Nutrient Removal Efficiency and Growth of Watercress (Nasturtium officinale) under Different Harvesting Regimes in Integrated Recirculating Aquaponic Systems for Rearing Common Carp (Cyprinus carpio L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Fish and Plants
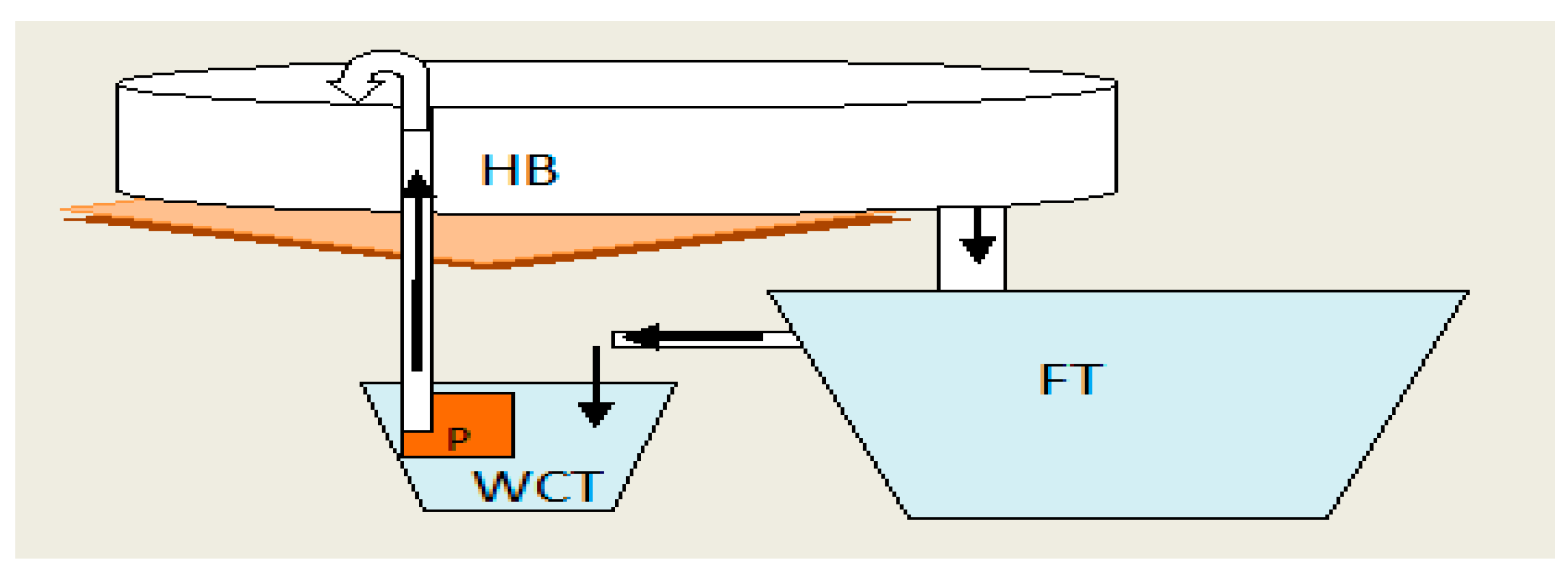
2.2. Design of Systems
2.3. Experimental Setup and Rearing Conditions
2.4. Water Quality Parameters
2.5. Fish and Plant Growth Parameters
2.6. Statistical Analysis
3. Results
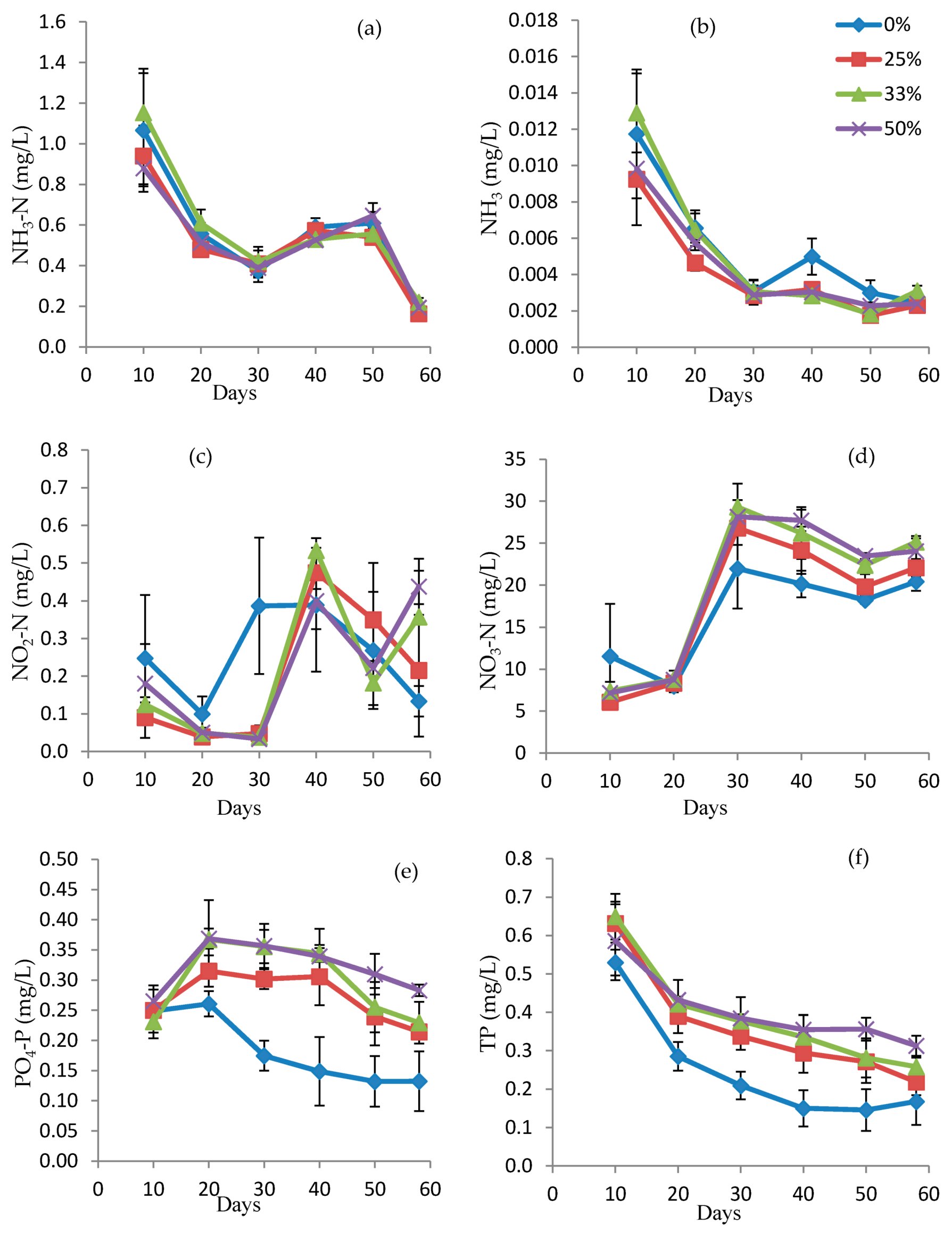
3.1. Water Quality Parameters in Fish Tanks
3.2. Nutrient Removal Rates
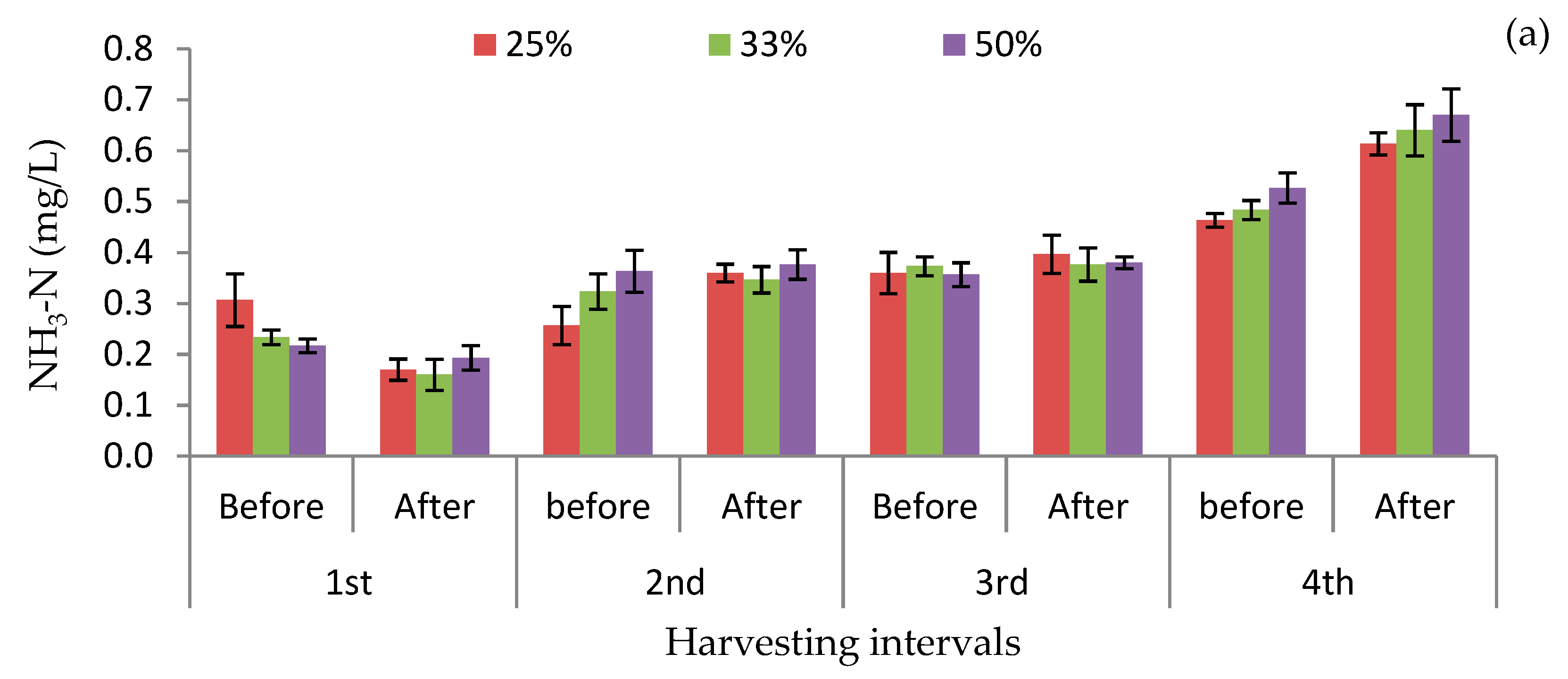
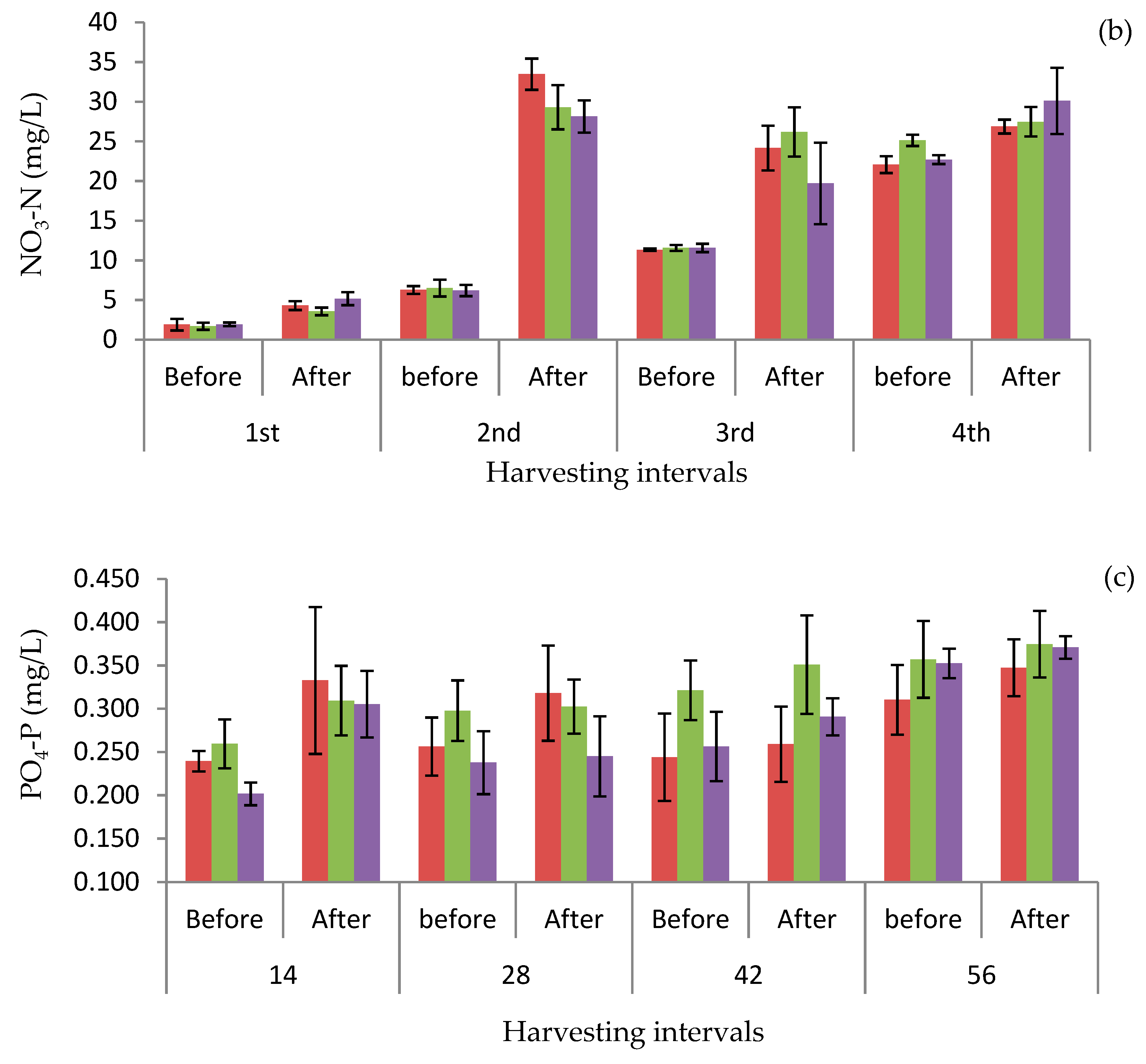
3.3. Concentrations of Nutrients Before and After Harvesting Plants
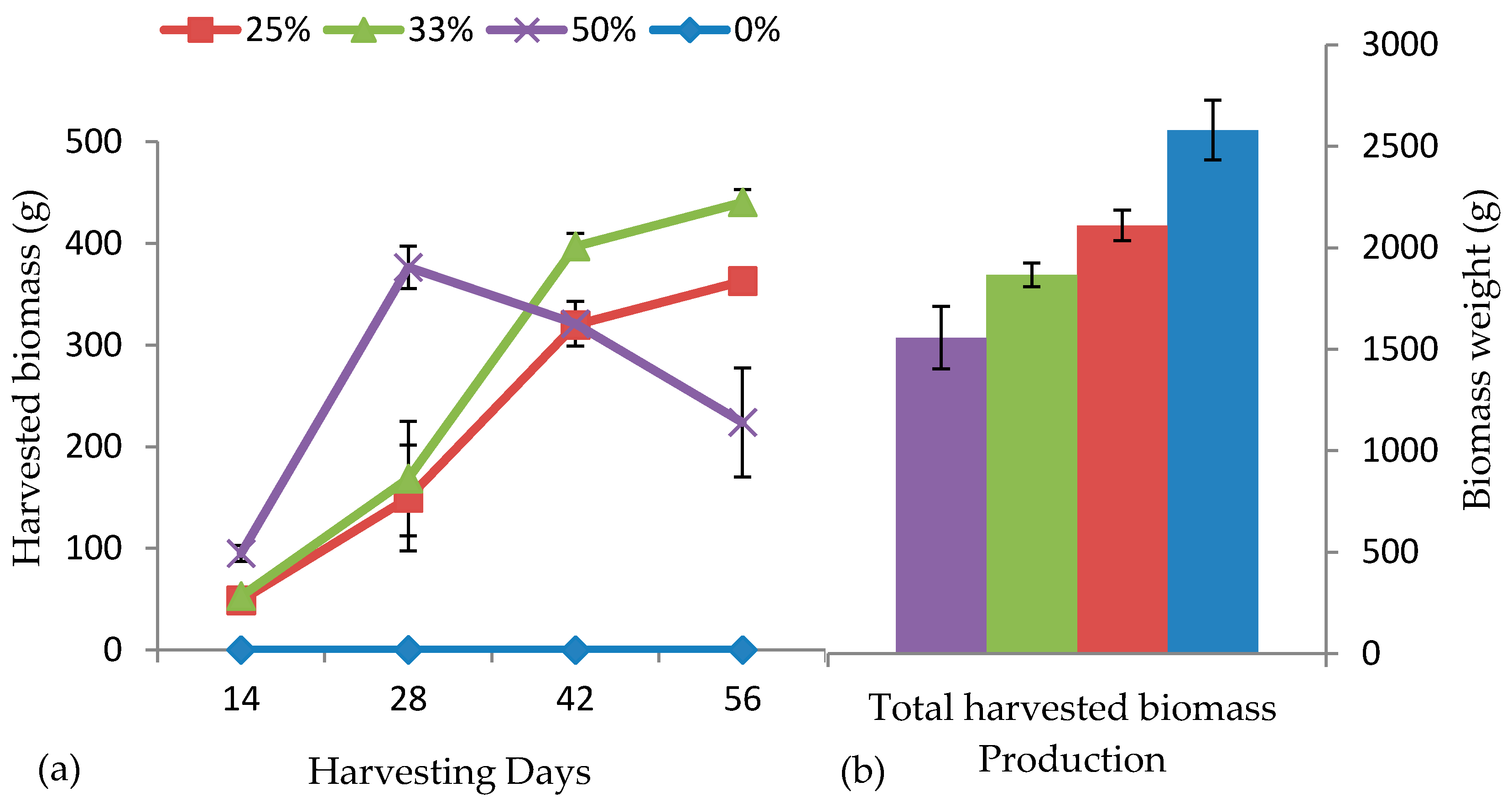
3.4. Fish and Plant Growth Performance
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Ethical Approval
References
- Rakocy, J.E.; Masser, M.P.; Losordo, T.M. Recirculating Aquaculture Tank Production Systems: Aquaponics-Integrating Fish and Plant Culture; Southern Regional Aquaculture Center: Stoneville, MS, USA, 2016; pp. 1–16. [Google Scholar]
- Love, D.C.; Fry, J.P.; Li, X.; Hill, E.S.; Genello, L.; Semmens, K.; Thompson, R.E. Commercial aquaponics production and profitability: Findings from an international survey. Aquaculture 2015, 435, 67–74. [Google Scholar] [CrossRef]
- Wongkiew, S.; Hu, Z.; Chandran, K.; Lee, J.W.; Khanal, S.K. Nitrogen transformations in aquaponic systems: A review. Aquac. Eng. 2017, 76, 9–19. [Google Scholar] [CrossRef]
- Estim, A.; Saufie, S.; Mustafa, S. Water quality remediation using aquaponics sub-systems as biological and mechanical filters in aquaculture. J. Water Process. Eng. 2019, 30, 100566. [Google Scholar] [CrossRef]
- Nhan, H.T.; Tai, N.T.; Liem, P.T.; Ut, V.N.; Ako, H. Effects of different stocking densities on growth performance of Asian swamp eel Monopterus albus, water quality and plant growth of watercress Nasturtium officinale in an aquaponic recirculating system. Aquaculture 2019, 503, 96–104. [Google Scholar] [CrossRef]
- Smith, E.N. Watercress (Nasturtium officinale) Production Utilizing Brook Trout (Salvelinus fontinalis) Flow-Through Aquaculture Effluent. Master’s Thesis, West Virginia University, Morgantown, WV, USA, 2007. [Google Scholar]
- Woynarovich, A.; Bueno, P.B.; Altan, Ö.; Jeney, Z.; Reantaso, M.; Xinhua, Y.; Van Anrooy, R. Better Management Practices for Carp Production in Central and Eastern Europe, the Caucasus and Central Asia; FAO, Fisheries and Aquaculture Technical Paper: Ankara, Turkey, 2011; p. 153. [Google Scholar]
- Vymazal, J.; Kröpfelová, L.; Švehla, J.; Štíchová, J. Can multiple harvest of aboveground biomass enhance removal of trace elements in constructed wetlands receiving municipal sewage? Ecol. Eng. 2010, 36, 939–945. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, Q.; Zhang, J.; Xie, H.; Feng, S. Effect of plant harvesting on the performance of constructed wetlands during summer. Water 2016, 8, 24. [Google Scholar] [CrossRef]
- Verhofstad, M.J.J.M.; Poelen, M.V.; Van Kempen, M.M.L.; Bakker, E.S.; Smolders, A.J.P. Finding the harvesting frequency to maximize nutrient removal in a constructed wetland dominated by submerged aquatic plants. Ecol. Eng. 2017, 106, 423–430. [Google Scholar] [CrossRef]
- Kim, S.Y.; Geary, P.M. The impact of biomass harvesting on phosphorus uptake by wetland plants. Water Sci. Technol. 2001, 44, 61–67. [Google Scholar] [CrossRef]
- Wang, Q.; Xie, H.; Zhang, J.; Liang, S.; Ngo, H.H.; Guo, W.; Liu, C.; Zhao, C.; Li, H. Effect of plant harvesting on the performance of constructed wetlands during winter: Radial oxygen loss and microbial characteristics. Environ. Sci. Pollut. Res. 2014, 22, 7476–7484. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, X.C.; Ge, Y.; Dzakpasu, M.; Zhao, Y.; Xiong, J. Effects of annual harvesting on plants growth and nutrients removal in surface-flow constructed wetlands in northwestern China. Ecol. Eng. 2015, 83, 268–275. [Google Scholar] [CrossRef]
- Álvarez, J.A.; Bécares, E. The effect of vegetation harvest on the operation of a surface flow constructed wetland. Water SA 2008, 34, 645–650. [Google Scholar] [CrossRef]
- Zheng, Y.; Dzakpasu, M.; Wang, X.; Zhang, L.; Ngo, H.H.; Guo, W.; Zhao, Y. Molecular characterization of long-term impacts of macrophytes harvest management in constructed wetlands. Bioresour. Technol. 2018, 268, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Gao, L.; He, S.; Huang, J.; Zhou, W. Nitrogen removal in response to plants harvesting in two kinds of enhanced hydroponic root mats treating secondary effluent. Sci. Total Environ. 2019, 670, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Bartodziej, W.M.; Blood, S.L.; Pilgrim, K. Aquatic plant harvesting: An economical phosphorus removal tool in an urban shallow lake. J. Aquat. Plant Manag. 2017, 55, 26–34. [Google Scholar]
- HACH. DR/3900 Spectrophotometer Procedure Manual; HACH Company: Loveland, CO, USA, 2019. [Google Scholar]
- Gichana, Z.; Meulenbroek, P.; Ogello, E.; Drexler, S.; Zollitsch, W.; Liti, D.; Akoll, P.; Waidbacher, H. Growth and Nutrient Removal Efficiency of Sweet Wormwood (Artemisia annua) in a Recirculating Aquaculture System for Nile Tilapia (Oreochromis niloticus). Water 2019, 11, 923. [Google Scholar] [CrossRef]
- Jinadasa, K.; Tanaka, N.; Sasikala, S.; Werellagama, D.; Mowjood, M.; Ng, W. Impact of harvesting on constructed wetlands performance—A comparison between Scirpus grossus and Typha angustifolia. J. Environ. Sci. Health A 2008, 43, 664–671. [Google Scholar] [CrossRef]
- Jeke, N.N.; Zvomuya, F.; Cicek, N.; Ross, L.; Badiou, P. Nitrogen and phosphorus phytoextraction by Cattail (Typha spp.) during wetland-based phytoremediation of an end-of-life municipal lagoon. J. Environ. Qual. 2019, 48, 24–31. [Google Scholar] [CrossRef]
- Saeed, T.; Sun, G. A review on nitrogen and organics removal mechanisms in subsurface flow constructed wetlands: Dependency on environmental parameters, operating conditions and supporting media. J. Environ. Manag. 2012, 112, 429–448. [Google Scholar] [CrossRef]
- Knaus, U.; Palm, H.W. Effects of the fish species choice on vegetables in aquaponics under spring-summer conditions in northern Germany (Mecklenburg Western Pomerania). Aquaculture 2017, 473, 62–73. [Google Scholar] [CrossRef]
- Irhayyim, T.; Fotedar, R. Effects of fish size and biofiltration techniques on water quality and nitrogen removal efficiency in recirculating aquaculture systems. AACL Bioflux 2019, 12, 1606–1616. [Google Scholar]
- Hu, Z.; Lee, J.W.; Chandran, K.; Kim, S.; Brotto, A.C.; Khanal, S.K. Effect of plant species on nitrogen recovery in aquaponics. Bioresour. Technol. 2015, 188, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.Y.; Babourina, O.; Rengel, Z.; Yang, X.E.; Pu, P.M. Ammonium and nitrate uptake by the floating plant Landoltia punctata. Ann. Bot. 2007, 99, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Bunce, J.T.; Ndam, E.; Ofiteru, I.D.; Moore, A.; Graham, D.W. A review of phosphorus removal technologies and their applicability to small-scale domestic wastewater treatment systems. Front. Environ. Sci. 2018, 6, 1–15. [Google Scholar] [CrossRef]
- Midlen, A.; Redding, T. Environmental Management for Aquaculture; Chapman and Hall: London, UK, 1998. [Google Scholar]
- Jones, C.; Olson-rutz, K.; Dinkins, C. Nutrient Uptake Timing by Crops, to Assist with Fertilizing Decisions; Montana State University: Bozeman, MT, USA, 2015; p. 8. [Google Scholar]
- Horváth, L.; Tamas, G.Y.; Seagrave, C. Carp and Pond Fish Culture, 2nd ed.; Blackwell science Ltd.: Oxford, UK, 2002. [Google Scholar]
- Timmons, M.B.; Ebeling, J.M.; Wheaton, F.W.; Summerfelt, S.T.; Vinci, B.J. Recirculating Aquaculture Systems, 2nd ed.; Cayuga Aqua Ventures: Ithaca, NY, USA, 2002. [Google Scholar]
- Biswas, J.K.; Sarkar, D.; Chakraborty, P.; Bhakta, J.N.; Jana, B.B. Density dependent ambient ammonium as the key factor for optimization of stocking density of common carp in small holding tanks. Aquaculture 2006, 261, 952–959. [Google Scholar] [CrossRef]
- Kroupova, H.; Prokes, M.; Macova, S.; Penaz, M.; Barus, V.; Novotny, L.; Machova, J. Effect of nitrite on early-life stages of common carp (Cyprinus carpio L). Environ. Toxicol. Chem. 2010, 29, 535–540. [Google Scholar] [CrossRef]
- Iqbal, F.; Qureshi, I.Z.; Ali, M. Histopathological changes in kidney of common carp, Cyprinus carpio, following nitrate exposure. J. Res. Sci. 2004, 15, 411–418. [Google Scholar]
- Kim, E.; Yoo, S.; Ro, H.Y.; Han, H.J.; Baek, Y.W.; Eom, I.C.; Kim, H.M.; Kim, P.; Choi, K. Aquatic toxicity assessment of phosphate compounds. Environ. Health Toxicol. 2013, 28, 1–7. [Google Scholar] [CrossRef]
- Simplício, N.; Muniz, D.; Rocha, F.; Martins, D.; Dias, Z.; Farias, B.; Oliveira-Filho, E.C. Comparative analysis between ecotoxicity of nitrogen-, phosphorus-, and potassium-based fertilizers and their active ingredients. Toxics 2017, 5, 2. [Google Scholar] [CrossRef]
- Suzuki, Y.; Maruyama, T.; Numata, H.; Sato, H.; Asakawa, M. Performance of a closed recirculating system with foam separation, nitrification and denitrification units for intensive culture of eel: Towards zero emission. Aquac. Eng. 2003, 29, 165–182. [Google Scholar] [CrossRef]
- Rakocy, J.E.; Bailey, D.S.; Shultz, R.C.; Thoman, E.S. Update on tilapia and vegetable production in the UVI aquaponic system. In New Dimensions on Farmed Tilapia, Proceedings of the Sixth International Symposium on Tilapia in Aquaculture, Manila, Philippines, September 12–16; Remedios, B., Graham, M., Kevin, F., Eds.; Center Roxas Boulevard: Manila, Philippines, 2004. [Google Scholar]
- Colt, J. Water quality requirements for reuse systems. Aquac. Eng. 2006, 34, 143–156. [Google Scholar] [CrossRef]
- Ardiansyah, A.; Fotedar, R. Water quality, growth and stress responses of juvenile barramundi (Lates calcarifer Bloch), reared at four different densities in integrated recirculating aquaculture systems. Aquaculture 2016, 458, 113–120. [Google Scholar] [CrossRef]
- Maucieri, C.; Nicoletto, C.; Zanin, G.; Birolo, M.; Trocino, A.; Sambo, P.; Borin, M.; Xiccato, G. Effect of stocking density of fish on water quality and growth performance of European Carp and leafy vegetables in a low-tech aquaponic system. PLoS ONE 2019, 14. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Verma, A.K.; Tiwari, V.K.; Prakash, C.; Rathore, G.; Shete, A.P.; Nuwansi, K.K.T. Optimizing koi carp, Cyprinus carpio var. koi (Linnaeus, 1758), stocking density and nutrient recycling with spinach in an aquaponic system. J. World Aquac. Soc. 2014, 45, 652–661. [Google Scholar] [CrossRef]
- Hussain, T.; Verma, A.K.; Tiwari, V.K.; Prakash, C.; Rathore, G.; Shete, A.P.; Saharan, N. Effect of water flow rates on growth of Cyprinus carpio var. koi (Cyprinus carpio L., 1758) and spinach plant in aquaponic system. Aquac. Int. 2015, 23, 369–384. [Google Scholar] [CrossRef]
- Shete, A.P.; Verma, A.K.; Chadha, N.K.; Prakash, C.; Peter, R.M.; Ahmad, I.; Nuwansi, K.K.T. Optimization of hydraulic loading rate in aquaponic system with Common carp (Cyprinus carpio) and Mint (Mentha arvensis). Aquac. Eng. 2016, 72, 53–57. [Google Scholar] [CrossRef]
- Shete, A.P.; Verma, A.K.; Chadha, N.K.; Prakash, C.; Chandrakant, M.H.; Nuwansi, K.K.T. Evaluation of different hydroponic media for mint (Mentha arvensis) with common carp (Cyprinus carpio) juveniles in an aquaponic system. Aquac. Int. 2017, 25, 1291–1301. [Google Scholar] [CrossRef]


| Water Parameters | Harvested Biomass | |||
|---|---|---|---|---|
| 0% | 25% | 33% | 50% | |
| Fish Tanks | ||||
| NH3-N (mg/L) | 0.56 ± 0.08 a | 0.52 ± 0.06 a | 0.58 ± 0.07 a | 0.53 ± 0.05 a |
| NH3 (mg/L) | 0.005 ± 0.001 a | 0.004 ± 0.001 a | 0.005 ± 0.001 a | 0.004 ± 0.001 a |
| NO2-N (mg/L) | 0.25 ± 0.05 a | 0.20 ± 0.05 a | 0.21 ± 0.04 a | 0.22 ± 0.04 a |
| NO3-N (mg/L) | 16.71 ± 1.68 a | 17.87 ± 3.1.98 a | 19.86 ± 2.17 a | 19.87 ± 2.12 a |
| PO4-P (mg/L) | 0.18 ± 0.02 b | 0.27 ± 0.01 a | 0.29 ± 0.02 a | 0.32 ± 0.01 a |
| TP (mg/L) | 0.25 ± 0.04 b | 0.36 ± 0.04 a | 0.39 ± 0.03 a | 0.40 ± 0.03 a |
| Dissolved oxygen (mg/L) | 8.16 ± 0.07 a | 8.19 ± 0.06 a | 8.24 ± 0.06 a | 8.34 ± 0.06 a |
| pH | 7.41 ± 0.02 a | 7.35 ± 0.04 a | 7.37 ± 0.03 a | 7.40 ± 0.04 a |
| Temperature (°C) | 14.71 ± 0.35 a | 14.45 ± 0.34 a | 14.29 ± 0.33 a | 14.21 ± 0.33 a |
| Bed Units | ||||
| Dissolved oxygen (mg/L) | 8.16 ± 0.07 a | 8.15 ± 0.07 a | 8.22 ± 0.07 a | 8.20 ± 0.08 a |
| pH | 7.41 ± 0.03 a | 7.38 ± 0.04 a | 7.37 ± 0.04 a | 7.40 ± 0.04 a |
| Temperature (°C) | 14.44 ± 0.34 a | 14.37 ± 0.34 a | 14.26 ± 0.33 a | 14.16 ± 0.33 a |
| Removal Rates | Harvested Biomass | |||
|---|---|---|---|---|
| 0% | 25% | 33% | 50% | |
| NH3-N (%) | 36.20 ± 3.19 a | 35.06 ± 3.82 a | 31.80 ± 2.74 a | 31.88 ± 2.93 a |
| NO2-N (%) | 42.43 ± 3.24 a | 41.67 ± 3.96 a | 47.14 ± 3.10 a | 42.58 ± 4.23 a |
| NO3-N (%) | 63.58 ± 2.36 a | 59.67 ± 2.78 ab | 54.26 ± 2.33 bc | 49.49 ± 2.78 c |
| PO4-P (%) | 31.09 ± 1.19 a | 28.44 ± 0.94 ab | 26.33 ± 1.34 bc | 24.97 ± 1.13 c |
| TP (%) | 47.31 ± 1.25 a | 46.56 ± 0.93 ab | 44.76 ± 0.83 b | 43.06 ± 1.17 b |
| Growth Parameters | Harvested Biomass | |||
|---|---|---|---|---|
| 0% | 25% | 33% | 50% | |
| Initial fish weight (g/fish) | 33.71 ± 0.10 a | 33.75 ± 0.08 a | 33.64 ± 0.04 a | 33.55 ± 0.99 a |
| Final fish weight (g/fish) | 60.20 ± 0.83 a | 60.37 ± 1.42 a | 61.35 ± 1.68 a | 60.63 ± 1.13 a |
| Fish weight gain (g/fish/58 days) | 26.49 ± 0.73 a | 26.62 ± 1.39 a | 27.70 ± 1.71 a | 27.07 ± 1.03 a |
| Specific growth rate (%/day) | 1.00 ± 0.02 a | 1.00 ± 0.04 a | 1.03 ± 0.05 a | 1.02 ± 0.03 a |
| Feed consumption (g/fish/day) | 0.67 ± 0.00 a | 0.67 ± 0.00 a | 0.67 ± 0.00 a | 0.67 ± 0.00 a |
| Fish weight gain (g/fish/day) | 0.46 ± 0.01 a | 0.46 ± 0.03 a | 0.48 ± 0.03 a | 0.47 ± 0.02 a |
| Feed conversion ratio | 1.46 ± 0.04 a | 1.46 ± 0.07 a | 1.40 ± 0.08 a | 1.43 ± 0.06 a |
| Survival (%) | 100 ± 0.00 a | 100 ± 0.00 a | 100 ± 0.00 a | 100 ± 0.00 a |
| Growth Parameters | Harvested Biomass | |||
|---|---|---|---|---|
| 0% | 25% | 33% | 50% | |
| Stocking biomass (g) | 240 ± 0.00 a | 240 ± 0.00 a | 240 ± 0.00 a | 240 ± 0.00 a |
| Final biomass (g) | 2580.00 ± 180.00 a | 2110.14 ± 8.36 ab | 1865.97 ± 71.67 bc | 1556.90 ± 187.95 c |
| Biomass gain (g) | 2340.0 ± 180.0 a | 1870.14 ± 8.36 ab | 1625.97 ± 71.67 bc | 1316.90 ± 187.95 c |
| Specific growth rate of the plants (%/day) | 4.090 ± 0.120 a | 3.745 ± 0.005 ab | 3.535 ± 0.065 bc | 3.210 ± 0.210 c |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Irhayyim, T.; Fehér, M.; Lelesz, J.; Bercsényi, M.; Bársony, P. Nutrient Removal Efficiency and Growth of Watercress (Nasturtium officinale) under Different Harvesting Regimes in Integrated Recirculating Aquaponic Systems for Rearing Common Carp (Cyprinus carpio L.). Water 2020, 12, 1419. https://doi.org/10.3390/w12051419
Irhayyim T, Fehér M, Lelesz J, Bercsényi M, Bársony P. Nutrient Removal Efficiency and Growth of Watercress (Nasturtium officinale) under Different Harvesting Regimes in Integrated Recirculating Aquaponic Systems for Rearing Common Carp (Cyprinus carpio L.). Water. 2020; 12(5):1419. https://doi.org/10.3390/w12051419
Chicago/Turabian StyleIrhayyim, Tareq, Milán Fehér, Judit Lelesz, Miklós Bercsényi, and Péter Bársony. 2020. "Nutrient Removal Efficiency and Growth of Watercress (Nasturtium officinale) under Different Harvesting Regimes in Integrated Recirculating Aquaponic Systems for Rearing Common Carp (Cyprinus carpio L.)" Water 12, no. 5: 1419. https://doi.org/10.3390/w12051419
APA StyleIrhayyim, T., Fehér, M., Lelesz, J., Bercsényi, M., & Bársony, P. (2020). Nutrient Removal Efficiency and Growth of Watercress (Nasturtium officinale) under Different Harvesting Regimes in Integrated Recirculating Aquaponic Systems for Rearing Common Carp (Cyprinus carpio L.). Water, 12(5), 1419. https://doi.org/10.3390/w12051419





