Urban Tree Species Capturing Anthropogenic Volatile Organic Compounds—Impact on Air Quality
Abstract
1. Introduction
2. Materials and Methods
2.1. Tree Species Selection
2.2. Sampling Sites
2.3. Analytical Treatment
2.4. Statistical Analysis
2.5. Estimation of Photochemical Reactivity
2.6. Reactivity Control Index
3. Results
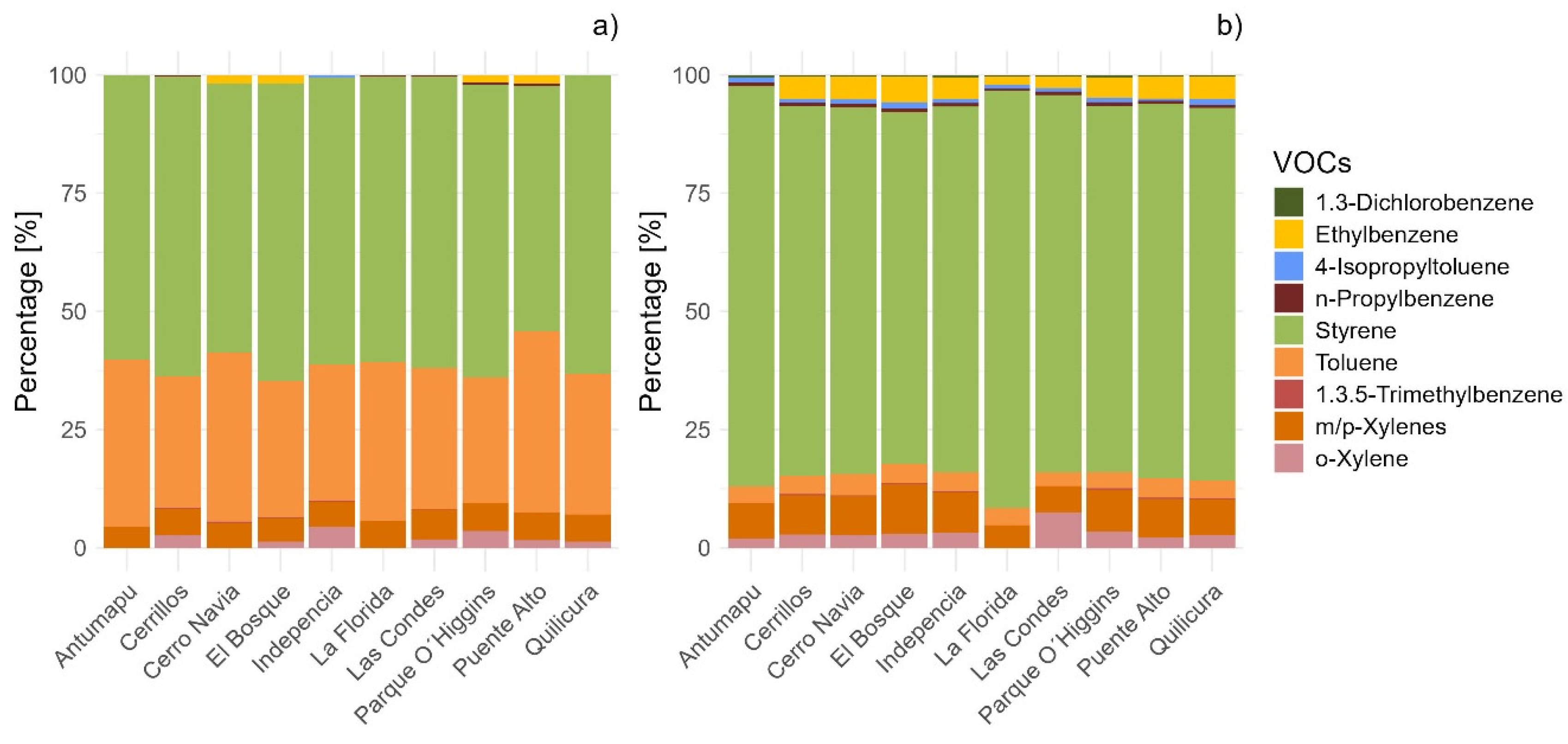
3.1. Quantification of the Anthropogenic Volatile Organic Compounds in the Foliar Material of the Two Tree Species
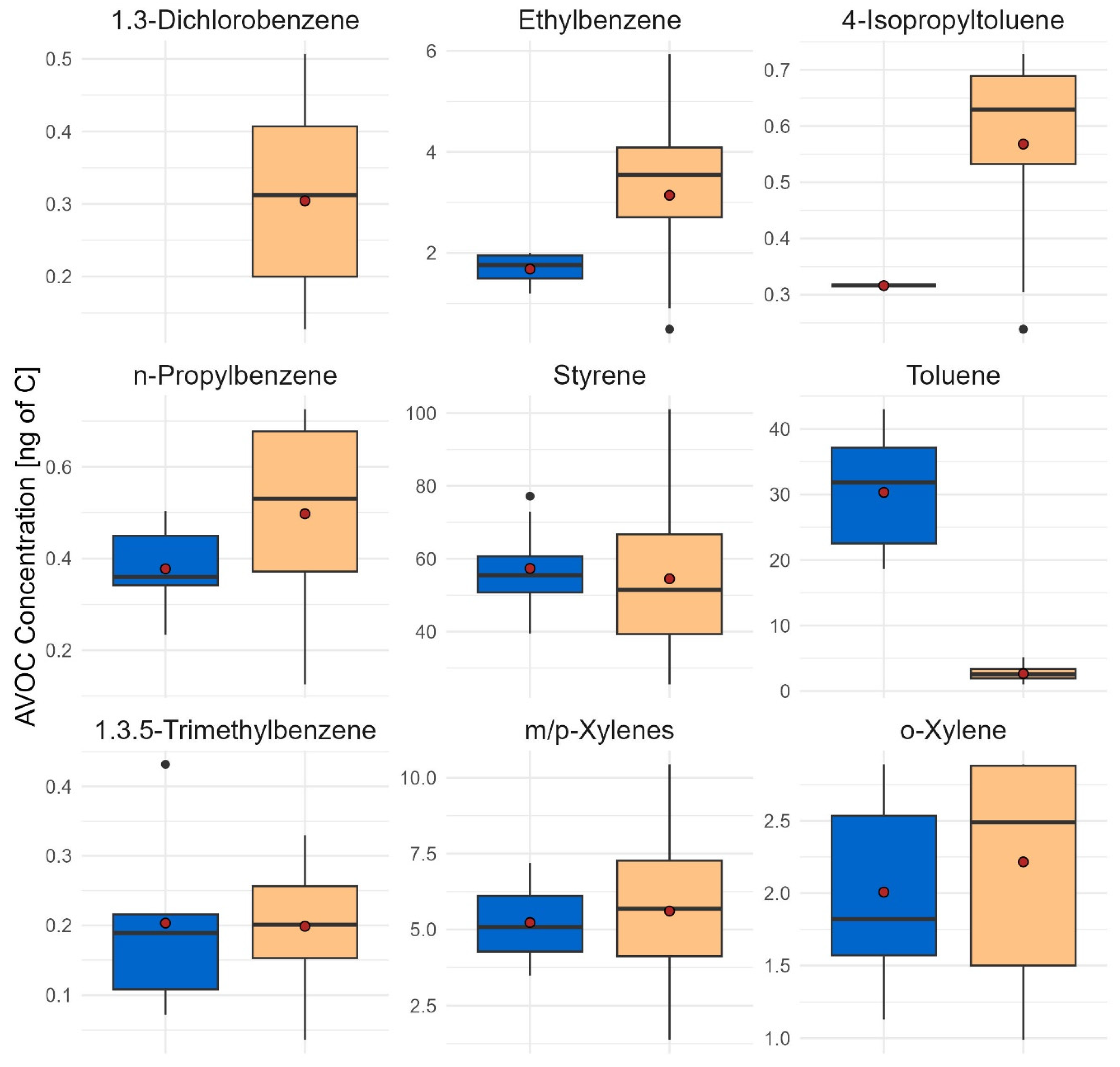
3.2. Variability in the Concentration of Anthropogenic Volatile Organic Compounds in the Foliar Material of the Two Tree Species
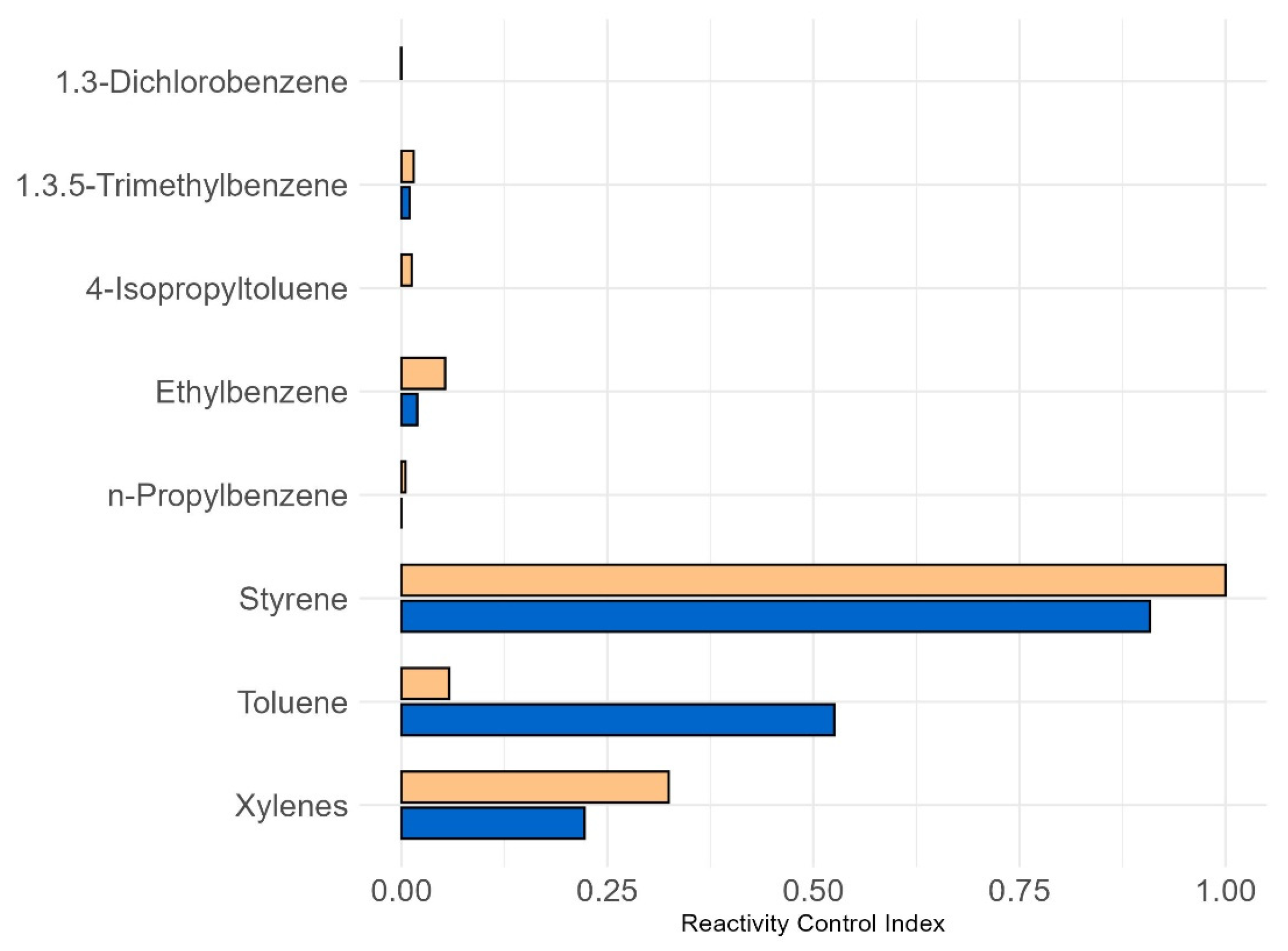
3.3. Ozone Formation Potentials and Reactivity Control Index
4. Discussion
Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UN United Nations. Informe Mundial de las Ciudades. 2022. Available online: https://unhabitat.org/sites/default/files/2022/06/wcr_2022.pdf (accessed on 20 June 2024).
- Shao, P.; An, J.; Xin, J.; Wu, F.; Wang, J.; Ji, D.; Wang, Y. Source apportionment of VOCs and the contribution to photochemical ozone formation during summer in the typical industrial area in the Yangtze River Delta, China. Atmos. Res. 2016, 176–177, 64–74. [Google Scholar] [CrossRef]
- Li, B.; Ho, S.S.; Xue, Y.; Huang, Y.; Wang, L.; Cheng, Y.; Dai, W.; Zhong, H.; Cao, J.; Lee, S. Characterizations of volatile organic compounds (VOCs) from vehicular emissions at roadside environment: The first comprehensive study in Northwestern China. Atmos. Environ. 2017, 161, 1–12. [Google Scholar] [CrossRef]
- Kountouriotis, A.; Aleiferis, P.G.; Charalambides, A.G. Numerical investigation of VOC levels in the area of petrol stations. Sci. Total Environ. 2014, 470, 1205–1224. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Wang, X.L.; Ho, K.F.; Gao, Y.; Liu, C.; Ho, S.S.; Li, H.W.; Lee, S.C.; Wang, X.M.; Jiang, B.Q.; et al. Decrease of VOC emissions from vehicular emissions in Hong Kong from 2003 to 2015: Results from a tunnel study. Atmos. Environ. 2018, 177, 64–74. [Google Scholar] [CrossRef]
- BCN 2021 Biblioteca del Congreso Nacional, (Library of the National Congress). Division Política-Administrativa. Available online: https://www.bcn.cl/siit/nuestropais_29_01_2021/div_pol-adm.htm (accessed on 15 January 2025).
- Rutllant, J.; Garreaud, R. Meteorological air pollution potential for Santiago, Chile: Towards an objective episode forecasting. Environ. Monit. Assess. 1995, 34, 223–244. [Google Scholar] [CrossRef]
- Préndez, M.; Alvarado, G.; Serey, I. Chapter 15: Some Guidelines to Improve Air Quality Management in Santiago, Chile. In Air Quality Monitoring Assessment and Management; Mazzeo, N., Ed.; INTECH Open Access Publisher: London, UK, 2011; pp. 305–328. [Google Scholar]
- Muñoz, R.C.; Garreaud, R.; Rutllant, J.A.; Seguel, R.; Corral, M. New Observations of the Meteorological Conditions Associated with Particulate Matter Air Pollution Episodes in Santiago, Chile. Atmosphere 2023, 14, 1454. [Google Scholar] [CrossRef]
- Préndez, M.; Araya, M.; Criollo, C.; Egas, C.; Farías, I.; Fuentealba, R.; Gonzalez, E. Chapter 8 “Urban trees and its relationships with air pollution by particulate matter and ozone in Santiago de Chile. In Urban Climates in Latin America; Henríquez, C., Romero, H., Eds.; Springer Nature Switzerland AG: Cham, Switzerland, 2019; pp. 167–206. [Google Scholar] [CrossRef]
- Jorquera, H. Ambient particulate matter in Santiago, Chile: 1989–2018: A tale of two size fractions. J. Environ. Manag. 2020, 258, 110035. [Google Scholar] [CrossRef]
- Farías, I.; Préndez, M.; Bown, H.E. Leaf Fluxes of Carbon Dioxide, Methane and Biogenic Volatile Organic Compounds of the Urban Trees Platanus × acerifolia and Schinus molle in Santiago, Chile. Atmosphere 2022, 13, 298. [Google Scholar] [CrossRef]
- Astudillo, P.; Mancilla, P.; Olmos, C.; Reyes, Á. Epidemiology of pediatric respiratory consultations in Santiago de Chile, from 1993 to 2009. Rev. Panam. Salud Pública 2012, 32, 5. [Google Scholar] [CrossRef]
- Romieu, I.; Gouveia, N.; Cifuentes, L.A.; de Leon, A.P.; Junger, W.; Vera, J.; Strappa, V.; Hurtado-Díaz, M.; Miranda-Soberanis, V.; Rojas-Bracho, L.; et al. Committee HEIHR, Multicity study of air pollution and mortality in Latin America (the ESCALA study). Res. Rep. 2012, 5–86. [Google Scholar]
- Criollo, C.; Assar, R.; Cáceres, D.; Préndez, M. Arbolado urbano, calidad del aire y afecciones respiratorias en seis comunas de la provincia de Santiago, Chile. Rev. Chil. Enfermedades Respir. 2016, 32, 77–86. [Google Scholar] [CrossRef]
- Prieto-Parra, L.; Yohannessen, K.; Brea, C.; Vidal, D.; Ubilla, C.A.; Ruiz-Rudolph, P. Air pollution, PM(2.5) composition, source factors, and respiratory symptoms in asthmatic and nonasthmatic children in Santiago, Chile. Environ. Int. 2017, 101, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Molina, C.; Manzano, C.A.; Toro, A.R.; Leiva, G.M. The oxidative potential of airborne particulate matter in two urban areas of Chile: More than meets the eye. Environ. Int. 2023, 173, 107866. [Google Scholar] [CrossRef] [PubMed]
- Álamos, N.; Huneeus, N.; Opazo, M.; Osses, M.; Puja, S.; Pantoja, N.; Denier van der Gon, H.; Schueftan, A.; Reyes, R.; Calvo, R. High-resolution inventory of atmospheric emissions from transport, industrial, energy, mining and residential activities in Chile. Earth Syst. Sci. Data 2022, 14, 361–379. [Google Scholar] [CrossRef]
- Inventario de Emisiones para el Sector Residencial de la Región Metropolitana para el año 2022. Versión 2. December 2023. Available online: https://airerm.mma.gob.cl/wp-content/uploads/2024/03/2023_Inventario-Residencial.pdf (accessed on 11 January 2025).
- MMA, Ministerio del Medio Ambiente. Informe del Estado de Medio Ambiente. 2022. Available online: https://iema.mma.gob.cl/calidad-atmosferica/presion (accessed on 22 January 2025).
- Ghosh, B.; Padhy, P.K.; Niyogi, S.; Patra, P.K.; Hecker, M.A. Comparative Study of Heavy Metal Pollution in Ambient Air and the Health Risks Assessment in Industrial, Urban and Semi-Urban Areas of West Bengal, India: An Evaluation of Carcinogenic, Non-Carcinogenic, and Additional Lifetime Cancer Cases. Environments 2023, 10, 190. [Google Scholar] [CrossRef]
- Cape, J.N.; Leith, I.D.; Binnie, J.; Content, J.; Donkin, M.; Skewes, M.; Price, D.N.; Brown, A.R.; Sharpe, A.D. Effects of VOCs on herbaceous plants in an open-top chamber experiment. Environ. Pollut. 2003, 124, 341–353. [Google Scholar] [CrossRef]
- Tian, L.; Yin, S.; Ma, Y.; Kang, H.; Zhang, X.; Tan, H.; Meng, H.; Liu, C. Impact factor assessment of the uptake and accumulation of polycyclic aromatic hydrocarbons by plant leaves: Morphological characteristics have the greatest impact. Sci. Total Environ. 2019, 652, 1149–1155. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, D.; Kumar, K.; Singh, B.B.; Kumar, J.V. Distribution of VOCs in urban and rural atmospheres of subtropical India: Temporal variation, source attribution, ratios, OFP and risk assessment. Sci. Total Environ. 2018, 613–614, 492–501. [Google Scholar] [CrossRef]
- Amaral, L.; Duarte, N.; Rückert, V.; Oliveira, R.; Pacheco, F. Effects of gasoline composition on engine performance, exhaust gases and operational costs. Renew. Sustain. Energy Rev. 2021, 135, 110196. [Google Scholar] [CrossRef]
- Gummin, D.D. Hydrocarbons. In Emergency Medicine WB Saunders; Elsevier: Amsterdam, The Netherlands, 2013; pp. 1299–1307. [Google Scholar]
- Liu, P.; Wu, Y.; Li, Z.; Lv, Z.; Zhang, J.; Liu, Y.; Song, A.; Wang, T.; Wu, L.; Mao, H.; et al. Tailpipe volatile organic compounds (VOCs) emissions from Chinese gasoline vehicles under different vehicle standards, fuel types, and driving conditions. Atmos. Environ. 2024, 323, 120348. [Google Scholar] [CrossRef]
- Li, H.; Sun, Q.Y.; Li, F.; Wang, B.S.; Zhu, B.L. Metabolomics of benzene exposure and development of biomarkers for exposure hazard assessment. Metabolites 2024, 14, 377. [Google Scholar] [CrossRef] [PubMed]
- Rong, X.; Cao, Q.; Gao, Y.; Du, X.; Dou, H.; Yan, M.; Li, S.; Wang, Q.; Zhang, Z.; Chen, B. Performance optimization and kinetic analysis of HNO3 coupled with microwave rapidly modifiedcoconut shell activated carbon for VOCs adsorption. Front. Energy Res. 2023, 10, 1047254. [Google Scholar] [CrossRef]
- Enesca, A. Enhancing the photocatalytic activity of SnO2-TiO2 and ZnO-TiO2tandem structures toward indoor air decontamination. Front. Chem. 2020, 8, 583270. [Google Scholar] [CrossRef]
- Le, M.C.; Le, T.H.; Thi, T.H.B.; Nguyen, Q.D.; Thi, T.H.; Thi, M.N.T. Synthesizing and evaluating the photocatalytic and antibacterial ability of TiO2/SiO2 nanocomposite for silicate coating. Front. Chem. 2021, 9, 738969. [Google Scholar] [CrossRef]
- Xing, X.; Wang, Y.; Hao, M.; Li, Z.; Liu, D.; Yan, K. Simultaneous catalytic oxidation of benzene and toluene over Pd-CeZrOx catalysts. Atmosphere 2024, 15, 1301. [Google Scholar] [CrossRef]
- Escobedo, F.J.; Wagner, J.E.; Nowak, D.J. Analyzing the cost effectivenes s of Santiago, Chile’s policy of using urban forests to improve air quality. J. Environ. Manag. 2008, 86, 148–157. [Google Scholar] [CrossRef]
- Dzierzanowski, K.; Popek, R.; Gawronska, H.; Sæbø, A.; Gawronski, S.W. Deposition of particulate matter of different size fractions on leaf surfaces and in waxes of urban forest species. Int. J. Phytoremediation 2011, 13, 1037–1046. [Google Scholar] [CrossRef]
- Marando, F.; Salvatori, E.; Fusaro, L.; Manes, F. Removal of PM10 by forests as a nature-based solution for air quality improvement in the metropolitan city of Rome. Forests 2016, 7, 150. [Google Scholar] [CrossRef]
- Muñoz, D.; Aguilar, B.; Fuentealba, R.; Préndez, M. Environmental studies in two communes of Santiago de Chile by the analysis of magnetic properties of particulate matter deposited on leaves of roadside trees. Atmos. Environ. 2017, 152, 617–627. [Google Scholar] [CrossRef]
- Duan, X.; Gu, H.; Lam, S.S.; Sonne, C.; Lu, W.; Li, H.; Chen, X.; Peng, W. Recent progress on phytor;mediation of urban air pollution. Chemosphere 2024, 349, 140821. [Google Scholar] [CrossRef]
- Dover, J. Green Infrastructure. Incorporating Plants and Enhancing Biodiversity in Buildings and Urban Environments; Routledge: London, UK, 2015. [Google Scholar]
- Vásquez, A.E. Infraestructura verde, servicios ecosistémicos y sus aportes para enfrentar el cambio climático en ciudades: El caso del corredor ribereño del río Mapocho en Santiago de Chile. Rev. Geogr. Norte Gd. 2016, 86, 63–86. [Google Scholar] [CrossRef]
- Balany, F.; Ng, A.W.M.; Muttil, N.; Muthukumaran, S.; Wong, M.S. Green Infrastructure as an Urban Heat Island Mitigation Strategy—A Review. Water 2020, 12, 3577. [Google Scholar] [CrossRef]
- Fang, X.; Li, J.; Ma, Q. Integrating green infrastructure, ecosystem services and nature-based solutions for urban sustainability: A comprehensive literature review. Sustain. Cities Soc. 2023, 98, 104843. [Google Scholar] [CrossRef]
- Guerrieri, R.; Cáliz, J.; Mattana, S.; Barceló, A.; Candela, M.; Elustondo , D.; Fortmann, H.; Hellsten, S.; Koenig, N.; Lindroos, A.-J.; et al. Substantial contribution of tree canopy nitrifiers to nitrogen fluxes in European forests. Nat. Geosci. 2024, 17, 130–136. [Google Scholar] [CrossRef]
- Araya, M.; Seelenfreund, D.; Buscaglia, M.; Peña-Ahumada, B.; Vera, J.; Egas, C.; Préndez, M. Assessment of Anthropogenic Volatile Organic Compounds in Leaves of Two Urban Tree Species in Santiago de Chile. Front. For. Glob. Change 2019, 2, 42. [Google Scholar] [CrossRef]
- Elshorbany, Y.F.; Kleffmann, J.; Kurtenbach, R.; Rubio, M.; Lissi, E.; Villena, G.; Gramsch, E.; Rickard, A.R.; Pilling, M.J.; Wiesen, P. Summertime photochemical ozone formation in Santiago, Chile. Atmos. Environ. 2009, 43, 6398–6407. [Google Scholar] [CrossRef]
- Seguel, R.J.; Gallardo, L.; Fleming, Z.L.; Landeros, S. Two decades of ozone standard exceedances in Santiago de Chile. Air Qual. Atmos. Health 2020, 13, 593–605. [Google Scholar] [CrossRef]
- Kansal, A. Sources and reactivity of NMHCs and VOCs in the atmosphere: A review. J. Hazard. Mater. 2009, 166, 17–26. [Google Scholar] [CrossRef]
- Kesselmeier, J.; Staudt, M. Biogenic volatile organic compouds (VOC): An overview on emission, physiology and ecology. J. Atmos. Chem. 1999, 33, 23–88. [Google Scholar] [CrossRef]
- Jurán, S.; Grace, J.; Urban, O. Temporal Changes in Ozone Concentrations and Their Impact on Vegetation. Atmosphere 2021, 12, 82. [Google Scholar] [CrossRef]
- INE Instituto Nacional de Estadísticas. 2023. Available online: https://www.ine.gob.cl/estadisticas/economia/transporte-y-comunicaciones/permiso-de-circulacion (accessed on 20 January 2025).
- Keymeulen, R.; Görgényi, M.; Héberger, K.; Priksane, A.; Van Langenhove, H. Benzene, toluene, ethyl benzene and xylenes in ambient air and Pinus sylvestris L. needles: A comparative study between Belgium, Hungary and Latvia. Atmos. Environ. 2001, 35, 6327–6335. [Google Scholar] [CrossRef]
- Wannomai, T.; Kemacheevakul, P.; Thiravetyan, P. Removal of trimethylamine from indoor air using potted plants under light and dark conditions. Aerosol. Air Qual. Res. 2019, 19, 1105–1113. [Google Scholar] [CrossRef]
- Dela Cruz, M.; Christensen, J.H.; Thomsen, J.D.; Müller, R. Can ornamental potted plants remove volatile organic compounds from indoor air?—A review. Environ. Sci. Pollut. Res. 2014, 21, 13909–13928. [Google Scholar] [CrossRef]
- Sriprapat, W.; Suksabye, P.; Areephak, S.; Klantup, P.; Waraha, A.; Sawattan, A.; Thiravetyan, P. Uptake of toluene and ethylbenzene by plants: Removal of volatile indoor air contaminants. Ecotoxicol. Environ. Saf. 2014, 102, 147–151. [Google Scholar] [CrossRef]
- Shen, X.; Sun, Q.; Mosey, G.; Ma, J.; Wang, L.; Ge, M. Benchmark of plant-based VOCs control effect for indoor air quality: Green wall case in smith campus at Harvard University. Sci. Total Environ. 2024, 906, 166269. [Google Scholar] [CrossRef]
- Brilli, F.; Fares, S.; Ghirardo, A.; de Visser, P.; Calatayud, V.; Muñoz, A.; Annesi-Maesano, I.; Sebastiani, F.; Alivernini, A.; Varriale, V.; et al. Plants for sustainable improvement of indoor air quality. Trends Plant Sci. 2018, 23, 507–512. [Google Scholar] [CrossRef]
- Hernández, H.J.; Villaseñor, N.R. Twelve-year change in tree diversity and spatial segregation in the Mediterranean city of Santiago, Chile. Urban For. Urban Green. 2018, 29, 10–18. [Google Scholar]
- BCN 2009. Biblioteca del Congreso Nacional, (National Library). Decreto 68 Ministerio de Agricultuta (MA) Establece, Aprueba y Oficializa Nómina de Especies Arbóreas y Arbustivas Originarias del Pais. Available online: https://www.bcn.cl/portal/resultado-busqueda?texto=Decreto%2068%20Ministerio%20de%20Agricultuta%20(MA)%20Establece,%20Aprueba%20y%20Oficializa%20N%C3%B3mina%20de%20Especies%20Arb%C3%B3reas%20y%20Arbustivas%20Origi-narias%20del%20Pais%20&dc_source=&npagina=1&tipo_recurso= (accessed on 11 January 2025).
- Baldini, A.; Alvarado Ojeda, A.; Guajardo Becchi, F. Árboles Urbanos de Chile. 2012. Programa de Arborización: Un Chileno, Un Arbol. Guía de Reconocimeinto. Available online: https://cifag.cl/wp-content/uploads/2022/10/ARBOLES_URBANOS_DE_CHILE.pdf (accessed on 11 January 2025).
- Donoso, S.; Peña, K.; Pacheco, C.; Luna, G.; Aguirre, A. Respuesta fisiológica y de crecimiento en plantas de Quillaja saponaria y Cryptocarya alba sometidas a restricción hídrica. Bosque 2011, 32, 187–195. [Google Scholar] [CrossRef]
- Bown, H.E.; Fuentes, J.P.; Martínez, A.M. Assessing water use and soil water balance of planted native tree species under strong water limitations in Northern Chile. New For. 2018, 49, 871–892. [Google Scholar] [CrossRef]
- Rodríguez, R.R.; Matthei, S.O.; Quezada, M.M. Arboreal Flora of Chile; Editorial Universidad de Concepción: Concepción, Chile, 1983; 408p. [Google Scholar]
- Retamales, H.; Morales, N. Árboles de Chile. Taxonomía, ecología y conservación de todas las especies arbóreas nativas de Chile; Bosque Chileno: Providencia, Chile, 2022; Available online: https://bibliotecadigital.infor.cl/handle/20.500.12220/32667 (accessed on 15 June 2023).
- Sitzia, T.; Cierjacks, A.; de Rigo, D.; Caudullo, G. Robinia pseudoacacia in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Publications Office of the EU: Luxembourg, 2016; pp. 166–167. [Google Scholar]
- Acuña-Ruz, T.; Mattar, C.; Hernández, H.J. Caracterización espectral de Quillaja saponaria (Mol.). Rev. Teledetección Asoc. Española Teledetección 2016, 47, 65–73. [Google Scholar] [CrossRef][Green Version]
- Moreira, N.; Lopes, P.; Cabral, M.; De Pinho, P.G. HS-SPME/GC-MS methodologies for the analysis of volatile compounds in cork material. Eur. Food Res. Technol. 2016, 242, 457–466. [Google Scholar] [CrossRef]
- Vázquez, A.M.; Aimar, M.L.; Demmel, G.I.; Decarlini, M.-F.; Díaz-Panero, M.; Cantero, J.J. Multivariate optimization of a HS-SPME/GC-MS technique for the characterization of volatile compounds present in Hedeoma multiflorum Benth. Boletín Latinoam. Del. Caribe Plantas Med. Aromáticas 2019, 18, 492–503. [Google Scholar]
- Chem Service, U.S.A. Available online: https://www.chemservice.com/volatile-organic-compounds-mixture-sharp2-60-components-m-voc2am5-1ml.html?srsltid=AfmBOorM06K1WcJ8-_cd0ENa7rCkuXiWa4ThuOkxKrGLtg9z0ExCgS67 (accessed on 5 November 2023).
- Guo, H.; Ling, Z.H.; Cheng, H.R.; Simpson, I.J.; Lyu, X.P.; Wang, X.M.; Shao, M.; Lu, H.X.; Ayoko, G.; Zhang, Y.L.; et al. Tropospheric volatile organic compounds in China. Sci. Total Environ. 2016, 574, 1021–1043. [Google Scholar] [CrossRef]
- Chameides, W.L.; Fehsenfeld, F.; Rodgers, M.O.; Cardelino, C.; Martinez, J.; Parrish, D.; Lonneman, W.; Lawson, D.R.; Rasmussen, R.A.; Zimmerman, P.; et al. Ozone precursor relationships in the ambient atmosphere. J. Geophys. Res. 1992, 97, 6037–6055. [Google Scholar] [CrossRef]
- Cai, C.-J.; Geng, F.-H.; Tie, X.-X.; Yu, Q.; Li, P.; Zhou, G.-Q. Characteristics of ambient volatile organic compounds (VOCs) measured in Shanghai, China. Sensors 2010, 10, 7843–7862. [Google Scholar] [CrossRef]
- Wu, W.; Zhao, B.; Wang, S.; Hao, J. Ozone and secondary Organic aerosol formation potential from anthropogenic volatile organic compounds emissions in China. J. Environ. Sci. 2017, 53, 224–237. [Google Scholar] [CrossRef]
- Atkinson, R.; Arey, J. Atmospheric degradation of volatile Organic compounds. Chem. Rev. 2003, 103, 4605–4638. [Google Scholar] [CrossRef]
- Fu, S.; Guo, M.; Luo, J.; Han, D.; Chen, X.; Jia, H.; Jin, X.; Liao, H.; Wang, X.; Fan, L.; et al. Improving VOCs control strategies based on source characteristics and chemical reactivity in a typical coastal city of South China through measurement and emission inventory. Sci. Total Environ. 2020, 744, 140825. [Google Scholar] [CrossRef]
- Ram, S.S.; Majumder, S.; Chaudhuri, P.; Chanda, S.; Santra, S.C.; Chakraborty, A.; Sudarshan, M. A review on air pollution monitoring and management using plants with special reference to foliar dust adsorption and physiological stress responses. Crit. Rev. Environ. Sci. Technol. 2015, 45, 2489–2522. [Google Scholar] [CrossRef]
- Klingberg, J.; Strandberg, B.; Sjöman, H.; Taube, M.; Wallin, G.; Pleijel, H. Polycyclic aromatic hydrocarbon (PAH) accumulation in Quercus palustris and Pinus nigra in the urban landscape of Gothenburg, Sweden. Sci. Total Environ. 2022, 805, 15016. [Google Scholar] [CrossRef]
- Asgari, K.; Amini, H. Biomonitoring of Trace Element in Air and Soil Pollution by Using Acacia. J. Rech. Agric. Sci. 2011, 7, 115–124. [Google Scholar]
- Turan, D.; Kocahakimoglu, C.; Kavcar, P.; Gaygisiz, H.; Atatanir, L.; Turgut, C.; Sofuoglu, S.C. The use of olive tree (Olea europea L.) leaves as a bioindicator for envionmental pollution in the province of Aydin, Turkey. Environ. Sci. Pollut. Res. 2011, 18, 355–364. [Google Scholar] [CrossRef]
- Konanç, M.U. Monitoring trace element concentrations with environmentally friendly biomonitors in Artvin, Turkey. Environ. Monit. Assess. 2023, 195, 1001. [Google Scholar] [CrossRef] [PubMed]
- Petrova, S.T. Biomonitoring study of air pollution with Betula pendula Roth from Plovdiv, Bulgaria. Ecol. Balk. 2011, 3, 1–10. [Google Scholar]
- Zhang, Y.; Zheng, Q.; Tyree, M.T. Factors controlling plasticity of leaf morphology in Robinia pseudoacacia L. I: Height-associated variation in leaf structure. Ann. For. Sci. 2012, 69, 29–37. [Google Scholar] [CrossRef]
- Egas, C.; Bown, H.; Godoy, N.; Hernández, H.J.; Naulin, P.; Ponce, M.; Préndez, M. Pollution induced leaf morphoanatomical changes of Quillaja saponaria in Santiago, Chile. Austin Environ. Sci. 2020, 5, 1041–1047. [Google Scholar]
- Préndez, M.; Carvallo, C.; Godoy, N.; Egas, ·C.; Aguilar Reyes, B.O.; Calzolai, G.; Fuentealba, R.; Lucarelli, F.; Nava, S. Magnetic and elemental characterization of the particulate matter deposited on leaves of urban trees in Santiago, Chile. Environ. Geochem. Health 2023, 45, 2629–2643. [Google Scholar] [CrossRef]


| Analyte | CAS No. | Qualifier Ion Q (m/z) | Qualifier Ion q1 (m/z) | Qualifier Ion q2 (m/z) | Retention Time (min) |
|---|---|---|---|---|---|
| cis-1,2-Dichloroethylene | 156-59-2 | 61 | 96 | 98 | 2.13 |
| trans-1,2-Dichloroethylene | 156-60-5 | 61 | 96 | 98 | 2.43 |
| cis-1,3-Dichloropropene | 1061-01-5 | 75 | 77 | 110 | 4.72 |
| trans-1,3-Dichloropropene | 1061-02-6 | 75 | 77 | 110 | 4.72 |
| Benzene | 71-43-2 | 78 | 77 | 51 | 3.06 |
| Bromobenzene | 108-86-1 | 77 | 156 | 158 | 12.34 |
| Bromochloromethane | 74-97-5 | 49 | 130 | 128 | 2.54 |
| Bromodichloromethane | 75-27-4 | 83 | 85 | 47 | 3.84 |
| sec-Butylbenzene | 135-98-8 | 105 | 120 | 77 | 15.45 |
| tert-Butylbenzene | 98-06-6 | 119 | 91 | 41 | 15.11 |
| Chlorobenzene | 108-90-7 | 112 | 77 | 114 | 8.47 |
| 2-Chlorotoluene | 95-49-8 | 91 | 126 | 89 | 13.17 |
| 4-Chlorotoluene | 106-43-4 | 126 | 128 | 89 | 13.43 |
| 1,2-Dibromo-3-chloropropane | 96-12-8 | 157 | 75 | 155 | 16.38 |
| Dibromochloromethane | 124-48-1 | 127 | 129 | 131 | 6.53 |
| 1,2-Dibromoethane | 106-93-4 | 107 | 109 | 93 | 6.91 |
| Dibromomethane | 74-95-3 | 93 | 174 | 95 | 3.73 |
| 1,1-Dichloro-1-propene | 563-58-6 | 75 | 39 | 110 | 2.98 |
| 1,2-Dichlorobenzene | 95-50-1 | 146 | 148 | 111 | 15.86 |
| 1,3-Dichlorobenzene | 541-73-1 | 146 | 148 | 111 | 15.39 |
| 1,4-Dichlorobenzene | 106-46-7 | 146 | 148 | 111 | 15.53 |
| 1,1-Dichloroethane | 75-34-3 | 63 | 65 | 83 | 2.22 |
| 1,2-Dichloroethane | 107-06-2 | 62 | 64 | 49 | 2.90 |
| 1,1-Dichloroethylene | 75-35-4 | 61 | 96 | 98 | 1.90 |
| Dichloromethane | 75-09-2 | 49 | 84 | 86 | 1.97 |
| 1,2-Dichloropropane | 78-87-5 | 63 | 62 | 41 | 3.68 |
| 1,3-Dichloropropane | 142-28-9 | 76 | 41 | 78 | 6.22 |
| 2,2-Dichloropropane | 594-20-7 | 77 | 41 | 79 | 2.52 |
| Ethylbenzene | 100-41-4 | 91 | 106 | 51 | 9.18 |
| Hexachloro-1,3-butadiene | 87-68-3 | 225 | 227 | 223 | 17.56 |
| Isopropylbenzene | 98-82-8 | 105 | 120 | 77 | 12.22 |
| 4-Isopropyltoluene | 99-87-6 | 119 | 134 | 91 | 15.70 |
| Naphthalene | 91-20-3 | 128 | 102 | 127 | 17.31 |
| n-Propylbenzene | 103-65-1 | 120 | 65 | 78 | 13.35 |
| Styrene | 100-42-5 | 104 | 78 | 51 | 10.69 |
| 1,1,1,2-Tetrachloroethane | 630-20-6 | 131 | 133 | 117 | 8.65 |
| 1,1,2,2-Tetrachloroethane | 79-34-5 | 83 | 85 | 61 | 11.85 |
| Tetrachloroethylene | 127-18-4 | 166 | 164 | 131 | 7.05 |
| Tetrachloromethane | 56-23-5 | 117 | 119 | 121 | 3.06 |
| Toluene | 108-88-3 | 91 | 92 | 65 | 5.55 |
| Tribromomethane | 75-25-2 | 173 | 171 | 175 | 10.24 |
| 1,2,3-Trichlorobenzene | 87-61-6 | 180 | 182 | 74 | 17.25 |
| 1,2,4-Trichlorobenzene | 120-82-1 | 180 | 145 | 184 | 17.24 |
| 1,1,1-Trichloroethane | 71-55-6 | 97 | 99 | 61 | 2.85 |
| 1,1,2-Trichloroethane | 79-00-5 | 97 | 83 | 99 | 5.75 |
| Trichloroethylene | 79-01-6 | 95 | 130 | 132 | 3.69 |
| Trichloromethane | 67-66-3 | 83 | 85 | 47 | 2.54 |
| 1,2,3-Trichloropropane | 96-18-4 | 75 | 105 | 77 | 12.06 |
| α,α,α-Trifluorotoluene (EI) | 98-08-8 | 146 | 127 | 96 | 3.96 |
| 1,2,4-Trimethylbenzene | 95-63-6 | 105 | 120 | 77 | 13.98 |
| 1,3,5-Trimethylbenzene | 108-67-8 | 105 | 120 | 77 | 13.50 |
| m-Xylene | 108-38-3 | 91 | 106 | 105 | 9.58 |
| o-Xylene | 95-47-6 | 91 | 106 | 105 | 10.80 |
| p-Xylene | 106-42-3 | 91 | 106 | 105 | 9.58 |
| Sampling Site | Coordinatess | 1,3-Dichlorobenzene | Ethylbenzene | 4-Isopropyltoluene | ||||
|---|---|---|---|---|---|---|---|---|
| Q. saponaria | R. pseudoacacia | Q. saponaria | R. pseudoacacia | Q. saponaria | R. pseudoacacia | |||
| Latitude | Longitude | Value (ng of C) | Value (ng of C) | Value (ng of C) | ||||
| Antumapu | −33.570876 | −70.633697 | <LOQ | 0.32 ± 0.03 | <LOQ | <LOQ | <LOQ | 0.52 ± 0.02 |
| Cerrillos | −33.492879 | −70.719397 | <LOQ | 0.31 ± 0.05 | 4.08 ± 0.03 | 3.55 ± 0.19 | <LOQ | 0.66 ± 0.02 |
| Cerro Navia | −33.433088 | −70.732082 | <LOQ | 0.36 ± 0.03 | 2.70 ± 0.03 | 2.70 ± 0.13 | <LOQ | 0.61 ± 0.03 |
| El Bosque | −33.546913 | −70.666728 | <LOQ | 0.33 ± 0.03 | 2.43 ± 0.03 | 4.25 ± 0.13 | <LOQ | 0.64 ± 0.03 |
| Independencia | −33.422242 | −70.651155 | <LOQ | 0.40 ± 0.04 | <LOQ | 0.50 ± 0.05 | 0.32 ± 0.05 | 0.73 ± 0.01 |
| La Florida | −33.516623 | −70.588089 | <LOQ | 0.13 ± 0.03 | <LOQ | 0.90 ± 0.05 | <LOQ | 0.30 ± 0.03 |
| Las Condes | −33.377673 | −70.52326 | <LOQ | 0.22 ± 0.03 | <LOQ | 0.05 ± 0.05 | <LOQ | 0.70 ± 0.04 |
| Parque O’Higgins | −33.463379 | −70.658585 | <LOQ | 0.46 ± 0.03 | 1.26 ± 0.06 | 3.61 ± 0.60 | <LOQ | 0.57 ± 0.04 |
| Puente Alto | −33.591361 | −70.594768 | <LOQ | 0.51 ± 0.03 | 1.95 ± 0.03 | 5.91 ± 0.15 | <LOQ | 0.70 ± 0.03 |
| Quilicura | −33.34966 | −70.723693 | <LOQ | 0.20 ± 0.09 | < LOQ | 2.71 ± 0.15 | <LOQ | 0.70 ± 0.03 |
| Sampling site | Coordinates | n-Propylbenzene | Styrene | 1,3,5-TMB | ||||
| Q. saponaria | R. pseudoacacia | Q. saponaria | R. pseudoacacia | Q. saponaria | R. pseudoacacia | |||
| Latitude | Longitude | Value (ng of C) | Value (ng of C) | Value (ng of C) | ||||
| Antumapu | −33.570876 | −70.633697 | <LOQ | 0.46 ± 0.01 | 58.19 ± 1.43 | 45.08 ± 1.43 | <LOQ | <LOQ |
| Cerrillos | −33.492879 | −70.719397 | 0.28 ± 0.05 | 0.62 ± 0.01 | 50.30 ± 8.46 | 67.57 ± 8.46 | 0.21 ± 0.05 | 0.27 ± 0.025 |
| Cerro Navia | −33.433088 | −70.732082 | <LOQ | 0.61 ± 0.02 | 60.68 ± 0.74 | 57.54 ± 2.54 | 0.23 ± 0.05 | 0.20 ± 0.043 |
| El Bosque | −33.546913 | −70.666728 | <LOQ | 0.37 ± 0.13 | 52.81 ± 1.03 | 37.37 ± 0.34 | <LOQ | 0.20 ± 0.043 |
| Independencia | −33.422242 | −70.651155 | <LOQ | 0.70 ± 0.01 | 39.51 ± 1.75 | 70.35 ± 1.78 | <LOQ | 0.33 ± 0.034 |
| La Florida | −33.516623 | −70.588089 | 0.50 ± 0.10 | 0.13 ± 0.05 | 77.18 ± 8.21 | 25.54 ± 0.54 | <LOQ | <LOQ |
| Las Condes | −33.377673 | −70.52326 | 0.31 ± 0.05 | 0.70 ± 0.03 | 60.70 ± 8.38 | 31.04 ± 0.93 | 0.19 ± 0.06 | <LOQ |
| Parque O’Higgins | −33.463379 | −70.658585 | 0.43 ± 0.02 | 0.70 ± 0.03 | 49.06 ± 3.21 | 64.11 ± 5.53 | <LOQ | 0.29 ± 0.049 |
| Puente Alto | −33.591361 | −70.594768 | 0.37 ± 0.01 | 0.73 ± 0.01 | 52.14 ± 1.64 | 101.04 ± 5.17 | 0.36 ± 0.02 | 0.36 ± 0.02 |
| Quilicura | −33.34966 | −70.723693 | <LOQ | 0.39 ± 0.14 | 72.91 ± 2.20 | 45.41 ± 2.12 | 0.14 ± 0.016 | 0.14 ± 0.016 |
| Sampling site | Coordinates | Toluene | m/p-Xylene | o-Xylene | ||||
| Q. saponaria | R. pseudoacacia | Q. saponaria | R. pseudoacacia | Q. saponaria | R. pseudoacacia | |||
| Latitude | Longitude | Value (ng of C) | Value (ng of C) | Value (ng of C) | ||||
| Antumapu | −33.570876 | −70.633697 | 34.11 ± 1.94 | 1.93 ± 0.37 | 4.29 ± 0.27 | 4.03 ± 0.27 | <LOQ | 0.99 ± 0.09 |
| Cerrillos | −33.492879 | −70.719397 | 21.97 ± 0.57 | 3.33 ± 0.02 | 4.27 ± 1.63 | 7.14 ± 0.20 | 2.19 ± 0.15 | 2.49 ± 0.15 |
| Cerro Navia | −33.433088 | −70.732082 | 38.12 ± 0.40 | 3.36 ± 0.26 | 5.50 ± 0.18 | 6.09 ± 0.28 | <LOQ | 2.01 ± 0.18 |
| El Bosque | −33.546913 | −70.666728 | 24.28 ± 1.02 | 1.99 ± 0.26 | 4.17 ± 0.17 | 5.27 ± 0.48 | 1.13 ± 0.09 | 1.48 ± 0.08 |
| Independencia | −33.422242 | −70.651155 | 18.64 ± 1.13 | 3.59 ± 0.25 | 3.48 ± 0.27 | 7.83 ± 0.15 | 2.89 ± 0.10 | 2.89 ± 0.10 |
| La Florida | −33.516623 | −70.588089 | 29.52 ± 6.01 | 6.01 ± 0.04 | 7.19 ± 1.64 | 1.38 ± 0.05 | <LOQ | <LOQ |
| Las Condes | −33.377673 | −70.52326 | 21.01 ± 2.28 | 2.94 ± 0.74 | 6.16 ± 1.60 | 2.21 ± 0.05 | 1.82 ± 0.03 | 2.88 ± 0.12 |
| Parque O’Higgins | −33.463379 | −70.658585 | 21.01 ± 2.28 | 2.28 ± 0.04 | 4.66 ± 0.71 | 7.31 ± 0.43 | 2.88 ± 0.12 | 2.88 ± 0.12 |
| Puente Alto | −33.591361 | −70.594768 | 10.21 ± 1.64 | 5.17 ± 0.21 | 5.94 ± 0.16 | 10.44 ± 0.37 | 1.64 ± 0.05 | 2.82 ± 0.15 |
| Quilicura | −33.34966 | −70.723693 | 34.20 ± 1.15 | 2.17 ± 0.39 | 6.61 ± 0.38 | 4.37 ± 0.36 | 1.50 ± 0.13 | 1.50 ± 0.13 |
| Prop-Equiv(i), ppbC | OFP(i), ppbC | |||
|---|---|---|---|---|
| Q. saponaria | R. pseudoacacia | Q. saponaria | R. pseudoacacia | |
| 1,3-Dichlorobenzene | 0.0 | 0.0 | 0.0 | 0.1 |
| Ethylbenzene | 0.5 | 0.9 | 5.2 | 9.7 |
| 4-Isopropyltoluene | 0.0 | 0.0 | 1.4 | 2.6 |
| n-Propylbenzene | 0.0 | 0.0 | 0.0 | 0.0 |
| Styrene | 128.8 | 122.4 | 101.2 | 96.2 |
| 1,3,5-TMB | 0.5 | 0.5 | 2.8 | 2.7 |
| Toluene | 6.6 | 0.6 | 123.7 | 10.9 |
| Xylenes | 4.6 | 5.1 | 51.1 | 58.5 |
| Total AVOC | 141.1 | 129.6 | 286.3 | 181.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araya, M.; Vera, J.; Préndez, M. Urban Tree Species Capturing Anthropogenic Volatile Organic Compounds—Impact on Air Quality. Atmosphere 2025, 16, 356. https://doi.org/10.3390/atmos16040356
Araya M, Vera J, Préndez M. Urban Tree Species Capturing Anthropogenic Volatile Organic Compounds—Impact on Air Quality. Atmosphere. 2025; 16(4):356. https://doi.org/10.3390/atmos16040356
Chicago/Turabian StyleAraya, Mauricio, Javier Vera, and Margarita Préndez. 2025. "Urban Tree Species Capturing Anthropogenic Volatile Organic Compounds—Impact on Air Quality" Atmosphere 16, no. 4: 356. https://doi.org/10.3390/atmos16040356
APA StyleAraya, M., Vera, J., & Préndez, M. (2025). Urban Tree Species Capturing Anthropogenic Volatile Organic Compounds—Impact on Air Quality. Atmosphere, 16(4), 356. https://doi.org/10.3390/atmos16040356





