | SUVA254 | E2/E3 | FI | BIX | C1 | C2 | C3 | C4 | C1% | C2% | C3% | C4% | DOC | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SR | 1 | ** | ** | ** | ** | ** | ** | * | ||||||
| SUVA254 | - | 1 | * | ** | ** | ** | ** | ** | ** | |||||
| E2/E3 | −0.631 | −0.369 | 1 | * | * | ** | * | ** | ** | ** | ** | ** | ||
| FI | - | - | −0.311 | 1 | ||||||||||
| BIX | - | −0.682 | - | - | 1 | ** | ** | ** | ** | ** | ||||
| C1 | - | 0.793 | −0.301 | - | −0.816 | 1 | ** | ** | ** | ** | ||||
| C2 | 0.435 | 0.616 | −0.679 | - | −0.545 | 0.716 | 1 | ** | ** | ** | ** | ** | ** | ** |
| C3 | - | 0.78 | - | - | −0.852 | 0.982 | 0.692 | 1 | ** | ** | ||||
| C4 | - | 0.784 | −0.303 | - | −0.819 | 0.981 | 0.737 | 0.97 | 1 | ** | ||||
| C1% | −0.53 | - | 0.671 | - | - | - | −0.727 | - | - | 1 | ** | ** | ** | ** |
| C2% | 0.555 | - | −0.671 | - | - | - | 0.679 | - | - | −0.97 | 1 | ** | ** | ** |
| C3% | −0.445 | - | 0.551 | - | - | - | −0.538 | - | - | 0.854 | −0.931 | 1 | ** | * |
| C4% | −0.615 | - | 0.697 | - | - | - | −0.664 | - | - | 0.903 | −0.949 | 0.854 | 1 | ** |
| DOC | 0.307 | 0.502 | −0.65 | - | −0.574 | 0.75 | 0.861 | 0.737 | 0.745 | −0.507 | 0.443 | −0.338 | −0.47 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, F.; Ji, F.; Zhang, Q.; Shen, Q. Contrasting the Optical Characterization of Dissolved Organic Matter in Water and Sediment from a Nascent River-Type Lake (Chongqing, China). Water 2021, 13, 70. https://doi.org/10.3390/w13010070
Niu F, Ji F, Zhang Q, Shen Q. Contrasting the Optical Characterization of Dissolved Organic Matter in Water and Sediment from a Nascent River-Type Lake (Chongqing, China). Water. 2021; 13(1):70. https://doi.org/10.3390/w13010070
Chicago/Turabian StyleNiu, Fengxia, Fangying Ji, Qian Zhang, and Qiushi Shen. 2021. "Contrasting the Optical Characterization of Dissolved Organic Matter in Water and Sediment from a Nascent River-Type Lake (Chongqing, China)" Water 13, no. 1: 70. https://doi.org/10.3390/w13010070
APA StyleNiu, F., Ji, F., Zhang, Q., & Shen, Q. (2021). Contrasting the Optical Characterization of Dissolved Organic Matter in Water and Sediment from a Nascent River-Type Lake (Chongqing, China). Water, 13(1), 70. https://doi.org/10.3390/w13010070




