Evaluating Sampling Materials for Atmospheric Volatile Organosulfur Compounds Measurement and Application in the Power Battery Recycling Industry
Abstract
1. Introduction
2. Materials and Methods
2.1. Information on Sampling Materials
2.2. Experimental Scheme
2.3. Volatile Organosulfur Compounds Analysis
3. Results and Discussion
3.1. Blank Background of Sampling Materials
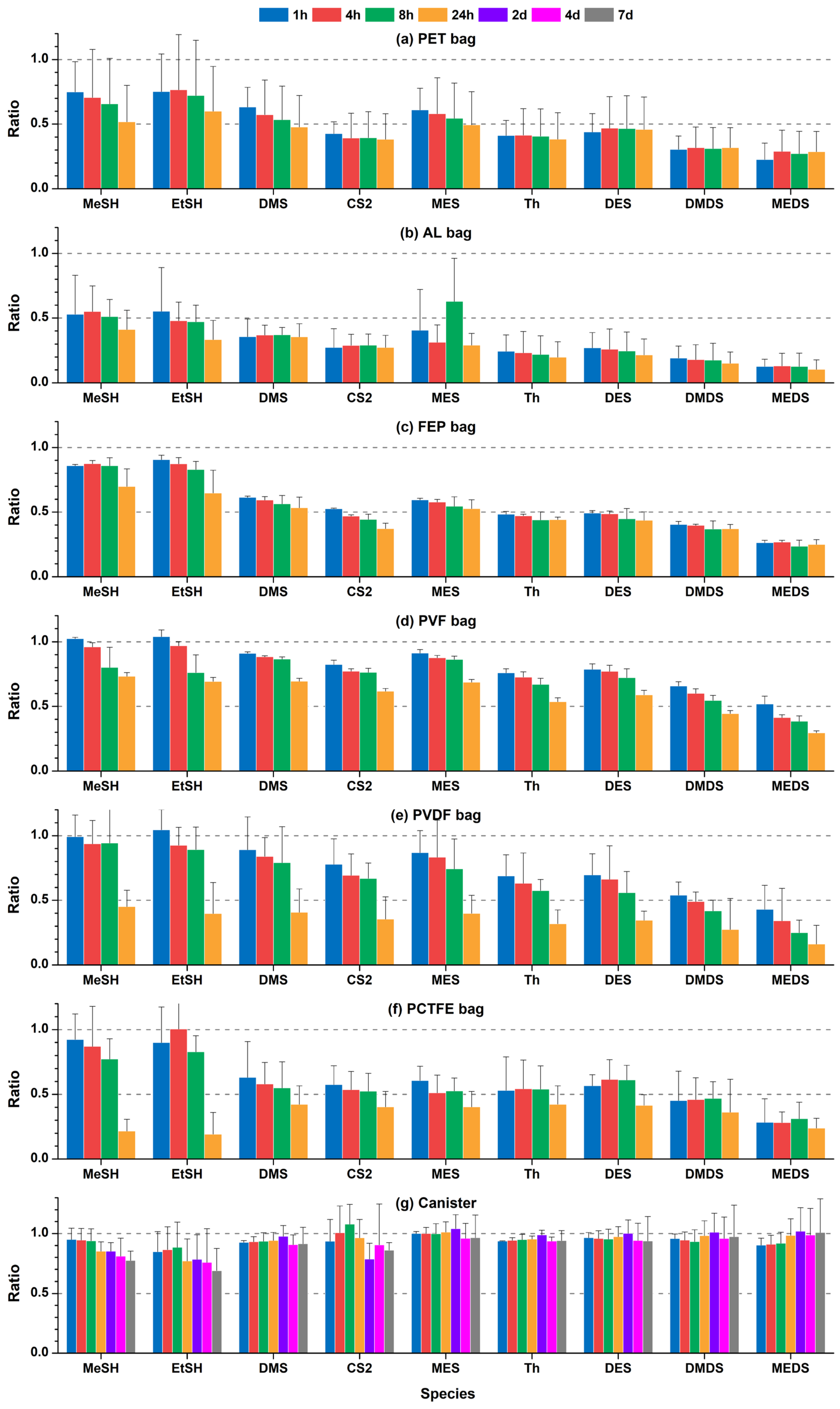
3.2. Stability of Volatile Organosulfur Compounds in Sampling Bags and Canisters
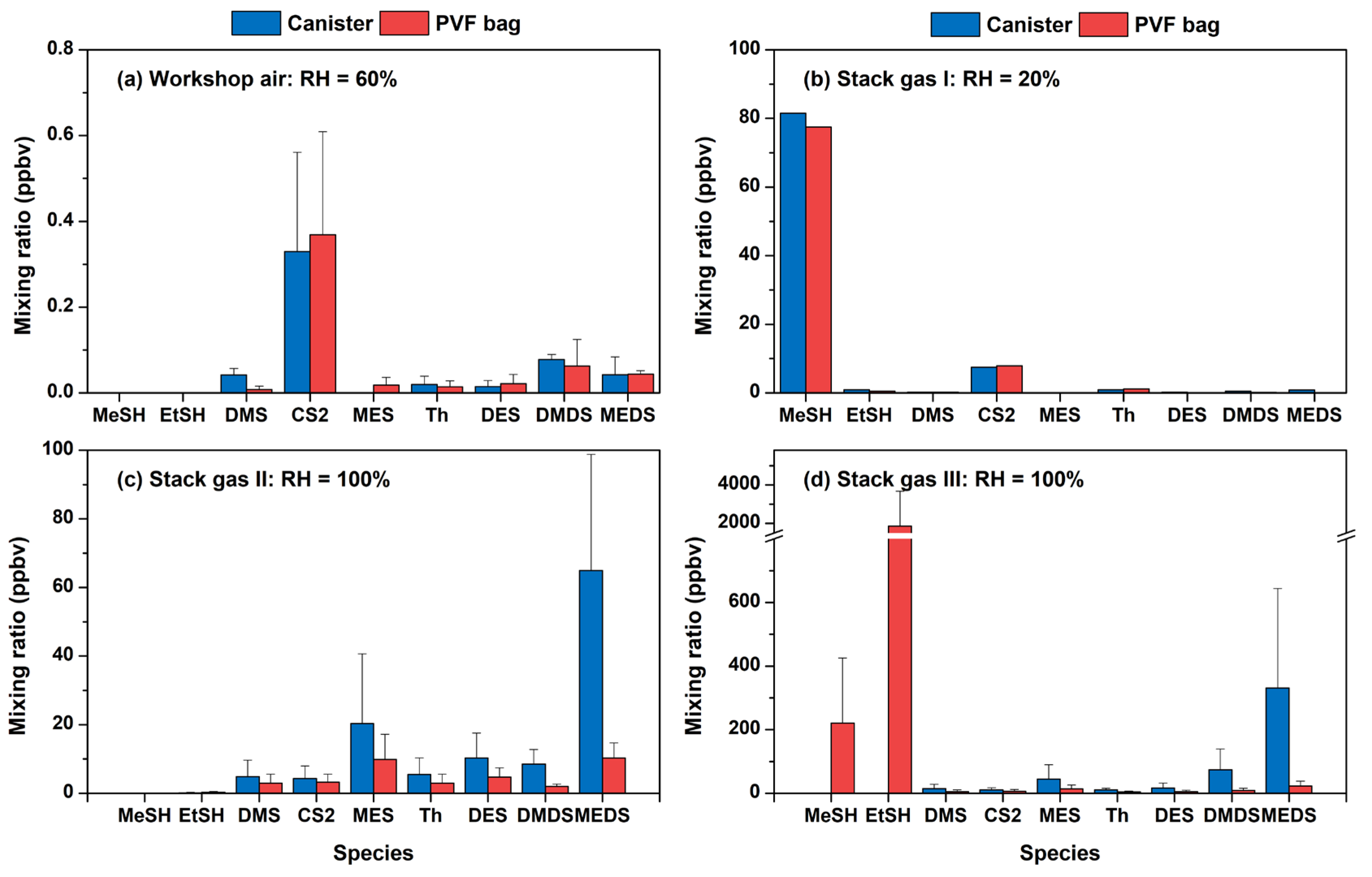
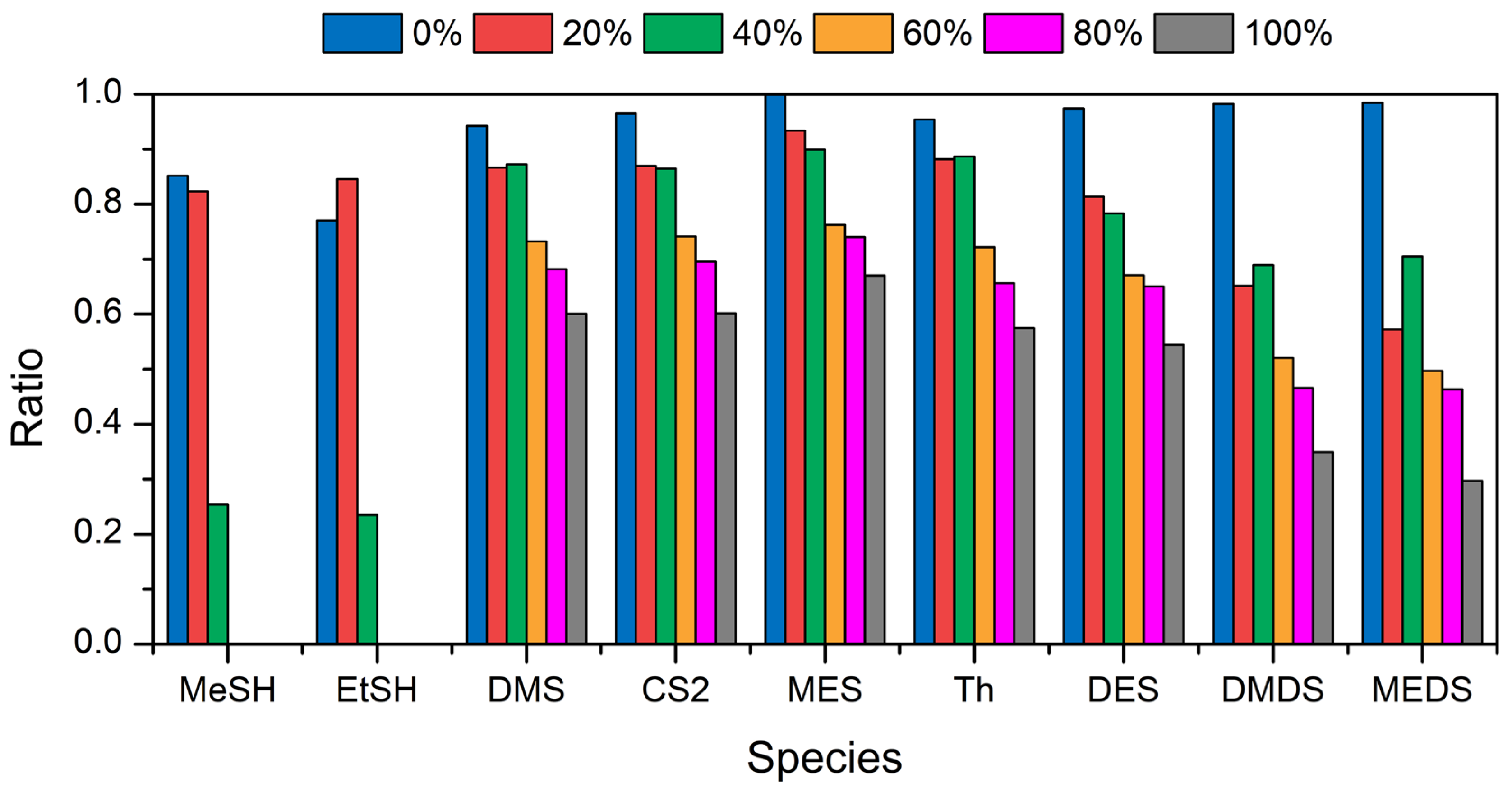
3.3. Field Comparison and the Impact of Humidity
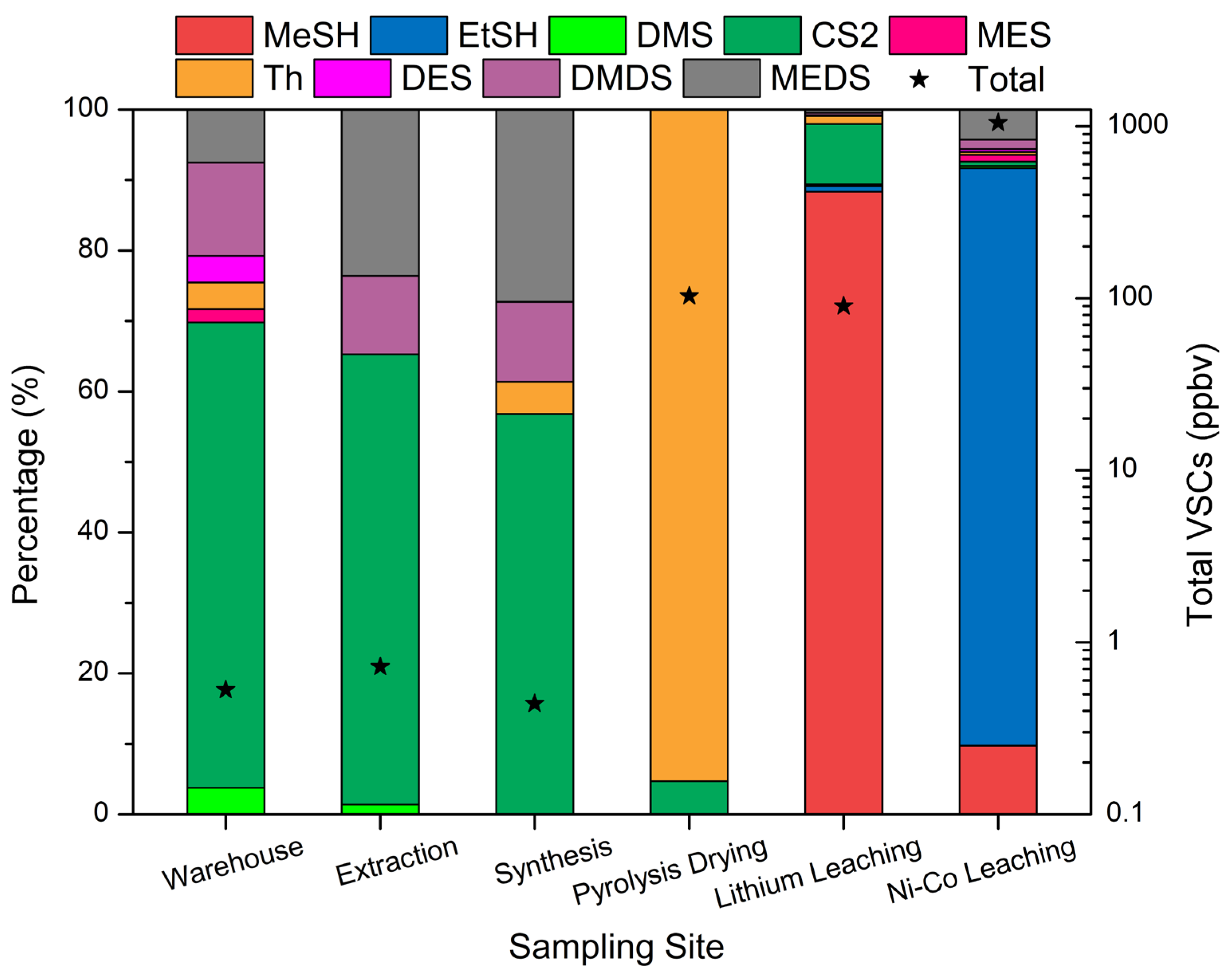
3.4. VSCs Emissions from Power Battery Recycling Industry
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chin, M.; Davis, D.D. Global sources and sinks of OCS and CS2 and their distributions. Glob. Biogeochem. Cycle 1993, 7, 321–337. [Google Scholar] [CrossRef]
- Charlson, R.J.; Lovelock, J.E.; Andreae, M.O.; Warren, S.G. Oceanic phytoplankton, atmospheric sulphur, cloud albedo and climate. Nature 1987, 326, 655–661. [Google Scholar] [CrossRef]
- Susaya, J.; Kim, K.-H.; Phan, N.-T.; Kim, J.-C. Assessment of reduced sulfur compounds in ambient air as malodor components in an urban area. Atmos. Environ. 2011, 45, 3381–3390. [Google Scholar] [CrossRef]
- Leonardos, G.; Sullivan, F.; Levine, S.P.; Stordeur, R.T.; Harvey, T.M.; Schuetzle, D. A Comparison of Polymer Adsorbent and Bag Sampling Techniques for Paint Bake Oven Odorous Emissions. J. Air Pollut. Control Assoc. 1980, 30, 22–29. [Google Scholar] [CrossRef]
- Hellman, T.M.; Small, F.H. Characterization of the Odor Properties of 101 Petrochemicals Using Sensory Methods. J. Air Pollut. Control Assoc. 1974, 24, 979–982. [Google Scholar] [CrossRef]
- Wohl, C.; Villamayor, J.; Galí, M.; Mahajan, A.S.; Fernández, R.P.; Cuevas, C.A.; Bossolasco, A.; Li, Q.; Kettle, A.J.; Williams, T.; et al. Marine emissions of methanethiol increase aerosol cooling in the Southern Ocean. Sci. Adv. 2024, 10, eadq2465. [Google Scholar] [CrossRef] [PubMed]
- Jacob, L.S.D.; Giorio, C.; Archibald, A.T. Extension, development, and evaluation of the representation of the OH-initiated dimethyl sulfide (DMS) oxidation mechanism in the Master Chemical Mechanism (MCM) v3.3.1 framework. Atmos. Chem. Phys. 2024, 24, 3329–3347. [Google Scholar] [CrossRef]
- Barnes, I.; Hjorth, J.; Mihalopoulos, N. Dimethyl Sulfide and Dimethyl Sulfoxide and Their Oxidation in the Atmosphere. Chem. Rev. 2006, 106, 940–975. [Google Scholar] [CrossRef]
- Pandey, S.K.; Kim, K.-H. A Review of Methods for the Determination of Reduced Sulfur Compounds (RSCs) in Air. Environ. Sci. Technol. 2009, 43, 3020–3029. [Google Scholar] [CrossRef]
- Kim, K.-H. Some Insights into the Gas Chromatographic Determination of Reduced Sulfur Compounds (RSCs) in Air. Environ. Sci. Technol. 2005, 39, 6765–6769. [Google Scholar] [CrossRef]
- Polvara, E.; Gallego, E.; Invernizzi, M.; Perales, J.F.; Sironi, S. Chemical characterization of odorous emissions: A comparative performance study of different sampling methods. Talanta 2023, 253, 124110. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, Z.; Zhang, H.; Zhang, Y.; Wang, X. High time resolution online detection of reduced sulfur compounds (RSCs) in ambient air in Guangzhou by proton transfer reaction time-of-flight mass spectrometry ( PTR-ToF-MS). Environ. Chem. 2018, 37, 2425–2432. [Google Scholar]
- Perraud, V.; Meinardi, S.; Blake, D.R.; Finlayson-Pitts, B.J. Challenges associated with the sampling and analysis of organosulfur compounds in air using real-time PTR-ToF-MS and offline GC-FID. Atmos. Meas. Tech. 2016, 9, 1325–1340. [Google Scholar] [CrossRef]
- Ghimenti, S.; Lomonaco, T.; Bellagambi, F.G.; Tabucchi, S.; Onor, M.; Trivella, M.G.; Ceccarini, A.; Fuoco, R.; Di Francesco, F. Comparison of sampling bags for the analysis of volatile organic compounds in breath. J. Breath Res. 2015, 9, 047110. [Google Scholar] [CrossRef]
- Li, X.; Sun, Q.; Yu, L.; Wang, X.; Feng, L.; Wang, K. Influence of Field Sampling Methods on Measuring Volatile Organic Compounds in a Swine Facility Using SUMMA Canisters. Atmosphere 2024, 15, 1021. [Google Scholar] [CrossRef]
- Khan, M.A.H.; Whelan, M.E.; Rhew, R.C. Analysis of low concentration reduced sulfur compounds (RSCs) in air: Storage issues and measurement by gas chromatography with sulfur chemiluminescence detection. Talanta 2012, 88, 581–586. [Google Scholar] [CrossRef]
- Jo, S.-H.; Kim, K.-H.; Shon, Z.-H.; Parker, D. Identification of control parameters for the sulfur gas storability with bag sampling methods. Anal. Chim. Acta 2012, 738, 51–58. [Google Scholar] [CrossRef]
- Mochalski, P.; Wzorek, B.; Śliwka, I.; Amann, A. Suitability of different polymer bags for storage of volatile sulphur compounds relevant to breath analysis. J. Chromatogr. B 2009, 877, 189–196. [Google Scholar] [CrossRef]
- Trabue, S.; Scoggin, K.; Mitloehner, F.; Li, H.; Burns, R.; Xin, H. Field sampling method for quantifying volatile sulfur compounds from animal feeding operations. Atmos. Environ. 2008, 42, 3332–3341. [Google Scholar] [CrossRef]
- Sulyok, M.; Haberhauer-Troyer, C.; Rosenberg, E.; Grasserbauer, M. Investigation of the storage stability of selected volatile sulfur compounds in different sampling containers. J. Chromatogr. A 2001, 917, 367–374. [Google Scholar] [CrossRef]
- Meng, J.; Zhai, Z.; Liu, Y.; Zhang, J.; Han, M. Influence factors and mechanism study on bag sampling method for determination of reduced sulfide compound by gas chromaography-mass spectrometry. Rock Miner. Anal. 2019, 38, 179–185. [Google Scholar] [CrossRef]
- Borrás, E.; Tortajada-Genaro, L.A.; Muñoz, A. Determination of reduced sulfur compounds in air samples for the monitoring of malodor caused by landfills. Talanta 2016, 148, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Andersen, K.B.; Hansen, M.J.; Feilberg, A. Minimisation of artefact formation of dimethyl disulphide during sampling and analysis of methanethiol in air using solid sorbent materials. J. Chromatogr. A 2012, 1245, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Zhang, H.-H.; Zhong, X.-S.; Yan, S.-B.; Zhang, J.-W.; Yang, G.-P.; Ma, X.; Chen, Z.-H. Revealing the Marine Cycles of Volatile Sulfur Compounds and Their Biogeochemical Controls: A Case of the Western North Pacific. Environ. Sci. Technol. 2024, 58, 3235–3245. [Google Scholar] [CrossRef] [PubMed]
- Lehnert, A.-S.; Cooper, R.E.; Ignatz, R.; Ruecker, A.; Gomes-Alves, E.; Küsel, K.; Pohnert, G.; Trumbore, S.E. Dimethyl Sulfide Emissions From a Peatland Result More From Organic Matter Degradation Than Sulfate Reduction. J. Geophys. Res. Biogeosciences 2024, 129, e2023JG007449. [Google Scholar] [CrossRef]
- Yi, Z.G.; Wang, X.M.; Ouyang, M.G.; Zhang, D.Q.; Zhou, G.Y. Air-soil exchange of dimethyl sulfide, carbon disulfide, and dimethyl disulfide in three subtropical forests in south China. J. Geophys. Res. Atmos. 2010, 115, D18302. [Google Scholar] [CrossRef]
- Lee, C.-L.; Brimblecombe, P. Anthropogenic contributions to global carbonyl sulfide, carbon disulfide and organosulfides fluxes. Earth Sci. Rev. 2016, 160, 1–18. [Google Scholar] [CrossRef]
- Guo, H.; Simpson, I.J.; Ding, A.J.; Wang, T.; Saunders, S.M.; Wang, T.J.; Cheng, H.R.; Barletta, B.; Meinardi, S.; Blake, D.R.; et al. Carbonyl sulfide, dimethyl sulfide and carbon disulfide in the Pearl River Delta of southern China: Impact of anthropogenic and biogenic sources. Atmos. Environ. 2010, 44, 3805–3813. [Google Scholar] [CrossRef]
- Andreae, M.O.; Raemdonck, H. Dimethyl Sulfide in the Surface Ocean and the Marine Atmosphere: A Global View. Science 1983, 221, 744–747. [Google Scholar] [CrossRef]
- Yan, Y.; Li, R.; Peng, L.; Yang, C.; Liu, C.; Cao, J.; Yang, F.; Li, Y.; Wu, J. Emission inventory of carbonyl sulfide (COS) from primary anthropogenic sources in China. Environ. Pollut. 2019, 247, 745–751. [Google Scholar] [CrossRef]
- Zou, S.C.; Lee, S.C.; Chan, C.Y.; Ho, K.F.; Wang, X.M.; Chan, L.Y.; Zhang, Z.X. Characterization of ambient volatile organic compounds at a landfill site in Guangzhou, South China. Chemosphere 2003, 51, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Zhao, R.; Li, J.; Yang, Q.; Zou, K. Release characteristics and risk assessment of volatile sulfur compounds in municipal wastewater treatment plants. Environ. Pollut. 2024, 350, 123946. [Google Scholar] [CrossRef]
- Jongebloed, U.A.; Chalif, J.I.; Tashmim, L.; Porter, W.C.; Bates, K.H.; Chen, Q.; Osterberg, E.C.; Koffman, B.G.; Cole-Dai, J.; Winski, D.A.; et al. Dimethyl sulfide chemistry over the industrial era: Comparison of key oxidation mechanisms and long-term observations. Atmos. Chem. Phys. 2025, 25, 4083–4106. [Google Scholar] [CrossRef]
- Han, B.; Liu, Y.-t.; Wu, J.-h.; Feng, Y.-c. Characterization of industrial odor sources in Binhai New Area of Tianjin, China. Environ. Sci. Pollut. Res. 2018, 25, 14006–14017. [Google Scholar] [CrossRef]
- Han, B.; Wu, J.; Wang, F.; Zuo, M.; Feng, Y. Characterization of industrial odor sources. China Environ. Sci. 2013, 33, 416–422. [Google Scholar]
- Zhou, Y.; Cui, X.-D.; Lin, A.-J.; Dong, Y.; Duan, G.-L. Environmental life cycle assessment on the recycling processes of power batteries for new energy vehicles. J. Clean. Prod. 2025, 488, 144641. [Google Scholar] [CrossRef]
- Sun, J.; Peng, Q.; Peng, Z.; Qu, L.; Zhang, Z.; Liu, W.; Ho, S.S.H. Ambient volatile organic compounds in a typical industrial city in southern China: Impacts of aromatic hydrocarbons from new industry. Sci. Total Environ. 2024, 954, 176424. [Google Scholar] [CrossRef] [PubMed]
- Harper, G.; Sommerville, R.; Kendrick, E.; Driscoll, L.; Slater, P.; Stolkin, R.; Walton, A.; Christensen, P.; Heidrich, O.; Lambert, S.; et al. Recycling lithium-ion batteries from electric vehicles. Nature 2019, 575, 75–86. [Google Scholar] [CrossRef]
- Duan, X.; Zhu, W.; Ruan, Z.; Xie, M.; Chen, J.; Ren, X. Recycling of Lithium Batteries—A Review. Energies 2022, 15, 1611. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, J.; Wei, Z.; Yang, L.; Dai, W.; Luo, F.; Wang, C.; Zhou, L. Emission characteristics of gaseous pollutants from typical retired lithium battery recycling enterprises. J. Chem. Eng. Chin. Univ. 2023, 37, 989–997. [Google Scholar]
- Liu, Z.; Liu, C.; He, Z.; Mu, Y.; Zhang, C.; Zhang, Y.; Liu, P.; Wang, Y.; Liu, J. Evaluation of offline sampling for atmospheric C3-C11 non-methane hydrocarbons. J. Environ. Sci. 2022, 113, 132–140. [Google Scholar] [CrossRef]
- Wang, G.; Zhao, N.; Zhang, H.; Li, G.; Xin, G. Spatiotemporal Distributions of Ambient Volatile Organic Compounds in China: Characteristics and Sources. Aerosol Air Qual. Res. 2022, 22, 210379. [Google Scholar] [CrossRef]
- Colman, J.J.; Swanson, A.L.; Meinardi, S.; Sive, B.C.; Blake, D.R.; Rowland, F.S. Description of the Analysis of a Wide Range of Volatile Organic Compounds in Whole Air Samples Collected during PEM-Tropics A and B. Anal. Chem. 2001, 73, 3723–3731. [Google Scholar] [CrossRef]
- Trabue, S.L.; Anhalt, J.C.; Zahn, J.A. Bias of Tedlar Bags in the Measurement of Agricultural Odorants. J. Environ. Qual. 2006, 35, 1668–1677. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Zou, L.; Ou, Z.; Luo, D.; Liu, Z.; Huang, Z.; Fei, L.; Wang, X. Intermediate-volatility aromatic hydrocarbons from the rubber products industry in China. Sci. Total Environ. 2023, 898, 165583. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Wang, X.; Lü, S.; Huang, Z.; Huang, X.; Yang, W.; Wang, Y.; Zhang, Q. Spatiotemporal patterns and source implications of aromatic hydrocarbons at six rural sites across China’s developed coastal regions. J. Geophys. Res. Atmos. 2016, 121, 6669–6687. [Google Scholar] [CrossRef]
- Wu, T.; Wang, X.; Li, D.; Yi, Z. Emission of volatile organic sulfur compounds (VOSCs) during aerobic decomposition of food wastes. Atmos. Environ. 2010, 44, 5065–5071. [Google Scholar] [CrossRef]
- Zhang, D.; Huang, X.; Xiao, S.; Zhang, Z.; Zhang, Y.; Wang, X. Characterization and Sources of VOCs during PM2.5 Pollution Periods in a Typical City of the Yangtze River Delta. Atmosphere 2024, 15, 1162. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Zhang, Y.; Lü, S.; Huang, Z.; Huang, X.; Wang, Y. Ambient air benzene at background sites in China’s most developed coastal regions: Exposure levels, source implications and health risks. Sci. Total Environ. 2015, 511, 792–800. [Google Scholar] [CrossRef]
- Sulyok, M.; Haberhauer-Troyer, C.; Rosenberg, E. Observation of sorptive losses of volatile sulfur compounds during natural gas sampling. J. Chromatogr. A 2002, 946, 301–305. [Google Scholar] [CrossRef]
- Zheng, J.; Yu, Y.; Mo, Z.; Zhang, Z.; Wang, X.; Yin, S.; Peng, K.; Yang, Y.; Feng, X.; Cai, H. Industrial sector-based volatile organic compound (VOC) source profiles measured in manufacturing facilities in the Pearl River Delta, China. Sci. Total Environ. 2013, 456–457, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Harreveld, A.P.v. Odor Concentration Decay and Stability in Gas Sampling Bags. J. Air Waste Manage. Assoc. 2003, 53, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-H.; Kim, K.-H.; Jo, S.-H.; Jeon, E.-C.; Sohn, J.R.; Parker, D.B. Comparison of storage stability of odorous VOCs in polyester aluminum and polyvinyl fluoride Tedlar® bags. Anal. Chim. Acta 2012, 712, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Li, G. Analysis of the stench sulfide components in the environmental air by GC/MS. Arid Environ. Monit. 2014, 28, 174–177+181. [Google Scholar]
| Name | Material | Volume | Manufacturer 1 |
|---|---|---|---|
| PET bag | polyethylene glycol terephthalate | 10 L | A/B/C |
| AL bag | aluminum foil composite film | 3 L | A/B/C |
| FEP bag | fluorinated ethylene propylene | 3 L | A/B/C |
| PVF bag | polyvinyl fluoride (Tedlar®) | 3 L | A/B/C |
| PVDF bag | polyvinylidene fluoride | 3 L | B |
| PCTFE bag | polychlorotrifluoroethylene | 3 L | A |
| Canister | stainless steel with passivated inner surface of a fused silica layer (Silonite®/Siltek®/InertSi®) | 6 L | D/E/F |
| Species | CAS No. | Formula | Abbr. | RT/min | SIM Ion | R2 | RSD/% | MDL/ppbv |
|---|---|---|---|---|---|---|---|---|
| Methanethiol | 74-93-1 | CH4S | MeSH | 7.39 | 47/48/45 | 0.994 | 11.6 | 0.059 |
| Ethanethiol | 75-08-1 | C2H6S | EtSH | 8.93 | 62/47/45 | 0.991 | 14.2 | 0.081 |
| Dimethyl sulfide | 75-18-3 | C2H6S | DMS | 9.34 | 62/47/45 | 0.999 | 6.3 | 0.018 |
| Carbon disulfide | 75-15-0 | CS2 | CS2 | 9.91 | 76/44 | 0.998 | 4.8 | 0.008 |
| Methyl ethyl sulfide | 624-89-5 | C3H8S | MES | 12.14 | 76/61/48 | 0.999 | 7.6 | 0.022 |
| Thiophene | 110-02-1 | C4H4S | Th | 14.11 | 84/58/45 | 0.999 | 4.5 | 0.013 |
| Diethyl sulfide | 352-93-2 | C4H10S | DES | 15.32 | 75/90/61 | 0.999 | 5.5 | 0.026 |
| Dimethyl disulfide | 624-92-0 | C2H6S2 | DMDS | 16.93 | 94/79/45 | 0.999 | 8.6 | 0.057 |
| Methyl ethyl disulfide | 20333-39-5 | C3H8S2 | MEDS | 20.48 | 108/80/45 | 0.999 | 13.5 | 0.105 |
| Site | Warehouse Workshop | Extraction Workshop | Synthesis Workshop | Pyrolysis Drying Stack | Lithium Leaching Stack | Ni-Co Leaching Stack |
|---|---|---|---|---|---|---|
| MeSH | BDL 1 | BDL | BDL | BDL | 79.52 | 102.10 (BDL-464.99) 2 |
| EtSH | BDL | BDL | BDL | BDL | 0.72 | 857.71 (BDL-3784.68) |
| DMS | 0.02 | 0.01 | BDL | BDL | 0.23 | 3.37 (0.24–16.43) |
| CS2 | 0.35 | 0.46 | 0.25 | 4.83 | 7.71 | 6.47 (0.51–30.41) |
| MES | 0.01 | BDL | BDL | BDL | BDL | 9.85 (1.00–55.20) |
| Th | 0.02 | BDL | 0.02 | 98.13 | 1.02 | 4.30 (0.74–18.69) |
| DES | 0.02 | BDL | BDL | BDL | 0.09 | 4.35 (0.64–22.76) |
| DMDS | 0.07 | 0.08 | 0.05 | BDL | 0.29 | 14.13 (0.32–80.27) |
| MEDS | 0.04 | 0.17 | 0.12 | BDL | 0.43 | 44.58 (1.25–250.68) |
| Total VSCs | 0.53 | 0.72 | 0.45 | 102.96 | 90.01 | 1046.86 (19.76–4313.04) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, T.; Zhang, Z.; Ou, Z.; Li, S.; Zhang, Y.; Wang, X. Evaluating Sampling Materials for Atmospheric Volatile Organosulfur Compounds Measurement and Application in the Power Battery Recycling Industry. Atmosphere 2025, 16, 1341. https://doi.org/10.3390/atmos16121341
Fang T, Zhang Z, Ou Z, Li S, Zhang Y, Wang X. Evaluating Sampling Materials for Atmospheric Volatile Organosulfur Compounds Measurement and Application in the Power Battery Recycling Industry. Atmosphere. 2025; 16(12):1341. https://doi.org/10.3390/atmos16121341
Chicago/Turabian StyleFang, Tianyu, Zhou Zhang, Zhongxiangyu Ou, Sheng Li, Yanli Zhang, and Xinming Wang. 2025. "Evaluating Sampling Materials for Atmospheric Volatile Organosulfur Compounds Measurement and Application in the Power Battery Recycling Industry" Atmosphere 16, no. 12: 1341. https://doi.org/10.3390/atmos16121341
APA StyleFang, T., Zhang, Z., Ou, Z., Li, S., Zhang, Y., & Wang, X. (2025). Evaluating Sampling Materials for Atmospheric Volatile Organosulfur Compounds Measurement and Application in the Power Battery Recycling Industry. Atmosphere, 16(12), 1341. https://doi.org/10.3390/atmos16121341







