Abstract
A novel biochar alginate composite adsorbent was synthesized and applied for removal of Zn2+ ions from aqueous solution. Kinetics, equilibrium and thermodynamic studies showed the suitability of the adsorbent. From a Langmuir isotherm study, the maximum monolayer adsorption capacity of the composite adsorbent was found to be 120 mg/g. To investigate the effect of process variables like initial Zn2+ concentration (25–100 mg/L), adsorbent dose (0.4–8 g/L) and temperature (298–318 K) on Zn2+ adsorption, response surface methodology (RSM) based on a three independent variables central composite design of experiments was employed. A quadratic model equation was developed to predict the relationship between the independent variables and response for maximum Zn2+ removal. The optimization study reveals that the initial Zn2+ concentration and adsorbent dose were the most effective parameters for removal of Zn2+ due to higher magnitude of F-statistic value which effects to a large extent of Zn2+ removal. The optimum physicochemical condition for maximum removal of Zn2+ was determined from the RSM study. The optimum conditions are 43.18 mg/L initial metal ion concentration, 0.062 g adsorbent dose and a system temperature of 313.5 K. At this particular condition, the removal efficiency of Zn2+ was obtained as 85%.
1. Introduction
In the era of industrialization contamination of water bodies by heavy metals have become an alarming threat for the mankind and aquatic flora and fauna [1]. The metals which are 6 times heavier than water are identified as heavy metals [2]. Among all, Zn2+ is one of the most commonly found heavy metal ions. Nevertheless, Zn2+ is a very vital micronutrient element for the human body and other natural flora, but its presence above a permissible limit become very hazardous and creates several health problems like skin problem, stomach and kidney diseases [3]. Various process industries like cosmetics, iron and brass, galvanizing, electroplating etc. are the primary sources of Zn2+ ions in wastewater [4]. Over the last few decades, a lot of methods have been reported for the elimination of such hazardous heavy metals from wastewater. Some widely used treatment methods are chemical precipitation [5], ion exchange [6], membrane technology [7] and adsorption [8]. However, some drawbacks are also encountered in these conventional techniques like huge sludge production and discarding, the high cost of operation, and high power intakes [9]. Therefore, biosorption attracts the attention of the researchers to find out a low-cost and environmentally safe method. Various adsorbents derived from various natural sources have already been adopted as an efficient alternative for heavy metal removal. The research article by Yagub et al. [10] also attracts the focus of the researchers on this field of research. In industrial application Commercial Grade Activated Carbon (CAC) has been extensively used for heavy metal ion removal from wastewater but its high cost and difficulties in reinforcement have motivated researchers to find substitutes to CAC but low-cost potential adsorbents. Therefore many low-cost and efficient bio adsorbent from various sources like an industrial solid waste, agricultural and dairy waste have been reported by many researchers [10]. Waste biomass from various sources converted into biochar by pyrolysis method has become an efficient adsorbent for removal of both heavy metals and organic pollutants from industrial effluent. Over the last few years, algae have become one of the valuable and efficient alternatives for bioremediation of heavy metals present in wastewater and attract the attention of the environment researchers. Moreover, the effectiveness of heavy metal removal by nonliving algae has been observed by many researchers because it has cellulose, glycoproteins etc. like biopolymeric structure. The non-living algae contain many active chemical groups like CH−, OH−, and COO−. The presence the active groups play a very significant role in heavy metal removal. The active groups present in the algae attract positively charged metal ions and create a surface complex. It also enhances the electrostatic charge separation. Therefore, we have previously reported sustainable cost-effective novel Ca-alginate-biochar composite (CABC) adsorbent and tested its synergetic effectiveness in the removal of aqueous phase Zn2+ metal ions [11]. Various process parameters have been identified but the experimental determination of a wide range of optimum process parameters are not a feasible option. Therefore Response Surface Methodology (RSM) has been employed here to model statistically and optimize the process variable for removal of Zn2+ metal ions by this novel CABC adsorbent which is a new aspect here. Although RSM is a very old optimization tool, its application in the field of Zn2+ adsorption by Ca-alginate-biochar composite adsorbent is limited and hence this study was undertaken. In recent years a number of statistical design and optimization techniques are successfully adopted in the various industrial sector for optimizing the process variables of leaching and beneficiation of coal, condition optimization for preparation of activated carbon and many more [12,13,14,15,16,17]. The optimization of process variables for adsorptive removal of heavy metal or other organic pollutants is also reported [18,19,20]. However, optimization of adsorption of Zn2+ on such novel composite adsorbent has not been reported yet. The conventional techniques use one factor at a time [16]. For a multivariable system use of conventional technique requires a huge number of experiments to determine the optimum conditions which require a long time. Moreover, it does not represent the interaction effect among the independent variables. Therefore, the technique may not be highly reliable. Hence, the primary objective of the design of the experiment is to understand the interaction effect which helps in optimizing the experimental parameters and provide a reliable statistical model which has wide applications.
Therefore, the current work investigates the combined effects of independent variables on aqueous phase Zn2+ removal on the composite adsorbent. Statistical analysis has been carried out to observe the significant effect of process variables such as temperature, contact time, metal dose, and adsorbent dose on Zn2+ removal using the composite adsorbent. Moreover, the main focus of the research is to determine the optimum condition of those process variables to obtain the maximum Zn2+ removal efficiency for its application in large-scale operation.
2. Materials and Method
Laboratory reagent grade Zn(NO3)2 6H2O salt was dissolved in ultrapure water to make a stock solution of the Zn2+ metal ion. All of the working solutions for the present experiments were made by a serial dilution process from the stock solution. The pH of the aqueous Zn2+ solution was controlled using 0.1 N HCl and 0.1 N NaOH solution. The alginate biochar composite was prepared by mixing 4:1 (w/v %) Na-Alginate and Biochar with a magnetic stirrer for 6 h and bubbles were eradicated by sonication. Then the homogeneous mixture of biochar and alginate was put dropwise in freshly prepared 3 (w/v %) chilled CaCl2·2H2O to obtain the composite adsorbent. All the laboratory reagent grade chemicals were procured from Sigma Aldrich Co.
A batch experiment has been conducted to investigate the kinetics and removal efficiency of Zn2+ on the novel composite adsorbent. For the experiment, 100 mL of a Zn2+ solution of a wide concentration range (25–100 mg/L) was prepared and the pH of the aqueous solution was maintained at 5.5. The calculated amount of composite adsorbent was mixed with the metal solution in a series of bottles representing small reactors. A constant temperature shaker was used to shake the mixture at 120 rpm for 180 min to achieve the equilibrium. At different time interval, liquid samples were collected, filtered with 45-micron filter paper and measured the concentration retained in the working solution. ICP-OES (Inductively coupled Plasma–Optical Emission spectrometry) model no Optima 8300, Perkin Elmer was employed to quantify the residual metal ion concentration.
The following equation has been used to compute the efficiency of the adsorbent removal efficiency (%)
where and are the initial concentration and concentration at any time of Zn2+, mg/L.
For the regression analysis of the actual data set obtained from the experiment, the software Design-Expert version 7.1 has been used in this present investigation. With the help of Analysis of variance or ANOVA (Analysis Of Variance), statistical parameters were determined. The significance of statistical analysis was checked in terms of F-test. The precision of the fitted polynomial model was confirmed from the high correlation coefficient (R2) value, 95% confidence interval had been used to evaluate the p-value to check the significance of the model.
3. Theory of Response Surface Methodology (RSM) Optimization Technique
The response surface methodology (RSM) can be defined as a set of mathematical and statistical tool or technique to build some empirical models. The objective of the optimization tool is to define a regression-based model and optimize an output variable (called response), which is governed by a number of independent input variables. Set of experiments were performed by changing all the input variables to identify the reason for output or response variable change. The RSM tool is employed in design optimization to minimize or reduce the cost of expensive analysis (e.g., finite element method or CFD analysis) and the numerical noise associated with the analysis.
Here, an attempt has been made to analyze the effect of operating parameters for Zn2+ removal by Alginate biochar composite adsorbent by the response surface methodology (RSM) system by the central composite design (CCD) technique. For a quadratic surface fitting, this method is very suitable and it optimizes the independent system variables with less number of experimental data set. Moreover, this method is efficient to find the interacting effects between the operating parameters.
The pictorial view of the CCD technique has been represented in Figure 1. From the Figure, it is understandable that in this method there are 2n factorial runs (n is the no of independent variables) improved with 2n axial runs and nc is the central runs to estimate the experimental errors. As this is a three-factor experiment the experiment is designed and coded as (±1) notation and all the axial points are (±α, 0, 0, 0), (0, ±α, 0, 0), (0, 0, ±α, 0), (0, 0, 0, ±α) respectively. All of the center points are at (0, 0, 0, 0) coordinate. This CCD model predicts the optimum condition more accurately over the normal factorial design because of axial points data and central point data [16]. In addition, this model is further improved with compared to Box-Behnken model due to more data points used for optimization calculation.
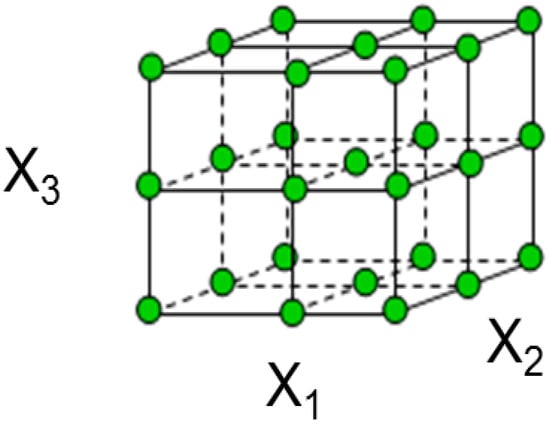
Figure 1.
A pictorial view of CCD design.
All of the independent variables were examined at two levels. According to the theory as the numbers of independent parameters (n) increase, total numbers of experimental runs increase rapidly for every full repetition of the design. It has been reported that discrete 2nd Order effect cannot be projected distinctly by 2n factorial design only. Hence, the CCD technique was adopted for investigating the quadratic effect and develops the model equation for the removal of aqueous phase Zn2+ ion by the composite adsorbent.
The output or response (removal efficiency) with conforming independent system variables was modeled to optimize the system variables for the preferred response using the statistical analysis. ANOVA was used to compute all the statistical constraints with the benefit of a response surface technique.
There are three major steps involved in the process optimization by RSM technique. The steps are, statistically experiment design, estimation of coefficients in the mathematical model and prediction of the response and check the accuracy within the range of experimental variables. In the current research, three independent operating parameters were selected for the statistical analysis. The parameters are the initial metal ion dose (, mg/L), adsorbent dose (, mg) and system temperature (, K). The level and range of the factor change consequently with the experimental design. All the independent parameters at their specific ranges were observed as significant parameters for efficient Zn2+ ion removal from wastewater on the composite adsorbent. Therefore, the response is represented as a function of all independent variables.
where is the response or output variables and are independent variables. An empirical model equation has been developed which correlate the response, removal efficiency with process variables by a second-degree polynomial equation and reproduced below in Equation (3):
where Y is the predicted response, A0, Ai, Aii, Aij are constant, linear, quadratic, and interaction coefficient respectively. Xi, Xi2, Xj are level of independent variables.
A number of tests (N) can be performed by considering the typical factorial design consisting origin at the center as mentioned earlier. A quadratic term was generated by the axial fixing of the 2n points at the distance α from the center. Independent variable was defined as (n). In a CCD design for three independent variables in these present experiments, there are 8 factorial points, 6 axial points, and 6 replicate at the central points. Hence, the total number of tests (N) necessary for 3 independent process variables can be estimated from the Equation (4):
4. Results and Discussion
From elaborate kinetic and isotherm study, it has been found that Zn2+ metal removal efficiency reduces with increasing the initial concentration of Zn2+. As the concentration of initial metal ion increases, there are excess Zn2+ ions with respect to available active sites on the adsorbent. Therefore, the increase of initial metal ion concentration inhibits the removal efficiency. The experimental data shows that for an initial Zn2+ dose of 25 mg/L, percentage removal was almost 90% and when the concentration of Zn2+ increases to 100 mg/L percentage removal was only 70%.
The adsorbent dose is one of the most significant parameters for the removal of any metal ion from its aqueous solution. It is the parameter which decides the capacity of any adsorbent and plays a significant role for design development and process optimization of any large scale adsorption column. In this present study, the effect of adsorbent dose for removal of Zn2+ has been investigated by using 50 mg/L metal ion solution, system temperature was fixed at 298 K and pH was adjusted at 5.5. The adsorbent dose was varied from 0.04 g to 0.8 g per 100 mL of working solution. From the experimental results, a sharp increase in percentage removal from 31% to 88% was obtained when the adsorbent dose was increased from 0.04 g to 0.8 g keeping the other parameters constant.
Effect of system temperature revealed that the removal efficiency of Zn2+ on the novel composite adsorbent is endothermic. When the system temperature raises from 298 K to 308 K percentage removal increases from 71% up to 86%. With further increase in temperature the removal efficiency increases but the change is not much significant i.e., only 4% increase is obtained. Therefore it can be concluded that the use of the adsorbent is advantageous for industrial application at room temperature. In order to develop the model and optimize the operating parameters, we have designed the experiment as suggested by the Design Expert software and all the details experimental runs are tabulated in Table 1. From this table, it is evident that maximum percentage removal was 89% at run number 9 where the operating conditions were, initial concentration 25 mg/L, adsorbent dose 0.42 g and system temperature 308 K. At the same time, the minimum percentage removal was obtained at run 2 which was 72%, for this run the operating parameters were, initial metal ion concentration 84.80 mg/L, adsorbent dose 0.19 g and system temperature 302.5 K. In order to carry out this present work and as per the design method we have taken the data sets of all the highest and lowest point of all the parameters at the same time we have taken many data points in between. This makes the experimental results more reliable and software also able to provide a very accurate model for better understanding of the work. The proper design saves experimental time and experimental cost also.

Table 1.
Detail Experimental runs for central composite design with factor values in the coded form and respective responses/output.
4.1. Model Development
The statistical parameters were assessed from the analysis of variance. In this Zn2+ removal study by the composite adsorbent, the ranges of independent experimental variables and coded variables are presented in Table 1, Table 2 and Table 3.

Table 2.
The coded form and the actual level of the independent variables.

Table 3.
Detail scope of experiments and range of the operating parameters.
The effects of all model terms were evaluated. Statistical parameters like F-value, , adj , predicted and lack of fit were evaluated and compared with the experimental results for the reliability of the model. 95% confidence interval was taken into consideration for finding the significance of factors and their interactions on the responses. Based on all the analysis a quadratic model equation has been developed and all the ANOVA results are tabulated in Table 4.

Table 4.
ANOVA table for Zn2+ removal efficiency on the composite adsorbent.
Final Equation in Terms of Code Factors:
Zn2+ Removal
Final Equation in Terms of Actual Factors:
Zn2+ Removal (%)
where, concentration stands for, initial metal ion concentration (mg/L), dose is amount adsorbent used (g), temp is system temperature (K) respectively.
In a model like this, lack of fit is undesirable. Basically, lack of fit is a comparison between the residual error and the pure error [21]. Hence, a small value of F and probability more than 0.1 are desired for the prediction of response.
In this present study, the F value of the model is obtained as 30.13 indicating that the model is significant. In addition, , , are also significant model terms with significant F-values.
The ratio between signals to noise was quantified in term of adequacy precision, including the expected values at different design points and the average predicted errors. A desirable value of adequacy precision ratio is reported as 4 [22]. The value obtained in the present study is much higher than the desired value. Therefore, the developed model is suitable for governing the design space. Also, this is the principal part of the predicted model to validate the present experimental data analysis. The normal probability versus the studentized residual plot is presented in Figure 2 for Zn2+ removal from wastewater. Figure 2 reveals that response alteration is not there and no major problem with the normality.
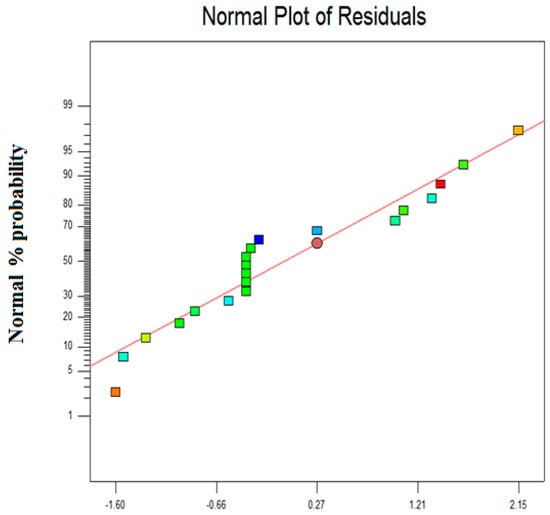
Figure 2.
The normal probability vs studentized residual plot.
Figure 3 represents the studentized residual versus predicted values for Zn2+ removal. This indicates that all the response values, distributed plot, the variance of the original observations are constant. This also indicates that there is no requirement of actual data transformations.
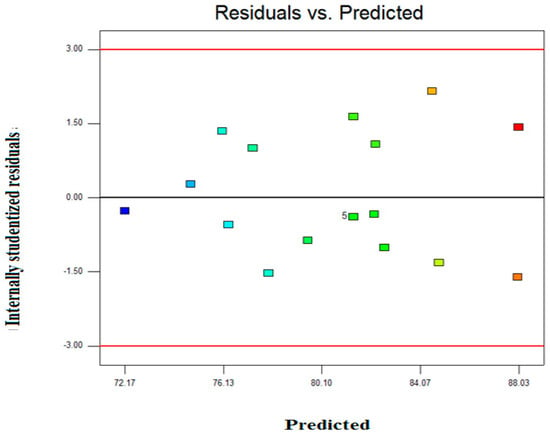
Figure 3.
Internally studentized residual versus predicted Zn2+ removal.
The actual vs model predicted percentage for Zn2+ removal is presented in Figure 4. From Figure 4, it was found that the regression coefficient and adjusted were 97% and 93%, respectively. These values of illustrates to what extent model can perfectly estimate the experimental data and adjusted signifies the variation of mean described by the developed model. In addition, the predicted value is close to value and therefore experimental data for Zn2+ removal by novel composite adsorbent are well in line with the predicted values from the model equation.
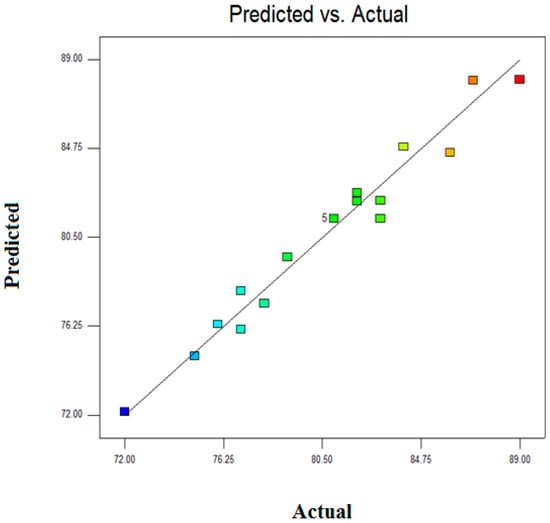
Figure 4.
Actual vs predicted Zn2+ removal.
The statistical results obtained from the design expert software have been summarized in Table 5.

Table 5.
Statistical result for the removal of Zn2+ on the novel adsorbent.
4.2. Combined Effect of Independent Parameters on Zn2+ Removal
For the investigation of individual effect and combined effects of three factors on Zn2+, response surface methodology has been used. From the ANOVA results, the effects of various experimental factors in terms of the three-dimensional response surfaces are represented in Figure 5, Figure 6 and Figure 7. The response model is presented in Equation (5). Initial metal ion concentration, adsorbent dose and temperature affect significantly on aqueous phase Zn2+. The analysis reveals that initial concentration () has a major effect on Zn2+ removal with respect to other process variables. This is because of the high F value (116.14) for initial concentration. However, the combined effects of the process variables are not significant for this present case.
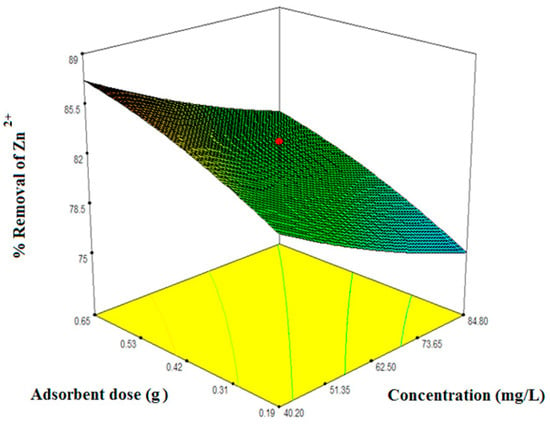
Figure 5.
Interacting effects of metal ions concentration and adsorbent dose at temperature 308 K.
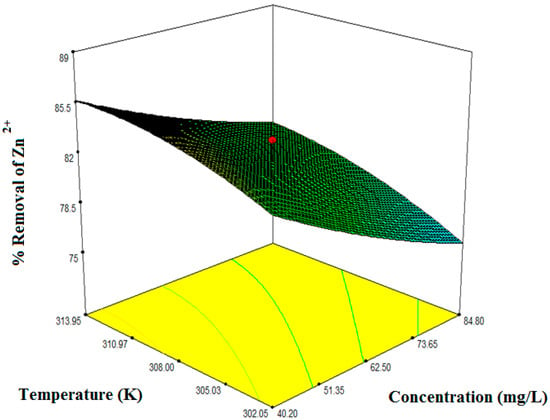
Figure 6.
Interacting effects of metal ions concentration and temperature at an adsorbent dose 0.42 g.
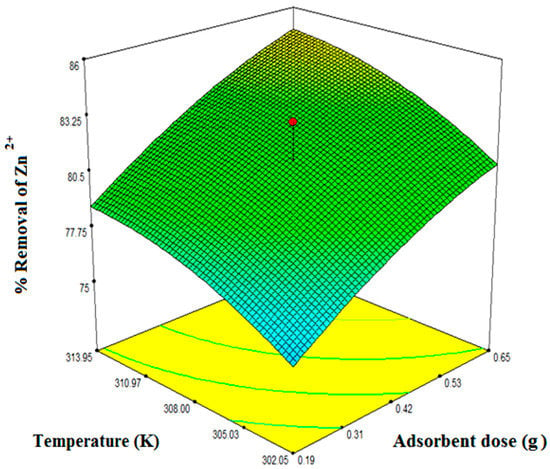
Figure 7.
Interacting effects of temperature and adsorbent dose at an initial concentration of 42.13 mg/L.
The response surface method has been used to analyze the three-dimensional response plot produced from the effect of all the process variables on Zn2+ adsorption on novel composite adsorbent from aqueous solution. It is evident from the ANOVA table that all the individual process variables are significant in increasing the effectiveness of the composite adsorbent in terms of percentage removal (Y).
Figure 5, Figure 6 and Figure 7 illustrate the collective effect of all the independent parameters on the percentage removal of Zn2+. All of the values on the axis of the figures represent the real values. Figure 5 shows the interacting effect of the initial Zn2+ concentration () and adsorbent dose () on Zn2+ removal at 180 min and the system pH 5.50.1. Percentage removal shows a sharply increasing trend with an increase in adsorbent dose and decreasing trend with an increase in initial metal ion concentration. As the initial concentration increases, there are excess ions in bulk solution with respect to active sites. Therefore, the percentage of removal gets reduced. On the other hand, increasing adsorbent dose increases the available surface area for the metal ion. Hence, the percentage of removal efficiency increases. Maximum removal obtained for the interacting effect of initial Zn2+ concentration and the adsorbent dose was 83%. Figure 6 demonstrates the mutual effect of the initial Zn2+ concentration () and system temperature (). As the system is endothermic, high temperature favors the adsorption process. Also, with increasing temperature the activity of the functional groups increases which enhance the surface complex formation and reduces the mass transfer resistance. Therefore, system temperature affects synergistically on the adsorption of Zn2+. Maximum removal efficiency for the combined effect of initial metal ion concentration and system temperature was found from the optimization study as 82%. In Figure 5 and Figure 6, one effect was synergistic and another was antagonistic. Figure 7 represents the combined effect of adsorbent dose () and system temperature (). Here, both parameters affect synergistically for aqueous phase Zn2+ removal. With increasing any of the two independent variables, removal efficiency increases. Practically, when both of these two parameters were at their maximum condition removal efficiency were maximum at any particular concentration. And this has been observed during the elaborate experimental trial. Here, we have represented only the experimental data required by the design expert software to optimize the system. Therefore, maximum removal efficiency obtained from the 3D plot is 86%. However, it is evident from the ANOVA table as well the 3D response plot, that initial concentration has most control on removal efficiency as compared with the other independent parameters.
4.3. Response Surface Modeling and Process Optimization
To find out the optimum condition for Zn2+ removal from aqueous solution by the novel composite adsorbent is the most important objective of the current work. Therefore optimization of adsorption variables is investigated using the RSM technique. The response surface plots and contour plots at an optimized condition for the highest removal efficiency are presented in Figure 8 and Figure 9, respectively.
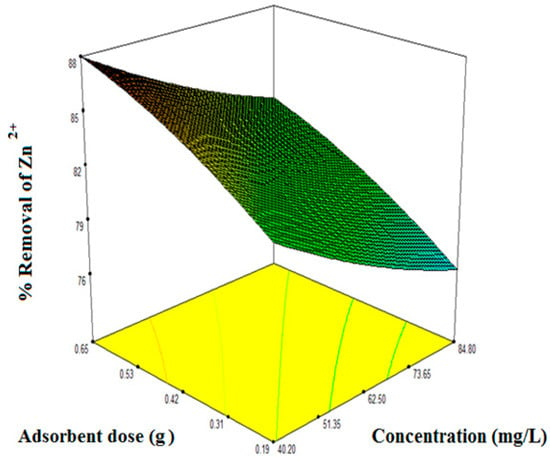
Figure 8.
3D plot at an optimized region on the adsorbent dose and concentration for the highest removal efficiency of Zn2+.
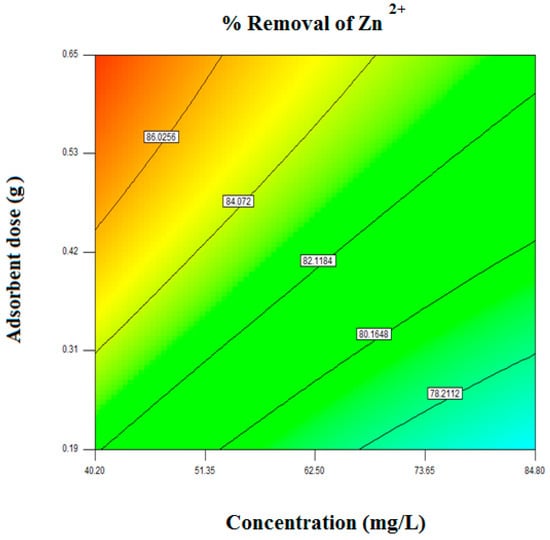
Figure 9.
Contour plot of adsorbent and concentration for the highest removal efficiency of Zn2+.
The optimum operating condition was found at the initial concentration of metal ion 43.18 mg/L, adsorbent dose 0.62 g and system temperature 313.15 K. The experimental and predicted removal efficiency at the optimum condition was found as 86.05% and 85.02%, respectively. The error obtained between model predicted and the actual value is less than 1.18% which indicates that the model is accurate for Zn2+ removal. The optimum conditions in detailed are produced in Table 6.

Table 6.
Optimum conditions for the removal of aqueous phase Zn2+ on the composite adsorbent.
5. Conclusions
In the present study, three variables central composite design based The response surface method (RSM) has been employed to investigate the individual as well as the combined effect of independent variables on Zn2+ adsorption. This particular method has been chosen over other methods like normal factorial design or Box-Behnken method because of its improved computation incorporating axial data points and center points. The study revealed that all the parameters, i.e., initial Zn2+ concentration, adsorbent dose, and system temperature individually affect the adsorption process significantly compared to the combined effect. With the help of design expert, the regression analysis and variables optimization were calculated to predict the significant response in the experimental domain. A large number of experimental data at a wide range of independent variables were employed to carry out the regression analysis, statistical importance, and response surface analysis. A simple second order quadratic model equation was developed using Design Expert software for predicting the response (percentage removal of Zn2+) on overall experimental regions and correlate the operating parameters and removal efficiency of Zn2+. The coefficients of the developed model were calculated for each response, and the high acceptability of the postulated model was proven by presenting the statistical specifications of them. The reliability of the developed model has been ensured from the high magnitude of the correlation coefficient between the experimental and model predicted values. The response surface was derived based on the developed model. Initial metal ion concentration was found as the most significant factor among all the process variables. The optimum condition for maximum Zn2+ removal was initial concentration 43.18 mg/L, adsorbent dose 0.62 g and system temperature 313.15 K. This response surface method is a conventional optimization technique used for many industrial applications, but very less implementation have been reported in adsorption-based water treatment technology. Therefore, using this tool in large scale adsorption based technology can be an advantageous one from time-saving and economical point of view.
Author Contributions
This work is an original partial work on S.B.’s Ph.D. thesis work and therefore S.B. has written full manuscript draft preparation based on his experimental design, investifation, data collection and analysis etc. T.K.S. and B.C.M. both are his Ph.D. supervisors and mostly involved with problem identification, guidence and manuscript review and editing etc. The contribution of other two co-authors, namely M.B. and S.K.B. are minor and their very initial assistance are on the application of software called “Design Expert”.
Funding
This research received no external funding.
Acknowledgments
The authors acknowledge Chemical Engineering of Curtin University-Perth, Australia and Department of Chemical Engineering of Indian Institute of Technology, Kharagpur, India for necessary research support and one of the authors also thanks for scholarship support under Curtin-IITKGP collaborative Ph.D. program.
Conflicts of Interest
The authors declare no conflict of interest.
References
- He, J.; Chen, J.P.A. Comprehensive Review on Biosorption of Heavy Metals by Algal Biomass: Materials, Performances, Chemistry, and Modeling Simulation Tools. Bioresour. Technol. 2014, 160, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Kara, İ.; Yilmazer, D.; Akar, S.T. Metakaolin Based Geopolymer as an Effective Adsorbent for Adsorption of Zinc(II) and Nickel(II) Ions from Aqueous Solutions. Appl. Clay Sci. 2017, 139, 54–63. [Google Scholar] [CrossRef]
- Hojati, S.; Landi, A. Kinetics and Thermodynamics of Zinc Removal from a Metal-Plating Wastewater by Adsorption onto an Iranian Sepiolite. Int. J. Environ. Sci. Technol. 2015, 12, 203–210. [Google Scholar] [CrossRef]
- Arias, F.; Sen, T.K. Removal of Zinc Metal Ion (Zn2+) from Its Aqueous Solution by Kaolin Clay Mineral: A Kinetic and Equilibrium Study. Colloids Surf. A Physicochem. Eng. Asp. 2009, 348, 100–108. [Google Scholar] [CrossRef]
- Charerntanyarak, L. Heavy Metals Removal by Chemical Coagulation and Precipitation. Water Sci. Technol. 1999, 39, 135–138. [Google Scholar] [CrossRef]
- Dabrowski, A.; Hubicki, Z.; Podkoscielny, P.; Robens, E. Selective Removal of the Heavy Metal Ions from Waters and Industrial Wastewaters by Ion-Exchange Method. Chemosphere 2004, 56, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Qdais, H.A.; Moussa, H. Removal of Heavy Metals from Wastewater by Membrane Processes: A Comparative Study. Desalination 2004, 164, 105–110. [Google Scholar] [CrossRef]
- Dawood, S.; Sen, T.K.; Phan, C. Synthesis and Characterisation of Novel-Activated Carbon from Waste Biomass Pine Cone and Its Application in the Removal of Congo Red Dye from Aqueous Solution by Adsorption. Water Air. Soil Pollut. 2014, 225. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Chan, G.Y.S.; Lo, W.H.; Babel, S. Physico-Chemical Treatment Techniques for Wastewater Laden with Heavy Metals. Chem. Eng. J. 2006, 118, 83–98. [Google Scholar] [CrossRef]
- Yagub, M.T.; Sen, T.K.; Afroze, S.; Ang, H.M. Dye and Its Removal from Aqueous Solution by Adsorption: A Review. Adv. Colloid Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef]
- Biswas, S.; Sen, T.K.; Yeneneh, A.M.; Meikap, B.C. Synthesis and Characterization of a Novel Ca-Alginate-Biochar Composite as Efficient Zinc (Zn2+) Adsorbent: Thermodynamics, Process Design, Mass Transfer and Isotherm Modeling. Sep. Sci. Technol. 2018, 1–19. [Google Scholar] [CrossRef]
- Behera, S.K.; Meena, H.; Chakraborty, S.; Meikap, B.C. Application of Response Surface Methodology (RSM) for Optimization of Leaching Parameters for Ash Reduction from Low-Grade Coal. Int. J. Min. Sci. Technol. 2018, 28, 621–629. [Google Scholar] [CrossRef]
- Tripathy, A.; Biswal, S.K.; Meikap, B.C. Statistical Modelling and Optimization Study for Beneficiation of Indian High Ash Semi-Coking Coal Using Allflux Separator. Adv. Powder Technol. 2016, 27, 1488–1493. [Google Scholar] [CrossRef]
- Das, D.; Meikap, B.C. Optimization of Process Condition for the Preparation of Amine-Impregnated Activated Carbon Developed for CO 2 Capture and Applied to Methylene Blue Adsorption by Response Surface Methodology. J. Environ. Sci. Heal. Part A 2017, 52, 1164–1172. [Google Scholar] [CrossRef] [PubMed]
- Panda, L.; Banerjee, P.K.; Biswal, S.K.; Venugopal, R.; Mandre, N.R. Modelling and Optimization of Process Parameters for Beneficiation of Ultrafine Chromite Particles by Selective Flocculation. Sep. Purif. Technol. 2014, 132, 666–673. [Google Scholar] [CrossRef]
- Aghaie, E.; Pazouki, M.; Hosseini, M.R.; Ranjbar, M.; Ghavipanjeh, F. Response Surface Methodology (RSM) Analysis of Organic Acid Production for Kaolin Beneficiation by Aspergillus Niger. Chem. Eng. J. 2009, 147, 245–251. [Google Scholar] [CrossRef]
- Gratuito, M.K.B.; Panyathanmaporn, T.; Chumnanklang, R.A.; Sirinuntawittaya, N.; Dutta, A. Production of Activated Carbon from Coconut Shell: Optimization Using Response Surface Methodology. Bioresour. Technol. 2008, 99, 4887–4895. [Google Scholar] [CrossRef] [PubMed]
- Saranya, N.; Nakeeran, E.; Giri Nandagopal, M.S.; Selvaraju, N. Optimization of Adsorption Process Parameters by Response Surface Methodology for Hexavalent Chromium Removal from Aqueous Solutions Using Annona Reticulata Linn Peel Microparticles. Water Sci. Technol. 2017, 75, 2094–2107. [Google Scholar] [CrossRef] [PubMed]
- Niad, M.; Zaree, S.; Tahanzadeh, N. Response Surface Methodology for Optimization of Cd (II) Biosorption by Cystoseria Myricaas. J. Biomed. 2016. [Google Scholar] [CrossRef]
- Sarkar, M.; Majumdar, P. Application of Response Surface Methodology for Optimization of Heavy Metal Biosorption Using Surfactant Modified Chitosan Bead. Chem. Eng. J. 2011. [Google Scholar] [CrossRef]
- Ferreira, S.L.C.; Bruns, R.E.; da Silva, E.G.P.; dos Santos, W.N.L.; Quintella, C.M.; David, J.M.; de Andrade, J.B.; Breitkreitz, M.C.; Jardim, I.C.S.F.; Neto, B.B. Statistical Designs and Response Surface Techniques for the Optimization of Chromatographic Systems. J. Chromatogr. A. 2007. [Google Scholar] [CrossRef] [PubMed]
- Isar, J.; Agarwal, L.; Saran, S.; Saxena, R.K. A Statistical Method for Enhancing the Production of Succinic Acid from Escherichia Coli under Anaerobic Conditions. Bioresour. Technol. 2006. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).