Evaluation of Effects of Municipal Sludge Leachates on Water Quality
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling and Creation of Mixtures
2.2. Leaching Tests and Parameters Tested
2.3. Statistical Analyses
3. Results
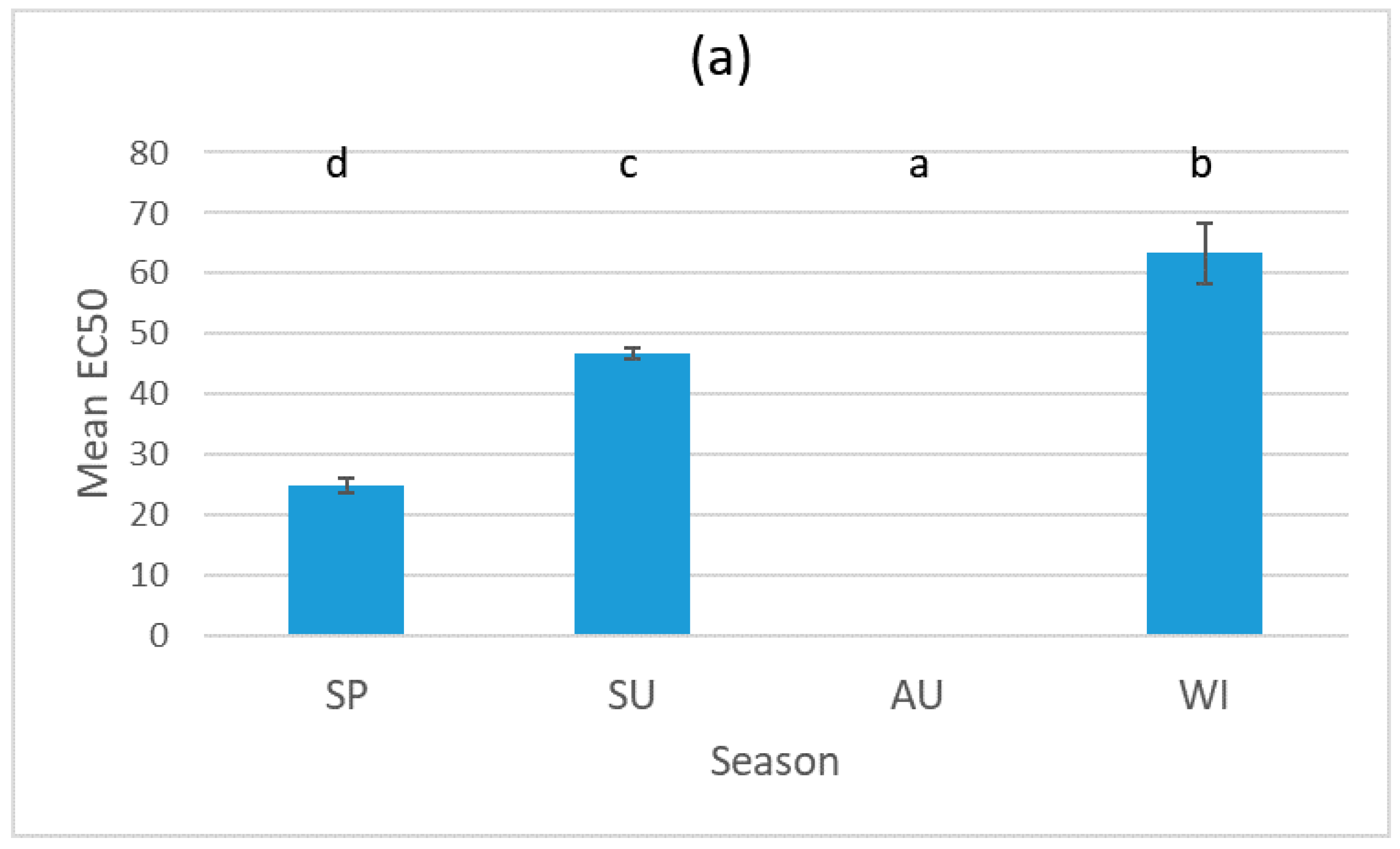
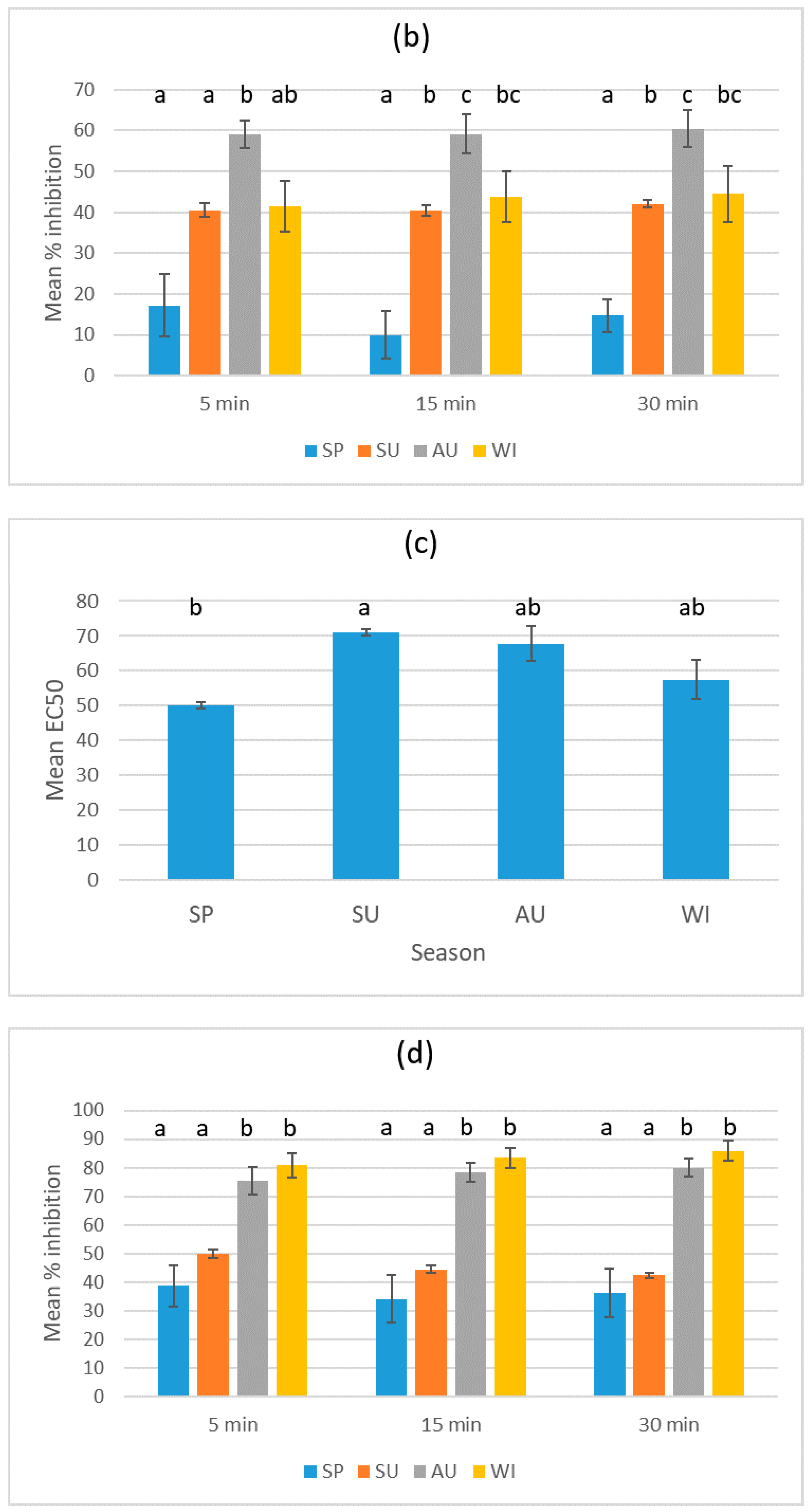
3.1. Results for the Sludge Samples
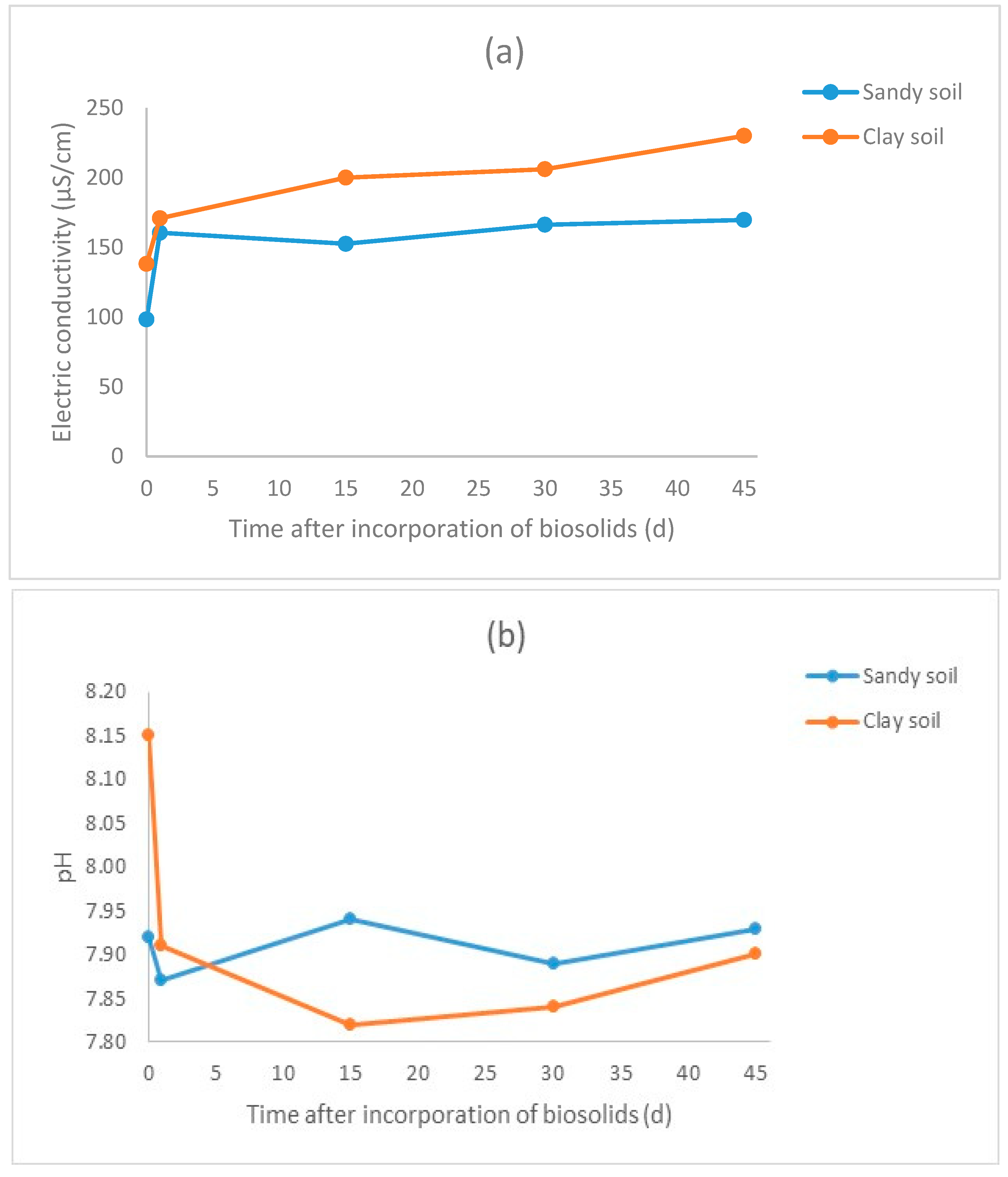
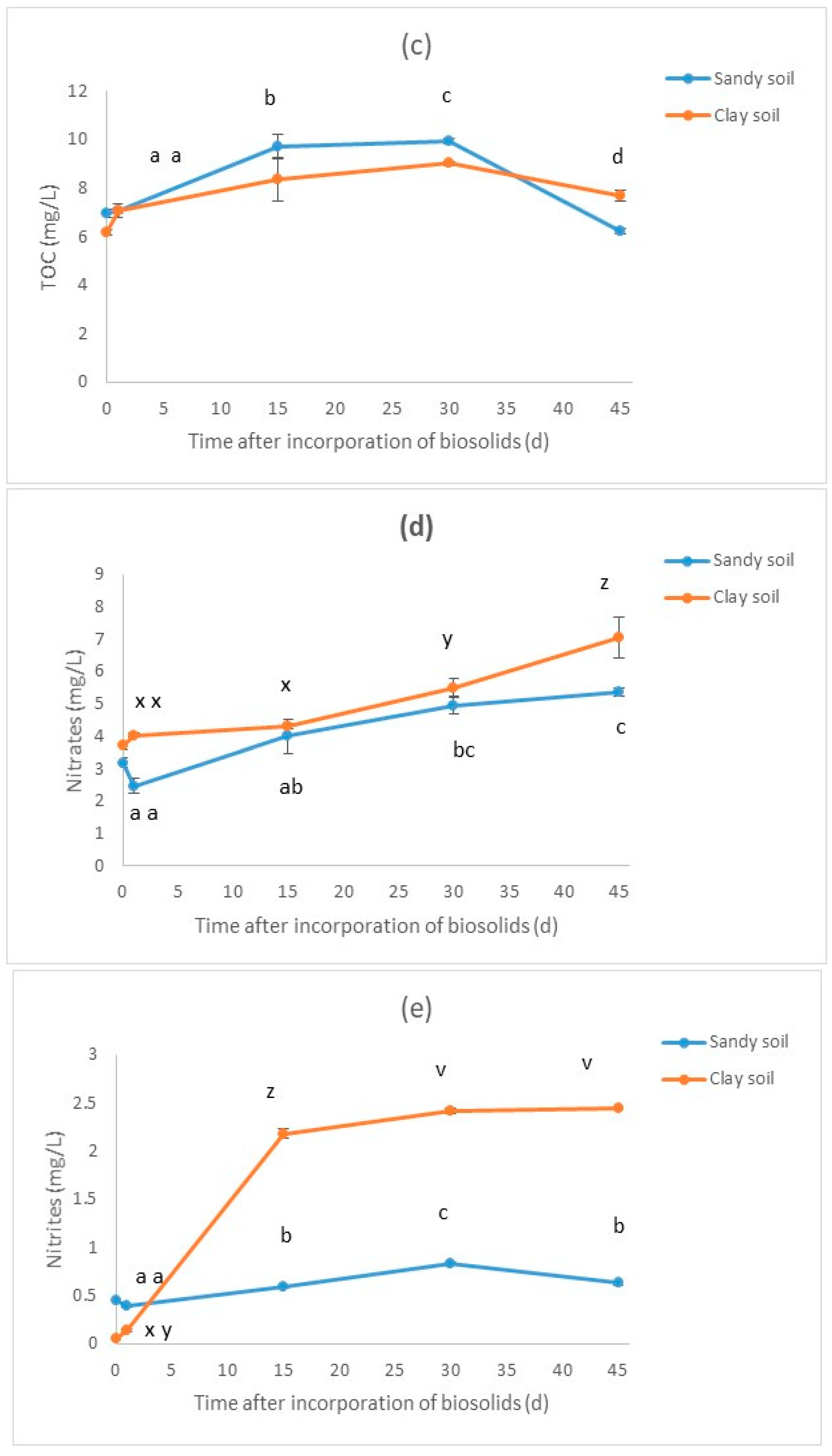
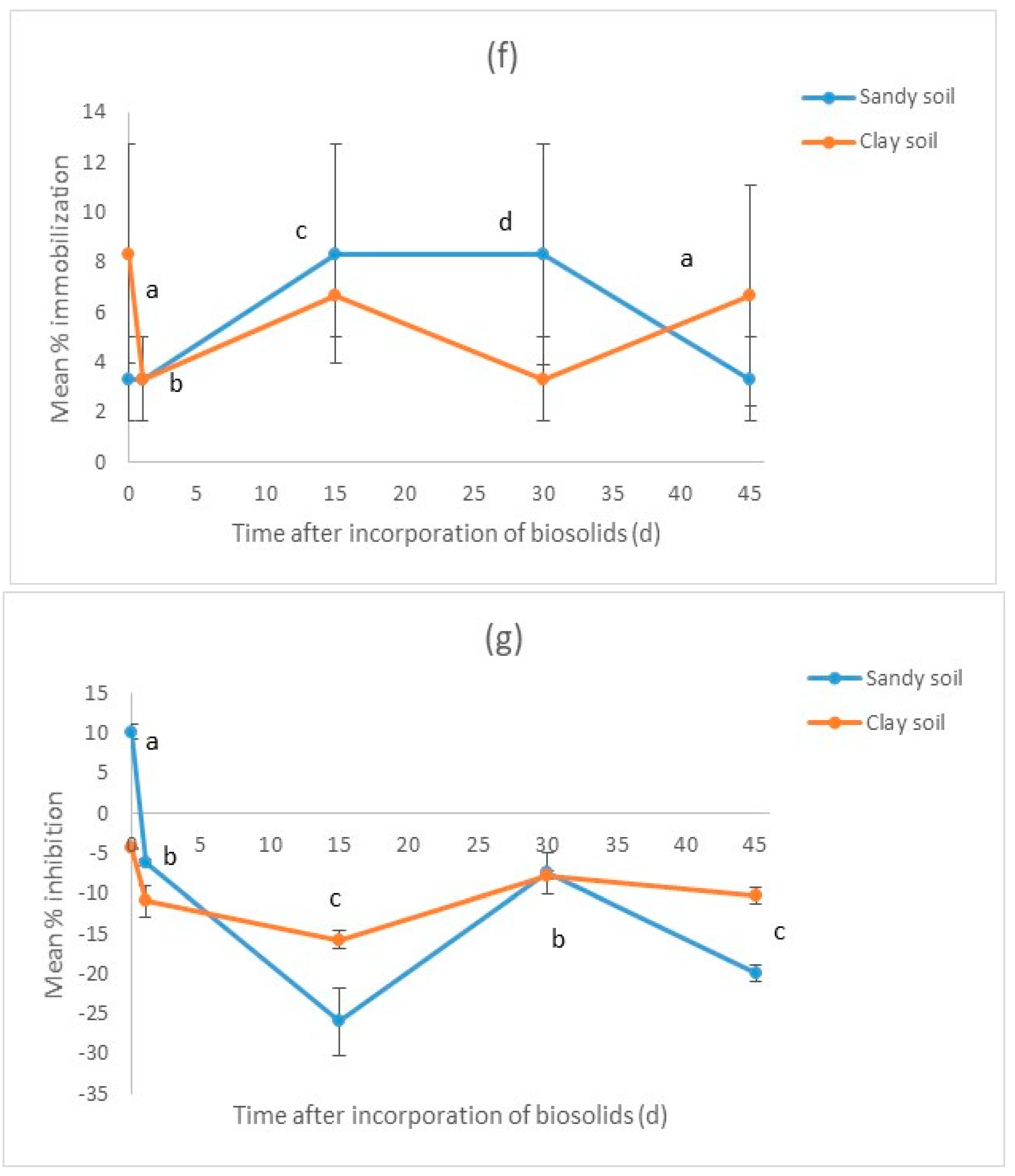
3.2. Results for the Biosolid–Soil Mixtures
4. Discussion
5. Conclusions
Author Contributions
Funding

Conflicts of Interest
References
- Torri, S.I.; Cabrera, M.N. The environmental impact of biosolids’ land application. In Organic Waste: Management Strategies, Environmental Impact and Emerging Regulations; Nova Science Publishers: New York, NY, USA, 2017. [Google Scholar]
- Romanowska-Duda, Z.; Pszczólkowski, W.; Pszczólkowska, A.; Wysokiñska, Z.; Grzesik, M. Use of Sewage Sludge in the Production of Plant Biomass for Energy: Biological and Economic Conditions. Comp. Econ. Res. 2012, 15, 105–122. [Google Scholar] [CrossRef]
- European Commission; DG Environment. Final Report Part III: Project Interim Reports In Environmental, Economic and Social Impacts of the Use of Sewage Sludge on Land; Milieu Ltd.: Brussels, Belgium, 2010. [Google Scholar]
- Council Directive 91/271/EEC of 21 May 1991 Concerning Urban Waste-Water Treatment; Official Journal of the European Union: Aberdeen, UK, 1991.
- Directive 2008/98/EC of the European Parliament and of the Council of 19 November 2008 on Waste and Repealing Certain DIRECTIVES; Official Journal of the European Union: Aberdeen, UK, 2008.
- Amendment and approval of the National Waste Management Plan & the National Strategic Plan on Waste Prevention; Official Government Gazette: Athens, Greece, 2015. (In Greek)
- Zaman, M.; Kim, M.; Nakhla, G.; Singh, A.; Yang, F. Enhanced biological phosphorus removal using thermal alkaline hydrolyzed municipal wastewater biosolids. J. Environ. Sci. 2019, 86, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Groth, V.A.; Carvalho-Pereira, T.; da Silva, E.M.; Niemeyer, J.C. Ecotoxicological assessment of biosolids by microcosms. Chemosphere 2016, 161, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Clarke, R.; Peyton, D.; Healy, M.G.; Fenton, O.; Cummins, E. A quantitative risk assessment for metals in surface water following the application of biosolids to grassland. Sci. Total Environ. 2016, 566–567, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Council Directive 86/278/EEC of 12 June 1986 on the Protection of the Environment, and in Particular of the Soil, when Sewage Sludge Is Used in Agriculture; Official Journal of the European Union: Aberdeen, UK, 1986.
- Joint Research Centre Institute for Environment and Sustainability Soil and Waste Unit. Organic Contaminants in Sewage Sludge for Agricultural Use; European Commission, Joint Research Centre Institute for Environment and Sustainability: Brussels, Belgium, 2001. [Google Scholar]
- Smith, S.R. Organic contaminants in sewage sludge (biosolids) and their significance for agricultural recycling. Phil. Trans. R. Soc. A 2009, 367, 4005–4041. [Google Scholar] [CrossRef] [Green Version]
- Verlicchi, P.; Zambello, E. Pharmaceuticals and personal care products in untreated and treated sewage sludge: Occurrence and environmental risk in the case of application on soil—A critical review. Sci. Total Environ. 2015, 538, 750–767. [Google Scholar] [CrossRef]
- EC.europa.eu. 2020. Available online: http://ec.europa.eu/environment/waste/sludge/index.htm (accessed on 17 July 2020).
- Van der Sloot, H.A.; Comans, R.N.J.; Meeussen, J.C.L.; Dijkstra, J.J. Leaching methods for soil, sludge and treated biowaste. Horiz. Desk Study 2003, 23, 16. [Google Scholar]
- Luczkiewicz, A. Soil and groundwater contamination as a result of sewage sludge land application. Pol. J. Environ. Stud. 2006, 15, 869–876. [Google Scholar]
- Alexakis, D.; Tsakiris, G. Drought impacts on karstic spring annual water potential. Application on Almyros (Heraklion Crete) brackish spring. Des. Water Treat. 2010, 16, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Alexakis, D. Assessment of water quality in the Messolonghi–Etoliko and Neochorio region (West Greece) using hydrochemical and statistical analysis methods. Environ. Monit. Assess. 2011, 182, 397–413. [Google Scholar] [CrossRef]
- Stamatis, G.; Alexakis, D.; Gamvroula, D.; Migiros, G. Groundwater quality assessment in Oropos–Kalamos basin, Attica, Greece. Environ. Earth Sci. 2011, 64, 973–988. [Google Scholar] [CrossRef]
- Gamvroula, D.; Alexakis, D.; Stamatis, G. Diagnosis of groundwater quality and assessment of contamination sources in the Megara basin (Attica, Greece). Arab. J. Geosci. 2013, 6, 2367–2381. [Google Scholar] [CrossRef]
- Correa, R.S.; White, R.E.; Weatherley, A.J. Risk of nitrate leaching from two soils amended with biosolids. Water Res. 2006, 33, 453–462. [Google Scholar] [CrossRef]
- Weyer, P.J.; Cerhan, J.R.; Kross, B.C.; Hallberg, G.R.; Kantamneni, J.; Breuer, G.; Jones, M.P.; Zheng, W.; Lynch, C.F. Municipal drinking water nitrate level and cancer risk in older women: The Iowa women’s health study. Epidemiology 2001, 12, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Gupta, R.C.; Seth, A.K.; Gupta, A.B.; Bassin, J.K.; Gupta, A. Methaemoglobinaemia in areas with high nitrate concentration in drinking water. Natl. Med. J. India. 2000, 13, 58–61. [Google Scholar]
- WHO. Chemical fact sheets. In Guidelines for Drinking Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2017; pp. 296–461. [Google Scholar]
- Smith, S.R.; Woods, V.; Evans, T.D. Nitrate dynamics in biosolids-treated soils. I. Influence of biosolids type and soil type. Biores. Technol. 1998, 66, 139–149. [Google Scholar] [CrossRef]
- Papadimitriou, C.A.; Haritou, I.; Samaras, P.; Zouboulis, A.I. Evaluation of leaching and ecotoxicological properties of sewage sludge–fly ash mixtures. Environ. Res. 2008, 106, 340–348. [Google Scholar] [CrossRef]
- CEN EN12457-2. Characterization of Waste–Leaching–Compliance Test for Leaching of Granular Waste Material and Sludge-Part 2; European Committee for Standardization: Brussels, Belgium, 2002. [Google Scholar]
- NEN 7341. Leaching tests, Determination of the Availability of Inorganic Components for Leaching. In Leaching Characteristics of Solid Earthy and Stony Building Materials; Netherlands Standards: Delft, The Netherlands, 1995. [Google Scholar]
- Rudde, H. Method of Determining a Concentration of Nitrate. U.S. Patent 9,052,292, 9 June 2015. [Google Scholar]
- Patton, C.; Kryskalla, J. Colorimetric determination of nitrate plus nitrite in water by enzymatic reduction, automated discrete analyzer methods. In Techniques and Methods 5-B8; US Geological Survey: Reston, VA, USA, 2011; pp. 1–34. [Google Scholar]
- Dinh, T.; Choi, I.; Son, Y.; Kim, J. A review on non-dispersive infrared gas sensors: Improvement of sensor detection limit and interference correction. Sens. Actuators B Chem. 2016, 231, 529–538. [Google Scholar] [CrossRef]
- ISO 6341. Water Quality-Determination of the Inhibition of the Mobility of Daphnia MAGNA STRAUSS; Cladocera, Crustacea, International Organization for Standardization: Geneva, Switzerland, 1996. [Google Scholar]
- Microbics Corporation. Microtox Manual; AZUR Environmental: Carlsbad, CA, USA, 1992. [Google Scholar]
- Persoone, G.; Marsalek, B.; Blinova, I.; Törökne, A.; Zarina, D.; Manusadzianas, L.; Nałęcz-Jawecki, G.; Tofan, L.; Stepanova, N.; Tothova, L.; et al. A practical and user-friendly toxicity classification system with microbiotests for natural waters and wastewaters. Environ. Toxicol. 2003, 18, 395–402. [Google Scholar] [CrossRef]
- Collivignarelli, M.C.; Abbà, A.; Frattarola, A.; Miino, M.C.; Padovani, S.; Katsoyiannis, I.; Torretta, V. Legislation for the reuse of biosolids on agricultural land in Europe: Overview. Sustainability 2019, 11, 6015. [Google Scholar] [CrossRef] [Green Version]
- Garg, S.; Kansal, D. Use of Bioassay Test as a Performance Indicator in Effluent Treatment Systems including Role in encouraging shift towards use of Green Chemicals. Int. J. Mult. Res. Dev. 2014, 1, 142–145. [Google Scholar]
- Shoji, R.; Mohri, S.; Sakai, Y.; Yamada, M. Ecotoxicity assessment of sludge and leaching test eluates of sludge. J. Environ. Sci. Health A Environ. Sci. Eng. Toxic/Hazard. Subst. Control 2008, 43, 1042–1047. [Google Scholar] [CrossRef]
- Wlodarczyk, Ε.; Próba, M.; Wolny, L. Ecotoxicity Assessment of Stabilized Sewage Sludge from Municipal Sewage Treatment Plant. Civ. Environ. Eng. Rep. 2016, 22, 157–166. [Google Scholar] [CrossRef] [Green Version]
- Abbas, M.; Adil, M.; Ehtisham-ul-Haque, S.; Munir, B.; Yameen, M.; Ghaffar, A.; Shar, G.A.; Asif Tahir, M.; Iqbal, M. Vibrio fischeri bioluminescence inhibition assay for ecotoxicity assessment: A review. Sci. Total Environ. 2018, 626, 1295–1309. [Google Scholar] [CrossRef] [PubMed]
- Davies, P.S.; Murdoch, F. The Increasing Importance of Assessing Toxicity in Determining Sludge Health and Management Policy. Meas. Control 2002, 35, 238–242. [Google Scholar] [CrossRef]
- Mikola, A.M.K.; Vahala, R.; Rautiainen, J.A. Factors affecting the quality of the plant influent and its suitability for prefermentation and the biological nutrient removal process. J. Environ. Eng. 2011, 137, 1185–1192. [Google Scholar] [CrossRef] [Green Version]
- Kasina, M.; Kowalski, P.R.; Michalik, M. Seasonal changes in chemical and mineralogical composition of sewage sludge incineration residues and their potential for metallic elements and valuable components recovery. Energy Procedia 2017, 125, 34–40. [Google Scholar] [CrossRef]
- Zhang, B.; Yu, Q.; Yan, G.; Zhu, H.; Xu, X.Y.; Zhu, L. Seasonal bacterial community succession in four typical wastewater treatment plants: Correlations between core microbes and process performance. Sci. Rep. 2018, 8, 4566. [Google Scholar] [CrossRef] [PubMed]
- Epstein, E. Land Application of Sewage Sludge and Biosolids; CRC Press: Boca Raton, FL, USA, 2002; pp. 15–23. [Google Scholar]
- Tabei, Y.; Era, M.; Ogawa, A.; Morita, H. Interactions between bicarbonate, potassium, and magnesium, and sulfur-dependent induction of luminescence in Vibrio fischeri. J. Basic Microbiol. 2012, 52, 350–359. [Google Scholar] [CrossRef]
- Abreu-Junior, C.H.; de Lima Brossi, M.J.; Monteiro, R.T.; Cardoso, P.H.S.; da Silva Mandu, T.; Nogueira, T.A.R.; Ganga, A.; Filzmoser, P.; de Oliveira, F.C.; Firme, L.P.; et al. Effects of sewage sludge application on unfertile tropical soils evaluated by multiple approaches: A field experiment in a commercial Eucalyptus plantation. Sci. Total Environ. 2019, 655, 1457–1467. [Google Scholar] [CrossRef]
- Malara, A.; Oleszczuk, P. Application of a battery of biotests for the determination of leachate toxicity to bacteria and invertebrates from sewage sludge-amended soil. Environ. Sci. Pollut. Res. Int. 2013, 20, 3435–3446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gebert, S. Assessing Ecological Impacts of Land-Applied Municipal Biosolids: Effect of Run-Off and Tile Drainage on the Aquatic Organisms Daphnia Magna, Hyalella azteca and Lemna minor. Master’s Thesis, Ryerson University, Ontario, ON, Canada, 2010. [Google Scholar]
- McIvor, K.; Cogger, C.; Brown, S. Effects of biosolids based soil products on soil physical and chemical properties in urban gardens. Compost Sci. Util. 2012, 20, 199–206. [Google Scholar] [CrossRef]
- Arduini, I.; Cardelli, R.; Pampana, S. Biosolids affect the growth, nitrogen accumulation and nitrogen leaching of barley. Plant Soil Environ. 2018, 64, 95–101. [Google Scholar]
- Van Cleemput, O.; Samater, A.H. Nitrite in soils: Accumulation and role in the formation of gaseous N compounds. Fertil. Res. 1995, 45, 81–89. [Google Scholar] [CrossRef]
- Wendland, F.; Bergmann, S.; Eisele, M.; Gömann, H.; Herrmann, F.; Kreins, P.; Kunkel, R. Model-Based Analysis of Nitrate Concentration in the Leachate—The North Rhine-Westfalia Case Study, Germany. Water 2020, 12, 550. [Google Scholar] [CrossRef] [Green Version]
| Sample | Moisture (%) | pH | EC (mS/cm) |
|---|---|---|---|
| SP | 77.1 | 7.35 1/5.53 2 | 2.91 1/4.55 2 |
| SU | 67.1 | 8.18 1/6.23 2 | 1.49 1/5.08 2 |
| AU | 66.7 | 7.83 1/5.78 2 | 2.42 1/4.81 2 |
| WI | 67.3 | 7.85 1/5.79 2 | 1.84 1/5.26 2 |
| Leaching Test | Organism Tested | SP | SU | AU | WI |
|---|---|---|---|---|---|
| ΕΝ 12457-2 | D. magna | 4 | 2.1 | 0.7 | 1.5 |
| V.fischeri 5 min | 0.3 | 0.8 | 1.3 | 0.8 | |
| V.fischeri 15 min | 0.2 | 0.8 | 1.5 | 0.9 | |
| V.fischeri 30 min | 0.3 | 0.8 | 1.5 | 0.9 | |
| NEN 7341 | D. magna | 2 | 1.4 | 1.5 | 1.8 |
| V.fischeri 5 min | 0.8 | 1 | 2.6 | 3.7 | |
| V.fischeri 15 min | 0.7 | 0.9 | 2.6 | 4.2 | |
| V.fischeri 30 min | 0.7 | 0.8 | 2.9 | 4.6 |
| Treatment * | pH | Electric Conductivity (μS/cm) | TOC (mg/L) | Nitrates (mg/L) | Nitrites (mg/L) | D. magna Immobilization (% in Relation to Blank) | V. fischeri Inhibition (% in Relation to Blank) |
|---|---|---|---|---|---|---|---|
| SA-C | 7.92 | 98.3 | 6.93 ± 0.10 | 3.19 ± 0.09 | 0.44 ± 0.01 | 3.33 ±1.67 | 10.19 ± 0.96 |
| SA-1 | 7.87 | 160.4 | 7.06 ± 0.17 | 2.47 ± 0.13 | 0.39 ± 0.01 | 3.33 ± 1.67 | −6.18 ± 0.37 |
| SA-15 | 7.94 | 152.5 | 9.72 ± 0.28 | 4 ± 0.31 | 0.59 ± 0.01 | 8.33 ± 4.41 | −25.99 ± 4.19 |
| SA-30 | 7.89 | 166.2 | 9.94 ± 0.07 | 4.96 ± 0.16 | 0.83 ± 0.01 | 8.33 ± 4.41 | −7.41 ± 2.55 |
| SA-45 | 7.93 | 169.6 | 6.21 ± 0.07 | 5.36 ± 0.07 | 0.63 ± 0.01 | 3.33 ± 1.67 | −19.88 ± 1.01 |
| CL-C | 8.15 | 138.1 | 6.18 ± 0.06 | 3.72 ± 0.08 | 0.05 ± 0.01 | 8.33 ± 4.41 | −4.38 ± 0.18 |
| CL-1 | 7.91 | 170.8 | 7.07 ± 0.06 | 4.03 ± 0.03 | 0.13 ± 0.01 | 3.33 ± 1.67 | −10.91 ± 1.94 |
| CL-15 | 7.82 | 200 | 8.36 ± 0.53 | 4.32 ± 0.04 | 2.18 ± 0.03 | 6.67 ± 1.67 | −15.78 ± 1.12 |
| CL-30 | 7.84 | 206 | 9.02 ± 0.04 | 5.5 ± 0.17 | 2.41 ± 0.02 | 3.33 ± 1.67 | −7.77 ± 0.53 |
| CL-45 | 7.9 | 230 | 7.69 ± 0.12 | 7.06 ± 0.36 | 2.44 ± 0.01 | 6.67 ± 1.67 | −10.28 ± 1.05 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannakis, I.; Emmanouil, C.; Kungolos, A. Evaluation of Effects of Municipal Sludge Leachates on Water Quality. Water 2020, 12, 2046. https://doi.org/10.3390/w12072046
Giannakis I, Emmanouil C, Kungolos A. Evaluation of Effects of Municipal Sludge Leachates on Water Quality. Water. 2020; 12(7):2046. https://doi.org/10.3390/w12072046
Chicago/Turabian StyleGiannakis, Ioannis, Christina Emmanouil, and Athanasios Kungolos. 2020. "Evaluation of Effects of Municipal Sludge Leachates on Water Quality" Water 12, no. 7: 2046. https://doi.org/10.3390/w12072046
APA StyleGiannakis, I., Emmanouil, C., & Kungolos, A. (2020). Evaluation of Effects of Municipal Sludge Leachates on Water Quality. Water, 12(7), 2046. https://doi.org/10.3390/w12072046







