Removal of Polycyclic Aromatic Hydrocarbons (PAHs) from Produced Water by Ferrate (VI) Oxidation
Abstract
1. Introduction
2. Experimental Work
2.1. Materials/Chemicals
2.2. Fe(VI) Oxidation and GC-MS Analysis
2.3. Development of Experimental Design Using RSM
3. Results and Discussion
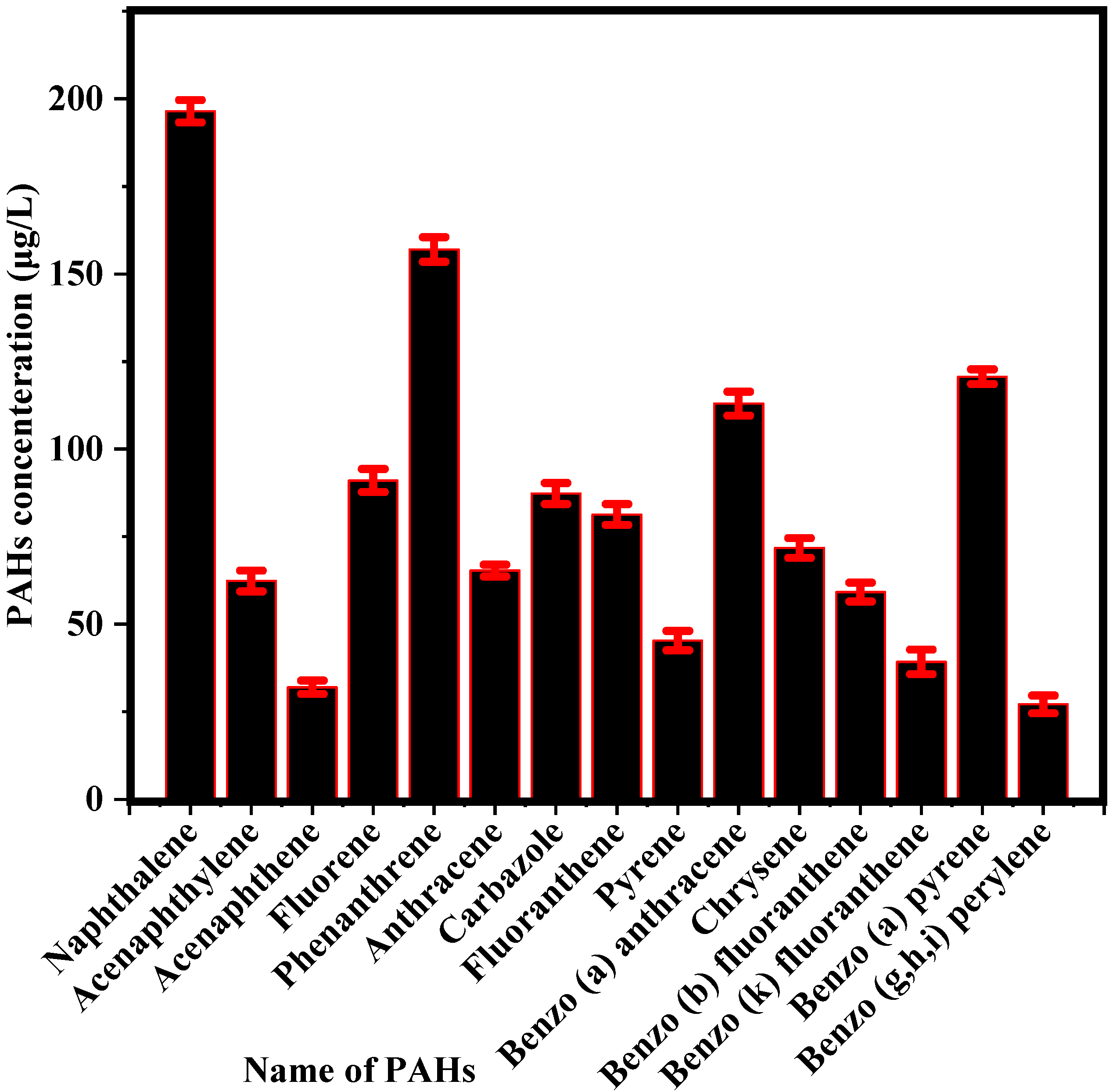
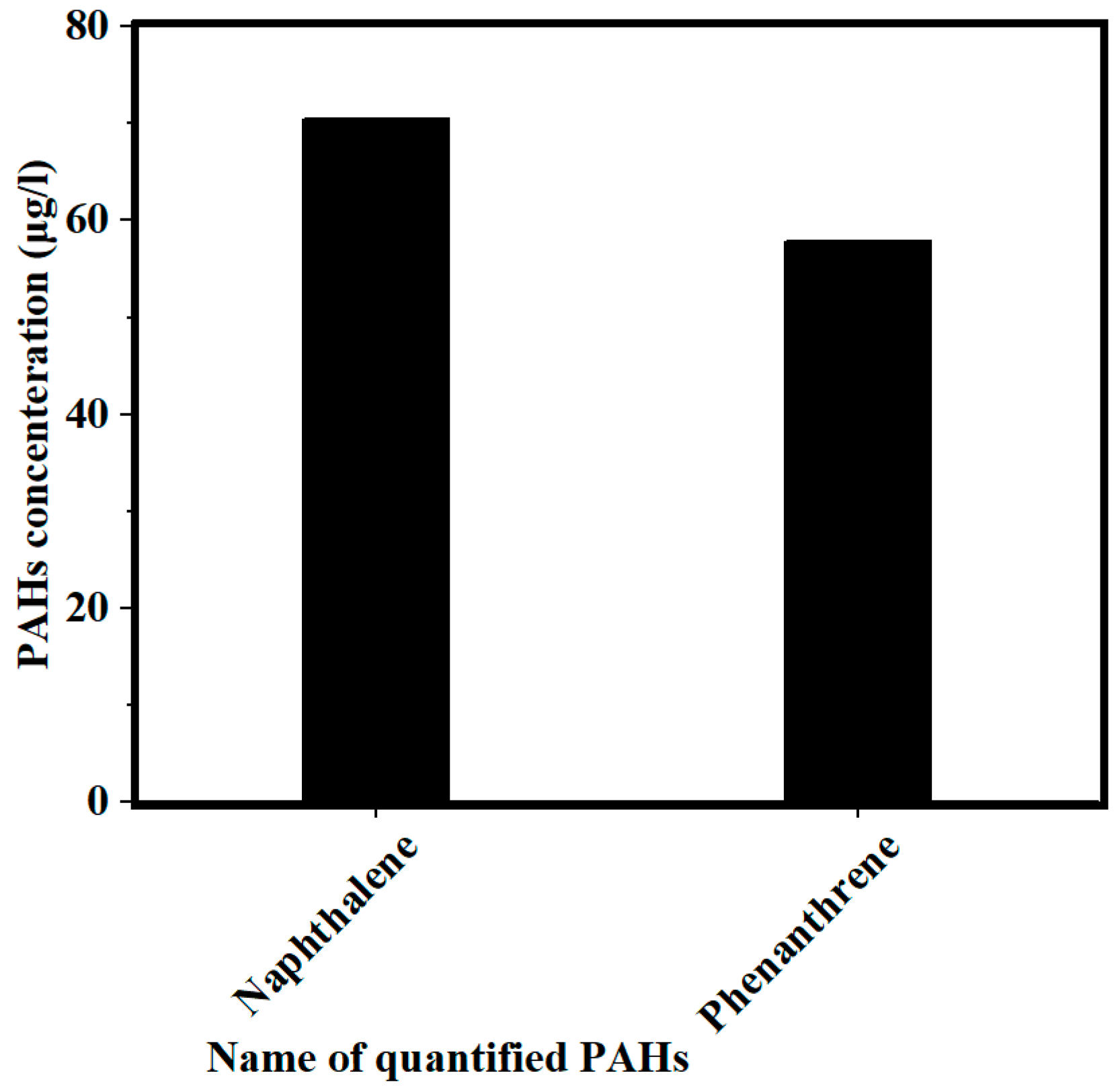
3.1. Concentration of PAHs and COD in PW
3.2. Screening Batch Experiments
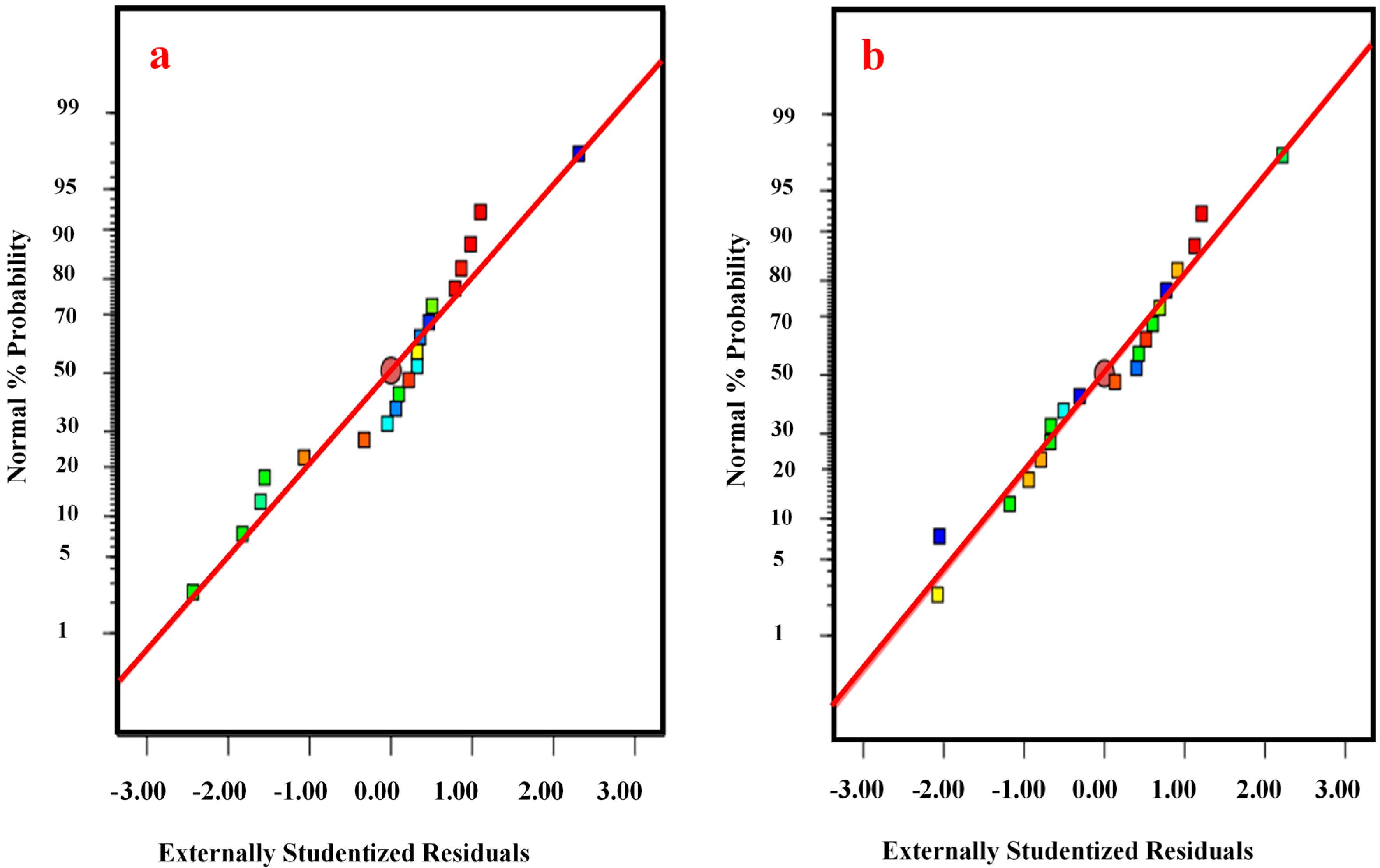
3.3. Analysis of Variance (ANOVA)
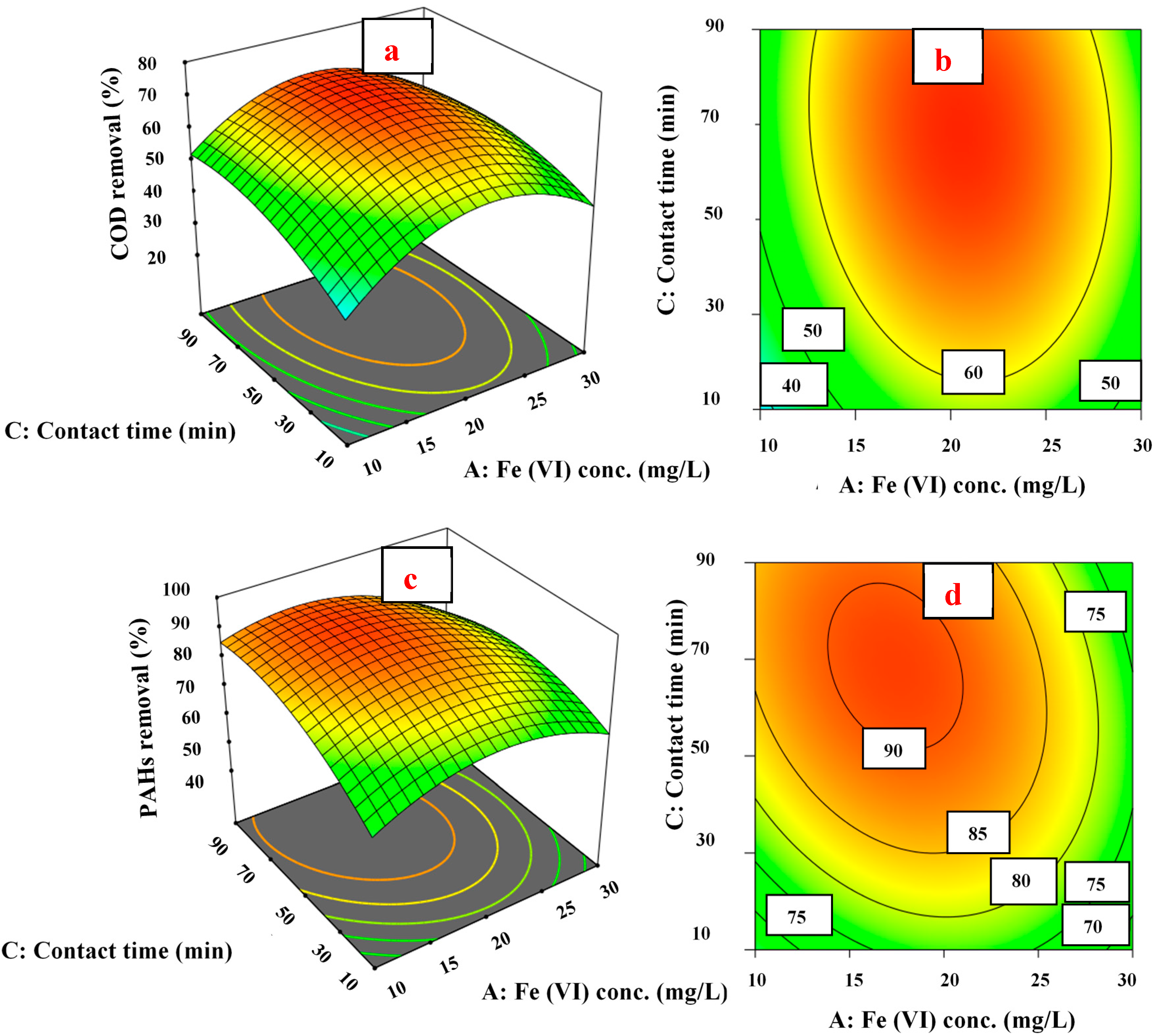
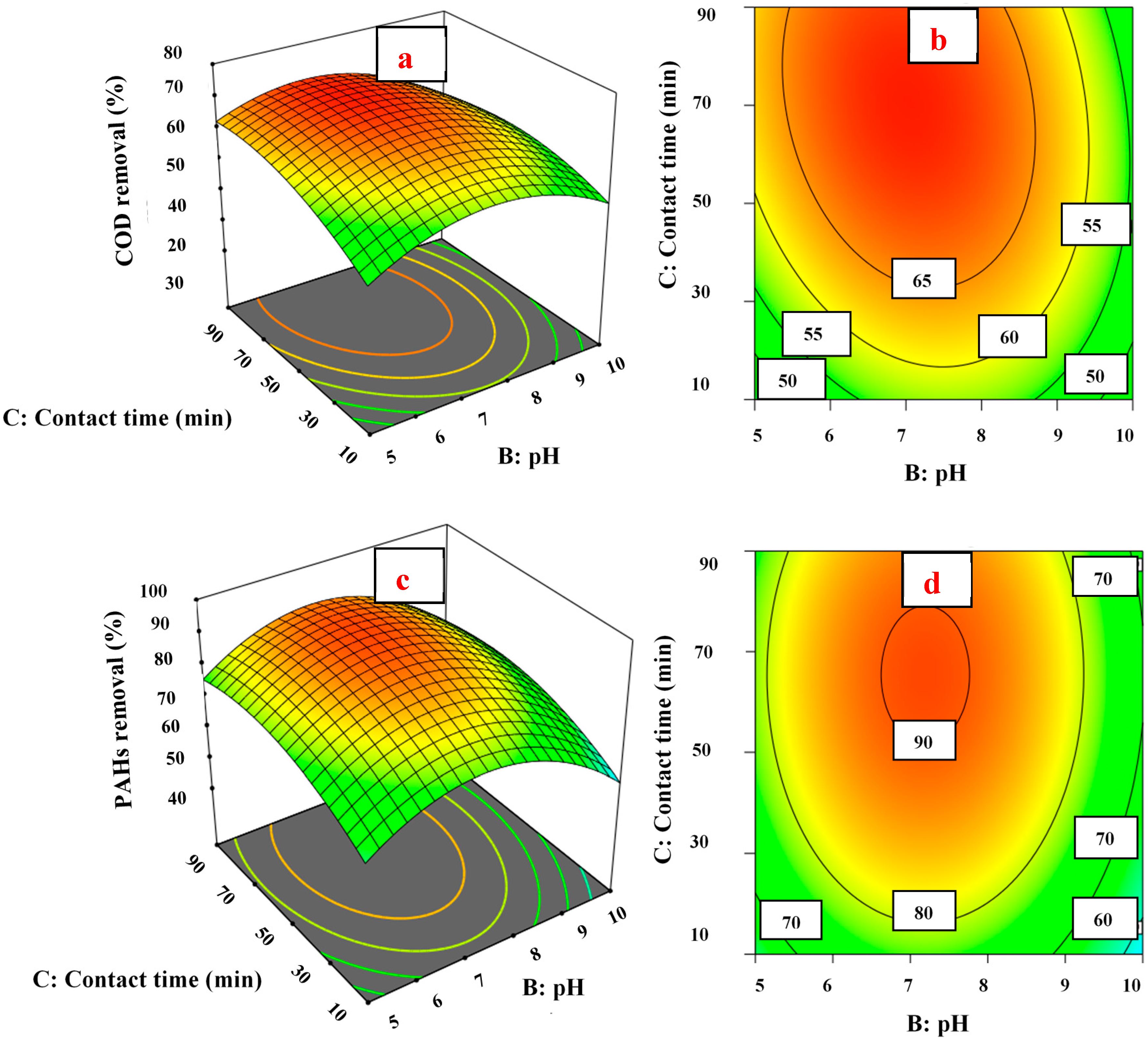
3.4. Response Surface and Contour Plots
3.4.1. pH Effect on COD and PAH Removal
3.4.2. Fe (VI) Concentration Effect on COD and PAH Removal
3.4.3. Contact Time Effect on COD and PAH Removal
3.5. Optimization and Validation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yaqub, A.; Isa, M.H.; Kutty, S.R.M.; Ajab, H.E. lectrochemical degradation of PAHs in produced water using Ti/Sb2O5-SnO2-IrO2 anode. Electrochem. Commun. 2014, 82, 979–984. [Google Scholar] [CrossRef]
- Vela, N.; Martínez-Menchón, M.; Navarro, G.; Pérez-Lucas, G.; Navarro, S. Removal of polycyclic aromatic hydrocarbons (PAHs) from groundwater by heterogeneous photocatalysis under natural sunlight. J. Photochem. Photobiol. 2012, 232, 32–40. [Google Scholar] [CrossRef]
- Liu, B.; Chen, B.; Zhang, B.; Song, X.; Zeng, G.; Lee, K. Photocatalytic ozonation of offshore produced water by TiO2 nanotube arrays coupled with UV-LED irradiation. J. Hazard. Mater. 2020, 402, 123456. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, S.; Micó, M.M.; Arnaldos, M.; Medina, F.; Contreras, S. State of the art of produced water treatment. Chemosphere 2018, 192, 186–208. [Google Scholar]
- Nawaz, R.; Kait, C.F.; Chia, H.Y.; Isa, M.H.; Huei, L.W. Glycerol-mediated facile synthesis of colored titania nanoparticles for visible light photodegradation of phenolic compounds. Nanomaterials 2019, 9, 1586. [Google Scholar] [CrossRef]
- Haneef, T.; Ul Mustafa, M.R.; Rasool, K.; Ho, Y.C.; Mohamed Kutty, S.R. Removal of polycyclic aromatic hydrocarbons in a heterogeneous Fenton like oxidation system using nanoscale zero-valent iron as a catalyst. Water 2020, 12, 2430. [Google Scholar] [CrossRef]
- Haneef, T.; Mustafa, M.R.U.; Farhan Yasin, H.M.; Farooq, S.; Hasnain Isa, M. Study of Ferrate(VI) oxidation for COD removal from wastewater. IOP Conf. Ser. Earth Environ. Sci. 2020, 442, 1–8. [Google Scholar] [CrossRef]
- Smol, M.; Włodarczyk, M.M. The effectiveness in the removal of PAHs from aqueous solutions in physical and chemical processes: A review. Polycycl. Aromat. Comp. 2017, 37, 292–313. [Google Scholar] [CrossRef]
- Sahrin, N.T.; Nawaz, R.; Fai Kait, C.; Lee, S.L.; Wirzal, M.D.H. Visible light photodegradation of formaldehyde over TiO2 nanotubes synthesized via electrochemical anodization of titanium foil. Nanomaterials 2020, 10, 128. [Google Scholar] [CrossRef]
- Manilal, A.M.; Soloman, P.A.; Basha, C.A. Removal of Oil and Grease from Produced Water Using Electrocoagulation. J. Hazard. Toxic Radioact. Waste 2020, 24, 04019023. [Google Scholar] [CrossRef]
- Chang, H.; Li, T.; Liu, B.; Vidic, R.D.; Elimelech, M.; Crittenden, J.C. Potential and implemented membrane-based technologies for the treatment and reuse of flowback and produced water from shale gas and oil plays: A review. Desalination 2019, 455, 34–57. [Google Scholar] [CrossRef]
- Campos, J.C.; Borges, R.M.H.; Oliveira Filho, A.M.D.; Nobrega, R.; Sant’Anna, G.L., Jr. Oilfield wastewater treatment by combined microfiltration and biological processes. Water Res. 2002, 36, 95–104. [Google Scholar] [CrossRef]
- Hao, H.; Huang, X.; Gao, C.; Gao, X. Application of an integrated system of coagulation and electrodialysis for treatment of wastewater produced by fracturing. Desalin. Water Treat. 2015, 55, 2034–2043. [Google Scholar] [CrossRef]
- Clay, L.; Pichtel, J. Treatment of simulated oil and gas produced water via pilot-scale rhizofiltration and constructed wetlands. Int. J. Environ. Sci. Technol. 2019, 13, 185–198. [Google Scholar] [CrossRef]
- Rasool, K.; Pandey, R.P. Water treatment and environmental remediation applications of two-dimensional metal carbides (MXenes). Mater. Today 2019, 30, 80–102. [Google Scholar] [CrossRef]
- Jiang, J.Q.; Stanford, C.; Petri, M. Practical application of ferrate (VI) for water and wastewater treatment–site study’s approach. Water-Energy Nexus 2018, 1, 42–46. [Google Scholar] [CrossRef]
- Gombos, E.; Barkács, K.; Felföldi, T.; Vértes, C.; Makó, M.; Palkó, G.; Záray, G. Removal of organic matters in wastewater treatment by ferrate (VI)-technology. Microchem. J. 2013, 107, 115–120. [Google Scholar] [CrossRef]
- Alsheyab, M.; Jiang, J.Q.; Stanford, C. On-line production of ferrate with an electrochemical method and its potential application for wastewater treatment—A review. J. Environ. Manag. 2009, 90, 1350–1356. [Google Scholar] [CrossRef]
- Sun, S.; Pang, S.; Jiang, J.; Ma, J.; Huang, Z.; Zhang, J.; Liu, Y.; Xu, C.; Liu, Q.; Yuan, Y. The combination of ferrate (VI) and sulfite as a novel advanced oxidation process for enhanced degradation of organic contaminants. Chem. Eng. J. 2018, 333, 11–19. [Google Scholar] [CrossRef]
- Jiang, J.Q. Research progress in the use of ferrate(VI) for the environmental remediation. J. Hazard. Mater. 2007, 146, 617–623. [Google Scholar] [CrossRef]
- Guan, W.; Xie, Z.; Zhang, J. Preparation and aromatic hydrocarbon removal performance of potassium Ferrate. Int. J. Spectrosc. 2014, 22, 1–8. [Google Scholar] [CrossRef][Green Version]
- Sharma, V.K.; Rivera, W.; Smith, J.O.; O’Brien, B. Ferrate (VI) oxidation of aqueous cyanide. J. Environ. Sci. Technol. 1998, 32, 2608–2613. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Ismail, S.; Bhatia, S. Optimization of coagulation-flocculation process for palm oil mill effluent using response surface methodology. Environ. Sci. Technol. 2005, 39, 2828–2834. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.O.; Azizli, K.; Isa, M.H.; Bashir, M.J. Application of CCD in RSM to obtain optimize treatment of POME using Fenton oxidation process. J. Water Process. Eng. 2015, 8, 7–16. [Google Scholar] [CrossRef]
- Wang, C.; Klamerth, N.; Huang, R.; Elnakar, H.; Gamal El-Din, M. Oxidation of oil sands process-affected water by potassium Ferrate(VI). Environ. Sci. Technol. 2016, 50, 4238–4247. [Google Scholar] [CrossRef]
- Han, H.; Li, J.; Ge, Q.; Wang, Y.; Chen, Y.; Wang, B. Green Ferrate(VI) for multiple treatments of fracturing wastewater: Demulsification, visbreaking, and chemical oxygen demand removal. Int. J. Mol. Sci. 2019, 20, 1857. [Google Scholar] [CrossRef]
- Ciabatti, I.; Tognotti, F.; Lombardi, L. Treatment and reuse of dyeing effluents by potassium ferrate. Desalination 2010, 250, 222–228. [Google Scholar] [CrossRef]
- Malik, S.N.; Ghosh, P.C.; Vaidya, A.N.; Waindeskar, V.; Das, S.; Mudliar, S.N. Comparison of coagulation, ozone and ferrate treatment processes for color, COD and toxicity removal from complex textile wastewater. Water Sci. Technol. 2017, 76, 1001–1010. [Google Scholar] [CrossRef]
- Li, Y.N.; Duan, Z.H.; Wang, Y.F.; Yuan, Z.J.; Wang, G.Y. Preliminary treatment of phenanthrene in coking wastewater by a combined potassium ferrate and Fenton process. Int. J. Sci. Environ. Technol. 2018, 16, 4483–4492. [Google Scholar] [CrossRef]
- Kozik, V.; Barbusinski, K.; Thomas, M.; Sroda, A.; Jampilek, J.; Sochanik, A.; Smolinski, A.; Bak, A. Taguchi method and response surface methodology in the treatment of highly contaminated tannery wastewater using commercial potassium Ferrate. Materials 2019, 12, 3784. [Google Scholar] [CrossRef]
- Tan, X.M.; Ji, F.Y.; Wang, X.D.; Zhang, G.Z. Preparation of potassium Ferrate and Ferrate(VI) oxidation of phenanthrene. Adv. Mater. Res. 2012, 523, 784–789. [Google Scholar] [CrossRef]
- Eaton, A.D. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 1915. [Google Scholar]
- Perez, S.; Guillamon, M.; Barceló, D. Quantitative analysis of polycyclic aromatic hydrocarbons in sewage sludge from wastewater treatment plants. J. Chromatogr. A 2001, 938, 57–65. [Google Scholar] [CrossRef]
- Zahid, M.; Shafiq, N.; Isa, M.H.; Gil, L. Statistical modeling and mix design optimization of fly ash based engineered geopolymer composite using response surface methodology. J. Clean. Prod. 2018, 194, 483–498. [Google Scholar] [CrossRef]
- Rai, P.K.; Lee, J.; Kailasa, S.K.; Kwon, E.E.; Tsang, Y.F.; Ok, Y.S.; Kim, K.H. A critical review of ferrate (VI)-based remediation of soil and groundwater. Environ. Res. 2018, 160, 420–448. [Google Scholar] [CrossRef]
- Jiang, J.Q.; Lloyd, B. Progress in the development and use of ferrate(VI) salt as an oxidant and coagulant for water and wastewater treatment. Water Res. 2001, 36, 1397–1408. [Google Scholar] [CrossRef]
- Matin, A.R.; Yousefzadeh, S.; Ahmadi, E.; Mahvi, A.; Alimohammadi, M.; Aslani, H.; Nabizadeh, R. A comparative study of the disinfection efficacy of H2O2/Ferrate and UV/H2O2/Ferrate processes on inactivation of Bacillus subtilis spores by response surface methodology for modeling and optimization. Food Chem. Toxicol. 2018, 116, 129–137. [Google Scholar] [CrossRef]
- Karim, A.V.; Krishnan, S.; Pisharody, L.; Malhotra, M. Application of Ferrate for Advanced Water and Wastewater Treatment. In Advanced Oxidation Processes-Applications, Trends, and Prospects; IntechOpen: London, UK, 2020. [Google Scholar]
- Sun, X.; Zhang, Q.; Liang, H.; Ying, L.; Xiangxu, M.; Sharma, V.K. Ferrate(VI) as a greener oxidant: Electrochemical generation and treatment of phenol. J. Hazard. Mater. 2016, 319, 130–136. [Google Scholar] [CrossRef]
- Graham, N.; Jiang, C.C.; Li, X.Z.; Jiang, J.Q.; Ma, J. The influence of pH on the degradation of phenol and chlorophenols by potassium ferrate. Chemosphere 2004, 56, 949–956. [Google Scholar] [CrossRef]
- Karaatli, T. Disinfection of Surface Waters by Ferrate. Master’s Thesis, Middle East Technical University, Ankara, Turkey, 1998. [Google Scholar]
- Song, Y.; Men, B.; Wang, D.; Ma, J. On line batch production of ferrate with an chemical method and its potential application for greywater recycling with Al(III) salt. Int. J. Environ. Sci. Technol. 2017, 52, 1–7. [Google Scholar] [CrossRef]
- Jiang, J.Q. Advances in the development and application of ferrate (VI) for water and wastewater treatment. J. Chem. Technol. 2014, 89, 165–177. [Google Scholar] [CrossRef]
- Miao, Z.C.; Wang, F.; Deng, D.; Wang, L.; Yang, J.Z. Removal effect of potassium Ferrate to COD in different wastewater. Adv. Mater. Res. 2012, 553, 2288–2290. [Google Scholar] [CrossRef]
- Duraisamy, R.T.; Beni, A.H.; Henni, A. State of the art treatment of produced water. Water Treat. 2013, 233, 199–222. [Google Scholar]
- Talaiekhozani, A.; Eskandari, Z.; Bagheri, M.; Talaie, M.R. Removal of H2S and COD using UV, Ferrate and UV/Ferrate from municipal wastewater. J. Hum. Environ. Health Prom. 2016, 2, 1–8. [Google Scholar] [CrossRef][Green Version]


| Oxidant | E° (Volt) | Oxidant | E° (Volt) |
|---|---|---|---|
| Permanganate | 1.67 | Dissolved oxygen | 1.22 |
| Ozone | 2.07 | Perchlorate | 1.38 |
| Chlorine dioxide | 0.95 | Hypochlorite | 1.48 |
| Chlorine | 1.35 | Ferrate (VI) | 2.20 |
| Factors | Independent Parameters | Low | Medium | High |
|---|---|---|---|---|
| −1 | 0 | 0 | ||
| A | Fe (VI) concentration (mg/L) | 10 | 20 | 30 |
| B | pH | 5.0 | 7.5 | 10.0 |
| C | Contact time (min) | 10 | 50 | 90 |
| Experiment No. | Parameters | ||
|---|---|---|---|
| Fe (VI) Concentration (mg/L) | Contact Time (min) | pH | |
| 1 | 10 | 10 | 10.0 |
| 2 | 20 | 50 | 7.5 |
| 3 | 10 | 50 | 7.5 |
| 4 | 10 | 90 | 10.0 |
| 5 | 20 | 50 | 5.0 |
| 6 | 20 | 50 | 7.5 |
| 7 | 30 | 50 | 7.5 |
| 8 | 30 | 90 | 5.0 |
| 9 | 10 | 90 | 5.0 |
| 10 | 30 | 10 | 5.0 |
| 11 | 20 | 10 | 7.5 |
| 12 | 20 | 50 | 7.5 |
| 13 | 20 | 50 | 7.5 |
| 14 | 20 | 50 | 7.5 |
| 15 | 30 | 10 | 10.0 7.5 |
| 16 | 20 | 90 | |
| 17 | 10 | 10 | 5.0 |
| 18 | 20 | 50 | 7.5 |
| 19 | 20 | 50 | 10.0 |
| 20 | 30 | 90 | 10.0 |
| Source | * SS | * DF | * MS | F-Value | p-Value | Model’s Status |
|---|---|---|---|---|---|---|
| Model | 4446.85 | 9 | 494.09 | 55.95 | 0.0001 | Significant |
| A | 60.02 | 1 | 60.02 | 6.80 | 0.0262 | |
| B | 67.60 | 1 | 67.60 | 7.65 | 0.0199 | |
| C | 276.60 | 1 | 276.68 | 31.33 | 0.0002 | |
| AB | 45.13 | 1 | 45.13 | 5.11 | 0.0473 | |
| AC | 32.00 | 1 | 32.00 | 3.62 | 0.0861 | |
| BC | 72.00 | 1 | 72.00 | 8.15 | 0.0171 | |
| A2 | 646.00 | 1 | 646.04 | 73.16 | 0.0001 | |
| B2 | 349.70 | 1 | 349.74 | 39.60 | 0.0001 | |
| C2 | 85.54 | 1 | 85.54 | 9.69 | 0.0110 | |
| Residual | 88.31 | 10 | 8.83 | |||
| Lack of Fit | 60.20 | 5 | 12.04 | 2.14 | 0.2116 | Non-significant |
| Model summary: | R2 | R2adj. | R2pred. | |||
| 98.05% | 96.30% | 89.23% |
| Source | * SS | * DF | * MS | F-Value | p-Value | Model’s Status |
|---|---|---|---|---|---|---|
| Model | 5104.18 | 9 | 567.13 | 30.93 | 0.0001 | Significant |
| A | 110.89 | 1 | 110.89 | 6.05 | 0.0337 | |
| B | 158.40 | 1 | 158.40 | 8.64 | 0.0148 | |
| C | 305.81 | 1 | 305.81 | 16.68 | 0.0022 | |
| AB | 113.25 | 1 | 113.25 | 6.18 | 0.0323 | |
| AC | 150.51 | 1 | 150.51 | 8.21 | 0.0168 | |
| BC | 0.01 | 1 | 0.01 | 0.00 | 0.9807 | |
| A2 | 295.88 | 1 | 295.88 | 16.13 | 0.0025 | |
| B2 | 723.74 | 1 | 723.74 | 39.47 | 0.0001 | |
| C2 | 141.48 | 1 | 141.48 | 7.71 | 0.0195 | |
| Residual | 183.39 | 10 | 18.34 | |||
| Lack of Fit | 73.30 | 5 | 14.66 | 0.6669 | 0.6669 | Non-significant |
| Model summary: | R2 | R2adj. | R2pred. | |||
| 96.50% | 93.40% | 79.72% |
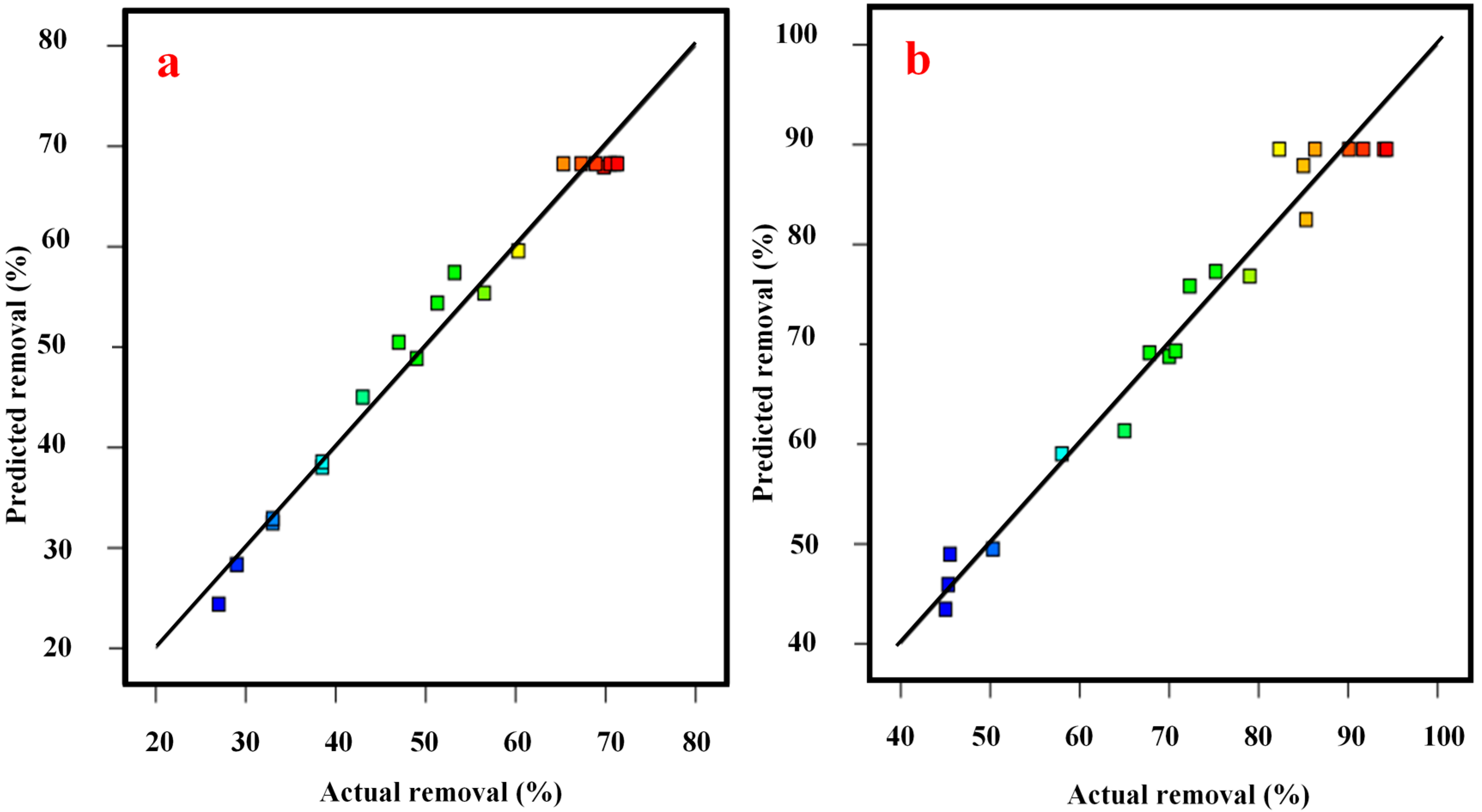
| Experimental Run | Actual PAHs Removal | Predicted PAHs Removal | Actual COD Removal | Predicted COD Removal |
|---|---|---|---|---|
| 1 | 45.50 | 48.98 | 27.00 | 24.39 |
| 2 | 82.30 | 89.54 | 71.30 | 68.26 |
| 3 | 85.30 | 82.50 | 47.00 | 50.48 |
| 4 | 70.00 | 68.79 | 33.00 | 32.91 |
| 5 | 75.20 | 77.30 | 60.30 | 59.58 |
| 6 | 94.30 | 89.54 | 67.30 | 68.26 |
| 7 | 72.30 | 75.84 | 56.50 | 55.38 |
| 8 | 65.00 | 61.34 | 43.00 | 45.01 |
| 9 | 67.80 | 69.15 | 49.00 | 48.86 |
| 10 | 58.00 | 59.03 | 33.00 | 32.49 |
| 11 | 79.00 | 76.84 | 53.20 | 57.42 |
| 12 | 90.10 | 89.54 | 65.30 | 68.26 |
| 13 | 91.70 | 89.54 | 68.90 | 68.26 |
| 14 | 86.30 | 89.54 | 70.50 | 68.26 |
| 15 | 45.00 | 43.47 | 38.50 | 38.04 |
| 16 | 85.00 | 87.90 | 69.80 | 67.94 |
| 17 | 50.30 | 49.49 | 29.00 | 28.34 |
| 18 | 94.00 | 89.54 | 71.00 | 68.26 |
| 19 | 70.70 | 69.34 | 51.30 | 54.38 |
| 20 | 45.30 | 45.93 | 38.50 | 38.56 |
| Dependent Variables | Fe (VI) Conc. (mg/L) | pH | Contact Time (min) | Predicted Removal (%) | Observed Removal (%) |
|---|---|---|---|---|---|
| COD | 19.35 | 7.1 | 68.34 | 69.69 | 73.41 |
| PAHs | 19.35 | 7.1 | 68.34 | 91.00 | 89.73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haneef, T.; Mustafa, M.R.U.; Wan Yusof, K.; Isa, M.H.; Bashir, M.J.K.; Ahmad, M.; Zafar, M. Removal of Polycyclic Aromatic Hydrocarbons (PAHs) from Produced Water by Ferrate (VI) Oxidation. Water 2020, 12, 3132. https://doi.org/10.3390/w12113132
Haneef T, Mustafa MRU, Wan Yusof K, Isa MH, Bashir MJK, Ahmad M, Zafar M. Removal of Polycyclic Aromatic Hydrocarbons (PAHs) from Produced Water by Ferrate (VI) Oxidation. Water. 2020; 12(11):3132. https://doi.org/10.3390/w12113132
Chicago/Turabian StyleHaneef, Tahir, Muhammad Raza Ul Mustafa, Khamaruzaman Wan Yusof, Mohamed Hasnain Isa, Mohammed J.K. Bashir, Mushtaq Ahmad, and Muhammad Zafar. 2020. "Removal of Polycyclic Aromatic Hydrocarbons (PAHs) from Produced Water by Ferrate (VI) Oxidation" Water 12, no. 11: 3132. https://doi.org/10.3390/w12113132
APA StyleHaneef, T., Mustafa, M. R. U., Wan Yusof, K., Isa, M. H., Bashir, M. J. K., Ahmad, M., & Zafar, M. (2020). Removal of Polycyclic Aromatic Hydrocarbons (PAHs) from Produced Water by Ferrate (VI) Oxidation. Water, 12(11), 3132. https://doi.org/10.3390/w12113132









