Effect of Different Radiation Sources and Noble Metal Doped onto TiO2 for Contaminants of Emerging Concern Removal
Abstract
1. Introduction
2. Materials and Methods
2.1. Photocatalysts Preparation and Chemicals
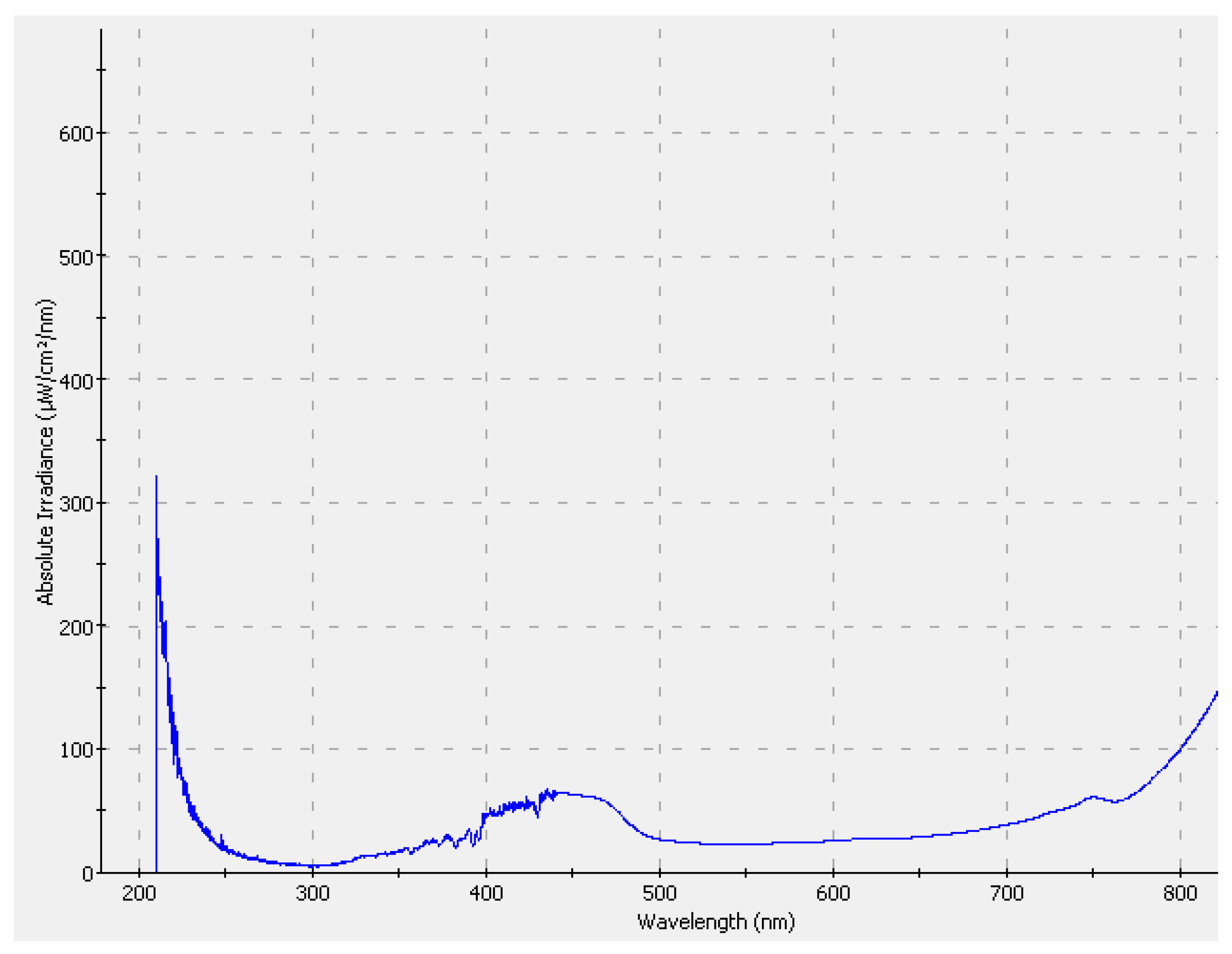
2.2. Experimental Methodology
2.3. Analytical Methods
3. Results
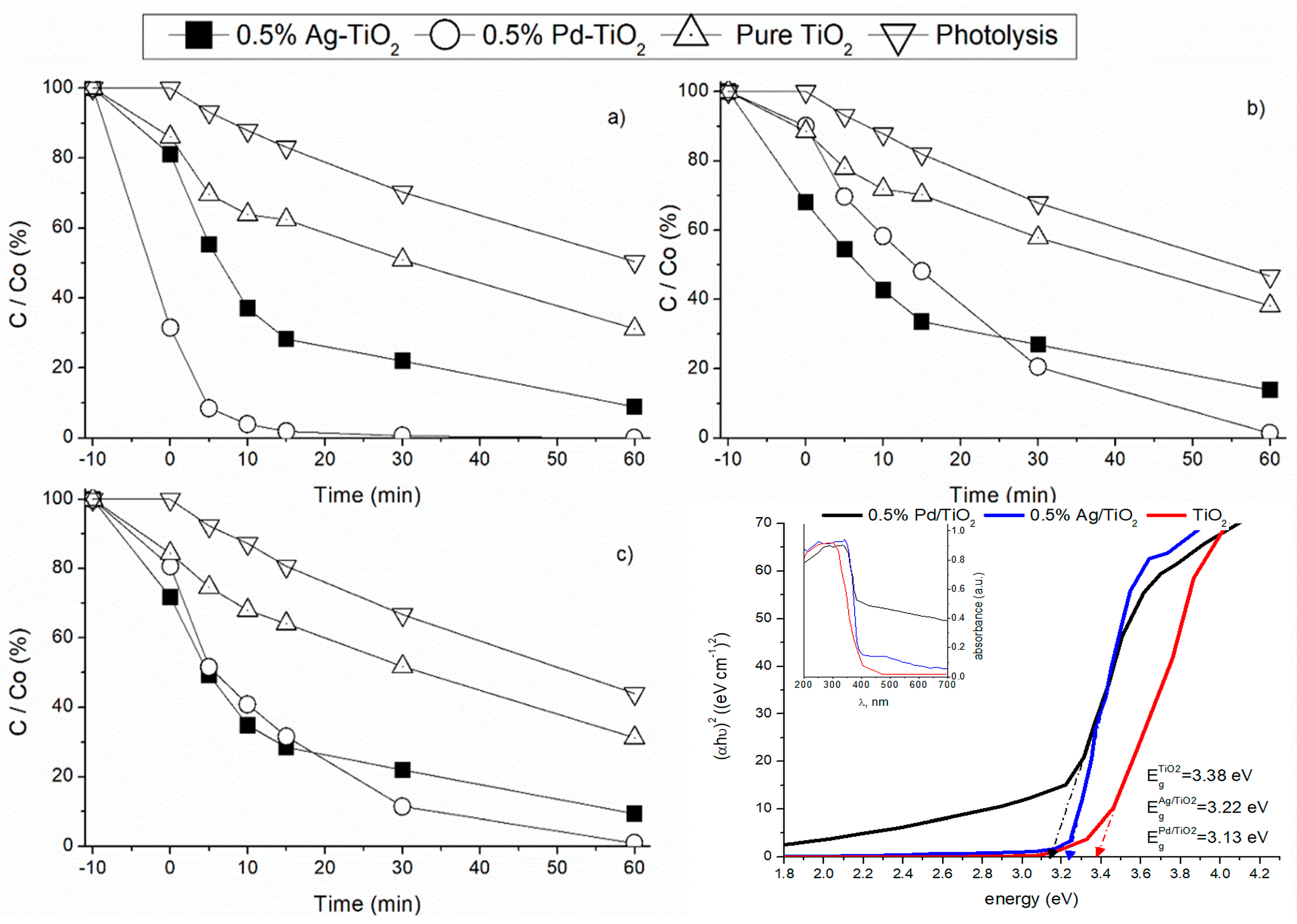
3.1. Effect of Noble Metals Doping on Photocatalytic Oxidation
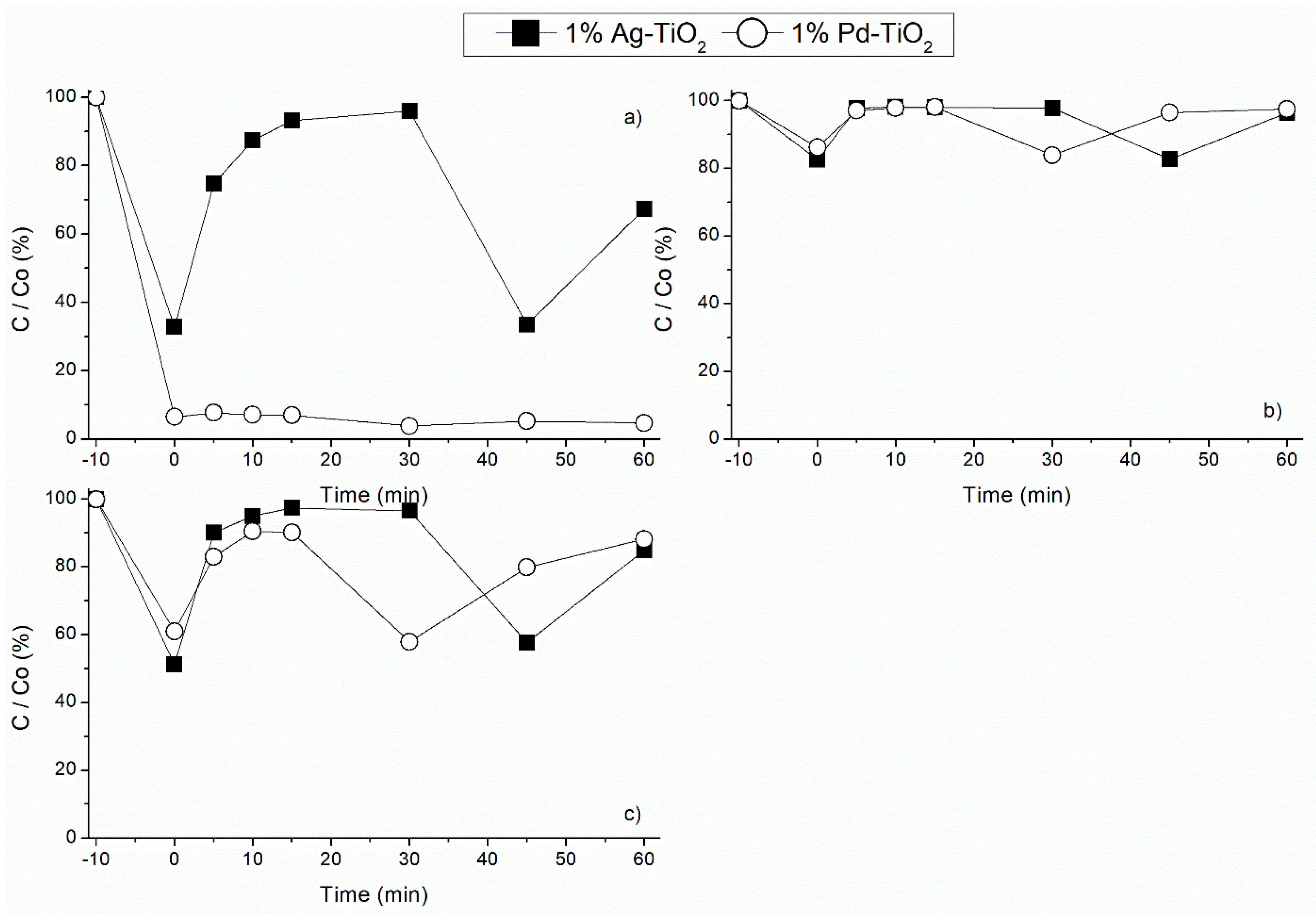
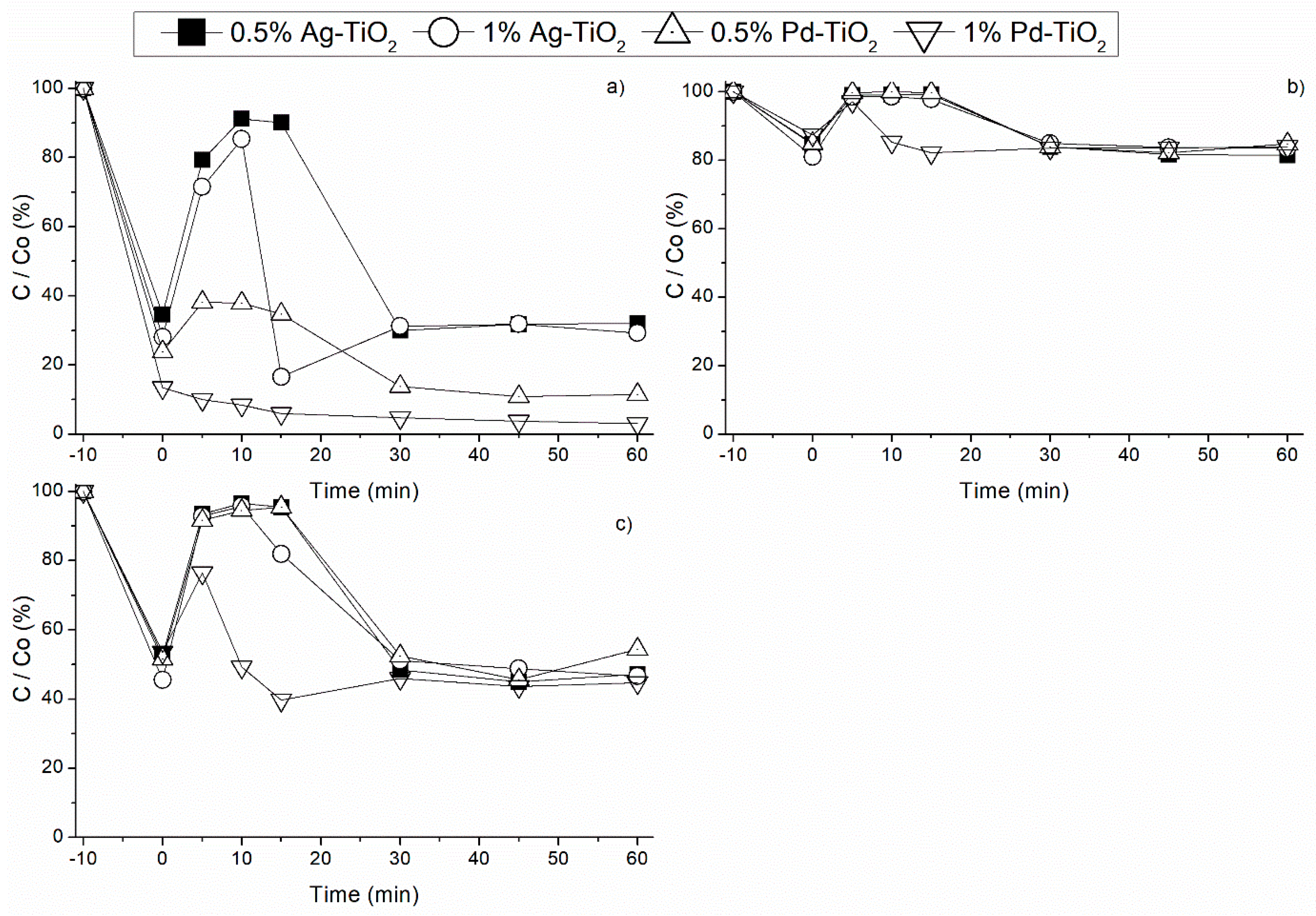
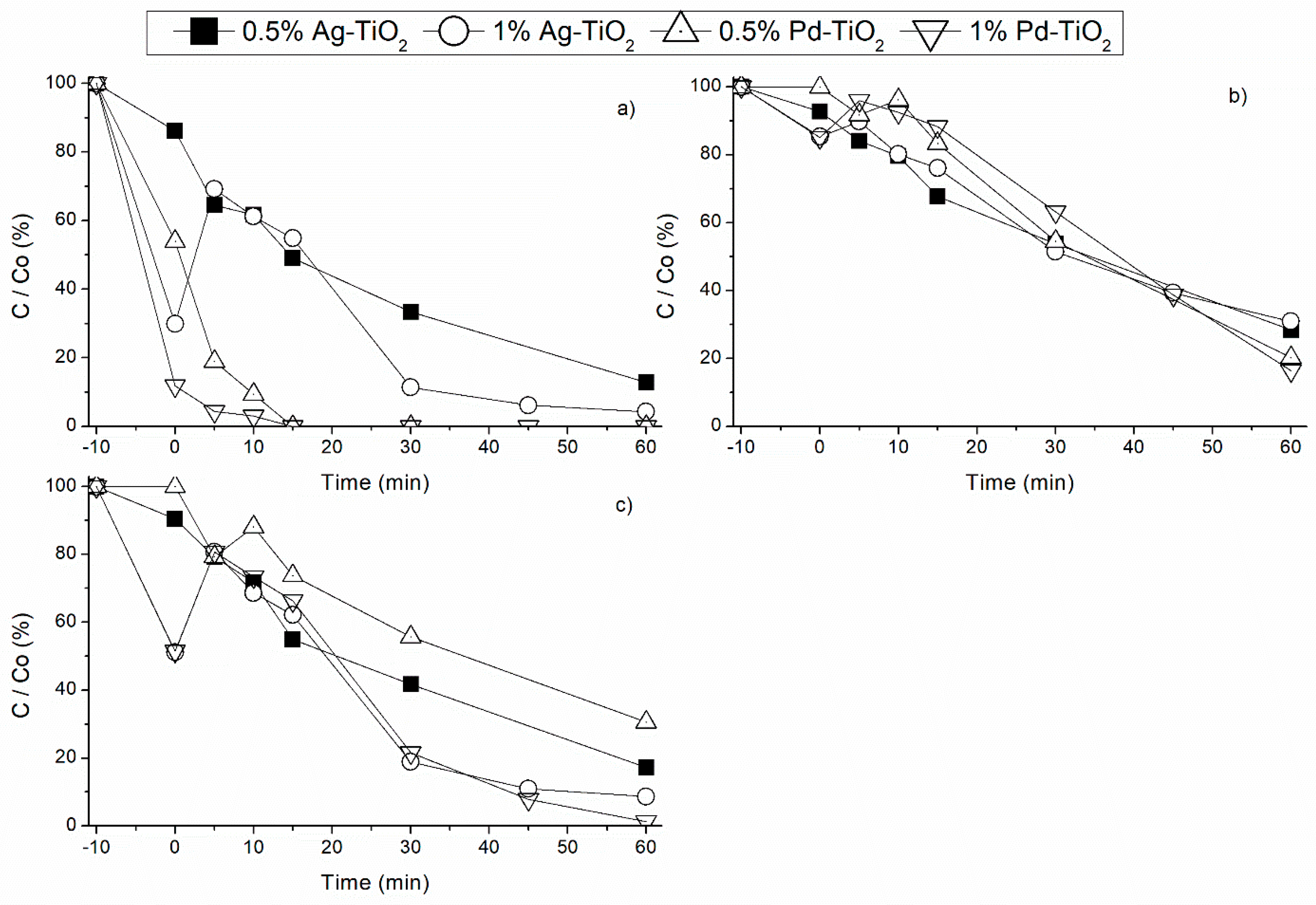
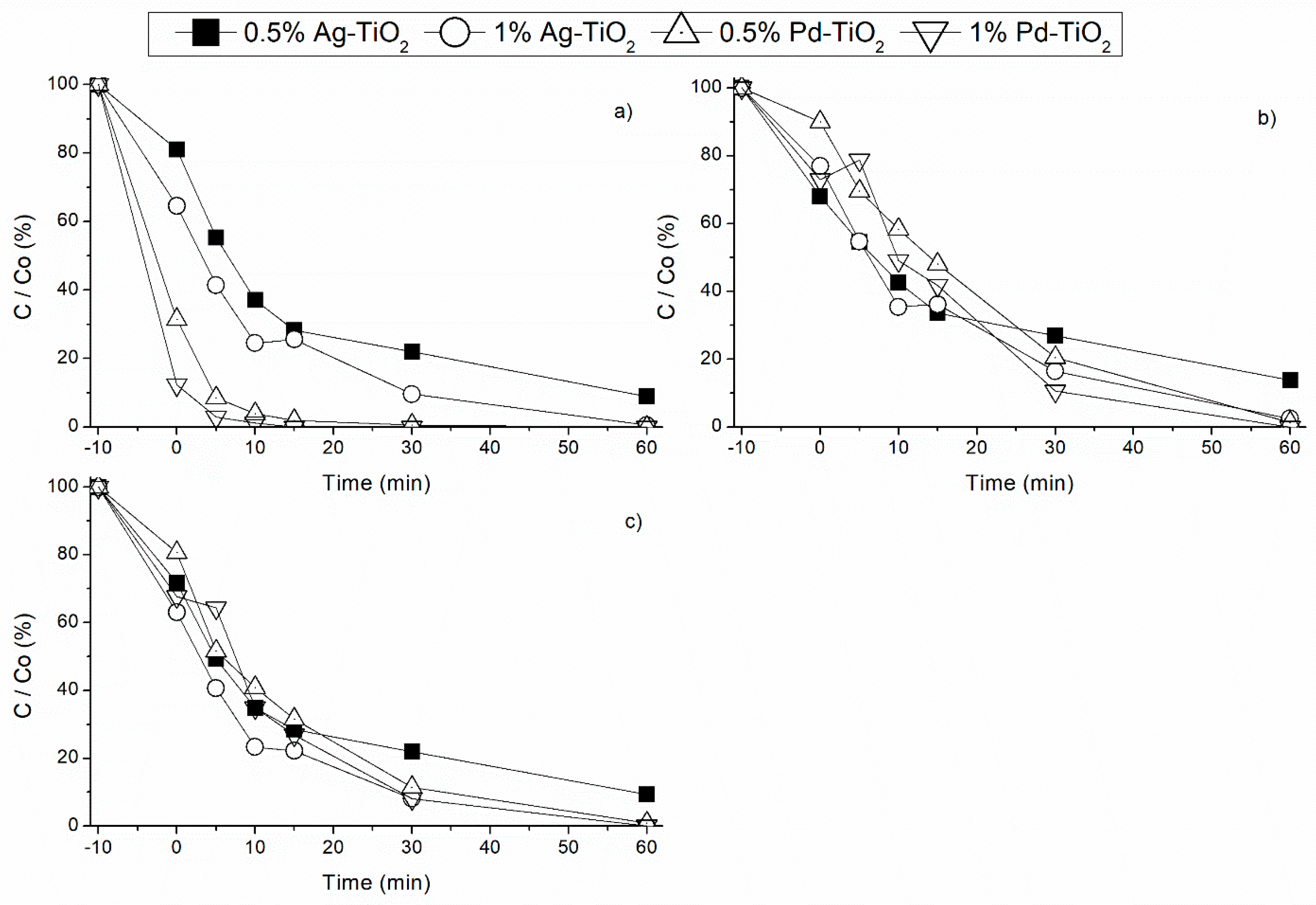
3.2. Noble Metal Load Effect under Different Radiation Sources
3.2.1. Adsorption Effect
3.2.2. Effect of Visible Light Radiation
3.2.3. Effect of UVA Radiation
3.2.4. Sunlight Radiation
3.3. Decontamination Kinetic Studies
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bosio, M.; Souza, B.; Saggioro, E.; Dezotti, A.; Bassin, J.P.; Quinta-Ferreira, E.; Quinta-Ferreira, R. Pharmaceutical compounds electrotreatment by Pt anodes and effect on synaptic function. Energy Procedia 2018, 163, 461–465. [Google Scholar] [CrossRef]
- Directive 2013/39/EU of the European Parliament and of the Council of 12 August 2013 Amending Directives 2000/60/EC and 2008/105/EC as Regards Priority Substances in the Field of Water Policy. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2013:226:0001:0017:EN:PDF (accessed on 24 April 2019).
- Commission Implementing Decision (EU) 2015/495 of 20 March 2015 Establishing a Watch List of Substances for Union-Wide Monitoring in the Field of Water Policy Pursuant to Directive 2008/105/EC of the European Parliament and of the Council. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32015D0495&from=PT (accessed on 24 April 2019).
- Yu, Y.; Huang, Q.; Wang, Z.; Zhang, K.; Tang, C.; Cui, J.; Feng, J.; Peng, X. Occurrence and behavior of pharmaceuticals, steroid hormones, and endocrine-disrupting personal care products in wastewater and the recipient river water of the Pearl River Delta, South China. J. Environ. Monit. 2011, 13, 871–878. [Google Scholar] [CrossRef]
- Valcárcel, Y.; Martínez, F.; González-Alonso, S.; Segura, Y.; Catalá, M.; Molina, R.; Montero-Rubio, J.C.; Mastroianni, N.; López de Alda, M.; Postigo, C.; Barceló, D. Drugs of abuse in surface and tap waters of the Tagus River basin: Heterogeneous photo-Fenton process is effective in their degradation. Environ. Int. 2012, 41, 35–43. [Google Scholar] [CrossRef]
- Rivera-Utrilla, J.; Sánchez-Polo, M.; Ferro-García, M.; Prados-Joya, G.; Ocampo-Pérez, R. Pharmaceuticals as emerging contaminants and their removal from water. A review. Chemosphere 2013, 93, 1268–1287. [Google Scholar] [CrossRef]
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci. Total Environ. 2014, 473–474, 619–641. [Google Scholar] [CrossRef] [PubMed]
- Heberer, T. Tracking persistent pharmaceutical residues from municipal sewage to drinking water. J. Hydrol. 2002, 266, 175–189. [Google Scholar] [CrossRef]
- Mompelat, S.; Le Bot, B.; Thomas, O. Occurrence and fate of pharmaceutical products and by-products, from resource to drinking water. Environ. Int. 2009, 35, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Bixio, D.; Thoeye, C.; Koning, J.; Joksimovic, S.; Savic, D.; Wintgens, T.; Melin, T. Wastewater reuse in Europe. Desalination 2006, 187, 89–101. [Google Scholar] [CrossRef]
- Gomes, J.; Costa, R.; Quinta-Ferreira, R.M.; Martins, R.C. Application of ozonation for pharmaceuticals and personal care products removal from water. Sci. Total Environ. 2017, 586, 265–283. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.; Lincho, J.; Domingues, E.; Quinta-Ferreira, R.M.; Martins, R.C. N–TiO2 Photocatalysts: A Review of Their Characteristics and Capacity for Emerging Contaminants Removal. Water 2019, 11, 373. [Google Scholar] [CrossRef]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.; Hamilton, J.W.; Byrne, J.A.; O’Shea, K.; Entezari, M.H.; Dionysiou, D.D. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef]
- Gomes, J.; Leal, I.; Bednarczyk, K.; Gmurek, M.; Stelmachowski, M.; Zaleska-Medynska, A.; Bastos, F.C.; Quinta-Ferreira, M.E.; Costa, R.; Quinta-Ferreira, R.M.; Martins, R.C. Detoxification of Parabens Using UV-A enhanced by Noble Metals – TiO2 Supported Catalysts. J. Environ. Chem. Eng. 2017, 5, 3065–3074. [Google Scholar] [CrossRef]
- Zheng, Z.K.; Huang, B.B.; Qin, X.Y.; Zhang, X.Y.; Dai, Y.; Whangbo, M.H. Facile in situ synthesis of visible-light plasmonic photocatalysts M@TiO2 (M = Au, Pt, Ag) and evaluation of their photocatalytic oxidation of benzene to phenol. J. Mater. Chem. 2011, 21, 9079–9087. [Google Scholar] [CrossRef]
- Wang, P.; Huang, B.; Dai, Y.; Whangbo, M. Plasmonic photocatalysts: Harvesting visible light with noble metal nanoparticles. Phys. Chem. Chem. Phys. 2012, 14, 9813–9825. [Google Scholar] [CrossRef] [PubMed]
- Petala, A.; Frontistis, Z.; Antonopoulou, M.; Konstantinou, I.; Kondarides, D.I.; Mantzavinos, D. Kinetics of ethyl paraben degradation by simulated solar radiation in the presence of N-doped TiO2 catalysts. Water Res. 2015, 81, 157–166. [Google Scholar] [CrossRef]
- Grabowska, E. Noble metal modified TiO2 microspheres: Surface properties and photocatalytic activity under UV–vis and visible light. J. Mol. Catal. A Chem. 2016, 423, 191–206. [Google Scholar] [CrossRef]
- Foszpańczyk, M.; Bednarczyk, K.; Drozdek, E.; Martins, R.C.; Ledakowicz, S.; Gmurek, M. Comparison of Photocatalytic and Photosensitized Oxidation of Paraben Aqueous Solutions under Sunlight. Water Air Soil Pollut. 2018, 229, 362. [Google Scholar] [CrossRef]
- Sousa, M.A.; Gonçalves, C.; Pereira, J.H.O.S.; Vilar, V.J.P.; Boaventura, R.A.R.; Alpendurada, M.F. Photolytic and TiO2-assisted photocatalytic oxidation of theanxiolytic drug lorazepam (Lorenin® pills) under artificial UV light and natural sunlight: A comparative and comprehensive study. Sol. Energy 2013, 87, 219–228. [Google Scholar] [CrossRef]
- Oros-Ruiz, S.; Zanella, R.; Prado, B. Photocatalytic degradation of trimethoprim by metallic nanoparticles supported on TiO2-P25. J. Hazar. Mater. 2013, 263, 28–35. [Google Scholar] [CrossRef]
- Mohamed, M.M.; Khairou, K.S. Preparation and characterization of nano-silver/mesoporous titania photocatalysts for herbicide degradation. Microporous Mesoporous Mater. 2011, 142, 130–138. [Google Scholar] [CrossRef]
- Peng, J.; Wang, S. Performance and characterization of supported metal catalysts for complete oxidation of formaldehyde at low temperature. Appl. Catal. B Environ. 2007, 73, 282–291. [Google Scholar] [CrossRef]
- Gomes, J.; Bednarczyk, K.; Gmurek, M.; Stelmachowski, M.; Zaleska-Medynska, A.; Bastos, F.C.; Quinta-Ferreira, M.E.; Costa, R.; Quinta-Ferreira, R.M.; Martins, R.C. Noble metal—TiO2 Supported Catalysts for the Catalytic Ozonation of Parabens Mixtures. Process Saf. Environ. Protect. 2017, 111, 148–159. [Google Scholar] [CrossRef]
- Gomes, J.; Leal, I.; Bednarczyk, K.; Gmurek, M.; Stelmachowski, M.; Diak, M.; Quinta-Ferreira, M.E.; Costa, R.; Quinta-Ferreira, R.M.; Martins, R.C. Photocatalytic Ozonation using doped TiO2 Catalysts for the Removal of Parabens in Water. Sci. Total Environ. 2017, 609, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.; Lopes, A.; Bednarczyk, K.; Gmurek, M.; Stelmachowski, M.; Zaleska-Medynska, A.; Quinta-Ferreira, M.E.; Costa, R.; Quinta-Ferreira, R.M.; Martins, R.C. Effect of noble metals (Ag, Pd, Pt) loading over the efficiency of TiO2 during photocatalytic ozonation on the toxicity of parabens. Chemengineering 2018, 2, 4. [Google Scholar] [CrossRef]
- Kuhn, H.J.; Braslavsky, S.E.; Schmidt, R. Chemical Actinometry (IUPAC Technical Report). Pure Appl. Chem. 2004, 76, 2105–2146. [Google Scholar] [CrossRef]
- Martins, R.; Quinta-Ferreira, R. Catalytic ozonation of phenolic acids over a Mn-Ce-O catalyts. Appl. Catal. B Environ. 2009, 90, 268–277. [Google Scholar] [CrossRef]
- Velegraki, T.; Hapeshi, E.; Fatta-Kassinos, D.; Poulios, I. Solar-induced heterogeneous photocatalytic degradation of methyl-paraben. Appl. Catal. B Environ. 2015, 178, 2–11. [Google Scholar] [CrossRef]
- Bouarioua, A.; Zerdaoui, M. Photocatalytic activities of TiO2 layers immobilized on glass substrates by dip-coating technique towards decolorization of methyl orange as a model pollutant. J. Environ. Chem. Eng. 2017, 5, 1565–1574. [Google Scholar] [CrossRef]
- Luo, S.; Wei, Z.; Spinney, R.; Zhang, Z.; Dionysiou, D.D.; Gao, L.; Chai, L.; Wang, D.; Xiao, R. UV direct photolysis of sulfamethoxazole and ibuprofen: An experimental and modelling study. J. Hazar. Mater. 2018, 343, 132–139. [Google Scholar] [CrossRef]
- Bahruji, H.; Bowker, M.; Davies, P.R.; Al-Mazroai, L.S.; Dickinson, A.; Greaves, J.; James, D.; Millard, L.; Pedrono, F. Sustainable H2 gas production by photocatalysis. J. Photochem. Photobiol. A Chem. 2010, 216, 115–118. [Google Scholar] [CrossRef]
- Zanella, R.; Avella, E.; Ramírez-Zamora, R.M.; Castillón-Barraza, F.; Durán-Álvarez, J.C. Enhanced photocatalytic degradation of sulfamethoxazoleby deposition of Au, Ag and Cu metallic nanoparticles on TiO2. Environ. Technol. 2018, 39, 2353–2364. [Google Scholar] [CrossRef]
- Gaya, U.I.; Abdullah, A.H. Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: A review of fundamentals, progress and problems. J. Photochem. Photobiol. C Photochem. Rev. 2008, 9, 1–12. [Google Scholar] [CrossRef]
- Zhang, L.; Tse, M.S.; Tan, O.K. Facile in situ synthesis of visible light-active Pt/C−TiO2 nanoparticles for environmental remediation. J. Environ. Chem. Eng. 2014, 2, 1214–1220. [Google Scholar] [CrossRef]
- Kryukova, G.N.; Zenkovets, G.A.; Shutilov, A.A.; Wilde, M.; Gunther, K.; Fassler, D.; Richter, K. Structural peculiarities of TiO2 and Pt/TiO2 catalysts for the photocatalytic oxidation of aqueous solution of acid Orange 7 Dye upon ultraviolet light. Appl. Catal. B Environ. 2007, 71, 169–176. [Google Scholar] [CrossRef]
- Kowalska, E.; Remita, H.; Colbeau-Justin, C.; Hupka, J.; Belloni, J. Modification of Titanium Dioxide with Platinum Ions and Clusters: Application in Photocatalysis. J. Phys. Chem. C. 2008, 112, 1124–1131. [Google Scholar] [CrossRef]
- Zielińska, A.; Kowalska, E.; Sobczak, J.W.; Łacka, I.; Gazda, M.; Ohtani, B.; Hupka, J.; Zaleska, A. Silver-doped TiO2 prepared by microemulsion method: Surface properties, bio- and photoactivity. Sep. Purif. Technol. 2010, 72, 309–318. [Google Scholar] [CrossRef]
- Martínez, C.; Canle, L.M.; Fernández, M.I.; Santaballa, J.A.; Faria, J. Kinetics and mechanism of aqueous degradation of carbamazepine by heterogeneous photocatalysis using nanocrystalline TiO2, ZnO and multi-walled carbon nanotubes anatase composites. Appl. Catal. B Environ. 2011, 102, 563–571. [Google Scholar] [CrossRef]
- Xekoukoulotakis, N.P.; Drosou, C.; Brebou, C.; Chatzisymeon, E.; Hapeshi, E.; Fatta-Kassinos, D.; Mantzavinos, D. Kinetics of UV-A/TiO2 photocatalytic degradation and mineralization of the antibiotic sulfamethoxazole in aqueous matrices. Catal. Today 2011, 161, 163–168. [Google Scholar] [CrossRef]
- Bayarri, B.; Abellán, M.N.; Giménez, J.; Esplugas, S. Study of the wavelength effect in the photolysis and heterogeneous photocatalysis. Catal. Today 2007, 129, 231–239. [Google Scholar] [CrossRef]
- Abellán, M.N.; Bayarri, B.; Giménez, J.; Costa, J. Photocatalytic degradation of sulfamethoxazole in aqueous suspension of TiO2. Appl. Catal. B Environ. 2007, 74, 233–241. [Google Scholar] [CrossRef]
- Rizzo, L.; Meric, S.; Guida, M.; Kassinos, D.; Belgiorno, V. Heterogenous photocatalytic degradation kinetics anddetoxification of an urban wastewater treatment plant effluent contaminated with pharmaceuticals. Water Res. 2009, 43, 4070–4078. [Google Scholar] [CrossRef]
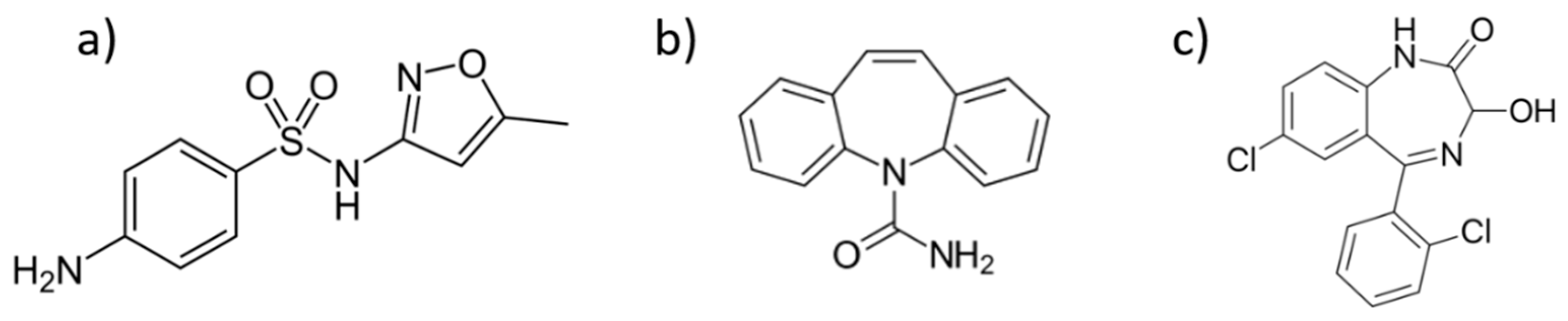

| Catalyst | UVA Radiation | Sunlight Radiation | ||||
|---|---|---|---|---|---|---|
| k’SMX (min−1) (R2) | k’CBZ (min−1) (R2) | k’LRZ (min−1) (R2) | k’SMX (min−1) (R2) | k’CBZ (min−1) (R2) | k’LRZ (min−1) (R2) | |
| 0.5%Pd–TiO2 | 0.203 (0.993) | 0.023 (0.921) | 0.020 (0.991) | 0.239 (0.994) | 0.047 (0.991) | 0.066 (0.989) |
| 1%Pd–TiO2 | 0.179 (0.980) | 0.024 (0.940) | 0.048 (0.940) | 0.274 (0.998) | 0.052 (0.920) | 0.064 (0.954) |
| 0.5%Ag–TiO2 | 0.032 (0.978) | 0.019 (0.993) | 0.028 (0.987) | 0.059 (0.930) | 0.033 (0.940) | 0.050 (0.920) |
| 1%Ag–TiO2 | 0.054 (0.934) | 0.020 (0.992) | 0.047 (0.960) | 0.074 (0.967) | 0.057 (0.971) | 0.080 (0.980) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, R.C.; Domingues, E.; Bosio, M.; Quina, M.J.; Gmurek, M.; Quinta-Ferreira, R.M.; Gomes, J. Effect of Different Radiation Sources and Noble Metal Doped onto TiO2 for Contaminants of Emerging Concern Removal. Water 2019, 11, 894. https://doi.org/10.3390/w11050894
Martins RC, Domingues E, Bosio M, Quina MJ, Gmurek M, Quinta-Ferreira RM, Gomes J. Effect of Different Radiation Sources and Noble Metal Doped onto TiO2 for Contaminants of Emerging Concern Removal. Water. 2019; 11(5):894. https://doi.org/10.3390/w11050894
Chicago/Turabian StyleMartins, Rui C., Eva Domingues, Morgana Bosio, Margarida J. Quina, Marta Gmurek, Rosa M. Quinta-Ferreira, and João Gomes. 2019. "Effect of Different Radiation Sources and Noble Metal Doped onto TiO2 for Contaminants of Emerging Concern Removal" Water 11, no. 5: 894. https://doi.org/10.3390/w11050894
APA StyleMartins, R. C., Domingues, E., Bosio, M., Quina, M. J., Gmurek, M., Quinta-Ferreira, R. M., & Gomes, J. (2019). Effect of Different Radiation Sources and Noble Metal Doped onto TiO2 for Contaminants of Emerging Concern Removal. Water, 11(5), 894. https://doi.org/10.3390/w11050894










