An Overview of Per- and Polyfluoroalkyl Substances (PFAS) in the Environment: Source, Fate, Risk and Regulations
Abstract
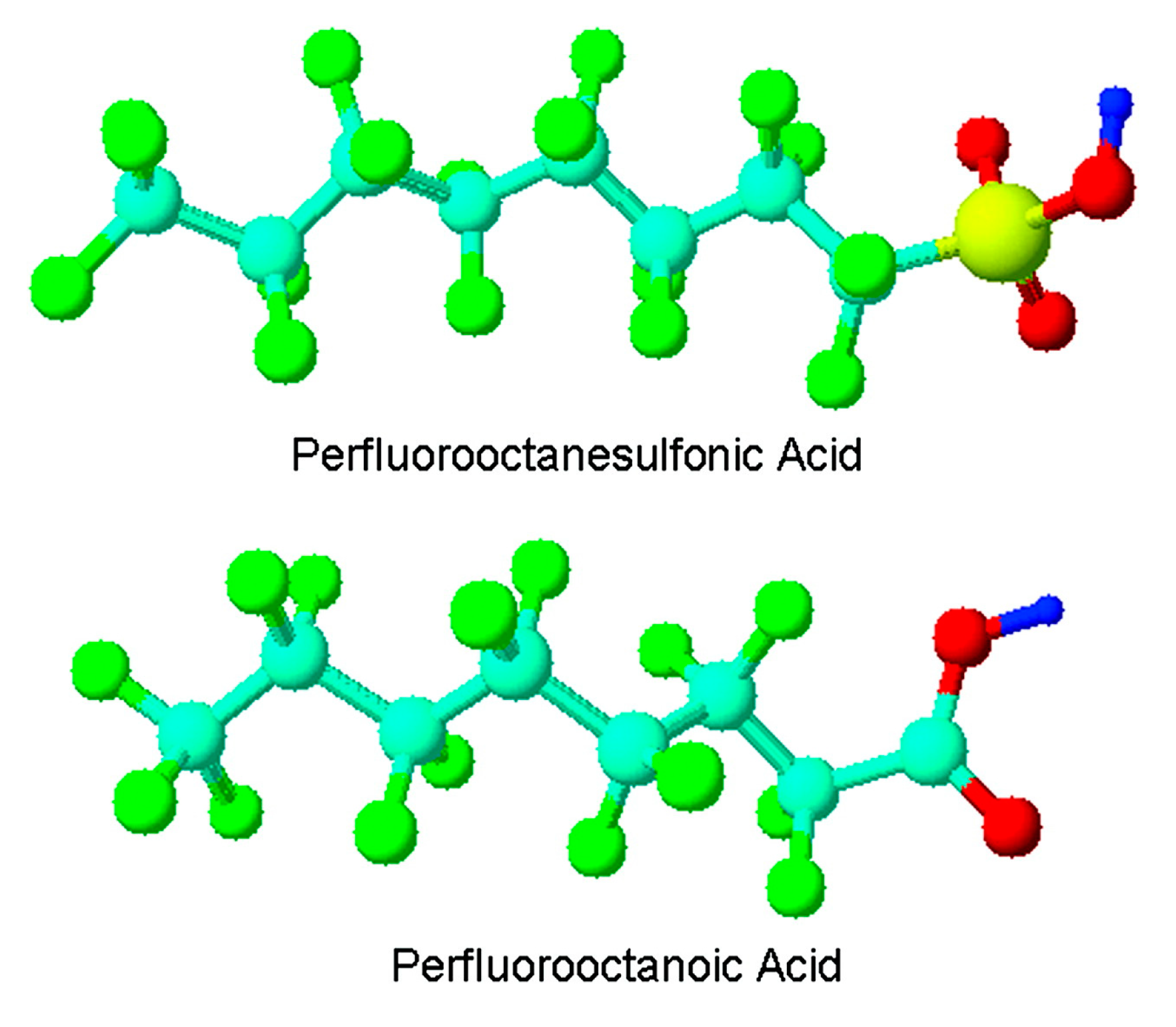
1. Introduction
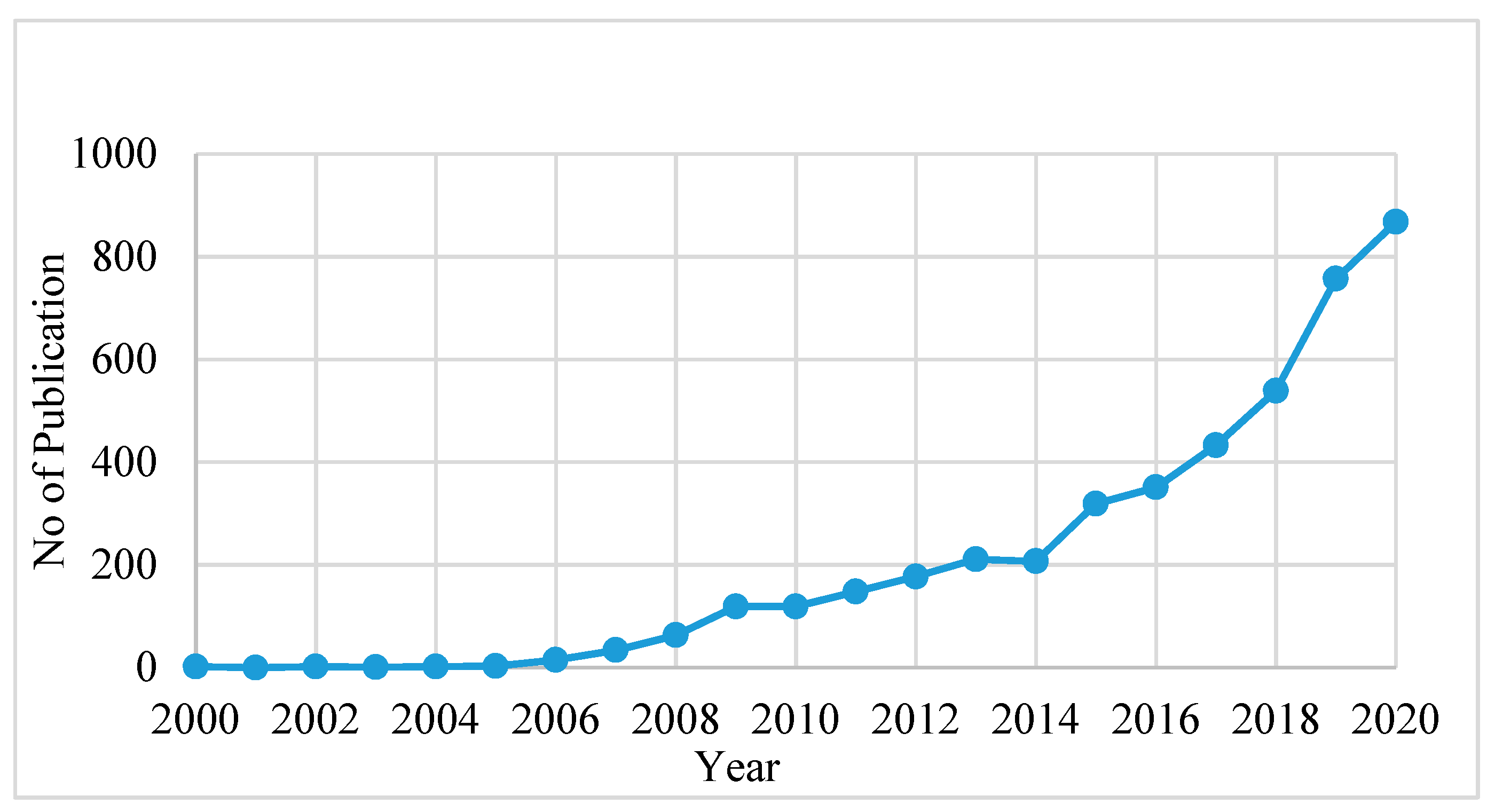
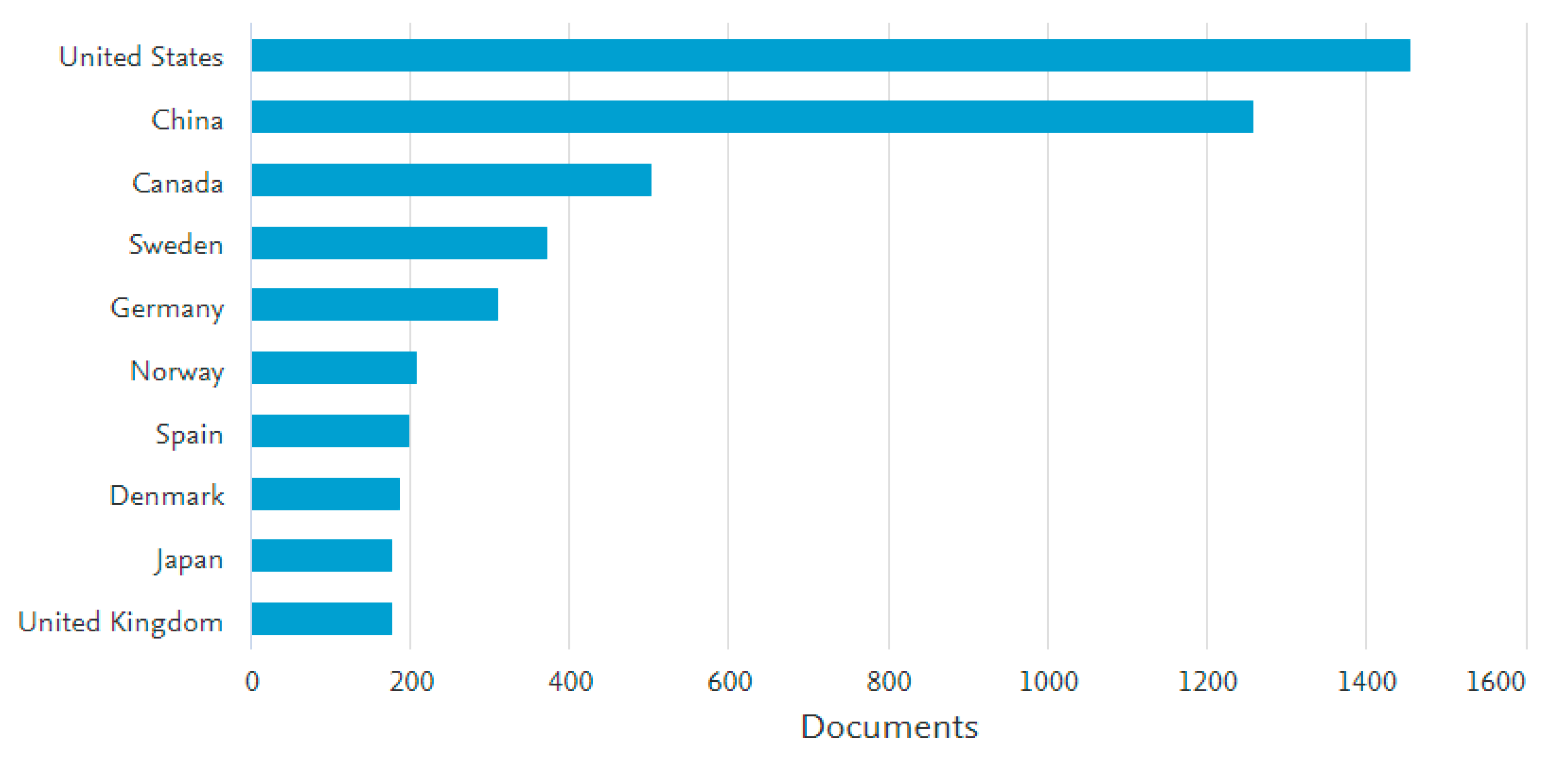
2. The Developing Trend in PFAS Research
3. PFASs Occurrence and Transformation
3.1. PFAS in Environment
3.1.1. PFASs in Water
3.1.2. PFASs in Soil
4. PFAS Treatment and Clean Up: Challenges and Achievement
5. The Key Knowledge Gaps and Future Research
5.1. Risks Associated by PFAS
Human Exposure Pathways
6. PFAS Water Quality Guidelines
6.1. Current Llegislations and Practices in Various Countries
6.2. EPA-US Guidelines
7. Factors Contributing to Variation in PFAS Guideline Levels
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Buck, R.C.; Franklin, J.; Berger, U.; Conder, J.M.; Cousins, I.T.; de Voogt, P.; Jensen, A.A.; Kannan, K.; Mabury, S.A.; van Leeuwen, S.P. Perfluoroalkyl and Polyfluoroalkyl Substances in the Environment: Terminology, Classification, and Origins. Integr. Environ. Assess. Manag. 2011, 7, 513–541. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Wang, T.; Wang, P.; Fu, Q.; Lu, Y. Effects of age, gender and region on serum concentrations of perfluorinated compounds in general population of Henan, China. Chemosphere 2014, 110, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Clout, L.; Priddle, D.; Spafford, P.J. The Coalition against PFAS. 2018. Available online: https://cappfas.com/what-is-pfas/ (accessed on 19 December 2020).
- ITRC. PFAS Technichal and Regulatory Guidanace Document and Fact Sheet PFAS-1; ITRC: Washington, DC, USA, 2020. [Google Scholar] [CrossRef]
- Cordner, A.; de la Rosa, V.Y.; Schaider, L.A.; Rudel, R.A.; Richter, L.; Brown, P. Guideline levels for PFOA and PFOS in drinking water: The role of scientific uncertainty, risk assessment decisions, and social factors. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Giesy, J.P.; Kannan, K. Global Distribution of Perfluorooctane Sulfonate in Wildlife. Environ. Sci. Technol. 2001, 35, 1339–1342. [Google Scholar] [CrossRef] [PubMed]
- USEPA. Drinking Water Health Advisories for PFOA and PFOS.; USEPA: Washington, DC, USA, 2016; pp. 1–4. Available online: https://www.epa.gov/sites/production/files/201605/documents/pfos_health_advisory_final-plain.pdf (accessed on 10 October 2020).
- ATSDR. Toxicological Profile for Perfluoroalkyls (Draft for Public Comment); Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2018. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=1117&tid=237 (accessed on 28 October 2020).
- Fàbrega, F.; Kumar, V.; Schuhmacher, M. PBPK modeling for PFOS and PFOA: Validation with human experimental data. Toxicol. Lett. 2014, 230, 244–251. [Google Scholar] [CrossRef]
- Lake, N.; Cao, Y.; Cao, X.; Wang, H.; Wan, Y.; Wang, S. Assessment on the distribution and partitioning of perfluorinated compounds in the water and sediment. Environ. Monit. Assess. 2015, 611, 611. [Google Scholar] [CrossRef]
- Zareitalabad, P.; Siemens, J.; Hamer, M.; Amelung, W. Perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS) in surface waters, sediments, soils and wastewater—A review on concentrations and distribution coefficients. Chemosphere 2013, 91, 725–732. [Google Scholar] [CrossRef]
- Post, G.B.; Louis, J.B.; Cooper, K.R.; Boros-Russo, B.J.; Lippincott, R.L. Occurrence and Potential Significance of Perfluorooctanoic Acid (PFOA) Detected in New Jersey Public Drinking Water Systems. Environ. Sci. Technol. 2009, 43, 4547–4554. [Google Scholar] [CrossRef]
- Jain, R.B. Time trends over 2003–2014 in the concentrations of selected perfluoroalkyl substances among US adults aged ≥ 20 years: Interpretational issues. Sci. Total Environ. 2018, 645, 946–957. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, H.; Li, J.; Dong, W. Perfluoroalkyl acids (PFAAs) in sediments from rivers of the Pearl River Delta, southern China. Environ. Monit. Assess. 2017, 189, 213. [Google Scholar] [CrossRef]
- Dunn, A.M.; Hofmann, O.S.; Waters, B.; Witchel, E. Cloaking malware with the trusted platform module. In Proceedings of the 20th USENIX Security Symposium, San Francisco, CA, USA, 8–12 August 2011; pp. 395–410. [Google Scholar]
- Vedagiri, U.K.; Anderson, R.H.; Loso, H.M.; Schwach, C.M. Ambient levels of PFOS and PFOA in multiple environmental media. Remediat. J. 2018, 28, 9–51. [Google Scholar] [CrossRef]
- Goosey, E.; Harrad, S. Perfluoroalkyl compounds in dust from Asian, AustralianEuropean, and North American homes and UK cars, classrooms, and offices. Environ. Int. 2011, 37, 86–92. [Google Scholar] [CrossRef] [PubMed]
- ITRC. Environmental Fate and Transport for per-and Polyfluoroalkyl Substances; ITRC: Washington, DC, USA, 2018; Available online: https://pfas-1.itrcweb.org/wp-content/uploads/2018/03/pfas_fact_sheet_fate_and_transport__3_16_18.pdf (accessed on 28 October 2020).
- Kucharzyk, K.H.; Darlington, R.; Benotti, M.; Deeb, R.; Hawley, E. Novel treatment technologies for PFAS compounds: A critical review. J. Environ. Manag. 2017, 204, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, P.; Meng, J.; Liu, S.; Lu, Y.; Khim, J.S.; Giesy, J.P. A review of sources, multimedia distribution and health risks of perfluoroalkyl acids (PFAAs) in China. Chemosphere 2015, 129, 87–99. [Google Scholar] [CrossRef]
- Page, D.; Vanderzalm, J.; Kumar, A.; Cheng, K.Y.; Kaksonen, A.H.; Simpson, S. Risks of perfluoroalkyl and polyfluoroalkyl substances (PFAS) for sustainable water recycling via aquifers. Water 2019, 11, 1737. [Google Scholar] [CrossRef]
- Gallen, C.; Drage, D.; Eaglesham, G.; Grant, S.; Bowman, M.; Mueller, J.F. Australia-wide assessment of perfluoroalkyl substances (PFASs) in landfill leachates. J. Hazard. Mater. 2017, 331, 132–141. [Google Scholar] [CrossRef]
- Cousins, I.T.; Vestergren, R.; Wang, Z.; Scheringer, M.; McLachlan, M.S. The precautionary principle and chemicals management: The example of perfluoroalkyl acids in groundwater. Environ. Int. 2016, 94, 331–340. [Google Scholar] [CrossRef]
- Gallen, C.; Eaglesham, G.; Drage, D.; Nguyen, T.H.; Mueller, J.F. A mass estim Figure 2020 ate of perfluoroalkyl substance (PFAS) release from Australian wastewater treatment plants. Chemosphere 2018, 208, 975–983. [Google Scholar] [CrossRef]
- Domingo, J.L.; Nadal, M. Human exposure to per-and polyfluoroalkyl substances (PFAS) through drinking water: A review of the recent scientific literature. Environ. Res. J. 2019, 177, 108648. [Google Scholar] [CrossRef]
- Gonzalez, D.; Thompson, K.; Quiñones, O.; Dickenson, E.; Bott, C. Assessment of PFAS fate transport, and treatment inhibition associated with a simulated AFFF release within a WASTEWATER treatment plant. Chemosphere 2021, 262, 127900. [Google Scholar] [CrossRef]
- Simon, J.A.; Cassidy, D.; Cherry, J.; Bryant, D.; Cox, D. PFAS Experts Symposium: Statements on regulatory policy, chemistry and analytics, toxicology, transport/fate, and remediation for per- and polyfluoroalkyl substances (PFAS) contamination issues. Remediat. J. 2019, 29, 31–48. [Google Scholar] [CrossRef]
- Cerveny, D.; Grabic, R.; Fedorova, G.; Grabicova, K.; Turek, J.; Zlabek, V.; Randak, T. Fate of perfluoroalkyl substances within a small stream food web affected by sewage effluent. Water Res. 2018, 134, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Brusseau, M.L. Assessing the potential contributions of additional retention processes to PFAS retardation in the subsurface. Sci. Total Environ. 2018, 613–614, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Trudel, D.; Horowitz, L.; Wormuth, M.; Scheringer, M.; Cousins, I.T.; Hungerb, K. Estimating Consumer Exposure to PFOS and PFOA. Risk Anal. 2008, 28, 13–15. [Google Scholar] [CrossRef]
- Sznajder-katarzy, K.; Surma, M.; Cie, I. A Review of Perfluoroalkyl Acids (PFAAs) in terms of Sources, Applications, Human Exposure, Dietary Intake, Toxicity, Legal Regulation, and Methods of Determination. J. Chem. 2019, 2019. [Google Scholar] [CrossRef]
- Uebelacker, L.A. A Review of the Pathways of Human Exposure to Poly- and Perfluoroalkyl Substances (PFASs) and Present Understanding of Health Effects Elsie. Physiol. Behav. 2017, 176, 139–148. [Google Scholar] [CrossRef]
- Pelch, K.E.; Reade, A.; Wol, T.A.M.; Kwiatkowski, C.F. Review article PFAS health e ff ects database: Protocol for a systematic evidence map. Environ. Int. J. 2019, 130, 104851. [Google Scholar] [CrossRef]
- Zhao, L.; Zhu, L.; Zhao, S.; Ma, X. Sequestration and bioavailability of perfluoroalkyl acids (PFAAs) in soils: Implications for their underestimated risk. Sci. Total Environ. 2016, 572, 169–176. [Google Scholar] [CrossRef]
- Yan, H.; Cousins, I.T.; Zhang, C.; Zhou, Q. Perfluoroalkyl acids in municipal landfill leachates from China: Occurrence, fate during leachate treatment and potential impact on groundwater. Sci. Total Environ. 2015, 524–525, 23–31. [Google Scholar] [CrossRef]
- Eriksson, U.; Kärrman, A.; Rotander, A.; Mikkelsen, B.; Dam, M. Perfluoroalkyl substances (PFASs) in food and water from Faroe Islands, Environ. Sci. Pollut. Res. 2013, 20, 7940–7948. [Google Scholar] [CrossRef]
- Lu, Z.; Lu, R.; Zheng, H.; Yan, J.; Song, L.; Wang, J.; Yang, H. Risk exposure assessment of per-and polyfluoroalkyl substances (PFASs) in drinking water and atmosphere in central eastern China. Environ. Sci. Pollut. Res. 2018, 25, 9311–9320. [Google Scholar] [CrossRef] [PubMed]
- Mastrantonio, M.; Bai, E.; Uccelli, R.; Cordiano, V.; Screpanti, A.; Crosignani, P. Drinking water contamination from perfluoroalkyl substances (PFAS): An ecological mortality study in the Veneto Region, Italy. Eur. J. Public Health 2017, 28, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Domingo, J.L.; Ericson-jogsten, I.; Perello, G.; Nadal, M.; van Bavel, B.; Ka, A. Human Exposure to Perfluorinated Compounds in Catalonia, Spain: Contribution of Drinking Water and Fish and Shellfish. Agric. Food Chem. 2012, 60, 4408–4415. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, M.; Bergmann, S.; Dieter, H.H. Occurrence of perfluorinated compounds (PFCs) in drinking water of North Rhine-Westphalia, Germany and new approach to assess drinking water contamination by shorter-chained C4–C7 PFCs. Int. J. Hyg. Environ. Health 2010, 213, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Schwanz, T.G.; Llorca, M.; Farré, M.; Barceló, D. Perfluoroalkyl substances assessment in drinking waters from Brazil, France and Spain. Sci. Total Environ. 2016, 539, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Quinete, N.; Wu, Q.; Zhang, T.; Yun, S.H.; Moreira, I.; Kannan, K. Specific profiles of perfluorinated compounds in surface and drinking waters and accumulation in mussels, fish, and dolphins from southeastern Brazil. Chemosphere 2009, 77, 863–869. [Google Scholar] [CrossRef]
- Russell, M.H.; Berti, W.R.; Buck, R.C. Investigation of the Biodegradation Potential of a Fluoroacrylate Polymer Product in Aerobic Soils. Environ. Sci. Technol. 2008, 42, 800–807. [Google Scholar] [CrossRef]
- Mak, Y.L.; Taniyasu, S.; Yeung, L.W.Y.; Lu, G.; Jin, L.; Yang, Y.; Lam, P.K.S.; Kannan, K.; Yamashita, N. Perfluorinated Compounds in Tap Water from China and Several Other Countries. Environ. Sci. Technol. 2009, 43, 4824–4829. [Google Scholar] [CrossRef]
- Ghisi, R.; Manzetti, S. Accumulation of perfluorinated alkyl substances (PFAS) in agricultural plants: A review. Environ. Res. J. 2019, 169, 326–341. [Google Scholar] [CrossRef]
- Bartolomé, M.; Gallego-picó, A.; Cutanda, F.; Huetos, O.; Esteban, M.; Pérez-Gómez, B.; Castaño, A. Perfluorinated alkyl substances in Spanish adults: Geographical distribution and determinants of exposure. Sci. Total Environ. 2017, 603–604, 352–360. [Google Scholar] [CrossRef]
- Boiteux, V.; Dauchy, X.; Bach, C.; Colin, A.; Hemard, J.; Sagres, V.; Rosin, C.; Munoz, J. Science of the Total Environment Concentrations and patterns of perfluoroalkyl and polyfluoroalkyl substances in a river and three drinking water treatment plants near and far from a major production source. Sci. Total Environ. 2017, 583, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Loos, R.; Wollgast, J.; Huber, T. Polar herbicides, pharmaceutical products, perfluorooctanesulfonate (PFOS), perfluorooctanoate (PFOA), and nonylphenol and its carboxylates and ethoxylates in surface and tap waters around Lake Maggiore in Northern Italy. Anal. Bioanal. Chem. 2007, 387, 1469–1478. [Google Scholar] [CrossRef] [PubMed]
- Lindstrom, A.B.; Strynar, M.J.; Libelo, E.L. Polyfluorinated compounds: Past, present, and future. Environ. Sci. Technol. 2011, 45, 7954–7961. [Google Scholar] [CrossRef] [PubMed]
- Gellrich, V.; Brunn, H.; Stahl, T. Perfluoroalkyl and polyfluoroalkyl substances (PFASs) in mineral water and tap water. J. Environ. Sci. Health Part A Toxic/Hazard. Subst. Environ. Eng. 2013, 48, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Horst, J.; Mcdonough, J.; Ross, I.; Dickson, M.; Miles, J.; Hurst, J.; Storch, P. Advances in Remediation Solutions Water Treatment Technologies for PFAS: The Next Generation, Gr. Water Monit. Remediat. 2018, 38. [Google Scholar] [CrossRef]
- Zhang, X.; Lohmann, R.; Dassuncao, C.; Hu, X.C.; Weber, A.K.; Vecitis, C.D.; Sunderland, E.M. Source Attribution of Poly- and Perfluoroalkyl Substances (PFASs) in Surface Waters from Rhode Island and the New York Metropolitan Area. Environ. Sci. Technol. Lett. 2016, 3, 316–321. [Google Scholar] [CrossRef]
- Egeghy, P.P.; Judson, R.; Gangwal, S.; Mosher, S.; Smith, D.; Vail, J.; Cohen, E.A. The exposure data landscape for manufactured chemicals. Sci. Total Environ. 2012, 414, 159–166. [Google Scholar] [CrossRef]
- Schultz, M.M.; Higgins, C.P.; Huset, C.A.; Luthy, R.G.; Barofsky, D.F.; Field, J.A. Fluorochemical mass flows in a municipal wastewater treatment facility. Environ. Sci. Technol. 2006, 40, 7350–7357. [Google Scholar] [CrossRef]
- Yin, T.; Chen, H.; Reinhard, M.; Yi, X.; He, Y.; Gin, K.Y.H. Perfluoroalkyl and polyfluoroalkyl substances removal in a full-scale tropical constructed wetland system treating landfill leachate. Water Res. 2017, 125, 418–426. [Google Scholar] [CrossRef]
- Bolan, N.; Sarkar, B.; Yan, Y.; Li, Q.; Wijesekara, H.; Kannan, K.; Tsang, D.C.W.; Schauerte, M.; Bosch, J.; Noll, H.; et al. Remediation of poly-and perfluoroalkyl substances (PFAS) contaminated soils–To mobilize or to immobilize or to degrade? J. Hazard. Mater. 2021, 401. [Google Scholar] [CrossRef]
- Lee, H.; Tevlin, A.G.; Mabury, S.A.; Mabury, S.A. Fate of Polyfluoroalkyl Phosphate Diesters and Their Metabolites in Biosolids-Applied Soil: Biodegradation and Plant Uptake in Greenhouse and Field Experiments. Environ. Sci. Technol. 2014, 48. [Google Scholar] [CrossRef] [PubMed]
- Karnjanapiboonwong, A.; Deb, S.K.; Subbiah, S.; Wang, D.; Anderson, T.A. Perfluoroalkylsulfonic and carboxylic acids in earthworms (Eisenia fetida): Accumulation and effects results from spiked soils at PFAS concentrations bracketing environmental relevance. Chemosphere 2018, 199, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Lechner, M.; Knapp, H. Carryover of Perfluorooctanoic Acid (PFOA) and Perfluorooctane Sulfonate (PFOS) from Soil to Plant and Distribution to the Different Plant Compartments Studied in Cultures of Carrots (Daucus carota ssp. Sativus), Potatoes (Solanum tuberosum), and cucumbers (Cucumis sativus). J. Agric. Food Chem. 2011, 11011–11088. [Google Scholar] [CrossRef]
- Stahl, T.; Heyn, J.; Thiele, H.; Hüther, J.; Failing, K.; Georgii, S.; Brunn, H. Carryover of Perfluorooctanoic Acid (PFOA) and Perfluorooctane Sulfonate (PFOS) from Soil to Plants. Arch. Environ. Contam. Toxicol. 2009, 57, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Chen, H.; Yuan, R.; Wang, F.; Chen, Z.; Zhou, B. Toxicity of perfluorinated compounds to soil microbial activity: Effect of carbon chain length, functional group and soil properties. Sci. Total Environ. 2019, 690, 1162–1169. [Google Scholar] [CrossRef] [PubMed]
- Qiao, W.; Xie, Z.; Zhang, Y.; Liu, X.; Xie, S.; Huang, J.; Yu, L. Perfluoroalkyl substances (PFASs) influence the structure and function of soil bacterial community: Greenhouse experiment. Sci. Total Environ. 2018, 642, 1118–1126. [Google Scholar] [CrossRef]
- Cai, Y.; Chen, H.; Yuan, R.; Wang, F.; Chen, Z.; Zhou, B. Metagenomic analysis of soil microbial community under PFOA and PFOS stress. Environ. Res. 2020, 188, 109838. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, T.; Peng, X.; Wang, P.; Lu, Y. Bacterial community compositions in sediment polluted by perfluoroalkyl acids (PFAAs) using Illumina high-throughput sequencing. Environ. Sci. Pollut. Res. 2016, 23, 10556–10565. [Google Scholar] [CrossRef]
- Li, B.; Bao, Y.; Xu, Y.; Xie, S.; Huang, J. Vertical distribution of microbial communities in soils contaminated by chromium and perfluoroalkyl substances. Sci. Total Environ. 2017, 599–600, 156–164. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Q.; Cai, Y.; Yuan, R.; Wang, F.; Zhou, B.; Chen, Z. Effect of perfluorooctanoic acid on microbial activity in wheat soil under different fertilization conditions. Environ. Pollut. 2020, 264, 114784. [Google Scholar] [CrossRef]
- Perkola, N.; Sainio, P. Survey of perfluorinated alkyl acids in Finnish effluents, storm water, landfill leachate and sludge. Environ. Sci. Pollut. Res. 2013, 20, 7979–7987. [Google Scholar] [CrossRef] [PubMed]
- Busch, J.; Ahrens, L.; Sturm, R.; Ebinghaus, R. Polyfluoroalkyl compounds in landfill leachates. Environ. Pollut. 2010, 158, 1467–1471. [Google Scholar] [CrossRef] [PubMed]
- Robey, N.M.; da Silva, B.F.; Annable, M.D.; Townsend, T.G.; Bowden, J.A. Concentrating Per- And Polyfluoroalkyl Substances (PFAS) in Municipal Solid Waste Landfill Leachate Using Foam Separation. Environ. Sci. Technol. 2020, 54, 12550–12559. [Google Scholar] [CrossRef] [PubMed]
- Dauchy, X.; Boiteux, V.; Colin, A.; Hémard, J.; Bach, C.; Rosin, C.; Munoz, J.F. Deep seepage of per-and polyfluoroalkyl substances through the soil of a firefighter training site and subsequent groundwater contamination. Chemosphere 2019, 214, 729–737. [Google Scholar] [CrossRef]
- Høisæter, Å.; Pfaff, A.; Breedveld, G.D. Leaching and transport of PFAS from aqueous film-forming foam (AFFF) in the unsaturated soil at a firefighting training facility under cold climatic conditions. J. Contam. Hydrol. 2019, 222, 112–122. [Google Scholar] [CrossRef]
- Gao, Y.; Liang, Y.; Gao, K.; Wang, Y.; Wang, C.; Fu, J.; Wang, Y.; Jiang, G.; Jiang, Y. Levels, spatial distribution and isomer profiles of perfluoroalkyl acids in soil, groundwater and tap water around a manufactory in China. Chemosphere 2019, 227, 305–314. [Google Scholar] [CrossRef]
- Wang, Y.; Fu, J.; Wang, T.; Liang, Y.; Pan, Y.; Cai, Y.; Jiang, G. Distribution of perfluorooctane sulfonate and other perfluorochemicals in the ambient environment around a manufacturing facility in china. Environ. Sci. Technol. 2010, 44, 8062–8067. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, H.; Yu, Y.; Xie, L.; Li, J.; Wang, X.; Dong, W. Perfluorinated Compounds (PFCs) in Soil of the Pearl River Delta, China: Spatial Distribution, Sources, and Ecological Risk Assessment. Arch. Environ. Contam. Toxicol. 2020, 78, 182–189. [Google Scholar] [CrossRef]
- Chen, S.; Jiao, X.C.; Gai, N.; Li, X.J.; Wang, X.C.; Lu, G.H.; Piao, H.T.; Rao, Z.; Yang, Y.L. Perfluorinated compounds in soil, surface water, and groundwater from rural areas in eastern China. Environ. Pollut. 2016, 211, 124–131. [Google Scholar] [CrossRef]
- Dalahmeh, S.; Tirgani, S.; Komakech, A.J.; Niwagaba, C.B.; Ahrens, L. Per-and polyfluoroalkyl substances (PFASs) in water, soil and plants in wetlands and agricultural areas in Kampala, Uganda. Sci. Total Environ. 2018, 631–632, 660–667. [Google Scholar] [CrossRef]
- Armstrong, D.L.; Lozano, N.; Rice, C.P.; Ramirez, M.; Torrents, A. Temporal trends of perfluoroalkyl substances in limed biosolids from a large municipal water resource recovery facility. J. Environ. Manag. 2016, 165, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Herzke, D.; Olssonb, E.; Posner, S. Perfluoroalkyl and polyfluoroalkyl substances (PFASs) in consumer products in Norway—A pilot study. Chemosphere 2012, 88, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Clarke, B.O.; Anumol, T.; Barlaz, M.; Snyder, S.A. Investigating landfill leachate as a source of trace organic pollutants. Chemosphere 2015, 127, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Benskin, J.P.; Li, B.; Ikonomou, M.G.; Grace, J.R.; Li, L.Y. Per-and Polyfl uoroalkyl Substances in Landfill Leachate: Patterns, Time Trends, and Sources. Environ. Sci. Technol. 2012, 46, 11532–11540. [Google Scholar] [CrossRef]
- Eggen, T.; Moeder, M.; Arukwe, A. Municipal landfill leachates: A signifi cant source for new and emerging pollutants. Sci. Total Environ. 2010, 408, 5147–5157. [Google Scholar] [CrossRef]
- Fuertes, I.; Gómez-Lavín, S.; Elizalde, M.P.; Urtiaga, A. Perfluorinated alkyl substances (PFASs) in northern Spain municipal solid waste landfill leachates. Chemosphere 2017, 168, 399–407. [Google Scholar] [CrossRef]
- Huset, C.A.; Barlaz, M.A.; Barofsky, D.F.; Field, J.A. Quantitative determination of fluorochemicals in municipal landfill leachates. Chemosphere 2011, 82, 1380–1386. [Google Scholar] [CrossRef]
- Garg, S.; Kumar, P.; Mishra, V.; Guijt, R.; Singh, P.; Dumée, L.F.; Sharma, R.S. A review on the sources, occurrence and health risks of per-/poly-fluoroalkyl substances (PFAS) arising from the manufacture and disposal of electric and electronic products. J. Water Process Eng. 2020, 38, 101683. [Google Scholar] [CrossRef]
- Loos, R.; Carvalho, R.; Anto, D.C.; Locoro, G.; Tavazzi, S.; Paracchini, B.; Ghiani, M.; Lettieri, T.; Blaha, L.; Jarosova, B.; et al. EU-wide monitoring survey on emerging polar organic contaminants in wastewater treatment plant effluents. Water Res. 2013, 7, 6475–6487. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, W.L.; Liang, Y.N. Adsorption of perfluoroalkyl and polyfluoroalkyl substances (PFASs) from aqueous solution—A review. Sci. Total Sci. Total Environ. 2020, 748, 142354. [Google Scholar] [CrossRef]
- Xiao, F. Emerging poly-and perfluoroalkyl substances in the aquatic environment: A review of current literature. Water Res. 2017, 124, 482–495. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Herrera, V.; Sierra-Alvarez, R. Removal of perfluorinated surfactants by sorption onto granular activated carbon, zeolite and sludge. Chemosphere 2008, 72, 1588–1593. [Google Scholar] [CrossRef]
- Tang, H.; Xiang, Q.; Lei, M.; Yan, J.; Zhu, L.; Zou, J. Efficient degradation of perfluorooctanoic acid by UV–Fenton process. Chem. Eng. J. 2012, 184, 156–162. [Google Scholar] [CrossRef]
- Gorenflo, A.; Veliizquez-Padon, D.; Frimmel, F.H. Nanofiltration of a German groundwater of high hardness and NOM content: Performance and costs. Desalination 2002, 151, 253–265. [Google Scholar] [CrossRef]
- Hori, H.; Yamamoto, A.R.I. Efficient Decomposition of Environmentally Persistent Perfluorocarboxylic Acids by Use of Persulfate as a Photochemical Oxidant. Environ. Sci. Technol. 2005, 39, 2383–2388. [Google Scholar] [CrossRef] [PubMed]
- Vecitis, C.D.; Wang, Y.; Cheng, J.I.E.; Park, H.; Mader, B.T. Sonochemical Degradation of Perfluorooctanesulfonate in Aqueous Film-Forming Foams. Environ. Sci. Technol. 2010, 44, 432–438. [Google Scholar] [CrossRef]
- Franke, V.; McCleaf, P.; Lindegren, K.; Ahrens, L. Efficient removal of per-And polyfluoroalkyl substances (PFASs) in drinking water treatment: Nanofiltration combined with active carbon or anion exchange. Environ. Sci. Water Res. Technol. 2019, 5, 1836–1843. [Google Scholar] [CrossRef]
- Zhang, D.; Luo, Q.; Gao, B.; Chiang, S.D.; Woodward, D.; Huang, Q. Sorption of perfluorooctanoic acid, perfluorooctane sulfonate and perfluoroheptanoic acid on granular activated carbon. Chemosphere 2016, 144, 2336–2342. [Google Scholar] [CrossRef]
- Li, J.; Li, Q.; Li, L.; Xu, L. Removal of perfluorooctanoic acid from water with economical mesoporous melamine-formaldehyde resin microsphere. Chem. Eng. J. 2017, 320, 501–509. [Google Scholar] [CrossRef]
- Du, Z.; Deng, S.; Chen, Y.; Wang, B.; Huang, J.; Wang, Y.; Yu, G. Removal of perfluorinated carboxylates from washing wastewater of perfluorooctanesulfonyl fluoride using activated carbons and resins. J. Hazard. Mater. 2015, 286, 136–143. [Google Scholar] [CrossRef]
- Gao, Y.; Deng, S.; Du, Z.; Liu, K.; Yu, G. Adsorptive removal of emerging polyfluoroalky substances F-53B and PFOS by anion-exchange resin: A comparative study. J. Hazard. Mater. 2017, 323, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Franke, V.; Schäfers, D.; Lindberg, J.J.; Ahrens, L. Removal of per-and polyfluoroalkyl substances (PFASs) from tap water using heterogeneously catalyzed ozonation. Environ. Sci. Water Res. Technol. 2019, 5, 1887–1896. [Google Scholar] [CrossRef]
- Andres, V.; Espana, A.; Mallavarapu, M.; Naidu, R. Treatment technologies for aqueous perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA): A critical review with an emphasis on field testing. Environ. Technol. Innov. 2015, 4, 168–181. [Google Scholar] [CrossRef]
- Collings, A.F.; Gwan, P.B. Large scale environmental applications of high power ultrasound. Ultrason. Sonochem. 2010, 17, 1049–1053. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, N.A.; Rodriguez-Freire, L.; Keswani, M.; Sierra-Alvarez, R. Effect of chemical structure on the sonochemical degradation of perfluoroalkyl and polyfluoroalkyl substances (PFASs)†. Environ. Sci. Water Res. Technol. 2016, 975–983. [Google Scholar] [CrossRef]
- Zhao, L.; Bian, J.; Zhang, Y.; Zhu, L.; Liu, Z. Comparison of the sorption behaviors and mechanisms of perfluorosulfonates and perfluorocarboxylic acids on three kinds of clay minerals. Chemosphere 2014, 114, 51–58. [Google Scholar] [CrossRef]
- Zhang, R.; Yan, W.; Jing, C. Mechanistic study of PFOS adsorption on kaolinite and montmorillonite, Colloids Surfaces A Physicochem. Eng. Asp. 2014, 462, 252–258. [Google Scholar] [CrossRef]
- Wu, T.; Wu, Z.; Ma, D.; Xiang, W.; Zhang, J.; Liu, H.; Deng, Y.; Tan, S.; Cai, X. Fabrication of Few-Layered Porous Graphite for Removing Fluorosurfactant from Aqueous Solution. Langmuir 2018, 34, 15181–15188. [Google Scholar] [CrossRef]
- Chen, X.; Xia, X.; Wang, X.; Qiao, J.; Chen, H. A comparative study on sorption of perfluorooctane sulfonate (PFOS) by chars, ash and carbon nanotubes. Chemosphere 2011, 83, 1313–1319. [Google Scholar] [CrossRef]
- Zhang, Q.; Deng, S.; Yu, G.; Huang, J. Removal of perfluorooctane sulfonate from aqueous solution by crosslinked chitosan beads: Sorption kinetics and uptake mechanism. Bioresour. Technol. 2011, 102, 2265–2271. [Google Scholar] [CrossRef]
- Fagbayigbo, B.O.; Opeolu, B.O.; Fatoki, O.S. Adsorption of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) from water using leaf biomass (Vitis vinifera) in a fixed-bed column study. J. Environ. Health Sci. Eng. 2020, 18, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Stebel, E.K.; Pike, K.A.; Nguyen, H.; Hartmann, H.A.; Klonowski, M.J.; Lawrence, M.G.; Collins, R.M.; Hefner, C.E.; Edmiston, P.L. Absorption of short-chain to long-chain perfluoroalkyl substances using swellable organically modified silica. Environ. Sci. Water Res. Technol. 2019, 5, 1854–1866. [Google Scholar] [CrossRef]
- Qian, J.; Shen, M.; Wang, P.; Wang, C.; Hu, J.; Hou, J.; Ao, Y.; Zheng, H.; Li, K.; Liu, J. Co-adsorption of perfluorooctane sulfonate and phosphate on boehmite: Influence of temperature, phosphate initial concentration and pH. Ecotoxicol. Environ. Saf. 2017, 137, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Jian, J.M.; Zhang, C.; Wang, F.; Lu, X.W.; Wang, F.; Zeng, E.Y. Effect of solution chemistry and aggregation on adsorption of perfluorooctanesulphonate (PFOS) to nano-sized alumina. Environ. Pollut. 2019, 251, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Sunderland, E.M.; Hu, X.C.; Dassuncao, C.; Tokranov, A.K.; Wagner, C.C.; Allen, J.G. A review of the pathways of human exposure to poly-and perfluoroalkyl substances (PFASs) and present understanding of health effects. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Gebbink, W.A.; Berger, U.; Cousins, I.T. Estimating human exposure to PFOS isomers and PFCA homologues: The relative importance of direct and indirect (precursor) exposure Estimating human exposure to PFOS isomers and PFCA homologues: The relative importance of direct and indirect (precursor). Environ. Int. 2015, 74, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Skaar, J.S.; Ræder, E.M.; Lyche, J.L.; Ahrens, L.; Kallenborn, R. Elucidation of contamination sources for poly-and perfluoroalkyl substances (PFASs) on Svalbard (Norwegian Arctic). Environ. Sci. Pollut. Res. 2019, 26, 7356–7363. [Google Scholar] [CrossRef]
- Andersen, M.E.; Butenhoff, J.L.; Chang, S.; Farrar, D.G.; Kennedy, G.L.; Lau, C.; Olsen, G.W.; Seed, J.; Wallace, K.B. Perfluoroalkyl Acids and Related Chemistries—Toxicokinetics and Modes of Action. Toxicol. Sci. 2008, 102, 3–14. [Google Scholar] [CrossRef]
- Hoffman, K.; Webster, T.F.; Bartell, S.M.; Weisskopf, M.G.; Fletcher, T. Private Drinking Water Wells as a Source of Exposure to Perfluorooctanoic Acid Private Drinking Water Wells as a Source of Exposure to Perfluorooctanoic Acid (PFOA) in Communities Surrounding a Fluoropolymer Production Facility. Environ. Health Perspect. 2010, 119, 92–97. [Google Scholar] [CrossRef]
- Longpré, D.; Lorusso, L.; Levicki, C.; Carrier, R.; Cureton, P. PFOS, PFOA, LC-PFCAS, and certain other PFAS: A focus on Canadian guidelines and guidance for contaminated sites management. Environ. Technol. Innov. 2020, 18, 100752. [Google Scholar] [CrossRef]
- Seyoum, A.; Pradhan, A.; Jass, J.; Olsson, P. Perfluorinated alkyl substances impede growth, reproduction, lipid metabolism and lifespan in Daphnia magna. Sci. Total Environ. 2020, 737, 139682. [Google Scholar] [CrossRef] [PubMed]
- Skogheim, T.S.; Villanger, G.D.; Weyde, K.V.F.; Engel, S.M.; Surén, P.; Øie, M.G.; Skogan, A.H.; Biele, G.; Zeiner, P.; Øvergaard, K.R.; et al. Prenatal exposure to perfluoroalkyl substances and associations with symptoms of attention-deficit/hyperactivity disorder and cognitive functions in preschool children. Int. J. Hyg. Environ. Health 2020, 223, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Liang, H.; Tian, Y.; Yuan, W.; Xiao, H.; Hu, H.; Sun, X.; Song, X.; Wen, S.; Yang, L.; et al. Prenatal plasma concentrations of Perfluoroalkyl and polyfluoroalkyl substances and neuropsychological development in children at four years of age. Environ. Health 2019, 18, 53. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, C.; Kappleman, W.; DiGuiseppi, W. Ecological Considerations of Per-and Polyfluoroalkyl Substances (PFAS). Curr. Pollut. Reports. 2017, 3, 289–301. [Google Scholar] [CrossRef]
- Cordner, A.; College, W.; Ave, B.; Walla, W.; States, U.; Richter, L.; Brown, P. Can Chemical Class Approaches Replace Chemical-by-Chemical Strategies? Lessons from Recent U.S. FDA Regulatory Action on per-and Polyfluoroalkyl Substances. Environ. Sci. Technol. 2016, 50, 12584–12591. [Google Scholar] [CrossRef] [PubMed]
- Australian-Government. Health Based Guidance Values for PFAS; Australian-Government, Department of Health: Canberra, Australia, 2019.
| Header | Å. Høisæter, et al. [71] ng/g PFASS in Soil at Various Depths | Cai et al. [63] ng/g Dry Weight in | Chen et al. [69] ng/g Dry Weight | Cai et al. [61] ng/g Dry Weight | Gao et al. [72] ng/g Dry Weigh from 32 Samples | Wang et al. [73] The Mean Values ng/g Dry Weight | Liu et al. [74] ng/g Dry Weight | Chen et al. [75] ng/g Dry Weight | Dalahmeh et al. [76] ng/g Dry Weight | Armstrong et al. [77] | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–1.0 m | 1–2 m | 2–3 m | 3–4 m | Dry soil in China | Tot.PFC 0.34–65.8 | PFAS in soil at varying distances | |||||||
| PFOS | 500–3000 | 1000–6500 | 1000–3500 | 1000–1200 | 130 | 70.5 | 8.6–10.4 | 0.06 | 2583, | 87 | 0–2 | 0.6–3 | 23 |
| PFOA | NA | NA | NA | NA | NA | 93 | 3.3–47.5 | 0.32 | 50 | 0.3–8 | 63 | 0.5–0.9 | 24 |
| PFHxS | NA | NA | NA | - | NA | 61 | NA | 0.19 | 36 | 65 | |||
| ∑PFCs | NA | NA | NA | NA | NA | - | NA | - | NA | 99 | NA | 8 | 126–809 |
| PFHxA | NA | NA | NA | NA | NA | NA | NA | 0.09 | NA | NA | NA | 0.2–0.5 | 8 |
| PFAA | NA | NA | NA | NA | NA | NA | NA | 1.30–913 | NA | NA | NA | NA | NA |
| PFBS | NA | NA | NA | NA | NA | NA | NA | 0.05 | NA | NA | NA | NA | NA |
| Header | Gallen et al. [22] (ng L−1) | Busch et al. [68] (ng L−1) | Herzke et al. [78] (ng L−1) | Clarke et al. [79] (ng L−1) | Yin et al. [55] (ng L−1) | Benskin et al. [80] (ng L−1) | Eggen et al. [81] (ng L−1) | Robey et al. [69] (ng L−1) | Fuertes et al. [82] (ng L−1) | Eggen et al. [81] (ng L−1) | Huset et al. [83] (ng L−1) | Garg et al. [84] (ng L−1) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| landfill (>50% SW) | landfill (>50% C&D) | Compounds in landfill leachates | Coated textiles, Teflon waste, fire-fighting foam, papers, and furniture | Leachate from CW outlet system (Max. level) | Municipal landfill leachate | Municipal land fill leachates | Foam produced via the bubble aeration of landfill leachate | Raw Leachate in MSW landfill | Treated Leachate in MSW landfill | PFCs analysis-untreated leachate Water | PFCs analysis -untreated leachate Particles | Leachates from six landfill | Manufacture and disposal of electric and electronic products | ||
| PFOS | 300 | 1100 | 235 | 570 | 187 | 439 | 4400 | 2920 | 104 | 25 | NA | 2920 | 34 | 56–160 | 128,670 |
| PFOA | 510 | 1200 | 926 | 9500 | 516 | 3457 | 1500 | 767 | 951 | 590 | 520 | 767 | 4 | 380–1100 | 118.3 |
| PFHxS | 940 | 3700 | 178 | - | 143 | 308 | 190 | 281 | 2058 | 630 | 870 | 281 | ND* | 120–700 | 133,330 |
| PFDS | - | - | - | NA | 0.72 | 63 | <14 | ND | - | - | 0–16 | - | |||
| PFHxA | 1300 | 5000 | 2509 | - | 697 | 868 | 2500 | 757 | 2178 | 65 | 77 | 757 | ND | 270–2200 | 76 |
| PFHpA | 360 | 760 | 280 | NA | 486 | 690 | 277 | 454 | - | - | 277 | ND | 100–2800 | 9 | |
| PFNA | 29 | 98 | 80 | - | 62 | 100 | 450 | 539 | 64 | - | - | 539 | ND | 19–140 | 8 |
| PFDA | 22 | 46 | 51 | - | NA* | 27 | 1100 | 75 | 87 | - | - | 70 | ND | 0.3–64 | 8 |
| PFAA | NA | NA | NA | - | - | 55 | - | - | - | - | - | - | - | - | - |
| PFBS | 1350 | NA | 112 | 1916 | 190 | - | - | - | - | <5 | ND | 280–2300 | - | ||
| PFPeA | 11 | 325 | - | - | - | ||||||||||
| Mechanism | Treatment Process |
|---|---|
| Destructive Treatment | Advance oxidation processes |
| Electrochemical oxidation | |
| Incinerations | |
| Sono-chemical | |
| Biodegradation | |
| Photolysis | |
| Non-Destructive treatment | Adsorption |
| Ion exchange | |
| Fractionation |
| Process | Treatment Mechanism | Operation Conditions | Performance | References |
|---|---|---|---|---|
| UV-Fenton | Oxidation | 30.0 mM of H2O2, 2.0 mM of Fe2+, pH 3.0. and 9 W UV lamp (max = 254 nm) | >95% PFOA destruction from 8.2 mg/L and defluorination efficiency of 53.2% | [88] |
| Oxidation | 30.0 mM of H2O2, 2.0 mM of Fe2+, pH 3.0. and 9 W UV lamp (max = 254 nm) | PFOA treatment >95% from 8.2 mg/L while defluorination effectiveness = 53% | Removal efficiency 100% (PFOA 559 mg/L) | [90] |
| Oxidation | Light-activated persulfate at 50 mM & radiation of 4 h of | Removal efficiency 100% (PFOA 559 mg/L) | 73% removal efficiency of PFOS throughout 120 min | [91] |
| Sonolysis | PFOS level from 65 μg/L to 13,100 μg/L) were treated through ultrasonic at frequency 505 kHz and power density 187.5 W/L). | 73% removal of PFOS within 120 min | 55–98% removals for different analyzed PFASs. Ozonation can create potentially toxic transformation products | [92] |
| Oxidation | Tested for 18 analyzed PFASs3 h of ozonation | 55–98% removals for different analyzed PFASs. Ozonation can create potentially toxic transformation products which needs to be investigated in future research. | Adsorption capacity 41.3 mg/g of PFOA and 72.2 mg/g of PFOS | [93] |
| Adsorption | 10 mg/L of PFOA; surface area: 534 m2/g; time of equilibrium 24 h; pH 5 | Adsorption capacity 41.3 mg/g of PFOA and 72.2 mg/g of PFOS | Adsorption capacity 510 mg/g of PFOA | [94] |
| Adsorption | 700 mg/L of PFOA; surface area: 1539 m2/g; time of equilibrium 24 h; pH 7 | Adsorption capacity 510 mg/g of PFOA | Adsorption capacity 166 mg/g of PFHxA | [95] |
| Ion exchange using IRA 67 | Particle size: 3–1.2 mm; 120 mg/L of PFHxA; time of equilibrium 12.5 h; pH 4 | Adsorption capacity 166 mg/g of PFHxA | Adsorption capacity 2390 mg/g mg/g of PFOS | [96] |
| Ion exchange using IRA 67 | Particle size: 3–1.2 mm; 200 mg/L of PFOS; time of equilibrium 20 h; pH 3 | Adsorption capacity 2390 mg/g mg/g of PFOS |
| Sorbent | Adsorbate | Operation Conditions | Adsorption Capacity | References |
|---|---|---|---|---|
| Clay minerals (surface area: 67.52 m2/g) | PFOS | Initial concentration of adsorbent 400 mg/L; pH7; concentration of adsorbate 0.2 mg/L | 0.29–0.31 mg/g | [101] |
| Kaolinite (surface area: 11.9 m2/g) | PFOS | Initial concentration of adsorbent 5000 mg/L; pH7; concentration of adsorbate 0.95 mg/L | 0.08 mg/g | [102] |
| Alumina (surface area: 88.6 m2/g) | PFOA | Initial concentration of adsorbent 10000 mg/L; pH4.3; concentration of adsorbate 0.1 mg/L | 0.16 ×10−3 mg/g | [63] |
| Porous graphite (surface area: 2870 m2/g) | PFOS | Initial concentration of adsorbent 100 mg/L; pH5; concentration of adsorbate 100 mg/L | 1240 mg/g | [103] |
| Biochar from maize straw (surface area: 7.21 m2/g) | PFOS | Initial concentration of adsorbent 200–1200 mg/L; pH7; concentration of adsorbate 100 mg/L | 91.6 mg/g | [104] |
| Chitosan (surface area: 2870 m2/g) | PFOS | Initial concentration of adsorbent 1350 mg/L; pH3; concentration of adsorbate 50 mg/L | 645 mg/g | [105] |
| Zeolite) NaY80 surface area, 780 m2/g | PFOS | Initial concentration of adsorbent 1000 mg/L; concentration of adsorbate 150 mg/L, Particle size: 3–1.2 mm | 114.7 mg/g | [87] |
| Activated carbon from leaf biomass | PFOA PFOS | Modified activated carbons (AC-H3PO4) produced from leaf, uniform particle size of ˃64 μm | 159.61 mg/g 208.64 mg/g | [106] |
| Modified silica | PFOS | Surface area 650 m2/g, Particle size: 250–450 µm, Pore volume: 1.03 mL/g | 55 mg/g | [107] |
| Boehmite | PFOS | Surface area 299 m2/g, Average particle size 37.02 µm | 0.1529 µg/m2 | [108] |
| Alumina nanoparticles | PFOS | Surface area: 83 m2/g, Particle size: 13 nm | At 30 °C: 589 mg/g, at 40 °C: 485 mg/g, at 50 °C: 447 mg/g | [109] |
| Sources | Exposure Pathways | Receptors |
|---|---|---|
| Industrial and wastewater effluents Packaging Consumer products Landfills Fire-fighting foams | Soil Biosolids Dust Sediment Surface water Groundwater Drinking water Biota (including foods) | Ecological Aquatic Benthic Terrestrial Avian Human |
| Guideline | Advisory Level | Reference Dose | ||
|---|---|---|---|---|
| PFAO (ng/L) | PFOS (ng/L) | PFAO (ng/kg-Day) | PFOS (ng/kg-Day) | |
| U.S. EPAa, 2016, Health Advisory Level | 70 | 70 | 20 | 20 |
| Alaska DECb, 2016, Groundwater cleanup level | 400 | 400 | 20 | 20 |
| Maine DEPb, 2016, Remedial action guideline | 130 | 560 | 6 | 80 |
| Minnesota DOH, 2017, Noncancer health-based level | 35 | 27 | 18 | 5.1 |
| New Jersey DEP, 2017, Maximum contaminant level | 14 | 13 | 2 | 1.8 |
| North Carolina DENRb, 2012, Interim maximum allowable concentration | 1000 | - | N/A | NA |
| Texas CEQb, 2017, Protective concentration level | 290 | 560 | 15 | 20 |
| Vermonta DEC/DOH,6 2016, Primary groundwater enforcement standard | 20 | 20 | 20 | 20 |
| PFAS Name | Acronym | Drinking Water Screening Value (ng/L) |
|---|---|---|
| perfluorobutanoate | PFBA | 30 |
| perfluorobutane sulfonate | PFBS | 15 |
| perfluorohexanesulfonate | PFHxS | 0.6 |
| perfluoropentanoate | PFPeA | 0.2 |
| perfluorohexanoate | PFHxA | 0.2 |
| perfluoroheptanoate | PFHpA | 0.2 |
| perfluorononanoate | PFNA | 0.02 |
| fluorotelomer sulfonate | 6:2 FTS | 0.2 |
| fluorotelomer sulfonate | 8:2 FTS | 0.2 |
| Health Based Guideline Value | PFOS and PFHxS (ng) | PFOA (ng) | PFAO (ng) |
|---|---|---|---|
| Tolerable daily intake | 20 | 160 | 0.16 |
| Guideline for drinking water quality | 70 | 560 | 0.56 |
| Guideline value for Recreational water quality | 2000 | 10,000 | 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abunada, Z.; Alazaiza, M.Y.D.; Bashir, M.J.K. An Overview of Per- and Polyfluoroalkyl Substances (PFAS) in the Environment: Source, Fate, Risk and Regulations. Water 2020, 12, 3590. https://doi.org/10.3390/w12123590
Abunada Z, Alazaiza MYD, Bashir MJK. An Overview of Per- and Polyfluoroalkyl Substances (PFAS) in the Environment: Source, Fate, Risk and Regulations. Water. 2020; 12(12):3590. https://doi.org/10.3390/w12123590
Chicago/Turabian StyleAbunada, Ziyad, Motasem Y. D. Alazaiza, and Mohammed J. K. Bashir. 2020. "An Overview of Per- and Polyfluoroalkyl Substances (PFAS) in the Environment: Source, Fate, Risk and Regulations" Water 12, no. 12: 3590. https://doi.org/10.3390/w12123590
APA StyleAbunada, Z., Alazaiza, M. Y. D., & Bashir, M. J. K. (2020). An Overview of Per- and Polyfluoroalkyl Substances (PFAS) in the Environment: Source, Fate, Risk and Regulations. Water, 12(12), 3590. https://doi.org/10.3390/w12123590







