A MATLAB-Based Application for Modeling and Simulation of Solar Slurry Photocatalytic Reactors for Environmental Applications
Abstract
1. Introduction
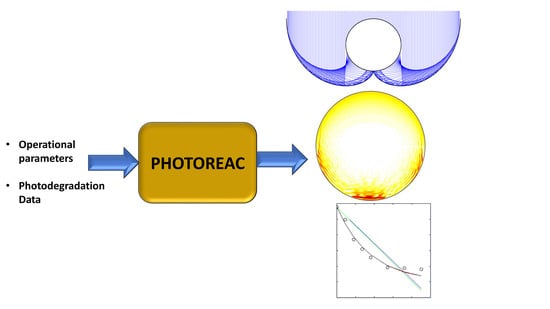
2. Solar Photoreactors Modeling by PHOTOREAC
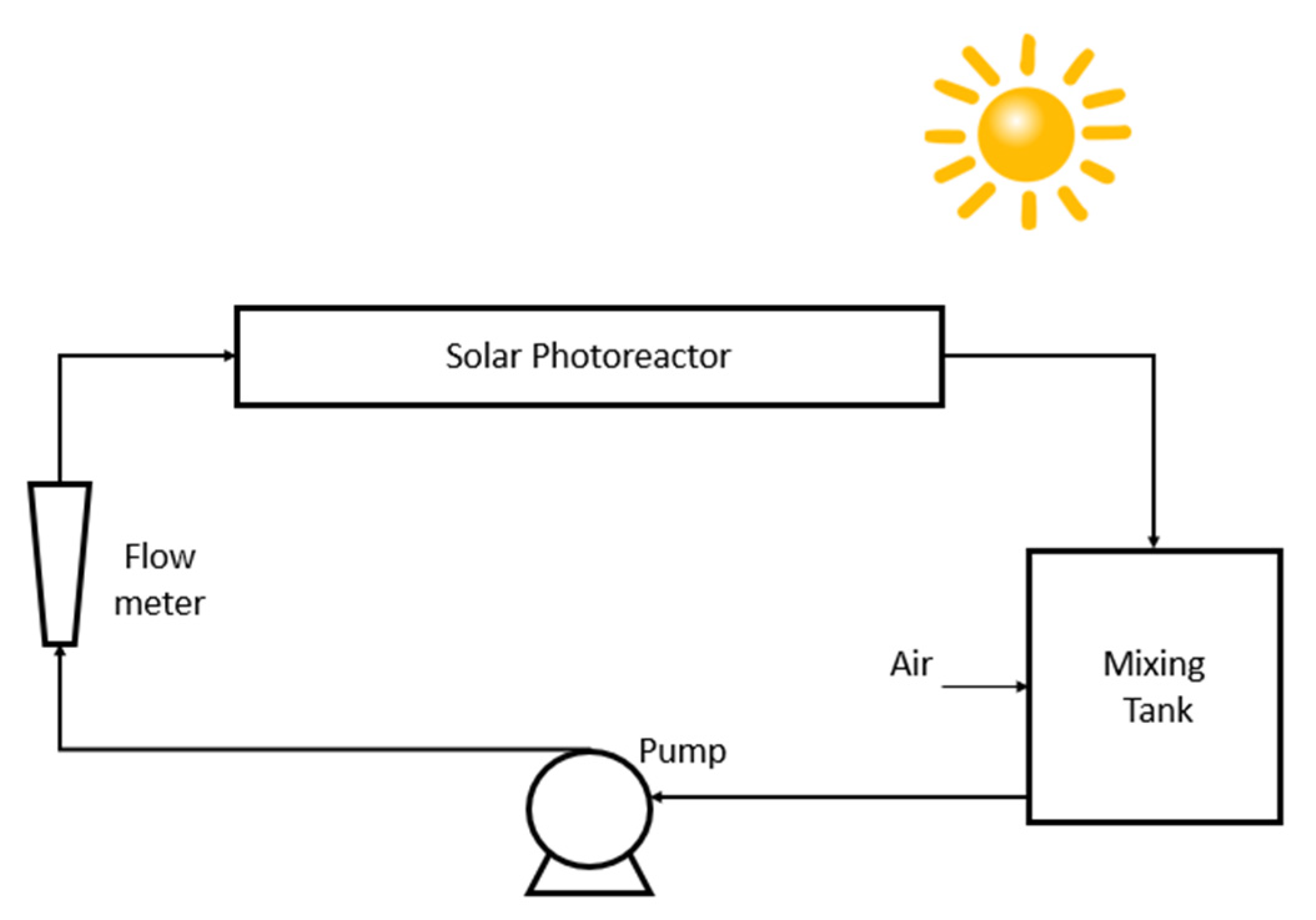
2.1. The Photoreactors Set-Up in PHOTOREAC
2.2. The Input Data for the Use of PHOTOREAC
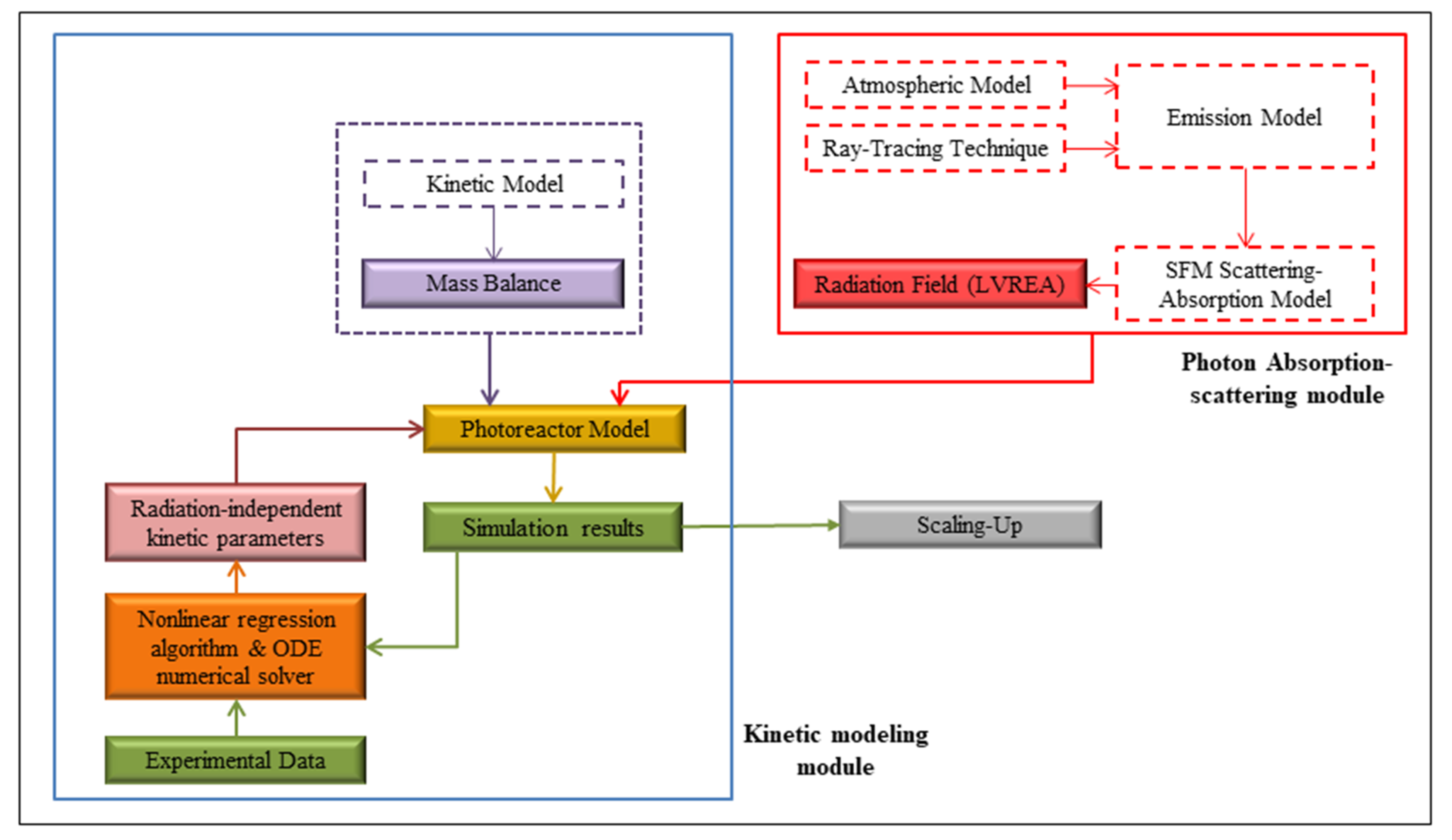
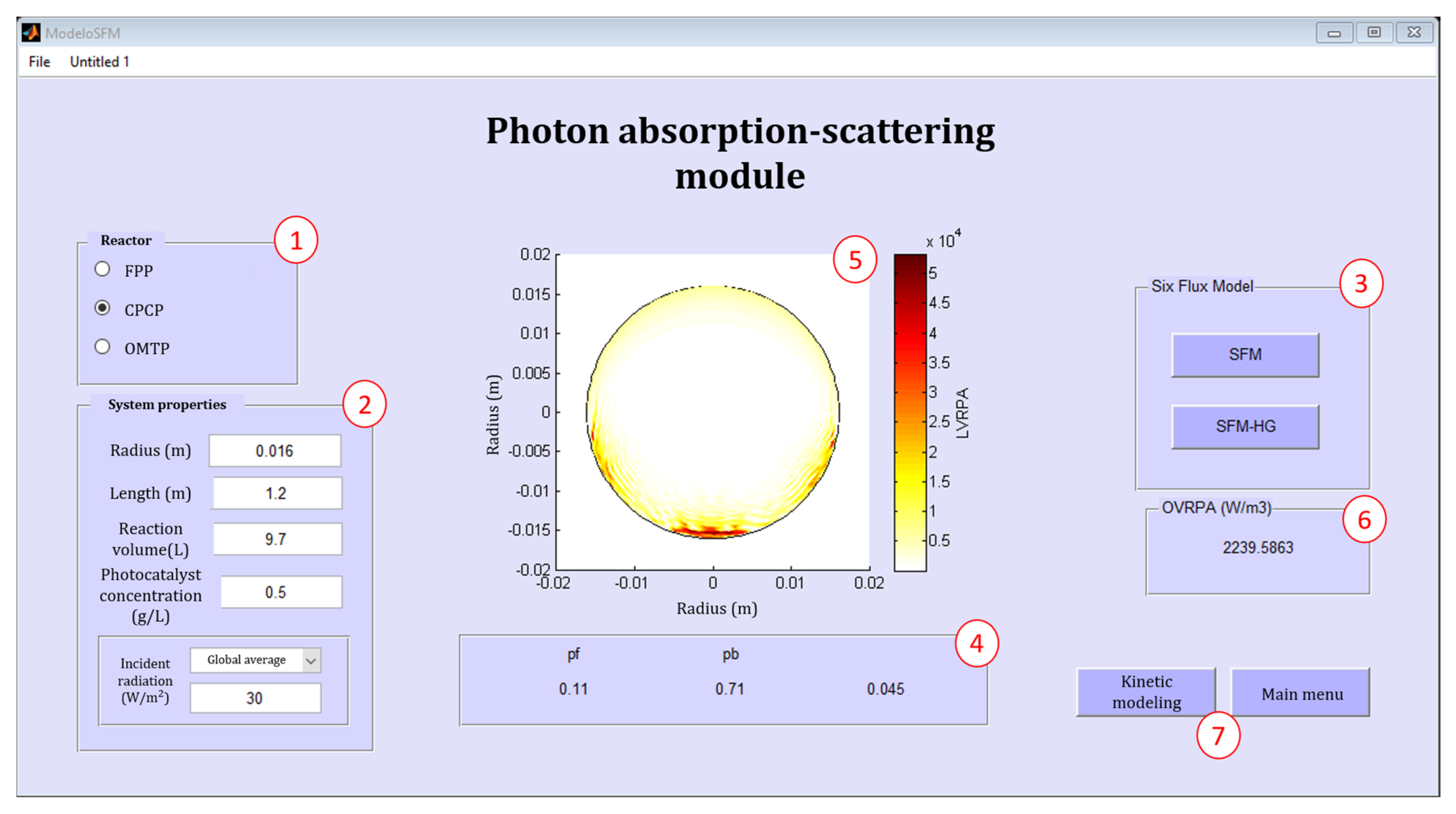
2.3. The PHOTOREAC Photon Absorption-Scattering Module
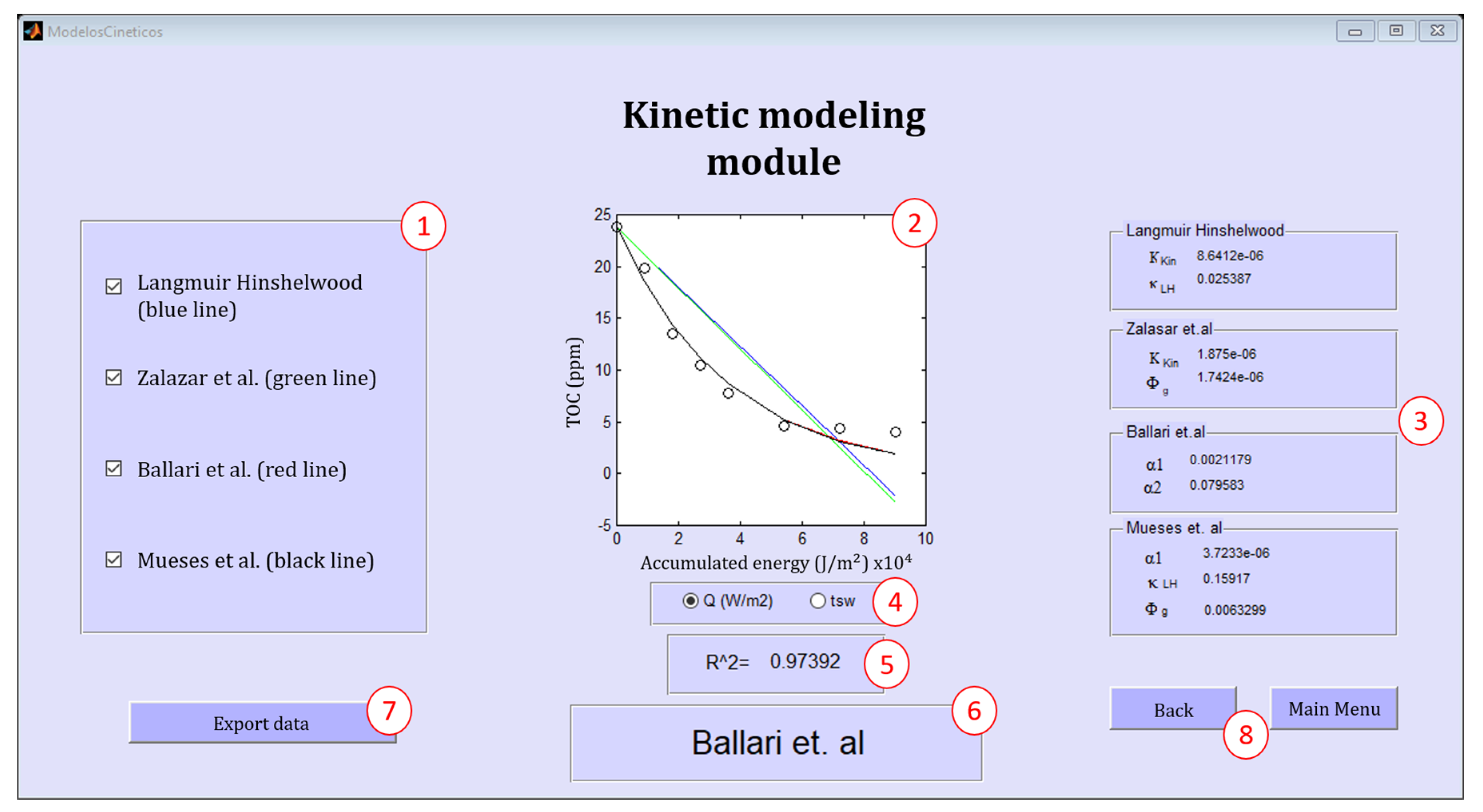
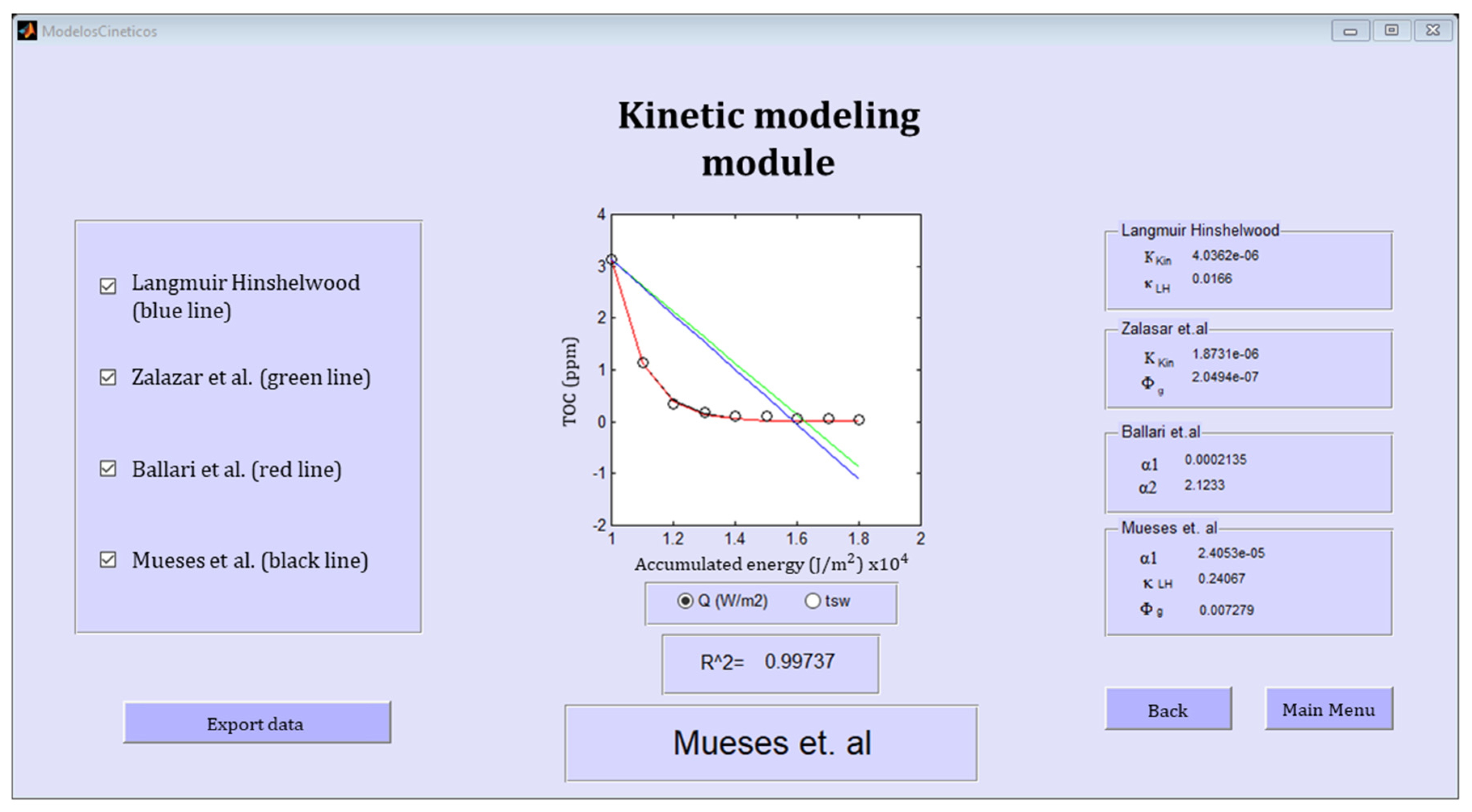
2.4. The PHOTOREAC Kinetic Modeling Module
The Kinetic Modeling Module Database
3. Implementation of PHOTOREAC in Solar Photoreactors
3.1. Example Case I: Solar Photodegradation of Dichloroacetic Acid (DCA) in a CPCP
3.2. Example Case II: Solar Photodegradation of Methylene Blue in an OMTP
3.3. Example Case III: Radiation Field Modeling in a Flat Plate Photoreactor (FPP)
4. PHOTOREAC Implementation in Research Projects in Heterogeneous Photocatalysis and Photoreactor Engineering by Chemical Engineering Undergraduates
5. Analysis of the Overall Performance of PHOTOREAC
6. Limitations and Future Work
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Byrne, C.; Subramanian, G.; Pillai, S.C. Recent advances in photocatalysis for environmental applications. J. Environ. Chem. Eng. 2018, 6, 3531–3555. [Google Scholar] [CrossRef]
- Kumaravel, V.; Mathew, S.; Bartlett, J.; Pillai, S.C. Photocatalytic hydrogen production using metal doped TiO2: A review of recent advances. Appl. Catal. B Environ. 2019, 244, 1021–1064. [Google Scholar] [CrossRef]
- Spasiano, D.; Marotta, R.; Malato, S.; Fernandez-Ibañez, P.; Di Somma, I. Solar photocatalysis: Materials, reactors, some commercial, and pre-industrialized applications. A comprehensive approach. Appl. Catal. B Environ. 2015, 170–171, 90–123. [Google Scholar] [CrossRef]
- Cassano, A.E.; Alfano, O.M. Reaction engineering of suspended solid heterogeneous photocatalytic reactors. Catal. Today 2000, 58, 167–197. [Google Scholar] [CrossRef]
- Li Puma, G.; Brucato, A. Dimensionless analysis of slurry photocatalytic reactors using two-flux and six-flux radiation absorption-scattering models. Catal. Today 2007, 122, 78–90. [Google Scholar] [CrossRef]
- Grčić, I.; Li Puma, G. Photocatalytic degradation of water contaminants in multiple photoreactors and evaluation of reaction kinetic constants independent of photon absorption, irradiance, reactor geometry, and hydrodynamics. Environ. Sci. Technol. 2013, 47, 13702–13711. [Google Scholar] [CrossRef]
- Marugán, J.; van Grieken, R.; Pablos, C.; Satuf, M.L.; Cassano, A.E.; Alfano, O.M. Rigorous kinetic modelling with explicit radiation absorption effects of the photocatalytic inactivation of bacteria in water using suspended titanium dioxide. Appl. Catal. B Environ. 2011, 102, 404–416. [Google Scholar] [CrossRef]
- Colina-Marquez, J.; Castilla-Caballero, D.; Machuca-Martinez, F. Modeling of a falling-film photocatalytic reactor: Fluid dynamics for turbulent regime. Appl. Math. Model. 2016, 40, 4812–4821. [Google Scholar] [CrossRef]
- Malato, S.; Maldonado, M.I.; Fernández-Ibáñez, P.; Oller, I.; Polo, I.; Sánchez-Moreno, R. Decontamination and disinfection of water by solar photocatalysis: The pilot plants of the Plataforma Solar de Almeria. Mater. Sci. Semicond. Process. 2016, 42, 15–23. [Google Scholar] [CrossRef]
- Ochoa-Gutiérrez, K.S.; Tabares-Aguilar, E.; Mueses, M.Á.; Machuca-Martínez, F.; Li Puma, G. A Novel Prototype Offset Multi Tubular Photoreactor (OMTP) for solar photocatalytic degradation of water contaminants. Chem. Eng. J. 2018, 341, 628–638. [Google Scholar] [CrossRef]
- Manassero, A.; Satuf, M.L.; Alfano, O.M. Evaluation of UV and visible light activity of TiO2 catalysts for water remediation. Chem. Eng. J. 2013, 225, 378–386. [Google Scholar] [CrossRef]
- Mueses, M.A.; Machuca-Martinez, F.; Li Puma, G. Effective quantum yield and reaction rate model for evaluation of photocatalytic degradation of water contaminants in heterogeneous pilot-scale solar photoreactors. Chem. Eng. J. 2013, 215–216, 937–947. [Google Scholar] [CrossRef]
- Colina-Márquez, J.; Machuca-Martínez, F.; Li Puma, G. Photocatalytic mineralization of commercial herbicides in a pilot-scale solar CPC reactor: Photoreactor modeling and reaction kinetics constants independent of radiation field. Environ. Sci. Technol. 2009, 43, 8953–8960. [Google Scholar] [CrossRef] [PubMed]
- Colina-Marquez, J.; Machuca-Martínez, F.; Puma, G.L. Radiation absorption and optimization of solar photocatalytic reactors for environmental applications. Environ. Sci. Technol. 2010, 44, 5112–5120. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Herazo, R.; Monterroza-Romero, J.; Mueses, M.A.; Machuca-Martínez, F.; Li Puma, G. Coupling the Six Flux Absorption-Scattering Model to the Henyey-Greenstein scattering phase function: Evaluation and optimization of radiation absorption in solar heterogeneous photoreactors. Chem. Eng. J. 2016, 302, 86–96. [Google Scholar] [CrossRef]
- Otálvaro-Marín, H.L.; Mueses, M.A.; Machuca-Martínez, F. Boundary layer of photon absorption applied to heterogeneous photocatalytic solar flat plate reactor design. Int. J. Photoenergy 2014, 2014. [Google Scholar] [CrossRef]
- Zalazar, C.S.; Romero, R.L.; Martín, C.A.; Cassano, A.E. Photocatalytic intrinsic reaction kinetics I: Mineralization of dichloroacetic acid. Chem. Eng. Sci. 2005, 60, 5240–5254. [Google Scholar] [CrossRef]
- De Los Ballari, M.M.; Alfano, O.O.; Cassano, A.E. Photocatalytic degradation of dichloroacetic acid. A kinetic study with a mechanistically based reaction model. Ind. Eng. Chem. Res. 2009, 48, 1847–1858. [Google Scholar] [CrossRef]
- Otálvaro-Marín, H.L.; González-Caicedo, F.; Arce-Sarria, A.; Mueses, M.A.; Crittenden, J.C.; Machuca-Martinez, F. Scaling-up a heterogeneous H2O2/TiO2/solar-radiation system using the DamkÖhler number. Chem. Eng. J. 2019, 364, 244–256. [Google Scholar] [CrossRef]
- Molano, M.; Mueses, M.A.; Fiderman, M.M. Modelado y evaluación experimental de un reactor solar fotocatalítico no isotérmico: Efecto de la temperatura sobre la cinética de la velocidad de reacción. Ing. Compet. 2017, 19, 143–154. [Google Scholar] [CrossRef][Green Version]
- Casado, C.; García-Gil, Á.; van Grieken, R.; Marugán, J. Critical role of the light spectrum on the simulation of solar photocatalytic reactors. Appl. Catal. B Environ. 2019, 252, 1–9. [Google Scholar] [CrossRef]
- Casado, C.; Marugán, J.; Timmers, R.; Muñoz, M.; van Grieken, R. Comprehensive multiphysics modeling of photocatalytic processes by computational fluid dynamics based on intrinsic kinetic parameters determined in a differential photoreactor. Chem. Eng. J. 2017, 310, 368–380. [Google Scholar] [CrossRef]
- Boyjoo, Y.; Ang, M.; Pareek, V. Some aspects of photocatalytic reactor modeling using computational fluid dynamics. Chem. Eng. Sci. 2013, 101, 764–784. [Google Scholar] [CrossRef]




| Parameter | Symbol | Units | Belonging to PHOTOREAC Module a |
|---|---|---|---|
| Photoreactor radius (CPCP and OMTP) | R | m | PASM |
| Water film thickness (FFP) | δ | m | PASM |
| Photoreactor length | L | m | PASM |
| Solar incident radiation | I0 | W/m2 | PASM |
| Reaction volume | VR | L | PASM/KMM |
| Photocatalyst concentration | Ccat | g/L | PASM/KMM |
| Total volume | VT | L | KMM |
| Number of experimental photodegradation data | N | Dimensionless | KMM |
| Concentration vs. accumulated energy data b | Ci vs. ξAE | ppm vs. J/m2 | KMM |
| Concentration vs. standard time data b | Ci vs. t | ppm vs. min | KMM |
| Kinetic Model | Mathematical Expression | Fitting Parameters | Refs. |
|---|---|---|---|
| Langmuir–Hinshelwood | kL−H (L/mol), Kkin (mol L−1 s−1 W−0.5) | [13] | |
| Zalazar et al. | (mol s−1 watts−1), Kkin (mole s kg2 m−9) | [17] | |
| Ballari et al. | α1 (cm s−1), α2 (mol watts−1 cm−1) | [18] | |
| Mueses et al. | α1 (mol m−2 s−1), (mol s−1 watts−1), kL−H(m3 mol−1) | [12] |
| Water Contaminant | Photoreactor Configuration | Initial Concentration of the Contaminant, ppm | Photocatalyst Concentration, g/L |
|---|---|---|---|
| Dichloroacetic acid (DCA) | CPCP | 30 | 0.1, 0.5 |
| 60 | 0.1, 0.35 | ||
| 120 | 0.1, 0.35, 0.5 | ||
| OMTP | 60 | 0.35 | |
| 120 | 0.35 | ||
| Phenol (PH) | CPCP | 60 | 0.1 |
| 120 | 0.1 | ||
| OMTP | 60 | 0.1 | |
| 120 | 0.1 | ||
| 4-chlorophenol (4-CP) | CPCP | 60 | 0.5 |
| 120 | 0.5 | ||
| OMTP | 60 | 0.5 | |
| 120 | 0.5 | ||
| Methylene Blue (MB) | CPCP | 10 | 0.25 |
| OMTP | 10 | 0.2, 0.25, 0.3, 0.35 | |
| Amoxicillin (AMX) | CPCP | 20 | 0.3, 0.6, 0.9, 1.0 |
| Year | Title of the Final Degree Project | Related Publication/Ref. | PHOTOREAC Impact on the Project Perceived by the Students | PHOTOREAC Implementation in the Project |
|---|---|---|---|---|
| 2015 | Design and evaluation of a modified compound parabolic collector solar reactor | A Novel Prototype Offset Multi Tubular Photoreactor (OMTP) for solar photocatalytic degradation of water contaminants/ref. [10] | 30% | Modeling the radiation field and kinetics of methylene blue for both the CPCP and OMTP |
| 2015 | Effect of oxygen transfer from the air on the photocatalytic degradation of dichloroacetic acid using a flat plate reactor | -- | 50% | Modeling the radiation field and kinetics of dichloroacetic acid in an FFP |
| 2016 | Radiant field modeling in heterogeneous photoreactors implementing Monte Carlo simulation: Modification of the Six Flux Model to new phase functions | Coupling the Six Flux Absorption-Scattering Model to the Henyey– Greenstein scattering phase function: Evaluation and optimization of radiation absorption in solar heterogeneous photoreactors/ref. [15] | 60% | Modeling the radiation field for the FFP and a CPCP |
| 2016 | Evaluation of the temperature effect on the heterogeneous photocatalytic degradation kinetics | Modeling and experimental evaluation of a non-isothermal photocatalytic solar reactor: temperature effect on the reaction rate kinetics/ref. [20] | 20% | The learning process for modeling CPCP |
| 2016 | Solar heterogeneous photocatalytic degradation of organic pollutants in a pilot-scale modified tubular collector | A Novel Prototype Offset Multi Tubular Photoreactor (OMTP) for solar photocatalytic degradation of water contaminants/ref. [10] | 50% | Modeling the radiation field and kinetics of DCA, PH and 4-CP for both the CPCP and OMTP |
| 2016 | Simulation of in series and in parallel arrangements of solar reactors (CPCP) for wastewater treatment | -- | 30% | The learning process for modeling CPCP |
| 2016 | Experimental evaluation and mathematical modeling of the performance of TiO2-P25 reuse in heterogeneous solar photocatalytic degradation of acetaminophen | -- | 30% | The learning process for modeling CPCP |
| 2017 | Solar photocatalytic ozonation applied to amoxicillin degradation in wastewater at pilot-plant scale | -- | 30% | The learning process for modeling CPCP |
| 2018 | Mathematical modeling and simulation of photocatalytic hydrogen production | -- | 35% | Radiation field modeling of an FFP |
| 2018 | Experimental evaluation and mathematical modeling of the regeneration of commercial TiO2 by the photocatalytic degradation of glyphosate | -- | 40% | Modeling the radiation field and the kinetics glyphosate in a CPCP |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acosta-Herazo, R.; Cañaveral-Velásquez, B.; Pérez-Giraldo, K.; Mueses, M.A.; Pinzón-Cárdenas, M.H.; Machuca-Martínez, F. A MATLAB-Based Application for Modeling and Simulation of Solar Slurry Photocatalytic Reactors for Environmental Applications. Water 2020, 12, 2196. https://doi.org/10.3390/w12082196
Acosta-Herazo R, Cañaveral-Velásquez B, Pérez-Giraldo K, Mueses MA, Pinzón-Cárdenas MH, Machuca-Martínez F. A MATLAB-Based Application for Modeling and Simulation of Solar Slurry Photocatalytic Reactors for Environmental Applications. Water. 2020; 12(8):2196. https://doi.org/10.3390/w12082196
Chicago/Turabian StyleAcosta-Herazo, Raúl, Briyith Cañaveral-Velásquez, Katrin Pérez-Giraldo, Miguel A. Mueses, María H. Pinzón-Cárdenas, and Fiderman Machuca-Martínez. 2020. "A MATLAB-Based Application for Modeling and Simulation of Solar Slurry Photocatalytic Reactors for Environmental Applications" Water 12, no. 8: 2196. https://doi.org/10.3390/w12082196
APA StyleAcosta-Herazo, R., Cañaveral-Velásquez, B., Pérez-Giraldo, K., Mueses, M. A., Pinzón-Cárdenas, M. H., & Machuca-Martínez, F. (2020). A MATLAB-Based Application for Modeling and Simulation of Solar Slurry Photocatalytic Reactors for Environmental Applications. Water, 12(8), 2196. https://doi.org/10.3390/w12082196