Abstract
Extreme weather events are projected to increase in frequency and intensity as climate change continues. Heterotrophic bacteria play a critical role in lake ecosystems, yet little research has been done to determine how they are affected by such extremes. The purpose of this study was to use high-throughput sequencing to explore the bacterial community composition of a humic oligotrophic lake on the North Atlantic Irish coast and to assess the impacts on composition dynamics related to extreme weather events. Samples for sequencing were collected from Lough Feeagh on a fortnightly basis from April to November 2018. Filtration was used to separate free-living and particle-associated bacterial communities and amplicon sequencing was performed for the 16S rRNA V4 region. Two named storms, six high discharge events, and one drought period occurred during the sampling period. These events had variable, context-dependent effects on bacterial communities in Lough Feeagh. The particle-associated community was found to be more likely to respond to physical changes, such as mixing, while the free-living population responded to changes in nutrient and carbon concentrations. Generally, however, the high stability of the bacterial community observed in Lough Feeagh suggests that the bacterial community is relatively resilient to extreme weather events.
Keywords:
extreme weather event; storm; drought; bacteria; free-living; particle-associated; humic lake 1. Introduction
The frequency and intensity of extreme weather events are anticipated to increase as global temperatures rise and climates continue to change [,,]. These events can have profound impacts on freshwater systems which provide numerous ecological services, including provision of drinking water, carbon cycling, and the preservation of biodiversity []. Extreme weather events can include heatwaves, droughts, storms, and high precipitation events with resulting floods. There is no strict standard for determining when a weather event is extreme. The Intergovernmental Panel on Climate Change (IPCC) has defined an extreme weather event based on ‘values above or below a threshold near the upper (or lower) ends (‘tails’) of the range of observed values of that variable’ []. Such definitions will result in differing threshold values dependent on location. In the limnological context, extreme weather events can cause disturbances in the stability of the thermal structure of lakes, as well as changes in nutrient loadings and concentrations [,]. High precipitation events and increased flow events can lead to increases in allochthonous dissolved organic matter (commonly quantified as dissolved organic carbon (DOC)) and nutrients [,]. Additionally, these changes in lake stability and nutrient concentrations can ultimately lead to biological changes [].
Freshwater lakes contain distinct bacterial populations that play a critical role in biogeochemical cycles, including carbon, nitrogen, and phosphorus cycling. Large fractions of organic carbon in lakes are respired and consumed by microorganisms [] and can then be accessed by higher trophic levels through the food web []. Studies of high precipitation events in lakes have found decreases in primary production and increases in respiration following these events [,,], increasing the availability of particulate and dissolved organic matter (DOM) for bacterial communities. Stratified lakes harbor distinct bacterial communities in different water layers [,] and mixing of the water column can, therefore, lead to disruptions across the water column. However, studies from lakes in Taiwan [,], found that bacterial communities were able to rapidly return to pre-storm conditions after individual typhoon-induced mixing events. This suggests that bacterial communities can be predictable and resilient even after more extreme weather events.
Peatlands are a vital long-term sink for carbon in terrestrial systems []. Disruptions in these systems, including those related to weather extremes, can cause peatlands to become a carbon source and climate change has the potential to threaten the delicate balance between net primary productivity (NPP) and decay []. High flow events increase the loading of both particulate and dissolved carbon to lakes in these humic systems [], which are lakes with a high amount of humic material that leads to a brown color and generally lower pH. Microbial dynamics in a small number of humic lakes have been examined [,,,]. One of those studies, Linz et al. (2017) [], reported on bacterial dynamics over 5 years in several humic lakes in Wisconsin and found that seasonal and episodic lake mixing stimulated differential planktonic bacterial community dynamics. Such studies are scarcer in humic lakes in upland peat catchments such as those found on the western fringe of Europe, including Ireland. One study in Lough Feeagh did include the effects of extreme precipitation events []. Sparber et al. (2015) [] found that bacteria dominated the pelagic biomass and their biomass was significantly impacted by the flooding that resulted from an extreme precipitation event. This study used the DAPI (4′,6-diamidino-2-phenylindole) staining method to observe bacterial abundance but did not look at the dynamics of bacterial community composition.
In addition to the effects of local climate and subsequent in-lake changes, bacterial communities are also determined by lifestyle, with free-living bacteria showing different spatio-temporal dynamics from particle-associated bacteria [,,]. Particulate organic matter is composed of a range of organic material, including live or dead organisms that have turned into detritus [], or pollen entering the system in spring and summer []. The high concentration of bioavailable organic matter and highly heterogeneous environment associated with particles is favored by copiotrophic bacteria that rapidly colonize and exploit the nutrients via production of a plethora of extracellular enzymes. Free-living bacteria, in contrast, do not directly attach or exploit the particulate organic matter but may indirectly benefit from the release of small, mainly dissolved organic molecules from the particle as it is decomposed []. The nature of particulate and DOM fractions influences the specific nature of the microbial communities present, which in turn can impact how much of the total organic matter pool is remineralized and how much is exported to the sediments []. There are, however, no high throughput sequencing studies that simultaneously focus on the impact of extreme, episodic weather events on the dynamics of both free-living and particle-associated bacterial fractions in humic systems, especially not in Ireland.
Extreme weather events are common in the west of Ireland. A recent 13-year study of Lough Feeagh by Andersen et al. (2020) [] found that, on average, there were four storms per year at Lough Feeagh during the stratified period (May–September), with at least one storm occurring every year. It was also notable that the threshold wind speed value (top 2.5%) for a storm at this coastal site was 10.6 m s−1, much higher than that reported for example in mainland Europe []. There is limited research on the bacterial communities in peatland catchments on the Atlantic fringe of Europe. Changes to these communities could disrupt the microbial loop [] and ultimately impact ecosystem services. Understanding the impacts of extreme weather events on bacterial communities can aid in the application of future management strategies. In the current study, we aimed to explore the response of bacterial communities to extreme weather events in Lough Feeagh. Specifically, we describe the community composition for both particulate and free-living bacteria in Lough Feeagh over the period from April to November 2018 and identify the role of 1) extreme weather events and 2) changes in lake conditions, including shifts in nutrient concentrations and physical variables, on the bacterial community composition over this period.
2. Materials and Methods
2.1. Site Description and High Frequency Monitoring
Lough Feeagh is the largest freshwater lake in the Burrishoole catchment, located on the west coast of Ireland in Co. Mayo (Figure 1). Lough Feeagh has a surface area of 3.95 km2, a maximum depth of 45 m, and an average depth of 14.5 m. It has an average retention time of 172 days []. Two sub-catchments serve as the main inflows for Lough Feeagh, the Black and Glenamong rivers. Lough Feeagh also has two outflows to the south, the Mill Race and the Salmon Leap, which cut through the terminal moraine that separates Lough Feeagh from the brackish Lough Furnace. Lough Feeagh is a humic lake due to low levels of nutrients and primary production but high levels of dissolved organic carbon (DOC) and color. Blanket bog comprises 64% of land cover in the catchment. Commercial afforestation covers 23% of the catchment while the remaining area is comprised of agricultural land, natural grasslands, and native oak woodlands [].
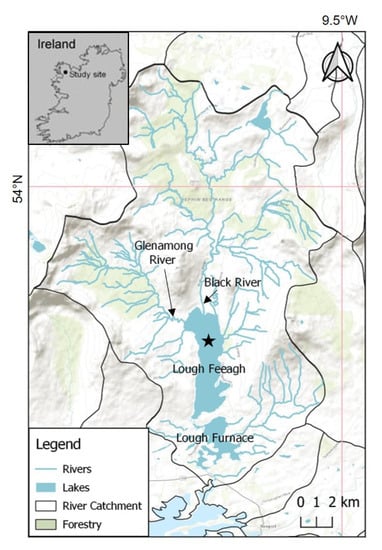
Figure 1.
Map of Burrishoole catchment. The star on Lough Feeagh indicates the sample site (Esri base map []).
An Automatic Water Quality Monitoring Station (AWQMS) is situated at the deepest point of Lough Feeagh (Coordinates: 53.95 N, 9.58 W) [] and is maintained by the Marine Institute (MI). This station collects high-frequency sensor information every 2 min, which is transmitted back to the research station via GPRS (http://burrishoole.marine.ie). A multiparameter probe (Hydrolab Data Sonde 5x, OTT Hydromet, UK) at the AWQMS is suspended in open water at 0.9 m and measures dissolved oxygen (DO) and temperature. The AWQMS sensors are cleaned fortnightly and the sonde is calibrated once a month. Vertical temperature profiles were measured with 12 platinum resistance thermometers (PRTs: Labfacility PT100 1/10DIN 4 wire sensor, www.labfacility.co.uk, Labfacility Ltd., Bognor Regis, UK) at 2.5, 5, 8, 11, 14, 16, 18, 20, 22, 27, 32, and 42 m. An anemometer (Vector Instruments A100L2-WR, Windspeed Ltd., UK) on the AWQMS measured wind speed.
Automatic River Monitoring Stations (ARMS) are located on the Black River (Coordinates: 53.97 N, 9.58 W) and Glenamong River. A sensor measuring chromorphic dissolved organic carbon (CDOM) fluorescence, a proxy for DOC concentration, was situated on the Black River ARMS (Seapoint CDOM UV fluorometer (http://www.seapoint.com/suvf.htm; Seapoint, Exeter, NH 03833, USA)). The fluorometer output in mV was corrected for the fluorescence reading when deployed in distilled deionized water and then corrected for the temperature quenching effect [,]. Water level was measured on the Glenamong River every 15 min (Coordinates: 53.97 N, 9.58 W) using a data logger (Orpheus mini, OTT Hydromet, Germany). The water level was converted to stream flow using an established rating curve. Black River discharge was estimated using the drainage area ratio method [] and based on the Glenamong River discharge. Minimum and maximum air temperature were obtained from the Met Éireann automatic weather station located on the shore of Lough Feeagh (Coordinates: 53.92 N, 9.58 W) [].
The sums of stream discharge data from both the Black and Glenamong Rivers were used to define extreme flow events as any date when the daily combined discharge was greater than the 90th percentile for the study period from April 17 to November 12, 2018. The change in CDOM fluorescence during the rising limb (the difference between the level at the peak of the hydrograph and the level on the date prior to the start of the event []) was used to define whether each extreme flow event was associated with an increase or decrease in the stream DOM concentration. These are referred to as either DOM flushing events (where concentration increased and more DOM was exported from the catchment at peak flow relative to the pre-event conditions) or DOM dilution events (where concentration declined, and less DOM was exported at peak flow relative to the pre-event conditions) (Table 1). Negative values indicated dilution events and positive values indicated flushing events.

Table 1.
Weather events that took place between mid-April and mid-November 2018. Dissolved organic matter (DOM) Dilution/Flushing were defined based on the concentrations of DOM in the Black River.
2.2. Sample Collection
Water samples were collected from Lough Feeagh fortnightly from mid-April to mid-November 2018 during daylight hours (10:00–16:00 h). The lake samples were collected next to the AWQMS. Integrated water samples were collected over the lake surface (0 m) to 6 m depth using a plastic tube (length: 6 m, diameter: 70 mm) for nutrient analyses. Three acid-washed 10 L HDPB bottles were pre-rinsed with lake water and 30 L of lake water were collected and placed in the bottles. Surface water samples (1000 mL) were collected for sequencing and were also placed in pre-rinsed bottles. All water samples were kept in the dark at 4 °C until further analysis (within 24–48 h of collection).
2.3. Chlorophyll a, Nutrient Analyses, and Schmidt Stability
Chlorophyll a (Chl a) analysis was performed on 2 L samples from the integrated water sample that were filtered through GF/F filters, followed by ethanol extraction and measurement carried out in triplicate at 665 and 750 nm on a spectrophotometer (UV-1800, Shimadzu, Germany) []. The analysis for total phosphorus (TP) was carried out separately in triplicate on unfiltered water following a persulfate oxidation digestion method and analyzed using the molybdenum blue method with absorbance read at 882 nm (UV-1800, Shimadzu, Germany) []. Ammonia (NH4-N) and nitrate (NO3-N) measurements were taken within 24 h and were carried out on 0.45 µm membrane filtered samples. Analyses for ammonia (NH4-N) were done following the salicylate method [] and absorbance was measured at 662 nm on a spectrophotometer (UV-1800, Shimadzu, Germany) []. Nitrate (NO3-N) concentrations were determined by ion chromatography [] using a Dionex® ICS-2000 system (electrochemical suppressed conductivity system with an anion exchange column). NH4-N and NO3-N measurements were carried out in triplicate.
Dissolved organic carbon (DOC) was analyzed on the integrated water samples filtered through 0.45 µm membrane filters and analyzed following a persulfate oxidation under UV illumination using a total organic carbon (TOC) analyzer (Sievers® TOC Analyzer 5310C, Sievers Instruments Inc, USA). Color was measured from the same samples that were filtered for DOC analysis with a HACH Dr 2000 spectrophotometer at 455 nm. Total suspended solids (TSS) were measured by filtering water samples through GF/C filters, drying the filters for 24 h, and weighing the filters.
The high-frequency data collected on the AWQMS were used to calculate Schmidt stability. A QA/QC was first applied to account for data gaps, calibration periods, or any sensor problems []. Data was then transformed from sub-daily (recorded every two minutes) to daily averages using the subdaily2daily function from the hydroTSM package in R []. Schmidt stability was calculated using lake water temperature profile data, Lough Feeagh bathymetry, and wind speed as input files to the rLakeAnalyzer package in R [].
2.4. Filtration, DNA Extraction, and Sequencing
The surface water samples (1000 mL) were successively filtered through 5.0 µm polycarbonate membranes for particle-associated bacteria, and through 0.22 µm membrane filters for free-living bacteria []. The filters were stored at −80 °C until further analysis.
DNA extraction was performed based on Nercessian et al. (2005) []. Cell lysis made using small (100–500 um) glass beads in the presence of lysozyme, proteinase K and cetyltrimethyl ammonium bromide (CTAB). DNA purification was facilitated by the addition of Phenol-Chloroform and polyethylene glycol (PEG). Amplification of the 16S rRNA V4 (515F/806R) region, library preparation and sequencing were carried out at Molecular Research Laboratories (Mr. DNA), Shallowater, TX, USA, on an Illumina MiSeq instrument.
2.5. Bioinformatics and Statistical Analyses
Demultiplexing, removal of primer and adapter sequences was performed using Cutadapt []. Additional quality filtering and trimming, formation of contiguous sequences and identification of unique amplicon sequence variants (ASVs) was performed using the DADA2 pipeline in R []. Taxonomy was assigned to ASVs using the TaxAss workflow [] incorporating the Freshwater Microbial Field Guide (https://github.com/McMahonLab/FWMFG, accessed on August 2019) and SILVA 132 release taxonomy [].
Alpha and beta diversity analyses were performed using PRIMER6 []. Specifically, raw ASVs were subjected to Hellinger transformation, and Bray–Curtis similarities visualized as a Principal Coordinate Analysis (PCoA). The PCoA was carried out to examine variability between samples. Distance-based linear modelling was performed using normalized environmental variables, and significant variables visualized via dbRDA plots. The dbRDA was carried out to compare the bacterial communities to different environmental variables (including precipitation, wind speed, DO, color, DOC, NO3-N, NH4-N, TP, Chl a, Schmidt stability, and TSS). Coefficient of variation (CV) analysis was performed for abundant taxa at the family level [].
3. Results
3.1. Weather Event Characteristics
The study year 2018 was an unprecedented year for Lough Feeagh and its catchment due to an exceptional heat wave and an abnormally dry summer. Two named storms (Hector and Ali), one drought period, and six high-discharge events occurred during the study period (Table 1). The first high-discharge event was on May 1 (Figure 2A), with an average of 5.88 m3 s−1 of discharge flowing from the Black river. A second high-discharge event occurred on May 20 and 21. The peak discharge from the Black river during this event was 6.02 m3 s−1. The lake also began to stratify in May, causing the Schmidt stability to increase (Figure 2D). The drought period began on May 22 and was interrupted by Storm Hector on June 13. During this first half of the drought period, the Schmidt stability increased from 92.8 to 548.3 J m−2. Storm Hector then arrived, bringing wind speeds up to 8.89 m s−1 (Figure 2C) and a total of 64 mm of rain over a seven-day period, with 33.2 mm falling on June 19 (Figure 2A). Storm Hector caused a disturbance in Schmidt stability, which decreased from 453.6 J m−2 on June 12 to 208.9 J m−2 on June 22. Storm Hector was followed by the second half of the drought period, which continued until July 13. Peaks in air temperature, 29.8 °C (Figure 2B), and Schmidt stability, 669.4 J m−2 (Figure 2D), were recorded during this period. The next high-discharge event occurred on August 1, with a peak in discharge of 11.42 m3 s−1 flowing from the Black River. Another precipitation and discharge event occurred on August 17 and 18 with 56.3 mm of total precipitation over the two days and a peak in discharge of 10.43 m3 s−1. The Schmidt stability began to decrease in August and continued to decrease until the lake was fully mixed in September. The second named storm, Ali, then arrived on September 17. A total of 52.6 mm of rain fell over the three days of September 17, 18, and 19, with 28.8 mm of rain falling on the first day (Figure 2A), and wind speeds reached up to 9.51 m s−1 (Figure 2C). The lake was almost fully mixed when Storm Ali arrived and was totally mixed by September 19. The event from October 7 to 9 brought 47.2 mm of rain and wind speeds up to 10.33 m s−1. This precipitation and discharge event had the highest levels of discharge of the whole sampling period, with 30.2 m3 s−1 of discharge coming from the Black River (Figure 2A). The final high discharge event occurred on November 2 and 3, with a peak in discharge of 19.32 m3 s−1 flowing from the Black River.
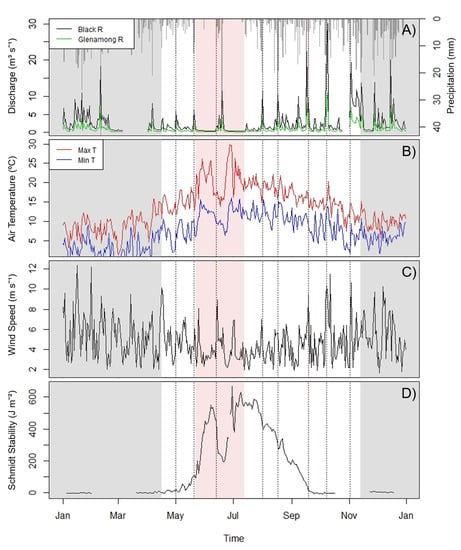
Figure 2.
Environmental variables for the entire year of 2018: (A) the discharge of the inflow rivers and daily precipitation, (B) minimum and maximum daily air temperature, (C) wind speed, and (D) Schmidt stability. Shaded grey areas indicate dates outside the study period. Dotted vertical lines represent weather events. The pale red shaded area indicates the drought period.
Based on the data from the CDOM sensor on the Black River, the largest inflow to Lough Feeagh, the four precipitation events from May 1 to early August were flushing events, that is, the CDOM fluorescence levels increased on the rising limb of the peak in discharge (Table 1). In contrast, the four events from August 17 to 18 to the end of the study period on November 12 were dilution events, that is, the CDOM fluorescence decreased in the Black River on the rising limb.
3.2. Nutrient Dynamics
Of the dates sampled, the DOC concentrations were highest (12.18 mg DOC L−1) on May 1 (Figure 3A), the same day a weaker weather event occurred that had a peak discharge of 5.88 m3 s−1 from the Black River (Table 1). The DOC concentrations decreased by the next sample date (May 15) to 7.54 mg DOC L−1 and remained lower through the first half of the drought period and through Storm Hector. They then increased again on June 26, during the second half of the drought period, to 11.02 mg DOC L−1 (Figure 3A). The DOC concentrations were lower in July (8.13 to 8.83 mg L−1) and remained generally higher from August to September (10.32 to 11.47). The DOC concentrations were then lower again from October onwards, ranging from 8.15 to 9.48 mg L−1. Water color was between 60 and 75 mg Pt L−1 from mid-April to July 24. In August, the color levels began to increase and reached 104 mg Pt L−1 on October 1 and 105 mg Pt L−1 on October 30 (Figure 3A).
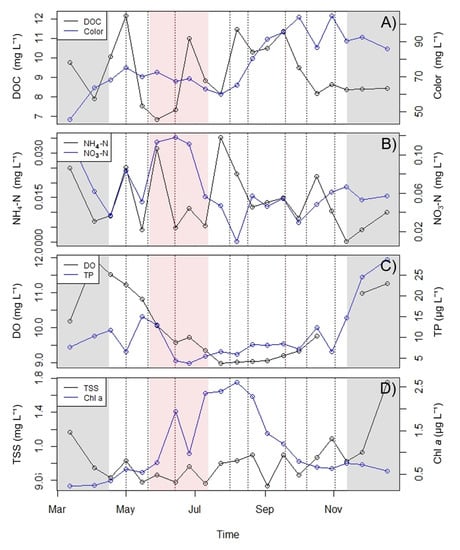
Figure 3.
Nutrients recorded in 2018: (A) Lough Feeagh DOC and color, (B) Lough Feeagh average NH4-N and NO3-N, (C) average daily dissolved oxygen (DO) and total phosphorus (TP), and (D) total suspended solids (TSS) and Chlorophyll a (Chl a). Shaded grey areas indicate dates outside the study period. Dotted vertical lines represent weather events. The pale red shaded area indicates the drought period.
Throughout the study period, NH4-N concentrations were relatively low and there were only four peaks above 0.015 mg L−1 (Figure 3B). The first peak was 0.025 mg L−1 on May 1, the same date of the first high-discharge event. The next peak was 0.031 mg L−1 on May 28, during the first half of the drought period. The third peak was the highest at 0.035 mg L−1 on 24 July and the final peak was the lowest at 0.022 mg L−1 on October 17 (Figure 3B). NO3-N concentrations were between 0.036 and 0.084 mg L−1 from April 17 to May 15 (Figure 3B). Concentrations then increased to 0.113 mg L−1 on May 28 and remained between 0.111 and 0.118 mg L−1 until June 26. NO3-N concentrations then dropped to 0.056 mg L−1 on July 10 and, with the exception of dropping below the limit of detection on August 7, were between 0.029 and 0.067 mg L−1 until the end of the study period (Figure 3B).
Total phosphorus (TP) concentrations decreased on the date of the first discharge event on May 1 to 6.52 µg L−1 from 11.76 µg L−1 on April 17 (Figure 3C). The TP concentration then peaked on May 15 with 14.98 µg L−1. After this peak, the TP concentration dropped to its lowest point of 3.70 µg L−1 on June 26. The TP then remained between 5.52 and 8.49 µg L−1 from July 10 to October 1 until peaking again on October 17 with 12.31 µg L−1 and 14.62 µg L−1 on November 12 (Figure 3C). Lough Feeagh dissolved oxygen (DO) concentrations remained between 8.97 and 11.51 mg L−1 during the study period (Figure 3C). DO was highest at the beginning of the study period when the lake was fully mixed, with a concentration of 11.51 mg L− on April 17. The DO concentrations then began to decrease and reached 9.57 mg L−1 by June 14. Following Storm Hector, there was a slight increase in the DO concentration on June 26 to 9.71 mg L−1 (Figure 3C). The lowest DO concentration was reached on July 24, at 8.97 mg L−1. Concentrations remained low and began to increase in September as the lake began to mix (Figure 3C).
The concentrations of total suspended solids (TSS) in Lough Feeagh were low throughout the sample period, ranging from 0.533 to 1.09 mg L−1 (Figure 3D). TSS concentrations were lowest on September 3 with 0.533 mg L−1, and peaked on October 30 with 1.089 mg L−1. Chl a concentrations were relatively low and followed a similar trend to Schmidt stability (Figure 3D). From the beginning of the sample period the Chl a increased until it reached a peak on June 14, during Storm Hector (1.93 µg L−1). After the storm, Chl a concentrations dropped to 0.98 µg L−1 and then increased again, peaking at 2.61 µg L−1 on August 7. Chl a then gradually decreased for the rest of the sample period (Figure 3D).
3.3. Bacterial Community Composition
3.3.1. Free-Living
The free-living bacterial population in Lough Feeagh generally contained large stable proportions of the LD12 clade (alfV), and of the Actinobacteria clades acI and acIV (Figure 4 and Figure 5E). In the first half of the study period, bacterial communities were more variable while the populations were more consistent after July (Figure 4). On May 4, there was a decrease in the proportion of Actinobacter clades acI and acIV and LD12 and an increase in the proportion of the Betaproteobacteria clades betI, betII, and betIII, and Patescibacteria. This shift occurred after a May 1 discharge event and peaks in TSS and NH4-N. By May 15, communities once again resembled those sampled on May 1. On May 28, there was a decrease in LD12 and Actinobacteria clades acI and acIV. There was an increase in the Patescibacteria, bacI, and betII populations. This population shift coincided with a peak in NH4-N concentrations. This shift also occurred during the first drought period (May 22–June 12). The free-living population then returned to pre-disturbance conditions and remained relatively stable until July 24. On July 24, a shift in the population was characterized by a decrease in LD12 and an increase in the Actinobacter clades acI and acIV. This population change coincided with a peak in NH4-N on July 24. By the next sample date, the relative abundances returned to pre-disturbance abundances and remained stable for the remainder of the sample period. The free-living bacterial community did not appear to be impacted by weather events that occurred in the late summer and autumn.
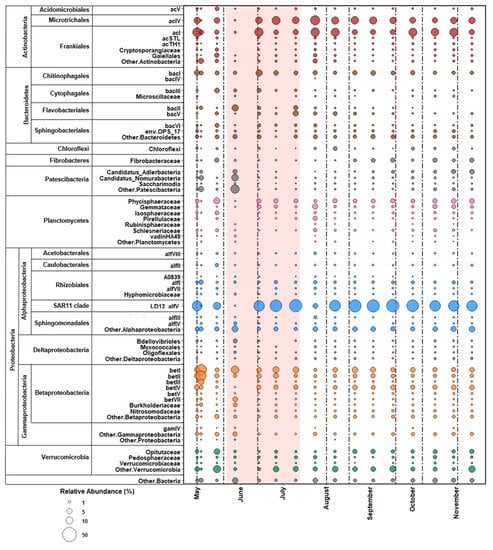
Figure 4.
Bubble plot of the free-living bacterial abundance in Lough Feeagh. Dotted vertical lines indicate the onset of weather events. The pale red shaded area indicates the drought period.
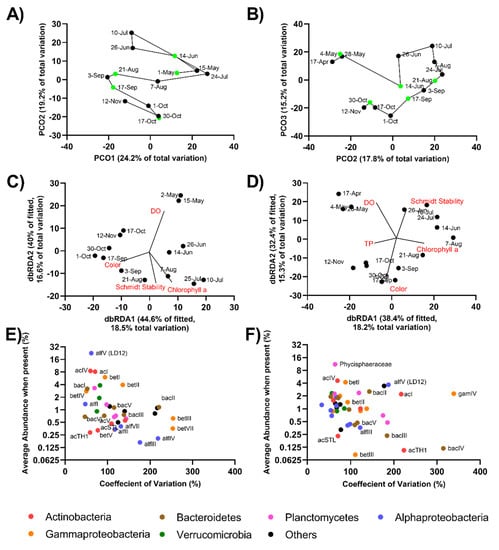
Figure 5.
Temporal dynamics of free-living (A,C,E) and particle-associated (B,D,F) communities in Lough Feeagh. (A,B) Principal Coordinate analysis depicting Bray–Curtis similarities (green points represent weather events (Table 1)). (C,D) Distance-based linear models depicting the relative contribution of environmental variables to the respective bacterial community composition. (E,F) Relative abundance of freshwater families as a function of their coefficient of variation.
A distinct seasonality in the free-living community was observed in the PCO and dbRDA plots (Figure 5A,C). A seasonality in the correlation between bacteria and environmental factors was also seen (Figure 5C). The major environmental variables associated with the free-living community were color, DO, Chl a, and Schmidt stability. The May 4 and May 28 samples were removed from the PCO and dbRDA plots in order to avoid skewing what might be the “normal” community distribution. DO appeared to be the main driver in spring (May), Chl a and Schmidt stability were associated with samples taken in summer and early autumn (June and September), and color was correlated with samples taken in later in autumn (mid-September and October).
3.3.2. Particle-Associated
The particle-associated bacterial population in Lough Feeagh contained a large stable proportion of Planctomycetes, specifically Phycisphaeraceae (Figure 5F and Figure 6). On May 1 there was an increase in the proportion of Bacteroidetes clade bacII, Candidatus Adlerbacteria, Candidatus Nomurabacteria, Betaproteobacteria clades betI, betII, and betIV, and Gammaproteobacteria clade gamIV populations. There were decreases in the relative abundances of Phycispaeriaceae and Isophaeraceae. The May 1 shift coincided with a small peak in river discharge, with an average water inflow of 5.89 m3 s−1 from the Black River versus 0.61 m3 s−1 from the previous day, April 30. The relative abundances returned to proportions similar to the April 17 sample date, by the next sample date (May 28). Similar population shifts to those observed for the May 1 sample were also seen for the June 14 sample, which was taken after Storm Hector made landfall, with wind speeds of 6.37 m s−1 on June 13 and 8.89 m s−1 on June 14. After this shift, the populations returned to pre-disturbance conditions by the next sample date, two weeks later, and remained relatively stable for the remainder of the study period.
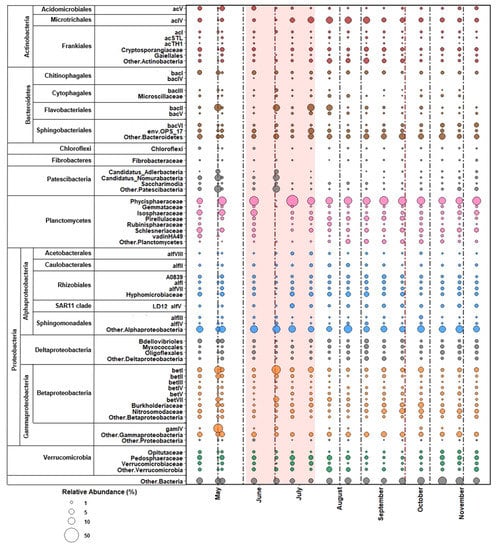
Figure 6.
Bubble plot of the particle-associated bacterial abundance in Lough Feeagh. Dotted vertical lines indicate the onset of weather events. The pale red shaded area indicates the drought period.
As with the free-living community, a seasonality could be seen in the particle-associated community in the PCO and dbRDA plots (Figure 5B,D). The environmental variables correlated with the particle-associated community were DO, Schmidt stability, Chl a, color, and TP concentration (Figure 5D). The May 1 sample was removed from the ordination plots to avoid skewing what might be the distribution of the “normal” community. DO was the main environmental factor correlated with the spring samples (April and May), Schmidt stability was correlated with the summer (June and July), Chl a was correlated with late summer/early autumn samples (August), color was correlated with autumn samples (September and October), and TP was correlated with late autumn samples (November).
4. Discussion
Understanding the responses of the lake biota, including bacterial communities, to extreme weather events will be critical for the future management of lakes and the ecosystem services they provide. We have shown that the bacterial community in a humic lake on the Atlantic coast of Europe appeared to be relatively resistant to the effects of storms. This potential resistance was apparent in both the free-living and particle-associated communities, however, there was a high degree of variability in the responses from the bacterial communities to the different weather events that occurred during the study period. Additionally, there was a seasonality observed in both the free-living and particle-associated communities, with more variability in both communities in the spring and early summer than in the autumn.
This study is the first to explore the microbial community of Lough Feeagh. The bacterial phylum with the highest relative abundance in the free-living population was LD12. This was not surprising as the alfV clade has been described as the most abundant member of Alphaproteobacteria in several freshwater lakes [,]. LD12, acI, and acIV also had stable populations in the free-living community. The most variable groups were betIII and betVII. In the particle-associated community, which appeared to be less variable than the free-living community overall, Phycisphaeraceae (Planctomycetes) had the highest relative abundance and was one of the most stable populations. The Gammaproteobacteria clade gamIV was one of the most variable populations in the particle-associated fraction, with relatively higher abundances when present. This clade is generally associated with terrestrial environments and was likely flushed into Lough Feeagh after higher precipitation events. Actinobacteria, Proteobacteria, and Bacteroidetes have previously been described as abundant phyla in humic lakes [,]. Limnohabitans (betI) and Polynucleobacter (betII) are also common freshwater bacteria [] and were relatively abundant when present and stable in both free-living and particle-associated fractions. One study with samples from a bog lake over a five-year period [] was unable to detect repeatable annual trends in the bacterial community composition over the study period. Because the bacterial community composition of Lough Feeagh has not been previously explored, it is impossible to know if the community seen during the study period was similar to other years.
The bacterial communities of Lough Feeagh exhibited seasonality in their composition. The environmental variables correlated with the both the free-living and particle-associated bacterial populations of Lough Feeagh were DO, Chl a, Schmidt stability, and color, while the particle-associated samples were also correlated with TP. The availability of DO was associated with samples taken in the spring, when DO was highest and the lake was fully mixed and slowly began to stratify []. High values for Chl a and Schmidt stability defined the bacterial community in the summer, when the lake was unusually stable and phytoplankton biomass reached a relatively high peak []. Color was correlated with the samples taken in the autumn. At this time of the year, color was highest, and rainfall and river discharge were more constant. TP was particularly correlated with particle-associated samples taken toward the end of the study period but was not correlated with the free-living samples. TP was highest at the beginning and end of the study period, when the lake was fully mixed and there was higher terrestrial input of phosphorus, allowing more phosphorus to be available to the bacteria on the surface of the lake. It is important to note that TP could also include phosphorus from bacterial biomass, as well as particulate matter. Three outlying dates were excluded from the dbRDA analysis in order to avoid skewing what might be the “normal” environmental drivers. For the free-living population, this was May 4 and May 28, and for the particle-associated population, it was May 1. May 1 and 4 samples were taken after a May 1 high discharge event that led to increases in NH4-N and NO3-N. The May 28 date was during the first half of the drought period and was also associated with peaks in NH4-N and NO3-N. These increases in nitrogen sources may have driven the shifts in the bacterial populations on those dates. It was particularly notable that the period when there was the greatest change in the community, i.e., earlier summer, coincided with a time when increases in discharge led to a flushing of DOM from the catchment area into the lake. Conversely, the time during which the bacterial community composition was more stable coincided with the period when high stream discharge diluted the organic matter in the streams. A similar seasonal pattern for CDOM fluorescence has been reported for other years in this peatland catchment [,] and from other upland peat sites []. The allochthonous carbon from the catchment basin most likely originates from long-term peat stores, and therefore will be less labile than carbon from, for example, algal biomass. However, a substantial portion of it can be also available to the bacterial community and therefore can stimulate growth [], although we note that color, a proxy for DOC concentrations, was only significantly increased in the lake in autumn.
Beyond these seasonal dynamics, shifts in bacterial community composition were observed in relation to nine “extreme” weather events during this study period. These events consisted of two named storm events (high wind speed and precipitation), six high discharge events, and one drought period. The year 2018 was also extreme for Lough Feeagh due to a heatwave [] and increased Schmidt stability compared to earlier years []. Previously, Jones et al. (2008) [] found that the bacterial community in a lake in Taiwan was surprisingly resilient to disturbances caused by typhoons. A similar potential resilience to disturbances was also observed in the bacterial communities in Lough Feeagh in the current study. Interestingly, disturbance events earlier in the year were more likely to result in shifts in both the free-living and particle-associated bacterial community composition. The first weather event occurred on May 1 and was characterized by high discharge, peaks in DOC, color, NH4-N, and NO3-N concentrations. Shifts in particle-associated bacterial community composition were defined by decreases in the relative abundances of Phycisphaeraceae and Isophaeraceae and increases in Bacteroidetes clade bacII, Candidatus Adlerbacteria, Candidatus Nomurabacteria, Betaproteobacterales clades betI, betII, and betIV, and Gammaproteobacteria clade gamIV. Decreases in the typically phytoplankton-associated Phycisphaeraceae [] as well as increases in TSS and terrestrially linked gamIV [] suggest that allochthonous particles might have been flushed into the lake from the surrounding peatland. We only observed an impact on the free-living bacterial community on May 4, which was notable as there appeared to be a delay in the response following the May 1 discharge event. On this date (May 4), we observed similar increases in betI and betII taxa, as was observed for the particle-associated community, suggesting that terrestrial runoff in parallel with allochthonous particles may have seeded these free-living taxa, at least in the short term. We also observed increases in free-living Patescibacteria and clade betIII, which were not dominant in the particle-associated community but may have benefited from the provision of dissolved inorganic nutrients that increased during this period. Both the particle-associated and free-living communities had returned to pre-disturbance conditions by the next sample date, May 15.
Following the drought conditions from May 22 to June 12 (the first half of the drought period), elevated temperatures resulted in the onset of stratification within Lough Feeagh. Around this time (May 28), we observed a disturbance in the relative abundance of the free-living bacteria despite no clear attributable weather event. On May 28, decreases in the abundance of LD12 and Actinobacteria clades acI and acIV and increases in Patescibacteria, bacII, and betI, were similar to what was observed in early May, coinciding once again with a peak in ammonium concentrations. In the absence of an external perturbation it is possible that such a shift might be due to the onset of the stratification itself creating unstable conditions for plankton communities in the lake, resulting in some lysis and release of ammonium which then stimulated a subset of the free-living bacterial community, however, this is speculative. Previously, Shade et al. (2010) [] found a significant relationship between the Schmidt stability of a lake in Taiwan and the differences in bacterial communities in the epilimnion and hypolimnion. Calderó et al. (2020) [] saw a decrease in the biovolume of Cyanobacteria and an increase in diatoms in Lough Feeagh on May 28 in the same study year. Both the high stability and the changes in phytoplankton biomass presumably have also influenced the shift in the free-living bacterial community.
Throughout the sampling period, there were three mixing events associated with extreme weather. Of these, only the first event, Storm Hector, which made landfall on June 13, appeared to significantly impact the microbial community composition of Lough Feeagh. Storm Hector brought high winds on June 13 and 14, reaching up to 8.89 m s−1. This caused a mixing event, disrupting the high stability that had been observed during the onset of the drought period. There was a shift in the relative abundances of particle-associated bacteria on June 14. The population shift was similar to the shift seen on May 1, with relative decreases in Planctomycetes groups (Phycisphaeraceae and Isophaeraceae) and increases in Patescibacteria. While the relative abundances of the free-living population did not appear to be immediately impacted by this storm event, a disruption of the seasonality of the PCO suggests that the community may have seen some disturbance. Extreme weather events and mixing events have been found to trigger shifts in lake processes, including biological processes [,]. Calderó et al. (2020) [] also described a cascade effect observed after Storm Hector in Lough Feeagh in 2018 and it is likely that the free-living bacterial population was impacted as well. Following Storm Hector, the second half of the drought period continued until July 14 and was characterized by the highest temperatures of the study period, up to 29.8 °C, and high stability. There was a shift in the relative abundances of free-living bacteria on July 24, characterized by a decrease in LD12. This shift also coincided with a peak in phytoplankton []. This was a last major shift in the relative abundances of the bacterial communities observed during the study period.
With a single exception (Storm Hector), following an extreme weather event or another induced disturbance, the bacterial communities in Lough Feeagh were able to recover to pre-disturbance states by the next sampling period, approximately two weeks later. The Lough Feeagh populations also appeared to be relatively resistant to some disturbance events, with no obvious shifts in relative abundances occurring. While shifts in relative abundances were not observed, absolute bacterial abundances were not characterized and, therefore, may have shifted without impacting the relative abundances. There were varying lake conditions surrounding each weather event, including nutrient dynamics and physical conditions (i.e., stability). Because of this variability, it is difficult to determine the exact drivers of the bacterial population shifts without applying higher sampling frequencies. The variability in lake conditions also led to a lack of repetition, making it impossible to accurately predict how the bacterial communities would respond to future weather events. A 2018 study by Martin-Platero et al. [] explored 93 consecutive days of time series data that revealed almost daily fluctuations in ASVs as well as rapid turnover in bacterial communities. Unlike this study, Lough Feeagh samples were taken fortnightly, and it is, therefore, unknown how quickly the communities were able to recover from disturbances. It is also possible that responses to weather events later in the study period were delayed and consequently were not captured by our sampling frequency. Giling et al., 2016 [], demonstrated that whole-ecosystem metabolism could be impacted by extreme weather events even weeks after stratification was re-established. This suggests that the impacts of these events can be long-lasting and, even with fortnightly sampling, we could still expect to see changes in the bacterial community after weather events. It should also be noted that this study utilized surface water samples, and therefore any impacts to bacteria deeper in the lake were not characterized.
This study adds to the limited knowledge of bacterial community dynamics in humic lakes. In addition, it gives us new insight into changes in bacterial community composition related to extreme weather events. While some shifts in the bacterial community composition of free-living and particle-associated bacteria were seen earlier in the study period, the communities appeared to be relatively resilient to extreme weather events. This was, however, an extreme year at Lough Feeagh that included a heatwave and higher than average stability in addition to the extreme weather events. These extremes, coupled with the variability of the weather events, make it difficult to predict how the microbial communities would respond to future disturbance events. More studies on Lough Feeagh and elsewhere utilizing next-generation sequencing are necessary to help distinguish the “normal” bacterial community dynamics and seasonal shifts from those driven by short-term extreme weather events. Such studies could also explore bacterial communities at depth as well as determine the mechanisms of community shifts triggered by disturbance events. More insight into how these events impact communities and ecosystem services can ultimately aid in the application of management strategies in future climate scenarios.
Author Contributions
This study was conceptualized by A.H., H.-P.G., V.M., E.d.E., E.G., and E.J. Data collection was carried out by E.G., M.C.-P., E.d.E., and M.D. Laboratory analyses were carried out by E.G. and M.C.-P. Physical metrics were computed by M.C.-P. and E.d.E. Bioinformatic analyses were carried out by A.H., J.W., and L.Z. The manuscript was written by A.H., revised by H.-P.G., V.M., E.d.E., and E.J., and approved by all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This project has received funding from the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement No 722518.
Acknowledgments
The authors wish to thank Allison Murdock, Patricia Antunes, and Solvig Pinnow for technical support and assistance in laboratory analyses.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, S.; McGrath, R.; Hanafin, J.; Lynch, P.; Semmler, T.; Nolan, P. The impact of climate change on storm surges over Irish waters. Ocean Model. Online 2008, 25, 83–94. [Google Scholar] [CrossRef]
- Lindner, M.; Maroschek, M.; Netherer, S.; Kremer, A.; Barbati, A.; Garcia-Gonzalo, J.; Seidl, R.; Delzon, S.; Corona, P.; Kolström, M.; et al. Climate change impacts, adaptive capacity, and vulnerability of European forest ecosystems. For. Ecol. Manag. 2010, 259, 698–709. [Google Scholar] [CrossRef]
- Frieler, K.; Lange, S.; Piontek, F.; Reyer, C.P.; Schewe, J.; Warszawski, L.; Zhao, F.; Chini, L.; Denvil, S.; Emanuel, K.; et al. Assessing the impacts of 1.5 °C global warming—Simulation protocol of the Inter-Sectoral Impact Model Intercomparison Project (ISIMIP2b). Geosci. Model. Dev. 2017, 10, 4321–4345. [Google Scholar] [CrossRef]
- Stockwell, J.D.; Doubek, J.P.; Adrian, R.; Anneville, O.; Carey, C.C.; Carvalho, L.; Domis, L.N.D.S.; Dur, G.; Frassl, M.A.; Grossart, H.; et al. Storm impacts on phytoplankton community dynamics in lakes. Glob. Change Biol. 2020, 26, 2756–2784. [Google Scholar] [CrossRef] [PubMed]
- Seneviratne, S.I.; Nicholls, N.; Easterling, D.; Goodess, C.M.; Kanae, S.; Kossin, J.; Luo, Y.; Marengo, J.; McInnes, K.; Rahimi, M.; et al. Changes in climate extremes and their impacts on the natural physical environment. In Managing the Risks of Extreme Events and Disasters to Advance Climate Change Adaptation. A Special Report of Working Groups I and II of the Intergovernmental Panel on Climate Change (IPCC); Field, C.B., Barros, V., Stocker, T.F., Qin, D., Dokken, D.J., Ebi, K.L., Mastrandrea, M.D., Mach, K.J., Plattner, G.K., Allen, et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2012; pp. 109–230. [Google Scholar]
- Jennings, E.; Jones, S.; Arvola, L.; Staehr, P.A.; Gaiser, E.; Jones, I.D.; Weathers, K.C.; Weyhenmeyer, G.A.; Chiu, C.Y.; De Eyto, E. Effects of weather-related episodic events in lakes: An analysis based on high-frequency data. Freshw. Biol. 2012, 57, 589–601. [Google Scholar] [CrossRef]
- Kasprzak, P.; Shatwell, T.; Gessner, M.O.; Gonsiorczyk, T.; Kirillin, G.; Selmeczy, G.; Padisák, J.; Engelhardt, C. Extreme weather event triggers cascade towards extreme turbidity in a clear-water lake. Ecosystems 2017, 20, 1407–1420. [Google Scholar] [CrossRef]
- Weyhenmeyer, G.E.; Willén, E.; Sonesten, L. Effects of an extreme precipitation event on water chemistry and phytoplankton in the Swedish Lake Mälaren. Boreal Env. Res. 2004, 9, 409–420. [Google Scholar]
- Sadro, S.; Melack, J.M. The effect of an extreme rain event on the biogeochemistry and ecosystem metabolism of an oligotrophic high-elevation lake. Arctic Antarct. Alp. Res. 2012, 44, 222–231. [Google Scholar] [CrossRef]
- Tranvik, L.J.; Downing, J.A.; Cotner, J.B.; Loiselle, S.A.; Striegl, R.G.; Ballatore, T.J.; Dillon, P.; Finlay, K.; Fortino, K.; Knoll, L.B. Lakes and reservoirs as regulators of carbon cycling and climate. Limnol. Oceanogr. 2009, 54, 2298–2314. [Google Scholar] [CrossRef]
- Azam, F.; Fenchel, T.; Field, J.; Gray, J.; Meyer-Reil, L.; Thingstad, T. The ecological role of water-column microbes in the sea. Mar. Ecol. Prog. Ser. 1983, 10, 257–263. [Google Scholar] [CrossRef]
- Foreman, C.M.; Wolf, C.F.; Priscu, J.C. Impact of episodic warming events on the physical, chemical and biological relationships of lakes in the McMurdo Dry Valleys. Antarct. Aquat. Geochem. 2004, 10, 239–268. [Google Scholar] [CrossRef]
- De Eyto, E.; Jennings, E.; Ryder, E.; Sparber, K.; Dillane, M.; Dalton, C.; Poole, R. Response of a humic lake ecosystem to an extreme precipitation event: Physical, chemical, and biological implications. Inland Waters 2016, 6, 483–498. [Google Scholar] [CrossRef]
- Jones, S.E.; Chiu, C.Y.; Kratz, T.K.; Wu, J.T.; Shade, A.; McMahon, K.D. Typhoons initiate predictable change in aquatic bacterial communities. Limnol. Oceanogr. 2008, 53, 1319–1326. [Google Scholar] [CrossRef]
- Garcia, S.L.; Salka, I.; Grossart, H.P.; Warnecke, F. Depth-discrete profiles of bacterial communities reveal pronounced spatio-temporal dynamics related to lake stratification. Environ. Microbiol. Rep. 2013, 5, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Shade, A.; Chiu, C.Y.; McMahon, K.D. Seasonal and episodic lake mixing stimulate differential planktonic bacterial dynamics. Microb. Ecol. 2009, 59, 546–554. [Google Scholar] [CrossRef]
- Yu, Z.C. Northern peatland carbon stocks and dynamics: A review. Biogeosciences 2012, 9, 5073–5107. [Google Scholar] [CrossRef]
- Wilson, R.M.; Hopple, A.M.; Tfaily, M.M.; Sebestyen, S.D.; Schadt, C.W.; Pfeifer-Meister, L.; Medvedeff, C.; McFarlane, K.J.; Kostka, J.E.; Kolton, M.; et al. Stability of peatland carbon to rising temperatures. Nat. Commun. 2016, 7, 1–10. [Google Scholar] [CrossRef]
- Ryder, E.; De Eyto, E.; Dillane, M.; Poole, R.; Jennings, E. Identifying the role of environmental drivers in organic carbon export from a forested peat catchment. Sci. Total. Environ. 2014, 490, 20–36. [Google Scholar] [CrossRef]
- Newton, R.J.; Kent, A.D.; Triplett, E.W.; McMahon, K.D. Microbial community dynamics in a humic lake: Differential persistence of common freshwater phylotypes. Environ. Microbiol. 2006, 8, 956–970. [Google Scholar] [CrossRef]
- Kent, A.D.; Yannarell, A.C.; Rusak, J.A.; Triplett, E.W.; McMahon, K.D. Synchrony in aquatic microbial community dynamics. ISME J. 2007, 1, 38–47. [Google Scholar] [CrossRef]
- Grossart, H.P.; Jezbera, J.; Horňák, K.; Hutalle, K.M.L.; Buck, U.; Šimek, K. Top-down and bottom-up induced shifts in bacterial abundance, production and community composition in an experimentally divided humic lake. Environ. Microbiol. 2008, 10, 635–652. [Google Scholar] [CrossRef] [PubMed]
- Linz, A.M.; Crary, B.C.; Shade, A.; Owens, S.; Gilbert, J.A.; Knight, R.; McMahon, K.D. Bacterial community composition and dynamics spanning five years in freshwater bog lakes. mSphere 2017, 2, e00169-17. [Google Scholar] [CrossRef] [PubMed]
- Sparber, K.; Dalton, C.; de Eyto, E.; Jennings, E.; Lenihan, D.; Cassina, F. Contrasting pelagic plankton in temperate Irish lakes: The relative contribution of heterotrophic, mixotrophic, and autotrophic components, and the effects of extreme rainfall events. Inland Waters 2015, 5, 295–310. [Google Scholar] [CrossRef]
- Allgaier, M.; Grossart, H.P. Seasonal dynamics and phylogenetic diversity of free-living and particle-associated bacterial communities in four lakes in northeastern Germany. Aquat. Microb. Ecol. 2006, 45, 115–128. [Google Scholar] [CrossRef]
- Rösel, S.; Allgaier, M.; Grossart, H.P. Long-term characterization of free-living and particle-associated bacterial communities in lake tiefwaren reveals distinct seasonal patterns. Microb. Ecol. 2012, 64, 571–583. [Google Scholar] [CrossRef]
- Grossart, H.P. Ecological consequences of bacterioplankton lifestyles: Changes in concepts are needed. Environ. Microbiol. Rep. 2010, 2, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.; Grossart, H.; Schweitzer, B.; Ploug, H. Microbial ecology of organic aggregates in aquatic ecosystems. Aquat. Microb. Ecol. 2002, 28, 175–211. [Google Scholar] [CrossRef]
- Rösel, S.; Rychła, A.; Wurzbacher, C.; Grossart, H.-P. Effects of pollen leaching and microbial degradation on organic carbon and nutrient availability in lake water. Aquat. Sci. 2011, 74, 87–99. [Google Scholar] [CrossRef]
- Smith, D.C.; Simon, M.; Alldredge, A.L.; Azam, F. Intense hydrolytic enzyme activity on marine aggregates and implications for rapid particle dissolution. Nature 1992, 359, 139–142. [Google Scholar] [CrossRef]
- Andersen, M.R.; De Eyto, E.; Dillane, M.; Poole, R.; Jennings, E. 13 years of storms: An analysis of the effects of storms on lake physics on the atlantic fringe of Europe. Water 2020, 12, 318. [Google Scholar] [CrossRef]
- Perga, M.-E.; Bruel, R.; Rodriguez, L.; Guénand, Y.; Bouffard, D. Storm impacts on alpine lakes: Antecedent weather conditions matter more than the event intensity. Glob. Change Biol. 2018, 24, 5004–5016. [Google Scholar] [CrossRef] [PubMed]
- Fealy, R.M.; Buckley, C.; Mechan, S.; Melland, A.; Mellander, P.E.; Shortle, G.; Wall, D.; Jordan, P. The Irish Agricultural Catchments Programme: Catchment selection using spatial multi-criteria decision analysis. Soil Use Manag. 2010, 26, 225–236. [Google Scholar] [CrossRef]
- Esri. Topographic [Basemap]. Scale Not Given. World Topographic Map. 2012. Available online: http://www.arcgis.com/home/item.html?id=30e5fe3149c34df1ba922e6f5bbf808f (accessed on 31 August 2020).
- De Eyto, E.; Dillane, M.; Cooney, J.; Hughes, P.; Murphy, M.; Nixon, P.; Sweeney, D.; Poole, R.; Rouen, M. Water Quality and Meteorological Data from the Lough Feeagh Automatic Water Quality Monitoring Station (AWQMS), 2004–2017 Marine Institute, Ireland. Available online: http://data.marine.ie/geonetwork/srv/eng/catalog.search#/metadata/ie.marine.data:dataset.3757 (accessed on 6 April 2020).
- Watras, C.J.; Hanson, P.; Stacy, T.; Morrison, K.; Mather, J.; Hu, Y.H.; Milewski, P.A. A temperature compensation method for CDOM fluorescence sensors in freshwater. Limnol. Oceanogr. Methods 2011, 9, 296–301. [Google Scholar] [CrossRef]
- Ryder, E.; Jennings, E.; De Eyto, E.; Dillane, M.; NicAonghusa, C.; Pierson, D.C.; Moore, K.; Rouen, M.; Poole, R. Temperature quenching of CDOM fluorescence sensors: Temporal and spatial variability in the temperature response and a recommended temperature correction equation. Limnol. Oceanogr. Methods 2012, 10, 1004–1010. [Google Scholar] [CrossRef]
- Doyle, B.C.; De Eyto, E.; Dillane, M.; Poole, R.; McCarthy, V.; Ryder, E.; Jennings, E. Synchrony in catchment stream colour levels is driven by both local and regional climate. Biogeosciences 2019, 16, 1053–1071. [Google Scholar] [CrossRef]
- Met Éireann. Display and Download Historical Data from Current Stations. Available online: https://www.met.ie/climate/available-data/historical-data (accessed on 6 April 2020).
- Butturini, A.; Álvarez, M.; Bernal, S.; Vázquez, E.; Sabater, F. Diversity and temporal sequences of forms of DOC and NO3-discharge responses in an intermittent stream: Predictable or random succession? J. Geophys. Res. Space Phys. 2008, 113 (Suppl. G3), 721. [Google Scholar] [CrossRef]
- FWE; APHA. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Eisenreich, S.J.; Bannerman, R.T.; Armstrong, D.E. A simplified phosphorus analysis technique. Environ. Lett. 1975, 9, 43–53. [Google Scholar] [CrossRef]
- Stainton, M.P.; Capel, M.J.; Armstrong, F.A.J. The Chemical Analysis of Fresh Water, 2nd ed.; Canadian Fisheries and Marine Service Miscellaneous Special Publications: Winnipeg, MB, Canada, 1977; p. 180. [Google Scholar]
- Standing Committee of Analysts. Methods for the examination of Waters and Associated Materials: Ammonia in Waters; HMSO: London, UK, 1981. [Google Scholar]
- Clesceri, L.S.; Greenberg, A.E.; Trussell, R.R. Standard Methods for the Examination of Water and Wastewater, 17th ed.; American Public Health Association: Washington, DC, USA, 1989; pp. 4–144. [Google Scholar]
- Irish Marine Institute. BurrishooleLTER-Public. Available online: https://github.com/IrishMarineInstitute/BurishooleLTER-Public (accessed on 14 May 2020).
- Zambrano-Bigiarini, M. Time Series Management, Analysis and Interpolation for Hydrological Modeling; R Package Version 04-2-1; R Foundation for Statistical Computing: Vienna, Austria, 2014. [Google Scholar]
- Winslow, L.; Read, J.; Woolway, R.; Brentrup, J.; Leach, T.; Zwart, Z.; Albers, S.; Collinge, D. rLakeAnalyzer: Lake Physics Tools; R Package Version 1110; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Nercessian, O.; Noyes, E.; Kalyuzhnaya, M.G.; Lidstrom, M.E.; Chistoserdova, L. Bacterial populations active in metabolism of C1 compounds in the sediment of Lake Washington, a freshwater lake. Microb. Ecol. 2005, 71, 6885–6899. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet.journal 2011, 17, 10. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Rohwer, R.R.; Hamilton, J.J.; Newton, R.J.; McMahon, K.D. TaxAss: Leveraging a custom freshwater database achieves fine-scale taxonomic resolution. mSphere 2018, 3, e00327-18. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Clarke, K.R.; Warwick, R.M. Primer-6 Computer Program; Natural Environment Research: Plymouth, MA, USA, 2005. [Google Scholar]
- Woodhouse, J.N.; Ziegler, J.; Grossart, H.-P.; Neilan, B.A. Cyanobacterial community composition and bacteria–bacteria interactions promote the stable occurrence of particle-associated bacteria. Front. Microbiol. 2018, 9, 777. [Google Scholar] [CrossRef] [PubMed]
- Newton, R.J.; Jones, S.E.; Eiler, A.; McMahon, K.D.; Bertilsson, S. A guide to the natural history of freshwater lake bacteria. Microbiol. Mol. Biol. Rev. 2011, 75, 14–49. [Google Scholar] [CrossRef] [PubMed]
- Salcher, M.M.; Pernthaler, J.; Posch, T. Seasonal bloom dynamics and ecophysiology of the freshwater sister clade of SAR11 bacteria ‘that rule the waves’ (LD12). ISME J. 2011, 5, 1242–1252. [Google Scholar] [CrossRef]
- Calderó-Pascual, M.; De Eyto, E.; Jennings, E.; Dillane, M.; Andersen, M.R.; Kelly, S.; Wilson, H.L.; McCarthy, V. Effects of consecutive extreme weather events on a temperate dystrophic lake: A detailed insight into physical, chemical and biological responses. Water 2020, 12, 1411. [Google Scholar] [CrossRef]
- Jennings, E.; Allott, N.; Arvola, L.; Jarvinen, M.; Moore, K.; Naden, P.; Nic Aongusa, C.; Noges, T.; Weyhermeyer, G. Impacts of climate on the flux of dissolved organic carbon from catchments. In The Impact of Climate Change on European Lakes; George, D.G., Ed.; Springer: New York, NY, USA, 2010. [Google Scholar]
- Jennings, E.; de Eyto, E.; Moore, T.; Dillane, M.; Ryder, E.; Allott, N.; Aonghusa, C.N.; Rouen, M.; Poole, R.; Pierson, D. From highs to lows: Changes in dissolved organic carbon in a peatland catchment and lake following extreme flow events. Water 2020. submitted. [Google Scholar]
- Clark, J.M.; Lane, S.N.; Chapman, P.J.; Adamson, J.K. Link between DOC in near surface peat and stream water in an upland catchment. Sci. Total. Environ. 2008, 404, 308–315. [Google Scholar] [CrossRef]
- Guillemette, F.; McCallister, S.L.; Del Giorgio, P.A. Selective consumption and metabolic allocation of terrestrial and algal carbon determine allochthony in lake bacteria. ISME J. 2016, 10, 1373–1382. [Google Scholar] [CrossRef]
- Woolway, R.I.; Jennings, E.; Carrea, L. Impact of the 2018 European heatwave on lake surface water temperature. Inland Waters 2020, 1–11. [Google Scholar] [CrossRef]
- Wiegand, S.; Jogler, M.; Jogler, C. On the maverick planctomycetes. FEMS Microbiol. Rev. 2018, 42, 739–760. [Google Scholar] [CrossRef]
- Giling, D.P.; Nejstgaard, J.C.; Berger, S.A.; Grossart, H.P.; Kirillin, G.; Penske, A.; Lentz, M.; Casper, P.; Sareyka, J.; Gessner, M.O. Thermocline deepening boosts ecosystem metabolism: Evidence from a large-scale lake enclosure experiment simulating a summer storm. Glob. Chang. Biol. 2017, 23, 1448–1462. [Google Scholar] [CrossRef] [PubMed]
- Martín-Platero, A.M.; Cleary, B.; Kauffman, K.; Preheim, S.P.; McGillicuddy, D.J.; Alm, E.J.; Polz, M.F. High resolution time series reveals cohesive but short-lived communities in coastal plankton. Nat. Commun. 2018, 9, 266. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).