Abstract
Climate fluctuations are expected to drive a decline in the growth of many conifer and broadleaf species, especially in the Mediterranean region, where these species grow at or very near the southern limits of their distribution. Such trends have important implications not only for forest productivity but also for plant diversity, as shifts in species performance may alter competitive interactions and long-term community composition. Using tree-ring data sourced from two Abies cephalonica stands with different elevation in Mount Parnassus in Central Greece, we evaluate the growth responses of the species to climatic variability employing a dendroecological approach. We hypothesize that radial growth at higher elevations is more strongly influenced by climate variability than at lower elevations. Despite the moderate to relatively good common signal indicated by the expressed population signal (EPS: 0.645 for the high-altitude stand and 0.782 for the low-altitude stand), the chronologies for both sites preserve crucial stand-level growth patterns, providing an important basis for ecological insights. The calculation of the Average Tree-Ring Width Index (ARWI) for both sites revealed that fir in both altitudes exhibited a decline in growth rates from the late 1980s to the early 1990s, followed by a general recovery and increase throughout the late 1990s. They also both experienced a significant decline in growth between approximately 2018 and 2022. The best-fit model for annual ring-width variation at lower elevations was a simple autoregressive model of order one (AR1), where growth was driven exclusively by the previous year’s growth (p < 0.001). At the higher elevation, a more complex model emerged: while previous year’s growth remained significant (p < 0.001), other variables such as maximum growing season temperature (p = 0.041), annual temperature (inverse effect, p = 0.039), annual precipitation (p = 0.017), and evapotranspiration (p = 0.039) also had a statistically significant impact on tree growth. Our results emphasize the prominent role of carry-over effects in shaping their annual growth patterns.
1. Introduction
Understanding how trees respond to climatic factors and the suggested monitoring of tree species are increasingly crucial for evaluating forest ecosystem resilience, plant diversity and managing environmental changes, especially as global climate patterns shift.
Predicted increases in the frequency and severity of global drought events may have a considerable impact on tree growth [1]. Simultaneously, severe droughts characterized by elevated temperatures and reduced precipitation have been associated with widespread episodes of forest dieback throughout Europe in recent decades [2,3,4,5]. Direct abiotic disturbances, such as droughts and other climatic variables, negatively affect forests. As expected, this issue is more intense in Southern Europe, where the sensitivity of tree species’ growth to a warmer or drier climate varies depending on the species and local or previous management conditions [3,6,7,8,9,10,11,12]. These effects, in turn, lead to reduced forest productivity [13] increased tree mortality [14,15,16,17,18] and cascading impacts on plant diversity in Mediterranean forest ecosystems. The greater impact of non-biological stresses in Southern Europe is mainly climate-driven, due to increased drought and heat extremes that outweigh positive growth effects, with geographical factors acting as secondary modifiers [16,17]. In many studies, fir species together with pine in Europe and globally are selected to capture such cause-and-effect relationships as well as spatial variability, usually using dendroclimatic methodologies [19,20,21]. More specifically, Carrer et al. [8] reported that Abies alba species’ sensitivity to global change can lead to pronounced spatial variations, reflecting the complexity of the Mediterranean climate and causing substantial differences across the various regions of the basin.
Past research indicates that Abies cephalonica Loundon (Greek fir, endemic to Greece) populations in mainland Greece, including Mount Parnassus, are experiencing both direct and indirect impacts from changing climatic conditions, with evidence pointing toward a trend of decline rather than resilience in several respects. The first serious reports of Greek fir dieback come from Mount Taygetos (Peloponnese) and date back to the 1960s. The phenomenon re-emerged in the following decades, reaching its peak intensity between 1985 and 1989. On Mount Mainalo (Peloponnese), the situation in 1988–1989 was dramatic, with areas where dieback affected up to 70% of fir trees. This event progressed with characteristic speed: first, branches in the middle of the crown would die, followed by resin exudation, and within a few weeks, the tree would be completely dead. This was often followed by bark beetle epidemics, which further exacerbated the situation [22].
Similar dieback was observed on Mount Parnassus as early as 1977, with the most severe effects seen at lower altitudes and in areas of mixed vegetation, where fir trees were interspersed among other broadleaf species. The situation worsened in the late 1980s, with dieback rates reaching up to 40%, even in areas at relatively higher altitudes. Significant studies from that era highlighted that these diebacks were associated with unfavorable climatic and soil conditions, warmer and drier years, especially when combined with hot and dry summers [22]. This resulted in a weakening of tree vitality, primarily in shallow, rocky soils and, as expected, on south-facing exposures.
This pattern was not an isolated behavior of the past. It recurred in 2000, 2002, and 2009, with extensive dieback recorded in many mountain ranges of Central and Southern Greece.
Despite potentially receiving high levels of precipitation in autumn and winter, Greece’s fir forests are particularly vulnerable to summer droughts, especially in areas with poor soils. The literature agrees that water scarcity and temperature anomalies—particularly unusually high spring or low winter temperatures—are the main climatic factors limiting forest growth in Greece [23,24,25,26,27,28,29,30]. Similar relationships between water availability and reduced growth have been observed in fir species across other Mediterranean regions [31].
The decline of Mediterranean fir forests in Southern and Central Greece is mainly driven by extreme droughts, with abnormal temperatures—such as late frosts, severe cold, or prolonged winters—further hindering growth and recovery [32,33,34,35,36]. In general, the research has consistently demonstrated a strong link between the availability of water and the growth of trees, as measured by ring width and overall size, in different forests and/or species [37,38].
Furthermore, the decline of fir ecosystems has recently become increasingly pronounced by wildfires connected to climate, as has often occurred in the Peloponnese and Central Greece, where fires typically originate at lower altitudes and spread to higher ones. It is well known that the recovery of these ecosystems, particularly those dominated by Greek fir, can be slow and uncertain [39].
Given that future climatic conditions are expected to make the conditions even drier in the region, this study focuses on the dominant tree species growing at high elevations in the mountains of Central Greece, specifically on Mount Parnassus, which represent the southernmost limit of its natural distribution [40,41]. The Greek fir is genetically and morphologically distinct within the Pinaceae family and exhibits high ecological importance as a keystone species in its native range [42,43]. The particular species endemic to Greece can reach heights of up to 30 m and is primarily distributed in Central Greece (Sterea Ellada), with additional populations in the Peloponnese, Kefalonia, and Evia. It forms extensive forests at relatively high altitudes.
We applied a dendroecological approach to investigate unmanaged stands and assess the species’ growth responses to climate variability. For this purpose, we focus on aggregated climate parameters (annual and growing-season values) rather than monthly data, in order to capture broader temporal patterns influencing growth. We hypothesize that the radial growth of Greek fir at higher elevations is more strongly influenced by climate variability than that of the particular species at lower elevations. Our specific objectives were to: (i) examine the relationship between radial growth and key climatic factors, and (ii) assess the influence of internal growth trends (legacy effect) and/or past growth influence and compare growth responses between two altitudes.
2. Materials and Methods
2.1. Study Sites, Vegetation and Climate
The two study sites (S1538 and S1174) are situated in uneven-aged fir stands near the settlement of Livadi on Mount Parnassus, facing south and southeast, respectively (Figure 1). The elevations of the stands are 1538 m and 1174 m, respectively. Data for both stands were collected during the summers of 2024 and 2025. All sampled trees within each site were located at essentially the same elevation, with only minimal variation (less than 1–2 m). Therefore, there is no internal altitudinal differentiation within sites. In addition, to the best of our knowledge, all samples were collected from unmanaged forests, where trees grow under natural and healthy competition. This ensures that our measurements reflect intrinsic tree growth and are not affected by human interventions.
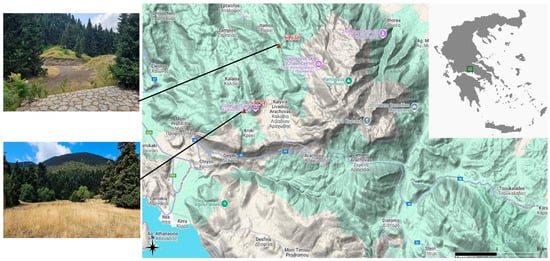
Figure 1.
Location of the study areas on Mount Parnassus: S1538 and S1174. The map was created with QGIS 3.22.3 [44]. Upper photo: fir stands near the first research area (Fterolakka, 1538 m). Lower photo: fir stands near the second research area (Corycian cave, 1174 m). Photos by Panagiotis P. Koulelis.
Regarding tree core sampling, we collected wood cores from 10 dominant or co-dominant Greek fir trees at breast height per stand, ensuring that sampled trees were at least 5 m apart, using a Mora three-threaded auger manufactured by Haglöf (Haglöf Sweden AB, Långsele, Sweden). To minimize the formation of reaction (tension) wood, we selected straight trees with the most symmetrical canopies possible. After lab preparation, we measured tree-ring width to 0.01 mm precision, using LignoVision software [45] (ver. 1.40). The mean tree age was 77.6 years at Fterolakka and 94.6 years at Corycian cave (Table 1).

Table 1.
Geographical and stand structural characteristics.
2.2. Vegetation
Mount Parnassus, in central Greece, is a predominantly calcareous, karstic massif with pronounced altitudinal zonation and high floristic diversity.
Both study sites lie within the Natura 2000 SAC GR2450005, where the regional vegetation mosaic is structured mainly by Greek fir (Abies cephalonica) forests on limestone substrates [46,47,48]. The massif supports ≥850 recorded taxa and a core of local endemics closely associated with limestone cliffs, screes, and the subalpine zone (e.g., Centaurea musarum, Erysimum parnassi, Genista parnassica, Silene guicciardii, Geocaryum pumilum, Paeonia parnassica) [48,49,50].
The Corycian cave site (1174 m) is embedded in a relatively closed Greek fir forest on limestone. In the broader surroundings, fir woodland grades into evergreen sclerophyll shrublands (Quercus coccifera), while rocky outcrops and cliff face support chasmophytic calcareous communities [46,47,48]. The sampled trees therefore represent the mid-montane fir belt, with a canopy dominated by A. cephalonica and limited evidence of recent anthropogenic disturbance. The conservation value of the Corycian cave landscape is reinforced by specialized, narrow-range taxa supported by cliff and scree microhabitats, including Natura 2000 habitat types 8210 and 8140 [48,49,50].
The Fterolakka site (1538 m) is positioned higher, within the upper montane fir zone and close to the local treeline. Fir remains dominant, but exposure and shallow soils increase the frequency of subalpine openings, grass–forb patches, and wind-shaped heaths; rocky ledges and screes are also common. Downslope, black pine (Pinus nigra) may co-occur with fir, whereas above the treeline the landscape transitions to oro-Mediterranean heaths and species-rich grasslands on exposed limestone [46,47,48]. The Fterolakka treeline/subalpine landscapes likewise derive high overall conservation importance from the presence of specialized taxa within 8210/8140 microhabitats [48,49,50].
2.3. Climate
Climate data was received from the ClimateEngine platform for the period 1964–2024 [51]. Specifically, we extracted monthly maximum and minimum air temperature (°C), precipitation (mm), and potential evapotranspiration (mm) from the TerraClimate dataset (4 km, monthly resolution). The mean annual air temperature for the period 1964–2024 was 8.78 ± 2.70 °C at Fterolakka and 10.81 ± 2.73 °C at Corycian cave. Mean annual precipitation was 904.7 ± 156.1 mm and 831.9 ± 142.1 mm for Fterolakka and Corycian cave, respectively, with most rainfall occurring in winter (36.8% and 37.7%). Summer was the driest season, receiving only 12.0% of annual precipitation at Fterolakka and 11.5% at Corycian cave. According to the modified Köppen–Geiger global climate classification [52], the climate of the region is temperate with warm and dry summers, classified as Csb.
The ombrothermic diagrams for the two study sites (Figure 2) indicate that Fterolakka experiences a short, low-intensity dry–hot period lasting two months (July–August). In contrast, the Corycian cave site exhibits a more intense dry–hot period, lasting approximately 15 days longer than at Fterolakka and covering both July and August.
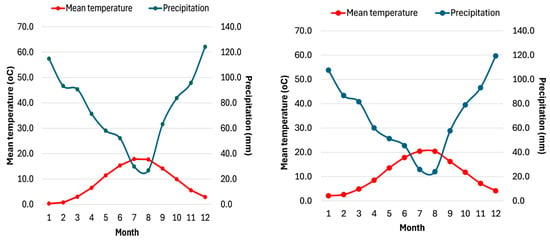
Figure 2.
Ombrothermic diagrams for the Fterolakka (left) and Corycian cave (right) sites for the period 1964–2024. Air temperature (°C) is depicted with the red line and precipitation (mm) with the blue one.
The Thornthwaite Aridity Index (AI) [53,54] was calculated using the UNEP [55] formula as:
where
- P = annual precipitation (mm), and
- PET = annual potential evapotranspiration (mm).
The calculated AI values were 1.52 ± 0.03 for Fterolakka and 1.25 ± 0.03 for the Corycian cave stand. According to the above classification, these values categorize the study area as humid.
Fady et al. [56] showed that Abies cephalonica typically grows in areas with a pronounced three-month summer drought and an overall humid climate receiving about 1000 mm of annual rainfall. These sites experience a broad temperature spectrum, from very cold winters (coldest-month minima around −7 °C) to cool conditions (0–3 °C), which is wide for a Mediterranean fir. Aussenac [57] further noted that the species performs well across a broad mean annual temperature range of 8–17 °C throughout its natural distribution [58]. Overall, our current climate characterization supports the suitability of the sites for A. cephalonica while revealing slightly less severe summer dryness than reported in earlier studies.
2.4. Cross-Dating, Detrending and Climate–Growth Relationships
Cross-dating of the tree-ring samples was performed through manual inspection, where growth patterns across all samples were visually identified and matched. This approach was supported by statistical validation to ensure the accuracy and consistency of the tree-ring chronologies. Bernabel et al. [59] highlighted that cross-dating can be evaluated through both statistical methods and visual inspection. Papadopoulos [60] further emphasized the use of standard dendrochronological techniques, particularly visual comparisons, for cross-dating samples from pine forests, underscoring the importance of visual assessment in the process. Similarly, Trouet et al. [61] noted that cross-dating frequently relies on visually comparing growth curves to match ring width series. Nevertheless, all series were then statistically verified using COFECHA (ver. 4.81c) [62] and TSAP-Win (ver. 6.06p) [63], where they were segmented into overlapping windows and correlated against a master chronology to detect misaligned rings or dating errors. The quality of the resulting chronologies was assessed through mean sensitivity, reflecting year-to-year growth variability, and quantifying the common growth signal among trees. Outlier series with low correlations or anomalous patterns were re-examined or excluded. Finally, following Wigley et al. [64] and Briffa and Jones [65], the Expressed Population Signal (EPS) was calculated by the standardized values of annual growth per tree and stand as:
where N is the number of trees that are included, and is the average inter-series correlation coefficient. To account for age-related growth trends, tree-ring series were detrended following the approach of Fritts [66]. After establishing appropriate curves to represent growth changes with increasing tree age, a moving average was applied to smooth the series and construct expected growth patterns. Radial growth indices are known to be valuable long-term measures of overall tree vigor [1], and dendroclimatological methods are frequently applied to identify the climatic factors most closely associated with variations in tree-ring parameters [66]. For the detrending of the time series, the measured ring widths (Wt) were converted into an average ring width index (ARWI) for the two stands by dividing each year’s width (Wt) by the expected growth (Yt) for year t. This division removes the trend in growth and scales the variance so that it is approximately the same throughout the entire length of time series. The formula that was used is:
This procedure was repeated for each altitude and stand. After constructing and solving the equations for all measurements, the master ring width indices were calculated for all stands.
Finally, we obtained time series data for soil moisture (SM), actual evapotranspiration (ActET), precipitation (PREC), climatic water deficit (ClimWD), mean maximum temperature (MaxTemp), and the Palmer Severity Index (PDSI) both annually and during the growing season of trees. These data were sourced from the climate engine and TERRACLIMATE dataset (4000 m, 1/24-degree) covering the years 1960 to 2024, as described by Huntington et al. [67]. For this study, the growing season is defined as April through October in the Northern Hemisphere. The selection of this period is well-documented through anatomical and modeling studies across multiple Mediterranean conifer species with reports indicating that cambial activity can continue until the end of October [68,69].
Although the ring width series were detrended following moving average to remove age-related growth trends, a significant autoregressive term remained, indicating persistent short-term growth inertia independent of climatic forcing. For this reason, we preferred the application of a method capable of accounting for the internal growth lag effect of the trees.
An autoregressive model, incorporating a lagged depended variable, was employed to effectively account for serial autocorrelation inherent in the tree growth data. Analysis of variance (ANOVA) was applied to these models to assess the overall fit and the contribution of the model to explain radial growth variation. The approach allows evaluation of the relative importance of internal growth persistence versus climatic effects. This approach seems to be crucial in the current analyses, where time series often exhibit temporal dependence, as current-year growth is influenced by the previous year’s conditions [21,66,70,71], which often leads to spurious correlations in simpler models.
By including lagged ARWI terms, the model’s Durbin–Watson statistic was considered, demonstrating that the serial correlation was appropriately modeled. Such augmentation in methodology enabled stronger testing of climate–growth relationships, separating the true effect of climatic variables from the spurious effect generated due to the time-series nature. The autoregression thus gave a valid and better framework to establish the true effect of climate on tree growth. The simple AR(1) model expresses the current value of a time series as a linear function of its immediately preceding value and a random error term. Its general form is:
where:
- is the value of the variable at time ,
- is a constant term,
- is the autoregressive parameter that measures the influence of the previous observation on the current one, and
- is a white noise error term.
The final AR(1) model for ARWI has the general form
where Xi,t are the considered climatic predictors: SM (annual soil moisture), SM_GS (growing season soil moisture), PREC (annual precipitation), PREC_GS (growing season precipitation), ActET (annual actual evapotranspiration), ActET_GS (growing season evapotranspiration), MaxTemp (annual mean maximum temperature), MaxTemp_GS (growing season mean maximum temperature), ClimWD (annual climate water deficit), ClimWD_GS (growing season climate water deficit), PDSI (annual Palmer Drought Severity Index), and PDSI_GS (growing season Palmer Drought Severity Index). Only statistically significant climatic variables are included in the final models.
The autoregressive analysis was performed using IBM SPSS Statistics (Version 23.0).
3. Results
3.1. Cross-Dating and Detrending Results
Cross-dating of the original raw tree-ring width series obtained directly from the increment cores using the longest single series as a master was conducted at the Fterolakka site (1538 m). 648 rings in the entire series were all measured, and 591 rings were dated as part of complete growth series to ensure they were properly dated. Based on results from both TSAP-Win (4.81c) and COFECHA (ver. 6.06p), 9 of the 10 increment core series available were included in the final chronology following very close examination of flagged segments and outliers. The mean inter-series correlation of 0.246 and the average mean sensitivity of 0.203 suggested a moderate common signal. Flagged segments, as detected by COFECHA diagnostics, were inspected visually and removed where they resulted in unacceptable time shifts in the series. The whole chronology’s stated population signal (EPS) was 0.645 [64,65], suggesting the existence of a moderate common signal. To incorporate as many of the series as was feasible, and since the series varied in length, the master site chronology was constructed from these nine series’ Average Ring Width Index (ARWIFTE) for the 1978–2024 period.
For the Corycian cave stand, the sample included 949 rings in total and 948 were successfully cross-dated within their respective series. The average series length was 94.9 years. The average inter-series correlation was 0.262, and the average mean sensitivity was 0.212. The EPS of the chronology was moderate at 0.782, accounting for a relatively coherent common growth signal. As was mentioned, COFECHA diagnostics recognized segments of potential issues, which were carefully investigated and adjusted where needed at the series level. Following this process and based on the outcome of CHAPWIN (ver. 4.81c) and COFECHA, 10 of the 10 original series were included in the final chronology. As in the case of the Fterolakka stand, and for as many series as possible to be incorporated, in the Corycian cave stand 10 increment core series were cross-dated and tested, yielding a master chronology (ARWICOR) 1964–2024.
It is important to underline that despite the EPS values for both sites indicating a moderate to relatively good common signal, the chronologies for both sites preserve stand-level growth patterns and provide a valuable basis for ecological interpretation.
3.2. Ring Width Indices
According to the method outlined, Figure 3 presents the annual fluctuation in the average radial growth between the two altitudes. The figure reveals that the standardized tree-ring width indices for the upper stand (FTE 1538 m) and lower stand (COR 1174 m) vary over time, yet always move similarly. The graph indicates periods of consistent growth and decline for both stands. Both, for example, have one period of declining growth indices from the late 1980s to the early 1990s and then a general trend upward through the late 1990s. There is also a notable decline in growth for both stands in the period from roughly 2018 to 2022. But there are some interesting differences too. Visually, the Fterolakka stand (red line), located at a higher elevation, appears to show more pronounced fluctuations in growth. It has some of the highest peak growth years, particularly in the mid-1970s and early 2010s, but also some of the lowest points, such as in the late 1980s and the early 2020s.
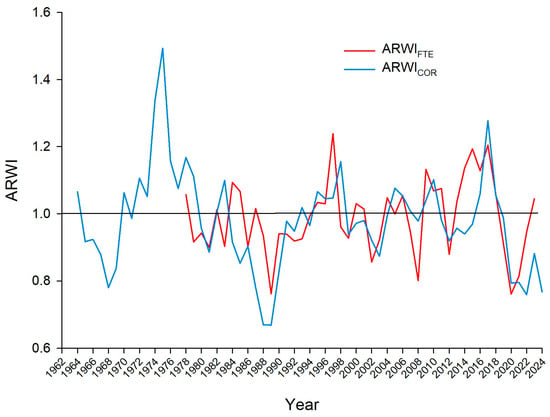
Figure 3.
Master average tree-ring width indices across time for the two stands (Fterolakka 1538 m and Corycian cave 1174 m.
The Corycian cave stand (blue line), at a lower altitude, shows a more stable growth pattern, though it still has periods of high and low growth. Its indices generally stay closer to the 1.0 average line, with less dramatic peaks and troughs compared to the Fterolakka stand.
3.3. Autoregressive Models
The autoregressive analysis for the Corycian cave stand reveals that climatic variables do not exert any significant influence on tree growth and previous-year growth is found to have a significant influence. The model successfully addresses the issue of serial autocorrelation, providing a more robust assessment of climate–growth relationships as its summary indicates a reasonably good fit, with an adjusted R2 of 0.397, which shows that close to 40% of the variance in the ARWI is explained by the predictors in the model (Table 2). The Durbin–Watson statistic of 1.802 is close to 2, indicating that serial correlation has been properly removed by adding the lagged variable ARWI (ARWIt−1) as described in the methodology.

Table 2.
Autoregressive model summary for the Corycian cave stand.
The ANOVA table revealed that the model as a whole is statistically significant (p < 0.001) (Table 3), primarily driven by the strong influence of the autoregressive term ARWIt−1. Analysis of the coefficients further elucidates these findings. The last variable, representing the previous year’s growth, has a highly significant positive effect (B = 0.691, p < 0.001). This suggests high biological inertia, where previous growth is the most powerful predictor of current growth. Conversely, none of the included climatic variables, including growing season (GS) and annual values for all the examined variables, have any statistically significant effect on tree growth (all p values > 0.05, Table 4). Focusing on the Corycian cave stand, the autoregressive term reflects the internal growth dynamics and constitutes the main factors of variability in tree growth. The assessed climatic factors seem to lack a meaningful and additional effect, which is likely to indicate that local and non-climatic factors, or even more complex climatic relations, are of greater significance.

Table 3.
Autoregressive model ANOVA for the Corycian cave stand.

Table 4.
Calculated standardized and unstandardized coefficients of the autoregressive model for the Corycian cave stand.
The final model of the AR(1) autoregressive model, where only the lagged dependent variable remains in the final model because the other predictors (the climatic variables) were not found to be statistically significant, can be expressed as:
where ARWIt is the tree growth index for the current year t, ARWIt−1 represents the previous year’s growth, and is the error term (residual) at time t.
Autoregressive analysis of the Fterolakka stand shows complex interaction between previous-year growth and a variety of climatic variables, though the overall model significance is marginal. The model summary (Table 5) shows an adjusted R2 of 0.217, and the predictors account for approximately 21.7% of the variation in the ARWI. The value of the Durbin–Watson statistic of 1.672 confirms that employing the lagged ARWI variable has addressed the issue of serial autocorrelation, so that climatic effects can be estimated more confidently.

Table 5.
Autoregressive model summary for the Fterolakka stand.
However, the ANOVA table (Table 6) reveals that the general model is only marginally significant (p = 0.045), suggesting that climatic relationships that have been found to be significant should be interpreted with caution. The lower adjusted R2 compared to the Corycian cave stand, despite having significant climate factors, suggests a weaker model.

Table 6.
Autoregressive model ANOVA for the Fterolakka stand.
Coefficient analysis provides further insight, revealing that the previous year’s growth (ARWIt−1) is a highly significant factor (B = 0.625, p < 0.001), confirming the presence of strong biological inertia, similar to the Corycian cave stand.
Unlike the Corycian cave, for Fterolakka and 1538 m elevation, there are certain climatic factors which are statistically significant predictors of tree growth (Table 7). Growing Season Maximum Temperature (MaxTemp_GS) has a statistically significant negative influence (B = −0.238, p = 0.041) and, conversely, annual precipitation (PREC) has a significant positive influence (p = 0.017). Annual Actual Evapotranspiration (ActET) also has a significant negative influence (B = −0.003, p = 0.039). In addition, Annual Maximum Temperature (MaxTemp) also has a significant positive impact (B = 0.291, p = 0.039). The fact that the growing season and the annual temperature effects have opposite signs suggests a complex relationship. The remaining climatic variables, including soil moisture, PDSI, and growing season precipitation, were not found to have a significant relationship with tree growth in this model.

Table 7.
Calculated standardized and unstandardized coefficients of the autoregressive model for the Fterolakka stand.
The final AR(1) model includes both the lagged dependent variable (ARWIt−1) and several statistically significant climatic variables. It can be expressed as:
where ARWIt is the tree growth index for the current year t, ARWIt−1 represents the previous year’s growth, the climatic variables are as defined above, and is the error term (residual) at time t.
Table 8 summarizes the results, highlighting differences in the effects of the examined variables on climatic sensitivity, the lagged growth effect, and their plausible biological interpretation based on the data.

Table 8.
Corycian cave vs. Fterolakka stands: Insights from two regions.
4. Discussion
The results of the current study indicate that the radial growth of fir trees in Mount Parnassus (at least based on our data) seems to be influenced by both biological memory and climatic drivers, with the relative importance of these drivers varying along the elevation gradient. At the lower elevation, the best-fit model for annual ring width variation was a simple autoregressive model of order one AR(1), where current-year growth depended solely on the growth of the previous year. This finding underlines the important contribution of carry-over effects—or the biological memory—in shaping annual growth patterns, particularly in Greek fir.
By contrast, at the higher elevation (Fterolakka), the best-fit model incorporated both the AR(1) term and four climate variables, indicating that growth at these sites is jointly controlled by biological memory and exogenous environmental drivers. The positive identification of climate effects at high elevations underscores the increasing sensitivity of trees to external forcing in more stressful environments. Different sites, and consequently different altitudes, soils, slopes, etc., revealed slightly different effects of climatic parameters. There are differences among specific sites elevations; thus, the climate sensitivity depends on other site characteristics, such as soil and slope, but probably also to other ecological drivers, such as competition, stand density, and water drainage, among others [72,73].
Higher elevation stands are often characterized by a shorter growing season, lower temperatures, greater radiation, and higher climatic variability that, singly or together, amplify the effect of interannual climate on radial growth. Under such conditions, the tree’s internal reserves and physiological state interact with the environmental signals, producing a growth response that reflects both memory effects and immediate climatic conditions. Conversely, the significant relationships at higher elevations indicate tighter climatic control, likely due to temperature limitations and/or the short length of the growing season. Consistently, focusing on three conifer species, Ettinger and HilleRisLambers [74], showed that conifer growth is strongly climate-sensitive at high elevations—where cold temperatures and heavy snowpack limit growth—but much less climate-driven in low-elevation, closed-canopy forests. As a result, climate change is expected to have smaller impacts on low-elevation communities, especially during early life stages, compared with the more climate-responsive high-elevation treeline zones.
In general, ecosystem state is significantly influenced by its past, making it crucial to account for these carry-over effects when modeling the future dynamics of the species. However, the magnitude and duration of these legacy effects, in relation to the influence of contemporary environmental drivers, are poorly understood [75]. Rathgeber et al. [76] published an in-depth review analyzing the interactions between environmental drivers, the physiological state of trees, and the developmental stage of forming xylem, all crucial to understanding how normal seasonal cycles create standard tree-ring structures and specialized anatomical features under exceptional conditions, such as extreme climatic events.
The memory effects have been widely documented in conifers, among other tree species, reflecting the capability of the tree to buffer against short-term environmental variability by drawing from internal reserves [66,77]. More specifically, Babst et al. [78] assessed the climate sensitivity of model-based forest productivity estimates for major European tree species, including conifers, and demonstrated that carry-over effects from the preceding growing season can significantly shape subsequent tree growth, especially in environments characterized by harsh climatic conditions. Moreover, the amount of annual stem growth explained by current-year conditions differs largely among tree species. Historic conditions explain a significant fraction of unexplained variance [79].
Other studies have discussed ecological stress memory (ESM) [80], a mechanism important in the acclimation of plants to repeated stress events. Despite the wide documentation of ESM, it remains uncertain whether this promotes tree resistance during recurrent stress in subsequent decades. The above discussion is consistent with previous studies conducted on Mediterranean and temperate conifer forests, where autocorrelation in ring width series reflects the carry-over of resources, hormonal status, and structural constraints from one year to the next [78,81].
The hypothesis that fir stands at higher altitudes would exhibit greater sensitivity to climate change compared with those at lower elevations is partially supported, but the relationship is more complex than a simple altitude-driven effect. In our case, the response of the Greek fir at higher elevation does show a stronger response to climatic factors; this does not imply that climate is inherently more important than biological factors, but rather that its influence is more consistent, predictable, and statistically measurable. In contrast, at low elevation, it seems that climatic effects may be less evident or obscured by other stresses, or the local climate may fall outside the species’ optimal range, so that annual growth is more dependent on the previous year’s tree viability than on short-term climate fluctuations. This latter finding demands more analysis, considering site-specific conditions and stand characteristics. Similar elevation-dependent shifts from memory-dominated toward climate-sensitive growth have been reported in other montane and alpine conifers, highlighting the complex interplay between endogenous and exogenous factors in determining annual ring formation [82].
One more observation that deserves discussion is the statistical significance of various climatic factors in Fterolakka. These include the positive influence of annual maximum temperature (MaxTemp) and precipitation (PREC), as well as the negative influence (indicated by their coefficients) of the maximum temperature during the growing season (MaxTemp_GS) and annual actual evapotranspiration (ActET). The marginal overall model significance (0.045) may be a result of a large number of predictors involved, suggesting that while these climatic effects are detectable, their overall contribution to explaining growth variability is limited. Many studies have investigated the relationship between tree-ring width and precipitation as well as temperature on a local scale for Greek fir [25,26,28,34,35] and identified cases of reduced tree growth unrelated to climate or weather events [25]. Generally, higher annual precipitation enhances soil moisture and water availability, promoting the formation of wider growth rings. However, while precipitation can be an important limiting factor for tree growth in those sites, the strength of this relationship varies with habitat, species, size of trees, and local hydrological conditions [83,84]. Our findings are consistent with several other studies, from around the world, where growth of conifers, such as Scots pine and Douglas fir, was mainly favored by winter temperature increase in particular the temperatures of January, February, and March of the current year [70]. Mountain ecosystems, especially those at the highest altitudes, are sensitive to ongoing climate variability. Radial growth of fir trees at high-elevation sites is promoted by normal or warmer summer temperatures (June–July) during the current growing season. Moreover, trees growing at high to mid elevations are sensitive to low winter temperatures, whereas those at low elevations exhibit little sensitivity to temperature fluctuations [85]. Another study demonstrated the positive effect of temperatures on tree growth, during late winter months [86] or the growth benefit from the increasing length of the growing season [87]. Moreover, the negative impact of rising maximum temperature on fir growth recorded in our study has also been documented in many other investigations, although most of them report only on the adverse impact of some particular month(s) of the growing season [23,24,25].
Finally, the negative coefficient between actual evapotranspiration (ActET) and fir growth indicates that higher water loss through evaporation and transpiration is associated with reduced water availability for physiological processes essential to growth. In other words, when atmospheric moisture demand is high—under warmer or drier conditions—fir trees experience increased water stress, resulting in narrower growth rings. More specifically, the statistical significance of this relationship pertains to annual evapotranspiration (warmer conditions outside the growing season, milder winters, or warmer early springs) rather than to in-season evapotranspiration.
This study employed climate variables aggregated at annual and growing-season scales, a deliberate choice aimed at identifying broad climatic influences on tree radial growth and at exploring the role of biological memory (carry over effect). While this approach is effective for detecting general patterns, it may not fully capture short-term or season-specific climatic events (e.g., prior-autumn precipitation or current-spring temperature variability) which can be important drivers in more detailed and specialized dendroclimatological studies. Consequently, the results should be interpreted as reflecting integrated climatic effects rather than detailed monthly climate–growth relationships. Furthermore, our detrending and modelling approach reflects a specific analytical perspective. Rather than removing autocorrelation to isolate a purely climate-driven signal—as is common in conventional dendroclimatic analyses—we treated it as an expression of biological memory. This approach is particularly well suited for capturing carry-over effects in tree growth, but it differs from other widely used methods, such as ARSTAN, which remove the influence of endogenous stand disturbances.
In general, the forests in Southern Europe have been impacted by direct abiotic disturbances, like droughts and other climatic variables [6,7,9,11], leading to reduced forest productivity [13] and increased tree mortality [14,15,18,19]. In the present study, the physiological state of fir trees seems to be driven more by internal physiological processes, which in turn may be affected by external factors.
5. Conclusions
For the high-elevation stand, the results support the idea that climate sensitivity exists and is probably more pronounced compared to low-elevation fir stands, likely due to specific limiting climatic factors at higher altitudes. However, the complex nature of this sensitivity is revealed by the opposite effects of growing season and annual temperatures, suggesting heat stress during the growing season and benefits from milder conditions in the rest of the year, thus underlining the complexity of climate–growth relationships.
In contrast, the Corycian cave stand’s lack of significant climate response is a notable statistical finding. It seems that at this site, the local, non-climatic factors, such as soil conditions, competition, site history that is not strongly evident, or others, may be more dominant drivers of tree growth variability rather than the climatic variables included in the current study.
Ultimately, for both regions, the internal biological inertia—the powerful effect of previous-year growth—is the single most important factor explaining the growth variability issue. The utilization of an autoregressive model correctly addresses the issue of serial autocorrelation, which often leads to spurious correlations in simpler models, and it accurately models the inherent temporal dependence in biological systems, such as tree growth.
The results underline the complexity of climate–growth relationships and point out the key role played by the internal biological mechanisms involved in the mediation of external environmental factors that affect the development of Mediterranean fir ecosystems. The autoregressive approach provides a methodologically robust framework for disentangling autocorrelation effects and understanding site-specific tree growth responses. This finding may be particularly relevant in the context of climate change, since rising temperature extremes, changes in precipitation variability, and increased frequency of drought events may disproportionately affect high-elevation fir Mediterranean ecosystems, which are usually found either at the southern limits of their distribution or very close to them.
This study is part of an extensive, multi-year research project focused on Greek fir on mountain in central Greece, which corroborates and builds partially upon our previous findings regarding its response to climate. Most of these studies are mentioned across the manuscript. The conclusions should be understood in the context of the region studied, as the observed role of biological memory and the climate variables identified are closely tied to local conditions and the scale of analysis, and may not apply beyond similar ecological settings. The relatively moderate common signal for both altitudes suggests a future study involving multiple species and broader geographic areas. Our aim is to increase the sample size in order to enhance the reliability of the signal and expand upon our results.
Regular assessment and monitoring underpin sustainable forestry through the provision of baseline data for adaptive silviculture, biodiversity conservation, and valuation of ecosystem services. This is especially important given the rising frequency of disturbances and the pressing need to meet both national and international policy commitments.
Author Contributions
Conceptualization, P.P.K. and A.S.; methodology, P.P.K., A.S. and A.B.; software, P.P.K.; validation, P.P.K.; formal analysis, P.P.K. and A.B.; investigation, P.P.K. and A.S.; writing—original draft preparation, P.P.K., A.S. and A.B.; writing—review and editing, P.P.K.; visualization, P.P.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Climate data is publicly available from the Climate engine platform at https://app.climateengine.org/login (accessed on 30 July 2025). Additional data and material used in this work can be obtained upon reasonable request to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dobbertin, M. Tree Growth as Indicator of Tree Vitality and of Tree Reaction to Environmental Stress: A Review. Eur. J. Forest Res. 2005, 124, 319–333. [Google Scholar] [CrossRef]
- Field, C.B.; Barros, V.; Stocker, T.F.; Dahe, Q. (Eds.) Managing the Risks of Extreme Events and Disasters to Advance Climate Change Adaptation: Special Report of the Intergovernmental Panel on Climate Change, 1st ed.; Cambridge University Press: Cambridge, UK, 2012; ISBN 978-1-107-02506-6. [Google Scholar]
- Gazol, A.; Camarero, J.J. Compound Climate Events Increase Tree Drought Mortality across European Forests. Sci. Total Environ. 2022, 816, 151604. [Google Scholar] [CrossRef]
- Giorgi, F.; Im, E.-S.; Coppola, E.; Diffenbaugh, N.S.; Gao, X.J.; Mariotti, L.; Shi, Y. Higher Hydroclimatic Intensity with Global Warming. J. Clim. 2011, 24, 5309–5324. [Google Scholar] [CrossRef]
- Peñuelas, J.; Lloret, F.; Montoya, R. Severe Drought Effects on Mediterranean Woody Flora in Spain. For. Sci. 2001, 47, 214–218. [Google Scholar] [CrossRef]
- Adams, H.D.; Zeppel, M.J.B.; Anderegg, W.R.L.; Hartmann, H.; Landhäusser, S.M.; Tissue, D.T.; Huxman, T.E.; Hudson, P.J.; Franz, T.E.; Allen, C.D.; et al. A Multi-Species Synthesis of Physiological Mechanisms in Drought-Induced Tree Mortality. Nat. Ecol. Evol. 2017, 1, 1285–1291. [Google Scholar] [CrossRef]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A Global Overview of Drought and Heat-Induced Tree Mortality Reveals Emerging Climate Change Risks for Forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Carrer, M.; Nola, P.; Motta, R.; Urbinati, C. Contrasting Tree-ring Growth to Climate Responses of Abies alba toward the Southern Limit of Its Distribution Area. Oikos 2010, 119, 1515–1525. [Google Scholar] [CrossRef]
- Choat, B.; Brodribb, T.J.; Brodersen, C.R.; Duursma, R.A.; López, R.; Medlyn, B.E. Triggers of Tree Mortality Under Drought. Nature 2018, 558, 531–539. [Google Scholar] [CrossRef]
- Manetti, M.C.; Cutini, A. Tree-Ring Growth of Silver Fir (Abies alba Mill.) in Two Stands under Different Silvicultural Systems in Central Italy. Dendrochronologia 2006, 23, 145–150. [Google Scholar] [CrossRef]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G.; et al. Mechanisms of Plant Survival and Mortality during Drought: Why Do Some Plants Survive While Others Succumb to Drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef] [PubMed]
- Sidor, C.G.; Popa, I.; Vlad, R.; Cherubini, P. Different Tree-Ring Responses of Norway Spruce to Air Temperature across an Altitudinal Gradient in the Eastern Carpathians (Romania). Trees 2015, 29, 985–997. [Google Scholar] [CrossRef]
- Ciais, P.; Reichstein, M.; Viovy, N.; Granier, A.; Ogée, J.; Allard, V.; Aubinet, M.; Buchmann, N.; Bernhofer, C.; Carrara, A.; et al. Europe-Wide Reduction in Primary Productivity Caused by the Heat and Drought in 2003. Nature 2005, 437, 529–533. [Google Scholar] [CrossRef]
- Colangelo, M.; Camarero, J.; Ripullone, F.; Gazol, A.; Sánchez-Salguero, R.; Oliva, J.; Redondo, M. Drought Decreases Growth and Increases Mortality of Coexisting Native and Introduced Tree Species in a Temperate Floodplain Forest. Forests 2018, 9, 205. [Google Scholar] [CrossRef]
- Navarro-Cerrillo, R.M.; Rodriguez-Vallejo, C.; Silveiro, E.; Hortal, A.; Palacios-Rodríguez, G.; Duque-Lazo, J.; Camarero, J.J. Cumulative Drought Stress Leads to a Loss of Growth Resilience and Explains Higher Mortality in Planted than in Naturally Regenerated Pinus Pinaster Stands. Forests 2018, 9, 358. [Google Scholar] [CrossRef]
- Lindner, M.; Maroschek, M.; Netherer, S.; Kremer, A.; Barbati, A.; Garcia-Gonzalo, J.; Seidl, R.; Delzon, S.; Corona, P.; Kolström, M.; et al. Climate Change Impacts, Adaptive Capacity, and Vulnerability of European Forest Ecosystems. For. Ecol. Manag. 2010, 259, 698–709. [Google Scholar] [CrossRef]
- Rita, A.; Camarero, J.J.; Nolè, A.; Borghetti, M.; Brunetti, M.; Pergola, N.; Serio, C.; Vicente-Serrano, S.M.; Tramutoli, V.; Ripullone, F. The Impact of Drought Spells on Forests Depends on Site Conditions: The Case of 2017 Summer Heat Wave in Southern Europe. Glob. Change Biol. 2020, 26, 851–863. [Google Scholar] [CrossRef]
- Navarro-Cerrillo, R.; Gazol, A.; Rodríguez-Vallejo, C.; Manzanedo, R.; Palacios-Rodríguez, G.; Camarero, J. Linkages between Climate, Radial Growth and Defoliation in Abies pinsapo Forests from Southern Spain. Forests 2020, 11, 1002. [Google Scholar] [CrossRef]
- Büntgen, U.; Frank, D.; Trouet, V.; Esper, J. Diverse Climate Sensitivity of Mediterranean Tree-Ring Width and Density. Trees 2010, 24, 261–273. [Google Scholar] [CrossRef]
- Kerhoulas, L.P.; Kane, J.M. Sensitivity of Ring Growth and Carbon Allocation to Climatic Variation Vary within Ponderosa Pine Trees. Tree Physiol. 2012, 32, 14–23. [Google Scholar] [CrossRef]
- Lee, E.H.; Wickham, C.; Beedlow, P.A.; Waschmann, R.S.; Tingey, D.T. A Likelihood-Based Time Series Modeling Approach for Application in Dendrochronology to Examine the Growth-Climate Relations and Forest Disturbance History. Dendrochronologia 2017, 45, 132–144. [Google Scholar] [CrossRef]
- Geotechnical Chamber of Greece (GEOTEE). Fir Forest Dieback: Proceedings of the Two-Day Conference, Tripoli, Greece, December 1989; GEOTEE: Thessaloniki, Greece, 1991. [Google Scholar]
- Kastridis, A.; Kamperidou, V.; Stathis, D. Dendroclimatological Analysis of Fir (A. borisii-regis) in Greece in the Frame of Climate Change Investigation. Forests 2022, 13, 879. [Google Scholar] [CrossRef]
- Koulelis, P.P.; Daskalakou, E.N.; Ioannidis, K.E. Impact of Regional Climatic Conditions on Tree Growth on Mainland Greece. Folia Oecologica 2019, 46, 127–136. [Google Scholar] [CrossRef]
- Koulelis, P.; Fassouli, V.; Petrakis, P.V.; Ioannidis, K.E.; Alexandris, S. The Impact of Selected Climatic Factors on the Growth of Greek Fir on Mount Giona in Mainland Greece Based on Tree Ring Analysis. Austrian J. For. Sci. 2022, 1, 1–30. [Google Scholar]
- Koulelis, P.P.; Petrakis, P.V. Brief Overview of Greek Fir Radial Growth in Response to Climate and European Fir Budworm: Three Case Studies from Giona Mountain, Central Greece. Climate 2023, 11, 78. [Google Scholar] [CrossRef]
- Koulelis, P.P.; Solomou, A.; Fassouli, V.; Petrakis, P.V.; Spanos, I. Radial Growth and Climatic Influences on Greek Fir: Tree Ring Analysis from Kirphi Mountain, Central Greece. Folia Oecologica 2025, 52, 113–123. [Google Scholar] [CrossRef]
- Koutavas, A. Late 20th Century Growth Acceleration in Greek Firs (Abies cephalonica) from Cephalonia Island, Greece: A CO2 Fertilization Effect? Dendrochronologia 2008, 26, 13–19. [Google Scholar] [CrossRef]
- Papadopoulos, A. Tree-Ring Patterns and Climate Response of Mediterranean Fir Populations in Central Greece. Dendrochronologia 2016, 40, 17–25. [Google Scholar] [CrossRef]
- Sarris, D.; Christodoulakis, D.; Körner, C. Recent Decline in Precipitation and Tree Growth in the Eastern Mediterranean. Glob. Change Biol. 2007, 13, 1187–1200. [Google Scholar] [CrossRef]
- Gentilesca, T.; Camarero, J.; Colangelo, M.; Nolè, A.; Ripullone, F. Drought-Induced Oak Decline in the Western Mediterranean Region: An Overview on Current Evidences, Mechanisms and Management Options to Improve Forest Resilience. iForest 2017, 10, 796–806. [Google Scholar] [CrossRef]
- Brofas, G.; Economidou, E. Le dépérissement du Sapin du Mont Parnasse (Grèce). Le rôle des conditions climatiques et écologiques. Ecol. Mediterr. 1994, 20, 1–8. [Google Scholar] [CrossRef]
- Markalas, S. Site and Stand Factors Related to Mortality Rate in a Fir Forest after a Combined Incidence of Drought and Insect Attack. For. Ecol. Manag. 1992, 47, 367–374. [Google Scholar] [CrossRef]
- Papadopoulos, A.M. Investigations dendroclimatologiques du Sapin de Céphalonie en Grèce Centrale. Geogr. Tech. 2009, 2, 34–38. [Google Scholar]
- Papadopoulos, A.; Raftoyannis, Y.; Pantera, A. Fir decline in Greece: A dendroclimatological approach. In Proceedings of the 10th International Conference on Environmental Science and Technology (CEST-2007), Kos, Greece, 5–7 September 2007; pp. 571–578. [Google Scholar]
- Tsopelas, P.; Angelopoulos, A.; Economou, A.; Soulioti, N. Mistletoe (Viscum album) in the Fir Forest of Mount Parnis, Greece. For. Ecol. Manag. 2004, 202, 59–65. [Google Scholar] [CrossRef]
- Sass-Klaassen, U.; Chowdhury, Q.; Sterck, F.; Zweifel, R. Effects of Water Availability on the Growth and Tree Morphology of Quercus Pubescens Willd. and Pinus sylvestris L. in the Valais, Switzerland. In Proceedings of the Dendrosymposium 2006, Tervuren, Belgium, 20–22 April 2006. [Google Scholar]
- Zheng, P.; Wang, D.; Jia, G.; Yu, X.; Liu, Z.; Wang, Y.; Zhang, Y. Variation in Water Supply Leads to Different Responses of Tree Growth to Warming. For. Ecosyst. 2022, 9, 100003. [Google Scholar] [CrossRef]
- Raftoyannis, Y.; Spanos, I. Central Greece University of Applied Sciences, Department of Forestry, Demokratias 3, GR-36100 Karpenisi, Greece Regeneration of Abies cephalonica Loudon after a Large Fire in Central Greece. SEEFOR 2014, 6, 54. [Google Scholar] [CrossRef]
- Fady, B.; Arbez, M.; Marpeau, A. Geographic Variability of Terpene Composition in Abies cephalonica Loudon and Abies Species around the Aegean: Hypotheses for Their Possible Phylogeny from the Miocene. Trees 1992, 6, 162–171. [Google Scholar] [CrossRef]
- Samaras, D.; Damianidis, C.; Fotiadis, G.; Tsiftsis, S. Effect of Climate Change on Fir Forest Communities in the Mountains of South-Central Greece. Eur. J. Environ. Sci. 2022, 12, 39–50. [Google Scholar] [CrossRef]
- Alizoti, P.G.; Fady, B.; Prada, M.A.; Vendramin, G.G. EUFORGEN Technical Guidelines for Genetic Conservation and Use of Mediterranean Firs (Abies Spp.); Bioversity International: Rome, Italy, 2011; p. 6. [Google Scholar]
- Papageorgiou, A.C.; Kostoudi, C.; Sorotos, I.; Varsamis, G.; Korakis, G.; Drouzas, A.D. Diversity in needle morphology and genetic markers in a marginal Abies cephalonica (Pinaceae) population. Ann. For. Res. 2015, 58, 217–234. [Google Scholar] [CrossRef]
- QGIS Development Team. QGIS Geographic Information System; Open-Source Geospatial Foundation: Beaverton, OR, USA, 2021; Version 3.22.3; Available online: https://qgis.org (accessed on 10 July 2025).
- Rinntech. LignoVision Software, version 1.40; Rinntech: St. Charles, IL, USA, 2024. [Google Scholar]
- European Environment Agency (EEA). Natura 2000 Site Factsheet: GR2450005 Notioanatolikos Parnassos—Ethnikos Drymos Parnassou—Dasos Tithoreas, Spilaio Varathro (Annex I Habitat List & Coverage). 2020. Available online: https://eunis.eea.europa.eu/sites/gr2450005 (accessed on 15 May 2025).
- National Natural Environment and Climate Change Agency (NECCA)—Management Unit of Parnassos & Oiti National Parks. Vegetation Types on Mt Parnassos—Actions for the Protection and Conservation of Parnassos National Park. 2021; p. 23. Available online: https://necca.gov.gr (accessed on 15 May 2025).
- Royal Botanic Gardens, Kew. Plants of the World Online. 2025. Available online: https://powo.science.kew.org (accessed on 15 May 2025).
- Dimopoulos, P.; Raus, T.; Bergmeier, E.; Constantinidis, T.; Iatrou, G.; Kokkini, S.; Strid, A.; Tzanoudakis, D. Vascular Plants of Greece: An Annotated Checklist; Botanic Garden and Botanical Museum Berlin-Dahlem & Hellenic Botanical Society: Berlin, Germany, 2013; ISBN 978-3-921800-88-1. [Google Scholar]
- Faltner, F.; Wessely, J.; Frajman, B. Phylogenetic Data Reveal a Surprising Origin of Euphorbia orphanidis (Euphorbiaceae) and Environmental Modeling Suggests That Microtopology Limits Its Distribution to Small Patches in Mt. Parnassus (Greece). Front. Plant Sci. 2023, 14, 1116496. [Google Scholar] [CrossRef]
- Climate Engine. Climate Engine; Desert Research Institute and University of California, Merced: Reno, NV, USA, 2025; Version 2.1; Available online: https://climateengine.org (accessed on 30 July 2025).
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World Map of the Köppen-Geiger Climate Classification Updated. Meteorol. Z. 2006, 15, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Thornthwaite, C.W.; Mather, J.R. The Water Balance; Laboratory of Climatology: Centerton, NJ, USA, 1955. [Google Scholar]
- Thornthwaite, C.W. An Approach toward a Rational Classification of Climate. Geogr. Rev. 1948, 38, 55. [Google Scholar] [CrossRef]
- UNEP. World Atlas of Desertification; Edward Arnold: London, UK, 1992. [Google Scholar]
- Fady, B.; Arbez, M.; Ferrandz, P. Variability of Juvenile Greek Firs (Abies cephalonica Loud.) and Stability of Characteristics with Age. Silvae Genet. 1991, 40, 91–100. [Google Scholar]
- Aussenac, G. Ecology and Ecophysiology of Circum-Mediterranean Firsin the Context of Climate Change. Ann. For. Sci. 2002, 59, 823–832. [Google Scholar] [CrossRef]
- Politi, P.-I.; Georghiou, K.; Arianoutsou, M. Reproductive Biology of Abies cephalonica Loudon in Mount Aenos National Park, Cephalonia, Greece. Trees 2011, 25, 655–668. [Google Scholar] [CrossRef]
- Bernabei, M.; Bontadi, J.; Nicolussi, K. Observations on Holocene Subfossil Tree Remains from High-Elevation Sites in the Italian Alps. Holocene 2018, 28, 2017–2027. [Google Scholar] [CrossRef]
- Papadopoulos, A.M. Resin Tapping History of an Aleppo Pine Forest in Central Greece. Open For. Sci. J. 2013, 6, 50–53. [Google Scholar] [CrossRef]
- Trouet, V.; Coppin, P.; Beeckman, H. Annual Growth Ring Patterns in Brachystegia spiciformis Reveal Influence of Precipitation on Tree Growth. Biotropica 2006, 38, 375–382. [Google Scholar] [CrossRef]
- COFECHA, version 4.81c; Laboratory of Tree-Ring Research, University of Arizona: Tucson, AZ, USA, 2023.
- Rinntech. TSAP-Win, version 6.06p; Rinntech: Heidelberg, Germany, 2019.
- Wigley, T.M.L.; Briffa, K.R.; Jones, P.D. On the Average Value of Correlated Time Series, with Applications in Dendroclimatology and Hydrometeorology. J. Clim. Appl. Meteor. 1984, 23, 201–213. [Google Scholar] [CrossRef]
- Briffa, K.R.; Jones, P.D. Basic chronology statistics and assessment. In Methods of Dendrochronology: Applications in the Environmental Sciences; Cook, E.R., Kairiukstis, L.A., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1990; pp. 137–152. [Google Scholar] [CrossRef]
- Fritts, H.C. Tree Rings and Climate; Academic Press: London, UK, 1976. [Google Scholar]
- Huntington, J.L.; Hegewisch, K.C.; Daudert, B.; Morton, C.G.; Abatzoglou, J.T.; McEvoy, D.J.; Erickson, T. Climate Engine: Cloud Computing and Visualization of Climate and Remote Sensing Data for Advanced Natural Resource Monitoring and Process Understanding. Bull. Am. Meteorol. Soc. 2017, 98, 2397–2410. [Google Scholar] [CrossRef]
- Pacheco, A.; Camarero, J.J.; Carrer, M. Linking Wood Anatomy and Xylogenesis Allows Pinpointing of Climate and Drought Influences on Growth of Coexisting Conifers in Continental Mediterranean Climate. Tree Physiol. 2016, 36, 502–512. [Google Scholar] [CrossRef]
- Valeriano, C.; Gutiérrez, E.; Colangelo, M.; Gazol, A.; Sánchez-Salguero, R.; Tumajer, J.; Shishov, V.; Bonet, J.A.; Martínez De Aragón, J.; Ibáñez, R.; et al. Seasonal Precipitation and Continentality Drive Bimodal Growth in Mediterranean Forests. Dendrochronologia 2023, 78, 126057. [Google Scholar] [CrossRef]
- Gauli, A.; Neupane, P.R.; Mundhenk, P.; Köhl, M. Effect of Climate Change on the Growth of Tree Species: Dendroclimatological Analysis. Forests 2022, 13, 496. [Google Scholar] [CrossRef]
- Carrer, M.; Urbinati, C. Age-Dependent Tree-Ring Growth Responses to Climate in Larix decidua and Pinus cembra. Ecology 2004, 85, 730–740. [Google Scholar] [CrossRef]
- Linares, J.C.; Camarero, J.J.; Carreira, J.A. Competition Modulates the Adaptation Capacity of Forests to Climatic Stress: Insights from Recent Growth Decline and Death in Relict Stands of the Mediterranean Fir Abies pinsapo. J. Ecol. 2010, 98, 592–603. [Google Scholar] [CrossRef]
- Pavão, D.C.; Jevšenak, J.; Engblom, J.; Borges Silva, L.; Elias, R.B.; Silva, L. Tree Growth-Climate Relationship in the Azorean Holly in a Temperate Humid Forest with Low Thermal Amplitude. Dendrochronologia 2023, 77, 126050. [Google Scholar] [CrossRef]
- Ettinger, A.K.; HilleRisLambers, J. Climate Isn’t Everything: Competitive Interactions and Variation by Life Stage Will Also Affect Range Shifts in a Warming World. Am. J. Bot. 2013, 100, 1344–1355. [Google Scholar] [CrossRef]
- Lian, X.; Piao, S.; Chen, A.; Wang, K.; Li, X.; Buermann, W.; Huntingford, C.; Peñuelas, J.; Xu, H.; Myneni, R.B. Seasonal Biological Carryover Dominates Northern Vegetation Growth. Nat. Commun. 2021, 12, 983. [Google Scholar] [CrossRef]
- Rathgeber, C.B.K.; Cuny, H.E.; Fonti, P. Biological Basis of Tree-Ring Formation: A Crash Course. Front. Plant Sci. 2016, 7, 734. [Google Scholar] [CrossRef]
- Vaganov, E.A.; Hughes, M.K.; Shashkin, A.V. Growth Dynamics of Conifer Tree Rings: Images of Past and Future Environments; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar] [CrossRef]
- Babst, F.; Poulter, B.; Trouet, V.; Tan, K.; Neuwirth, B.; Wilson, R.; Carrer, M.; Grabner, M.; Tegel, W.; Levanic, T.; et al. Site- and Species-Specific Responses of Forest Growth to Climate across the European Continent: Climate Sensitivity of Forest Growth across Europe. Glob. Ecol. Biogeogr. 2013, 22, 706–717. [Google Scholar] [CrossRef]
- Zweifel, R.; Sterck, F. A Conceptual Tree Model Explaining Legacy Effects on Stem Growth. Front. For. Glob. Chang. 2018, 1, 9. [Google Scholar] [CrossRef]
- Mu, Y.; Lyu, L.; Li, Y.; Fang, O. Tree-Ring Evidence of Ecological Stress Memory. Proc. R. Soc. B. 2022, 289, 20221850. [Google Scholar] [CrossRef] [PubMed]
- Monserud, R.A.; Marshall, J.D. Time-Series Analysis of 13C from Tree Rings. I. Time Trends and Autocorrelation. Tree Physiol. 2001, 21, 1087–1102. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Y.; Zhang, T.; Liu, K.; Guo, K.; Hou, T.; Song, J.; He, Z.; Liang, B. Vegetation Growth Carryover and Lagged Climatic Effect at Different Scales: From Tree Rings to the Early Xylem Growth Season. Forests 2025, 16, 1107. [Google Scholar] [CrossRef]
- Fritts, H.C. Relationships of Ring Widths in Arid-Site Conifers to Variations in Monthly Temperature and Precipitation. Ecol. Monogr. 1974, 44, 411–440. [Google Scholar] [CrossRef]
- Lopez, E.L.; Kerr, S.A.; Sauchyn, D.J.; Vanderwel, M.C. Variation in Tree Growth Sensitivity to Moisture across a Water-Limited Forest Landscape. Dendrochronologia 2019, 54, 87–96. [Google Scholar] [CrossRef]
- Fan, Z.-X.; Bräuning, A.; Cao, K.-F.; Zhu, S.-D. Growth–Climate Responses of High-Elevation Conifers in the Central Hengduan Mountains, Southwestern China. For. Ecol. Manag. 2009, 258, 306–313. [Google Scholar] [CrossRef]
- Lehejček, J.; Vávrů, G.; Wangchuk, S.; Svoboda, M.; Boonen, K. Himalayan Fir Growth in Central Bhutan Reflects Variability in Temperature and Precipitation. J. For. Sci. 2025, 71, 516–524. [Google Scholar] [CrossRef]
- Sperlich, D.; Nadal-Sala, D.; Gracia, C.; Kreuzwieser, J.; Hanewinkel, M.; Yousefpour, R. Gains or Losses in Forest Productivity under Climate Change? The Uncertainty of CO2 Fertilization and Climate Effects. Climate 2020, 8, 141. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.