Use of Bacteria and Synthetic Zeolites in Remediation of Soil and Water Polluted with Superhigh-Organic-Sulfur Raša Coal (Raša Bay, North Adriatic, Croatia)
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and Sample Preparation
2.2. Bioremediation Tests
2.3. Adsorption Experiment
2.4. Analysis of Sulfur and PTEs
2.5. Data Analysis
3. Results and Discussion
3.1. Bioremediation of SHOS Raša Coal and Polluted Soil
3.1.1. Geochemical Characterization of Coal and Soil
3.1.2. Desulfurization of Coal and Soil
3.1.3. Demineralization of Soil
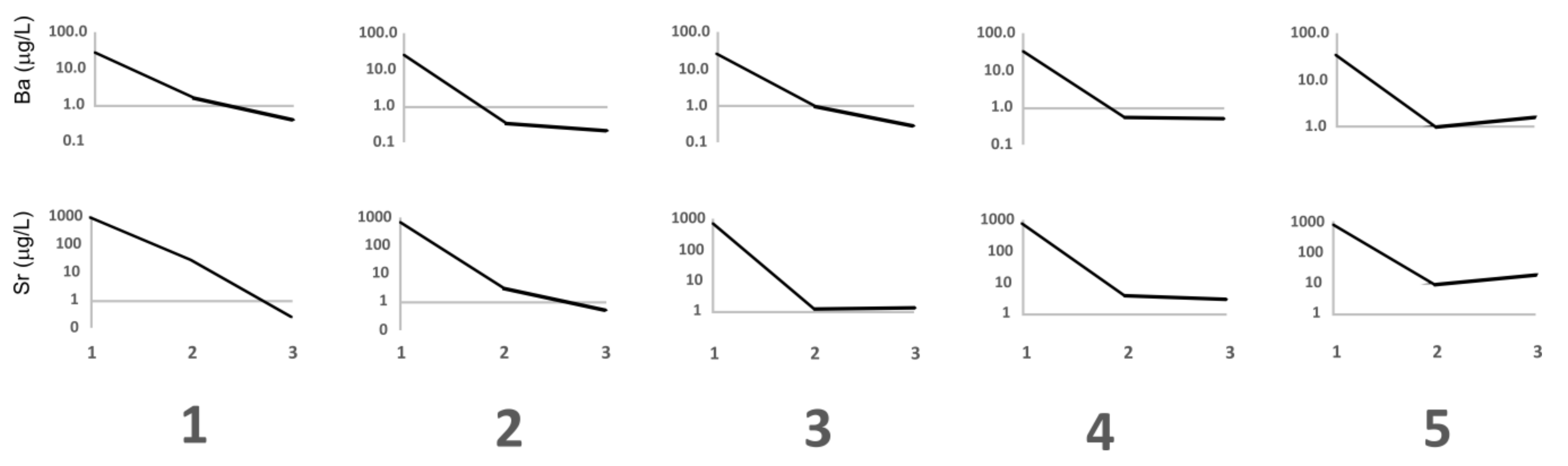
3.2. Zeolite Adsorptive Removal of PTEs from Coalmine and Stream Water Samples
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dai, S.; Yan, X.; Ward, C.R.; Hower, J.C.; Zhao, L.; Wang, X.; Zhao, L.; Ren, D.; Finkelman, R.B. Valuable elements in Chinese coals. Int. Geol. Rev. 2016. [Google Scholar] [CrossRef]
- Rađenović, A. Inorganic constituents in coal. Kem. Ind. 2006, 55, 65–71. [Google Scholar]
- Anggara, F.; Amijaya, D.H.; Harijoko, A.; Tambaria, T.N.; Sahri, A.A.; Asa, Z.A.N. Rare earth element and yttrium content of coal in the Banko coalfield, South Sumatra Basin, Indonesia: Contributions from tonstein layers. Int. J. Coal Geol. 2018, 196, 159–172. [Google Scholar] [CrossRef]
- Medunić, G.; Ahel, M.; Božičević Mihalić, I.; Gaurina Srček, V.; Kopjar, N.; Fiket, Ž.; Bituh, T.; Mikac, I. Toxic airborne S, PAH, and trace element legacy of the superhigh-organic-sulphur Raša coal combustion: Cytotoxicity and genotoxicity assessment of soil and ash. Sci. Total Environ. 2016, 566–567, 306–319. [Google Scholar] [CrossRef] [PubMed]
- Medunić, G.; Kuharić, Ž.; Krivohlavek, A.; Fiket, Ž.; Rađenović, A.; Gödel, K.; Kampić, Š.; Kniewald, G. Geochemistry of Croatian superhigh-organic-sulphur Raša coal, imported low-S coal and bottom ash: Their Se and trace metal fingerprints in seawater, clover, foliage and mushroom specimens. Int. J. Oil Gas Coal Technol. 2018, 18. [Google Scholar] [CrossRef]
- Saikia, J.; Saikia, P.; Boruah, R.; Saikia, B.K. Ambient air quality and emission characteristics in and around a non-recovery type coke oven using high sulphur coal. Sci. Total Environ. 2015, 530–531, 304–313. [Google Scholar] [CrossRef]
- Zacchini, M.; Pietrini, F.; Mugnozza, G.S.; Iori, V.; Pietrosanti, L.; Massacci, A. Metal tolerance, accumulation and translocation in poplar and willow clones treated with cadmium in hydroponics. Water Air Soil Pollut. 2009, 197, 23–34. [Google Scholar] [CrossRef]
- Pandey, V.C.; Singh, J.S.; Singh, R.P.; Singh, N.; Yunus, M. Arsenic hazards in coal fly ash and its fate in Indian scenario. Res. Conserv. Recycl. 2011, 55, 819–835. [Google Scholar] [CrossRef]
- Voltaggio, M.; Spadoni, M.; Sacchi, E.; Sanam, R.; Pujari, P.R.; Labhasetwar, P.K. Assessment of groundwater pollution from ash ponds using stable and unstable isotopes around the Koradi and Khaperkheda thermal power plants (Maharashtra, India). Sci. Total Environ. 2015, 518–519, 616–625. [Google Scholar] [CrossRef]
- Park, Y.-J.; Ko, J.-J.; Yun, S.-L.; Lee, E.Y.; Kim, S.-J.; Kang, S.-W.; Lee, B.-C.; Kim, S.-K. Enhancement of bioremediation by Ralstonia sp. HM-1 in sediment polluted by Cd and Zn. Bioresour. Technol. 2008, 99, 7458–7463. [Google Scholar] [CrossRef]
- Ojuederie, O.B.; Babalola, O.O. Microbial and Plant-Assisted Bioremediation of Heavy Metal Polluted Environments: A Review. Int. J. Environ. Res. Public Health 2017, 14, 1504. [Google Scholar] [CrossRef] [PubMed]
- Vimal, S.R.; Singh, J.S.; Arora, N.K.; Singh, S. Soil-Plant-Microbe Interactions in Stressed Agriculture Management: A Review. Pedosphere 2017, 27, 177–192. [Google Scholar] [CrossRef]
- Crawford, R.L.; Crawford, D.L. Bioremediation: Principles and Applications; Cambridge University Press: New York, NY, USA, 2005; 406p. [Google Scholar]
- Ralph, J.P.; Catcheside, D.E.A. Transformations of low rank coal by Phanerochaete chrysosporium and other wood-rot fungi. Fuel Process. Technol. 1997, 52, 79–93. [Google Scholar] [CrossRef]
- Sekhohola, L.M.; Igbinigie, E.E.; Cowan, A.K. Biological degradation and solubilisation of coal. Biodegradation 2013, 24, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.K.; Singh, A.L.; Kumar, A.; Singh, M.P. Mixed bacterial consortium as an emerging tool to remove hazardous tracemetals from coal. Fuel 2012, 102, 227–230. [Google Scholar] [CrossRef]
- Singh, A.L.; Singh, P.K.; Kumar, A.; Singh, M.P. Desulfurization of selected hard and brown coal samples from India and Indonesia with Ralstonia sp. and Pseudoxanthomonas sp. Energy Explor. Exploit. 2012, 30, 985–998. [Google Scholar] [CrossRef]
- Utturkar, S.M.; Bollmann, A.; Brzoska, R.M.; Klingeman, D.M.; Epstein, S.E.; Palumbo, A.V.; Brown, S.D. Draft Genome Sequence for Ralstonia sp. Strain OR214, a Bacterium with Potential for Bioremediation. Microbiol. Soc. Announc. 2013. [Google Scholar] [CrossRef]
- Ryan, M.P.; Pembroke, J.T.; Adley, C.C. Ralstonia pickettii in environmental biotechnology: Potential and applications. J. Appl. Microbiol. 2007. [Google Scholar] [CrossRef]
- Medunić, G.; Kuharić, Ž.; Krivohlavek, A.; Đuroković, M.; Dropučić, K.; Rađenović, A.; Oberiter, B.L.; Krizmanić, A.; Bajramović, M. Selenium, sulphur, trace metal, and BTEX levels in soil, water, and lettuce from the Croatian Raša Bay contaminated by superhigh organic sulphur coal. Geosciences 2018, 8, 408. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M. Industrial wastes as low-cost potential adsorbents for the treatment of wastewater laden with heavy metals. Adv. Colloid Interface Sci. 2011, 166, 36–59. [Google Scholar] [CrossRef]
- Fiket, Ž.; Galović, A.; Medunić, G.; Furdek Turk, M.; Ivanić, M.; Dolenec, M.; Biljan, I.; Šoster, A.; Kniewald, G. Adsorption of rare earth elements from aqueous solutions using geopolymers. Proceedings 2018, 2, 567. [Google Scholar] [CrossRef]
- Jha, V.K.; Matsuda, M.; Miyake, M. Sorption properties of the activated carbon-zeolite composite prepared from coal fly ash for Ni2+, Cu2+, Cd2+ and Pb2+. J. Hazard. Mater. 2008, 160, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Munthali, M.W.; Johan, E.; Aono, H.; Matsue, N. Cs+ and Sr2+ adsorption selectivity of zeolites in relation to radioactive decontamination. J. Asian Ceram. Soc. 2015, 3, 245–250. [Google Scholar] [CrossRef]
- Ćurković, L.; Cerjan-Stefanović, Š.; Filipan, T. Metal ion exchange by natural and modified zeolites. Water Res. 1997, 31, 1379–1382. [Google Scholar] [CrossRef]
- Medunić, G.; Rađenović, A.; Bajramović, M.; Švec, M.; Tomac, M. Once grand, now forgotten: What do we know about the superhigh-organic-sulphur Raša coal? Min. Geol. Pet. Eng. Bull. 2016, 34, 27–45. [Google Scholar] [CrossRef]
- Dvoršćak, M.; Stipičević, S.; Mendaš, G.; Drevenkar, V.; Medunić, G.; Stančić, Z.; Vujević, D. Soil burden by persistent organochlorine compounds in the vicinity of a coal-fired power plant in Croatia: A comparison study with urban-industrialized area. Environ. Sci. Pollut. Res. 2019. [Google Scholar] [CrossRef] [PubMed]
- Medunić, G.; Kuharić, Ž.; Fiket, Ž.; Bajramović, M.; Singh, A.L.; Krivovlahek, A.; Kniewald, G.; Dujmović, L. Selemium dan other potentially toxic elements in vegetables and tissues of three non-migratory birds exposed to soil, water and aquatic sediment contaminated with seleniferous Raša coal. Min. Geol. Pet. Eng. Bull. 2018, 33, 53–62. [Google Scholar] [CrossRef]
- Valković, V.; Makjanić, J.; Jakšić, M.; Popović, S.; Bos, A.J.J.; Vis, R.D.; Wiederspahn, K.; Verheul, H. Analysis of fly ash by X-ray emission spectroscopy and proton microbeam analysis. Fuel 1984, 63, 1357–1362. [Google Scholar] [CrossRef]
- Miko, S.; Durn, G.; Adamcová, R.; Čović, M.; Dubíková, M.; Skalský, R.; Kapelj, S.; Ottner, F. Heavy metal distribution in karst soils from Croatia and Slovakia. Environ. Geol. 2003, 45, 262–272. [Google Scholar] [CrossRef]
- ASTM International. Standard Test Methods for Total Sulfur in the Analysis Sample of Refuse Derived Fuel. Available online: https://www.astm.org/Standards/E775.htm (accessed on 7 September 2018).
- Kodom, K.; Preko, K.; Boamah, D. X-ray fluorescence (XRF) analysis of soil heavy metal pollution from an industrial area in Kumasi, Ghana. Soil Sediment Contam. 2012, 21, 1006–1021. [Google Scholar] [CrossRef]
- Fiket, Ž.; Mikac, N.; Kniewald, G. Mass Fractions of Forty-Six Major and Trace Elements, Including Rare Earth Elements, in Sediment and Soil Reference Materials Used in Environmental Studies. Geostand. Geoanal. Res. 2017, 41, 123–135. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. Available online: http://palaeo-electronica.org/2001_1/past/issue1_01.htm (accessed on 30 May 2019).
- Lemley, A.D. Guidelines for evaluating selenium data from aquatic monitoring and assessment studies. Environ. Monit. Assess. 1993, 28, 83–100. [Google Scholar] [CrossRef] [PubMed]
- Acharya, C.; Kar, R.N.; Sukla, L.B. Bacterial removal of sulfur from three different coals. Fuel 2001, 80, 2207–2216. [Google Scholar] [CrossRef]
- Berthelin, J. Microbial Weathering Processes in Natural Environments. In Physical and Chemical Weathering in Geochemical Cycles; Lerman, A., Meybeck, M., Eds.; Springer: Dordrecht, The Netherlands, 1988; Volume 251. [Google Scholar]
- Calkins, W.H. The chemical forms of sulfur in coal: A review. Fuel 1994, 73, 475–484. [Google Scholar] [CrossRef]
- Rađenović, A. Sulfur in coal. Kem. Ind. 2004, 53, 557–565. [Google Scholar]
- Boudou, J.; Boulègue, J.; Maléchaux, L. Identification of some sulphur species in a high organic sulphur coal. Fuel 1987, 66, 1558–1569. [Google Scholar] [CrossRef]
- Wrenn, B.A.; Venosa, A.D. Selective enumeration of aromatic and aliphatic hydrocarbon degrading bacteria by a most-probable-number procedure. Can. J. Microbiol. 1995, 42, 252–258. [Google Scholar] [CrossRef]
- Tripathy, S.S.; Kar, R.N.; Kumar Mishra, S.; Twardowska, I.; Sukla, L.B. Effect of chemical pretreatment on bacterial desulphurisation of Assam coal. Fuel 1998, 77, 859–864. [Google Scholar] [CrossRef]
- Singh, P.K.; Singh, A.L.; Kumar, A.; Singh, M.P. Control of different pyrite forms on desulfurization of coal with bacteria. Fuel 2013, 106, 876–879. [Google Scholar] [CrossRef]
- Aller, A.; Martinez, O.; de Linaje, J.A.; Moran, A. Biodesulphurisation of coal by microorganisms isolated from the coal itself. Fuel Process. Technol. 2001, 69, 45–57. [Google Scholar] [CrossRef]
- Cardona, I.C.; Márquez, M.A. Biodesulfurization of two Colombian coal with native microorganisms. Fuel Process. Technol. 2009, 90, 1099–1106. [Google Scholar] [CrossRef]
- Singh, A.L.; Singh, P.K.; Kumar, A.; Singh, M.P. Demineralization of Rajmahal Gondwana coals by bacteria: Revelations from X-ray diffraction (XRD) and Fourier Transform Infra Red (FTIR) studies. Energy Explor. Exploit. 2015, 33, 755–767. [Google Scholar] [CrossRef]
- Yudovich, Y.E.; Ketris, M.P. Selenium in coal: A review. Int. J. Coal Geol. 2006, 67, 112–126. [Google Scholar] [CrossRef]
- Reimann, C.; de Caritat, P. Chemical Elements in the Environment. Factsheets for the Geochemist and Environmental Scientist; Springer-Verlag: Berlin/Heidelberg, Germany, 1998; 398p. [Google Scholar]
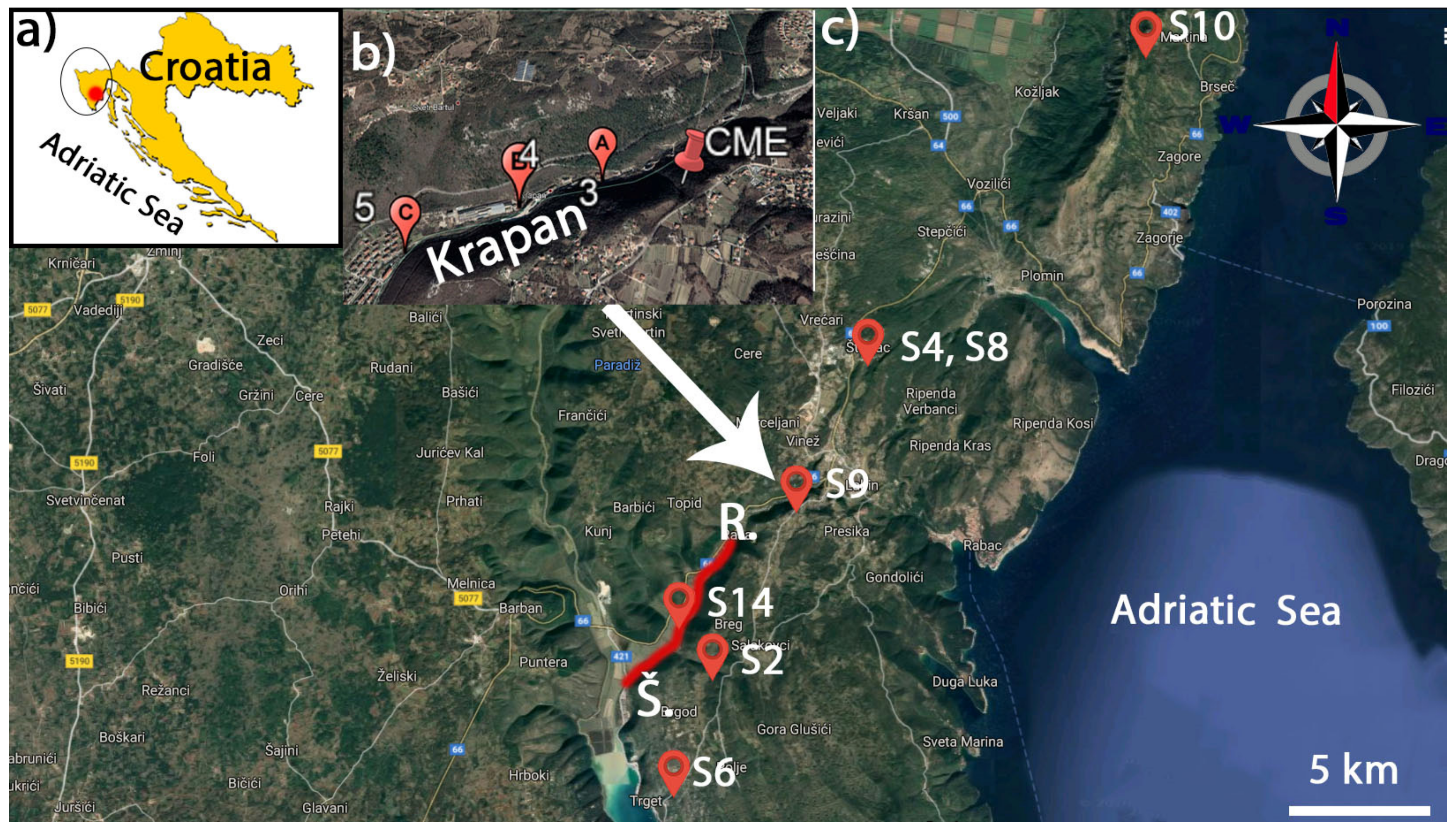
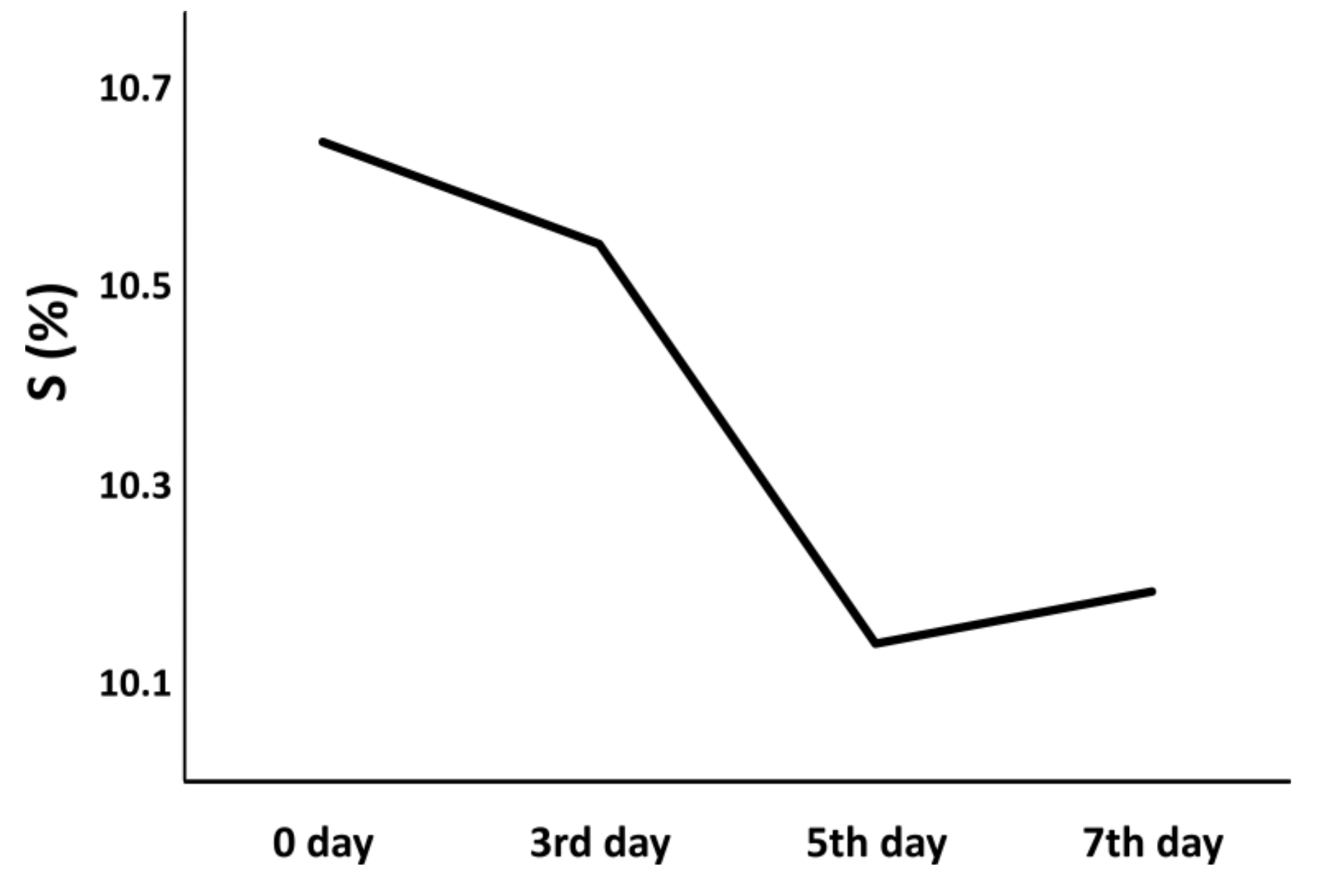
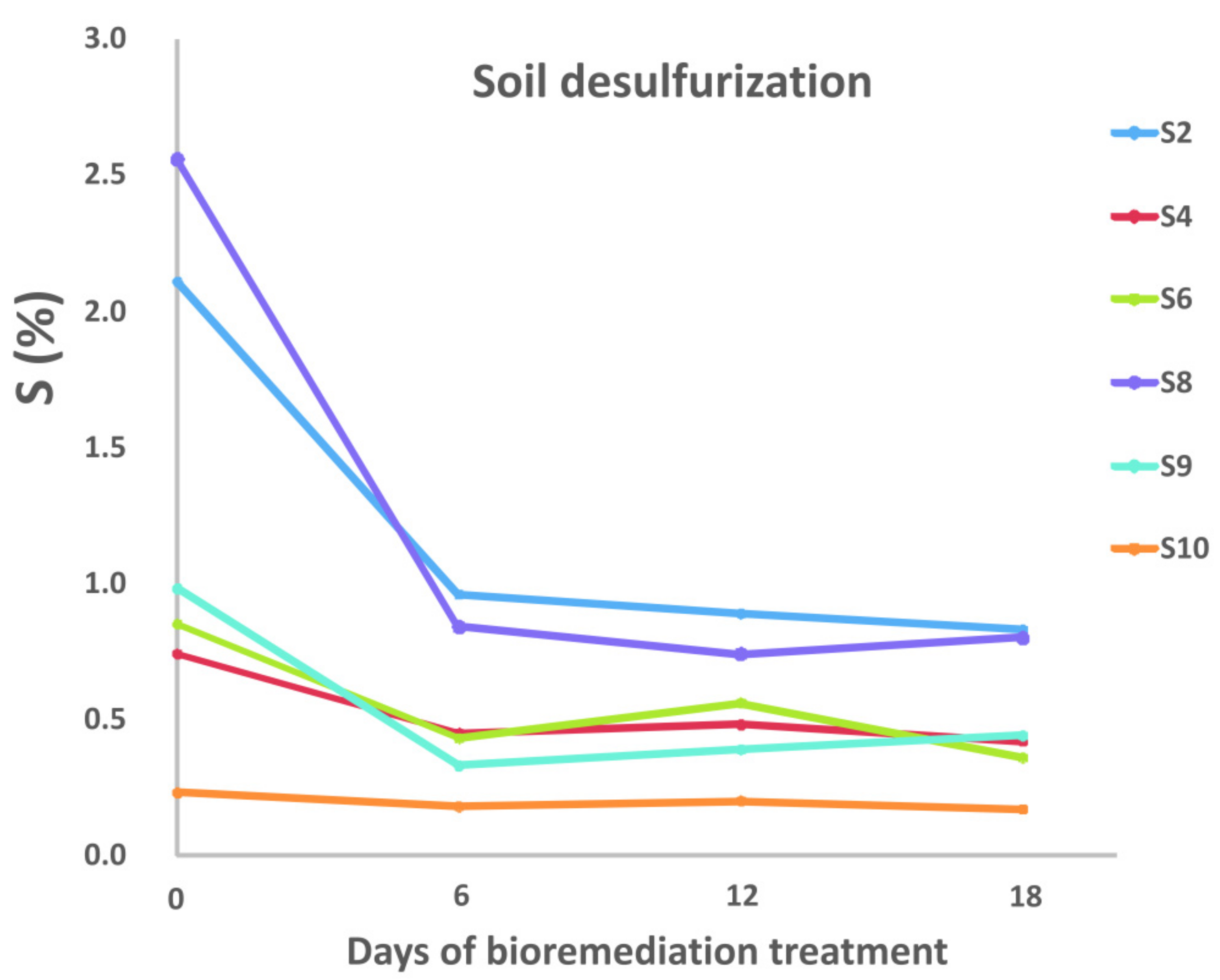
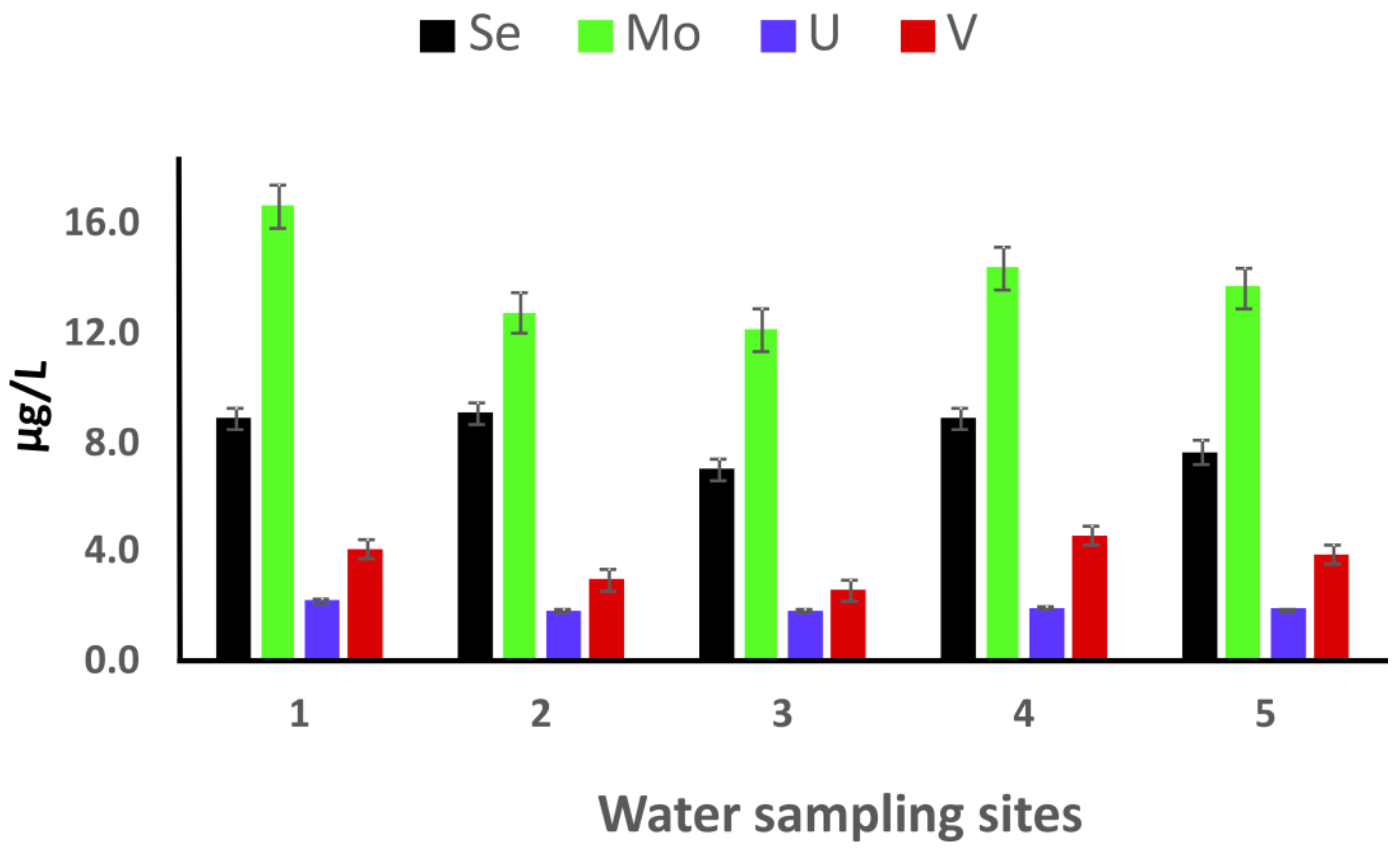
| Site | pH | CaCO3 | LOI | CEC | S | Se | V | U | Sr | Cr | Cu | Pb | Zn | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S2 | Vlaška | 7.6 | 56 | 15 | 23 | 2.1 | 10 | 318 | 11 | 655 | 73 | 13 | 75 | 11 |
| S4 | CCW | na | na | na | na | 0.7 | 3.3 | 118 | 5.7 | 285 | 89 | 1800 | 200 | 6580 |
| S6 | Trget | 7.6 | 36 | 19 | 6 | 0.8 | <DL | 52 | 5.0 | 169 | 45 | 625 | 210 | 936 |
| S8 | CCW | 7.2 | 39 | 25 | 22 | 2.5 | 1.8 | 72 | 1.8 | 277 | 52 | 111 | 69 | 334 |
| S9 | Krapan | 7.1 | 12 | 19 | 12 | 0.9 | <DL | 560 | 0.2 | 74 | 1860 | 1847 | 72 | 953 |
| S14 | Rail | 6.9 | 3 | 83 | 6 | 6.9 | 27 | 264 | 8.2 | 356 | 100 | 190 | 46 | 863 |
| S10 | Unpolluted | 6.8 | 0 | 15 | 20 | 0.2 | 1.5 | 229 | 4.3 | 82 | 135 | 41 | 56 | 169 |
| C | Coal | na | na | na | na | 10.6 | 23 | 80 | 14 | 290 | 27 | 6 | 3 | 32 |
| pH | CaCO3 | LOI | CEC | S | Se | V | U | Sr | Cr | Cu | Pb | Zn | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | 0.07 | 0.10 | 0.27 | 0.19 | 0.58 | 0.43 | 0.43 | 0.79 | 0.19 | 0.43 | 0.07 | 0.43 | |
| CaCO3 | 0.73 | 0.19 | 0.07 | 0.62 | 0.79 | 0.99 | 0.62 | 0.32 | 0.62 | 0.14 | 0.32 | 0.14 | |
| LOI | −0.66 | −0.52 | 0.27 | 0.19 | 0.41 | 0.79 | 0.79 | 0.79 | 0.79 | 0.79 | 0.07 | 0.79 | |
| CEC | 0.44 | 0.73 | −0.44 | 0.79 | 0.58 | 0.43 | 0.79 | 0.43 | 0.79 | 0.19 | 0.79 | 0.19 | |
| S | −0.52 | −0.20 | 0.52 | 0.10 | 0.24 | 0.85 | 0.85 | 0.57 | 0.85 | 0.34 | 0.03 | 0.18 | |
| Se | −0.22 | 0.10 | 0.33 | 0.22 | 0.41 | 0.43 | 0.05 | 0.01 | 0.43 | 0.24 | 0.24 | 0.43 | |
| V | −0.31 | 0 | −0.10 | 0.31 | 0.06 | 0.27 | 0.57 | 0.34 | 0.03 | 0.85 | 0.34 | 0.85 | |
| U | 0.31 | 0.20 | −0.10 | 0.10 | 0.06 | 0.69 | 0.20 | 0.01 | 0.85 | 0.18 | 0.85 | 0.34 | |
| Sr | 0.10 | 0.40 | 0.10 | 0.31 | 0.20 | 0.82 | 0.33 | 0.86 | 0.85 | 0.09 | 0.57 | 0.18 | |
| Cr | −0.52 | −0.20 | 0.10 | 0.10 | 0.06 | 0.27 | 0.73 | −0.06 | 0.06 | 0.34 | 0.34 | 0.57 | |
| Cu | −0.31 | −0.60 | 0.10 | −0.52 | −0.33 | −0.41 | 0.06 | −0.46 | −0.60 | 0.33 | 0.85 | 0.01 | |
| Pb | 0.73 | 0.40 | −0.73 | 0.10 | −0.73 | −0.41 | −0.33 | −0.06 | −0.20 | −0.33 | 0.06 | 0.57 | |
| Zn | −0.31 | −0.60 | 0.10 | −0.52 | −0.46 | −0.27 | −0.06 | −0.33 | −0.46 | 0.20 | 0.86 | 0.20 |
| S10 | S4 | S14 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Initial | Final | Removal (%) | Initial | Final | Removal (%) | Initial | Final | Removal (%) | |
| As | 30.8 | 23.3 | 20 | 8.82 | 5.55 | 30 | 46.5 | 5.02 | 80 |
| Ba | 227 | 174 | 20 | 392 | 135 | 60 | 147 | 48.8 | 60 |
| Co | 19.3 | 18.2 | 0 | 9.05 | 6.22 | 30 | 9.30 | 3.05 | 60 |
| Cr | 135 | 106 | 20 | 89.7 | 47.7 | 40 | 100 | 42.6 | 50 |
| Cu | 41.4 | 31.3 | 20 | 1800 | 3000 | - | 190 | 71.7 | 60 |
| Ni | 123 | 80.6 | 30 | 52.3 | 26.5 | 40 | 60.3 | 19.5 | 60 |
| Pb | 56.4 | 48.9 | 10 | 200 | 132 | 30 | 46.3 | 20.3 | 50 |
| Rb | 122 | 67.7 | 40 | 30.4 | 18.0 | 40 | 23.7 | 9.40 | 60 |
| Se | 1.50 | 1.35 | 10 | 3.31 | 1.00 | 60 | 27.5 | 27.0 | 0 |
| Sr | 82.2 | 63.0 | 20 | 285 | 328 | - | 356 | 127 | 60 |
| U | 4.30 | 4.18 | 0 | 5.76 | 4.23 | 20 | 8.20 | 7.07 | 10 |
| V | 229 | 167 | 20 | 118 | 58.2 | 50 | 264 | 93.6 | 60 |
| Zn | 169 | 112 | 30 | 6580 | 3320 | 40 | 863 | 331 | 60 |
| Cd | Pb | Cr | Fe | Ni | Cu | Zn | Sr | Ba | As | Se | Mo | U | V | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cd | 0.79 | 0.07 | 0.79 | 0.19 | 0.79 | 0.43 | 0.07 | 0.43 | 0.79 | 0.27 | 0.02 | 0.07 | 0.07 | |
| Pb | −0.10 | 0.62 | 0.05 | 0.32 | 0.01 | 0.14 | 0.62 | 0.62 | 0.32 | 0.79 | 0.99 | 0.62 | 0.62 | |
| Cr | 0.73 | 0.20 | 0.32 | 0.05 | 0.62 | 0.14 | 0.14 | 0.14 | 0.32 | 0.43 | 0.05 | 0.14 | 0.01 | |
| Fe | 0.10 | 0.80 | 0.40 | 0.62 | 0.05 | 0.32 | 0.99 | 0.32 | 0.14 | 0.79 | 0.62 | 0.99 | 0.32 | |
| Ni | 0.52 | 0.40 | 0.80 | 0.20 | 0.32 | 0.05 | 0.05 | 0.32 | 0.62 | 0.79 | 0.14 | 0.05 | 0.05 | |
| Cu | −0.10 | 0.99 | 0.20 | 0.80 | 0.40 | 0.14 | 0.62 | 0.62 | 0.32 | 0.79 | 0.99 | 0.62 | 0.62 | |
| Zn | 0.31 | 0.60 | 0.60 | 0.40 | 0.80 | 0.60 | 0.14 | 0.14 | 0.32 | 0.79 | 0.32 | 0.14 | 0.14 | |
| Sr | 0.73 | 0.20 | 0.60 | 0 | 0.80 | 0.20 | 0.60 | 0.62 | 0.99 | 0.79 | 0.05 | 0.01 | 0.14 | |
| Ba | 0.31 | 0.20 | 0.60 | 0.40 | 0.40 | 0.20 | 0.60 | 0.20 | 0.05 | 0.79 | 0.32 | 0.62 | 0.14 | |
| As | 0.10 | 0.40 | 0.40 | 0.60 | 0.20 | 0.40 | 0.40 | 0 | 0.80 | 0.79 | 0.62 | 0.99 | 0.32 | |
| Se | 0.44 | −0.10 | 0.31 | 0.10 | 0.10 | −0.10 | −0.10 | 0.10 | −0.10 | 0.10 | 0.43 | 0.79 | 0.43 | |
| Mo | 0.94 | 0 | 0.80 | 0.20 | 0.60 | 0 | 0.40 | 0.80 | 0.40 | 0.20 | 0.31 | 0.05 | 0.05 | |
| U | 0.73 | 0.20 | 0.60 | 0 | 0.80 | 0.20 | 0.60 | 0.99 | 0.20 | 0 | 0.10 | 0.80 | 0.14 | |
| V | 0.73 | 0.20 | 0.99 | 0.40 | 0.80 | 0.20 | 0.60 | 0.60 | 0.60 | 0.40 | 0.31 | 0.80 | 0.60 |
| Loc. | Tr. | Mo | Cd | Pb | U | V | Cr | Fe | Ni | Cu | Zn | As | Se | Ba | Sr |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 16.7 | 0.18 | 0.08 | 2.20 | 4.10 | 0.89 | 10.6 | 1.40 | 0.49 | 6.81 | 0.35 | 8.90 | 27.6 | 908 |
| 2 | 18.4 | 0.16 | 0.07 | 2.31 | 5.30 | 10.1 | 15.9 | 1.43 | 2.26 | 4.64 | 0.84 | 11.4 | 1.57 | 27 | |
| 3 | 32.4 | 0.26 | 0.21 | 3.11 | 14.5 | 2.93 | 46.0 | 0.78 | 1.14 | 7.55 | 2.30 | 14.9 | 0.39 | 0.24 | |
| 2 | 1 | 12.8 | 0.14 | 0.09 | 1.84 | 3.00 | 0.73 | 25.8 | 0.98 | 0.62 | 5.01 | 0.38 | 9.10 | 26.1 | 721 |
| 2 | 14.0 | 0.12 | 0.06 | 1.91 | 4.20 | 10.1 | 19.6 | 0.89 | 0.73 | 3.46 | 0.99 | 8.90 | 0.33 | 3.00 | |
| 3 | 13.9 | 0.12 | 0.14 | 1.37 | 7.00 | 1.23 | 5.00 | 0.41 | 0.50 | 3.57 | 1.77 | 9.30 | 0.21 | 0.54 | |
| 3 | 1 | 12.2 | 0.12 | 0.19 | 1.85 | 2.60 | 0.67 | 12.0 | 1.00 | 1.02 | 6.65 | 0.13 | 7.00 | 25.7 | 732 |
| 2 | 13.3 | 0.13 | 0.24 | 2.12 | 8.50 | 9.50 | 168 | 1.24 | 4.60 | 11.3 | 1.21 | 9.00 | 0.95 | 1.21 | |
| 3 | 13.1 | 0.12 | 0.20 | 1.76 | 10.4 | 1.20 | 29.1 | 0.65 | 1.97 | 6.85 | 1.75 | 9.50 | 0.29 | 1.29 | |
| 4 | 1 | 14.4 | 0.15 | 0.34 | 1.94 | 4.60 | 0.90 | 81.3 | 1.69 | 3.24 | 14.5 | 0.62 | 8.90 | 33.6 | 798 |
| 2 | 15.1 | 0.14 | 0.36 | 1.95 | 5.20 | 9.92 | 33.6 | 2.63 | 3.23 | 13.8 | 1.36 | 9.10 | 0.55 | 3.84 | |
| 3 | 14.9 | 0.14 | 0.17 | 1.65 | 8.20 | 1.32 | 20.7 | 1.60 | 3.24 | 17.1 | 1.77 | 7.10 | 0.50 | 2.94 | |
| 5 | 1 | 13.7 | 0.14 | 0.27 | 1.87 | 3.90 | 0.78 | 26.6 | 1.22 | 2.31 | 13.0 | 0.65 | 7.70 | 33.7 | 786 |
| 2 | 15.0 | 0.14 | 0.20 | 1.96 | 4.90 | 9.80 | 34.0 | 2.63 | 2.97 | 10.5 | 1.09 | 8.20 | 1.02 | 9.34 | |
| 3 | 15.0 | 0.14 | 0.89 | 1.72 | 8.10 | 1.39 | 32.2 | 1.29 | 2.50 | 10.9 | 1.82 | 7.30 | 1.65 | 18.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medunić, G.; Singh, P.K.; Singh, A.L.; Rai, A.; Rai, S.; Jaiswal, M.K.; Obrenović, Z.; Petković, Z.; Janeš, M. Use of Bacteria and Synthetic Zeolites in Remediation of Soil and Water Polluted with Superhigh-Organic-Sulfur Raša Coal (Raša Bay, North Adriatic, Croatia). Water 2019, 11, 1419. https://doi.org/10.3390/w11071419
Medunić G, Singh PK, Singh AL, Rai A, Rai S, Jaiswal MK, Obrenović Z, Petković Z, Janeš M. Use of Bacteria and Synthetic Zeolites in Remediation of Soil and Water Polluted with Superhigh-Organic-Sulfur Raša Coal (Raša Bay, North Adriatic, Croatia). Water. 2019; 11(7):1419. https://doi.org/10.3390/w11071419
Chicago/Turabian StyleMedunić, Gordana, Prakash Kumar Singh, Asha Lata Singh, Ankita Rai, Shweta Rai, Manoj Kumar Jaiswal, Zoran Obrenović, Zoran Petković, and Magdalena Janeš. 2019. "Use of Bacteria and Synthetic Zeolites in Remediation of Soil and Water Polluted with Superhigh-Organic-Sulfur Raša Coal (Raša Bay, North Adriatic, Croatia)" Water 11, no. 7: 1419. https://doi.org/10.3390/w11071419
APA StyleMedunić, G., Singh, P. K., Singh, A. L., Rai, A., Rai, S., Jaiswal, M. K., Obrenović, Z., Petković, Z., & Janeš, M. (2019). Use of Bacteria and Synthetic Zeolites in Remediation of Soil and Water Polluted with Superhigh-Organic-Sulfur Raša Coal (Raša Bay, North Adriatic, Croatia). Water, 11(7), 1419. https://doi.org/10.3390/w11071419





