Toledo and Climate Change: 30 Years of Clinical Aerobiology in the Center of Spain
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Aerobiological Study
2.3. Air Pollution Data and Meteorological Variables
2.4. Skin Tests
2.5. Statistical Analysis
3. Results
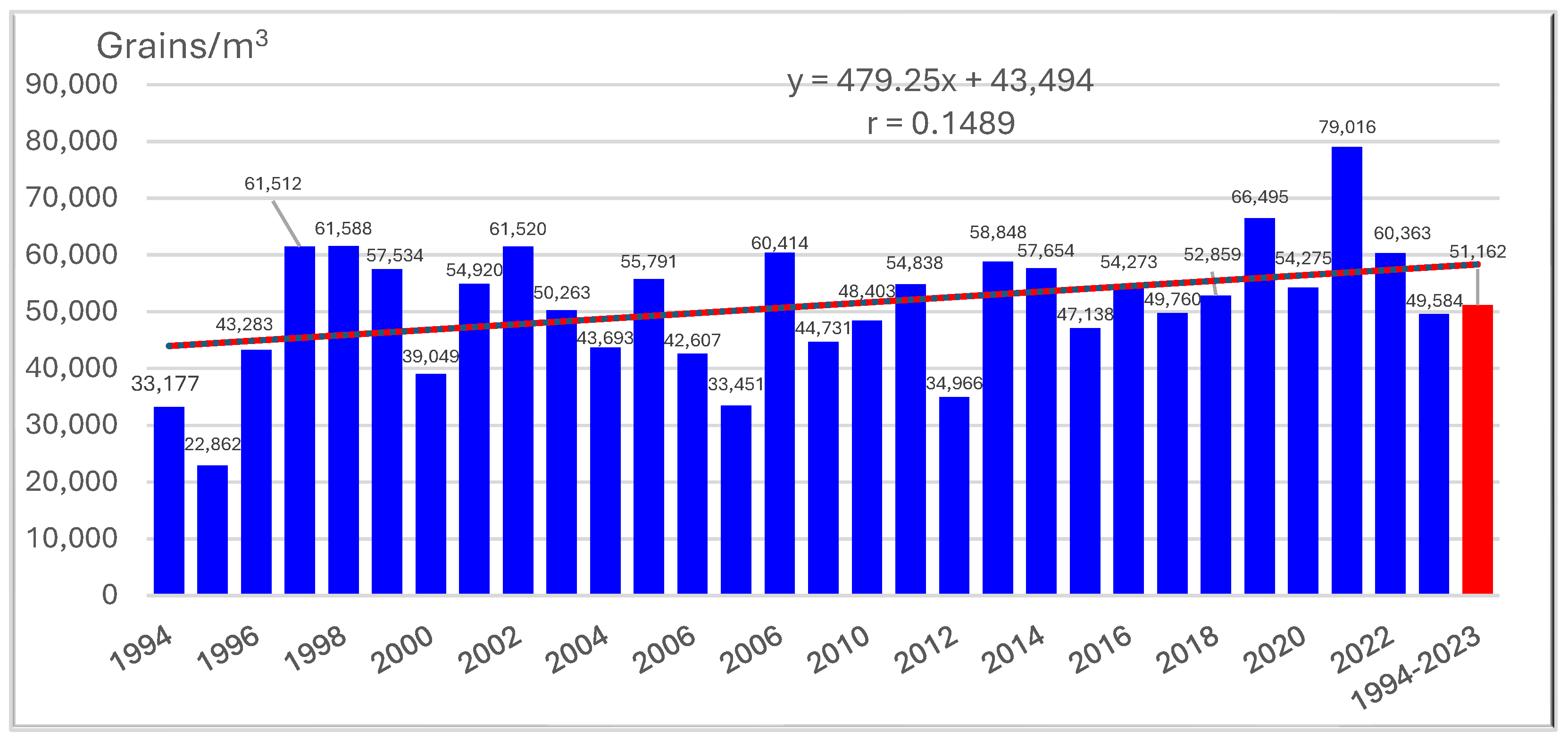
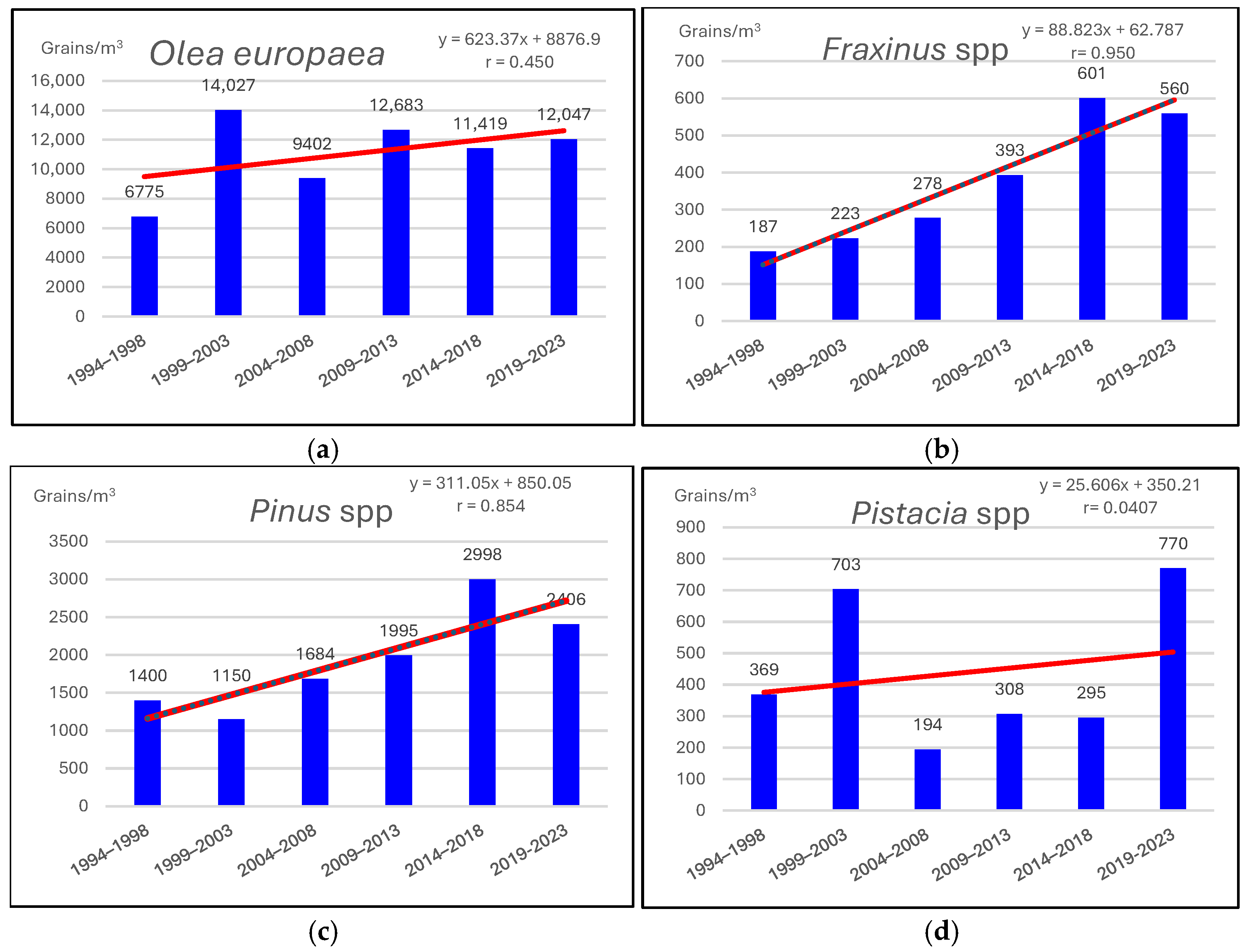
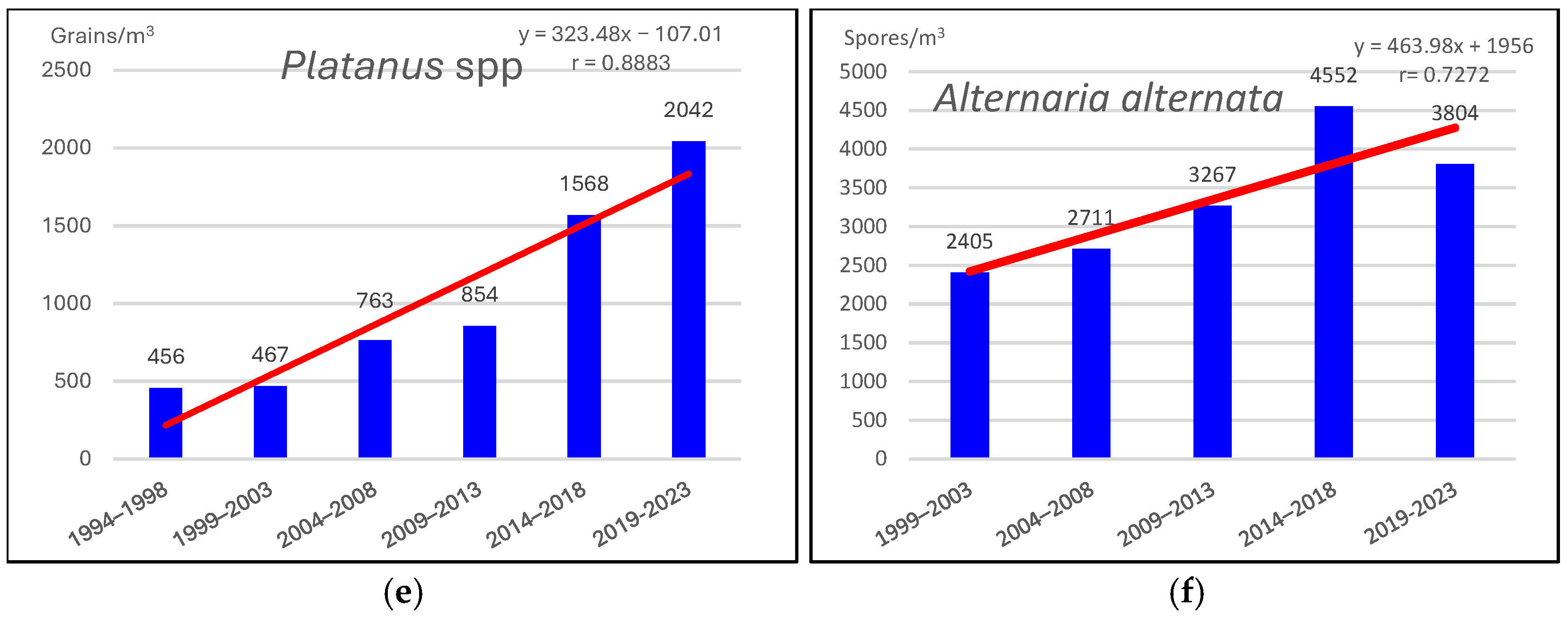
3.1. Pollen and Alternaria Spores (1994–2023)
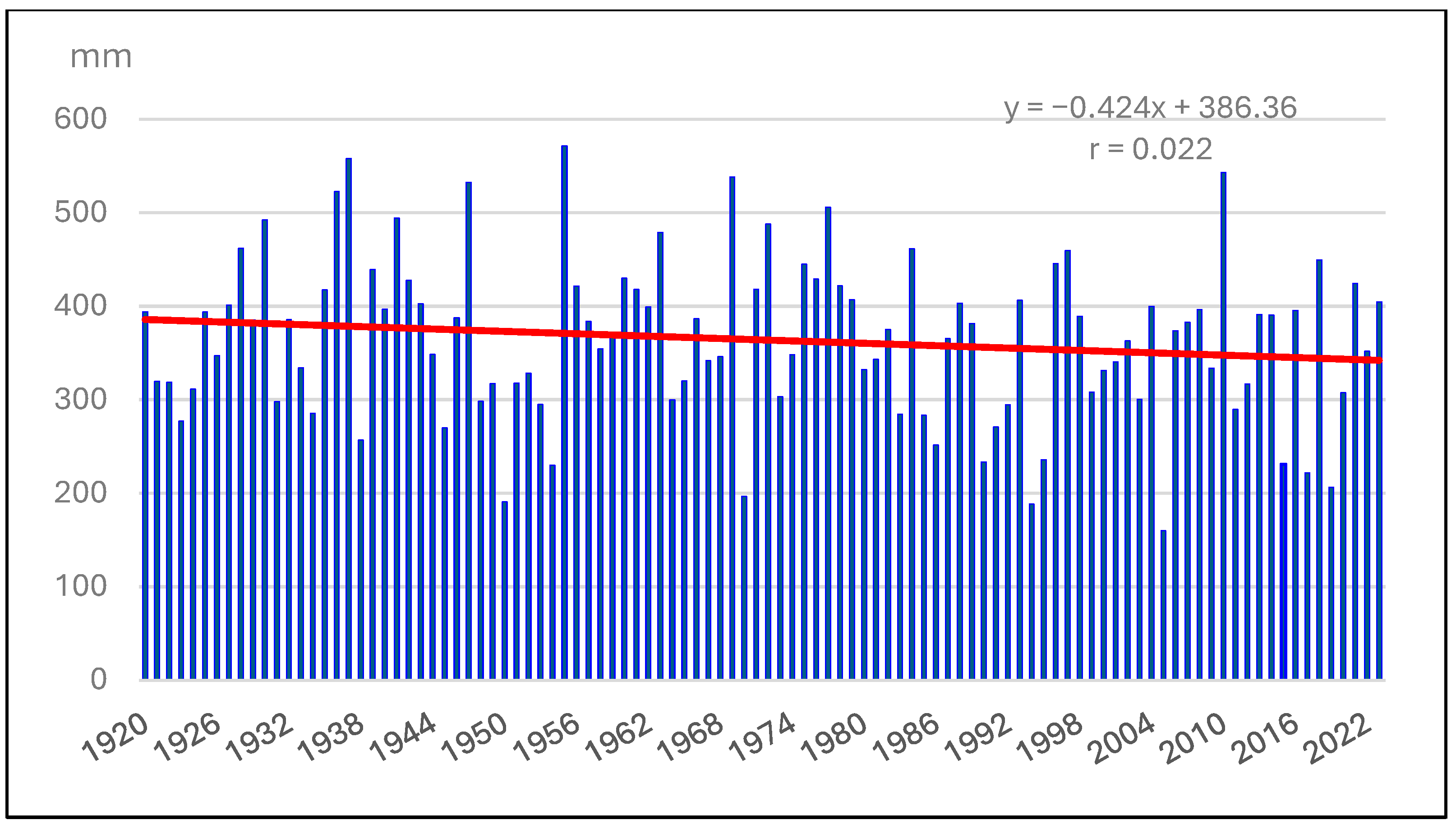
3.2. Meteorological Variables (1994–2023)
3.3. Chemical Pollutants (1999–2023)
3.4. Relationships Between Meteorological Variables and Chemical and Biological Pollutants
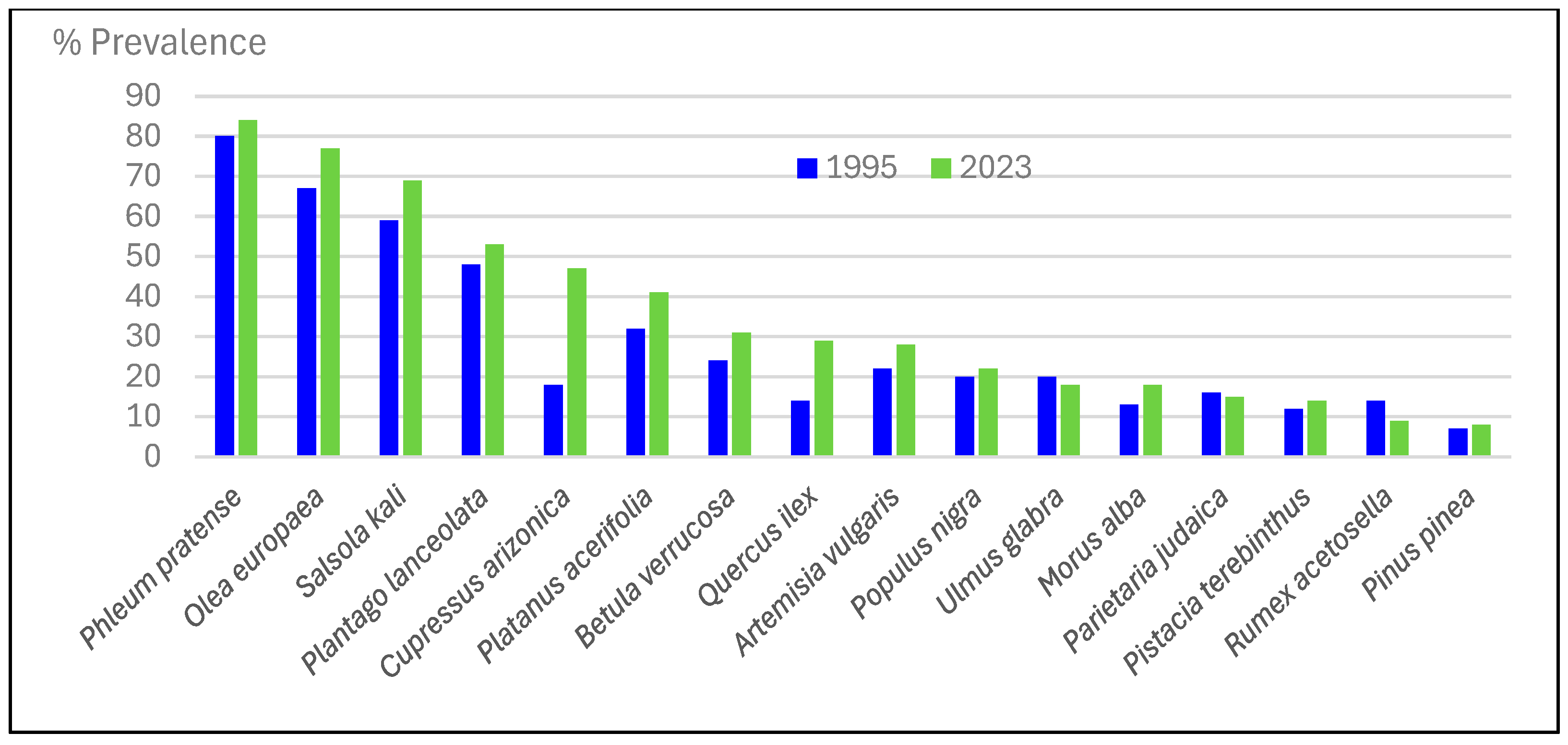
3.5. Prevalence of Pollen Sensitivity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PM10 | Particulate matter 10 |
| PM2.5 | Particulate matter 2.5 |
| NO2 | Nitrogen dioxide |
| NO | Nitrogen monoxide |
| O3 | Ozone |
References
- D’Amato, G.; Chong-Neto, H.J.; Monge Ortega, O.P.; Vitale, C.; Ansotegui, I.; Rosario, N.; Haahtela, T.; Galan, C.; Pawankar, R.; Murrieta-Aguttes, M.; et al. The effects of climate change on respiratory allergy and asthma induced by pollen and mold allergens. Allergy 2020, 75, 2219–2228. [Google Scholar] [CrossRef]
- Strachan, D.P.; Rutter, C.E.; Asher, M.I.; Bissell, K.; Chiang, C.; Sony, A.E.; Ellwood, E.; Ellwood, P.; García-Marcos, L.; Marks, G.B.; et al. Worldwide time trends in prevalence of symptoms of rhinoconjunctivitis in children: Global Asthma Network Phase I. Pediatr. Allergy Immunol. 2022, 33, e13656. [Google Scholar] [CrossRef] [PubMed]
- García-Mozo, H. Poace pollen as the leading aeroallergen worldwide: A review. Allergy 2017, 72, 1849–1858. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, M.; Subiza, J.; Fernández-Caldas, E.; Garzón García, B.; Moreno-Grau, S.; Subiza, J.L. Influence of environmental drivers on allergy to pollen grains in a case study in Spain (Madrid): Meteorological factors, pollutants, and airborne concentration of aeroallergens. Environ. Sci. Pollut. Res. 2021, 28, 53614–53628. [Google Scholar] [CrossRef]
- Feo-Brito, F.; Alfaya Arias, T.; Amo-Salas, M.; Somoza Alvarez, M.L.; Haroun Diaz, E.; Mayorga Mayorga, C.; Fernandez Santamaria, R.; Urra Ardanaz, J.M. Clinical impact and immunological alterations in asthmatic patients allergic to grass pollen subjected to high urban pollution in Madrid. Clin. Exp. Allergy 2022, 52, 530–539. [Google Scholar] [CrossRef]
- Schiavoni, G.; D’Amato, G.; Afferni, C. The dangerous liaison between pollens and pollution in respiratory allergy. Ann. Allergy Asthma Immunol. 2017, 118, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, S.E.; Guidos-Fogelbach, G.; Annesi-Maesano, I.; Pawankar, R.; D’ Amato, G.; Latour-Staffeld; Urrutia-Pereira, M.; Kesic, M.J.; Hernandez, M.L. Climate change and global issues in allergy and immunology. J. Allergy Clin. Immunol. 2021, 148, 1366–1377. [Google Scholar] [CrossRef]
- Verscheure, P.; Honnay, O.; Speybroeck, N.; Daelemans, R.; Bruffaerts, N.; Devleesschauwer, B.; Ceulemans, T.; Van Gerven, L.; Aerts, R.; Schrijvers, R. Impact of environmental nitrogen pollution on pollen allergy: A scoping review. Sci. Total Environ. 2023, 893, 164801. [Google Scholar] [CrossRef]
- Losol, P.; Sokolowska, M.; Hwang, Y.K.; Ogulur, I.; Mitamura, Y.; Yazici, D.; Pat, Y.; Radzikowska, U.; Ardicli, S.; Yoon, J.E.; et al. Epithelial Barrier Theory: The Role of Exposome, Microbiome, and Barrier Function in Allergic Diseases. Allergy Asthma Immunol. Res. 2023, 15, 705–724. [Google Scholar] [CrossRef]
- Stein, M.M.; Hrusch, C.L.; Gozdz, J.; Igartua, C.; Pivniouk, V.; Murray, S.E.; Ledford, J.G.; Marques dos Santos, M.; Anderson, R.L.; Metwali, N.; et al. Innate Immunity and Asthma Risk in Amish and Hutterite Farm Children. N. Engl. J. Med. 2016, 375, 411–421. [Google Scholar] [CrossRef]
- Lucas, J.A.; Gutierrez-Albanchez, E.; Alfaya, T.; Feo-Brito, F.; Gutiérrez-Mañero, F.J. Oxidative stress in ryegrass growing under different air pollution levels and its likely effects on pollen allergenicity. Plant Physiol. Biochem 2019, 135, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Visez, N.; Hamzé, M.; Vandenbossche, K.; Occelli, F.; de Nadaï, P.; Tobon, Y.; Hájek, T.; Choël, M. Uptake of ozone by allergenic pollen grains. Environ. Pollut. 2023, 331 Pt 1, 121793. [Google Scholar] [CrossRef] [PubMed]
- Lucas, J.A.; Gutierrez-Albanchez, E.; Alfaya, T.; Feo Brito, F.; Gutierrez-Mañero, F.J. Search for New Allergens in Lolium perenne Pollen Growing under Different Air Pollution Conditions by Comparative Transcriptome Study. Plants 2020, 9, 1507. [Google Scholar] [CrossRef]
- Cortegano, I.; Civantos, E.; Aceituno, E.; del Moral, A.; Lopez, E.; Lombardero, M.; Del Pozo, V.; Lahoz, C. Cloning and expression of a major allergen from Cupressus arizonica pollen, Cup a 3, a PR-5 protein expressed under polluted environment. Allergy 2004, 59, 485–490. [Google Scholar] [CrossRef]
- Montoro, J.; Antolín-Amérigo, D.; Artés, M.; Izquierdo-Domínguez, A.; Zapata, J.J.; Mur, P.; Carrillo, T.; Antépara, I.; Feo, F.; Moral, A.; et al. Impact of Climate Change-Related Environmental Factors on Allergen Production and the Epidemiology and Severity of Allergic Diseases. J. Investig. Allergol. Clin. Immunol. 2024, 34, 358–366. [Google Scholar] [CrossRef]
- Montoro, J.; Antolín-Amérigo, D.; Izquierdo-Domínguez, A.; Zapata, J.J.; García-Gallardo, M.V.; González, R.; Armentia, A.; Rondón, C.; Fernández, M.M.; Pedrero, S.G.; et al. Climate Change-Associated Environmental Factors and Pollutants: Impact on Allergic Diseases, Epidemiology, Severity, and Health Care Burden. J. Investig. Allergol. Clin. Immunol. 2025, 35, 240–250. [Google Scholar] [CrossRef]
- Moral de Gregorio, A.; Senent Sánchez, C.; Cabañes Higuero, N.; García Villamuza, Y.; Gomez-Serranillos Reus, M. Pólenes alergénicos y polinosis en Toledo durante 1995–1996. Rev. Esp. Alergol. Inmunol. Clin. 1998, 13, 107–119. [Google Scholar] [PubMed]
- Moral de Gregorio, A.; Senent Sanchez, C.; Garcia Gomez, E.; Perez-Badia, R. Manuel de Alergopalinología: Plantas, Pólenes y Proteínas, 1st ed.; MIlkPost: Toledo, OH, USA, 2016. [Google Scholar]
- Montagnani, C.; Gentili, R.; Citterio, S. Ragweed is in the Air: Ambrosia L. (Asteraceae) and Pollen Allergens in a Changing World. Curr. Protein Pept. Sci. 2023, 24, 98–111. [Google Scholar] [CrossRef]
- Lara, B.; Rojo, J.; Costa, A.R.; Burgos-Montero, A.M.; Antunes, C.M.; Pérez-Badia, R. Atmospheric pollen allergen load and environmental patterns in central and southwestern Iberian Peninsula. Sci. Total Environ. 2023, 858 Pt 2, 159630. [Google Scholar] [CrossRef]
- Subiza, J.; Feo Brito, F.; Pola, J.; Moral, A.; Fernández, J.; Jerez, M.; Ferreiro, M. Pólenes alergénicos y polinosis en 12 ciudades españolas. Rev. Esp. Alergol. Inmunol. Clin. 1998, 13, 45–58. [Google Scholar]
- Subiza, J.; Cabrera, M.; Cárdenas-Rebollo, J.M.; Craciunescu, J.C.; Narganes, M.J. Influence of climate change on airborne pollen concentrations in Madrid, 1979–2018. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2022, 52, 574–577. [Google Scholar] [CrossRef] [PubMed]
- Connell, J.T. Quantitative intranasal pollen challenges. 3. The priming effect in allergic rhinitis. J. Allergy 1969, 43, 33–44. [Google Scholar] [CrossRef]
- Galán, C.; Cariñanos; González, P.; Alcázar, P.; Rodríguez, E. Manual de Calidad y Gestión de la Red Española de Aerobiología; Servicio de Publicaciones de la Universidad de Córdoba: Córdoba, Spain, 2007; ISBN 978-84-690-6354-5. [Google Scholar]
- Pola, J.; Subiza, F.J.; García, J.M.; Porcel, S.L. Pólenes de interés en Alergología en nuestro medio. In Tratado de Alergología; Ergon: Madrid, Spain, 2015; pp. 247–275. ISBN 978-84-16270-36-1. [Google Scholar]
- Dreborg, S. The skin prick test in the diagnosis of atopic allergy. J. Am. Acad. Dermatol. 1989, 21, 820–821. [Google Scholar] [CrossRef]
- Pickrell, A.; Smith, M.; Rojo, J. Climate change related phenological decoupling in species belonging to the Betulaceae family. Int. J. Biometeorol. 2023, 67, 195–209. [Google Scholar] [CrossRef]
- Sedghy, F.; Sankian, M.; Mogadhan, M.; Ghasemi, Z.; Mahmoudi, M.; Varasteh, A. Impact of traffic-related air pollution on the expression of Platanus orientalis pollen allergens. Int. J. Biometeorol. 2017, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Wang, X.; Lu, S.; Yao, C.; Zhang, L.; Lao, R.; Liu, X.; Zhang, W.; Li, S.; Wang, W.; et al. Characterization of allergenicity of Platanus pollen allergen a 3 (Pla a 3) after exposure to NO2 and O3. Environ. Pollut. 2021, 278, 116913. [Google Scholar] [CrossRef]
- Galera, M.D.; Elvira-Rendueles, B.; Moreno, J.M.; Negral, L.; Ruiz-Abellón, M.C.; García-Sánchez, A.; Moreno-Grau, S. Analysis of airborne Olea pollen in Cartagena (Spain). Sci. Total Environ. 2018, 622–623, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Rojo, J.; Orlandi, F.; Pérez-Badia, R.; Aguilera, F.; Ben Dhiab, A.; Bouziane, H.; de la Guardia, C.D.; Galán, C.; Gutiérrez-Bustillo, A.M.; Moreno-Grau, S.; et al. Modeling olive pollen intensity in the Mediterranean region through analysis of emission sources. Sci. Total Environ. 2016, 551–552, 73–82. [Google Scholar] [CrossRef]
- Brito, F.F.; Gimeno, P.M.; Carnés, J.; Martín, R.; Fernández-Caldas, E.; Lara, P.; López-Fidalgo, J.; Guerra, F. Olea europaea pollen counts and aeroallergen levels predict clinical symptoms in patients allergic to olive pollen. Ann. Allergy Asthma Immunol. 2011, 106, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Penedos, C.; Salamanca, G.; Tavares, B.; Fonseca, J.; Carreiro-Martins, P.; Rodrigues-Alves, R.; Moral de Gregorio, Á.; Valero, A.; Branco Ferreira, M. Aerobiology of Olive Pollen (Olea europaea L.) in the Atmosphere of the Iberian Peninsula. Atmosphere 2024, 15, 1087. [Google Scholar] [CrossRef]
- Galan, C.; Antunes, C.; Brandao, R.; Torres, C.; Garcia-Mozo, H.; Caeiro, E.; Ferro, R.; Prank, M.; Sofiev, M.; Albertini, R.; et al. Airborne olive pollen counts are not representative of exposure to the major olive allergen Ole e 1. Allergy 2013, 68, 809–812. [Google Scholar] [CrossRef] [PubMed]
- Marques Mejías, M.A.; Tomás Pérez, M.; Hernández, I.; López, I.; Quirce, S. Asthma Exacerbations in the Pediatric Emergency Department at a Tertiary Hospital: Association With Environmental Factors. J. Investig. Allergol. Clin. Immunol. 2019, 29, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Montero, A.M.; Sánchez-García, A.M.; Feo-Brito, F.; Pérez-Badia, R. Primera aproximación a la caracterización del espectro polínico y presencia del alérgeno Sal k 1 en la atmósfera de Alcázar de San Juan (Ciudad Real). Rev. Salud Ambient. 2017, 17, 157–164. [Google Scholar]
- Villalba, M.; Barderas, R.; Mas, S.; Colás, C.; Batanero, E.; Rodríguez, R. Amaranthaceae pollens: Review of an emerging allergy in the mediterranean area. J. Investig. Allergol. Clin. Immunol. 2014, 24, 371–381. [Google Scholar] [PubMed]
- Nuñez Borque, E.; Betancor, D.; Fernández Bravo, S.; Gómez Cardeñosa, A.; Esteban, V.; Garrido Arandia, M.; de Las Heras, M.; Pastor Vargas, C.; Cuesta Herranz, J. Allergen Profile of London Plane Tree Pollen: Clinical and Molecular Pattern in Central Spain. J. Investig. Allergol. Clin. Immunol. 2022, 32, 367–374. [Google Scholar] [CrossRef]
- González-Parrado, Z.; Fernández-González, D.; Camazón, B.; Valencia-Barrera, R.M.; Vega-Maray, A.M.; Asturias, J.A.; Monsalve, R.I.; Mandrioli, P. Molecular aerobiology—Plantago allergen Pla l 1 in the atmosphere. Ann. Agric. Environ. Med. 2014, 21, 282–289. [Google Scholar] [CrossRef][Green Version]
- Fernandez-Caldas, E.; Swanson, M.C.; Pravda, J.; Welsh, P.; Yunginger, J.W.; Reed, C.E. Immunochemical Demonstration of Red Oak Pollen Aeroallergens outside the Oak Pollination Season. Grana 1989, 28, 205–209. [Google Scholar] [CrossRef]
- Prados, M.; Aragon, R.; Carranco, M.I.; Martinez, A.; Martinez, J. Assessment of sensitization to holm oak (Quercus ilex) pollen in the Mérida area (Spain). Allergy 1995, 50, 456–459. [Google Scholar] [CrossRef]
- Lluch-Bernal, M.; Pedrosa, M.; Domínguez-Ortega, J.; Colque-Bayona, M.; Correa-Borit, J.; Phillips-Anglés, E.; Gómez-Traseira, C.; Quirce, S.; Rodríguez-Pérez, R. Sensitization to Quercus ilex Pollen Is Clinically Relevant in Patients With Seasonal Pollen Allergy. J. Investig. Allergol. Clin. Immunol. 2024, 34, 338–340. [Google Scholar] [CrossRef]
- Adams-Groom, B.; Selby, K.; Derrett, S.; Frisk, C.A.; Pashley, C.H.; Satchwell, J.; King, D.; McKenzie, G.; Neilson, R. Pollen season trends as markers of climate change impact: Betula, Quercus and Poaceae. Sci. Total Environ. 2022, 831, 154882. [Google Scholar] [CrossRef]
- Keynan, N.; Tamir, R.; Waisel, Y.; Reshef, A.; Spitz, E.; Shomer-Ilan, A.; Geller-Bernstein, C. Allergenicity of the pollen of Pistacia. Allergy 1997, 52, 323–330. [Google Scholar] [CrossRef]
- Velasco-Jiménez, M.J.; Arenas, M.; Alcázar, P.; Galán, C.; Domínguez-Vilches, E. Aerobiological and phenological study of Pistacia in Córdoba city (Spain). Sci. Total Environ. 2015, 505, 1036–1042. [Google Scholar] [CrossRef] [PubMed]
- Jiménez Lara, M.; Moral de Gregorio, A.; Núñez Aceves, A.; Rodríguez, R.; Arenas, M.; Senent Sanchez, C. Pistacea terebinthus, una nueva polinosis en España. Rev. Esp. Alergol. Inmunol. Clínica 2003, 18, 152–153. [Google Scholar]
- Brito, F.F.; Alonso, A.M.; Carnés, J.; Martín-Martín, R.; Fernández-Caldas, E.; Galindo, P.A.; Alfaya, T.; Amo-Salas, M. Correlation between Alt a 1 levels and clinical symptoms in Alternaria alternata-monosensitized patients. J. Investig. Allergol. Clin. Immunol. 2012, 22, 154–159. [Google Scholar] [PubMed]
- Pulimood, T.B.; Corden, J.M.; Bryden, C.; Sharples, L.; Nasser, S.M. Epidemic asthma and the role of the fungal mold Alternaria alternata. J. Allergy Clin. Immunol. 2007, 120, 610–617. [Google Scholar] [CrossRef]
- Targonski, P.V.; Persky, V.W.; Ramekrishnan, V. Effect of environmental molds on risk of death from asthma during the pollen season. J. Allergy Clin. Immunol. 1995, 95, 955–961. [Google Scholar] [CrossRef]



| Pollen | Average Annual Pollen Integral (grains/m3) | Peak Concentration Day (grains/m3) | Maximum Annual Pollen Integral (grains/m3) (year) |
|---|---|---|---|
| Cupressaceae | 13.735 (26.53%) | 3.734 (26 January 2014) | 33.095 (2014) |
| Olea europaea | 11.059 (21.62%) | 5.064 (30 May 1999) | 21.893 (2013) |
| Quercus spp. | 10.806 (21.12%) | 3.456 (28 April 2002) | 25.111 (2002) |
| Poaceae | 5.272 (10.3%) | 1.320 (18 May 2003) | 9.747 (2016) |
| Pinus spp. | 1.939 (3.79%) | 420 (17 June 2018) | 4.232 (2018) |
| Urticaceae | 1.321 (2.58%) | 452 (8 March 2017) | 3.348 (2020) |
| Plantago spp. | 1.269 (2.48%) | 210 (24 May 2008) | 2.282 (2020) |
| Platanus spp. | 1.025 (2.00%) | 668 (8 April 2018) | 2.988 (2019) |
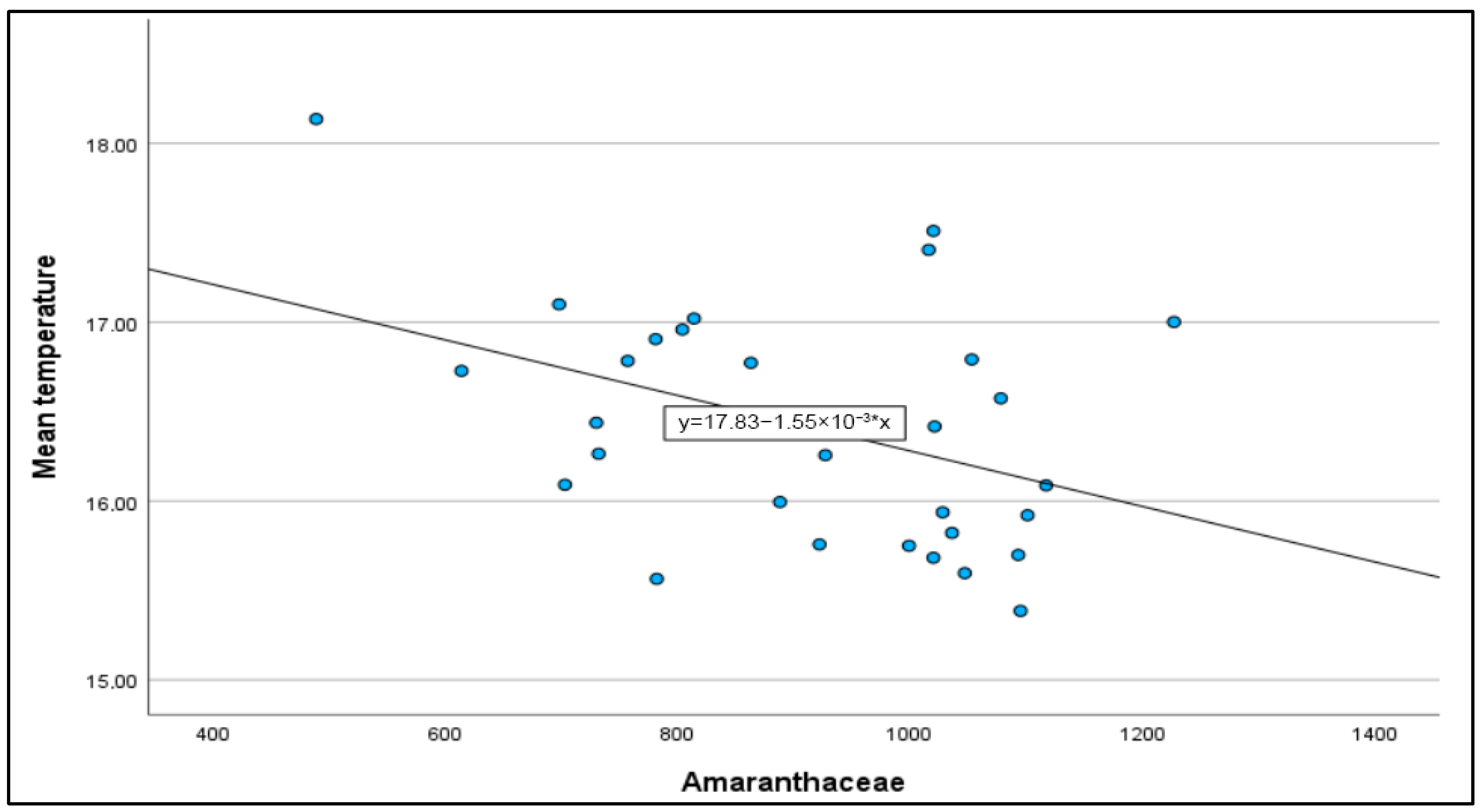
| Amaranthaceae | 932 (1.72%) | 62 (2 September 2007) | 1.129 (2022) |
| Rumex spp. | 861 (1.68%) | 164 (10 June 1998) | 2.287 (1998) |
| Morus spp. | 463 (0.90%) | 216 (7 April 1999) | 1.910 (2023) |
| Pistacia spp. | 440 (0.86%) | 401 (6 April 2001) | 2.630 (2023) |
| Populus spp. | 427 (0.83%) | 112 (20 February 2020) | 1.046 (2022) |
| Artemisia spp. | 382 (0.75%) | 92 (13 November 1998) | 1.073 (1998) |
| Fraxinus spp. | 374 (0.73%) | 92 (2 February 2016) | 962 (2019) |
| Ulmus spp. | 225 (0.44%) | 84 (9 February 2019) | 576 (2018) |
| Asteraceae (no Artemisia) | 214 (0.42%) | 72 (20 May 2000) | 414 (1996) |
| Alnus spp. | 190 (0.37%) | 124 (26 January 2013) | 566 (2013) |
| Carex spp. | 145 (0.27%) | 44 (28 May 1995) | 366 (1998) |
| Ericaceae | 83 (0.16%) | 34 (9 May 2021) | 238 (2023) |
| PM10 | PM2.5 | SO2 | NO2 | NO | NOx | CO | O3 | |
|---|---|---|---|---|---|---|---|---|
| Mean temp | 0.202 ** | 0.090 ** | −0.095 | −0.320 ** | −0.477 ** | −0.323 | −0.145 ** | 0.617 ** |
| <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Min temp | 0.142 ** | 0.041 | −0.112 ** | −0.388 ** | −0.521 ** | −0.378 ** | −0.145 ** | 0.616 ** |
| <0.001 | 0.004 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Max temp | 0.256 | 0.145 ** | −0.072 | −0.243 ** | −0.412 ** | −0.253 ** | −0.137 | 0.585 ** |
| <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Rain | −0.202 ** | −0.155 ** | −0.042 ** | −0.141 ** | −0.100 ** | −0.152 ** | 0.016 | −0.050 ** |
| <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.185 | <0.001 | |
| Wind Speeed | −0.110 ** | −0.233 ** | −0.069 ** | 0.452 ** | −0.439 ** | −0.512 ** | −0.051 ** | 0.471 ** |
| <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.015 | <0.001 | <0.001 | |
| Thermal range | 0.335 | 0.267 ** | 0.046 * | 0.143 ** | −0.026 ** | 0.113 ** | −0.057 ** | 0.241 ** |
| <0.001 | <0.001 | <0.001 | <0.001 | 0.015 | <0.001 | <0.001 | <0.001 | |
| Insolation | −0.155 ** | 0.072 ** | −0.032 | −0.015 ** | −0.247 ** | −0.121 ** | −0.118 ** | 0.510 ** |
| <0.001 | <0.001 | 0.003 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Mean pressure | 0.190 ** | 0.226 ** | 0.049 ** | 0.350 ** | 0.380 ** | 0.398 ** | 0.060 ** | −0.402 ** |
| <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Humidity | −0.177 ** | −0.025 | 0.058 ** | 0.199 * | 0.377 ** | 0.221 ** | 0.164 ** | −0.629 |
| <0.001 | 0.083 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Solar radiation | 0.172 ** | −0.009 | −0.001 | −0.184 ** | −0.366 ** | −0.265 ** | −0.084 ** | 0.681 ** |
| <0.001 | 0.604 | 0.933 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| (A) | |||||||
| Amarantaceae | Artemisia | Betula | Cupressaceae | Fraxinus | Olea | Plantago | |
| Mean temp | 0.684 ** | −0.005 | 0.029 ** | −0.547 ** | −0.362 ** | 0.359 | 0.211 ** |
| <0.001 | 0.471 | 0.002 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Min temp | 0.661 * | 0.001 | 0.008 | −0.565 ** | −0.374 ** | 0.337 ** | 0.172 ** |
| <0.001 | 0.919 | 0.401 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Max temp | 0.678 ** | −0.007 | 0.038 | −0.512 ** | −0.330 ** | 0.358 ** | 0.221 |
| <0.001 | 0.471 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Rain | −0.203 ** | −0.027 ** | −0.011 | 0.042 ** | −0.009 | −0.049 ** | −0.029 ** |
| <0.001 | 0.005 | 0.241 | <0.001 | 0.342 | <0.001 | 0.002 | |
| Wind Speeed | 0.097 ** | −0.119 ** | 0.015 | −0.083 ** | −0.043 ** | 0.178 ** | 0.151 ** |
| <0.001 | <0.001 | 0.114 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Thermal range | 0.389 | −0.021 ** | −0.021 * | −0.184 ** | −0.084 ** | 0.209 ** | 0.157 ** |
| <0.001 | 0.027 | 0.027 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Insolation | 0.494 ** | −0.079 ** | 0.078 ** | −0.316 ** | −0.171 ** | 0.338 ** | −0.144 ** |
| <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Mean pressure | −0.122 ** | 0.060 ** | −0.027 ** | 0.224 ** | 0.181 ** | −0.160 ** | 0.264 ** |
| <0.001 | <0.001 | 0.005 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Humidity | −0.596 ** | 0.070 | −0.047 ** | 0.375 * | 0.207 ** | −0.326 ** | −0.208 ** |
| <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Solar radiation | 0.553 ** | −0.126 ** | 0.115 ** | −0.429 ** | −0.261 ** | 0.468 ** | 0.387 ** |
| <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| (B) | |||||||
| Platanus | Pistacia | Poaceae | Quercus | Urticaceae | Total Pollen | Alternaria | |
| Mean temp | −0.083 ** | −0.026 ** | 0.333 ** | 0.080 ** | −0.070 ** | 0.026 ** | 0.386 ** |
| <0.001 | 0.007 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Min temp | −0.129 * | −0.056 ** | 0.277 ** | 0.040 ** | −0.127 ** | −0.047 ** | 0.370 ** |
| <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Max temp | −0.059 ** | −0.015 | 0.357 ** | 0.091 ** | −0.033 ** | 0.073 ** | 0.379 ** |
| <0.001 | 0.110 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Rain | 0.019 * | −0.040 ** | −0.135 ** | 0.045 ** | −0.070 ** | −0.091 ** | −0.054 ** |
| 0.048 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Wind Speeed | 0.048 ** | −0.063 ** | 0.188 ** | 0.139 ** | 0.036 ** | 0.141 ** | 0.080 ** |
| <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Thermal range | 0.038 ** | 0.020 * | 0.288 *** | 0.086 ** | 0.093 ** | 0.196 ** | 0.190 ** |
| <0.001 | 0.034 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Insolation | 0.017 | −0.034 ** | 0.420 ** | 0.150 ** | 0.092 ** | 0.213 ** | 0.290 ** |
| 0.077 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Mean pressure | −0.034 ** | −0.094 ** | −0.105 ** | −0.165 ** | 0.060 ** | 0.013 | −0.130 ** |
| <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.190 | <0.001 | |
| Humidity | 0.002 | 0.019 | −0.370 ** | −0.111 * | −0.017 ** | −0.135 ** | −0.280 ** |
| 0.832 | 0.074 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Solar radiation | 0.074 ** | 0.111 ** | 0.529 ** | 0.301 ** | 0.113 ** | 0.266 ** | 0.321 ** |
| <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| (A) | |||||||
| Amaranthaceae | Asteraceae | Betula | Cupressaceae | Fraxinus | Olea | Plantago | |
| PM10 | 0.186 ** | 0.076 | 0.025 | −0.118 | −0.066 | 0.032 | −0.058 ** |
| <0.001 | <0.001 | 0.024 | <0.001 | <0.001 | 0.004 | <0.001 | |
| PM2.5 | 0.058 ** | −0.003 | −0.045 ** | −0.002 | 0.028 * | 0.001 | −0.085 ** |
| <0.001 | 0.811 | 0.002 | 0.905 | 0.047 | 0.933 | <0.001 | |
| SO2 | −0.041 | 0.016 | 0.008 | −0.017 | −0.013 | 0.004 | 0.016 |
| <0.001 | 0.147 | 0.474 | 0.117 | 0.228 | 0.689 | 0.144 | |
| NO2 | −0.190 ** | −0.161 ** | −0.032 ** | 0.184 ** | 0.181 ** | −0.272 ** | −0.246 ** |
| <0.001 | <0.001 | 0.003 | <0.001 | <0.001 | <0.001 | <0.001 | |
| NO | −0.308 ** | −0.183 | −0.032 ** | 0.249 ** | 0.207 ** | −0.290 ** | −0.239 ** |
| <0.001 | <0.001 | 0.003 | <0.001 | <0.001 | <0.001 | <0.001 | |
| NOX | −0.181 | −0.209 | −0.049 * | 0.241 ** | 0.271 ** | −0.345 | −0.295 ** |
| <0.001 | <0.001 | 0.037 | <0.001 | <0.001 | <0.001 | <0.001 | |
| CO | −0.058 ** | −0.012 | 0.014 | 0.039 ** | 0.019 | −0.074 ** | −0.042 ** |
| <0.001 | 0.323 | 0.243 | 0.002 | 0.126 | <0.001 | <0.001 | |
| O3 | 0.421 ** | 0.288 ** | 0.083 ** | −0.316 ** | −0.227 ** | 0.443 ** | 0.362 ** |
| <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| (B) | |||||||
| Platanus | Pistacia | Poaceae | Quercus | Urticaceae | Total Pollen | Alternaria | |
| PM10 | −0.076 ** | −0.075 | 0.029 ** | −0.100 ** | −0.089 ** | −0.031 ** | −0.072 ** |
| <0.001 | <0.001 | 0.007 | <0.001 | <0.001 | 0.004 | <0.001 | |
| PM2.5 | −0.069 ** | −0.085 ** | −0.031 ** | −0.157 ** | −0.056 * | −0.040 ** | 0.057 ** |
| <0.001 | <0.001 | 0.031 | <0.001 | <0.001 | 0.005 | <0.001 | |
| SO2 | −0.033 ** | −0.018 | 0.012 | 0.003 | 0.009 | 0.012 | −0.094 ** |
| <0.002 | 0.094 | 0.283 | 0.770 | 0.415 | 0.268 | <0.001 | |
| NO2 | −0.052 ** | −0.105 ** | −0.218 ** | −0.225 ** | −0.043 ** | −0.119 ** | −0.204 ** |
| <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| NO | −0.074 ** | −0.087 | −0.256 ** | −0.191 ** | −0.037 ** | −0.103 ** | −0.266 ** |
| <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| NOX | −0.096 ** | −0.130 | −0.225 * | −0.251 ** | −0.046 | −0.081 ** | −0.214 ** |
| <0.001 | <0.001 | <0.001 | <0.001 | 0.052 | <0.001 | <0.001 | |
| CO | −0.004 ** | −0.016 | −0.037 ** | 0.011 | 0.007 | −0.033 ** | −0.160 ** |
| 0.770 | 0.198 | 0.003 | 0.354 | 0.585 | 0.008 | <0.001 | |
| O3 | 0.132 ** | 0.143 | 0.446 ** | 0.305 ** | −0.113 ** | 0.250 ** | 0.299 ** |
| <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moral de Gregorio, A.; Guzmán Rodríguez, R.; Senent Sánchez, C.; Feo Brito, F.; Beneyto Martin, P. Toledo and Climate Change: 30 Years of Clinical Aerobiology in the Center of Spain. Atmosphere 2025, 16, 981. https://doi.org/10.3390/atmos16080981
Moral de Gregorio A, Guzmán Rodríguez R, Senent Sánchez C, Feo Brito F, Beneyto Martin P. Toledo and Climate Change: 30 Years of Clinical Aerobiology in the Center of Spain. Atmosphere. 2025; 16(8):981. https://doi.org/10.3390/atmos16080981
Chicago/Turabian StyleMoral de Gregorio, Angel, Raúl Guzmán Rodríguez, Carlos Senent Sánchez, Francisco Feo Brito, and Pedro Beneyto Martin. 2025. "Toledo and Climate Change: 30 Years of Clinical Aerobiology in the Center of Spain" Atmosphere 16, no. 8: 981. https://doi.org/10.3390/atmos16080981
APA StyleMoral de Gregorio, A., Guzmán Rodríguez, R., Senent Sánchez, C., Feo Brito, F., & Beneyto Martin, P. (2025). Toledo and Climate Change: 30 Years of Clinical Aerobiology in the Center of Spain. Atmosphere, 16(8), 981. https://doi.org/10.3390/atmos16080981






