Catalytic Efficiency of Red Mud for the Degradation of Olive Mill Wastewater through Heterogeneous Fenton’s Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Simulated OMW Preparation
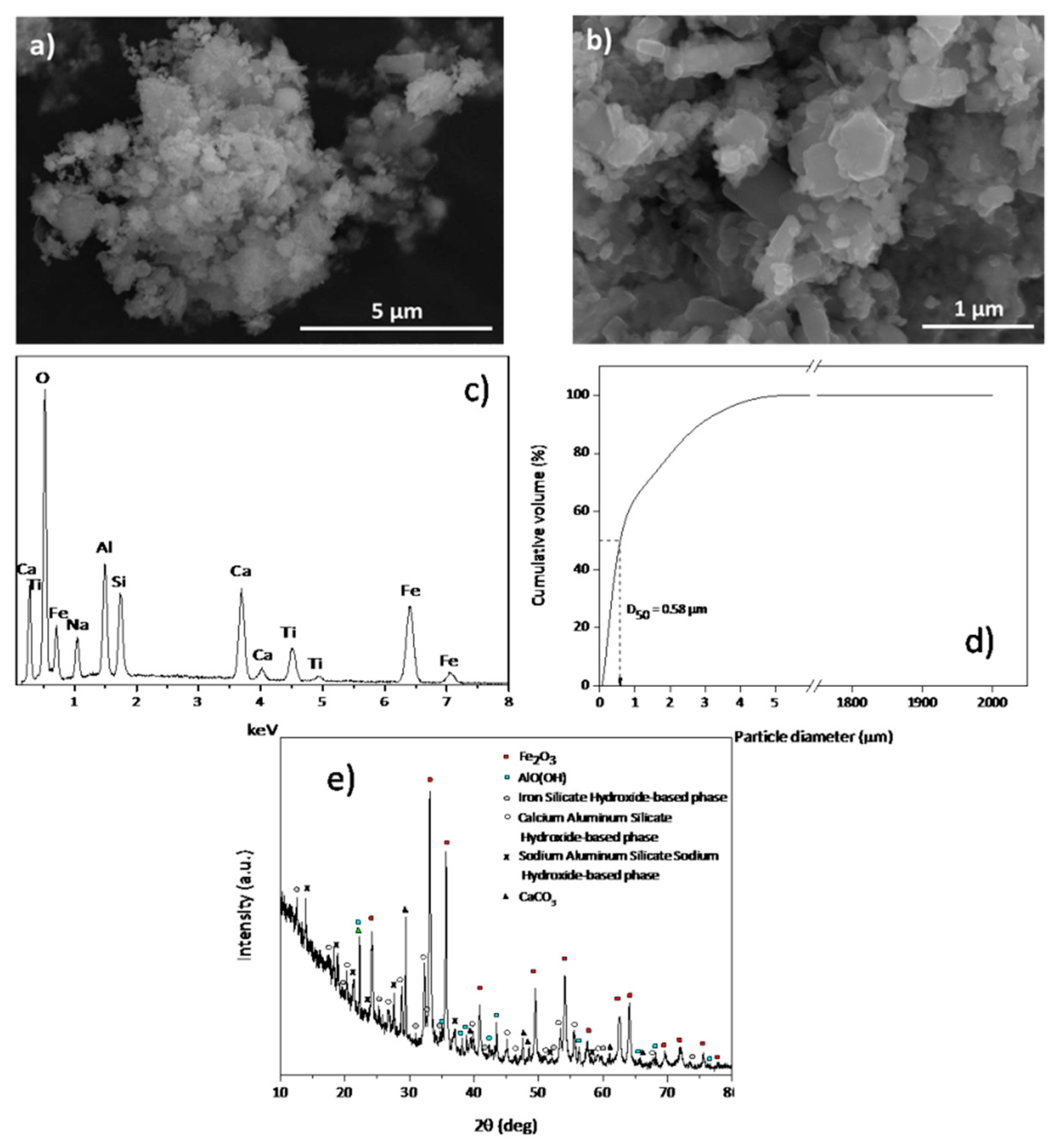
2.2. Red Mud Characterization
2.3. Fenton Experiments
2.4. Analytical Techniques
2.5. Toxicity Assessment
3. Results and Discussion
3.1. Red Mud Characterization
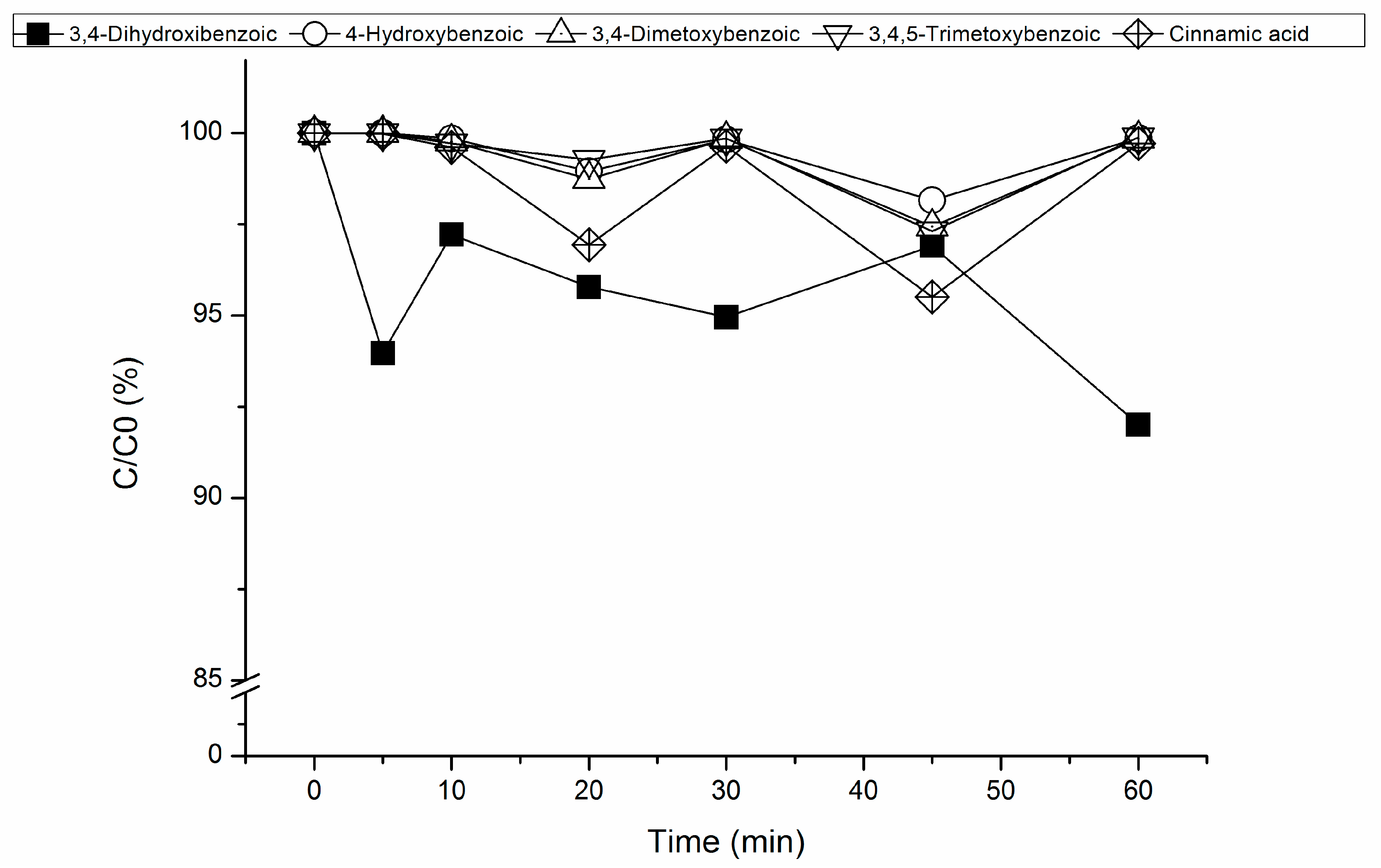
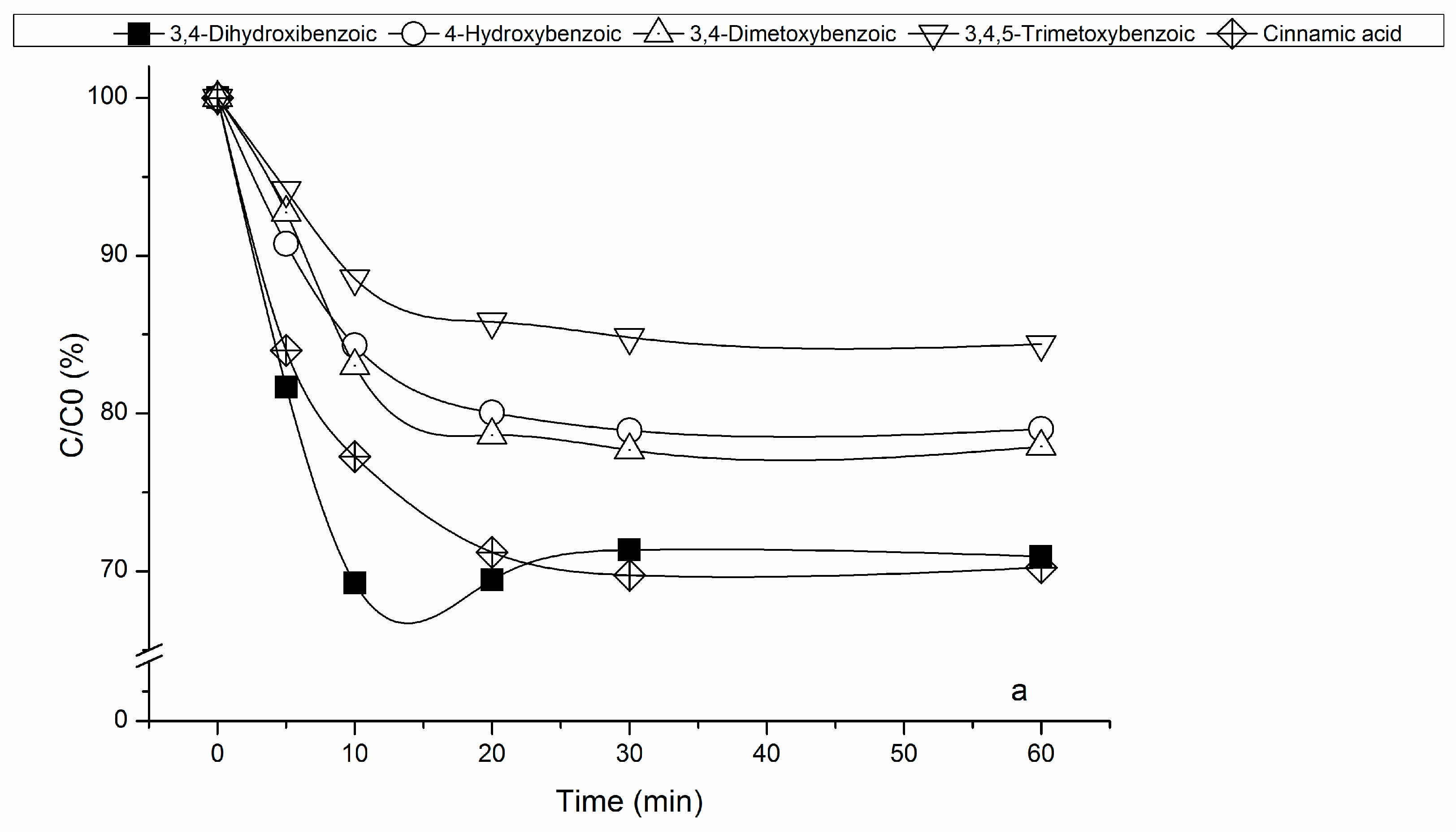
3.2. Fenton’s Process
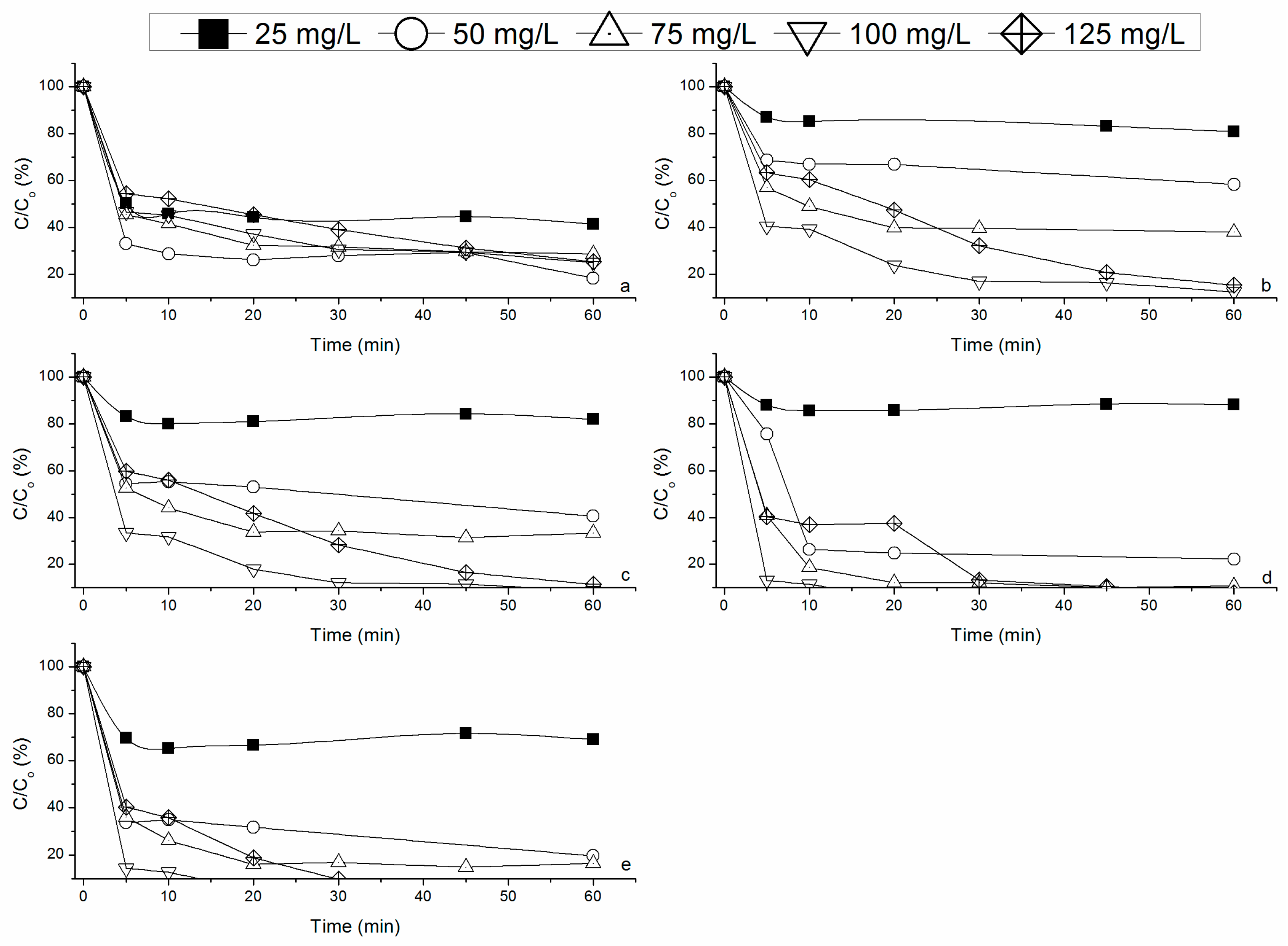
3.2.1. Effect of RM Loading on Fenton’s Reaction
3.2.2. Effect of Hydrogen Peroxide Concentration on Fenton’s Reaction
3.3. Mineralization Achieved by Heterogeneous Fenton’s Process
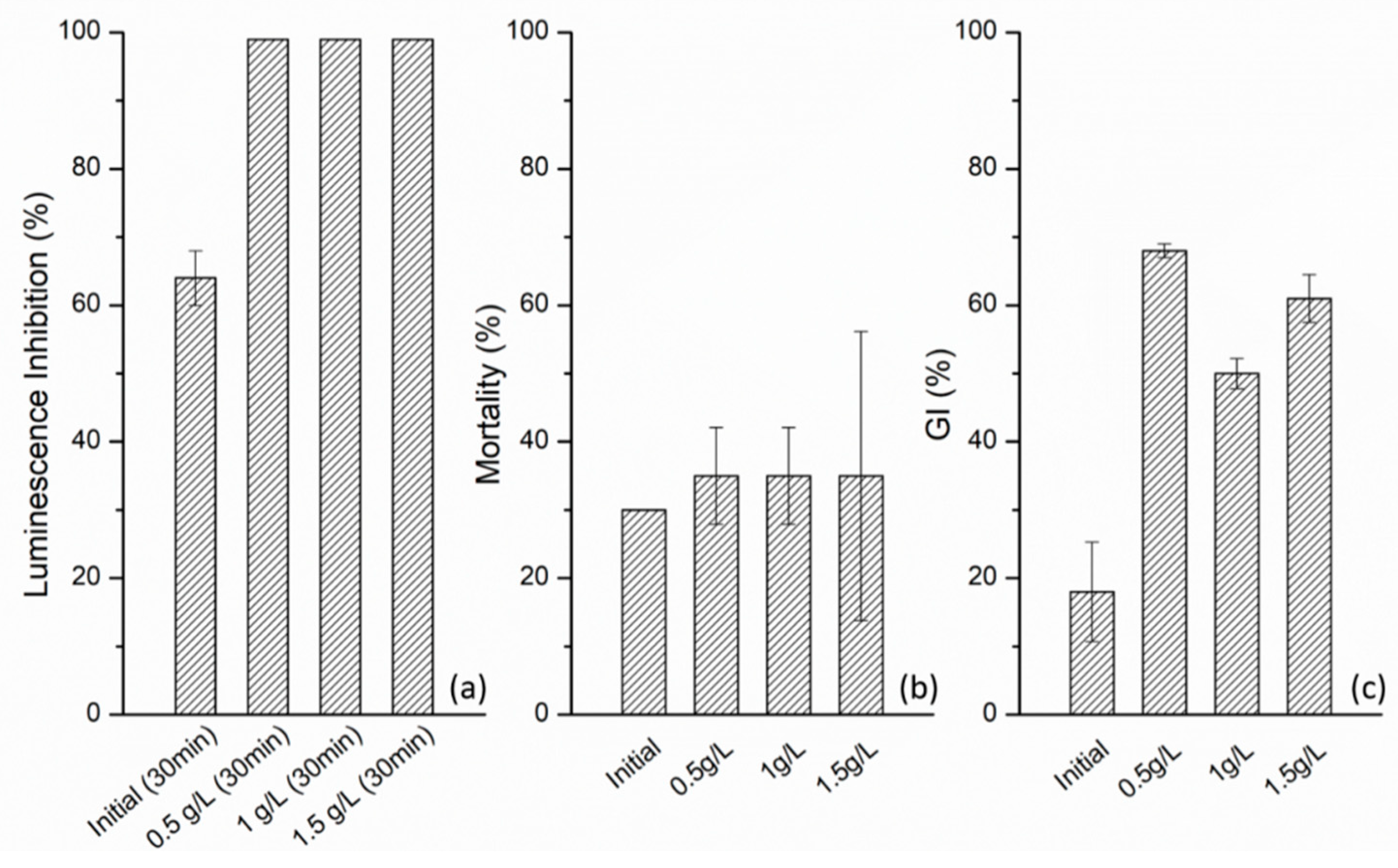
3.4. Toxicity Assessment
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Eroglu, E.; Eroglu, I.; Gündüz, U.; Türker, L.; Yücel, M. Biological hydrogen production from olive mill wastewater with two-stage processes. Int. J. Hydr. Energy 2006, 31, 1527–1535. [Google Scholar] [CrossRef]
- Pinho, I.A.; Lopes, D.V.; Martins, R.C.; Quina, M.J. Phytotoxicity assessment of olive mill solid wastes and the influence of phenolic compounds. Chemosphere 2017, 185, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Vlyssides, A.G.; Loukakis, H.N.; Karlis, P.K.; Barampouti, E.M.P.; Mai, S.T. Olive mill wastewater detoxification by applying pH related Fenton oxidation process. Fresenius Environ. Bull. 2004, 13, 501–504. [Google Scholar]
- Domingues, E.; Gomes, J.; Quina, M.J.; Quinta-Ferreira, R.M.; Martins, R.C. Detoxification of Olive Mill Wastewaters by Fenton’s Process. Catalysts 2018, 8, 662. [Google Scholar] [CrossRef]
- Guido Greco, J.; Colarieti, M.L.; Toscano, G.; Iamarino, G.; Rao, M.A.; Gianfreda, L. Mitigation of Olive Mill Wastewater Toxicity. J. Agric. Food Chem. 2006, 54, 6776–6782. [Google Scholar] [CrossRef]
- Saadi, I.; Laor, Y.; Raviv, M.; Medina, S. Land spreading of olive mill wastewater: Effects on soil microbial activity and potential phytotoxicity. Chemosphere 2007, 66, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Azbar, N.; Bayram, A.; Filibeli, A.; Muezzinoglu, A.; Sengul, F.; Ozer, A. A Review of Waste Management Options in Olive Oil Production. Crit. Rev. Environ. Sci. Technol. 2004, 34, 209–247. [Google Scholar] [CrossRef]
- Saez, L.; Perez, J.; Martinez, J. Low molecular weight phenolics attenuation during simulated treatment of wastewaters from olive mills in evaporating ponds. Water Res. 1992, 26, 1261–1266. [Google Scholar] [CrossRef]
- Beltran, F.J.; Garcia Araya, J.F.; Frades, J.; Alvarez, P.; Gimeno, O. Effects of single and combined ozonation with hydrogen peroxide or UV radiation on the chemical degradation and biodegradability of debittering table olive industrial watewater. Water Res. 1999, 33, 723–732. [Google Scholar] [CrossRef]
- Cañizares, P.; Lobato, J.; Paz, R.; Rodrigo, M.A.; Sáez, C. Advanced oxidation processes for the treatment of olive-oil mills wastewater. Chemosphere 2007, 67, 832–838. [Google Scholar] [CrossRef]
- Amaral-Silva, N.; Martins, R.C.; Nunes, P.; Castro-Silva, S.; Quinta-Ferreira, R.M. From a lab test to industrial application: Scale-up of Fenton process for real olive mill wastewater treatment. J. Chem. Technol. Biotechnol. 2017, 92, 1336–1344. [Google Scholar] [CrossRef]
- Amaral-Silva, N.; Martins, R.C.; Castro-Silva, S.; Quinta-Ferreira, R.M. Integration of traditional systems and advanced oxidation process technologies for the industrial treatment of olive mill wastewaters. Environ. Technol. (UK) 2016, 37, 2524–2535. [Google Scholar] [CrossRef] [PubMed]
- Peres, J.A.; Carvalho, L.M.; Boaventura, R.; Costa, C. Characteristics of p-hydroxybenzoic acid oxidation using Fenton’s reagent. J. Environ. Sci. Health Part A 2004, 39, 2897–2913. [Google Scholar] [CrossRef]
- Reis, P.; Marques, P.; Martins, R.C.; Gando-Ferreira, L.; Quinta-Ferreira, R.M. Integrating Fenton’s process and Ion-Exchange for Olive Mill Wastewater Treatment and Iron Recovery. Environ. Technol. 2018, 39, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Martins, P.; Reis, P.; Martins, R.C.; Gando-Ferreira, L.; Quinta-Ferreira, R.M. Iron recovery from the Fenton’s treatment of winery effluent using na ion-exchange resin. J. Mol. Liq. 2017, 242, 505–511. [Google Scholar] [CrossRef]
- Rossi, A.; Martins, R.C.; Quinta-Ferreira, R.M. Heterogeneous Fenton using ceria based catalysts: Effects of the calcination temperature in the process efficiency. Appl. Catal. B Environ. 2012, 111, 254–263. [Google Scholar] [CrossRef]
- Martins, R.C.; Henriques, L.R.; Quinta-Ferreira, R.M. Catalytic Activity of Low Cost Materials for Pollutants Abatement by Fenton’s Process. Chem. Eng. Sci. 2013, 100, 225–233. [Google Scholar] [CrossRef]
- Shi, X.; Tian, A.; You, J.; Yang, H.; Wang, Y.; Xue, X. Degradation of organic dyes by a new heterogeneous Fenton reagent—Fe2GeS4 nanoparticle. J. Hazard. Mater. 2018, 353, 182–189. [Google Scholar] [CrossRef]
- Qiang, Z.; Chang, J.; Huang, C. Electrochemical regeneration of Fe2+ in Fenton oxidation processes. Water Res. 2003, 37, 1308–1319. [Google Scholar] [CrossRef]
- Neyens, E.; Baeyens, J. A review of classic Fenton’s peroxidation as an advanced oxidation technique. J. Hazard. Mater. 2003, 98, 33–50. [Google Scholar] [CrossRef]
- Martins, R.C.; Rossi, A.; Quinta-Ferreira, R.M. Fenton’s oxidation process for phenolic wastewater remediation and biodegradability enhancement. J. Hazard. Mater. 2010, 180, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.A.S.; Tristão, J.C.; Ardisson, J.D.; Dias, A.; Lago, R.M. Production of nanostructured magnetic composites based on Fe0 nuclei coated with carbon nanofibers and nanotubes from red mud waste and ethanol. Appl. Catal. B 2011, 105, 163–170. [Google Scholar] [CrossRef]
- Dias, F.; Oliveira, A.; Arcanjo, A.; Moura, F.; Pacheco, J. Residue-based iron catalyst for the degradation of textile dye via heterogeneous photo-Fenton. Appl. Catal. B 2016, 186, 136–142. [Google Scholar] [CrossRef]
- Costa, R.; Moura, F.; Oliveira, P.; Magalhães, F.; Ardisson, J.; Lago, R. Controlled reduction of red mud waste to produce active systems for environmental applications: Heterogeneous Fenton reaction and reduction of Cr(VI). Chemosphere 2010, 78, 1116–1120. [Google Scholar] [CrossRef]
- Gomes, J.; Frasson, D.; Pereira, J.L.; Gonçalves, F.J.M.; Castro, L.M.; Quinta-Ferreira, R.M.; Martins, R.C. Ecotoxicity variation through parabens degradation by single and catalytic ozonation using volcanic rock. Chem. Eng. J. 2019, 360, 30–37. [Google Scholar] [CrossRef]
- Rivera-Ultrilla, J.; Bautista-Toledo, I.; Ferro-García, M.; Moreno-Castilla, C. Activated carbon surface modifications by adsorption of bacteria and their effect on aqueous lead adsorption. J. Chem. Technol. Biotechnol. 2001, 76, 1209–1215. [Google Scholar] [CrossRef]
- Ltaïef, A.H.; Pastrana-Martínez, L.M.; Ammar, S.; Gadri, A.; Faria, J.L.; Silva, A.M. Mined pyrite and chalcopyrite as catalysts for spontaneous acidic pH adjustment in Fenton and LED photo-Fenton-like processes. J. Chem. Technol. Biotechnol. 2018, 93, 1137–1146. [Google Scholar] [CrossRef]
- Seller, R. Spectrophotometric determination of hydrogen peroxide using potassium titanium (IV) oxalate. Analyst 1980, 105, 950–954. [Google Scholar] [CrossRef]
- Rosa, I.C.; Garrido, R.; Ré, A.; Gomes, J.; Pereira, J.L.; Gonçalves, F.; Costa, R. Sensitivity of the invasive bivalve Corbicula fluminea to candidate control chemicals: The role of dissolved oxygen conditions. Sci. Tot. Environ. 2015, 536, 825–830. [Google Scholar] [CrossRef]
- Gomes, J.; Pereira, J.L.; Rosa, I.C.; Saraiva, P.M.; Gonçalves, F.; Costa, R. Evaluation of candidate biocides to control the biofouling Asian clam in the drinking water treatment industry: An environmentally friendly approach. J. Great Lakes Res. 2014, 40, 421–428. [Google Scholar] [CrossRef]
- Trautmann, N.M.; Krasny, M.E. Composting in the classroom. In Nature Science Foundation; Cornell Waste Management Institute and Cornell Center for the Environment: New York, NY, USA, 1997. [Google Scholar]
- Khairul, M.A.; Zanganeh, J.; Moghtaderi, B. The composition, recycling andutilisation of Bayer red mud. Resour. Conserv. Recycl. 2019, 141, 483–498. [Google Scholar] [CrossRef]
- Gomes, J.; Leal, I.; Bednarczyk, K.; Gmurek, M.; Stelmachowski, M.; Diak, M.; Quinta-Ferreira, M.E.; Costa, R.; Quinta-Ferreira, R.M.; Martins, R.C. Photocatalytic Ozonation using doped TiO2 Catalysts for the Removal of Parabens in Water. Sci. Total Environ. 2017, 609, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, J.H.; Costa, C.A.; Madeira, L.M.; Mata, G.; Vicente, M.A.; Rojas-Cervantes, M.L. Fenton-like oxidation of Orange II solutions using heterogeneous catalysts based on saponite clay. Appl. Catal. B 2007, 71, 44–56. [Google Scholar] [CrossRef]
- Miralles-Cuevas, S.; Oller, I.; Agüera, A.; Sánchez Pérez, J.A.; Malato, S. Strategies for reducing cost by using solar photo-Fenton treatment combined with nanofiltration to remove microcontaminants in real municipal effluents: Toxicity and economic assessment. Chem. Eng. J. 2017, 318, 161–170. [Google Scholar] [CrossRef]
- Rosa, I.C.; Pereira, J.L.; Costa, R.; Gomes, J.; Pereira, M.L.; Gonçalves, F. Dispersal of Corbicula fluminea: Factors influencing the invasive clam’s drifting behaviour. Annal. Limnol. Int. J. Limnol. 2014, 50, 37–47. [Google Scholar] [CrossRef]
- Yadav, Y.C.; Srivastav, D.N.; Seth, A.K.; Saini, V.; Balaraman, R.; Ghelani, T.K. In Vivo antioxidantpotential of Lepidium sativum L. seeds in albino rats using cisplatininduced nephrotoxicity. Interact. J. Phytomed. 2010, 2, 292–298. [Google Scholar]
- Hwang, S.L.; Yen, G.C. Neuroprotective effects of the citrusflavanones against H2O2-induced cytotoxicity in PC12 cells. J. Agric. Food Chem. 2008, 56, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Al-Sheddi, E.S.; Farshori, N.N.; Al-Oqail, M.M.; Musarrat, J.; Al-Khedhairy, A.A.; Siddiqui, M.A. Protective effect of Lepidium sativum seed extract against hydrogenperoxide-induced cytotoxicity and oxidative stress in human liver cells(HepG2). Pharm. Biol. 2016, 54, 314–321. [Google Scholar] [CrossRef] [PubMed]
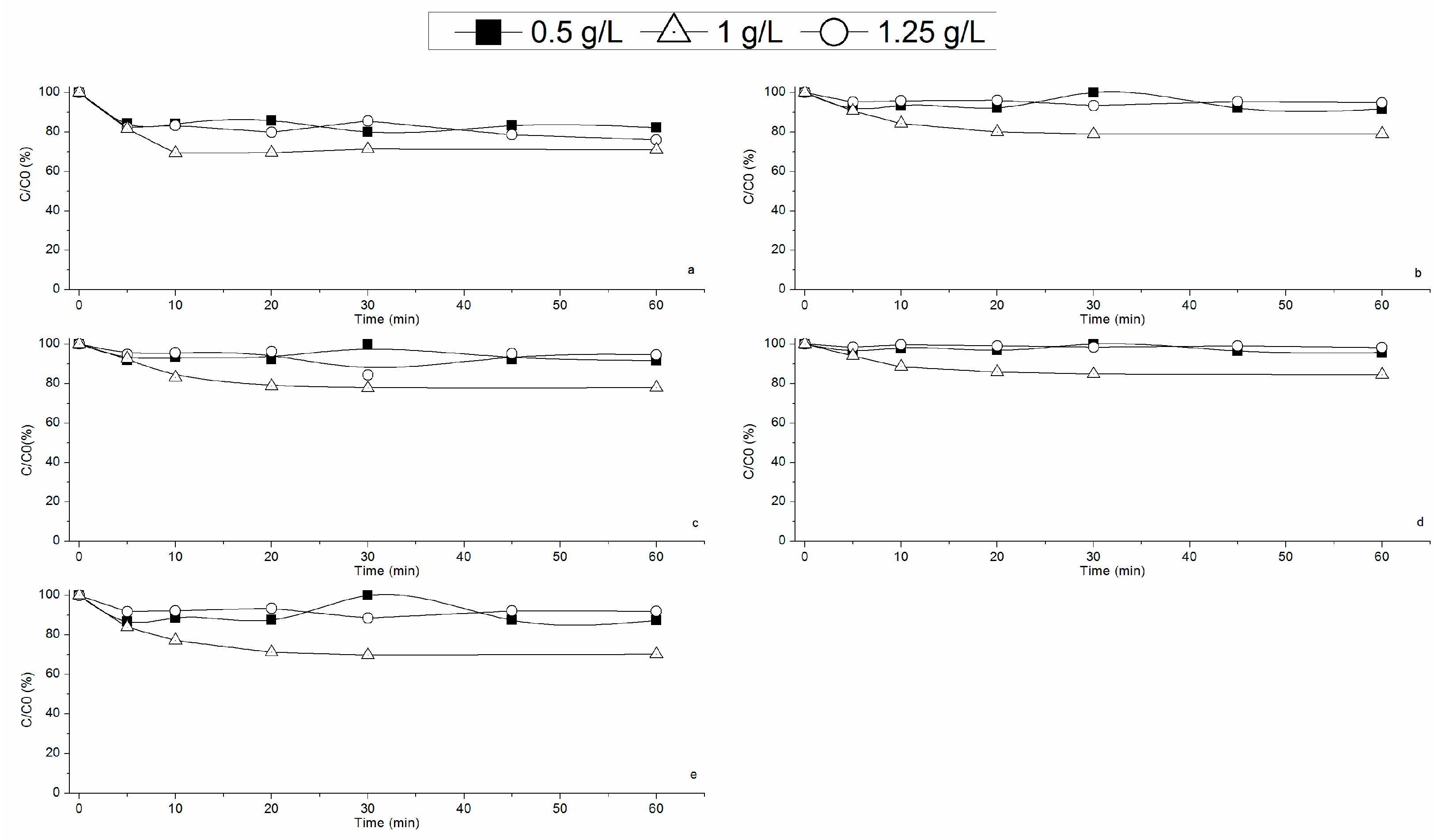
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domingues, E.; Assunção, N.; Gomes, J.; Lopes, D.V.; Frade, J.R.; Quina, M.J.; Quinta-Ferreira, R.M.; Martins, R.C. Catalytic Efficiency of Red Mud for the Degradation of Olive Mill Wastewater through Heterogeneous Fenton’s Process. Water 2019, 11, 1183. https://doi.org/10.3390/w11061183
Domingues E, Assunção N, Gomes J, Lopes DV, Frade JR, Quina MJ, Quinta-Ferreira RM, Martins RC. Catalytic Efficiency of Red Mud for the Degradation of Olive Mill Wastewater through Heterogeneous Fenton’s Process. Water. 2019; 11(6):1183. https://doi.org/10.3390/w11061183
Chicago/Turabian StyleDomingues, Eva, Nelson Assunção, João Gomes, Daniela V. Lopes, Jorge R. Frade, Margarida J. Quina, Rosa M. Quinta-Ferreira, and Rui C. Martins. 2019. "Catalytic Efficiency of Red Mud for the Degradation of Olive Mill Wastewater through Heterogeneous Fenton’s Process" Water 11, no. 6: 1183. https://doi.org/10.3390/w11061183
APA StyleDomingues, E., Assunção, N., Gomes, J., Lopes, D. V., Frade, J. R., Quina, M. J., Quinta-Ferreira, R. M., & Martins, R. C. (2019). Catalytic Efficiency of Red Mud for the Degradation of Olive Mill Wastewater through Heterogeneous Fenton’s Process. Water, 11(6), 1183. https://doi.org/10.3390/w11061183







