Effective Adsorption of Reactive Black 5 onto Hybrid Hexadecylamine Impregnated Chitosan-Powdered Activated Carbon Beads
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Preparation of Adsorbent
2.3. Characterization
2.4. Adsorption and Desorption Experiments
- qe = Adsorption capacity (mg/g)
- RE = Removal efficiency (%)
- C0 = Initial RB5 concentration (mg/L)
- Ce = Equilibrium RB5 concentration (mg/L)
- V = RB5 solution volume (L)
- W = Adsorbent mass (g)
- qt = RB5 adsorption at time t (mg/g)
- qe = RB5 adsorption at equilibrium (mg/g)
- k = PFO rate constant (1/min)
- v0 = PFO/PSO rate constant (g/mg/min)
- t = time (min)
- qt = Maximum adsorption capacity of the Ct-PAC-HDA beads (mg/g)
- qm = Maximum adsorption capacity of the adsorbent (mg/g)
- Ce = RB5 equilibrium concentration (mg/L)
- B = Affinity of Ct-PAC-HDA beads towards RB5 (L/g)
- KF = Freundlich constant (mg/g)
- N = Adsorption intensity
3. Results and Discussion
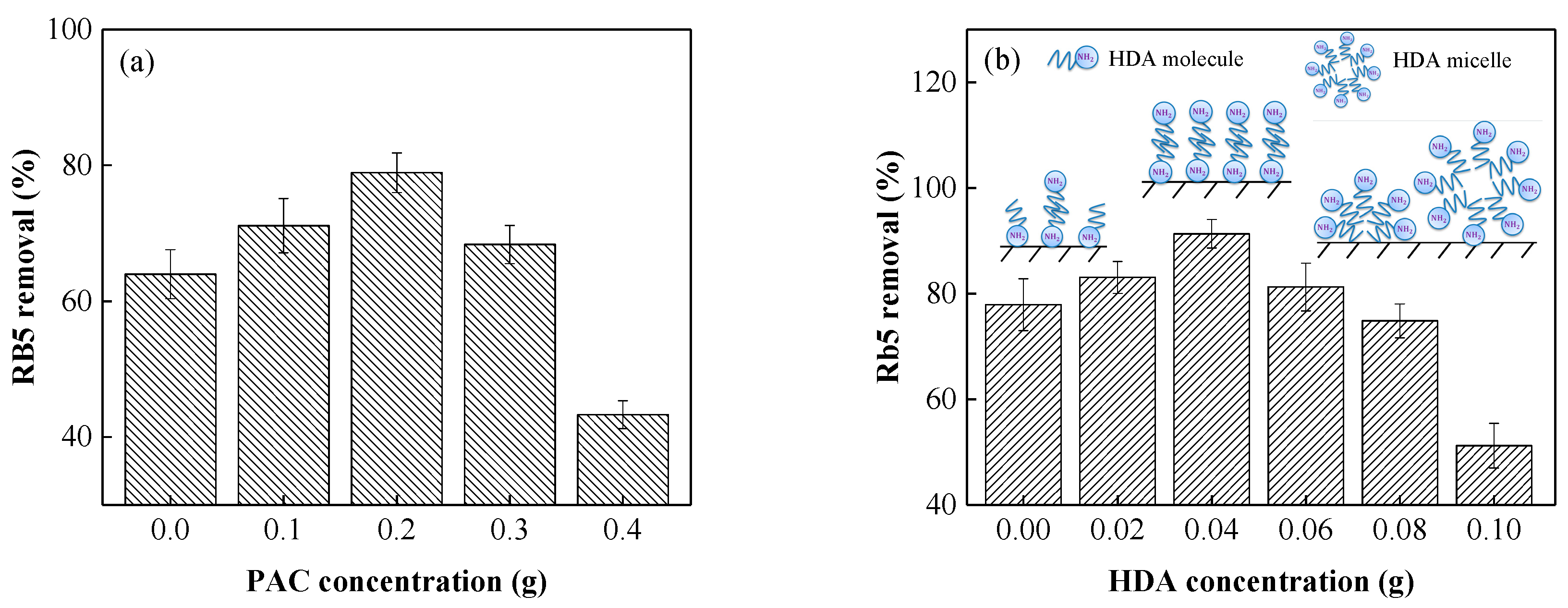
3.1. Effects of the Reaction Conditions
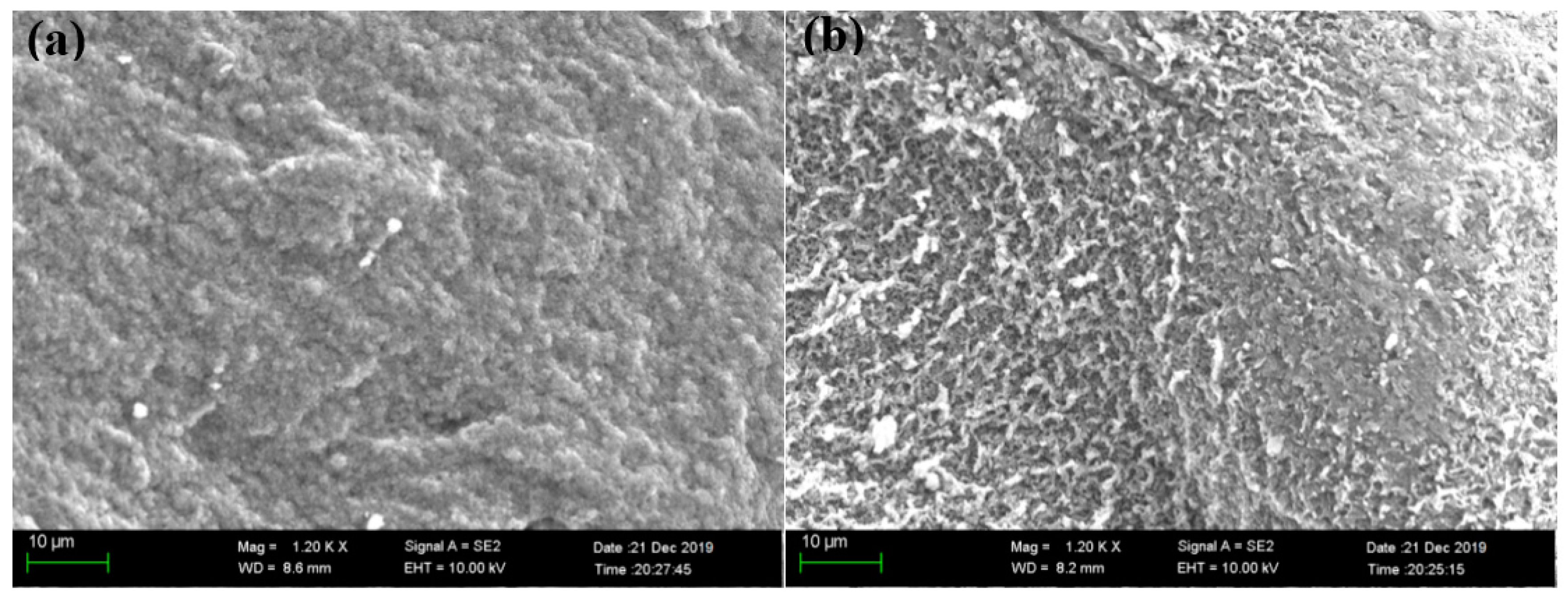
3.2. Adsorbent Characterization
3.3. RB5 Adsorption Experiments
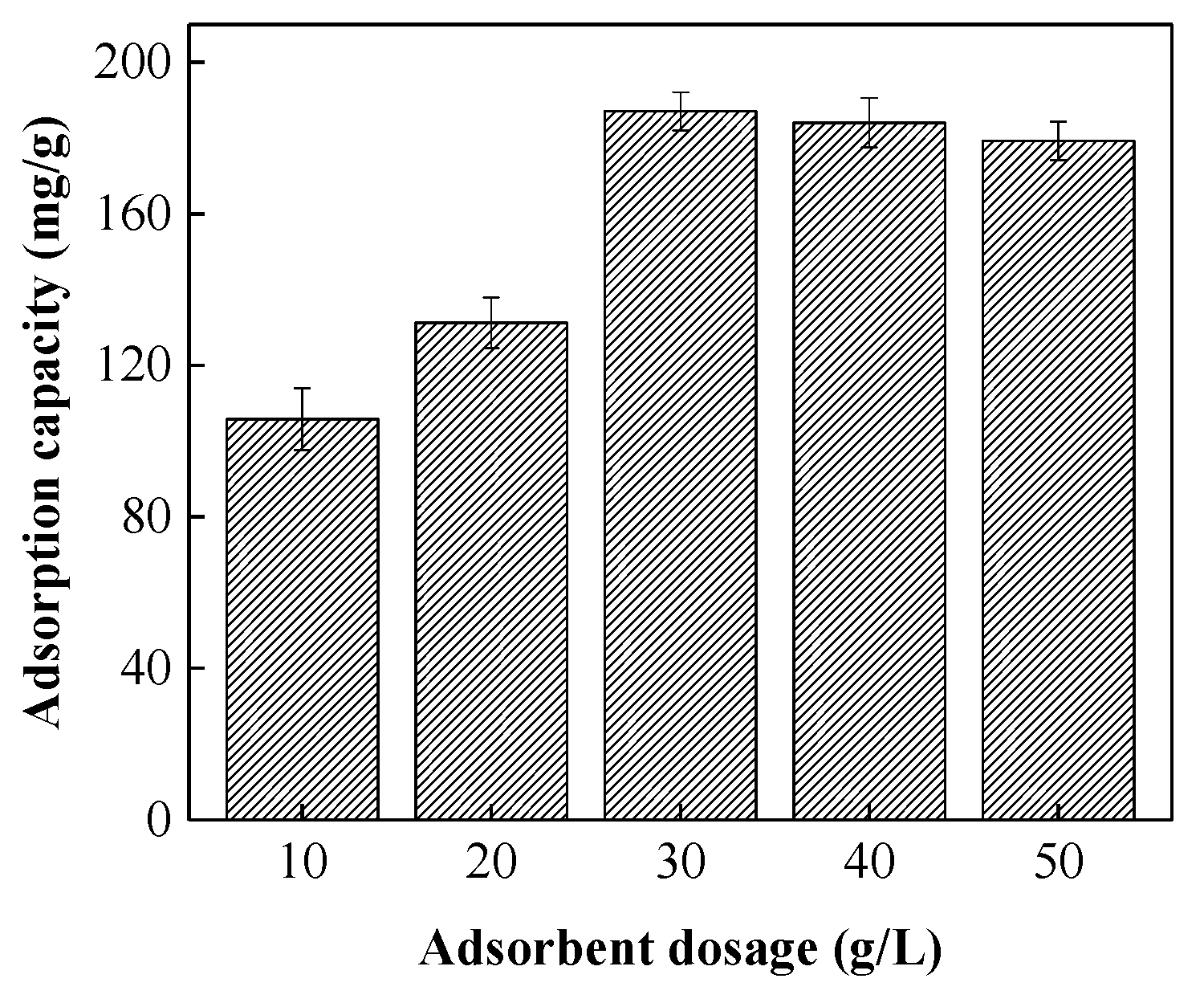
3.3.1. Effect of Adsorbent Dosage on RB5 Removal
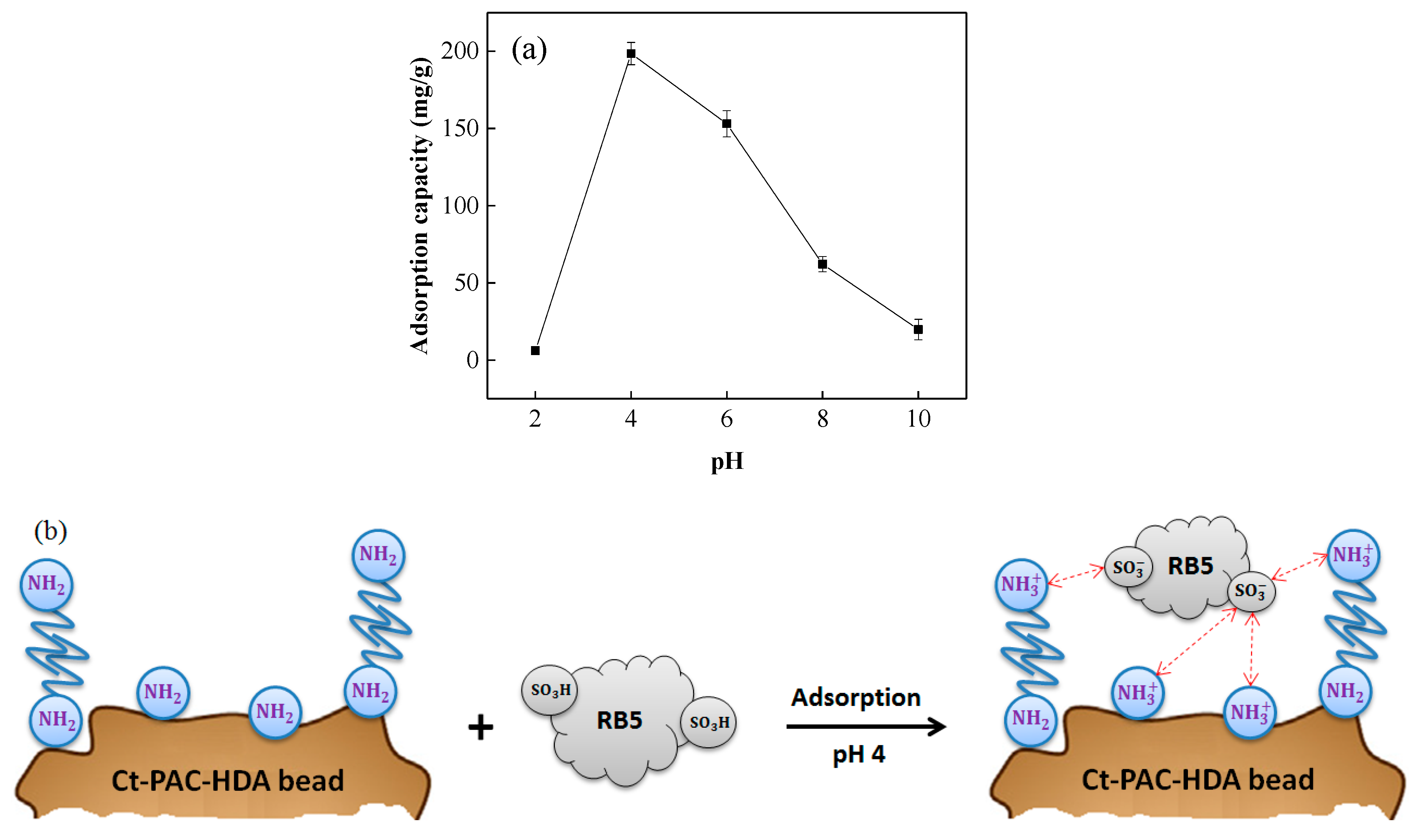
3.3.2. Effect of pH on RB5 Removal
3.3.3. Kinetic Study of RB5 Adsorption
3.3.4. Isotherm Study of RB5 Adsorption
3.4. Desorption and Reuse of Spent Ct-PAC-HDA Beads
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Farraji, H.; Robinson, B.; Mohajeri, P.; Abedi, T. Phytoremediation: Green technology for improving aquatic and terrestrial environments. Nippon. J. Environ. Sci. 2020, 1, 1–30. [Google Scholar]
- Vakili, M.; Rafatullah, M.; Salamatinia, B.; Abdullah, A.Z.; Ibrahim, M.H.; Tan, K.B.; Gholami, Z.; Amouzgar, P. Application of chitosan and its derivatives as adsorbents for dye removal from water and wastewater: A review. Carbohyd. Polym. 2014, 113, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Vakili, M.; Rafatullah, M.; Salamatinia, B.; Ibrahim, M.H.; Abdullah, A.Z. Elimination of reactive blue 4 from aqueous solutions using 3-aminopropyl triethoxysilane modified chitosan beads. Carbohyd. Polym. 2015, 132, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Homaeigohar, S. The nanosized dye adsorbents for water treatment. Nanomaterials 2020, 10, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.M. 9—The chemistry of reactive dyes and their application processes. In Handbook of Textile and Industrial Dyeing; Clark, M., Ed.; Woodhead Publishing: Sawston, UK, 2011; Volume 1, pp. 303–364. [Google Scholar]
- Nabil, G.M.; El-Mallah, N.M.; Mahmoud, M.E. Enhanced decolorization of reactive black 5 dye by active carbon sorbent-immobilized-cationic surfactant (AC-CS). J. Ind. Eng. Chem. 2014, 20, 994–1002. [Google Scholar] [CrossRef]
- Vakili, M.; Rafatullah, M.; Gholami, Z.; Farraji, H. Treatment of reactive dyes from water and wastewater through chitosan and its derivatives. Smart Mater. Waste Water Appl. 2016, 347–377. [Google Scholar] [CrossRef]
- Vakili, M.; Rafatullah, M.; Ibrahim, M.H.; Abdullah, A.Z.; Gholami, Z.; Salamatinia, B. Enhancing reactive blue 4 adsorption through chemical modification of chitosan with hexadecylamine and 3-aminopropyl triethoxysilane. J. Water Process. Eng. 2017, 15, 49–54. [Google Scholar] [CrossRef]
- Vakili, M.; Rafatullah, M.; Ibrahim, M.H.; Abdullah, A.Z.; Salamatinia, B.; Gholami, Z. Oil palm biomass as an adsorbent for heavy metals. Rev. Environ. Contam. T 2014, 232, 61–88. [Google Scholar]
- Vakili, M.; Mojiri, A.; Kindaichi, T.; Cagnetta, G.; Yuan, J.; Wang, B.; Giwa, A.S. Cross-linked chitosan/zeolite as a fixed-bed column for organic micropollutants removal from aqueous solution, optimization with RSM and artificial neural network. J. Environ. 2019, 250, 109434. [Google Scholar] [CrossRef]
- Vakili, M.; Amouzgar, P.; Cagnetta, G.; Wang, B.; Guo, X.; Mojiri, A.; Zeimaran, E.; Salamatinia, B. Ultrasound-Assisted Preparation of Chitosan/Nano-Activated Carbon Composite Beads Aminated with (3-Aminopropyl) Triethoxysilane for Adsorption of Acetaminophen from Aqueous Solutions. Polymers 2019, 11, 1701. [Google Scholar] [CrossRef]
- Vakili, M.; Mojiri, A.; Zwain, H.M.; Yuan, J.; Giwa, A.S.; Wang, W.; Gholami, F.; Guo, X.; Cagnetta, G.; Yu, G. Effect of beading parameters on cross-linked chitosan adsorptive properties. React. Funct. Polym. 2019, 144, 104354. [Google Scholar] [CrossRef]
- Qiu, W.; Vakili, M.; Cagnetta, G.; Huang, J.; Yu, G. Effect of high energy ball milling on organic pollutant adsorption properties of chitosan. Int. J. Biol. Macromol. 2020, 148, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Lan, W.; He, L.; Liu, Y. Preparation and Properties of Sodium Carboxymethyl Cellulose/Sodium Alginate/Chitosan Composite Film. Coatings 2018, 8, 1–17. [Google Scholar] [CrossRef]
- Ahmed, M.J.; Hameed, B.H.; Hummadi, E.H. Review on recent progress in chitosan/chitin-carbonaceous material composites for the adsorption of water pollutants. Carbohyd. Polym. 2020, 247, 116690. [Google Scholar] [CrossRef]
- Yadaei, H.; Beyki, M.H.; Shemirani, F.; Nouroozi, S. Ferrofluid mediated chitosan@mesoporous carbon nanohybrid for green adsorption/preconcentration of toxic Cd(II): Modeling, kinetic and isotherm study. React. Funct. Polym. 2018, 122, 85–97. [Google Scholar] [CrossRef]
- Arumugam, T.K.; Krishnamoorthy, P.; Rajagopalan, N.R.; Nanthini, S.; Vasudevan, D. Removal of malachite green from aqueous solutions using a modified chitosan composite. Int. J. Biol. Macromol. 2019, 128, 655–664. [Google Scholar] [CrossRef]
- Yan, M.; Huang, W.; Li, Z. Chitosan cross-linked graphene oxide/lignosulfonate composite aerogel for enhanced adsorption of methylene blue in water. Int. J. Biol. Macromol. 2019, 136, 927–935. [Google Scholar] [CrossRef]
- Vakili, M.; Rafatullah, M.; Salamatinia, B.; Ibrahim, M.H.; Ismail, N.; Abdullah, A.Z. Adsorption Studies of Methyl Tert-butyl Ether from Environment. Sep. Purif. Rev. 2017, 46, 273–290. [Google Scholar] [CrossRef]
- Lv, S.; Zhou, Z.; Xue, M.; Zhang, X.; Yang, Z. Adsorption characteristics of reactive blue 81 by powdered activated carbon: Role of the calcium content. J. Water Process. Eng. 2020, 36, 101247. [Google Scholar] [CrossRef]
- Zhou, J.; Ma, F.; Guo, H. Adsorption behavior of tetracycline from aqueous solution on ferroferric oxide nanoparticles assisted powdered activated carbon. Chem. Eng. J. 2020, 384, 123290. [Google Scholar] [CrossRef]
- Vakili, M.; Rafatullah, M.; Ibrahim, M.H.; Abdullah, A.Z.; Salamatinia, B.; Gholami, Z. Chitosan hydrogel beads impregnated with hexadecylamine for improved reactive blue 4 adsorption. Carbohyd. Polym. 2016, 137, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Amouzgar, P.; Vakili, M.; Chan, E.-S.; Salamatinia, B. Effects of beading parameters for development of chitosan-nano-activated carbon biocomposite for acetaminophen elimination from aqueous sources. Environ. Eng. Sci. 2017, 34, 805–815. [Google Scholar] [CrossRef]
- Mahaninia, M.H.; Wilson, L.D. Phosphate uptake studies of cross-linked chitosan bead materials. J. Colloid Interface Sci. 2017, 485, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Lee, D.S.; Lee, M.W.; Woo, S.H. Enhanced adsorption of congo red from aqueous solutions by chitosan hydrogel beads impregnated with cetyl trimethyl ammonium bromide. Bioresour. Technol. 2009, 100, 2803–2809. [Google Scholar] [CrossRef]
- Sutirman, Z.A.; Sanagi, M.M.; Abd Karim, K.J.; Ibrahim, W.A.W.; Jume, B.H. Equilibrium, kinetic and mechanism studies of Cu (II) and Cd (II) ions adsorption by modified chitosan beads. Int. J. Biol. Macromol. 2018, 116, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Nematollahzadeh, A.; Shojaei, A.; Karimi, M. Chemically modified organic/inorganic nanoporous composite particles for the adsorption of reactive black 5 from aqueous solution. React. Funct. Polym. 2015, 86, 7–15. [Google Scholar] [CrossRef]
- Cardoso, N.F.; Pinto, R.B.; Lima, E.C.; Calvete, T.; Amavisca, C.V.; Royer, B.; Cunha, M.L.; Fernandes, T.H.M.; Pinto, I.S. Removal of remazol black B textile dye from aqueous solution by adsorption. Desalination 2011, 269, 92–103. [Google Scholar] [CrossRef]
- Banerjee, S.; Chattopadhyaya, M.C. Adsorption characteristics for the removal of a toxic dye, tartrazine from aqueous solutions by a low cost agricultural by-product. Arab. J. Chem. 2017, 10, S1629–S1638. [Google Scholar] [CrossRef]
- Subramaniam, R.; Kumar Ponnusamy, S. Novel adsorbent from agricultural waste (cashew NUT shell) for methylene blue dye removal: Optimization by response surface methodology. Water Resour. Ind. 2015, 11, 64–70. [Google Scholar] [CrossRef]
- Huang, R.; Liu, Q.; Huo, J.; Yang, B. Adsorption of methyl orange onto protonated cross-linked chitosan. Arab. J. Chem. 2017, 10, 24–32. [Google Scholar] [CrossRef]
- El-Bindary, M.; El-Deen, I.; Shoair, A. Removal of anionic dye from aqueous solution using magnetic sodium alginate beads. J. Mater. Environ. Sci. 2019, 10, 604–617. [Google Scholar]
- Wong, S.; Ghafar, N.A.; Ngadi, N.; Razmi, F.A.; Inuwa, I.M.; Mat, R.; Amin, N.A.S. Effective removal of anionic textile dyes using adsorbent synthesized from coffee waste. Sci. Rep. 2020, 10, 2928. [Google Scholar] [CrossRef] [PubMed]
- Kasaai, M.R.; Arul, J.; Charlet, G. Fragmentation of Chitosan by Acids. Sci. World J. 2013, 2013, 508540. [Google Scholar] [CrossRef] [PubMed]
- Batool, F.; Akbar, J.; Iqbal, S.; Noreen, S.; Bukhari, S.N.A. Study of Isothermal, Kinetic, and Thermodynamic Parameters for Adsorption of Cadmium: An Overview of Linear and Nonlinear Approach and Error Analysis. Bioinorg. Chem. Appl. 2018, 2018, 3463724. [Google Scholar] [CrossRef] [PubMed]
- Dehmani, Y.; Alrashdi, A.A.; Lgaz, H.; Lamhasni, T.; Abouarnadasse, S.; Chung, I.-M. Removal of phenol from aqueous solution by adsorption onto hematite (α-Fe2O3): Mechanism exploration from both experimental and theoretical studies. Arab. J. Chem. 2020, 13, 5474–5486. [Google Scholar] [CrossRef]
- Mondal, M.; Manoli, K.; Ray, A.K. Removal of arsenic (III) from aqueous solution by concrete-based adsorbents. Can. J. Chem. Eng. 2020, 98, 353–359. [Google Scholar] [CrossRef]
- Al-Senani, G.M.; Al-Kadhi, N.S. Studies on Adsorption of Fluorescein Dye from Aqueous Solutions Using Wild Herbs. Int. J. Anal. Chem. 2020, 2020, 8019274. [Google Scholar] [CrossRef]
- El-Zawahry, M.M.; Abdelghaffar, F.; Abdelghaffar, R.A.; Hassabo, A.G. Equilibrium and kinetic models on the adsorption of Reactive Black 5 from aqueous solution using Eichhornia crassipes/chitosan composite. Carbohyd. Polym. 2016, 136, 507–515. [Google Scholar] [CrossRef]
- Felista, M.M.; Wanyonyi, W.C.; Ongera, G. Adsorption of anionic dye (Reactive black 5) using macadamia seed Husks: Kinetics and equilibrium studies. Sci. Afr. 2020, 7, e00283. [Google Scholar] [CrossRef]
- Tanyildizi, M.Ş. Modeling of adsorption isotherms and kinetics of reactive dye from aqueous solution by peanut hull. Chem. Eng. J. 2011, 168, 1234–1240. [Google Scholar] [CrossRef]
- Ziane, S.; Bessaha, F.; Marouf-Khelifa, K.; Khelifa, A. Single and binary adsorption of reactive black 5 and Congo red on modified dolomite: Performance and mechanism. J. Mol. Liq. 2018, 249, 1245–1253. [Google Scholar] [CrossRef]
- Ip, A.W.M.; Barford, J.P.; McKay, G. A comparative study on the kinetics and mechanisms of removal of Reactive Black 5 by adsorption onto activated carbons and bone char. Chem. Eng. J. 2010, 157, 434–442. [Google Scholar] [CrossRef]
- Chatterjee, S.; Chatterjee, T.; Woo, S.H. Influence of the polyethyleneimine grafting on the adsorption capacity of chitosan beads for Reactive Black 5 from aqueous solutions. Chem. Eng. J. 2011, 166, 168–175. [Google Scholar] [CrossRef]
- Eren, Z.; Acar, F.N. Equilibrium and kinetic mechanism for Reactive Black 5 sorption onto high lime Soma fly ash. J. Hazard. Mater. 2007, 143, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Peng, H.; Deng, S.; Yang, X.; Zhang, Y.; Li, Y. Preparation of activated carbon from edible fungi residue by microwave assisted K2CO3 activation—Application in reactive black 5 adsorption from aqueous solution. Bioresour. Technol. 2012, 111, 127–133. [Google Scholar] [CrossRef]
- Li, Z.; Sellaoui, L.; Dotto, G.L.; Lamine, A.B.; Bonilla-Petriciolet, A.; Hanafy, H.; Belmabrouk, H.; Netto, M.S.; Erto, A. Interpretation of the adsorption mechanism of Reactive Black 5 and Ponceau 4R dyes on chitosan/polyamide nanofibers via advanced statistical physics model. J. Mol. Liq. 2019, 285, 165–170. [Google Scholar] [CrossRef]
- Vakili, M.; Deng, S.; Cagnetta, G.; Wang, W.; Meng, P.; Liu, D.; Yu, G. Regeneration of chitosan-based adsorbents used in heavy metal adsorption: A review. Sep. Purif. Technol. 2019, 224, 373–387. [Google Scholar] [CrossRef]
- Vakili, M.; Deng, S.; Shen, L.; Shan, D.; Liu, D.; Yu, G. Regeneration of chitosan-based adsorbents for eliminating dyes from aqueous solutions. Sep. Purif. Rev. 2019, 48, 1–13. [Google Scholar] [CrossRef]
- Chen, A.-H.; Huang, Y.-Y. Adsorption of Remazol Black 5 from aqueous solution by the templated crosslinked-chitosans. J. Hazard. Mater. 2010, 177, 668–675. [Google Scholar] [CrossRef]

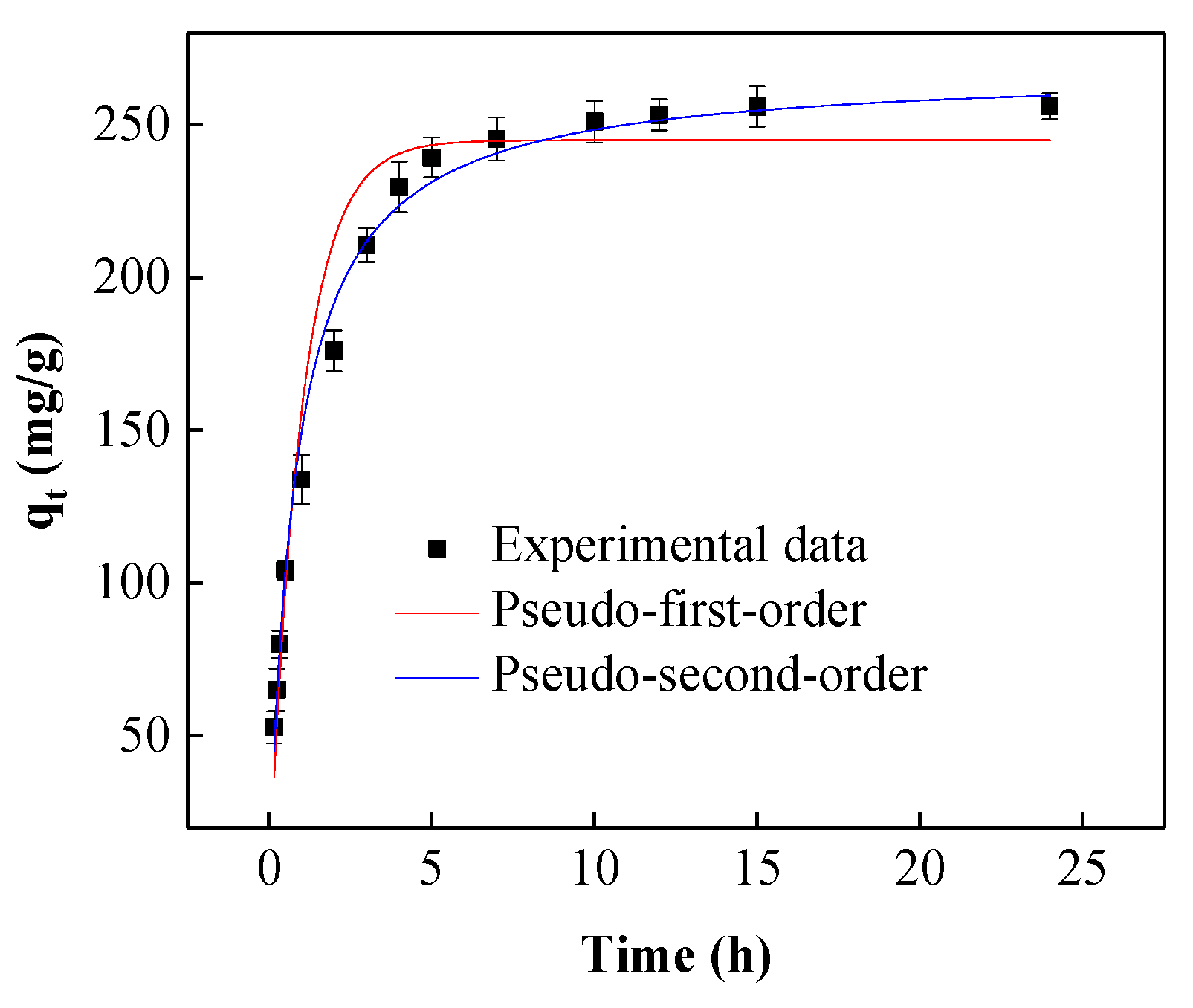

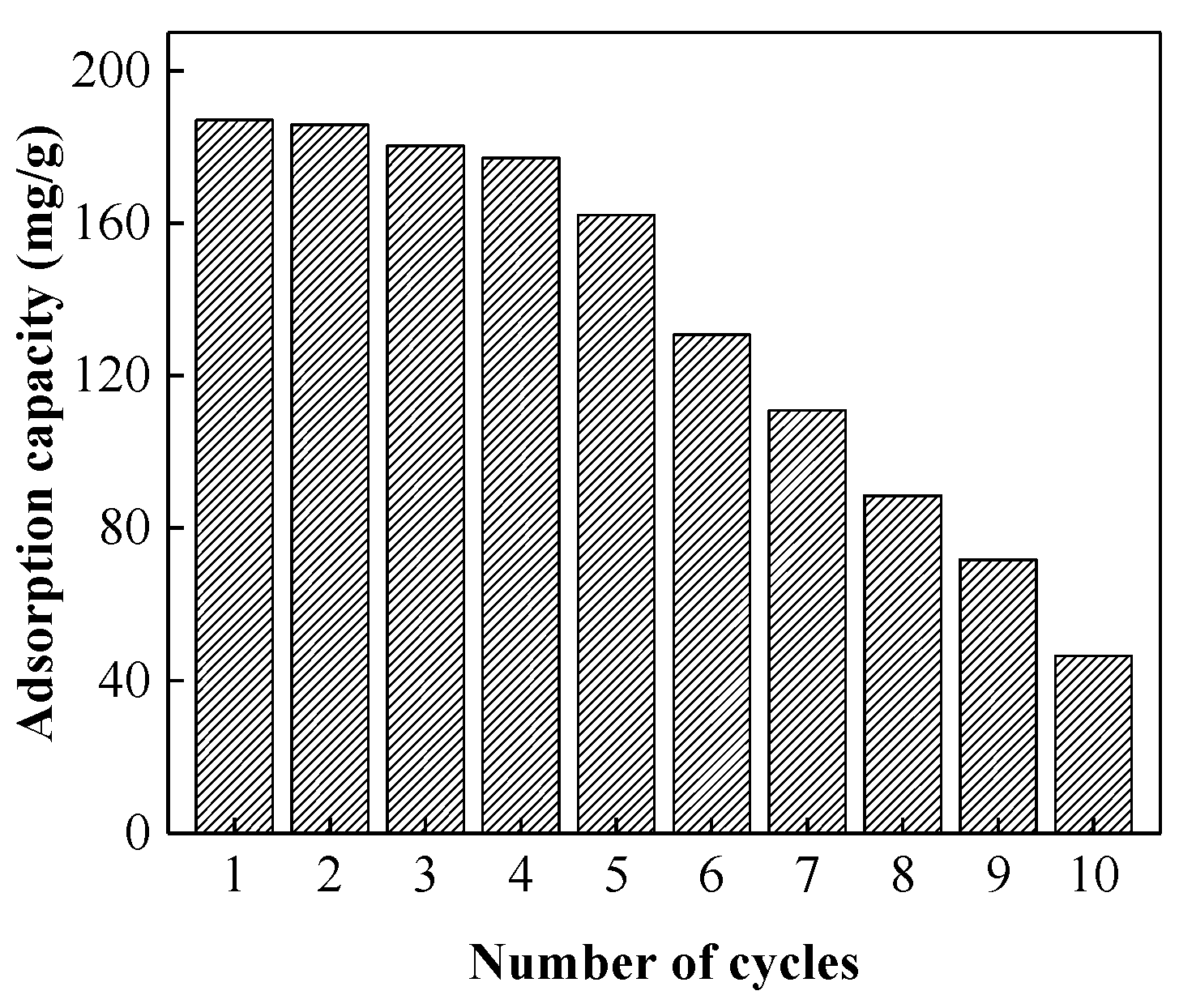
| Kinetic parameters | Values |
|---|---|
| Pseudo-first-order | |
| C0 (mg/L) | 200 |
| qe (mg/g) | 256.01 |
| qcal (mg/g) | 244.81 ± 5.939 |
| k (1/min) | 1.009 ± 0.0930 |
| χ2 | 7.57 |
| R2 | 0.968 |
| Slope | −0.366 |
| Intercept | 5.123 |
| Pseudo-second-order | |
| C0 (mg/L) | 200 |
| qe (mg/g) | 256.01 |
| qcal (mg/g) | 260.13 ± 3.851 |
| V0 (g/mg/min) | 335.24 ± 18.29 |
| χ2 | 1.26 |
| R2 | 0.994 |
| Slope | 0.004 |
| Intercept | 0.003 |
| Isotherm parameters | Values |
|---|---|
| Langmuir | |
| (mg/g) | 666.97 ± 38.14 |
| b (l/mg) | 327.82 ± 27.19 |
| χ2 | 0.225 |
| R2 | 0.997 |
| Slope | 0.002 |
| Intercept | 0.201 |
| Freundlich | |
| (mg/g) | 4.48 ± 0.495 |
| n | 1.3 ± 0.038 |
| χ2 | 0.074 |
| R2 | 0.999 |
| Slope | 0.686 |
| Intercept | 2.279 |
| Adsorbent | pH | Ref. | |
|---|---|---|---|
| Eichhornia crassipes/chitosan composite | 3 | 0.60 | [39] |
| Macadamia seed husks | 3 | 1.14 | [40] |
| Fly ash | 7 | 7.18 | [45] |
| Edible fungi activated carbon | 2 | 19.6 | [46] |
| Peanut hull | 6.4 | 55.55 | [41] |
| Pine-fruit shell | 2 | 74.6 | [28] |
| Dolomite | 6.9 | 80.9 | [42] |
| Bone char | 5.2 | 160.0 | [43] |
| Activated carbon F400 | 5.2 | 197.5 | [43] |
| Chitosan/polyamide nanofibers | 1 | 198.60 | [47] |
| Polyacrylamide/silica nanoporous composite | 2 | 389.58 | [27] |
| Polyethyleneimine/sodium dodecyl sulphate | 4 | 413.23 | [44] |
| Bamboo activated carbon | 5.2 | 441.7 | [37] |
| Pine-fruit shell activated carbon | 6 | 446.2 | [28] |
| Ct-PAC-HDA beads | 4 | 666.97 | Present study |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vakili, M.; Zwain, H.M.; Mojiri, A.; Wang, W.; Gholami, F.; Gholami, Z.; Giwa, A.S.; Wang, B.; Cagnetta, G.; Salamatinia, B. Effective Adsorption of Reactive Black 5 onto Hybrid Hexadecylamine Impregnated Chitosan-Powdered Activated Carbon Beads. Water 2020, 12, 2242. https://doi.org/10.3390/w12082242
Vakili M, Zwain HM, Mojiri A, Wang W, Gholami F, Gholami Z, Giwa AS, Wang B, Cagnetta G, Salamatinia B. Effective Adsorption of Reactive Black 5 onto Hybrid Hexadecylamine Impregnated Chitosan-Powdered Activated Carbon Beads. Water. 2020; 12(8):2242. https://doi.org/10.3390/w12082242
Chicago/Turabian StyleVakili, Mohammadtaghi, Haider M. Zwain, Amin Mojiri, Wei Wang, Fatemeh Gholami, Zahra Gholami, Abdulmoseen S. Giwa, Baozhen Wang, Giovanni Cagnetta, and Babak Salamatinia. 2020. "Effective Adsorption of Reactive Black 5 onto Hybrid Hexadecylamine Impregnated Chitosan-Powdered Activated Carbon Beads" Water 12, no. 8: 2242. https://doi.org/10.3390/w12082242
APA StyleVakili, M., Zwain, H. M., Mojiri, A., Wang, W., Gholami, F., Gholami, Z., Giwa, A. S., Wang, B., Cagnetta, G., & Salamatinia, B. (2020). Effective Adsorption of Reactive Black 5 onto Hybrid Hexadecylamine Impregnated Chitosan-Powdered Activated Carbon Beads. Water, 12(8), 2242. https://doi.org/10.3390/w12082242










