Research on the Emission of Biogenic Volatile Organic Compounds from Terrestrial Vegetation
Abstract
1. Introduction
2. Monitoring Methods
2.1. Off-Line Method
2.2. On-Line Method
2.3. Model Simulation
3. Emission Characteristics
3.1. BVOC Emission Source and Components
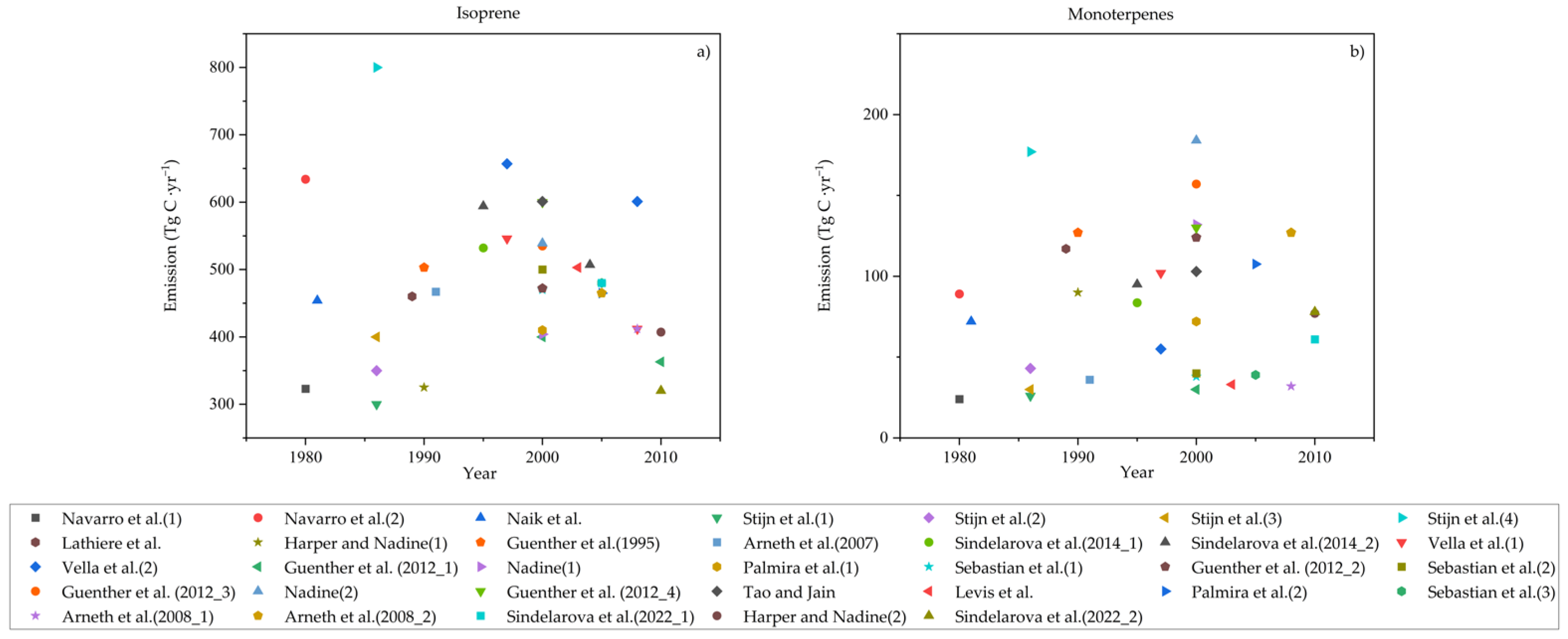
3.2. Emission Inventory
4. The Factors Affecting BVOC Emission
4.1. Intrinsic Factors
4.1.1. Tree Species
4.1.2. Gene
4.1.3. Tree Age
4.1.4. Growth Rhythms
4.2. External Factors
4.2.1. Temperature
4.2.2. Illumination
4.2.3. Water Conditions
5. Ecological and Environmental Impacts of BVOCs
6. Discussion and Prospect
- (1)
- The BVOC emission inventory has yet to be clarified
- (2)
- The climatic and environmental impacts of BVOCs needs to be further explored
- (3)
- The role of BVOCs in the carbon cycle of terrestrial ecosystems needs to be further investigated
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, X.; Xue, Z.; Li, H.; Yan, L.; Yang, Y.; Wang, Y.; Duan, J.; Li, L.; Chai, F.; Cheng, M.; et al. Ambient Volatile Organic Compounds Pollution in China. J. Environ. Sci. 2017, 55, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Cataldo, F.; Ursini, O.; Lilla, E.; Angelini, G. Ozonolysis of α-PINENE, β-PINENE, d- and l-Turpentine Oil Studied by Chirooptical Methods; Some Implications on the Atmospheric Chemistry of Biogenic Volatile Organic Compounds. Ozone Sci. Eng. 2010, 32, 274–285. [Google Scholar] [CrossRef]
- Kefauver, S.C.; Filella, I.; Peñuelas, J. Remote Sensing of Atmospheric Biogenic Volatile Organic Compounds (BVOCs) via Satellite-Based Formaldehyde Vertical Column Assessments. Int. J. Remote Sens. 2014, 35, 7519–7542. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, H.; Kang, T.; Wei, W.; Liu, X. Spatially Resolved Analysis of Speciated VOC Emissions and Their Contributions to Secondary Pollutant Formation: A Comparative Assessment of Anthropogenic and Biogenic Sources in China. Environ. Int. 2025, 202, 109627. [Google Scholar] [CrossRef] [PubMed]
- Qie, G.; Wang, C.; Peng, Z. Research advances on BVOCs emission from forest. Chin. J. Appl. Ecol. 2005, 16, 1151–1155. [Google Scholar]
- Lun, X.; Lin, Y.; Chai, F.; Fan, C.; Li, H.; Liu, J. Reviews of Emission of Biogenic Volatile Organic Compounds (BVOCs) in Asia. J. Environ. Sci. 2020, 95, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Sartelet, K.N.; Couvidat, F.; Seigneur, C.; Roustan, Y. Impact of Biogenic Emissions on Air Quality over Europe and North America. Atmos. Environ. 2012, 53, 131–141. [Google Scholar] [CrossRef]
- Guenther, A.B.; Jiang, X.; Heald, C.L.; Sakulyanontvittaya, T.; Duhl, T.; Emmons, L.K.; Wang, X. The Model of Emissions of Gases and Aerosols from Nature Version 2.1 (MEGAN2.1): An Extended and Updated Framework for Modeling Biogenic Emissions. Geosci. Model Dev. 2012, 5, 1471–1492. [Google Scholar] [CrossRef]
- Helmig, D.; Ortega, J.; Duhl, T.; Tanner, D.; Guenther, A.; Harley, P.; Wiedinmyer, C.; Milford, J.; Sakulyanontvittaya, T. Sesquiterpene Emissions from Pine Trees—Identifications, Emission Rates and Flux Estimates for the Contiguous United States. Environ. Sci. Technol. 2007, 41, 1545–1553. [Google Scholar] [CrossRef] [PubMed]
- Loreto, F.; Schnitzler, J.-P. Abiotic Stresses and Induced BVOCs. Trends Plant Sci. 2010, 15, 154–166. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, R.P.; Silfver, T.; Myller, K.; Oksanen, E.; Holopainen, J.K.; Mikola, J. BVOC Emissions from a Subarctic Ecosystem, as Controlled by Insect Herbivore Pressure and Temperature. Ecosystems 2022, 25, 872–891. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, F.; Chen, Y.; Yang, S.; Dai, Y.; Qin, Y.; Zhang, N.; Shu, Z.; Yan, H.; Ge, X.; et al. Impact of Temperature on the Biogenic Volatile Organic Compound (BVOC) Emissions in China: A Review. J. Environ. Sci. 2025; in press. [Google Scholar] [CrossRef]
- Cheng, R.; Yang, S.; Wang, D.; Qin, F.; Wang, S.; Meng, S. Advances in the Biosynthesis of Plant Terpenoids: Models, Mechanisms, and Applications. Plants 2025, 14, 1428. [Google Scholar] [CrossRef] [PubMed]
- Laothawornkitkul, J.; Taylor, J.E.; Paul, N.D.; Hewitt, C.N. Biogenic Volatile Organic Compounds in the Earth System. New Phytol. 2009, 183, 27–51. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Lyu, Y.; Yang, X.; Yuan, L.; Wang, Y.; Wang, L.; Liang, Y.; Qiao, Y.; Wang, S. Modeling Biogenic Volatile Organic Compounds Emissions and Subsequent Impacts on Ozone Air Quality in the Sichuan Basin, Southwestern China. Front. Ecol. Evol. 2022, 10, 924944. [Google Scholar] [CrossRef]
- Fitzky, A.C.; Sandén, H.; Karl, T.; Fares, S.; Calfapietra, C.; Grote, R.; Saunier, A.; Rewald, B. The Interplay Between Ozone and Urban Vegetation—BVOC Emissions, Ozone Deposition, and Tree Ecophysiology. Front. For. Glob. Change 2019, 2, 50. [Google Scholar] [CrossRef]
- Li, S.; Yuan, X.; Xu, Y.; Li, Z.; Feng, Z.; Yue, X.; Paoletti, E. Biogenic Volatile Organic Compound Emissions from Leaves and Fruits of Apple and Peach Trees during Fruit Development. J. Environ. Sci. 2021, 108, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Calfapietra, C.; Fares, S.; Manes, F.; Morani, A.; Sgrigna, G.; Loreto, F. Role of Biogenic Volatile Organic Compounds (BVOC) Emitted by Urban Trees on Ozone Concentration in Cities: A Review. Environ. Pollut. 2013, 183, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Guenther, A.; Jiang, X.; Shah, T.; Huang, L.; Kemball-Cook, S.; Yarwood, G. Model of Emissions of Gases and Aerosol from Nature Version 3 (MEGAN3) for Estimating Biogenic Emissions. In Air Pollution Modeling and Its Application XXVI; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Went, F.W. Organic Matter in the Atmosphere, and Its Possible Relation to Petroleum Formation. Proc. Natl. Acad. Sci. USA 1960, 46, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Zuo, S.; Wu, Z.; Qiu, Y.; Wang, J.; Lei, Y.; Liao, H.; Ren, Y. A Review of Research Hotspots and Trends in Biogenic Volatile Organic Compounds (BVOCs) Emissions Combining Bibliometrics with Evolution Tree Methods. Environ. Res. Lett. 2021, 16, 13003. [Google Scholar] [CrossRef]
- Cai, M.; An, C.; Guy, C. A Scientometric Analysis and Review of Biogenic Volatile Organic Compound Emissions: Research Hotspots, New Frontiers, and Environmental Implications. Renew. Sustain. Energy Rev. 2021, 149, 111317. [Google Scholar] [CrossRef]
- Bai, J.; Guenther, A.; Turnipseed, A.; Duhl, T. Seasonal and Interannual Variations in Whole–Ecosystem Isoprene and Monoterpene Emissions from a Temperate Mixed Forest in Northern China. Atmos. Pollut. Res. 2015, 6, 696–707. [Google Scholar] [CrossRef]
- Genard-Zielinski, A.C.; Boissard, C.; Fernandez, C.; Kalogridis, C.; Lathière, J.; Gros, V.; Bonnaire, N.; Ormeño, E. Variability of BVOC Emissions from a Mediterranean Mixed Forest in Southern France with a Focus on Quercus Pubescens. Atmos. Chem. Phys. 2015, 15, 431–446. [Google Scholar] [CrossRef]
- Mozaffar, A.; Zhang, Y.-L. Atmospheric Volatile Organic Compounds (VOCs) in China: A Review. Curr. Pollut. Rep. 2020, 6, 250–263. [Google Scholar] [CrossRef]
- Guenther, A.; Karl, T.; Harley, P.; Wiedinmyer, C.; Palmer, P.I.; Geron, C. Estimates of Global Terrestrial Isoprene Emissions Using MEGAN (Model of Emissions of Gases and Aerosols from Nature). Atmos. Chem. Phys. 2006, 6, 3181–3210. [Google Scholar] [CrossRef]
- Pihlatie, M.K.; Christiansen, J.R.; Aaltonen, H.; Korhonen, J.F.J.; Nordbo, A.; Rasilo, T.; Benanti, G.; Giebels, M.; Helmy, M.; Sheehy, J.; et al. Comparison of Static Chambers to Measure CH4 Emissions from Soils. Agric. For. Meteorol. 2013, 171–172, 124–136. [Google Scholar] [CrossRef]
- Baghi, R.; Helmig, D.; Guenther, A.; Duhl, T.; Daly, R. Contribution of Flowering Trees to Urban Atmospheric Biogenic Volatile Organic Compound Emissions. Biogeosci. Discuss. 2012, 9, 3145–3172. [Google Scholar] [CrossRef]
- Ortega, J.; Helmig, D. Approaches for Quantifying Reactive and Low-Volatility Biogenic Organic Compound Emissions by Vegetation Enclosure Techniques—Part A. Chemosphere 2008, 72, 343–364. [Google Scholar] [CrossRef] [PubMed]
- Joó, É.; Dewulf, J.; Demarcke, M.; Amelynck, C.; Schoon, N.; Müller, J.-F.; Šimpraga, M.; Steppe, K.; Van Langenhove, H. Quantification of Interferences in PTR-MS Measurements of Monoterpene Emissions from Fagus sylvatica L. Using Simultaneous TD-GC-MS Measurements. Int. J. Mass Spectrom. 2010, 291, 90–95. [Google Scholar] [CrossRef]
- Kato, S.; Miyakawa, Y.; Kaneko, T.; Kajii, Y. Urban Air Measurements Using PTR-MS in Tokyo Area and Comparison with GC-FID Measurements. Int. J. Mass Spectrom. 2004, 235, 103–110. [Google Scholar] [CrossRef]
- Liu, X.; Pawliszyn, R.; Wang, L.; Pawliszyn, J. On-Site Monitoring of Biogenic Emissions from Eucalyptus Dunnii Leaves Using Membrane Extraction with Sorbent Interface Combined with a Portable Gas Chromatograph System. Analyst 2004, 129, 55. [Google Scholar] [CrossRef] [PubMed]
- Bowling, D.R.; Turnipseed, A.A.; Delany, A.C.; Baldocchi, D.D.; Greenberg, J.P.; Monson, R.K. The Use of Relaxed Eddy Accumulation to Measure Biosphere-Atmosphere Exchange of Isoprene and Other Biological Trace Gases. Oecologia 1998, 116, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, C.; Turnipseed, A.; Shertz, S.; Karl, T.; Potosnak, M.; Bai, J.; Serça, D.; Bonal, D.; Burban, B.; Lopes, P.R.C.; et al. A Portable, Low-Cost Relaxed Eddy Accumulation (REA) System for Quantifying Ecosystem-Level Fluxes of Volatile Organics. Atmos. Environ. 2020, 242, 117764. [Google Scholar] [CrossRef]
- De Gouw, J.; Warneke, C.; Karl, T.; Eerdekens, G.; Van Der Veen, C.; Fall, R. Sensitivity and Specificity of Atmospheric Trace Gas Detection by Proton-Transfer-Reaction Mass Spectrometry. Int. J. Mass Spectrom. 2003, 223–224, 365–382. [Google Scholar] [CrossRef]
- Pallozzi, E.; Guidolotti, G.; Ciccioli, P.; Brilli, F.; Feil, S.; Calfapietra, C. Does the Novel Fast-GC Coupled with PTR-TOF-MS Allow a Significant Advancement in Detecting VOC Emissions from Plants? Agric. For. Meteorol. 2016, 216, 232–240. [Google Scholar] [CrossRef]
- Smith, D.; Španěl, P. Direct, Rapid Quantitative Analyses of BVOCs Using SIFT-MS and PTR-MS Obviating Sample Collection. TrAC Trends Anal. Chem. 2011, 30, 945–959. [Google Scholar] [CrossRef]
- Warneke, C.; De Gouw, J.A.; Lovejoy, E.R.; Murphy, P.C.; Kuster, W.C.; Fall, R. Development of Proton-Transfer Ion Trap-Mass Spectrometry: On-Line Detection and Identification of Volatile Organic Compounds in Air. J. Am. Soc. Mass Spectrom. 2005, 16, 1316–1324. [Google Scholar] [CrossRef]
- Steeghs, M.M.L.; Crespo, E.; Harren, F.J.M. Collision Induced Dissociation Study of 10 Monoterpenes for Identification in Trace Gas Measurements Using the Newly Developed Proton-Transfer Reaction Ion Trap Mass Spectrometer. Int. J. Mass Spectrom. 2007, 263, 204–212. [Google Scholar] [CrossRef]
- Xia, L.; Liu, Y.; Chen, R.T.; Weng, B.; Zou, Y. Advancements in Miniaturized Infrared Spectroscopic-Based Volatile Organic Compound Sensors: A Systematic Review. Appl. Phys. Rev. 2024, 11, 031306. [Google Scholar] [CrossRef]
- Pierce, T.E.; Waldruff, P.S. PC-BEIS: A Personal Computer Version of the Biogenic Emissions Inventory System. J. Air Waste Manag. Assoc. 1991, 41, 937–941. [Google Scholar] [CrossRef]
- Levis, S.; Wiedinmyer, C.; Bonan, G.B.; Guenther, A. Simulating Biogenic Volatile Organic Compound Emissions in the Community Climate System Model. J. Geophys. Res. 2003, 108, 46–59. [Google Scholar] [CrossRef]
- Pacifico, F.; Harrison, S.P.; Jones, C.D.; Arneth, A.; Sitch, S.; Weedon, G.P.; Barkley, M.P.; Palmer, P.I.; Serça, D.; Potosnak, M.; et al. Evaluation of a Photosynthesis-Based Biogenic Isoprene Emission Scheme in JULES and Simulation of Isoprene Emissions under Present-Day Climate Conditions. Atmos. Chem. Phys. 2011, 11, 4371–4389. [Google Scholar] [CrossRef]
- Sindelarova, K.; Markova, J.; Simpson, D.; Huszar, P.; Karlicky, J.; Darras, S.; Granier, C. High-Resolution Biogenic Global Emission Inventory for the Time Period 2000–2019 for Air Quality Modelling. Earth Syst. Sci. Data 2022, 14, 251–270. [Google Scholar] [CrossRef]
- Zimmer, W.; Steinbrecher, R.; Körner, C.; Schnitzler, J.P. The Process-Based SIM–BIM Model: Towards More Realistic Prediction of Isoprene Emissions from Adult Quercus Petraea Forest Trees. Atmos. Environ. 2003, 37, 1665–1671. [Google Scholar] [CrossRef]
- Bai, J.; Duhl, T. A Primary Generalized Empirical Model of BVOC Emissions for Some Typical Forests in China. Atmos. Pollut. Res. 2021, 12, 101–126. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, L.; Zhao, W.; Wang, X.; Jiang, L.; Liu, B.; Li, X.; Lu, H. Emission Characteristics of VOCs from Forests and Its Impact on Regional Air Quality in Beijing. China Environ. Sci. 2021, 41, 622–632. [Google Scholar]
- Diem, J.E.; Comrie, A.C. Integrating Remote Sensing and Local Vegetation Information for a High-Resolution Biogenic Emissions Inventory—Application to an Urbanized, Semiarid Region. J. Air Waste Manag. Assoc. 2000, 50, 1968–1979. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Wang, Z.; Bai, Y.; Xie, C.; Shao, M. Establishment of Vegetation VOC Emission Inventory in China. China Environ. Sci. 2005, 25, 110–114. [Google Scholar]
- Oderbolz, D.C.; Aksoyoglu, S.; Keller, J.; Barmpadimos, I.; Steinbrecher, R.; Skjøth, C.A.; Plaß-Dülmer, C.; Prévôt, A.S.H. A Comprehensive Emission Inventory of Biogenic Volatile Organic Compounds in Europe: Improved Seasonality and Land-Cover. Atmos. Chem. Phys. 2013, 13, 1689–1712. [Google Scholar] [CrossRef]
- Morfopoulos, C.; Müller, J.-F.; Stavrakou, T.; Bauwens, M.; De Smedt, I.; Friedlingstein, P.; Prentice, I.C.; Regnier, P. Vegetation Responses to Climate Extremes Recorded by Remotely Sensed Atmospheric Formaldehyde. Glob. Change Biol. 2022, 28, 1809–1822. [Google Scholar] [CrossRef] [PubMed]
- Moradi, A.; Abera, T.A.; Shayle, E.S.; Muhammed, M.A.; Zeuss, D. Modeling Long-Term Dynamics of Biogenic Volatile Organic Compounds (BVOCs) in Germany Based on Major Precursors. Environ. Sci. Technol. 2025, 59, 4587–4596. [Google Scholar] [CrossRef] [PubMed]
- Cui, B.; Xian, C.; Han, B.; Shu, C.; Qian, Y.; Ouyang, Z.; Wang, X. High-Resolution Emission Inventory of Biogenic Volatile Organic Compounds for Rapidly Urbanizing Areas: A Case of Shenzhen Megacity, China. J. Environ. Manag. 2024, 351, 119754. [Google Scholar] [CrossRef] [PubMed]
- Muhlemann, J.K.; Klempien, A.; Dudareva, N. Floral Volatiles: From Biosynthesis to Function. Plant Cell Environ. 2014, 37, 1936–1949. [Google Scholar] [CrossRef] [PubMed]
- Loreto, F.; Barta, C.; Brilli, F.; Nogues, I. On the Induction of Volatile Organic Compound Emissions by Plants as Consequence of Wounding or Fluctuations of Light and Temperature. Plant Cell Environ. 2006, 29, 1820–1828. [Google Scholar] [CrossRef] [PubMed]
- Babikova, Z.; Gilbert, L.; Bruce, T.J.A.; Birkett, M.; Caulfield, J.C.; Woodcock, C.; Pickett, J.A.; Johnson, D. Underground Signals Carried through Common Mycelial Networks Warn Neighbouring Plants of Aphid Attack. Ecol. Lett. 2013, 16, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Xu, Y.; Calatayud, V.; Li, Z.; Feng, Z.; Loreto, F. Emissions of Isoprene and Monoterpenes from Urban Tree Species in China and Relationships with Their Driving Factors. Atmos. Environ. 2023, 314, 120096. [Google Scholar] [CrossRef]
- Zorić, M.; Kostić, S.; Kladar, N.; Božin, B.; Vasić, V.; Kebert, M.; Orlović, S. Phytochemical Screening of Volatile Organic Compounds in Three Common Coniferous Tree Species in Terms of Forest Ecosystem Services. Forests 2021, 12, 928. [Google Scholar] [CrossRef]
- Sharkey, T.D.; Wiberley, A.E.; Donohue, A.R. Isoprene Emission from Plants: Why and How. Ann. Bot. 2007, 101, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, Q.; Wang, L.; Li, L.; Lun, X.; Chen, W.; Gao, Y.; Huang, L.; Wang, Q.; Liu, B. Seasonal Biogenic Volatile Organic Compound Emission Factors in Temperate Tree Species: Implications for Emission Estimation and Ozone Formation. Environ. Pollut. 2024, 361, 124895. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Lun, X.; Fan, C.; Ma, W. Emission Patterns of Biogenic Volatile Organic Compounds from Dominant Forest Species in Beijing, China. J. Environ. Sci. 2020, 95, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Sun, Z.; Kännaste, A.; Guo, M.; Zhou, G.; Niinemets, Ü. Isoprenoid and Aromatic Compound Emissions in Relation to Leaf Structure, Plant Growth Form and Species Ecology in 45 East-Asian Urban Subtropical Woody Species. Urban For. Urban Green. 2020, 53, 126705. [Google Scholar] [CrossRef]
- Fares, S.; Gentner, D.R.; Park, J.-H.; Ormeno, E.; Karlik, J.; Goldstein, A.H. Biogenic Emissions from Citrus Species in California. Atmos. Environ. 2011, 45, 4557–4568. [Google Scholar] [CrossRef]
- Aaltonen, H.; Pumpanen, J.; Pihlatie, M.; Hakola, H.; Hellen, H.; Kulmala, L.; Vesala, T.; Back, J. Boreal Pine Forest Floor Biogenic Volatile Organic Compound Emissions Peak in Early Summer and Autumn. Agric. For. Meteorol. 2011, 151, 682–691. [Google Scholar] [CrossRef]
- Peñuelas, J.; Llusià, J. Plant VOC Emissions: Making Use of the Unavoidable. Trends Ecol. Evol. 2004, 19, 402–404. [Google Scholar] [CrossRef] [PubMed]
- Jardine, K.J.; Meyers, K.; Abrell, L.; Alves, E.G.; Yanez Serrano, A.M.; Kesselmeier, J.; Karl, T.; Guenther, A.; Chambers, J.Q.; Vickers, C. Emissions of Putative Isoprene Oxidation Products from Mango Branches under Abiotic Stress. J. Exp. Bot. 2013, 64, 3697–3708. [Google Scholar] [CrossRef] [PubMed]
- Karl, T.G.; Christian, T.J.; Yokelson, R.J.; Artaxo, P.; Hao, W.M.; Guenther, A. The Tropical Forest and Fire Emissions Experiment: Method Evaluation of Volatile Organic Compound Emissions Measured by PTR-MS, FTIR, and GC from Tropical Biomass Burning. Atmos. Chem. Phys. 2007, 7, 5883–5897. [Google Scholar] [CrossRef]
- Guenther, A.; Hewitt, C.N.; Erickson, D.; Fall, R.; Geron, C.; Graedel, T.; Harley, P.; Klinger, L.; Lerdau, M.; Mckay, W.A.; et al. Global Model of Natural Volatile Organic Compound Emission. J. Geophys. Res. 1995, 100, 8873–8892. [Google Scholar] [CrossRef]
- Messina, P.; Lathière, J.; Sindelarova, K.; Vuichard, N.; Granier, C.; Messina, P.; Lathière, J.; Sindelarova, K.; Vuichard, N.; Ghattas, C.G.; et al. Global Biogenic Volatile Organic Compound Emissions in the ORCHIDEE and MEGAN Models and Sensitivity to Key Parameters. Atmos. Chem. Phys. 2016, 16, 14169–14202. [Google Scholar] [CrossRef]
- Sindelarova, K.; Granier, C.; Bouarar, I.; Guenther, A.; Tilmes, S.; Stavrakou, T.; Müller, J.F.; Kuhn, U.; Stefani, P.; Knorr, W. Global Data Set of Biogenic VOC Emissions Calculated by the MEGAN Model over the Last 30 Years. Atmos. Chem. Phys. 2014, 14, 9317–9341. [Google Scholar] [CrossRef]
- Unger, N. Human Land-Use-Driven Reduction of Forest Volatiles Cools Global Climate. Nat. Clim. Chang. 2014, 4, 907–910. [Google Scholar] [CrossRef]
- Rasmussen, R.A.; Went, F.W. Volatile Organic Material of Plant Origin in The Atmosphere. Proc. Natl. Acad. Sci. USA 1965, 53, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.-F. Geographical Distribution and Seasonal Variation of Surface Emissions and Deposition Velocities of Atmospheric Trace Gases. J. Geophys. Res. 1992, 97, 3787–3804. [Google Scholar] [CrossRef]
- Fehsenfeld, F.; Calvert, J.; Fall, R.; Goldan, P.; Guenther, A.B.; Hewitt, C.N.; Lamb, B.; Liu, S.; Trainer, M.; Westberg, H.; et al. Emissions of Volatile Organic Compounds from Vegetation and The Implications for Atmospheric Chemistry. Glob. Biogeochem. Cycles 1992, 6, 389–430. [Google Scholar] [CrossRef]
- Henrot, A.-J.; Stanelle, T.; Schröder, S.; Siegenthaler, C.; Taraborrelli, D.; Schultz, M.G. Implementation of the MEGAN (v2.1) Biogenic Emission Model in the ECHAM6-HAMMOZ Chemistry Climate Model. Geosci. Model Dev. 2017, 10, 903–926. [Google Scholar] [CrossRef]
- Tao, Z.; Jain, A.K. Modeling of Global Biogenic Emissions for Key Indirect Greenhouse Gases and Their Response to Atmospheric CO2 Increases and Changes in Land Cover and Climate. J. Geophys. Res. 2005, 110, 1–13. [Google Scholar] [CrossRef]
- Lathiere, J.; Hauglustaine, D.A.; Friend, A.D.; De Noblet-Ducoudre, N.; Viovy, N.; Folberth, G.A. Impact of Climate Variability and Land Use Changes on Global Biogenic Volatile Organic Compound Emissions. Atmos. Chem. Phys. 2006, 6, 2129–2146. [Google Scholar] [CrossRef]
- Weber, J.; King, J.A.; Sindelarova, K.; Martin, M.V. Updated Isoprene and Terpene Emission Factors for the Interactive BVOC (iBVOC) Emission Scheme in the United Kingdom Earth System Model (UKESM1.0). Geosci. Model Dev. 2023, 16, 3083–3101. [Google Scholar] [CrossRef]
- Szogs, S.; Arneth, A.; Anthoni, P.; Doelman, J.C.; Humpenöder, F.; Popp, A.; Pugh, T.A.; Stehfest, E. Impact of LULCC on the Emission of BVOCs during the 21st Century. Atmos. Environ. 2017, 165, 73–87. [Google Scholar] [CrossRef]
- Hantson, S.; Knorr, W.; Schurgers, G.; Pugh, T.A.M.; Arneth, A. Global Isoprene and Monoterpene Emissions under Changing Climate, Vegetation, CO2 and Land Use. Atmos. Environ. 2017, 155, 35–45. [Google Scholar] [CrossRef]
- Arneth, A.; Monson, R.K.; Schurgers, G.; Niinemets, Ü.; Palmer, P.I. Why Are Estimates of Global Terrestrial Isoprene Emissions so Similar (and Why Is This Not so for Monoterpenes)? Atmos. Chem. Phys. 2008, 8, 4605–4620. [Google Scholar] [CrossRef]
- Navarro, J.C.A.; Smolander, S.; Struthers, H.; Zorita, E.; Ekman, A.M.L.; Kaplan, J.O.; Guenther, A.; Arneth, A.; Riipinen, I. Global Emissions of Terpenoid VOCs from Terrestrial Vegetation in the Last Millennium. J. Geophys. Res. Atmos. 2014, 119, 6867–6885. [Google Scholar] [CrossRef] [PubMed]
- Vella, R.; Forrest, M.; Lelieveld, J.; Tost, H. Isoprene and Monoterpene Simulations Using the Chemistry–Climate Model EMAC (v2.55) with Interactive Vegetation from LPJ-GUESS (v4.0). Geosci. Model Dev. 2023, 16, 885–906. [Google Scholar] [CrossRef]
- Harper, K.L.; Unger, N. Global Climate Forcing Driven by Altered BVOC Fluxes from 1990 to 2010 Land Cover Change in Maritime Southeast Asia. Atmos. Chem. Phys. 2018, 18, 16931–16952. [Google Scholar] [CrossRef]
- Wang, H.; Wu, Q.; Liu, H.; Wang, Y.; Cheng, H.; Wang, R.; Wang, L.; Xiao, H.; Yang, X. Sensitivity of Biogenic Volatile Organic Compound Emissions to Leaf Area Index and Land Cover in Beijing. Atmos. Chem. Phys. 2018, 18, 9583–9596. [Google Scholar] [CrossRef]
- Arneth, A.; Miller, P.A.; Scholze, M.; Hickler, T.; Schurgers, G.; Smith, B.; Prentic, I.C. CO2 Inhibition of Global Terrestrial Isoprene Emissions: Potential Implications for Atmospheric Chemistry. Geophys. Res. Lett. 2007, 34, L18813. [Google Scholar] [CrossRef]
- Wang, H.; Wu, Q.; Guenther, A.B.; Yang, X.; Wang, L.; Xiao, T.; Li, J.; Feng, J.; Xu, Q.; Cheng, H. A Long-Term Estimation of Biogenic Volatile Organic Compound (BVOC) Emission in China from 2001–2016: The Roles of Land Cover Change and Climate Variability. Atmos. Chem. Phys. 2021, 21, 4825–4848. [Google Scholar] [CrossRef]
- Li, L.; Yang, W.; Xie, S.; Wu, Y. Estimations and Uncertainty of Biogenic Volatile Organic Compound Emission Inventory in China for 2008–2018. Sci. Total Environ. 2020, 733, 139301. [Google Scholar] [CrossRef] [PubMed]
- Li, L.Y.; Xie, S.D. Historical Variations of Biogenic Volatile Organic Compound Emission Inventories in China, 1981–2003. Atmos. Environ. 2014, 95, 185–196. [Google Scholar] [CrossRef]
- Li, J.; Li, L.Y.; Wu, R.R.; Li, Y.Q.; Bo, Y.; Xie, S.D. Inventory of Highly Resolved Temporal and Spatial Volatile Organic Compounds Emission in China. WIT Trans. Ecol. Environ. 2016, 207, 79–86. [Google Scholar] [CrossRef]
- Fu, Y.; Liao, H. Simulation of the Interannual Variations of Biogenic Emissions of Volatile Organic Compounds in China: Impacts on Tropospheric Ozone and Secondary Organic Aerosol. Atmos. Environ. 2012, 59, 170–185. [Google Scholar] [CrossRef]
- Li, L.Y.; Chen, Y.; Xie, S.D. Spatio-Temporal Variation of Biogenic Volatile Organic Compounds Emissions in China. Environ. Pollut. 2013, 182, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Cao, J.; Hao, Y. Spatial and Species-Specific Responses of Biogenic Volatile Organic Compound (BVOC) Emissions to Elevated Ozone from 2014–2020 in China. Sci. Total Environ. 2023, 868, 161636. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Yang, X.; Chen, D.; Gu, S.; Lu, Y.; Jiang, Q.; Wang, K.; Ou, Y.; Qian, Y.; Shao, P.; et al. Estimation of Biogenic VOC Emissions and Their Corresponding Impact on Ozone and Secondary Organic Aerosol Formation in China. Atmos. Res. 2020, 231, 104656. [Google Scholar] [CrossRef]
- Bao, X.; Zhou, W.; Xu, L.; Zheng, Z. A Meta-Analysis on Plant Volatile Organic Compound Emissions of Different Plant Species and Responses to Environmental Stress. Environ. Pollut. 2023, 318, 120886. [Google Scholar] [CrossRef] [PubMed]
- Smiatek, G.; Steinbrecher, R. Temporal and Spatial Variation of Forest VOC Emissions in Germany in the Decade 1994–2003. Atmos. Environ. 2006, 40, 166–177. [Google Scholar] [CrossRef]
- Zhao, J.; Bai, Y.; Wang, Z.; Zhang, S. Studies on the Emission Rates of Plants VOCs in China. China Environ. Sci. 2004, 24, 654–657. [Google Scholar]
- Duan, C.; Wu, Z.; Liao, H.; Ren, Y. Interaction Processes of Environment and Plant Ecophysiology with BVOC Emissions from Dominant Greening Trees. Forests 2023, 14, 523. [Google Scholar] [CrossRef]
- Thoss, V.; O’ Reilly-Wapstra, J.; Iason, G.R. Assessment and Implications of Intraspecific and Phenological Variability in Monoterpenes of Scots Pine (Pinus sylvestris) Foliage. J. Chem. Ecol. 2007, 33, 477–491. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-J.; Huang, Y.-L.; Chen, Y.-H.; Chang, S.-T.; Yeh, T.-F. Biogenic Volatile Organic Compounds and Protein Expressions of Chamaecyparis formosensis and Chamaecyparis obtusa var. formosana Leaves under Different Light Intensities and Temperatures. Plants 2022, 11, 1535. [Google Scholar] [CrossRef]
- Mutanda, I.; Saitoh, S.; Inafuku, M.; Aoyama, H.; Takamine, T.; Satou, K.; Akutsu, M.; Teruya, K.; Tamotsu, H.; Shimoji, M.; et al. Gene Expression Analysis of Disabled and Re-Induced Isoprene Emission by the Tropical Tree Ficus septica before and after Cold Ambient Temperature Exposure. Tree Physiol. 2016, 36, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Wuyun, T.; Hõrak, H.; Liu, B.; Talts, E.; Kilk, K.; Kaurilind, E.; Li, C.; Zhang, L.; Niinemets, Ü. Impacts of Methyl Jasmonate on Selaginella martensii: Volatiles, Transcriptomics, Phytohormones, and Gas Exchange. J. Exp. Bot. 2023, 74, 889–908. [Google Scholar] [CrossRef] [PubMed]
- Iwasa, Y.; Hayashi, R.; Satake, A. Optimal Seasonal Schedule for Producing Biogenic Volatile Organic Compounds for Tree Defense. J. Theor. Biol. 2025, 596, 111986. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.-J.; Armendariz, A.; Son, Y.-S.; Kim, J.-C. Seasonal Variations of Isoprene Emissions from Five Oak Tree Species in East Asia. Atmos. Environ. 2011, 45, 2202–2210. [Google Scholar] [CrossRef]
- Karlsson, T.; Rinnan, R.; Holst, T. Variability of BVOC Emissions from Commercially Used Willow (Salix spp.) Varieties. Atmosphere 2020, 11, 356. [Google Scholar] [CrossRef]
- Kim, J.-C.; Kim, K.-J.; Kim, D.-S.; Han, J.-S. Seasonal Variations of Monoterpene Emissions from Coniferous Trees of Different Ages in Korea. Chemosphere 2005, 59, 1685–1696. [Google Scholar] [CrossRef] [PubMed]
- Hakola, H.; Taipale, D.; Praplan, A.; Schallhart, S.; Thomas, S.; Tykkä, T.; Helin, A.; Bäck, J.; Hellén, H. Emissions of Volatile Organic Compounds from Norway Spruce and Potential Atmospheric Impacts. Front. For. Glob. Change 2023, 6, 1116414. [Google Scholar] [CrossRef]
- Baggesen, N.; Li, T.; Seco, R.; Holst, T.; Michelsen, A.; Rinnan, R. Phenological Stage of Tundra Vegetation Controls Bidirectional Exchange of BVOCs in a Climate Change Experiment on a Subarctic Heath. Glob. Change Biol. 2021, 27, 2928–2944. [Google Scholar] [CrossRef] [PubMed]
- Dani, K.G.S.; Fineschi, S.; Michelozzi, M.; Trivellini, A.; Pollastri, S.; Loreto, F. Diversification of Petal Monoterpene Profiles during Floral Development and Senescence in Wild Roses: Relationships among Geraniol Content, Petal Colour, and Floral Lifespan. Oecologia 2021, 197, 957–969. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, S.N.; Niwa, S.; Mochizuki, T.; Tani, A.; Kusumoto, D.; Utsumi, Y.; Enoki, T.; Hiura, T. Seasonal Variation in Basal Emission Rates and Composition of Mono- and Sesquiterpenes Emitted from Dominant Conifers in Japan. Atmos. Environ. 2013, 69, 124–130. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Tan, Y.; Tan, Y.; Bai, J.; Gu, D.; Ma, Z.; Du, J.; Han, Z. Effects of Light on the Emissions of Biogenic Isoprene and Monoterpenes: A Review. Atmos. Pollut. Res. 2022, 13, 101397. [Google Scholar] [CrossRef]
- Kivimäenpää, M.; Riikonen, J.; Valolahti, H.; Elina, H.; Holopainen, J.K.; Holopainen, T. Effects of Elevated Ozone and Warming on Terpenoid Emissions and Concentrations of Norway Spruce Depend on Needle Phenology and Age. Tree Physiol. 2022, 42, 1570–1586. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Holopainen, J.K.; Kivimaenpaa, M.; Virtanen, A.; Blande, J.D. Potential of Climate Change and Herbivory to Affect the Release and Atmospheric Reactions of BVOCs from Boreal and Subarctic Forests. Molecules 2021, 26, 2283. [Google Scholar] [CrossRef] [PubMed]
- Tiiva, P.; Faubert, P.; Michelsen, A.; Holopainen, T.; Holopainen, J.K.; Rinnan, R. Climatic Warming Increases Isoprene Emission from a Subarctic Heath. New Phytol. 2008, 180, 853–863. [Google Scholar] [CrossRef] [PubMed]
- Miyama, T.; Morishita, T.; Kominami, Y.; Noguchi, H.; Yasuda, Y.; Yoshifuji, N.; Okano, M.; Yamanoi, K.; Mizoguchi, Y.; Takanashi, S.; et al. Increases in Biogenic Volatile Organic Compound Concentrations Observed after Rains at Six Forest Sites in Non-Summer Periods. Atmosphere 2020, 11, 1381. [Google Scholar] [CrossRef]
- Mu, Z.; Llusià, J.; Zeng, J.; Zhang, Y.; Asensio, D.; Yang, K.; Yi, Z.; Wang, X.; Peñuelas, J. An Overview of the Isoprenoid Emissions from Tropical Plant Species. Front. Plant Sci. 2022, 13, 833030. [Google Scholar] [CrossRef] [PubMed]
- Lantz, A.T.; Allman, J.; Weraduwage, S.M.; Sharkey, T.D. Control of Rate and Physiological Role of Isoprene Emission from Plants. Plant Cell Environ. 2019, 42, 2808–2826. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Guenther, A.; Turnipseed, A.; Duhl, T.; Greenberg, J. Seasonal and Interannual Variations in Whole-Ecosystem BVOC Emissions from a Subtropical Plantation in China. Atmos. Environ. 2017, 161, 176–190. [Google Scholar] [CrossRef]
- Staudt, M.; Lhoutellier, L. Monoterpene and Sesquiterpene Emissions from Quercus coccifera Exhibit Interacting Responses to Light and Temperature. Biogeosciences 2011, 8, 2757–2771. [Google Scholar] [CrossRef]
- Li, L.; Guenther, A.B.; Gu, D.; Roger, S.; Sanjeevi, N. Impact of Short-Term Drought Stress on Volatile Organic Compounds Emissions from Pinus massoniana. China Environ. Sci. 2020, 40, 3776–3780. [Google Scholar]
- Yang, W.; Zhang, B.; Wu, Y.; Liu, S.; Kong, F.; Li, L. Effects of Soil Drought and Nitrogen Deposition on BVOC Emissions and Their O3 and SOA Formation for Pinus thunbergii. Environ. Pollut. 2023, 316, 120693. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Du, L.; Tsona, N.T.; Ge, M. Anthropogenic Effects on Biogenic Secondary Organic Aerosol Formation. Adv. Atmos. Sci. 2021, 38, 1053–1084. [Google Scholar] [CrossRef]
- Touhami, D.; Mofikoya, A.O.; Girling, R.D.; Langford, B.; Misztal, P.K.; Pfrang, C. Atmospheric Degradation of Ecologically Important Biogenic Volatiles: Investigating the Ozonolysis of (E)-β-Ocimene, Isomers of α and β-Farnesene, α-Terpinene and 6-Methyl-5-Hepten-2-One, and Their Gas-Phase Products. J. Chem. Ecol. 2024, 50, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Gagan, S.; Sarang, K.; Rudzinski, K.J.; Liu, R.; Szmigielski, R.; Zhang, Y. Synthetic Strategies for Oxidation Products from Biogenic Volatile Organic Compounds in the Atmosphere: A Review. Atmos. Environ. 2023, 312, 120017. [Google Scholar] [CrossRef]
- Fitzky, A.C.; Kaser, L.; Peron, A.; Karl, T.; Graus, M.; Tholen, D.; Halbwirth, H.; Trimmel, H.; Pesendorfer, M.; Rewald, B.; et al. Same, Same, but Different: Drought and Salinity Affect BVOC Emission Rate and Alter Blend Composition of Urban Trees. Urban For. Urban Green. 2023, 80, 127842. [Google Scholar] [CrossRef]
- Ghirardo, A.; Xie, J.; Zheng, X.; Wang, Y.; Grote, R.; Block, K.; Wildt, J.; Mentel, T.; Kiendler-Scharr, A.; Hallquist, M.; et al. Urban Stress-Induced Biogenic VOC Emissions and SOA-Forming Potentials in Beijing. Atmos. Chem. Phys. 2016, 16, 2901–2920. [Google Scholar] [CrossRef]
- Riva, M.; Heikkinen, L.; Bell, D.M.; Peräkylä, O.; Zha, Q.; Schallhart, S.; Rissanen, M.P.; Imre, D.; Petäjä, T.; Thornton, J.A.; et al. Chemical Transformations in Monoterpene-Derived Organic Aerosol Enhanced by Inorganic Composition. npj Clim. Atmos. Sci. 2019, 2, 2. [Google Scholar] [CrossRef]
- Khalaj, F.; Rivas-Ubach, A.; Anderton, C.R.; China, S.; Mooney, K.; Faiola, C.L. Acyclic Terpenes Reduce Secondary Organic Aerosol Formation from Emissions of a Riparian Shrub. ACS Earth Space Chem. 2021, 5, 1242–1253. [Google Scholar] [CrossRef]
- Zhao, D.; Schmitt, S.H.; Wang, M.; Acir, I.-H.; Tillmann, R.; Tan, Z.; Novelli, A.; Fuchs, H.; Pullinen, I.; Wegener, R.; et al. Effects of NOx and SO2 on the Secondary Organic Aerosol Formation from Photooxidation of α-Pinene and Limonene. Atmos. Chem. Phys. 2018, 18, 1611–1628. [Google Scholar] [CrossRef]
- Pinto, D.M.; Blande, J.D.; Souza, S.R.; Nerg, A.-M.; Holopainen, J.K. Plant Volatile Organic Compounds (VOCs) in Ozone (O3) Polluted Atmospheres: The Ecological Effects. J. Chem. Ecol. 2010, 36, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Laaksonen, A.; Kulmala, M.; O’Dowd, C.D.; Joutsensaari, J.; Vaattovaara, P.; Mikkonen, S.; Lehtinen, K.E.J.; Sogacheva, L.; Maso, M.D.; Aalto, P.; et al. The Role of VOC Oxidation Products in Continental New Particle Formation. Atmos. Chem. Phys. 2008, 8, 2657–2665. [Google Scholar] [CrossRef]
- Claeys, M.; Graham, B.; Vas, G.; Wang, W.; Vermeylen, R.; Pashynska, V.; Cafmeyer, J.; Guyon, P.; Andreae, M.O.; Artaxo, P.; et al. Formation of Secondary Organic Aerosols through Photooxidation of Isoprene. Sci. Am. Assoc. Adv. Sci. 2004, 303, 1173–1176. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, J. Measurements of Total Ozone Reactivity in a Suburban Forest in Japan. Atmos. Environ. 2021, 246, 117990. [Google Scholar] [CrossRef]
- VanReken, T.M.; Greenberg, J.P.; Harley, P.C.; Guenther, A.B.; Smith, J.N. Direct Measurement of Particle Formation and Growth from the Oxidation of Biogenic Emissions. Atmos. Chem. Phys. 2006, 12, 4403–4413. [Google Scholar] [CrossRef]
- Tunved, P.; Hansson, H.C.; Kerminen, V.M.; Strom, J.; Dal Maso, M.; Lihavainen, H.; Viisanen, Y.; Aalto, P.P.; Komppula, M.; Kulmala, M. High Natural Aerosol Loading over Boreal Forests. Science 2006, 312, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Mentel, T.F.; Wildt, J.; Kiendler-Scharr, A.; Kleist, E.; Tillmann, R.; Maso, M.D.; Fisseha, R.; Hohaus, T.; Spahn, H.; Uerlings, R.; et al. Photochemical Production of Aerosols from Real Plant Emissions. Atmos. Chem. Phys. 2009, 13, 4387–4406. [Google Scholar] [CrossRef]
- Naik, V.; Delire, C.; Wuebbles, D.J. Sensitivity of Global Biogenic Isoprenoid Emissions to Climate Variability and Atmospheric CO2. J. Geophys. Res. Atmos. 2004, 109, D06301. [Google Scholar] [CrossRef]
- Han, Z.; Zhang, Y.; Zhang, H.; Ge, X.; Gu, D.; Liu, X.; Bai, J.; Ma, Z.; Tan, Y.; Zhu, F.; et al. Impacts of Drought and Rehydration Cycles on Isoprene Emissions in Populus Nigra Seedlings. Int. J. Environ. Res. Public Health 2022, 19, 14528. [Google Scholar] [CrossRef] [PubMed]
- Saunier, A.; Ormeño, E.; Wortham, H.; Temime-Roussel, B.; Lecareux, C.; Boissard, C.; Fernandez, C. Chronic Drought Decreases Anabolic and Catabolic BVOC Emissions of Quercus Pubescens in a Mediterranean Forest. Front. Plant Sci. 2017, 8, 71. [Google Scholar] [CrossRef] [PubMed]
- Yue, X.; Unger, N.; Zheng, Y. Distinguishing the Drivers of Trends in Land Carbon Fluxes and Plant Volatile Emissions over the Past 3 Decades. Atmos. Chem. Phys. 2015, 15, 11931–11948. [Google Scholar] [CrossRef]
- Schurgers, G.; Hickler, T.; Miller, P.A.; Arneth, A. European Emissions of Isoprene and Monoterpenes from the Last Glacial Maximum to Present. Biogeosciences 2009, 6, 2779–2797. [Google Scholar] [CrossRef]
- Feng, Z.; Yuan, X.; Fares, S.; Loreto, F.; Li, P.; Hoshika, Y.; Paoletti, E. Isoprene Is More Affected by Climate Drivers than Monoterpenes: A Meta-analytic Review on Plant Isoprenoid Emissions. Plant Cell Environ. 2019, 42, 1939–1949. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, R.P.; Kivimäenpää, M.; Kasurinen, A.; Häikiö, E.; Holopainen, T.; Holopainen, J.K. Herbivore-Induced BVOC Emissions of Scots Pine under Warming, Elevated Ozone and Increased Nitrogen Availability in an Open-Field Exposure. Agric. For. Meteorol. 2017, 242, 21–32. [Google Scholar] [CrossRef]
- Peñuelas, J.; Staudt, M. BVOCs and Global Change. Trends Plant Sci. 2010, 15, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Loreto, F.; Sharkey, T.D. A Gas-Exchange Study of Photosynthesis and Isoprene Emission in Quercus rubra L. Planta 1990, 182, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, C.; Guenther, A.B.; Park, J.-H.; Seco, R.; Alves, E.; Batalha, S.; Santana, R.; Kim, S.; Smith, J.; Tóta, J.; et al. PTR-TOF-MS Eddy Covariance Measurements of Isoprene and Monoterpene Fluxes from an Eastern Amazonian Rainforest. Atmos. Chem. Phys. 2020, 20, 7179–7191. [Google Scholar] [CrossRef]
- Ye, C.; Yuan, B.; Lin, Y.; Wang, Z.; Hu, W.; Li, T.; Chen, W.; Wu, C.; Wang, C.; Huang, S.; et al. Chemical Characterization of Oxygenated Organic Compounds in the Gas Phase and Particle Phase Using Iodide CIMS with FIGAERO in Urban Air. Atmos. Chem. Phys. 2021, 21, 8455–8478. [Google Scholar] [CrossRef]
- Shang, F.; Yin, L.; Liu, M.; Liu, B.; Xu, T.; Li, M.; Cai, X.; Kang, L.; Zhang, H.; Yue, X.; et al. Impact of Oversimplified Parameters on BVOC Emissions Estimation in China: A Sensitivity Analysis Using the WRF-CLM4-MEGAN Model. J. Geophys. Res. Biogeosciences 2024, 129, e2024JG008038. [Google Scholar] [CrossRef]
- Zhang, C.; Guo, Y.; Shen, H.; Luo, H.; Pullinen, I.; Schmitt, S.H.; Wang, M.; Fuchs, H.; Kiendler Scharr, A.; Wahner, A.; et al. Contrasting Influence of Nitrogen Oxides on the Cloud Condensation Nuclei Activity of Monoterpene-Derived Secondary Organic Aerosol in Daytime and Nighttime Oxidation. Geophys. Res. Lett. 2023, 50, e2022GL102110. [Google Scholar] [CrossRef]
- Sporre, M.K.; Blichner, S.M.; Karset, I.H.H.; Makkonen, R.; Berntsen, T.K. BVOC-Aerosol-Climate Feedbacks Investigated Using NorESM. Atmos. Chem. Phys. 2019, 19, 4763–4782. [Google Scholar] [CrossRef]
- Lindwall, F.; Schollert, M.; Michelsen, A.; Blok, D.; Rinnan, R. Fourfold Higher Tundra Volatile Emissions Due to Arctic Summer Warming. J. Geophys. Res. Biogeosci. 2016, 121, 895–902. [Google Scholar] [CrossRef]
- Arneth, A.; Makkonen, R.; Olin, S.; Paasonen, P.; Holst, T.; Kajos, M.K.; Kulmala, M.; Maximov, T.; Miller, P.A.; Schurgers, G. Future Vegetation-Climate Interactions in Eastern Siberia: An Assessment of the Competing Effects of CO2 and Secondary Organic Aerosols. Atmos. Chem. Phys. 2016, 16, 5243–5262. [Google Scholar] [CrossRef]
- Bouvier-Brown, N.C.; Schade, G.W.; Misson, L.; Lee, A.; McKay, M.; Goldstein, A.H. Contributions of Biogenic Volatile Organic Compounds to Net Ecosystem Carbon Flux in a Ponderosa Pine Plantation. Atmos. Environ. 2012, 60, 527–533. [Google Scholar] [CrossRef]
- Ma, F.; Zhang, G.; Zhang, J.; Luo, X.; Liao, L.; Wang, H.; Tang, X.; Yi, Z. Isoprenoid Emissions from Schima superba and Cunninghamia lanceolata: Their Responses to Elevated Temperature by Two Warming Facilities. Sci. Total Environ. 2024, 930, 172669. [Google Scholar] [CrossRef] [PubMed]
- Gulden, L.E.; Yang, Z.-L. Development of Species-Based, Regional Emission Capacities for Simulation of Biogenic Volatile Organic Compound Emissions in Land-Surface Models: An Example from Texas, USA. Atmos. Environ. 2006, 40, 1464–1479. [Google Scholar] [CrossRef]
- Guenther, A. The Contribution of Reactive Carbon Emissions from Vegetation to the Carbon Balance of Terrestrial Ecosystems. Chemosphere 2002, 49, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Ito, A. Disequilibrium of Terrestrial Ecosystem CO2 Budget Caused by Disturbance-Induced Emissions and Non-CO2 Carbon Export Flows: A Global Model Assessment. Earth Syst. Dyn. 2019, 10, 685–709. [Google Scholar] [CrossRef]
- Nagalingam, S.; Seco, R.; Kim, S.; Guenther, A. Heat Stress Strongly Induces Monoterpene Emissions in Some Plants with Specialized Terpenoid Storage Structures. Agric. For. Meteorol. 2023, 333, 109400. [Google Scholar] [CrossRef]

| BVOC Class | Representative Compounds | Sources | Reactivity (Lifetime) |
|---|---|---|---|
| Isoprene (C5H8) | Isoprene (C5H8) | Broadleaf deciduous trees (e.g., oak, poplar) | Very high (~1.3 h) [8,59] |
| Monoterpenes (C10H16) | α-Pinene, β-Pinene, Limonene, Myrcene, Sabinene, 3-Carene, Camphene, Terpinolene | Coniferous trees (e.g., pine, spruce) | Moderate to high (~1–10 h) [14,59] |
| Sesquiterpenes (C15H24) | β-Caryophyllene, α-Humulene, Germacrene D, Longifolene, Farnesene, Bisabolene, Nerolidol | Tropical trees, grasses, and some shrubs | Extremely high (minutes to <1 h) [9] |
| Oxygenated Terpenoids | Linalool, 1,8-Cineole, Terpineol, Borneol, Geraniol, Verbenone | Flowering species, stress-induced emissions | Variable (hours to days) [10] |
| Green Leaf Volatiles (GLVs) | (Z)-3-Hexenal, (E)-2-Hexenal, (Z)-3-Hexen-1-ol, Hexanal, Hexenyl acetate | Leaf damage | High (minutes to a few hours) [64] |
| Aromatic Compounds | Benzaldehyde, Methyl salicylate, Styrene, p-Cymene, Eugenol, Cresol [65] | Floral emissions, understory vegetation | Variable [65] |
| Small Oxygenated VOCs | Methanol, Ethanol, Acetone, Acetaldehyde, Formaldehyde, Acetic acid | leaf development | Low (days) [66,67] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pei, D.; Wang, A.; Shen, L.; Wu, J. Research on the Emission of Biogenic Volatile Organic Compounds from Terrestrial Vegetation. Atmosphere 2025, 16, 885. https://doi.org/10.3390/atmos16070885
Pei D, Wang A, Shen L, Wu J. Research on the Emission of Biogenic Volatile Organic Compounds from Terrestrial Vegetation. Atmosphere. 2025; 16(7):885. https://doi.org/10.3390/atmos16070885
Chicago/Turabian StylePei, Dingyi, Anzhi Wang, Lidu Shen, and Jiabing Wu. 2025. "Research on the Emission of Biogenic Volatile Organic Compounds from Terrestrial Vegetation" Atmosphere 16, no. 7: 885. https://doi.org/10.3390/atmos16070885
APA StylePei, D., Wang, A., Shen, L., & Wu, J. (2025). Research on the Emission of Biogenic Volatile Organic Compounds from Terrestrial Vegetation. Atmosphere, 16(7), 885. https://doi.org/10.3390/atmos16070885







