Concentration Characteristics, Source Analysis, and Health Risk Assessment of Water-Soluble Heavy Metals in PM2.5 During Winter in Taiyuan, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and Measurement
2.1.1. Sample Collection
2.1.2. WSHMs Extraction and Measurements
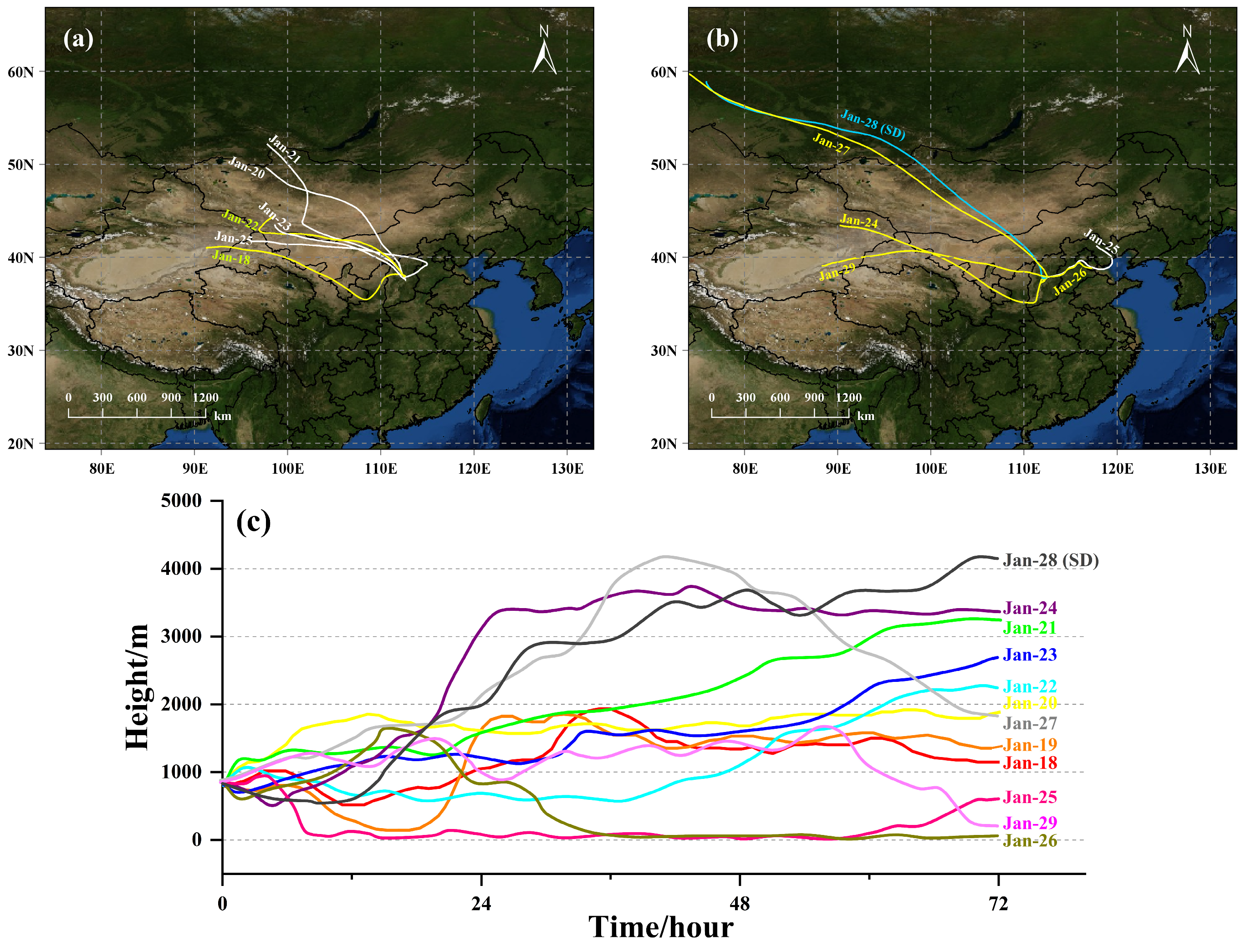
2.1.3. Auxiliary Data
2.2. Data Analysis
2.2.1. Principal Component Analysis (PCA)
2.2.2. Absolute Principal Component Score–Multiple Linear Regression (APCS-MLR)
2.2.3. Health Risk Assessment
3. Results and Discussion
3.1. Concentration Characteristics of WSHMs
3.1.1. Overview of WSHMs Mass Concentrations
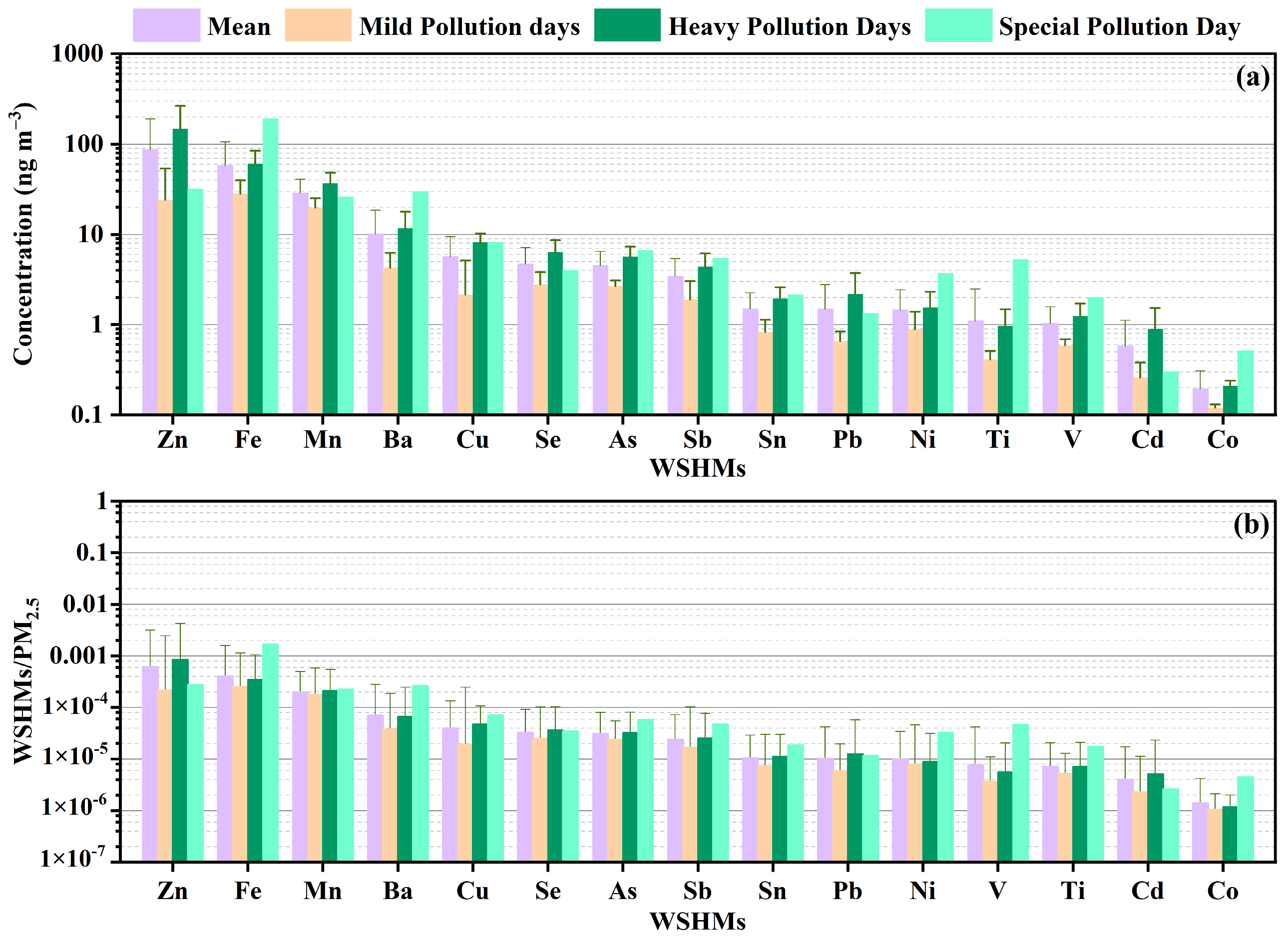
3.1.2. WSHMs in Different Pollution Conditions
3.2. Source Distribution of WSHMs in PM2.5
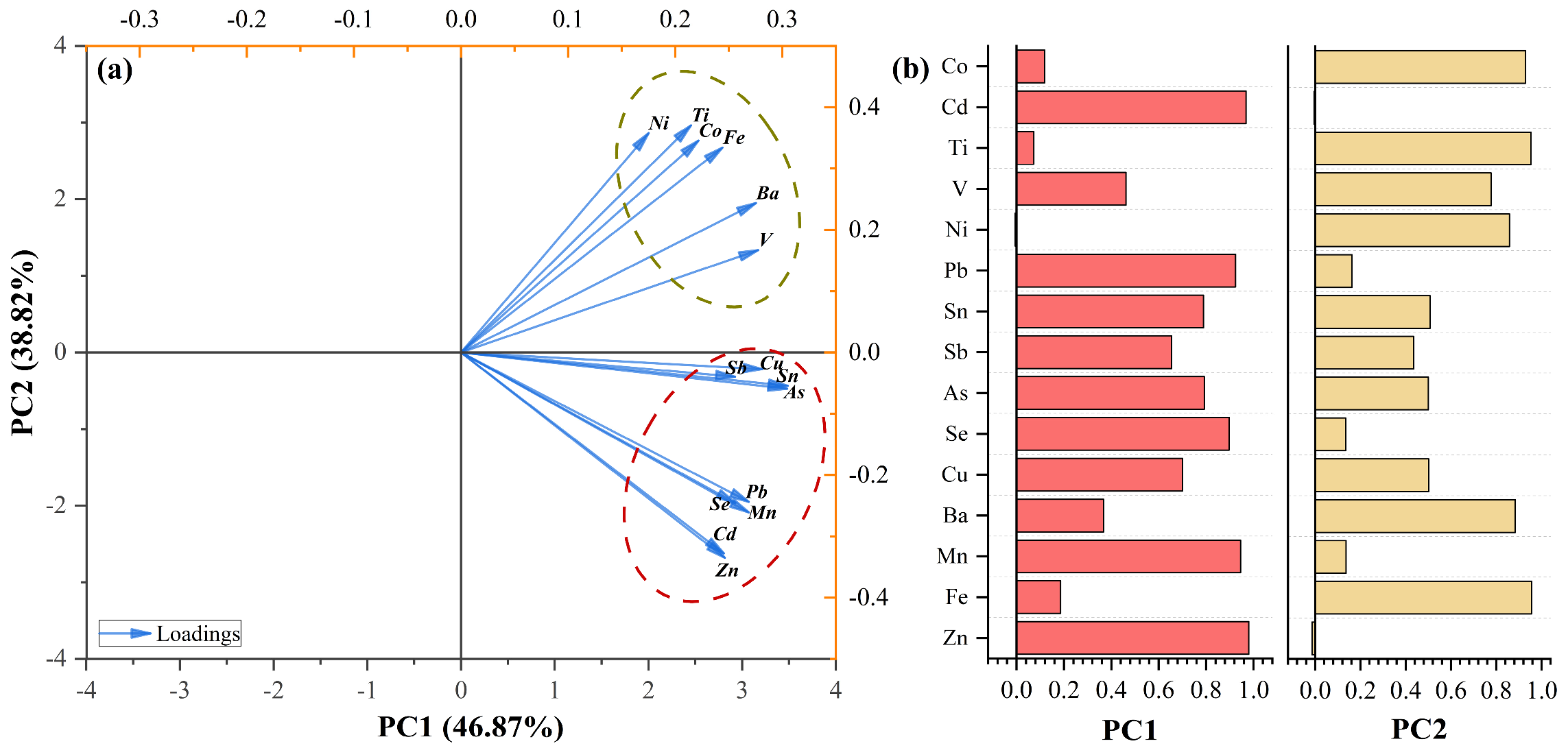
3.2.1. Source Analysis Based on PCA
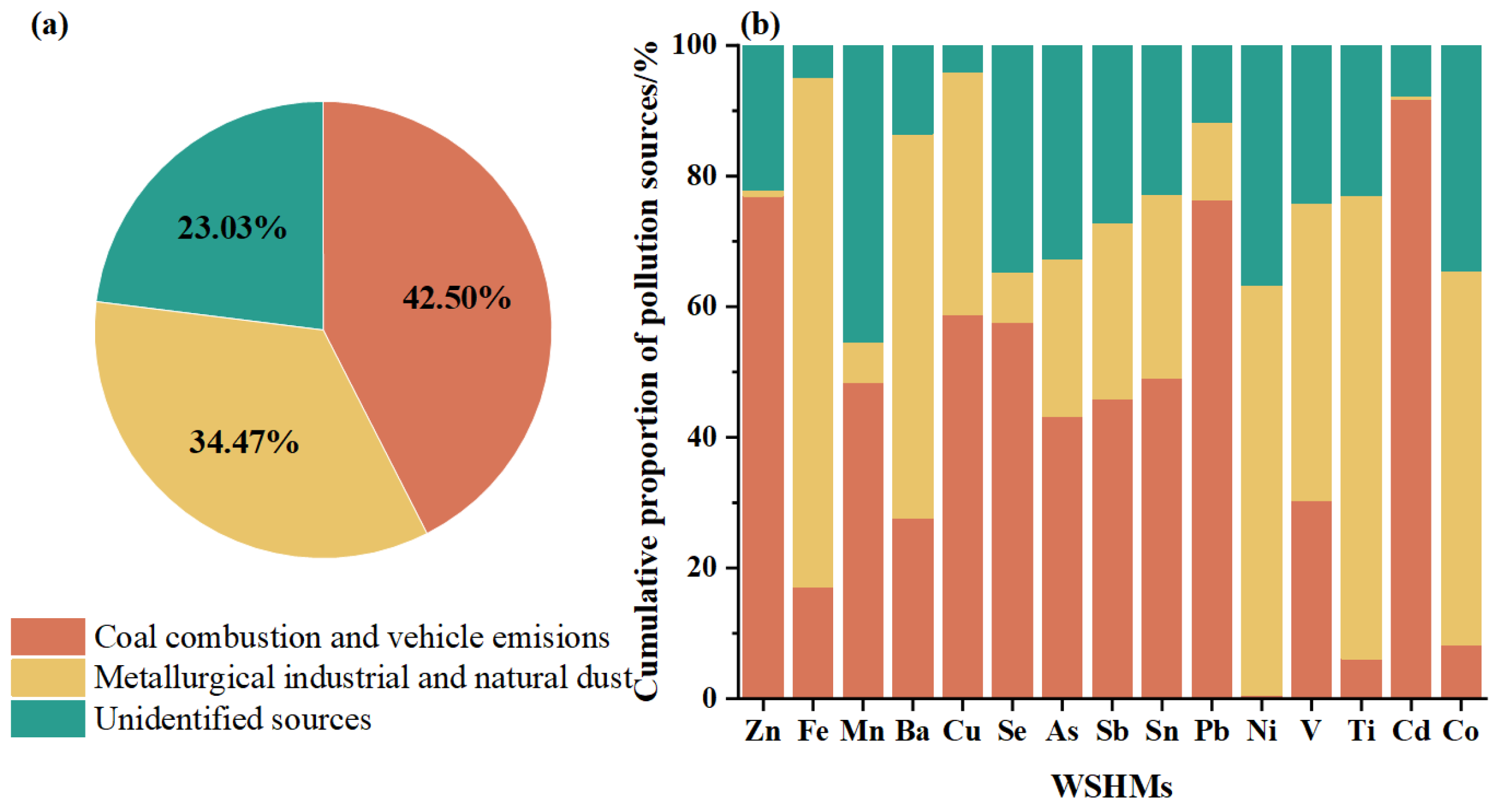
3.2.2. APCS-MLR Quantification of WSHM Sources
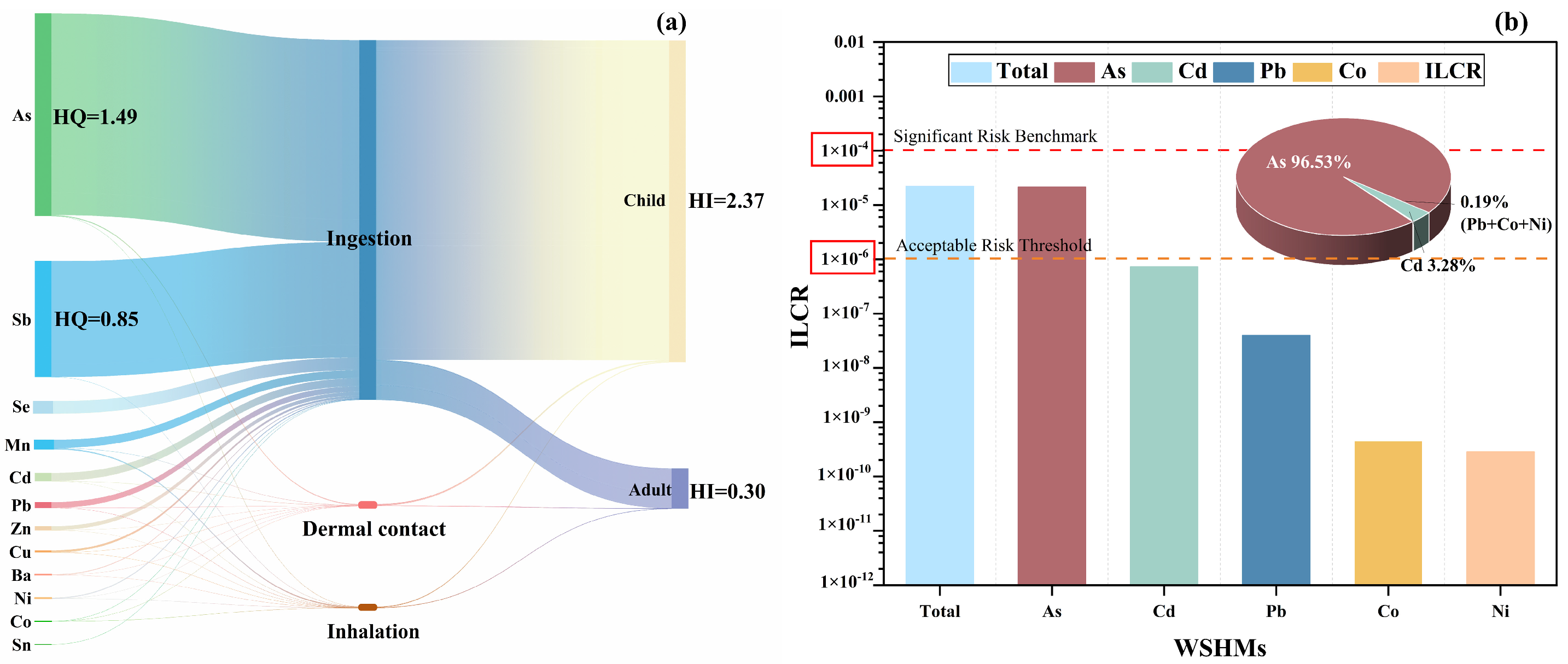
3.3. Health Risk Assessment of WSHMs in PM2.5
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, X.; Zhai, Y.; Zhu, Y.; Liu, Y.; Chen, H.; Li, P.; Peng, C.; Xu, B.; Li, C.; Zeng, G. Mass concentration and health risk assessment of heavy metals in size-segregated airborne particulate matter in Changsha. Sci. Total Environ. 2015, 517, 215–221. [Google Scholar] [CrossRef]
- Yin, H.; Xu, L. Comparative study of PM10/PM2.5-bound PAHs in downtown Beijing, China: Concentrations, sources, and health risks. J. Clean. Prod. 2018, 177, 674–683. [Google Scholar] [CrossRef]
- Cho, W.-S.; Duffin, R.; Poland, C.A.; Duschl, A.; Oostingh, G.J.; MacNee, W.; Bradley, M.; Megson, I.L.; Donaldson, K. Differential pro-inflammatory effects of metal oxide nanoparticles and their soluble ions in vitro and in vivo; zinc and copper nanoparticles, but not their ions, recruit eosinophils to the lungs. Nanotoxicology 2012, 6, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; He, Y.; Zhong, Y.; Li, X.; Li, S.; Ayitken, M. Source apportionment and health risk assessment of PM2.5-bound elements on winter pollution days in industrial cities on the northern slope of Tianshan Mountain, China. Atmos. Pollut. Res. 2024, 15, 102036. [Google Scholar] [CrossRef]
- He, L.; Wang, S.; Liu, M.; Chen, Z.; Xu, J.; Dong, Y. Transport and transformation of atmospheric metals in ecosystems: A review. J. Hazard. Mater. Adv. 2023, 9, 100218. [Google Scholar] [CrossRef]
- Mitra, S.; Chakraborty, A.J.; Tareq, A.M.; Emran, T.B.; Nainu, F.; Khusro, A.; Idris, A.M.; Khandaker, M.U.; Osman, H.; Alhumaydhi, F.A. Impact of heavy metals on the environment and human health: Novel therapeutic insights to counter the toxicity. J. King Saud Univ.-Sci. 2022, 34, 101865. [Google Scholar] [CrossRef]
- Burada, A.; Topa, C.M.; Georgescu, L.P.; Teodorof, L.; Nastase, C.; Seceleanu-Odor, D.; Iticescu, C. Heavy Metals Environment Accumulation in Somova—Parches Aquatic Complex from the Danube Delta Area. Rev. Chim.-Buchar.-Orig. Ed 2015, 66, 48–54. [Google Scholar]
- Chen, L.; Zhou, S.; Wu, S.; Wang, C.; He, D. Concentration, fluxes, risks, and sources of heavy metals in atmospheric deposition in the Lihe River watershed, Taihu region, eastern China. Environ. Pollut. 2019, 255, 113301. [Google Scholar] [CrossRef]
- Siddiqui, E.; Pandey, J. Atmospheric deposition: An important determinant of nutrients and heavy metal levels in urban surface runoff reaching to the Ganga River. Arch. Environ. Contam. Toxicol. 2022, 82, 191–205. [Google Scholar] [CrossRef]
- Duan, J.; Tan, J. Atmospheric heavy metals and arsenic in China: Situation, sources and control policies. Atmos. Environ. 2013, 74, 93–101. [Google Scholar] [CrossRef]
- Niu, J.; Rasmussen, P.E.; Hassan, N.M.; Vincent, R. Concentration distribution and bioaccessibility of trace elements in nano and fine urban airborne particulate matter: Influence of particle size. Water Air Soil Pollut. 2010, 213, 211–225. [Google Scholar] [CrossRef]
- Li, W.; Xu, L.; Liu, X.; Zhang, J.; Lin, Y.; Yao, X.; Gao, H.; Zhang, D.; Chen, J.; Wang, W. Air pollution–aerosol interactions produce more bioavailable iron for ocean ecosystems. Sci. Adv. 2017, 3, e1601749. [Google Scholar] [CrossRef] [PubMed]
- Mahowald, N.M.; Hamilton, D.S.; Mackey, K.R.; Moore, J.K.; Baker, A.R.; Scanza, R.A.; Zhang, Y. Aerosol trace metal leaching and impacts on marine microorganisms. Nat. Commun. 2018, 9, 2614. [Google Scholar] [CrossRef] [PubMed]
- Harris, E.; Sinha, B.; Van Pinxteren, D.; Tilgner, A.; Fomba, K.W.; Schneider, J.; Roth, A.; Gnauk, T.; Fahlbusch, B.; Mertes, S. Enhanced role of transition metal ion catalysis during in-cloud oxidation of SO2. science 2013, 340, 727–730. [Google Scholar] [CrossRef]
- Wang, W.; Liu, M.; Wang, T.; Song, Y.; Zhou, L.; Cao, J.; Hu, J.; Tang, G.; Chen, Z.; Li, Z. Sulfate formation is dominated by manganese-catalyzed oxidation of SO2 on aerosol surfaces during haze events. Nat. Commun. 2021, 12, 1993. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, W.; Lin, Q.; Yuan, Q.; Liu, L.; Zhang, J.; Zhang, Y.; Shao, L.; Niu, H.; Yang, S.; et al. Iron solubility in fine particles associated with secondary acidic aerosols in east China. Environ. Pollut. 2020, 264, 114769. [Google Scholar] [CrossRef]
- Slikboer, S.; Grandy, L.; Blair, S.L.; Nizkorodov, S.A.; Smith, R.W.; Al-Abadleh, H.A. Formation of light absorbing soluble secondary organics and insoluble polymeric particles from the dark reaction of catechol and guaiacol with Fe (III). Environ. Sci. Technol. 2015, 49, 7793–7801. [Google Scholar] [CrossRef]
- Tran, A.; Williams, G.; Younus, S.; Ali, N.N.; Blair, S.L.; Nizkorodov, S.A.; Al-Abadleh, H.A. Efficient formation of light-absorbing polymeric nanoparticles from the reaction of soluble Fe (III) with C4 and C6 dicarboxylic acids. Environ. Sci. Technol. 2017, 51, 9700–9708. [Google Scholar] [CrossRef]
- Al Nimer, A.; Rocha, L.; Rahman, M.A.; Nizkorodov, S.A.; Al-Abadleh, H.A. Effect of oxalate and sulfate on iron-catalyzed secondary brown carbon formation. Environ. Sci. Technol. 2019, 53, 6708–6717. [Google Scholar] [CrossRef]
- Badami, M.M.; Tohidi, R.; Jalali Farahani, V.; Sioutas, C. Size-segregated source identification of water-soluble and water-insoluble metals and trace elements of coarse and fine PM in central Los Angeles. Atmos. Environ. 2023, 310, 119984. [Google Scholar] [CrossRef]
- He, X.; Liu, P.; Zhao, W.; Xu, H.; Zhang, R.; Shen, Z. Size distribution of water-soluble metals in atmospheric particles in Xi’an, China: Seasonal variations, bioavailability, and health risk assessment. Atmos. Pollut. Res. 2021, 12, 101090. [Google Scholar] [CrossRef]
- von Schneidemesser, E.; Stone, E.A.; Quraishi, T.A.; Shafer, M.M.; Schauer, J.J. Toxic metals in the atmosphere in Lahore, Pakistan. Sci. Total Environ. 2010, 408, 1640–1648. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Tao, J.; Zhang, L.; Hu, B.; Liu, M.; Nie, F.; Lu, H.; Chen, L.; Wu, Y.; Chen, D.; et al. Influence of sources and atmospheric processes on metal solubility in PM2.5 in urban Guangzhou, South China. Sci. Total Environ. 2024, 951, 175807. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.Z.; Zhu, C.Y.; Gao, J.J.; Cheng, K.; Hao, J.M.; Wang, K.; Hua, S.B.; Wang, Y.; Zhou, J.R. Quantitative assessment of atmospheric emissions of toxic heavy metals from anthropogenic sources in China: Historical trend, spatial distribution, uncertainties, and control policies. Atmos. Chem. Phys. 2015, 15, 10127–10147. [Google Scholar] [CrossRef]
- Hien, T.T.; Chi, N.D.T.; Huy, D.H.; Le, H.A.; Oram, D.E.; Forster, G.L.; Mills, G.P.; Baker, A.R. Soluble trace metals associated with atmospheric fine particulate matter in the two most populous cities in Vietnam. Atmos. Environ. X 2022, 15, 100178. [Google Scholar] [CrossRef]
- Wang, Q.; Cao, J.; Shen, Z.; Tao, J.; Xiao, S.; Luo, L.; He, Q.; Tang, X. Chemical characteristics of PM2.5 during dust storms and air pollution events in Chengdu, China. Particuology 2013, 11, 70–77. [Google Scholar] [CrossRef]
- Wu, J.; Tou, F.; Guo, X.; Liu, C.; Sun, Y.; Xu, M.; Liu, M.; Yang, Y. Vast emission of Fe-and Ti-containing nanoparticles from representative coal-fired power plants in China and environmental implications. Sci. Total Environ. 2022, 838, 156070. [Google Scholar] [CrossRef]
- Yang, Q.; Yang, L.; Chen, C.; Yun, J.; Zhao, C.; Liu, G. Atmospheric emissions of Ti-containing nanoparticles from industrial activities in China. Environ. Sci. Nano 2024, 11, 3816–3825. [Google Scholar] [CrossRef]
- Contini, D.; Cesari, D.; Genga, A.; Siciliano, M.; Ielpo, P.; Guascito, M.R.; Conte, M. Source apportionment of size-segregated atmospheric particles based on the major water-soluble components in Lecce (Italy). Sci. Total Environ. 2014, 472, 248–261. [Google Scholar] [CrossRef]
- Wang, Q.; Ma, Y.; Tan, J.; Zheng, N.; Duan, J.; Sun, Y.; He, K.; Zhang, Y. Characteristics of size-fractionated atmospheric metals and water-soluble metals in two typical episodes in Beijing. Atmos. Environ. 2015, 119, 294–303. [Google Scholar] [CrossRef]
- Wu, S.-P.; Li, X.; Xiao, S.-H.; Zhang, J.; Schwab, J.J. Solubility of aerosol minor and trace elements in Xiamen Island, Southeast China: Size distribution, health risk and dry deposition. Sci. Total Environ. 2022, 844, 157100. [Google Scholar] [CrossRef]
- Fang, T.; Guo, H.; Zeng, L.; Verma, V.; Nenes, A.; Weber, R.J. Highly acidic ambient particles, soluble metals, and oxidative potential: A link between sulfate and aerosol toxicity. Environ. Sci. Technol. 2017, 51, 2611–2620. [Google Scholar] [CrossRef]
- Taira, M.; Sakakibara, K.; Saeki, K.; Ohira, S.-I.; Toda, K. Determination of oxoanions and water-soluble species of arsenic, selenium, antimony, vanadium, and chromium eluted in water from airborne fine particles (PM2.5): Effect of acid and transition metal content of particles on heavy metal elution. Environ. Sci. Process. Impacts 2020, 22, 1514–1524. [Google Scholar] [CrossRef] [PubMed]
- Manousakas, M.; Papaefthymiou, H.; Eleftheriadis, K.; Katsanou, K. Determination of water-soluble and insoluble elements in PM2.5 by ICP-MS. Sci. Total Environ. 2014, 493, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Xu, Y.; Liu, W.; Liu, Q.; Yu, S.; Liu, Y.; Wang, X.; Tao, S. Oxidative potential of ambient PM2.5 in the coastal cities of the Bohai Sea, northern China: Seasonal variation and source apportionment. Environ. Pollut. 2018, 236, 514–528. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, H.; Cai, X.; Song, Y.; Zhu, T. The impacts of the atmospheric boundary layer on regional haze in North China. NPJ Clim. Atmos. Sci. 2021, 4, 9. [Google Scholar] [CrossRef]
- Stull, R.B. An Introduction to Boundary Layer Meteorology; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; Volume 13. [Google Scholar]
- Yang, J.; Ma, L.; He, X.; Au, W.C.; Miao, Y.; Wang, W.-X.; Nah, T. Measurement Report: Abundance and fractional solubilities of aerosol metals in urban Hong Kong: Insights into factors that control aerosol metal dissolution in an urban site in South China. Atmos. Chem. Phys. Discuss. 2022, 23, 1403–1419. [Google Scholar] [CrossRef]
- Vione, D.; Maurino, V.; Minero, C.; Pelizzetti, E.; Harrison, M.A.; Olariu, R.-I.; Arsene, C. Photochemical reactions in the tropospheric aqueous phase and on particulate matter. Chem. Soc. Rev. 2006, 35, 441–453. [Google Scholar] [CrossRef]
- Wang, Q.-Q.; Ma, Y.-L.; Tan, J.-H.; Yang, F.-M.; Wei, L.-F.; Duan, J.-C.; He, K.-B. Characterization of water-soluble heavy metals of PM2.5 during winter in Beijing. China Environ. Sci. 2014, 34, 2204–2210. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency (U.S. EPA). Exposure Factors Handbook; PA/600/P-95/002F. EPA; Office of Research and Development: Washington, DC, USA, 1997.
- United States Environmental Protection Agency (U.S. EPA). Risk Assessment Guidance for Superfund (RAGS). Part A (1989): Human Health Evaluation Manual; Part E (2005): Supplemental Guidance for Dermal Risk Assessment; Part F (2009): Supplemental Guidance for Inhalation Risk Assessment. Available online: https://www.epa.gov/risk/risk-assessment-guidance-superfund-rags-part (accessed on 9 August 2025).
- Kogianni, E.; Kouras, A.; Samara, C. Indoor concentrations of PM2.5 and associated water-soluble and labile heavy metal fractions in workplaces: Implications for inhalation health risk assessment. Environ. Sci. Pollut. Res. 2021, 28, 58983–58993. [Google Scholar] [CrossRef]
- Galon-Negru, A.G.; Olariu, R.I.; Arsene, C. Size-resolved measurements of PM2.5 water-soluble elements in Iasi, north-eastern Romania: Seasonality, source apportionment and potential implications for human health. Sci. Total Environ. 2019, 695, 133839. [Google Scholar] [CrossRef] [PubMed]
- Dinu, A.; Bounegru, A.V.; Iticescu, C.; Georgescu, L.P.; Apetrei, C. Electrochemical detection of Cd2+, Pb2+, Cu2+ and Hg2+ with sensors based on carbonaceous nanomaterials and Fe3O4 nanoparticles. Nanomaterials 2024, 14, 702. [Google Scholar] [CrossRef]
- Xie, R.; Seip, H.M.; Wibetoe, G.; Nori, S.; McLeod, C.W. Heavy coal combustion as the dominant source of particulate pollution in Taiyuan, China, corroborated by high concentrations of arsenic and selenium in PM10. Sci. Total Environ. 2006, 370, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Li, P.; Wang, H.; Song, M. Quantifying the effects of air pollution control policies: A case of Shanxi province in China. Atmos. Pollut. Res. 2018, 9, 429–438. [Google Scholar] [CrossRef]
- Liu, K.; Shang, Q.; Wan, C.; Song, P.; Ma, C.; Cao, L. Characteristics and Sources of Heavy Metals in PM2.5 during a Typical Haze Episode in Rural and Urban Areas in Taiyuan, China. Atmosphere 2018, 9, 2. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Z.; Bai, H.; Zhang, S.; Mu, L.; Peng, L. Characteristics and health risk assessments of heavy metals in PM2.5 in Taiyuan and Yuci college town, China. Air Qual. Atmos. Health 2020, 13, 909–919. [Google Scholar] [CrossRef]
- Qin, S.; Li, B.; Wang, X.; Huang, H.; Zeng, M.; Xiao, F.; Xu, X. Metal Element Detection and Carcinogenicity Risk Assessment of PM2.5 Samples. Environ. Toxicol. Chem. 2020, 39, 1273–1276. [Google Scholar] [CrossRef]
- Ding, X.; Zhao, W.; Yan, T.; Wang, L. Response of Ecosystem Service Value to Spatio-Temporal Pattern Evolution of Land Use in Typical Heavy Industry Cities: A Case Study of Taiyuan City, China. Land 2022, 11, 2035. [Google Scholar] [CrossRef]
- Ma, H.; Zhen, Z.; Mi, M.; Wang, Q. Characteristics of nutrients pollution and ecological risk assessment of heavy metal in sediments of Fenhe River, Taiyuan section, China. Water Supply 2022, 22, 2596–2611. [Google Scholar] [CrossRef]
- Zhang, Y.; Ji, X.; Ku, T.; Li, G.; Sang, N. Heavy metals bound to fine particulate matter from northern China induce season-dependent health risks: A study based on myocardial toxicity. Environ. Pollut. 2016, 216, 380–390. [Google Scholar] [CrossRef]
- Zhao, W.; Xuan, L.; Li, W.; Wang, W.; Wang, X. Comprehensive Impact of Different Urban Form Indices on Land Surface Temperature and PM2.5 Pollution in Summer and Winter, Based on Urban Functional Zones: A Case Study of Taiyuan City. Sustainability 2025, 17, 6618. [Google Scholar] [CrossRef]
- Xu, Y.; Xue, W.; Lei, Y.; Zhao, Y.; Cheng, S.; Ren, Z.; Huang, Q. Impact of meteorological conditions on PM2.5 pollution in China during winter. Atmosphere 2018, 9, 429. [Google Scholar] [CrossRef]
- Shu, Z.; Zhao, T.; Liu, Y.; Zhang, L.; Ma, X.; Kuang, X.; Li, Y.; Huo, Z.; Ding, Q.; Sun, X. Impact of deep basin terrain on PM2.5 distribution and its seasonality over the Sichuan Basin, Southwest China. Environ. Pollut. 2022, 300, 118944. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Cheng, S.; Li, H.; Fu, K.; Xu, Y. Groundwater pollution source identification and apportionment using PMF and PCA-APCA-MLR receptor models in a typical mixed land-use area in Southwestern China. Sci. Total Environ. 2020, 741, 140383. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Chen, C.; Liu, D.; Wang, M.; Han, Q.; Zhang, X.; Feng, X.; Sun, A.; Mao, P.; Xiong, Q.; et al. Health risk assessment of PM2.5 heavy metals in county units of northern China based on Monte Carlo simulation and APCS-MLR. Sci. Total Environ. 2022, 843, 156777. [Google Scholar] [CrossRef] [PubMed]
- Larsen, R.K.; Baker, J.E. Source Apportionment of Polycyclic Aromatic Hydrocarbons in the Urban Atmosphere: A Comparison of Three Methods. Environ. Sci. Technol. 2003, 37, 1873–1881. [Google Scholar] [CrossRef]
- Jin, G.; Fang, W.; Shafi, M.; Wu, D.; Li, Y.; Zhong, B.; Ma, J.; Liu, D. Source apportionment of heavy metals in farmland soil with application of APCS-MLR model: A pilot study for restoration of farmland in Shaoxing City Zhejiang, China. Ecotoxicol. Environ. Saf. 2019, 184, 109495. [Google Scholar] [CrossRef]
- Sun, Y.; Hu, X.; Wu, J.; Lian, H.; Chen, Y. Fractionation and health risks of atmospheric particle-bound As and heavy metals in summer and winter. Sci. Total Environ. 2014, 493, 487–494. [Google Scholar] [CrossRef]
- Liu, M.; Wang, W.; Li, J.; Wang, T.; Xu, Z.; Song, Y.; Zhang, W.; Zhou, L.; Lian, C.; Yang, J.; et al. High fraction of soluble trace metals in fine particles under heavy haze in central China. Sci. Total Environ. 2022, 841, 156771. [Google Scholar] [CrossRef]
- Jiang, S.Y.N.; Yang, F.; Chan, K.L.; Ning, Z. Water solubility of metals in coarse PM and PM2.5 in typical urban environment in Hong Kong. Atmos. Pollut. Res. 2014, 5, 236–244. [Google Scholar] [CrossRef]
- Guieu, C.; Chester, R.; Nimmo, M.; Martin, J.M.; Guerzoni, S.; Nicolas, E.; Mateu, J.; Keyse, S. Atmospheric input of dissolved and particulate metals to the northwestern Mediterranean. Deep Sea Res. Part II Top. Stud. Oceanogr. 1997, 44, 655–674. [Google Scholar] [CrossRef]
- Morel, F.M.; Price, N.M. The biogeochemical cycles of trace metals in the oceans. Science 2003, 300, 944–947. [Google Scholar] [CrossRef]
- Karthikeyan, S.; Joshi, U.M.; Balasubramanian, R. Microwave assisted sample preparation for determining water-soluble fraction of trace elements in urban airborne particulate matter: Evaluation of bioavailability. Anal. Chim. Acta 2006, 576, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Alias, N.F.; Khan, M.F.; Sairi, N.A.; Zain, S.M.; Suradi, H.; Rahim, H.A.; Banerjee, T.; Bari, M.A.; Othman, M.; Latif, M.T. Characteristics, emission sources, and risk factors of heavy metals in PM2.5 from Southern Malaysia. ACS Earth Space Chem. 2020, 4, 1309–1323. [Google Scholar] [CrossRef]
- Mondal, S.; Singh, G. PM2.5-bound trace elements in a critically polluted industrial coal belt of India: Seasonal patterns, source identification, and human health risk assessment. Environ. Sci. Pollut. Res. 2021, 28, 32634–32647. [Google Scholar] [CrossRef]
- Charrier, J.G.; Anastasio, C. On dithiothreitol (DTT) as a measure of oxidative potential for ambient particles: Evidence for the importance of soluble transition metals. Atmos. Chem. Phys. 2012, 12, 9321–9333. [Google Scholar] [CrossRef]
- Lyu, Y.; Guo, H.; Cheng, T.; Li, X. Particle size distributions of oxidative potential of lung-deposited particles: Assessing contributions from quinones and water-soluble metals. Environ. Sci. Technol. 2018, 52, 6592–6600. [Google Scholar] [CrossRef]
- Li, J.-M.; Zhao, S.-M.; Wu, S.-P.; Jiang, B.-Q.; Liu, Y.-J.; Zhang, J.; Schwab, J.J. Size-segregated characteristics of water-soluble oxidative potential in urban Xiamen: Potential driving factors and implications for human health. Sci. Total Environ. 2024, 912, 168902. [Google Scholar] [CrossRef]
- Palleschi, S.; Rossi, B.; Armiento, G.; Montereali, M.R.; Nardi, E.; Tagliani, S.M.; Inglessis, M.; Gianfagna, A.; Silvestroni, L. Toxicity of the readily leachable fraction of urban PM2.5 to human lung epithelial cells: Role of soluble metals. Chemosphere 2018, 196, 35–44. [Google Scholar] [CrossRef]
- Liu, Q.; Baumgartner, J.; Zhang, Y.; Liu, Y.; Sun, Y.; Zhang, M. Oxidative potential and inflammatory impacts of source apportioned ambient air pollution in Beijing. Environ. Sci. Technol. 2014, 48, 12920–12929. [Google Scholar] [CrossRef]
- Tian, S.; Liang, T.; Li, K.; Wang, L. Source and path identification of metals pollution in a mining area by PMF and rare earth element patterns in road dust. Sci. Total Environ. 2018, 633, 958–966. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wu, L.; Zhang, X.; Wang, X.; Bai, W.; Ming, J.; Geng, C.; Yang, W. Comparison of PM2.5 chemical compositions during haze and non-haze days in a heavy industrial city in north China. Aerosol Air Qual. Res. 2020, 20, 1950–1960. [Google Scholar] [CrossRef]
- Banerjee, T.; Murari, V.; Kumar, M.; Raju, M. Source apportionment of airborne particulates through receptor modeling: Indian scenario. Atmos. Res. 2015, 164, 167–187. [Google Scholar] [CrossRef]
- Fussell, J.C.; Franklin, M.; Green, D.C.; Gustafsson, M.; Harrison, R.M.; Hicks, W.; Kelly, F.J.; Kishta, F.; Miller, M.R.; Mudway, I.S. A review of road traffic-derived non-exhaust particles: Emissions, physicochemical characteristics, health risks, and mitigation measures. Environ. Sci. Technol. 2022, 56, 6813–6835. [Google Scholar] [CrossRef]
- Hjortenkrans, D.S.; Bergbäck, B.G.; Häggerud, A.V. Metal emissions from brake linings and tires: Case studies of Stockholm, Sweden 1995/1998 and 2005. Environ. Sci. Technol. 2007, 41, 5224–5230. [Google Scholar] [CrossRef]
- Lin, Z.; Fan, X.; Chen, G.; Hong, Y.; Li, M.; Xu, L.; Hu, B.; Yang, C.; Chen, Y.; Shao, Z.; et al. Sources appointment and health risks of PM2.5-bound trace elements in a coastal city of southeastern China. J. Environ. Sci. 2024, 138, 561–571. [Google Scholar] [CrossRef]
- Coufalík, P.; Matoušek, T.; Křůmal, K.; Vojtíšek-Lom, M.; Beránek, V.; Mikuška, P. Content of metals in emissions from gasoline, diesel, and alternative mixed biofuels. Environ. Sci. Pollut. Res. 2019, 26, 29012–29019. [Google Scholar] [CrossRef]
- Liu, K.; Shang, Q.; Wan, C. Sources and health risks of heavy metals in PM2.5 in a campus in a typical suburb area of Taiyuan, North China. Atmosphere 2018, 9, 46. [Google Scholar] [CrossRef]
- Zhang, R.; Jing, J.; Tao, J.; Hsu, S.-C.; Wang, G.; Cao, J.; Lee, C.S.L.; Zhu, L.; Chen, Z.; Zhao, Y. Chemical characterization and source apportionment of PM2.5 in Beijing: Seasonal perspective. Atmos. Chem. Phys. 2013, 13, 7053–7074. [Google Scholar] [CrossRef]
- Senior, C.; Granite, E.; Linak, W.; Seames, W. Chemistry of trace inorganic elements in coal combustion systems: A century of discovery. Energy Fuels 2020, 34, 15141–15168. [Google Scholar] [CrossRef]
- Bartoňová, L.; Raclavská, H.; Čech, B.; Kucbel, M. Behavior of Cd during coal combustion: An overview. Processes 2020, 8, 1237. [Google Scholar] [CrossRef]
- Li, R.; Li, J.; Cui, L.; Wu, Y.; Fu, H.; Chen, J.; Chen, M. Atmospheric emissions of Cu and Zn from coal combustion in China: Spatio-temporal distribution, human health effects, and short-term prediction. Environ. Pollut. 2017, 229, 724–734. [Google Scholar] [CrossRef] [PubMed]
- Yadav, K.; Sunder Raman, R. Size-segregated chemical source profiles and potential health impacts of multiple sources of fugitive dust in and around Bhopal, central India. Environ. Pollut. 2021, 284, 117385. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.L.; Bi, X.H.; Wu, J.H.; Zhang, Y.F.; Wang, J.; Xu, H.; Yao, L.; Jiao, L.; Feng, Y.C. Characterization and Source Identification of Heavy Metals in Ambient PM10 and PM2.5 in an Integrated Iron and Steel Industry Zone Compared with a Background Site. Aerosol Air Qual. Res. 2015, 15, 875–887. [Google Scholar] [CrossRef]
- Yang, Y.; Weber, R.J. Ultrafiltration to characterize PM2.5 water-soluble iron and its sources in an urban environment. Atmos. Environ. 2022, 286, 119246. [Google Scholar] [CrossRef]
- Ogundele, L.T.; Owoade, O.K.; Olise, F.S.; Hopke, P.K. Source identification and apportionment of PM2.5 and PM2.5-10 in iron and steel scrap smelting factory environment using PMF, PCFA and UNMIX receptor models. Environ. Monit. Assess. 2016, 188, 574. [Google Scholar] [CrossRef]
- Mehta, U.H.; Kaul, D.S.; Westerdahl, D.; Ning, Z.; Zhang, K.; Sun, L.; Wei, P.; Gajjar, H.H.; Jeyaraman, J.D.; Patel, M.V. Understanding the Sources of Heavy Metal Pollution in Ambient Air of Neighboring a Solid Waste Landfill Site. Aerosol Sci. Eng. 2022, 6, 161–175. [Google Scholar] [CrossRef]
- Assessment, P.R. Risk Assessment Guidance for Superfund: Volume III-Part a. Process for Conducting Probabilistic Risk Assessment; Unites States Environmental Protection Agency: Washington, DC, USA, 2001. [Google Scholar]
- Lu, X.; Zhang, X.; Li, L.Y.; Chen, H. Assessment of metals pollution and health risk in dust from nursery schools in Xi’an, China. Environ. Res. 2014, 128, 27–34. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency (U.S. EPA). Exposure Factors Handbook 2011 Edition (Final); United States Environmental Protection Agency: Washington, DC, USA, 2011; Volume 414.

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Q.; Zhang, C.; Chen, Y.; Pei, N.; Zhao, Y.; Sun, L.; Lan, J.; Liu, F.; Guo, Z.; Mu, L.; et al. Concentration Characteristics, Source Analysis, and Health Risk Assessment of Water-Soluble Heavy Metals in PM2.5 During Winter in Taiyuan, China. Atmosphere 2025, 16, 980. https://doi.org/10.3390/atmos16080980
Hu Q, Zhang C, Chen Y, Pei N, Zhao Y, Sun L, Lan J, Liu F, Guo Z, Mu L, et al. Concentration Characteristics, Source Analysis, and Health Risk Assessment of Water-Soluble Heavy Metals in PM2.5 During Winter in Taiyuan, China. Atmosphere. 2025; 16(8):980. https://doi.org/10.3390/atmos16080980
Chicago/Turabian StyleHu, Qingyu, Chao Zhang, Yang Chen, Nan Pei, Yufeng Zhao, Lijuan Sun, Jie Lan, Fengxian Liu, Ziyong Guo, Ling Mu, and et al. 2025. "Concentration Characteristics, Source Analysis, and Health Risk Assessment of Water-Soluble Heavy Metals in PM2.5 During Winter in Taiyuan, China" Atmosphere 16, no. 8: 980. https://doi.org/10.3390/atmos16080980
APA StyleHu, Q., Zhang, C., Chen, Y., Pei, N., Zhao, Y., Sun, L., Lan, J., Liu, F., Guo, Z., Mu, L., Wang, J., & Bi, X. (2025). Concentration Characteristics, Source Analysis, and Health Risk Assessment of Water-Soluble Heavy Metals in PM2.5 During Winter in Taiyuan, China. Atmosphere, 16(8), 980. https://doi.org/10.3390/atmos16080980




