PM2.5 Chemical Compositions and Aerosol Optical Properties in Beijing during the Late Fall
Abstract
:1. Introduction
2. Experimental Section
2.1. Sampling
2.2. Gravimetric and Chemical Analysis
2.3. Quality Control
2.4. Measurements of Aerosol Optical and Meteorological Parameters
2.5. Data Analysis
2.5.1. Reconstruction of PM2.5 Mass
2.5.2. Reconstruction of the Light Extinction Coefficient
3. Results and Discussion
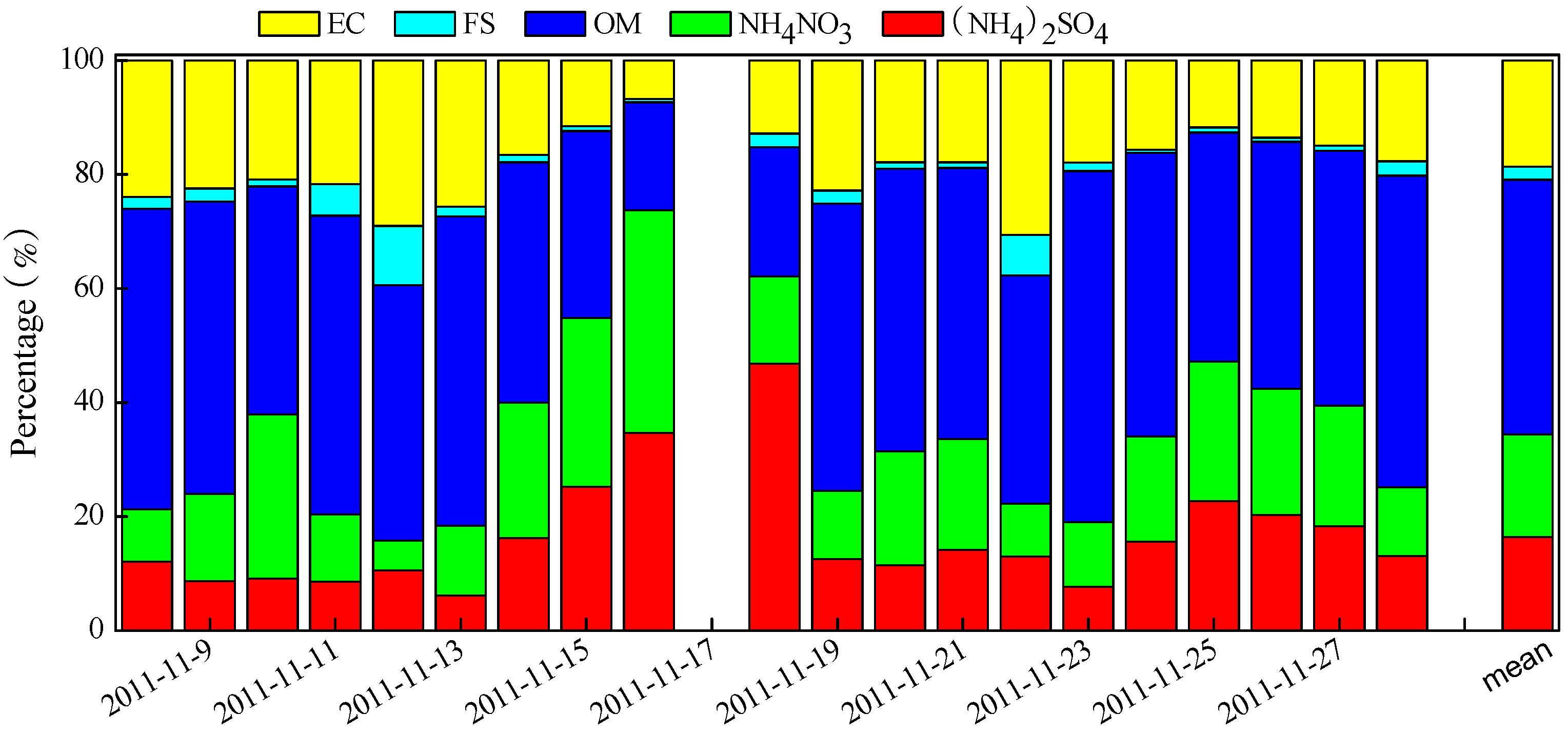
3.1. PM2.5 Chemical Compositions
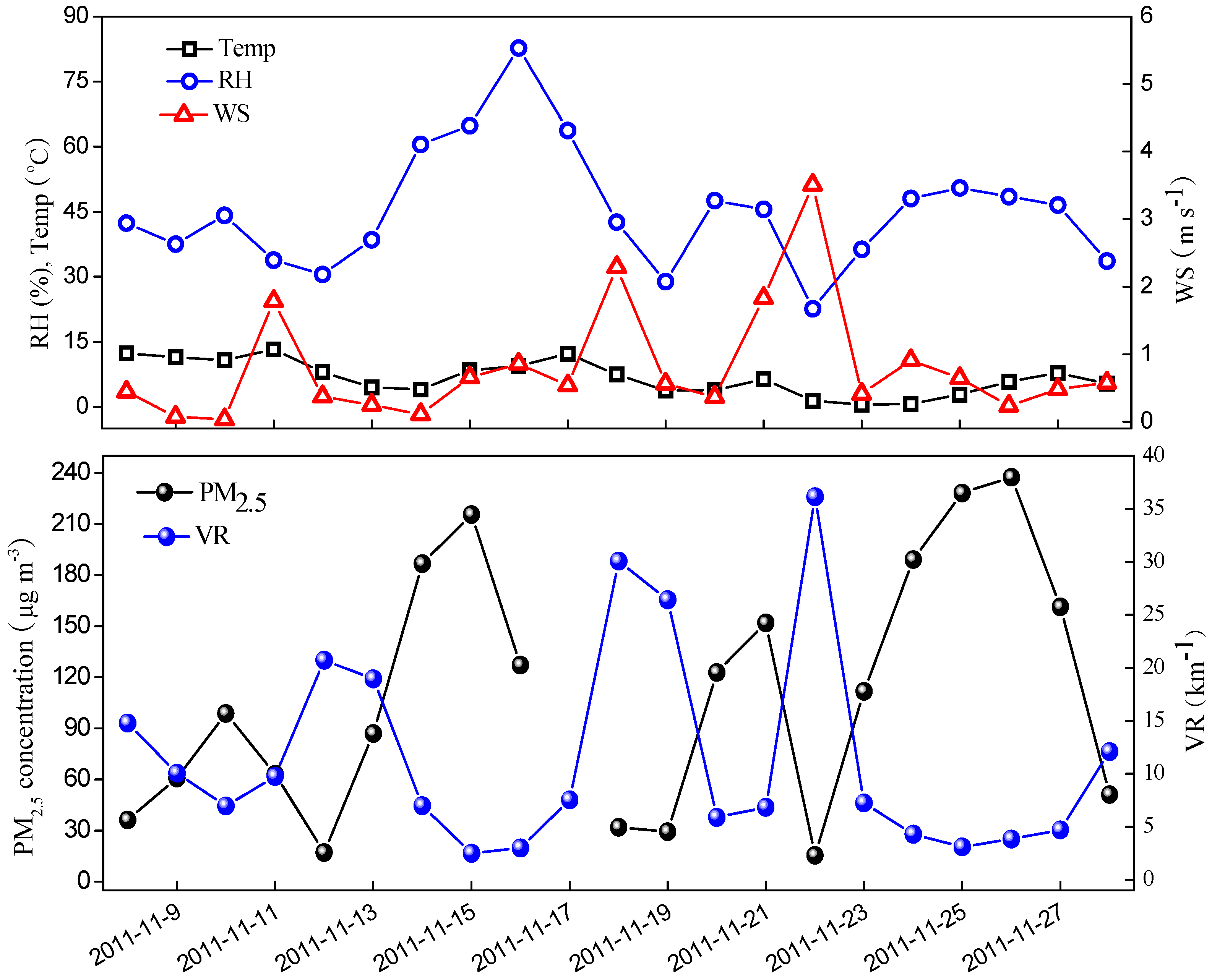

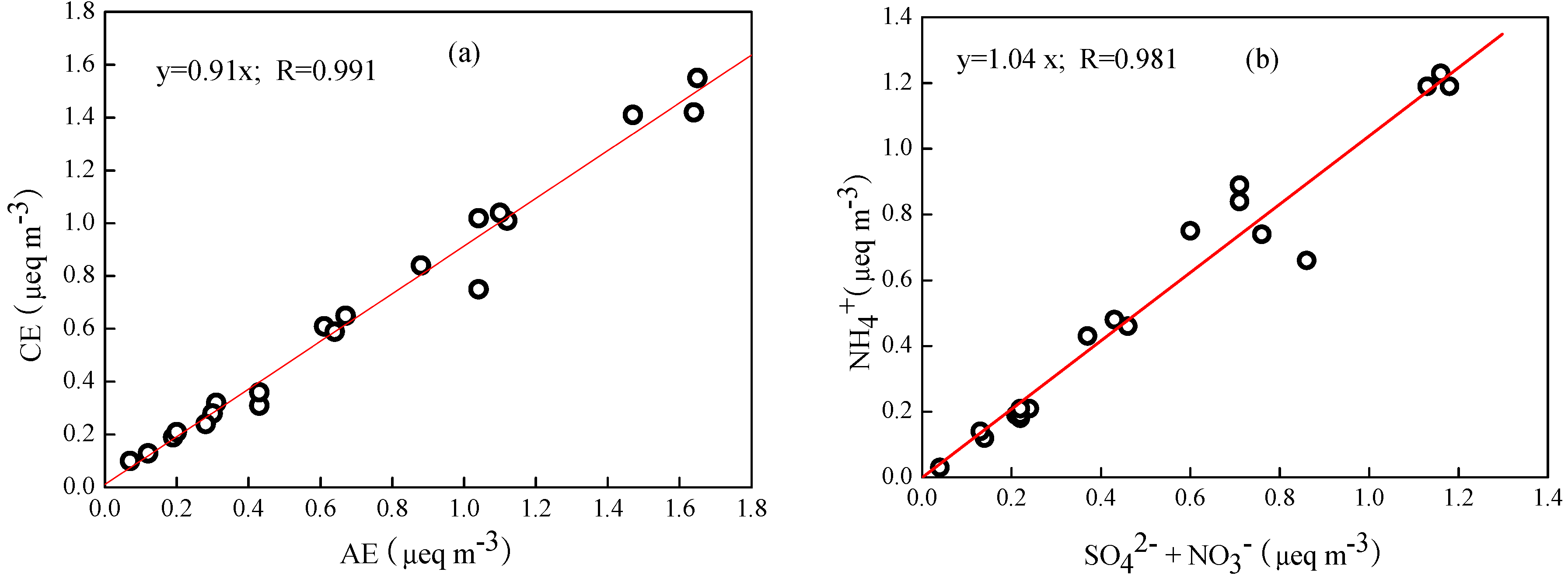
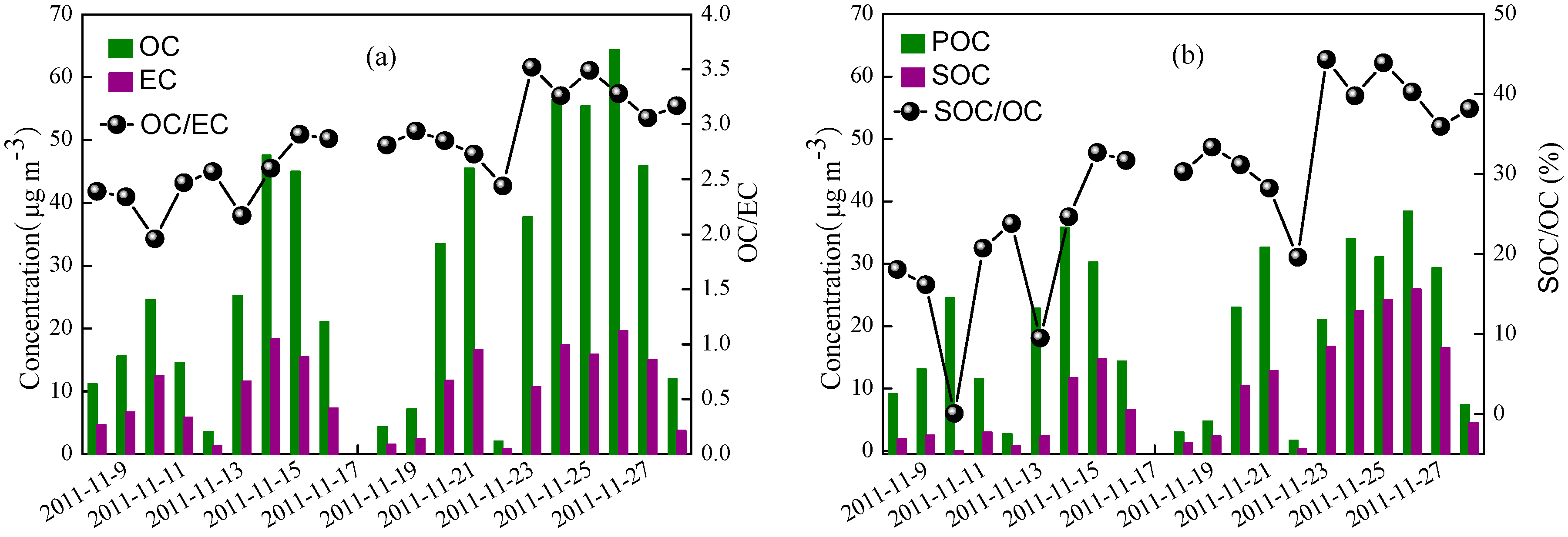
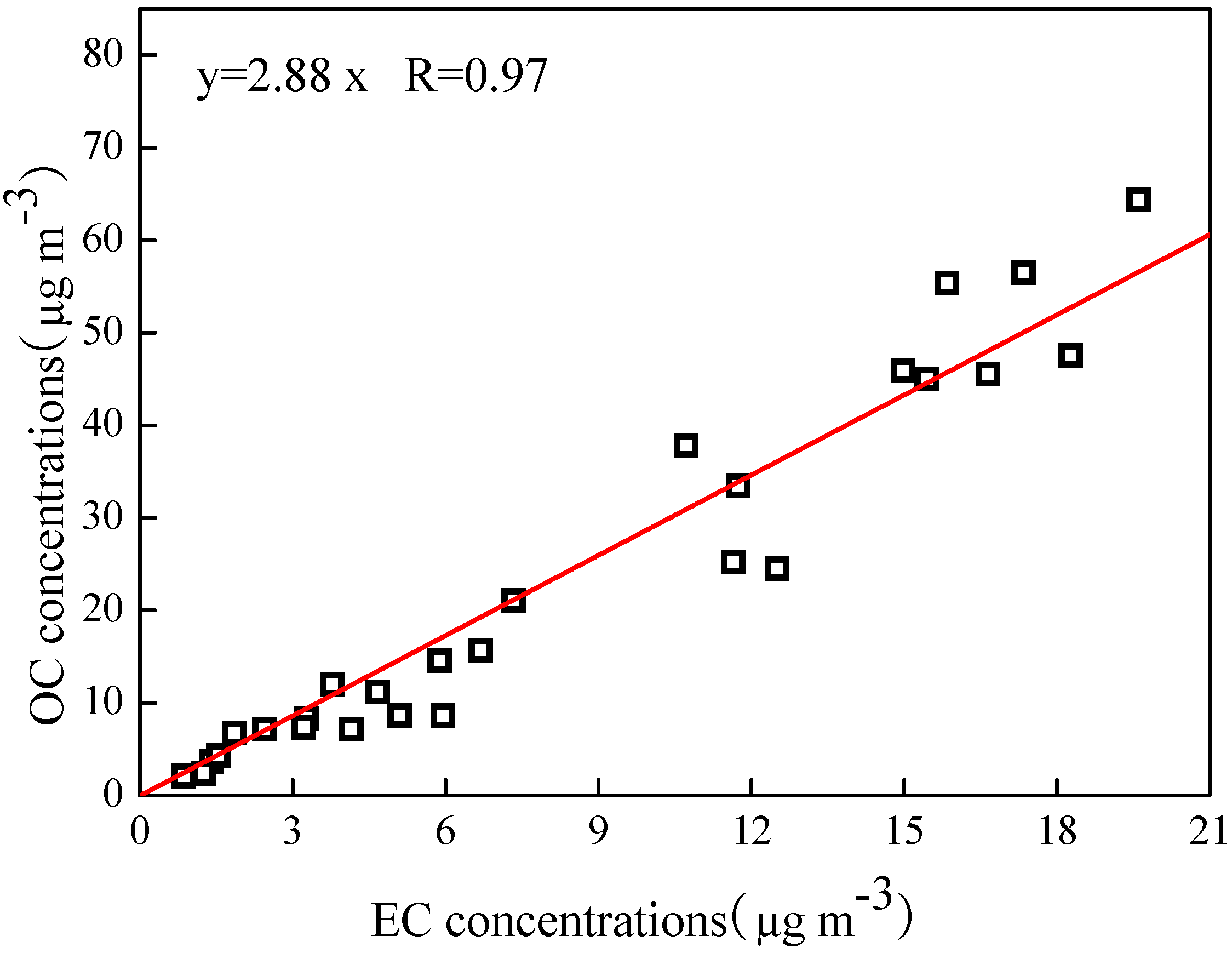
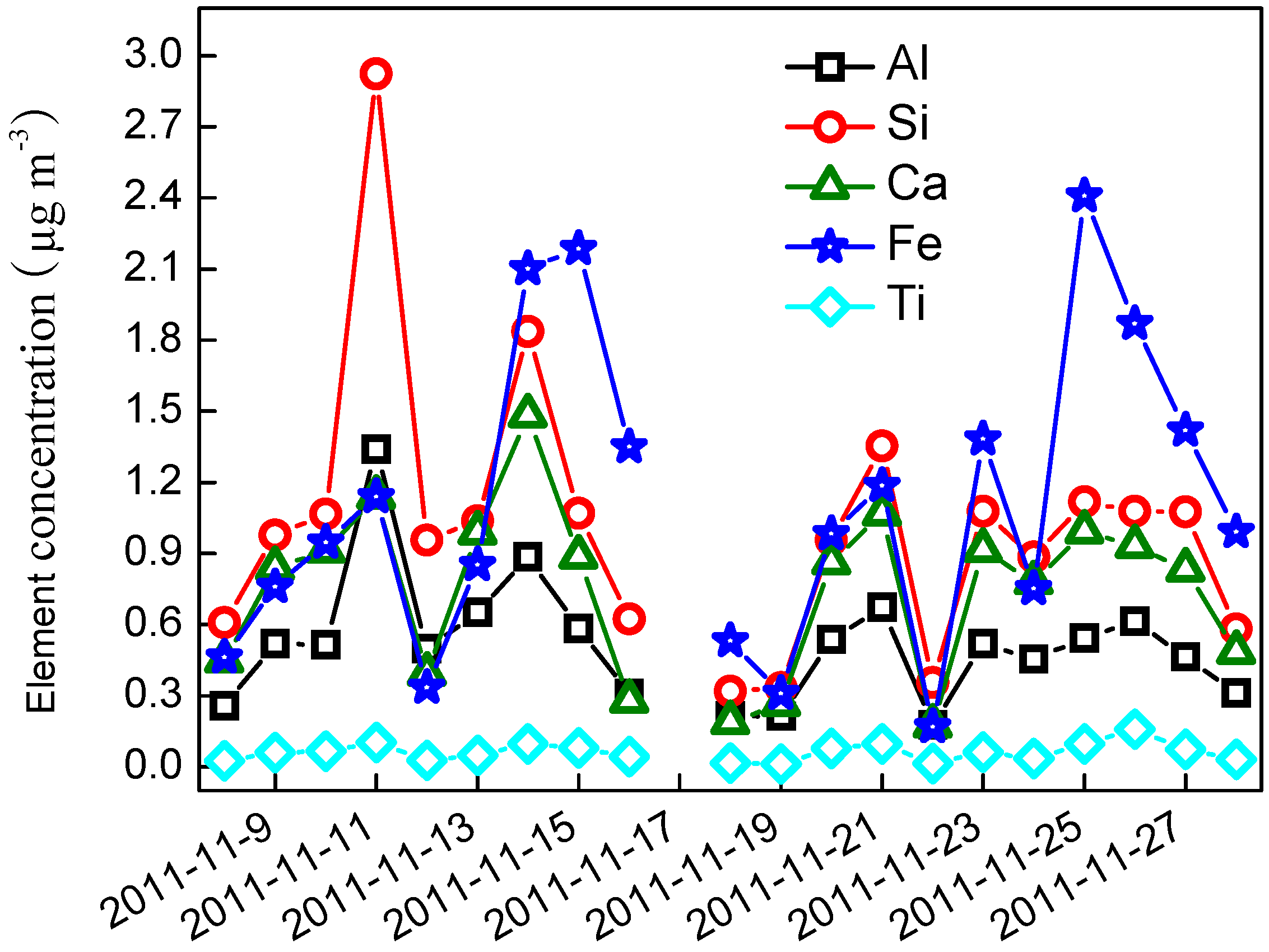
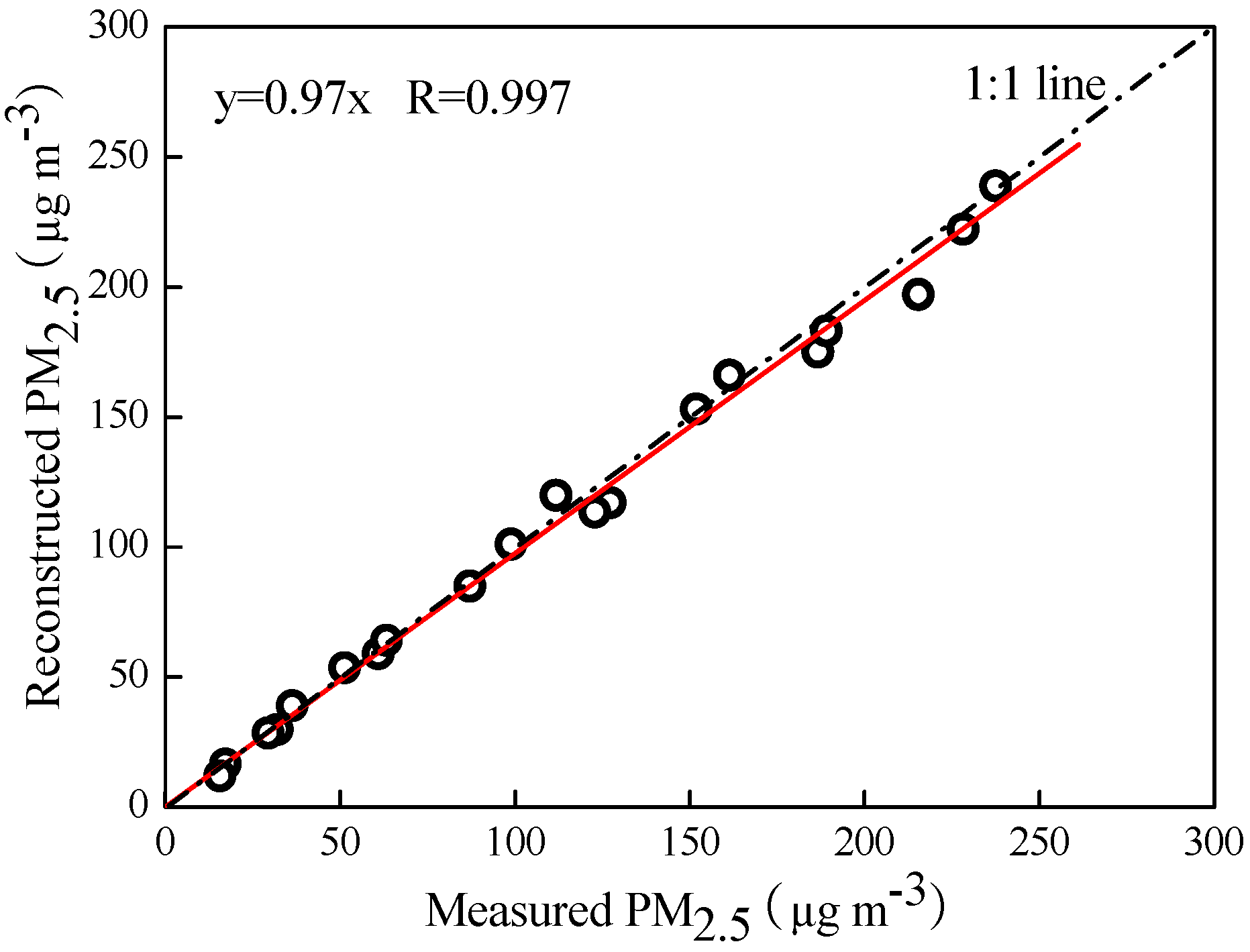
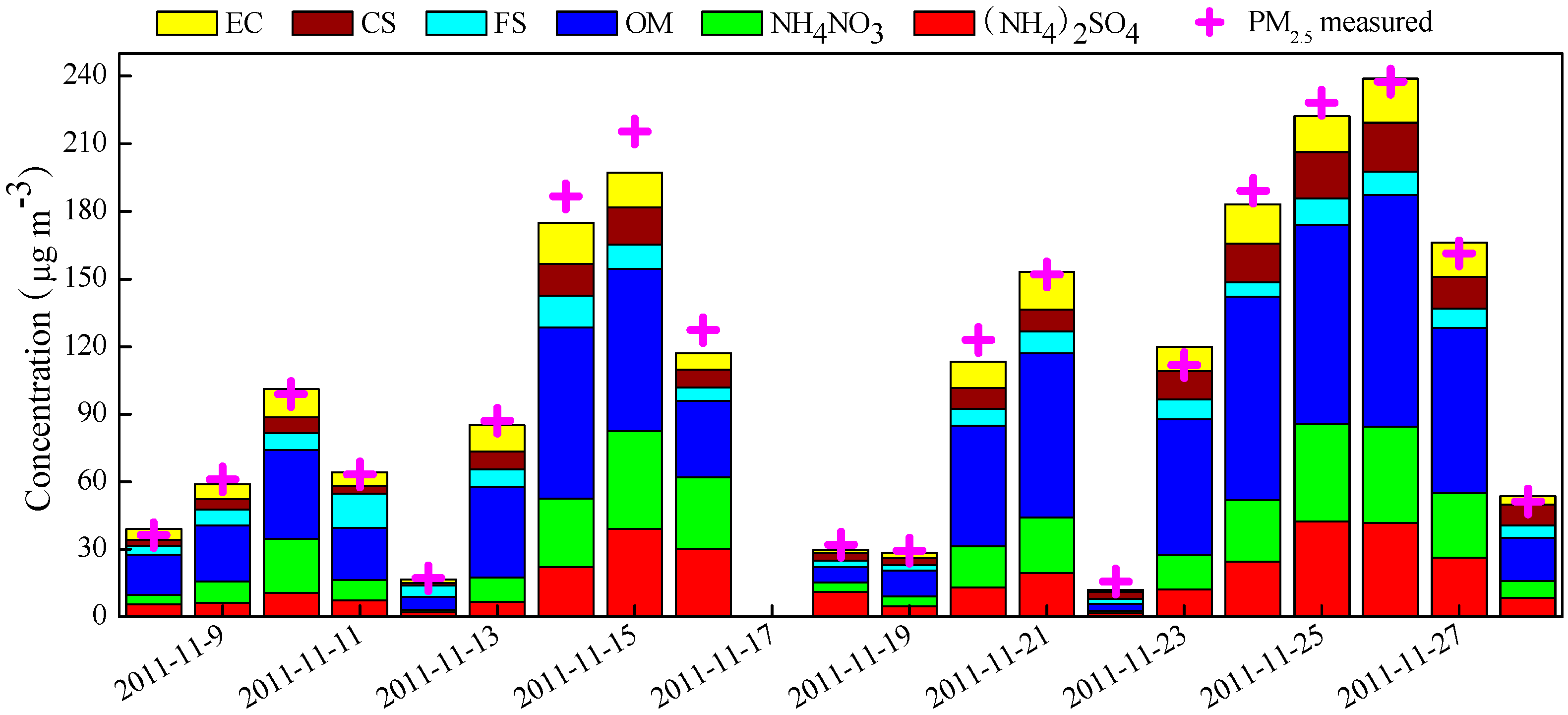
3.2. PM2.5 Mass Balance
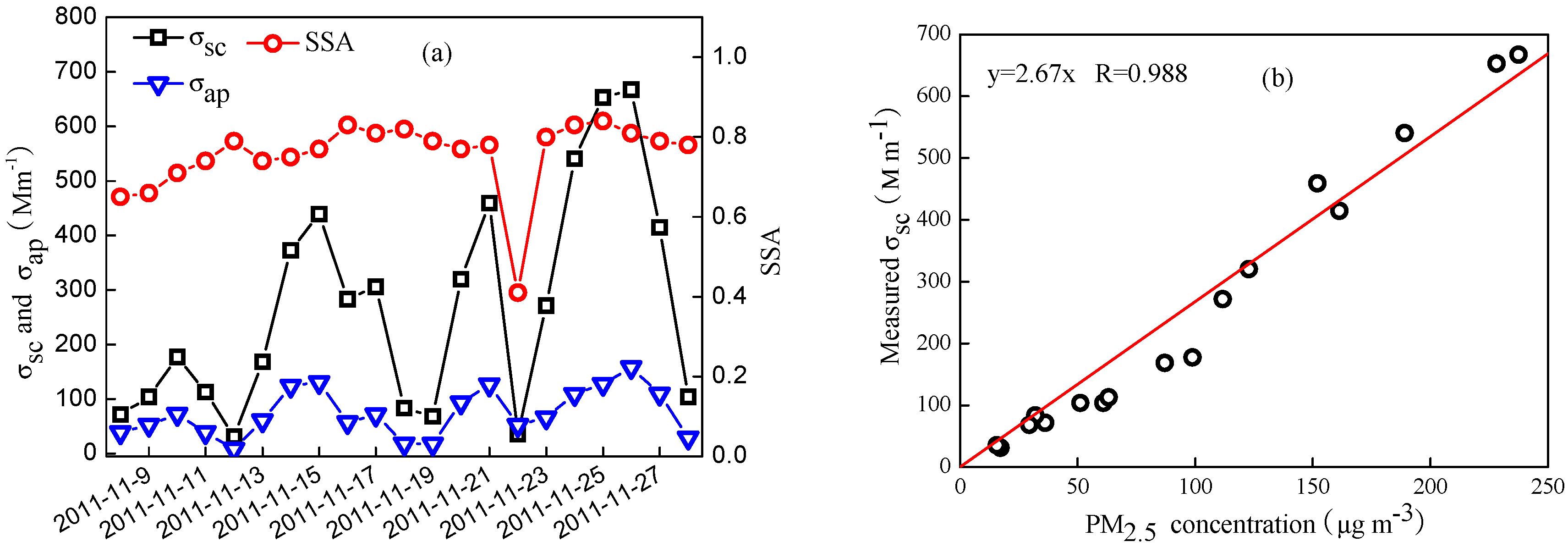
3.3. Analysis of Aerosol Optical Properties
3.4. Chemical Apportionment of the Aerosol Optical Parameters
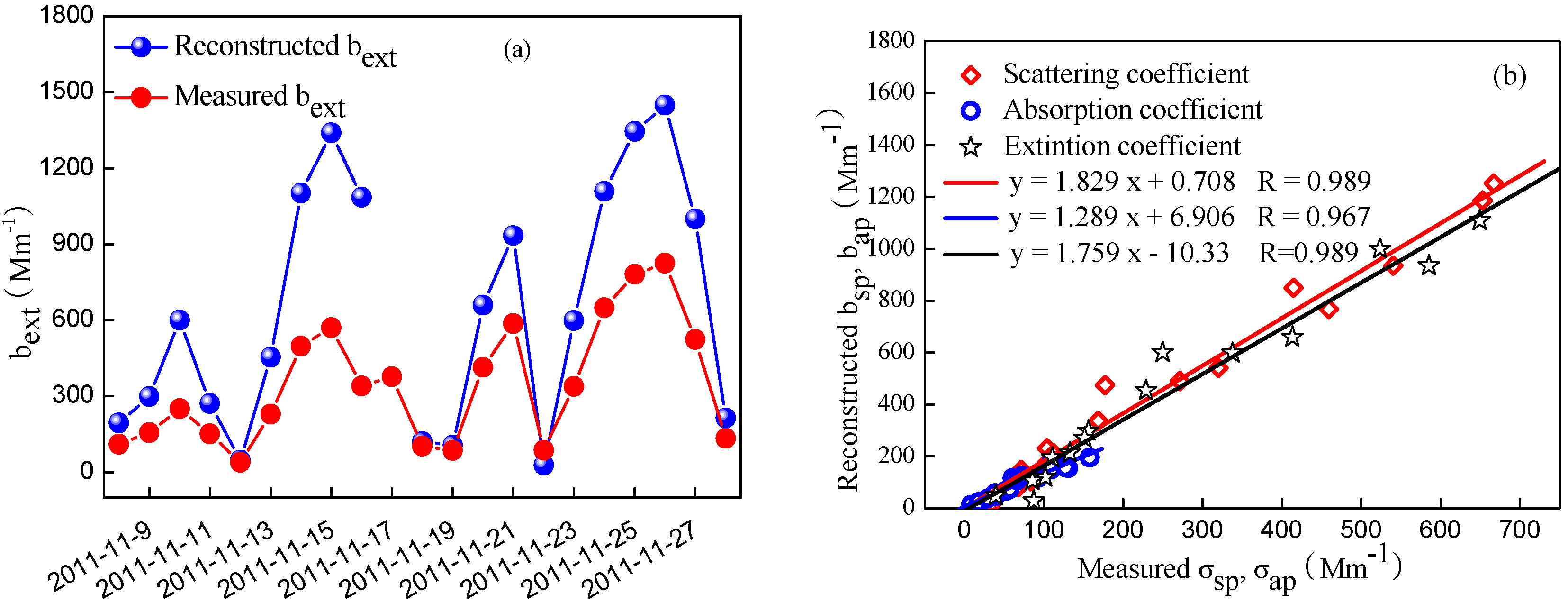
3.5. Typical Pollution Episodes
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, Q.Y.; Cao, J.J.; Tao, J.; Li, N.; Su, X.O.; Chen, L.W.A.; Wang, P.; Shen, Z.X.; Liu, S.X.; Dai, W.T. Long-term trends in visibility and at Chengdu, China. PLoS One 2013. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Wang, Y.Q.; Niu, T.; Zhang, X.C.; Gong, S.L.; Zhang, Y.M.; Sun, J.Y. Atmospheric aerosol compositions in China: Spatial/temporal variability, chemical signature, regional haze distribution and comparisons with global aerosols. Atmos. Chem. Phys. 2012, 12, 779–799. [Google Scholar] [CrossRef]
- Shen, G.F.; Xue, M.; Yuan, S.Y.; Zhang, J.; Zhao, Q.Y.; Li, B.; Wu, H.S.; Ding, A.J. Chemical compositions and reconstructed light extinction coefficients of particulate matter in a mega-city. In the western Yangtze River Delta, China. Atmos. Environ. 2014, 83, 14–20. [Google Scholar] [CrossRef]
- Tao, J.; Zhang, L.M.; Ho, K.F.; Zhang, R.J.; Lin, Z.J.; Zhang, Z.S.; Lin, M.; Cao, J.J.; Liu, S.X.; Wang, G.H. Impact of PM2.5 chemical compositions on aerosol light scattering in Guangzhou—The largest megacity in south China. Atmos. Res. 2014, 135, 48–58. [Google Scholar] [CrossRef]
- Chen, J.; Qiu, S.S.; Shang, J.; Wilfrid, O.M.F.; Liu, X.G.; Tian, H.Z.; Boman, J. Impact of relative humidity and water soluble constituents of PM2.5 on visibility impairment in Beijing, China. Aerosol Air Qual. Res. 2014, 14, 260–268. [Google Scholar]
- Ramanathan, V.; Feng, Y. Air pollution, greenhouse gases and climate change: Global and regional perspectives. Atmos. Environ. 2009, 43, 37–50. [Google Scholar] [CrossRef]
- Ma, X.; Yu, F.; Luo, G. Aerosol direct radiative forcing based on GEOS-Chem-APM and uncertainties. Atmos. Chem. Phys. 2012, 12, 5563–5581. [Google Scholar] [CrossRef]
- Yang, F.; Huang, L.; Duan, F.; Zhang, W.; He, K.; Ma, Y.; Brook, J.R.; Tan, J.; Zhao, Q.; Cheng, Y. Carbonaceous species in PM2.5 at a pair of rural/urban sites in Beijing, 2005–2008. Atmos. Chem. Phys. 2011, 11, 7893–7903. [Google Scholar] [CrossRef]
- Yang, F.M.; He, K.B.; Ma, Y.L.; Zhang, Q.; Cadle, S.H.; Chan, T.; Mulawa, P.A. Characterization of carbonaceous species of ambient PM2.5 in Beijing, China. J. Air Waste Manag. 2005, 55, 984–992. [Google Scholar] [CrossRef]
- Zhao, P.S.; Dong, F.; He, D.; Zhao, X.J.; Zhang, X.L.; Zhang, W.Z.; Yao, Q.; Liu, H.Y. Characteristics of concentrations and chemical compositions for PM2.5 in the region of Beijing, Tianjin, and Hebei, China. Atmos. Chem. Phys. 2013, 13, 4631–4644. [Google Scholar] [CrossRef]
- Yang, F.; Tan, J.; Zhao, Q.; Du, Z.; He, K.; Ma, Y.; Duan, F.; Chen, G.; Zhao, Q. Characteristics of PM2.5 speciation in representative megacities and across China. Atmos. Chem. Phys. 2011, 11, 5207–5219. [Google Scholar] [CrossRef]
- Zhang, R.; Jing, J.; Tao, J.; Hsu, S.C.; Wang, G.; Cao, J.; Lee, C.S.L.; Zhu, L.; Chen, Z.; Zhao, Y.; et al. Chemical characterization and source apportionment of PM2.5 in Beijing: Seasonal perspective. Atmos. Chem. Phys. 2013, 13, 7053–7074. [Google Scholar] [CrossRef]
- Xu, J.W.; Tao, J.; Zhang, R.J.; Cheng, T.T.; Leng, C.P.; Chen, J.M.; Huang, G.H.; Li, X.; Zhu, Z.Q. Measurements of surface aerosol optical properties in winter of Shanghai. Atmos. Res. 2012, 109, 25–35. [Google Scholar] [CrossRef]
- Yan, P.; Tang, J.; Huang, J.; Mao, J.T.; Zhou, X.J.; Liu, Q.; Wang, Z.F.; Zhou, H.G. The measurement of aerosol optical properties at a rural site in northern China. Atmos. Chem. Phys. 2008, 8, 2229–2242. [Google Scholar] [CrossRef]
- Zhao, X.J.; Zhang, X.L.; Pu, W.W.; Meng, W.; Xu, X.F. Scattering properties of the atmospheric aerosol in Beijing, China. Atmos. Res. 2011, 101, 799–808. [Google Scholar] [CrossRef]
- Jung, J.; Lee, H.; Kim, Y.J.; Liu, X.G.; Zhang, Y.H.; Hu, M.; Sugimoto, N. Optical properties of atmospheric aerosols obtained by in situ and remote measurements during 2006 Campaign of Air Quality Research in Beijing (CAREBeijing-2006). J. Geophys. Res.: Atmos. 2009. [Google Scholar] [CrossRef]
- Garland, R.M.; Yang, H.; Schmid, O.; Rose, D.; Nowak, A.; Achtert, P.; Wiedensohler, A.; Takegawa, N.; Kita, K.; Miyazaki, Y.; et al. Aerosol optical properties in a rural environment near the mega-city Guangzhou, China: Implications for regional air pollution, radiative forcing and remote sensing. Atmos. Chem. Phys. 2008, 8, 5161–5186. [Google Scholar] [CrossRef]
- Tian, P.; Wang, G.F.; Zhang, R.J. Impacts of aerosol chemical compositions on optical properties in urban Beijing, China. Particuology 2014. [Google Scholar] [CrossRef]
- Huang, G.H.; Cheng, T.T.; Zhang, R.J.; Tao, J.; Leng, C.P.; Zhang, Y.W.; Zha, S.P.; Zhang, D.Q.; Li, X.; Xu, C.Y. Optical properties and chemical composition of PM2.5 in Shanghai in the spring of 2012. Particuology 2014, 13, 52–59. [Google Scholar] [CrossRef]
- Pitchford, M.; Malm, W.; Schichtel, B.; Kumar, N.; Lowenthal, D.; Hand, J. Revised algorithm for estimating light extinction from improve particle speciation data. J. Air Waste Manag. 2007, 57, 1326–1336. [Google Scholar] [CrossRef]
- Chow, J.C.; Watson, J.G.; Chen, L.W.A.; Chang, M.C.O.; Robinson, N.F.; Trimble, D.; Kohl, S. The IMPROVE_A temperature protocol for thermal/optical carbon analysis: Maintaining consistency with a long-term database. J. Air Waste Manag. 2007, 57, 1014–1023. [Google Scholar] [CrossRef]
- Xu, H.M.; Cao, J.J.; Ho, K.F.; Ding, H.; Han, Y.M.; Wang, G.H.; Chow, J.C.; Watson, J.G.; Khol, S.D.; Qiang, J.; et al. Lead concentrations in fine particulate matter after the phasing out of leaded gasoline in Xi’an, China. Atmos. Environ. 2012, 46, 217–224. [Google Scholar] [CrossRef]
- Xing, L.; Fu, T.M.; Cao, J.J.; Lee, S.C.; Wang, G.H.; Ho, K.F.; Cheng, M.C.; You, C.F.; Wang, T.J. Seasonal and spatial variability of the OM/OC mass ratios and high regional correlation between oxalic acid and zinc in Chinese urban organic aerosols. Atmos. Chem. Phys. 2013, 13, 4307–4318. [Google Scholar] [CrossRef]
- Malm, W.C.; Sisler, J.F.; Huffman, D.; Eldred, R.A.; Cahill, T.A. Spatial and seasonal trends in particle concentration and optical extinction in the United States. J. Geophys. Res.: Atmos. 1994, 99, 1347–1370. [Google Scholar] [CrossRef]
- Barnard, J.C.; Kassianov, E.I.; Ackerman, T.P.; Johnson, K.; Zuberi, B.; Molina, L.T.; Molina, M.J. Estimation of a “radiatively correct” black carbon specific absorption during the Mexico City Metropolitan Area (MCMA) 2003 field campaign. Atmos. Chem. Phys. 2007, 7, 1645–1655. [Google Scholar] [CrossRef]
- Zhou, J.M.; Zhang, R.J.; Cao, J.J.; Chow, J.C.; Watson, J.G. Carbonaceous and ionic components of atmospheric fine particles in Beijing and their impact on atmospheric visibility. Aerosol Air Qual. Res. 2012, 12, 492–502. [Google Scholar]
- Sun, K.; Qu, Y.; Wu, Q.; Han, T.T.; Gu, J.W.; Zhao, J.J.; Sun, Y.L.; Jiang, Q.; Gao, Z.Q.; Hu, M.; et al. Chemical characteristics of size-resolved aerosols in winter in Beijing. J. Environ. Sci. 2014, 26, 1641–1650. [Google Scholar] [CrossRef]
- Arimoto, R.; Duce, R.A.; Savoie, D.L.; Prospero, J.M.; Talbot, R.; Cullen, J.D.; Tomza, U.; Lewis, N.F.; Jay, B.J. Relationships among aerosol constituents from Asia and the North Pacific during Pem-West A. J. Geophys. Res.: Atmos. 1996, 101, 2011–2023. [Google Scholar] [CrossRef]
- Duan, F.K.; He, K.B.; Ma, Y.L.; Yang, F.M.; Yu, X.C.; Cadle, S.H.; Chan, T.; Mulawa, P.A. Concentration and chemical characteristics of PM2.5 in Beijing, China: 2001–2002. Sci. Total Environ. 2006, 355, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.X.; Xing, J.; Chatani, S.; Hao, J.M.; Klimont, Z.; Cofala, J.; Amann, M. Verification of anthropogenic emissions of China by satellite and ground observations. Atmos. Environ. 2011, 45, 6347–6358. [Google Scholar] [CrossRef]
- Wang, Y.; Zhuang, G.S.; Tang, A.H.; Yuan, H.; Sun, Y.L.; Chen, S.A.; Zheng, A.H. The ion chemistry and the source of PM2.5 aerosol in Beijing. Atmos. Environ. 2005, 39, 3771–3784. [Google Scholar] [CrossRef]
- Wang, R.S.; Xiao, H.; Bai, T.; Wang, Y.J.; Qian, L.Y. The amount of vehicles by “China vehicle emission control annual report in 2013”. Environ. Sustain. Dev. 2014, 2014, 88–90. [Google Scholar]
- Cheng, Y.; Engling, G.; He, K.B.; Duan, F.K.; Ma, Y.L.; Du, Z.Y.; Liu, J.M.; Zheng, M.; Weber, R.J. Biomass burning contribution to Beijing aerosol. Atmos. Chem. Phys. 2013, 13, 7765–7781. [Google Scholar] [CrossRef]
- Yang, F.; He, K.; Ye, B.; Chen, X.; Cha, L.; Cadle, S.H.; Chan, T.; Mulawa, P.A. One-year record of organic and elemental carbon in fine particles in downtown Beijing and Shanghai. Atmos. Chem. Phys. 2005, 5, 1449–1457. [Google Scholar] [CrossRef]
- Cao, J.J.; Lee, S.C.; Chow, J.C.; Watson, J.G.; Ho, K.F.; Zhang, R.J.; Jin, Z.D.; Shen, Z.X.; Chen, G.C.; Kang, Y.M.; et al. Spatial and seasonal distributions of carbonaceous aerosols over China. J. Geophys. Res.: Atmos. 2007. [Google Scholar] [CrossRef]
- Turpin, B.J.; Huntzicker, J.J. Identification of secondary organic aerosol episodes and quantitation of primary and secondary organic aerosol concentrations during SCAQS. Atmos. Environ. 1995, 29, 3527–3544. [Google Scholar] [CrossRef]
- Watson, J.G.; Chow, J.C.; Houck, J.E. PM2.5 chemical source profiles for vehicle exhaust, vegetative burning, geological material, and coal burning in northwestern Colorado during 1995. Chemosphere 2001, 43, 1141–1151. [Google Scholar] [CrossRef] [PubMed]
- Chow, J.C.; Watson, J.G.; Lu, Z.Q.; Lowenthal, D.H.; Frazier, C.A.; Solomon, P.A.; Thuillier, R.H.; Magliano, K. Descriptive analysis of PM2.5 and PM10 at regionally representative locations during SJVAQS/AUSPEX. Atmos. Environ. 1996, 30, 2079–2112. [Google Scholar] [CrossRef]
- Castro, L.M.; Pio, C.A.; Harrison, R.M.; Smith, D.J.T. Carbonaceous aerosol in urban and rural European atmospheres: Estimation of secondary organic carbon concentrations. Atmos. Environ. 1999, 33, 2771–2781. [Google Scholar] [CrossRef]
- Sheehan, P.E.; Bowman, F.M. Estimated effects of temperature on secondary organic aerosol concentrations. Environ. Sci. Technol. 2001, 35, 2129–2135. [Google Scholar] [CrossRef] [PubMed]
- Jing, J.S.; Wu, Y.F.; Tao, J.; Che, H.Z. Observation and analysis of near-surface atmospheric aerosol optical properties in urban Beijing. Particuology 2014. [Google Scholar] [CrossRef]
- He, X.; Li, C.C.; Lau, A.K.H.; Deng, Z.Z.; Mao, J.T.; Wang, M.H.; Liu, X.Y. An intensive study of aerosol optical properties in Beijing urban area. Atmos. Chem. Phys. 2009, 9, 8903–8915. [Google Scholar] [CrossRef]
- Titos, G.; Foyo-Moreno, I.; Lyamani, H.; Querol, X.; Alastuey, A.; Alados-Arboledas, L. Optical properties and chemical composition of aerosol particles at an urban location: An estimation of the aerosol mass scattering and absorption efficiencies. J. Geophys. Res.: Atmos. 2012. [Google Scholar] [CrossRef]
- Cao, J.J.; Wang, Q.Y.; Chow, J.C.; Watson, J.G.; Tie, X.X.; Shen, Z.X.; Wang, P.; An, Z.S. Impacts of aerosol compositions on visibility impairment in Xi’an, China. Atmos. Environ. 2012, 59, 559–566. [Google Scholar] [CrossRef]
- Jung, J.; Lee, H.; Kim, Y.J.; Liu, X.G.; Zhang, Y.H.; Gu, J.W.; Fan, S.J. Aerosol chemistry and the effect of aerosol water content on visibility impairment and radiative forcing in Guangzhou during the 2006 Pearl River Delta campaign. J. Environ. Manag. 2009, 90, 3231–3244. [Google Scholar] [CrossRef]
- Li, X.H.; He, K.B.; Li, C.C.; Yang, F.M.; Zhao, Q.; Ma, Y.L.; Cheng, Y.; Ouyang, W.J.; Chen, G.C. PM2.5 mass, chemical composition, and light extinction before and during the 2008 Beijing Olympics. J. Geophys. Res.: Atmos. 2013, 118, 12158–12167. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Li, X.; Shi, G.; Cao, J.; Li, C.; Yang, F.; Ma, Y.; He, K. PM2.5 Chemical Compositions and Aerosol Optical Properties in Beijing during the Late Fall. Atmosphere 2015, 6, 164-182. https://doi.org/10.3390/atmos6020164
Wang H, Li X, Shi G, Cao J, Li C, Yang F, Ma Y, He K. PM2.5 Chemical Compositions and Aerosol Optical Properties in Beijing during the Late Fall. Atmosphere. 2015; 6(2):164-182. https://doi.org/10.3390/atmos6020164
Chicago/Turabian StyleWang, Huanbo, Xinghua Li, Guangming Shi, Junji Cao, Chengcai Li, Fumo Yang, Yongliang Ma, and Kebin He. 2015. "PM2.5 Chemical Compositions and Aerosol Optical Properties in Beijing during the Late Fall" Atmosphere 6, no. 2: 164-182. https://doi.org/10.3390/atmos6020164
APA StyleWang, H., Li, X., Shi, G., Cao, J., Li, C., Yang, F., Ma, Y., & He, K. (2015). PM2.5 Chemical Compositions and Aerosol Optical Properties in Beijing during the Late Fall. Atmosphere, 6(2), 164-182. https://doi.org/10.3390/atmos6020164







