Abstract
The environmental impact of livestock by-products presents significant challenges, particularly in regions with intensive farming and high pollution levels, such as the Po Valley. This study evaluated the effectiveness of biochar and wood vinegar in reducing gaseous emissions during the laboratory-scale storage of livestock slurry, digestate, and liquid fractions. Various types and applications of biochar, both with and without wood vinegar, were tested across three independent incubation periods of varying durations. The results showed that ammonia (NH3) emissions were lower from slurry compared to raw digestate and the liquid fraction, while methane (CH4) emissions exhibited the opposite trend. Pyrolysis biochar effectively reduced NH3 emissions by 47% on average when applied as a 5 cm surface layer. However, its effectiveness was inconsistent when mixed into the material or when produced via gasification. Biochar activated with wood vinegar significantly reduced NH3 emissions from both slurry and digestate by 61%, but it also led to increased emissions of CH4 and CO2. Nitrous oxide (N2O) emissions were detected only after at least one month of incubation and were higher when biochar was used as a cover alone or when activated with wood vinegar. Overall, applying biochar as a cover and activating it with wood vinegar proved effective in reducing NH3 emissions during the storage of livestock by-products. However, the effectiveness varied significantly depending on the type of biochar and its method of application, particularly with respect to CH4 emissions, highlighting the need for careful consideration when using wood vinegar-activated biochar.
1. Introduction
The livestock sector plays an important role in climate change, contributing 14.5% to all human-induced greenhouse gas (GHG) emissions, of which livestock waste storage and processing represent 10% [1]. The largest percentage (44%) is represented by methane (CH4) produced by enteric fermentation, whereas manure management is responsible for 5.7% and 4.3% of global livestock CH4 and nitrous oxide (N2O) emissions, respectively [2]. Additional nitrogen (N) losses occur through ammonia (NH3) emissions, which significantly harm human health and ecosystems by contributing to eutrophication, acidification, and fine particulate matter (PM2.5) formation. In Europe, 94% of NH3 emissions originate from agriculture, with livestock farming accounting for 87% of these emissions [3,4]. Manure management in the Po Valley faces distinct challenges due to high livestock density and intensive agriculture, which significantly impact air and water quality [5]. The Po Valley is highly vulnerable to nitrate leaching due to intensive agriculture and livestock density. To comply with the EU Nitrates Directive (91/676/EEC), regulations limit N application to 170 kg N/hectare in nitrate vulnerable zones (NVZs), promoting technologies like anaerobic digestion and combustion to manage manure efficiently [6].
On the other hand, livestock wastes are valuable sources of nutrients and organic matter (OM) that help maintain soil fertility and support crop production [7]. They provide readily available nutrients to crops, improve soil physical properties, stimulate soil biological activity by supplying easily degradable carbon (C) compounds, and contribute to the buildup of soil OM [8,9]. Their potential use as alternatives to mineral fertilizers presents new opportunities within a circular economy framework [10].
Currently, several techniques to mitigate emissions and preserve N during manure storage are available, including anaerobic digestion [11], composting [10], solid–liquid separation [12], and manure covers [13].
The environmental impact of these techniques is not straightforward, as gaseous emissions result from biological processes that occur under varying conditions. Effective mitigation of N losses in one form (e.g., NH3) is often offset by increased losses in other forms (e.g., nitrite, N2O, or nitrate). Similarly, reductions in CH4 emissions achieved through solid–liquid separation or aeration may lead to increased CO2 emissions or N losses [14]. Therefore, the interactive effects of different mitigation strategies on overall GHG and NH3 emissions from livestock waste must be carefully considered.
Biochar addition is a promising technique to reduce emissions from manure during storage. Studies have shown that biochar can reduce NH3 emissions from manure storage tanks, as both a floating cover and a manure additive to mitigate emissions [15,16]. Biochar’s effectiveness is influenced by its specific properties, application methods, and dosage [17]. The production process affects biochar characteristics such as particle size, surface area, porosity, pH, and nutrient content, which can in turn significantly affect its performance [18]. Pyrolysis (particularly slow pyrolysis) tends to produce biochar with larger, more structured particles due to slower decomposition and lower operating temperatures (typically 300–700 °C). Gasification operates at higher temperatures (often 700–1200 °C) with limited oxygen, leading to finer, more fragmented particles, with higher stability and surface areas.
Acidification or bio-acidification of livestock by-products are also successfully applied to reduce NH3 emissions [19]. Wood vinegar is a promising bio-acidifying agent that has been investigated in recent studies for its potential to reduce GHG and NH3 emissions and to preserve N, either on its own or in combination with biochar [20]. However, investigations on the overall environmental feedback of bio-acidification are still needed, since several interactive and contrasting effects are expected to occur [19]. Furthermore, comprehensive assessments of the net environmental impact, considering the combined effects on CH4, N2O, CO2, and NH3 emissions, remain limited, with studies reporting variable effects across different greenhouse gases [21].
The primary objective of this study was to evaluate the efficacy of different treatments in mitigating NH3 and GHG emissions during the storage of livestock by-products. We report results from three independent experiments comparing: (i) digestate versus slurry; (ii) raw digestate versus liquid fraction; (iii) biochar as a cover; (iv) biochar derived from pyrolysis versus gasification; (v) biochar application as a cover versus a mixture; and (vi) activation using wood vinegar.
The three experiments differed in lengths and treatments; therefore, not all combinations have been assessed. However, we believe the study provides a good overview of the main processes and mitigation options, considering a certain variability of tested materials.
2. Materials and Methods
2.1. Incubation Experiments
Livestock by-products were incubated in 30 L capacity tanks with hermetic seals, arranged in a completely randomized design using a random number generator. The experiment was conducted in climate-controlled greenhouses at Fondazione Minoprio. Each tank was filled with 10 L of livestock by-products, maintaining a diameter-to-height ratio representative of typical on-farm storage conditions in the Po Valley. The tanks were sealed with lids equipped with two valves for gas sampling, which were connected via tubing to a portable gas analyzer. The main characteristics of the livestock by-products and the applied mitigation treatments are summarized in Table 1.

Table 1.
Composition of livestock by-products and treatments. The following abbreviations are used: C org—Organic C; TN—Total nitrogen; N-NH4—Ammonium; H:Corg—Hydrogen–carbon ratio; Ac. Acid—Acetic acid. Bulk density of biochar was measured on raw, moist material.
Biochar produced by pyrolysis exhibited significantly larger particle sizes, with 77% exceeding 5 mm, whereas biochar from gasification was notably finer and powderier in texture. Furthermore, biochar from pyrolysis had a higher ash content (37%) than biochar from gasification (21%). Amounts of polycyclic aromatic hydrocarbons were below the maximum permissible levels for both biochar types (from pyrolysis and gasification). When activated with wood vinegar, biochar pH decreased to 7.9 and bulk density increased to 0.635 kg L−1.
The following incubations were set up in tri-replicates:
Incubation 1: Effect of biochar cover on digestate (raw and liquid fraction)
The incubation was conducted in July 2023 with 8 measurements in 14 days (0, 1, 2, 3, 4, 7, 10, 14). Eighteen tanks were filled with 10 L of livestock by-product covered with biochar from pyrolysis in layers of different heights. The tested treatments are reported in Table 2.

Table 2.
Livestock by-products and treatments tested in incubation 1.
Incubation 2: Effect of biochar cover and bio-acidification on raw digestate and slurry
The incubation was conducted in October 2023 with 15 measurements in 59 days (0, 1, 2, 4, 7, 10, 14, 17, 21, 24, 30, 38, 45, 52, 59). Eighteen tanks were filled with 10 L of livestock by-product covered with biochar from pyrolysis in a 5 cm layer. Biochar was activated with 1 L wood vinegar (BioDea, Italy, diluted with water 1:5) 2 h before the incubation start. The tested treatments are reported in Table 3.

Table 3.
Livestock by-products and treatments tested in incubation 2.
Incubation 3: Effect of pyrolysis or gasification biochar applied as cover or mixture and bio-acidification
The incubation was conducted in October 2024 with 22 measurements in 85 days (0, 1, 2, 5, 6, 7, 8, 11, 14, 19, 21, 26, 29, 32, 36, 43, 49, 57, 64, 71, 78, 85). Twenty-one tanks were filled with 10 L of livestock by-product treated with biochar from pyrolysis or gasification as cover or mixture. The gasification biochar cover and pyrolysis biochar mixture were activated with 1 L wood vinegar (BioDea, Italy, diluted with water 1:5) 2 h before the incubation start. The quantity of biochar added as cover or mixed was the same, respecting the ratio kg biochar/volume by-product = 3%, or volume biochar/volume by-product = 20%. The tested treatments are reported in Table 4.

Table 4.
Livestock by-products and treatments tested in incubation 3.
2.2. Gas Production Measurements
Gas measurements were conducted with a DX4040 FTIR Gas Analyzer (Gasmet Technologies Oy, Vantaa, Finland), which is based on infrared spectroscopy and Fourier transformation, resulting in an IR spectrum of the sample gas. The instrument is equipped with Teflon tubes with quick-connect fittings and an on-board sample pump, which has a neoprene diaphragm membrane; the maximum pressure is 1 bar and the maximum flow is 2 L min−1. The instrument simultaneously analyzes 25 gases, and was previously calibrated by the manufacturer. In our study, only H2O, CO2, CH4, N2O and NH3 fluxes were monitored. Prior to each use, the hardware status is checked, and a zero calibration is performed with pure N (99.999%). The instrument was connected to the lids with two Teflon tubes, creating a closed loop between the instrument and the tank, which allowed reliable NH3 measurements to be obtained [22]. To measure the flux for each gas, consecutive measurements of the individual gas concentrations over time were performed for 6 min, reading gas concentrations every 30 sec, for a total of 18 readings every measurement time, to measure the increase of gas concentrations within the tank. The tanks were left open between each measurement time to avoid saturation and were closed during measurements.
To convert gas concentration (ppm) into gas flux the following equation was used:
where:
Gas flux (µmol m−2 s−1) = [(dC/dt)] × [V/(Va × (T2/T1)] × 1/Vs
dC/dt is the rate of change of concentration of each gas [ppm] with time (min)
V is the volume of the vessel including cell, sample lines + headspace tank volume (free headspace was calculated for each tank)
V𝑎 is the molar volume for ideal gas at 273 K = 22.4 m3 mol−1
T1 is the air temperature (K)
T2 is the standard temperature (K)
Vs is the volume of substrate = 0.01 m3
Gas fluxes were then converted to g h−1 m−3 using molecular weight of each gas. Linear interpolation was used to calculate cumulative fluxes.
Temperature within the tanks was measured at every single measurement time with a portable instrument (Hanna thermometer, Part Code HI7661), to correct gas fluxes according to the Idela Gas Law.
pH of the substrates within the tanks was measured at every single measurement time with a pH meter (InLab 413, MP220, Mettler Toledo, Columbus, OH, USA).
2.3. Statistical Analysis
Significant differences in gas production between treatments were evaluated separately for each incubation using repeated measures ANOVA across all measurement times, followed by Fisher’s LSD post-hoc test (p < 0.05), performed with Statistica 7 software (StatSoft, Hamburg, Germany). The pH of substrates in incubations 2 and 3 was analyzed using two-way ANOVA, followed by Fisher LSD post-hoc test (p < 0.05).
Discriminant function analysis (DFA) was performed using NH3, CH4 and CO2 emissions as grouping variables. Squared Mahalanobis distances between group centroids were determined. Two significant discriminatory roots were derived and the results of DFA were graphically presented in two dimensions.
3. Results
3.1. Incubation 1
The liquid fraction of digestate (DLF) exhibited significantly higher NH3 emissions and lower CH4 and CO2 emissions (not statistically significant) compared to raw digestate (RD) throughout the incubation period (Figure 1 and Table 5). The application of a 5 cm biochar cover significantly reduced NH3 and CO2 emissions in both RD and DLF. These differences remained consistent over the entire incubation period for both gases. A non-significant increase in CH4 emissions was observed in the RD treatment with the 5 cm biochar layer after the first week. N2O emissions remained near zero, with high variability among replicates, and no significant differences were observed regardless of time, substrate, or treatment.
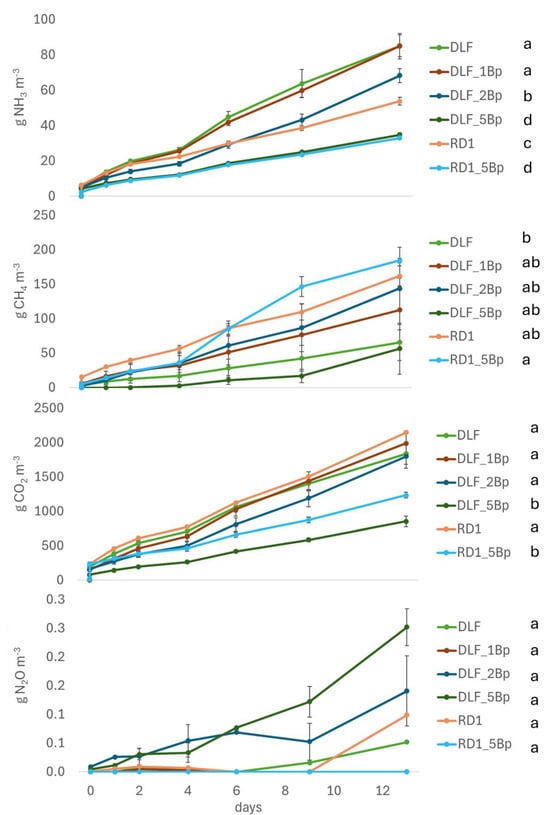
Figure 1.
Cumulative emissions of NH3, CH4, CO2 and N2O (from top to bottom) measured in incubation 1. Standard errors are reported for each measurement day. Different letters indicate significant differences between treatments at p < 0.05 (repeated ANOVA). According to Table 2, RD: Raw digestate; DLF: Digestate liquid fraction; 1Bp, 2Bp, 3Bp: cover layer (cm) of biochar from pyrolysis.

Table 5.
Percentage effects of mitigation strategies on cumulative gas production and pH at the beginning and at the end of incubations. Significant effects are reported with * (t test, p < 0.05). n.s. not significant, n.d. not determined.
3.2. Incubation 2
RD exhibited higher NH3 and CO2 emissions compared to slurry (SL), with a consistent trend throughout the incubation period (Figure 2 and Table 5). Methane (CH4) emissions were similar between the two by-products. The application of a biochar cover significantly reduced NH3 and CO2 emissions from both RD and SL as early as the first 24 h of incubation (Table 5), but had no significant effect on CH4 or N2O emissions. However, a non-significant increasing trend in CH4 emissions was observed after the first month. Wood vinegar (WV) significantly reduced NH3 emissions from both RD and SL from the beginning of the incubation period. A sharp increase in CH4 emissions from RD and SL was observed starting from the first week (p < 0.05). N2O emissions became detectable after one month in untreated RD and SL, although no significant differences were observed at any measurement time in response to either biochar or WV treatments.
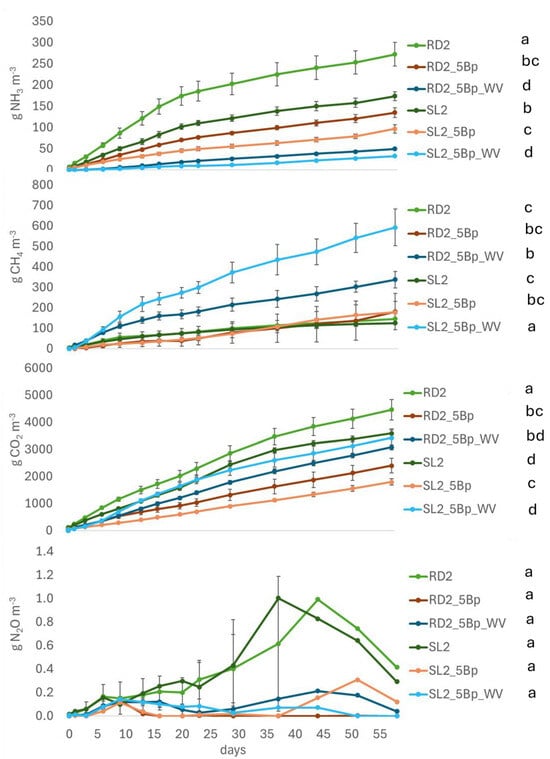
Figure 2.
Cumulative emissions of NH3, CH4, CO2 and N2O (from top to bottom) measured in incubation 2. Standard errors are reported for each measurement day. Different letters indicate significant differences between treatments at p < 0.05 (repeated ANOVA). According to Table 3, RD: Raw digestate; SL: Slurry; 5Bp: 5 cm biochar from pyrolysis; WV: Wood vinegar.
3.3. Incubation 3
Overall, incubation 3 showed higher values for all gases compared to the other incubations, even considering a comparable length. The NH3 emissions rate showed a sharp increase within the first month of storage, reaching a maximum of 14 g m−3 day−1. Afterward, the rate slowed down to approximately 7 g m−3 day−1. Pyrolysis biochar effectively reduced NH3 emissions from slurry (SL) when applied as a cover, but had no significant effect when mixed into the slurry throughout the incubation period (Figure 3 and Table 5). Methane and CO2 emissions were not significantly affected by pyrolysis biochar, either as cover or when mixed, at any measurement time. After the first month of incubation, N2O emissions showed a significant increase with pyrolysis biochar cover (p < 0.05). When added as a cover, biochar from gasification resulted in higher NH3, CH4 and CO2 emissions, compared to the pyrolysis biochar, with an opposite trend observed for N2O emissions. When mixed, biochar from gasification showed lower CH4 emissions than biochar from pyrolysis, with this trend consistent throughout the incubation period. Gasification biochar cover significantly increased CH4 and CO2 emissions after the first week of incubation. Wood vinegar had variable effects depending on its application: when combined with pyrolysis biochar cover, it reduced NH3 emissions while increasing CH4 and CO2 emissions; when combined with gasification biochar cover, it reduced NH3 and CH4 emissions but increased CO2 and N2O emissions; and when mixed with biochar, it only reduced CH4 emissions.
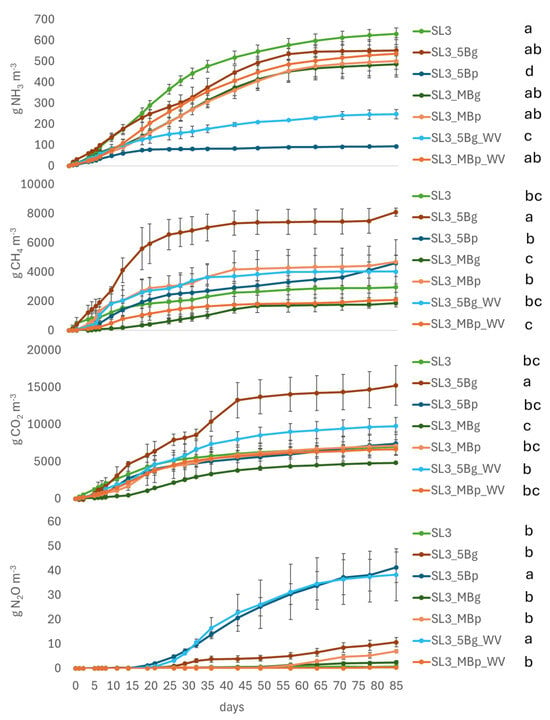
Figure 3.
Cumulative emissions of NH3, CH4, CO2 and N2O (from top to bottom) measured in incubation 3. Standard errors are reported for each measurement day. Different letters indicate significant differences between treatments at p < 0.05 (repeated ANOVA). According to Table 4, SL: Slurry; Bp: 5 cm biochar from pyrolysis: Bg: biochar from gasification; 5B: 5 cm biochar cover; MB: Biochar mixed; WV: Wood vinegar.
3.4. pH Variations
The pH of the tested by-products followed the order: DLF > RD > SL SL used in incubation 3 had significantly lower pH compared to SL used in incubation 2 (Table 1 and Table 4). At the start of the incubations, biochar cover increased the pH of RD and SL, while mixed biochar only increased pH when derived from gasification (Figure 4). pH was significantly lower at the beginning of incubation in RD and SL treated with wood vinegar, but no significant difference was observed at the end of the incubation (Figure 4).
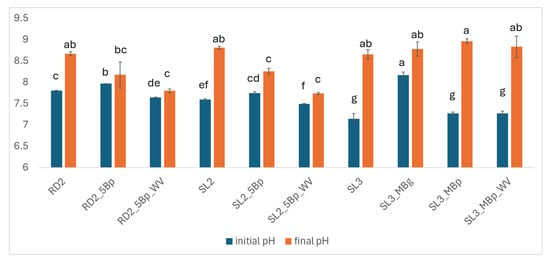
Figure 4.
pH values at the beginning and at the end of incubations 2 and 3. Standard errors are reported. Different letters indicate significant differences between treatments at p < 0.05.
3.5. Discriminant Function Analysis
Discriminant function analysis showed a clear separation between slurries used in incubation 2 and 3 and between digestates used in incubation 1 and 2 (Figure 5). Raw and liquid fractions of digestate used in incubation 1 were not different, and nor were those treated with 1 and 2 cm biochar cover. All by-products treated with a cover of 5 cm biochar from pyrolysis were well separated, forming a group with DLF, RD1, RD2 and SL2, and another group with SL3. The addition of wood vinegar further separated SL2 and RD2. By-products treated with mixed biochar from pyrolysis and with biochar from gasification formed distinct groups, with and without the addition of wood vinegar.
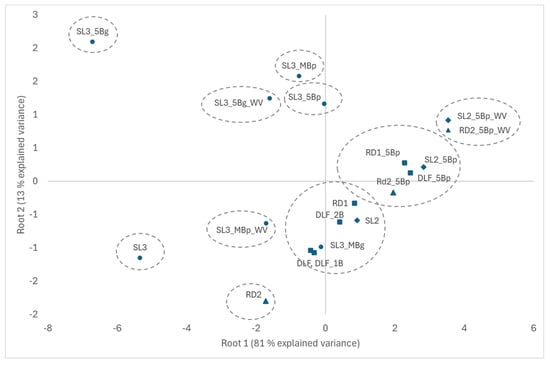
Figure 5.
Discriminant function analysis using NH3, CH4 and CO2 cumulative emissions of the three incubations at 14 days as independent variables. Explained variance of Root 1 and 2 is reported in the figure. Squared Mahalanobis distances are represented by dotted circles, indicating significant differences among treatments when not overlapping (p < 0.05).
4. Discussion
Overall, the three incubations revealed distinct patterns of gaseous emissions from livestock by-products, highlighting varied responses for each gas depending on the type of compound, storage duration, and applied treatment.
The duration of storage played a pivotal role in determining the environmental impact of livestock by-product storage. High rates of NH3, CH4, and CO2 emissions were observed during the first month of storage, aligning with previous findings by [23] for NH3 and by [24] for both NH3 and CH4. Prolonged storage also led to N2O emissions, which only became significantly different from zero after the first month in incubations 2 and 3. In line with this, several studies have reported negligible N2O emissions during short-term storage [25,26]. This suggests that reducing storage duration could be a highly effective strategy for mitigating emissions.
Processing slurry through anaerobic digestion represents a win–win strategy, offering several significant benefits: (i) it eliminates the need for prolonged storage between slurry production and soil application, (ii) it enables the utilization of a portion of organic matter as biofuel, and (iii) it produces an N-enriched material [27,28]. In fact, digestate exhibited a higher N-NH4 content, which was further concentrated in the liquid fraction (Table 1). However, NH3 emissions exhibited a distinct hierarchy: slurry < raw digestate < liquid fraction correlated to NH4 availability. Regardless of C concentration, an inverse trend emerged for both CH4 and CO2. This pattern underscores two critical requirements: (i) simultaneous measurements of all gases to ensure reliable estimates, and (ii) tailored strategies targeting the primary loss pathways for each by-product.
Biochar application is increasingly recognized as a promising strategy [28,29], particularly when activated with acids [30], for mitigating NH3 emissions during the storage of livestock by-products. This study further enhances our understanding of biochar’s effectiveness by examining the influence of biochar type and application method. Notably, the thickness of the biochar layer plays a crucial role: layers of 1 or 2 cm thickness did not significantly affect NH3 and GHG emissions, regardless of the treated by-product. However, a 5 cm thick surface layer proved effective in reducing NH3 emissions by up to 60% from the liquid fraction of digestate. The significant effect of the biochar cover was not influenced by the increase in pH, which was transient and observed only at the beginning of the incubation period. Similar results were reported by [31], who created a semi-porous crust layer over the surface of manure, affecting mass transfer to the headspace. Biochar’s porosity and sorption capacity, including its ability to absorb ammonium, are well-documented [32].
These characteristics help explain the differences in the effectiveness of pyrolysis and gasification biochar. Visual observations revealed that biochar produced via pyrolysis demonstrated superior performance, as its floating cap structure remained stable during storage, whereas the biochar derived from gasification tended to partially submerge over time. Accordingly, the cover with pyrolysis biochar proved more effective than the cover with gasification biochar in reducing NH3, CH4 and CO2 emissions. This difference can be attributed to the distinct physicochemical properties imparted by the two processes: pyrolysis biochar typically exhibits greater structural integrity and larger particle size, while gasification biochar is generally more powdery and less cohesive, leading to reduced buoyancy and stability during storage, in addition to potential problems related to being blown or moved by the wind if placed outdoors [18,33].
In contrast, when incorporated as a mixture, neither pyrolysis nor gasification biochar significantly mitigated gaseous emissions. When biochar is added as a cover, it may act as a physical barrier to gas diffusion, potentially limiting microbial activity beneath the cover. Conversely, mixing biochar into the substrate increases soil porosity and aeration, facilitating O2 diffusion throughout the substrate and supporting aerobic microbial processes. The observed increase in CH4 emissions when using gasification biochar as a surface cover, compared to its incorporation into the substrate, could be primarily attributed to the preservation of anaerobic conditions beneath the cover. These conditions, combined with the presence of nutrients and carbon, likely stimulated methanogenic communities under anaerobic conditions [34].
Pyrolysis biochar covers also increased N2O emissions from slurry at the end of incubation 3, compared to both incorporated pyrolysis biochar and gasification biochar. Limited data are available on N2O fluxes from stored livestock by-products, as long incubation periods are required to observe non-negligible fluxes [23]. N2O peaks are typically influenced by pH, oxygen, and substrate availability in localized hotspots that may have occurred in the biochar cover layer. However, based on our data, it is not possible to draw broad generalizations.
Wood vinegar-activated biochar cover was effective in reducing NH3 emissions by up to 66%, both for slurry and digestate, as reported by [20,35]. Wood vinegar is a pyrolysis by-product that is mainly constituted by acetic acid and phenols [36]. Wood vinegar demonstrated an acidification capacity (Table 1 and Table 5) that effectively reduced NH3 emissions while retaining N-NH4 content, highlighting its potential as a promising approach for producing N-rich organic fertilizers.
The surface application of wood vinegar-activated pyrolysis biochar significantly increased CH4 and CO2 emissions. Wood vinegar can stimulate methanogenesis in anaerobic environments, primarily due to its high content of readily biodegradable organics, especially acetic acid, which is a direct substrate for acetoclastic methanogenesis [37]. Furthermore, studies by [38] have shown that, at moderate organic loading rates, wood vinegar additions increase the abundance and activity of methanogenic archaea, particularly those associated with acetoclastic pathways. Slurry environments, characterized by high labile C content, abundant substrates, and anaerobic conditions, likely intensified acetoclastic methanogenesis, causing a peak in CH4 production. This CH4 surge offset NH3 mitigation efforts achieved through acidification. Notably, this trade-off occurred only with pyrolysis biochar covers, not with (i) incorporated biochar mixture or (ii) gasification biochar applications, which reduced CH4 emissions. Low H:C and high C:N ratios are reflective of biochar recalcitrance [39]. The differing effects of pyrolysis and gasification biochar when activated with wood vinegar likely arise from a complex interplay between biochar recalcitrance, substrate availability, and pH conditions. This interaction also influenced the observed increase in N2O emissions associated with wood vinegar-activated gasification biochar covers. Therefore, further research is necessary to fully elucidate the underlying mechanisms, as our current data did not provide a comprehensive explanation.
In conclusion, the results of this study suggest that using pyrolysis biochar as a cover layer, with a thickness of 5 cm (equivalent to 3% w/v), is more effective than incorporation. The pyrolysis biochar is preferable, as: (i) it provides the highest benefits, (ii) it creates a more stable cover, and (iii) the pyrolysis process produces both biochar and wood vinegar. For N-rich by-products, such as the liquid fraction of digestate, it is recommended to activate the biochar layer with wood vinegar or another organic acid. However, for slurry or substrates with high methanogenesis potential, adding acetic acid is not advised due to the potential negative impact on CH4 emissions.
Further research is warranted to explore the differences between pyrolysis and gasification biochar, as well as the potential benefits of activating biochar with wood vinegar or other organic acids. By investigating the nuances of pyrolysis versus gasification biochar and the activation process, we can unlock new pathways for improving soil health, reducing greenhouse gas emissions, and promoting a circular economy.
Author Contributions
Conceptualization, A.T., M.V. and A.L.; methodology, E.V., M.V. and S.R.; investigation, E.V., M.V., C.B., F.R. and S.R.; resources, A.T.; data curation, S.R., E.V. and A.L.; writing—original draft preparation, A.L., writing—review and editing, A.L., C.B., A.T. and M.V.; funding acquisition, A.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Regione Lombardia with Project AMMOCHAR (PSR 2014–2020, Operazione 1.2.01 of FEASR, Fondo europeo per l’agricoltura e lo sviluppo rurale).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| NH3 | Ammonia |
| CH4 | Methane |
| CO2 | Carbon dioxide |
| N2O | Nitrous oxide |
| GHG | Greenhouse gas |
| N | Nitrogen |
| PM | Particulate matter |
| NVZs | Nitrate vulnerable zones |
| OM | Organic matter |
| C | Carbon |
| C org | Organic C |
| TN | Total nitrogen |
| N-NH4 | Ammonium |
| H:Corg | Hydrogen carbon ratio |
| Ac. acid | Acetic acid |
| ANOVA | Analysis of variance |
| DFA | Discriminant function analysis |
References
- Nugrahaeningtyas, E.; Lee, J.S.; Park, K.H. Greenhouse gas emissions from livestock: Sources, estimation, and mitigation. J. Anim. Sci. Technol. 2024, 66, 1083–1098. [Google Scholar] [CrossRef]
- Gerber, P.J.; Steinfeld, H.; Henderson, B.; Mottet, A.; Opio, C.; Dijkman, J.; Falcucci, A.; Tempio, G. Tackling Climate Change Through Livestock: A Global Assessment of Emissions and Mitigation Opportunities; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2013; pp. 1–115. [Google Scholar]
- Gavrilova, O.; Leip, A.; Dong, H.; MacDonald, J.D.; Gomez Bravo, C.A.; Amon, B.; Barahona Rosales, R.; del Prado, A.; Oy-hantçabal, W.; van der Weerden, T.J.; et al. Chapter 10: Emissions from Livestock and Manure Management. In 2019 Refinement to the 2006 IPCC Guidelines for National Greenhouse Gas Inventories, Volume 4: Agriculture, Forestry and Other Land Use; IPCC National Greenhouse Gas Inventories Programme; Intergovernmental Panel on Climate Change (IPCC): Geneva, Switzerland, 2019. [Google Scholar]
- Groenestein, C.M.; Hutchings, N.J.; Haenel, H.D.; Amon, B.; Menzi, H.; Mikkelsen, M.H.; Misselbrook, T.H.; van Bruggen, C.; Kupper, T.; Webb, J. Comparison of ammonia emissions related to nitrogen use efficiency of livestock production in Europe. J. Clean. Prod. 2019, 211, 1162–1170. [Google Scholar] [CrossRef]
- Sutton, M.A.; Howard, C.M.; Erisman, J.W.; Billen, G.; Bleeker, A.; Grennfelt, P.; van Grinsven, H.; Grizzetti, B. The European Nitrogen Assessment: Sources, Effects and Policy Perspectives; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- ISPRA (Italian Institute for Environmental Protection and Research). Rapporto Rifiuti Speciali e Gestione degli Effluenti Zootecnici; ISPRA: Rome, Italy, 2023.
- Ogbuewu, I.P.; Odoemenam, V.U.; Omede, A.A.; Durunna, C.S.; Emenalom, O.O.; Uchegbu, M.C.; Ifeanyi Charles, O.; Iloeje, M.U. Livestock waste and its impact on the environment. Sci. J. Rev. 2012, 1, 17–32. [Google Scholar]
- Francioli, D.; Schulz, E.; Lentendu, G.; Wubet, T.; Buscot, F.; Reitz, T. Mineral vs. organic amendments: Microbial community structure, activity and abundance of agriculturally relevant microbes are driven by long-term fertilization strategies. Front. Microbiol. 2016, 7, 1446. [Google Scholar] [CrossRef] [PubMed]
- Zavattaro, L.; Bechini, L.; Grignani, C.; Van Evert, F.K.; Mallast, J.; Spiegel, H.; Sandén, T.; Pecio, A.; Giráldez Cervera, J.V.; Guzmán, G.; et al. Agronomic effects of bovine manure: A review of long-term European field experiments. Eur. J. Agron. 2017, 90, 127–138. [Google Scholar] [CrossRef]
- Bhunia, S.; Bhowmik, A.; Mallick, R.; Mukherjee, J. Agronomic efficiency of animal-derived organic fertilizers and their effects on biology and fertility of soil: A review. Agronomy 2021, 11, 823. [Google Scholar] [CrossRef]
- Möller, K. Effects of anaerobic digestion on soil carbon and nitrogen turnover, N emissions, and soil biological activity. A review. Agron. Sustain. Dev. 2015, 35, 1021–1041. [Google Scholar] [CrossRef]
- Carraro, G.; Tonderski, K.; Enrich-Prast, A. Solid-liquid separation of digestate from biogas plants: A systematic review of the techniques’ performance. J. Environ. Manage. 2024, 356, 120585. [Google Scholar] [CrossRef]
- Kupper, T.; Häni, C.; Neftel, A.; Kincaid, C.; Bühler, M.; Amon, B.; VanderZaag, A. Ammonia and greenhouse gas emissions from slurry storage-A review. Agric. Ecosyst. Environ. 2020, 300, 106963. [Google Scholar] [CrossRef]
- Petersen, S.O.; Sommer, S.G. Ammonia and nitrous oxide interactions: Roles of manure organic matter management. Anim. Feed Sci. Technol. 2011, 166, 503–513. [Google Scholar] [CrossRef]
- Scotto di Perta, E.; Giudicianni, P.; Mautone, A.; Grottola, C.M.; Cervelli, E.; Ragucci, R.; Pindozzi, S. An Effective bio-char application for reducing nitrogen emissions from buffalo digestate storage tank. Appl. Sci. 2024, 14, 6456. [Google Scholar] [CrossRef]
- Cao, X.; Mašek, O.; Cross, A. Biochar for ammonia removal: Mechanisms, key parameters and application. Environ. Pollut. 2020, 266, 115185. [Google Scholar]
- Sanchez-Monedero, M.A.; Cayuela, M.L.; Roig, A.; Jindo, K.; Mondini, C.; Bolan, N.J.B.T. Role of biochar as an additive in organic waste composting. Bioresour. Technol. 2018, 247, 1155–1164. [Google Scholar] [CrossRef]
- Fryda, L.; Visser, R. Biochar for Soil Improvement: Evaluation of Biochar from Gasification and Slow Pyrolysis. Agriculture 2015, 5, 1076–1115. [Google Scholar] [CrossRef]
- Sommer, S.G.; Clough, T.J.; Balaine, N.; Hafner, S.D.; Cameron, K.C. Transformation of organic matter and the emissions of methane and ammonia during storage of liquid manure as affected by acidification. J. Environ. Qual. 2017, 46, 514–521. [Google Scholar] [CrossRef]
- Wang, X.; Liu, X.; Wang, Z.; Sun, G.; Li, J. Greenhouse gas reduction and nitrogen conservation during manure composting by combining biochar with wood vinegar. J. Environ. Manag. 2022, 324, 116349. [Google Scholar] [CrossRef] [PubMed]
- Regueiro, I.; Gómez-Muñoz, B.; Lübeck, M.; Hjorth, M.; Jensen, L.S. Bio-acidification of animal slurry: Efficiency, stability and the mechanisms involved. Bioresour. Technol. Rep. 2022, 19, 101135. [Google Scholar]
- Holly, M.A.; Larson, R.A.; Powell, J.M.; Ruark, M.D.; Aguirre-Villegas, H. Greenhouse gas and ammonia emissions from digested and separated dairy manure during storage and after land application. Agric. Ecosyst. Environ. 2017, 239, 410–419. [Google Scholar] [CrossRef]
- Lee, C.; Hristov, A.N.; Cassidy, T.; Heyler, K. Nitrogen isotope fractionation and origin of ammonia nitrogen volatilized from cattle manure in simulated storage. Atmosphere 2011, 2, 256–270. [Google Scholar] [CrossRef]
- Misselbrook, T.; Hunt, J.; Perazzolo, F.; Provolo, G. Greenhouse gas and ammonia emissions from slurry storage: Impacts of temperature and potential mitigation through covering (pig slurry) or acidification (cattle slurry). J. Environ. Qual. 2016, 45, 1520–1530. [Google Scholar] [CrossRef]
- Rodhe, L.K.; Ascue, J.; Willén, A.; Persson, B.V.; Nordberg, Å. Greenhouse gas emissions from storage and field application of anaerobically digested and non-digested cattle slurry. Agric. Ecosyst. Environ. 2015, 199, 358–368. [Google Scholar] [CrossRef]
- Baral, K.R.; Jégo, G.; Amon, B.; Bol, R.; Chantigny, M.H.; Olesen, J.E.; Petersen, S.O. Greenhouse gas emissions during storage of manure and digestates: Key role of methane for prediction and mitigation. Agric. Syst. 2018, 166, 26–35. [Google Scholar] [CrossRef]
- Ahlberg-Eliasson, K.; Westerholm, M.; Isaksson, S.; Schnürer, A. Anaerobic digestion of animal manure and influence of organic loading rate and temperature on process performance, microbiology, and methane emission from digestates. Front. Energy Res. 2021, 9, 740314. [Google Scholar] [CrossRef]
- Silva, I.; Lapa, N.; Ribeiro, H.; Duarte, E. Pig Slurry Anaerobic Digestion: The Role of Biochar as an Additive Towards Biogas and Digestate Improvement. Appl. Sci. 2025, 15, 1037. [Google Scholar] [CrossRef]
- Akdeniz, N. A Systematic Review of Biochar Use in Animal Waste Composting. Waste Manag. 2019, 88, 291–300. [Google Scholar] [CrossRef]
- Baral, K.R.; McIlroy, J.; Lyons, G.; Johnston, C. The Effect of Biochar and Acid Activated Biochar on Ammonia Emissions During Manure Storage. Environ. Pollut. 2023, 317, 120815. [Google Scholar] [CrossRef]
- Maurer, D.L.; Koziel, J.A.; Kalus, K.; Andersen, D.S.; Opalinski, S. Pilot-Scale Testing of Non-Activated Biochar for Swine Manure Treatment and Mitigation of Ammonia, Hydrogen Sulfide, Odorous VOCs, and Greenhouse Gas Emissions. Sustainability 2017, 9, 929. [Google Scholar] [CrossRef]
- Wong, J.W.C.; Webber, J.B.W.; Ogbonnaya, U.O. Characteristics of Biochar Porosity by NMR and Study of Ammonium Ion Adsorption. J. Anal. Appl. Pyrolysis 2019, 143, 104687. [Google Scholar] [CrossRef]
- Brewer, C.E.; Schmidt-Rohr, K.; Satrio, J.A.; Brown, R.C. Characterization of biochar from fast pyrolysis and gasification systems. Environ. Prog. Sustain. Energy 2009, 28, 386–396. [Google Scholar] [CrossRef]
- Yu, L.; Tang, J.; Zhang, R.; Wu, Q.; Gong, M. Effects of Biochar Application on Soil Methane Emission at Different Soil Moisture Levels. Biol. Fertil. Soils 2013, 49, 119–128. [Google Scholar] [CrossRef]
- Wang, Q.; Awasthi, M.K.; Ren, X.; Zhao, J.; Li, R.; Wang, Z.; Wang, M.; Chen, H.; Zhang, Z. Combining Biochar, Zeolite and Wood Vinegar for Composting of Pig Manure: The Effect on Greenhouse Gas Emission and Nitrogen Conservation. Waste Manag. 2018, 74, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Zhang, S.; Hou, B.; Zheng, H.; Deng, W.; Liu, D.; Tang, W. Study on the Preparation of Wood Vinegar from Biomass Residues by Carbonization Process. Bioresour. Technol. 2015, 179, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Dai, K.; Wen, J.-L.; Zhang, F.; Zeng, R.J. Valuable Biochemical Production in Mixed Culture Fermentation: Fundamentals and Process Coupling. Appl. Microbiol. Biotechnol. 2017, 101, 6575–6586. [Google Scholar] [CrossRef] [PubMed]
- Hua, D.; Fan, Q.; Zhao, Y.; Xu, H.; Chen, L.; Si, H.; Li, Y. Continuous anaerobic digestion of wood vinegar wastewater from pyrolysis: Microbial diversity and functional genes prediction. Front. Bioeng. Biotechnol. 2020, 8, 923. [Google Scholar] [CrossRef]
- Bakshi, S.; Banik, C.; Laird, D.A. Quantification and Characterization of Chemically- and Thermally-Labile and Recalcitrant Biochar Fractions. Chemosphere 2018, 194, 247–255. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).