Abstract
Atmospheric carbonyl compounds have significant impacts on the atmospheric environment and human health, making the selection of appropriate analytical techniques crucial for accurately detecting these compounds in specific environments. Based on extensive literature research, this study summarized the development history, relevant features, and applicable scenarios of the main analytical techniques for atmospheric carbonyl compounds; pointed out the main problems and challenges in this field; and discussed the needs and prospects of future research and application. It was found that the direct sampling methods of atmospheric carbonyl compounds were applicable to low-molecular-weight carbonyl species with low reactivity, low boiling points, high polarity, and high volatility, while indirect sampling methods were suitable for a wider range and various types and phases of species. For formaldehyde, offline detection was primarily influenced by chemical reagents and reaction conditions, whereas online monitoring relied on sufficiently stable operating environments. For multiple carbonyl compounds, offline detection results were greatly influenced by detectors coupled with chromatography, whereas online monitoring techniques were applicable to all types of volatile organic compounds (VOCs), including some carbonyl compounds, providing higher temporal resolution and improved isomer identification with the development of online mass spectrometry. The combined use of proton transfer reaction-mass spectrometry (PTR-MS) and liquid chromatography-mass spectrometry (GC-MS) was suitable for the detection of carbonyl compounds in atmospheric photochemical smog chamber simulation studies. Currently, offline analytical techniques for carbonyl compounds require significant time and advanced experimental skills for multiple optimization experiments to detect a broader range of species. Online monitoring techniques face challenges such as poor stability and limited species coverage. In smog chamber simulation studies, the detection of carbonyl compounds is heavily influenced by both the sampling system and the chamber itself. Future efforts should focus on improving the environmental adaptability and automation of carbonyl compound analytical techniques, the synergistic use of various techniques, developing new sampling systems, and reducing the impact of the chamber itself on carbonyl compound detection, in order to enhance detection sensitivity, selectivity, time resolution, accuracy, and operability.
1. Introduction
Atmospheric carbonyl compounds are significant oxygenated volatile organic compounds (OVOCs) [1] that play essential roles as precursors and intermediates in photochemical reactions, contributing substantially to the formation of peroxyacetyl nitrate (PAN), ozone (O3), and secondary organic aerosols (SOAs) [2]. As precursors, carbonyl compounds can directly participate in atmospheric photochemical reactions, leading to the formation of secondary pollutants such as O3 and PAN [3]. These compounds can also undergo photolysis or react with OH radicals to affect the formation of SOAs by forming oligomers [4,5,6]. As intermediates in photochemical reactions, carbonyl compounds can undergo photolysis or further reactions with atmospheric radicals or oxidants [7], affecting the generation of photochemical pollutants [8]. These chemical reactions of carbonyl compounds play a crucial role in the comprehensive understanding of atmospheric auto-oxidation, gas-particle partitioning, and photochemical mechanisms [9]. Furthermore, carbonyl compounds have significant impacts on both the ecosystem and human health. As components of volatile organic compounds (VOCs), carbonyl compounds directly affect air quality and pose immediate health risks. Most of these compounds are highly irritating, potentially causing respiratory infections and posing sensitizing, carcinogenic, and mutagenic hazards [10]. Formaldehyde and acetaldehyde have been classified as carcinogenic and teratogenic by the World Health Organization (WHO) [11]. Acetone has been recognized as having significant effects on blood and kidney health [12], and crotonaldehyde has been found to be carcinogenic in animals [13]. Pollutants like PAN, O3, and SOA, formed from chemical reactions involving carbonyl compounds, can influence climate change and lead to photochemical smog and acid rain, thereby indirectly posing serious threats to the atmospheric environment, vegetation, and human health. Consequently, atmospheric carbonyl compounds have become a key focus in atmospheric environmental research.
Atmospheric carbonyl compounds exhibit a wide range of concentration variability, high reactivity, and instability, with typically short atmospheric lifetimes [14,15]. This places stringent demands on sampling and analytical techniques. Accurate measurement of the types and concentrations of atmospheric carbonyl compounds in various environments has become a critical prerequisite for studying their pollution characteristics, reaction mechanisms, environmental impacts, and health effects. Overall, the concentration levels and composition of carbonyl compounds vary across different atmospheric environments. Concentrations in rural areas are generally lower than those in urban and suburban areas, with even higher levels observed in large cities [16]. This variation necessitates analytical techniques with high sensitivity and selectivity to accurately measure a wide range of concentrations and multiple carbonyl species in diverse environments. Atmospheric carbonyl compounds are easily affected by other environmental highly oxidative species, leading to potential volatilization, degradation, or chemical reactions of certain compounds during sampling or storage [17]. Thus, choosing suitable sampling techniques, derivatization reagents, or adsorbents is crucial. Some carbonyl compounds possess strong polarity and good water solubility, allowing them to exist simultaneously in both the gas and aerosol phases. The atmospheric lifetime and impact of these compounds undergo significant changes between the gas and particle phases [18], requiring the use of accurate and appropriate methods for simultaneous detection of their gas- and particle-phase species and concentrations. Smog chamber simulation studies involve numerous experimental scenarios and complex chemical reactions, which generate various types of carbonyl compounds and unknown carbonyl species. Additionally, smog chambers differ in size, shape, materials, and light sources worldwide, all of which can affect the chemical reactions within the chamber [19]. Therefore, the identification of carbonyl compounds in smog chamber simulation studies requires detection technologies that meet high time resolution, high sensitivity, high stability, and high selectivity. Notably, formaldehyde is one of the most abundant, reactive, and volatile carbonyl compounds in the atmosphere. Ubiquitous and highly toxic, formaldehyde has significant impacts on daily life, requiring timely and accurate monitoring of its concentration levels [20,21]. To fully assess formaldehyde pollution in specific settings, it is often measured separately.
Analytical methods for carbonyl compounds can be categorized into offline and online analytical methods. Offline methods involve separate steps for sampling, pretreatment, and analysis of targeted carbonyl compounds, while online methods integrate sampling, pretreatment, and analysis into a unified system. The 2,4-dinitrophenylhydrazine (DNPH) derivatization method coupled with high-performance liquid chromatography/ultraviolet (HPLC/UV) is a common offline method for detecting carbonyl compounds. Coupling gas chromatography (GC) or liquid chromatography (LC) with mass spectrometry (MS) enables the offline detection of a wider range of carbonyl species [22]. Formaldehyde can also be detected offline using methods such as fluorescence, spectrophotometry, and electrochemistry [23,24]. Online analytical methods for carbonyl compounds primarily include online gas chromatography-flame ionization detector/mass spectrometry (GC-FID/MS) and proton transfer reaction-mass spectrometry (PTR-MS) [25]. Formaldehyde can be monitored online using techniques such as Fourier transform infrared spectroscopy (FTIR), laser-induced fluorescence (LIF), differential optical absorption spectroscopy (DOAS), tunable diode laser absorption spectroscopy (TDLAS), cavity-enhanced absorption spectroscopy (CEAS), cavity ring-down spectroscopy (CRDS), and gas sensors [21,26]. The selection of analytical techniques for carbonyl compounds depends on factors such as the specific application scenario, the properties of the targeted compounds, the detection objectives, and cost considerations.
Currently, carbonyl compound analytical methods are advancing rapidly, with both improvements in existing techniques and the emergence of new methods. The optimization of common analytical techniques for carbonyl compounds is frequent, with numerous studies focusing on technical advancements. Some researchers have conducted comprehensive reviews of specific techniques, such as Wu et al.’s review on spectral detection techniques for formaldehyde [27] and Chung et al.’s review on formaldehyde sensor technologies [26]. Other studies have reviewed analytical methods for multiple carbonyl compounds in various environments, but these reviews do not specifically focus on atmospheric analytical methods [28]. The reviews on analytical methods in the atmospheric environment often cover only the significant advancements and primary performance characteristics of certain techniques, with limited summaries on other common methods and their specific parameters [29], and lack a summary of detection techniques for particle-phase carbonyls and smog chamber simulation studies. Given the current lack of comprehensive reviews on the optimization history, applicability, and specific parameters of commonly used analytical techniques for atmospheric carbonyl compounds, this study provides a comprehensive overview of sampling methods for gas- and particle-phase carbonyl compounds based on extensive literature research and systematic analysis, examining the optimization process and suitable applications of online and offline methods for detecting formaldehyde and multiple carbonyl compounds and summarizing the detection technologies for carbonyl compounds involved in atmospheric photochemical smog chamber simulation studies. It also highlights the primary problems and challenges in current sampling and analytical techniques. Finally, future research needs and prospects for sampling and analytical techniques of atmospheric carbonyl compounds were discussed. The findings of this study will help clarify the optimization processes and applicability of commonly used carbonyl compound analytical techniques, providing a reference for the selection and optimization of current analytical methods and offering guidance for the improvement and innovation of future analytical methods.
2. Methodology
To comprehensively review and summarize the analytical methods for atmospheric carbonyl compounds, the databases of Web of Science, Google Scholar, and China National Knowledge Network (CNKI) were systematically searched. Keywords such as “carbonyls analytical methods”, “OVOCs analytical methods”, “monitoring techniques”, “derivatization”, “online mass spectrometry”, “formaldehyde gas sensor”, and “HCHO spectroscopy” were used to conduct the subject search, and 434 articles were obtained. By screening and analyzing the search results to eliminate duplicates and optimize keywords, it was determined that detailed information on 269 articles needed to be retrieved, of which 6 articles were excluded as they could not be obtained through available channels. Subsequently, based on the objectives of this review, the titles, abstracts, and full texts of the 263 articles were identified for further review. A total of 82 articles were excluded due to their irrelevance to the research topic, redundancy, and poor citation value resulting from incomplete or missing full text information. Finally, 180 research articles were obtained through the database. In addition, 36 research materials were obtained through other methods, such as websites, university and research institute sites, and citation searching. After deduplication and evaluation, 23 materials were included in this review. In total, through various methods, 202 eligible materials were selected and used in this review. The review followed the PRISMA flow methodological phases, as shown in Figure 1, together with the corresponding inclusion and exclusion criteria.
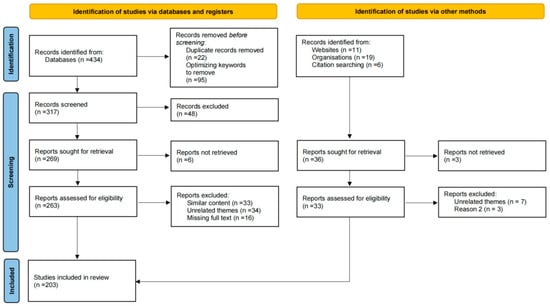
Figure 1.
PRISMA flow diagram.
As shown in Figure 2, the research materials used in this review included 72 domestic and 79 international articles and standards spanning the period from the 1970s to the 2020s. To further analyze the research materials, the majority of them were divided into three major sections based on the research subjects (formaldehyde, multiple carbonyl compounds, and chambers). The formaldehyde section included offline (derivatization) and online (spectroscopy and sensor), while the multiple carbonyl compounds section included offline (LC/GC-MS and DNPH-HPLC-UV) and online (GC-FID/MS and online MS). Additionally, due to the comprehensive nature of review articles, the unique source of standard documents, and the use of other types of documents in the Section 1, the remaining materials were categorized into reviews, standards, and others. It was observed that articles on carbonyl compounds analytical methods have increased rapidly since the 1990s. For formaldehyde, the research mainly focused on online analytical methods, while both online and offline methods for multiple carbonyl compounds were relatively abundant.
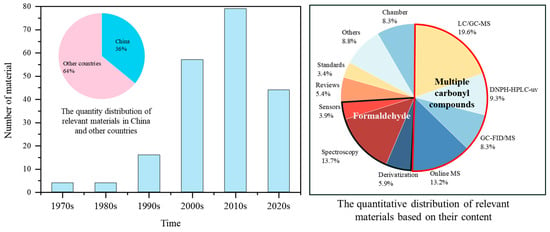
Figure 2.
Distribution of 151 materials on analytical methods for atmospheric carbonyl compounds from the perspectives of countries and content.
3. Sampling Methods for Atmospheric Carbonyl Compounds
Sampling methods for atmospheric carbonyl compounds can be categorized into direct and indirect sampling methods. Direct sampling methods include tube sampling, canister sampling, and cryogenic enrichment techniques. Tube sampling was applied to carbonyl compounds in the 1960s–1970s, while cryogenic enrichment was primarily used for sampling VOCs. Canister sampling began to be employed for VOCs in the 1970s–1980s. With advancements in new adsorbent materials, refrigeration techniques, canister materials, and analytical instruments, tube sampling was extended to other VOC types, and cryogenic enrichment enabled targeted sampling of carbonyl compounds in the 1980s–1990s. Canister sampling efficiency for carbonyl compounds significantly improved in the 1990s. Indirect sampling methods primarily rely on derivatization techniques, which originated in the 1960s–1970s. Various derivatization reagents and coating methods emerged in the 1980s–1990s. In the 2000s, the automation of various sampling techniques improved, and combined approaches began to be employed (Figure 3).
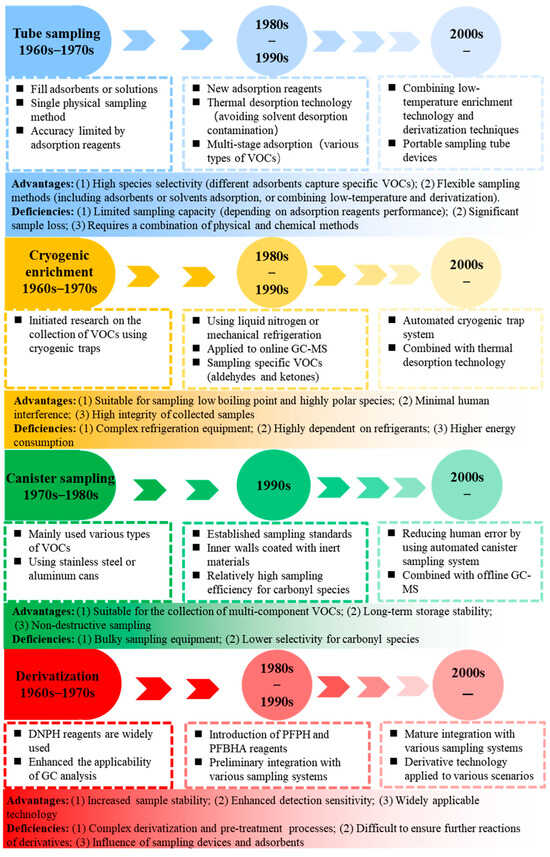
Figure 3.
Development of main sampling methods for atmospheric carbonyl compounds.
3.1. Direct Sampling Methods
Tube sampling techniques achieve efficient collection of carbonyl compounds by enriching them with solvents or adsorbents. Solvent absorption is simple and cost-effective but is only suitable for carbonyl compounds with low molecular weight and high concentration, such as formaldehyde. Adsorbents are more effective for aldehydes (>C5). The use of multi-layer adsorption tubes enhances the ability to sample C4 aldehydes effectively [30]. Under certain cryogenic conditions, low-boiling-point carbonyl compounds can also be adsorbed. Furthermore, highly polar carbonyl compounds may undergo irreversible adsorption in adsorption tubes [31]. Using cryogenic polytetrafluoroethylene (Teflon) sampling tubes can mitigate this issue [32], effectively improving the sampling efficiency of carbonyl compounds. However, different solvents or adsorbents are only suitable for capturing specific types of compounds, posing certain limitations in applications involving simultaneous sampling of multi-component carbonyl compounds. Currently, tube sampling methods often integrate physical collection with chemical derivatization, such as DNPH-coated silica gel or PFPH (pentafluorophenylhydrazine)-coated Tenax TA adsorbents, to collect a broader range of carbonyl compounds [33,34]. Solvent absorption or adsorbent collection alone is currently limited to formaldehyde sampling.
Canister sampling techniques use specially designed stainless steel or metal canisters to collect atmospheric samples. It is a standard VOC sampling method recommended by the U.S. Environmental Protection Agency (EPA) (TO-14A, TO-15), typically coupled with GC-MS for offline detection of various types of VOCs [35,36]. Its application to carbonyl compound sampling represents a natural extension of existing VOC analytical methods. Canister sampling is widely used due to its advantages of multi-component analysis, environmental friendliness, and repeatable sample injection. However, due to the high reactivity of carbonyl compounds, the storage stability of samples collected using canister sampling is relatively poor, making this method currently suitable only for a limited number of carbonyl compound species [37,38].
Cryogenic enrichment techniques create low-temperature environments to concentrate low-boiling-point atmospheric carbonyl compounds. These techniques are typically coupled with online GC-FID/MS for detecting VOCs, including carbonyl compounds. Currently, most applications utilize liquid nitrogen or other refrigerants to achieve ultra-low temperatures. Cryogenic trap-freezing techniques based on liquid nitrogen enhance the capture of polar carbonyl compounds [39]. When combined with thermal desorption, these methods improve collection efficiency and reduce losses of carbonyl compounds [32], enabling continuous monitoring of more than ten species. However, these techniques heavily rely on refrigerants, and the associated cryogenic traps are bulky and complex, limiting their use in remote areas. Mechanical refrigeration systems that do not require refrigerants are currently applicable to only a limited range of carbonyl compounds (C3–C6) [40]. In addition, the online spectroscopy and sensor-based monitoring technologies for formaldehyde sampling directly draw air through pipeline systems.
3.2. Indirect Sampling Methods
Indirect sampling methods primarily employ derivatization techniques, which reduce the polarity and reactivity of target analytes to enhance the stability of carbonyl compound samples [41]. Following sampling with this method, carbonyl compound samples require pretreatment before analysis with relevant instruments, which can result in higher sample loss and increased time consumption [42]. Derivatization techniques are typically used for the offline detection of formaldehyde or combined with chromatography for analyzing multiple carbonyl compounds. The choice of derivatization reagent requires careful evaluation to ensure stable reaction products compatible with the selected separation and detection procedures [43]. Common derivatization reagents include DNPH, PFPH, and PFBHA.
DNPH is suitable for derivatization detection using LC, and the most commonly used sampling method involves directly passing gas into a device containing a DNPH solution or a cartridge with an acidic solid adsorbent. The device with the DNPH solution is suitable for the enrichment of formaldehyde alone [44], while the cartridge with the adsorbent is suitable for multiple species, with its adsorption performance dependent on the type of adsorbent. Silica cartridges with DNPH have a cleaner background and better breakthrough resistance compared with C18 [45], and they can remain stable at <4 °C for up to six months. However, the background levels of formaldehyde and acetone may increase with prolonged storage times and elevated temperatures. Studies have found that using a gas-phase coating method [46] can reduce the background concentration levels of carbonyl compounds. DNPH is prone to react with oxidants in the environment, such as NOX and O3, generating interfering compounds. Attaching an IK column or a glass annular tube to the front of the sampling device can remove interference from O3 and other photochemical oxidants on the derivatives [47,48]. Furthermore, unsaturated carbonyl compound derivatives can further react with DNPH, forming dimers and trimers, and silica gel adsorbents may promote this reaction, leading to uncertainties in concentration measurements [49]. Kahnt et al. [50] compared denuders coated with XAD-4 resin and those simultaneously coated with XAD-4 and DNPH and found that using XAD-4 with DNPH improved the collection efficiency of carbonyl compounds. In contrast, XAD-4 resin alone had lower collection efficiency for high-volatility carbonyl compounds, such as formaldehyde, acetaldehyde, acetone, and methacrolein, but was less affected by atmospheric oxidants, including NO2 and O3.
The five fluorine atoms in O-(2,3,4,5,6-pentafluorobenzyl) hydroxylamine (PFBHA) and PFPH make the carbonyl-PFPH/PFBHA derivatives more thermally stable and volatile than DNPH derivatives, making them suitable for GC [51]. PFPH is relatively inexpensive compared with PFBHA and exhibits better derivatization performance. The compatible sampling systems include adsorption tubes, denuders, impactors, and cartridges. The most common and easy-to-operate method is to use derivatizing reagents coated on solid adsorbent tubes. Coating methods include solid-phase adsorption, gas-phase coating, and liquid-phase coating [52]. The same GC-MS analytical technique requires adjustments in coating methods based on different detection needs and analytical objectives [53]. Common adsorbents include silica gel, XAD series, and Tenax series adsorbents. XAD-2 and XAD-7 are thermally unstable, while C18 silica gel has lower efficiency than Tenax adsorbents in desorbing PFBHA derivatives [54]. Tenax TA adsorbents show varying absorption efficiencies and collection rates for different species under different derivatizing reagent coatings. Tenax TA adsorbents with PFBHA require lower rates and collect fewer species, while Tenax TA adsorbents with PFPH are widely used and suitable for C1-C10 species [55]. The combination of the same adsorbent with different derivatizing reagents can also affect the derivatization reaction. Tenax GR adsorbents with PFBHA remain stable for only one day, while Tenax TA adsorbents with PFBHA can last for ten days. Additionally, silica gel cartridges coated with PFPH can effectively capture carbonyl compounds in the air, with a lower detection limit compared with the classic HPLC-UV/visible spectrometry method [56].
Solid-phase microextraction (SPME) is a sampling and enrichment technique first developed by Arthur and Pawliszyn (1990) [57]. SPME fibers can be directly derivatized [58] and exposed to air or headspace during sampling to extract and retain analytes. Headspace solid-phase microextraction (HS-SPME) avoids direct contact with complex sample matrices by extracting the compounds in the sample’s headspace, while other SPME methods extracts compounds by direct immersion in the sample. The choice of method depends on the nature of the sample, the volatility of the target analytes, and the requirements for analytical sensitivity and selectivity. The advantages of SPME include high selectivity and sensitivity, the option for automation to minimize human error and solvent consumption, and increased analytical throughput due to fewer preparation steps and shorter extraction times [59]. However, due to the limited adsorption capacity of the fibers, competition between saturation effects and analytes may affect the performance and reproducibility of SPME. Fiber effects can alter the extraction efficiency [60], and cooling the fibers during extraction can improve the SPME efficiency [61]. Studies have found that the effectiveness of different derivatizing reagents on SPME fibers varies significantly. PFPH-coated SPME fibers remain stable for about 11 weeks, while PFBHA-coated SPME fibers remain stable for only about 3 days. Ketones react much more slowly than aldehydes with PFBHA-coated SPME fibers. Furthermore, the loading of PFPH-coated SPME fibers is at least five times greater than that of PFBHA, but the byproducts generated from PFPH derivatization are significantly more than those from PFBHA [62].
The sampling methods mentioned above lack the ability to differentiate between the gas phase and particle phase, making it difficult to simultaneously measure the gas- and particle-phase concentrations of carbonyl compounds. Traditional filter membrane sampling systems are a technique used to collect particulate matter and semi-volatile organic compounds (SVOCs) in the atmosphere. By combining with derivatization techniques, this system can facilitate the collection of carbonyl compounds. Particles are collected on quartz or PTFE filters, followed by sample extraction using solvent extraction, supercritical fluid extraction, or thermal desorption. The extracted samples are then analyzed using analytical techniques. Filtered samples typically require several hours or days of sampling, and compound loss may occur due to volatilization, gas-phase adsorption, and reactions during the collection process [9].
Collecting particle-phase samples on impact surfaces is an effective method for high-throughput sample collection. The thermally desorbed particle beam mass spectrometer (TDPB-MS) [63] focuses particles using an aerodynamic lens, then samples the cooled impact surface in a high-vacuum chamber. Williams et al. [64] proposed a new in situ instrument, the thermal desorption aerosol GC/MS-FID (TAG), collecting aerosols on impact surfaces, including both the gas and particle phases of carbonyl compounds. These methods offer high sample throughput, but they are not compatible with a range of different detectors. Hohaus et al. [65] developed an aerosol collection module (ACM) for sampling atmospheric aerosols, which allows derivatization during the collection process. This system is suitable for smoke chamber studies with limited sample volumes where large-volume collectors cannot be used. The ACM allows for the selection of detector types based on specific analytical capabilities, addressing different scientific questions without altering the sampling method. For example, it can be used with GC-MS to study isomers in samples or with PTR-MS for high-resolution detection of species.
The denuder sampling method has been proven effective for accurately measuring the gas-particle distribution of volatile organic compounds. Denuders coated with XAD-4 resin and PFBHA are an effective method for measuring the gas-particle distribution and partitioning of semi-volatile carbonyl compounds. Appropriate sampling flow rates can achieve satisfactory collection efficiency for mono-, di-, and aromatic carbonyl compounds, as well as carbonyl-containing furans and quinones. However, prolonged sampling times lead to a sharp decline in collection efficiency. Temime et al. [66] compared the absorption efficiency of denuders using adsorbents alone versus those using both adsorbents and derivatization reagents. They found that the PFBHA-coated denuder had a collection efficiency exceeding 90%, significantly higher than that of denuders using XAD-4 as the sole adsorbent. Compared with traditional filter sampling methods, the PFBHA-coated denuder–filter system reduces gas-phase adsorption onto the filter, thereby minimizing errors during the sampling process. Based on the denuder technique, Eichler et al. [67] proposed a novel modular injection system called “chemical analysis of aerosol online” (CHARON). It consists of a gas-phase denuder, an aerodynamic lens, and a thermal desorption device, aiming to provide online chemical characterization of semi-volatile submicron particles.
3.3. Applicability and Selection Principles of Sampling Methods
Direct sampling methods for atmospheric carbonyl compounds do not require chemical reactions, thus minimizing human-induced interference; however, they face challenges in ensuring the stability of the sampled compounds. Among them, tube sampling methods are suitable for collecting strong volatile low-molecular-weight carbonyl compounds, and when combined with chemical derivatization, a wider range of carbonyl compound species can be collected. Canister sampling methods are used for offline sampling of various VOCs, including less reactive carbonyl compound species; however, the storage stability of samples containing highly reactive carbonyl compounds is relatively poor. The cryogenic enrichment technique requires the rapid and stable capture and release of target compounds, making it suitable for online enrichment of low-boiling and highly polar carbonyl compound species.
Samples collected via indirect sampling methods are more stable but require sample pre-treatment, leading to higher sample loss and time consumption. The selection of derivatization reagents, adsorbents, coating methods for reagents, and sampling devices must be carefully evaluated according to different detection requirements and analytical objectives. For example, DNPH solid-phase adsorbent cartridge and denuder sampling are commonly combined with LC, while PFPH and PFBHA are typically used with various adsorbents and sampling devices for sampling, followed by GC analysis. Filters, impactors, and denuders generally use PFPH and PFBHA for derivatization, coupled with GC or PTR-MS for detecting particle-phase carbonyls.
4. Analytical Methods for Atmospheric Formaldehyde
Analytical methods for formaldehyde are classified into online and offline techniques. In the 1970s, derivatization-based fluorescence and spectrophotometry methods were introduced for offline formaldehyde detection, followed by efforts to develop novel derivatization reagents and optimize chemical reaction conditions. During the 1980s and 1990s, techniques such as DOAS, LIF, and semiconductor and electrochemical sensors were the primary methods for online formaldehyde monitoring. In the early 21st century, CEAS, CRDS, and photochemical sensors were introduced.
4.1. Offline Analytical Methods for Formaldehyde
Offline analytical methods for formaldehyde primarily include derivatization-based fluorescence and spectrophotometry methods. The fluorescence method is based on the Hantzsch reaction, quantifying formaldehyde by measuring the fluorescence intensity of the sample. Fluorescence intensity is influenced by reaction temperature, time, and reagents, with the choice of fluorescence reagents directly affecting detection sensitivity, selectivity, stability, and cost efficiency [68]. Dong et al. [69] designed and synthesized a fluorescence reagent with high selectivity for formaldehyde. This reagent operates under mild reaction conditions, exhibits vigorous chemical reactivity, and produces strong fluorescence intensity. Li et al. [70] developed a reagent applicable to both fluorescence and spectrophotometry methods. The fluorescence method offers a lower detection limit and higher sensitivity, while the spectrophotometry method is more economical and user-friendly.
The spectrophotometric method, also based on the Hantzsch reaction, detects formaldehyde by utilizing its light absorption characteristics at specific wavelengths. The results are significantly influenced by factors such as chromogenic temperature, reaction time, and chromogenic reagent [71]. By incorporating different reagents and optimizing analytical conditions, this method enables rapid detection of formaldehyde under various scenarios [23]. The phenol-reagent spectrophotometric method offers advantages such as low reagent cost and operational simplicity. Moreover, it demonstrates excellent overall performance in terms of detection limits, sensitivity, and anti-interference capabilities [44]. This method is widely used as an offline technique for detecting low concentrations of formaldehyde [72,73]. Ji et al. [74], following the experimental steps for formaldehyde detection specified in the national standard GB/T 18204.2—2014 [75], achieved low-concentration detection (0.061 μg) by optimizing the chromogenic reagent concentration, absorbent concentration, and chromogenic conditions. The acetylacetone-reagent method exhibits stable linearity and a wider detection limit range, making it suitable for detecting higher concentrations of formaldehyde with greater accuracy [72]. The AHMT-reagent method offers high selectivity and sensitivity [76], while spectrophotometric methods based on colorimetric probes demonstrate broad applicability [77]. Both approaches enable the accurate detection of formaldehyde concentrations in air. Ashraf et al. [78] developed a low-cost, highly sensitive, and selective offline analytical method for determining formaldehyde and acetaldehyde based on the DPD (N, N-diethyl-p-phenylenediamine) spectrophotometric method, achieving a resolution of at least 30 s.
4.2. Online Analytical Methods for Formaldehyde
Online analytical methods for formaldehyde primarily include spectroscopic and sensor-based methods (Table 1). Spectroscopy quantifies formaldehyde concentration based on the linear relationship between the light absorption or reflection intensity of formaldehyde or its derivatives and their concentrations. The detection limit is typically lower than that of sensor-based methods [24]. Spectroscopic techniques require a stable light source to minimize environmental interference and an extended optical path to achieve high sensitivity in field measurements [79,80], resulting in large and bulky equipment. Catoire et al. [81] demonstrated that the optical path length significantly affects TDLAS performance, with longer paths leading to lower detection limits and improved resolution. CRDS utilizes high-reflectivity mirrors, enabling light to reflect multiple times within the cavity to extend the effective optical path. However, this requires a large and complex cavity design [82,83]. Online application of fluorescence methods requires well-designed workflows for derivatization-based sample enrichment [84] and the ability to achieve low time resolution [85] to monitor formaldehyde in environments with significant concentration fluctuations [86].
Additionally, spectroscopic detection technologies have been used to detect di-carbonyl compounds, addressing the issue where PTR-MS is unable to effectively monitor acetaldehyde and methylglyoxal due to interference from similar fragment ions or isomers. The laser-induced phosphorescence of (methyl) GLyOxal spectrometry (LIPGLOS) method can be used for the simultaneous real-time quantification of acetaldehyde and methylglyoxal in the air, utilizing the unique phosphorescence lifetime of each molecule. At a single wavelength, acetaldehyde has a detection limit of 11 pptv in 5 min, and methylglyoxal has a detection limit of 243 pptv in 5 min [87]. The DOAS technique was first used for the direct measurement of acetaldehyde, with a detection sensitivity of 150 pptv over 2–15 min [88]. Improved technologies, such as long-path DOAS (LP-DOAS) [89] and passive multi-axis DOAS (MAX-DOAS) [90], have enhanced the sensitivity of acetaldehyde measurement, with detection limits of 100–200 ppt/10 min and 20 ppt/15 min, respectively. Light-emitting-diodes cavity-enhanced DOAS (LED-CE-DOAS) can also be used to measure acetaldehyde (detection limit, 28.5 ppt/min) and methylglyoxal (detection limit, 255 ppt/min) [91]. Another spectroscopic technique, incoherent broadband cavity-enhanced absorption spectroscopy (IBBCEAS), involves simultaneous detection across multiple wavelengths. When measuring acetaldehyde, it can achieve a detection limit of 29 ppt/min [92]. Compared with CRDS with wavelength-specific detection, IBBCEAS is better suited for humid environments and complex samples. Broadband cavity-enhanced absorption spectroscopy (BBCEAS) combined with a charge-coupled device (CCD) offers higher time resolution and lower detection limits (10 ppt/min), while coupling with a photomultiplier tube (PMT) provides a more cost-effective alternative [93].
Gas sensors can be categorized based on their detection principles into electrochemical, optical, and semiconductor gas sensors. These sensors are portable and exhibit high selectivity for formaldehyde but are susceptible to temperature and humidity fluctuations. Their performance is highly dependent on the sensor materials [94]. Electrochemical sensors quantify formaldehyde based on electrical signals generated by its adsorption onto sensor materials. However, these signals are prone to interference and lack stability [26]. Optical sensors rely on the optical properties of gases or their reaction products but are sensitive to dust interference [95,96]. Semiconductor gas sensors operate on the principle of changes in electrical properties caused by the adsorption of gas molecules onto semiconductor materials. Despite limitations such as a short linear range and susceptibility to interference, advancements in semiconductor materials and their diversity have made these sensors a research hot spot. Their advantages include fast response times, low cost, simple structures, long lifespan, miniaturization, and ease of operation [97]. Additionally, Warneke et al. [98] employed PTR-MS techniques to detect formaldehyde exclusively, enabling a rapid single 16 s data acquisition of formaldehyde in the troposphere and marine boundary layer from aircraft. The above technologies offer diverse solutions for real-time and accurate formaldehyde monitoring, ranging from portable environmental monitoring to high-precision industrial and airborne detection.

Table 1.
Main online monitoring methods for atmospheric formaldehyde 1.
Table 1.
Main online monitoring methods for atmospheric formaldehyde 1.
| Method | Principle | Optimization | LOD | TR | OP | Remarks | Ref. |
|---|---|---|---|---|---|---|---|
| DOAS | Spectral absorption characteristics | LP-DOAS | 0.45 ppb | 20 min | 5 km | Pathway located in the atmosphere; wide measurement range; no calibration required; poor time resolution | [99] |
| Xenon lamp as light source | 540–1200 ppt | 10–20 min | 4.9–8.2 km | [79] | |||
| Tungsten lamp as light source and different detectors | 198–1500 ppt | 5–20 min | 5–15 km | [100] | |||
| Low optical path | 3–4 ppt | - 2 | 426 m | [101] | |||
| MAX-DOAS | 0.7–4.2 ppb | 5 min | 40 km | [102] | |||
| FTIR | Infrared spectral absorption | Eight-mirror multi-reflection unit system | 4 ppb | 10 min | 1.08 km | Simultaneous analysis of multiple components; requires appropriate gas qualitative and quantitative prediction models | [103] |
| FTIR system equipment | 0.6 ppb | 5 min | 1 km | [80] | |||
| Commercial FTIR | 1.05–1.1 ppm | 0.2–1 s | 3.2–10 m | [104] | |||
| Hantzsch fluorimetry | Hantzsch reaction | Online analytical instrument based on Hantzsch reaction | 84 ppt | 120 s | - | Operate stably over the long term; significant differences between devices; requires independent design | [86] |
| 0.05 ppb | 18 min | - | [85] | ||||
| LIF | Fluorescence signal intensity at a specific wavelength of the laser | Frequency-doubled tunable dye laser | 50 ppt | - | - | Laser as the light source; relatively complex calibration process; larger size; high measurement accuracy | [105] |
| No sample collection, water extraction, or further chemical treatment required | 10 ppt | 100 s | - | [106] | |||
| No background detection and aerial surveying used | 36 ppt | 1 s | - | [107] | |||
| Direct in situ detection in a white multi-channel cell | 51 ppt | 1 s | - | [108] | |||
| Non-resonant-LIF (NR-LIF) | 261 ppt | 10 s | - | [109] | |||
| TDLAS | Molecular absorption spectroscopy | Lead salt laser | 0.75 ppb/300 ppt | 3 min | 33.5 m/153 m | High accuracy; relatively compact setup; suitable for airborne and other fields | [110,111] |
| Difference frequency generation (DFG) laser | 222 ppt | 1 min | 100 m | [112] | |||
| Quantum cascade laser (QCL) | 450 ppt/ 30–120 ppt | 1 min/1–10 s | 76 m/ 240 m | [113,114] | |||
| Interband cascade laser (ICL) | 207 ppt/ 153 ppt | 90 s/ 10 s | 54.6 m/ 96 m | [115,116] | |||
| CRDS | Vibrational absorption spectral characteristics | Pulsed CRDS | 300 ppb | - | - | Small sampling volume; unaffected by light source fluctuations. Interference by external environmental | [82] |
| OPO (optical parametric oscillator) light source | 112 ppt | 1 s | 300 m | [83] | |||
| System based on CRDS | 1–2 ppb/ 3 ppb | ~s | - | [117,118] | |||
| CEAS | Beer–Lambert law | IBBCEAS | 1.14 ppb | 30 s | 2.15 km | High sensitivity requires proper technological integration; complex system with poor stability. High stability and resolution be achieved by sacrificing optical path. | [119,120] |
| Mode-locked (ML)-CEAS | 3.3 ppb | - | - | [118] | |||
| V-shaped CEAS | 15 ppt | 10 s | 1.97 km | [121] | |||
| Pound–Drever–Hall (PDH) technique | 75 ppb | 1 s | 20 m | [122] | |||
| Electrochemical sensor | Electrical signal generated by formaldehyde adsorption on the sensor material | 20~50 ppb | 20 s to 30 min | Good repeatability; high resolution; high cost; poor stability | [26] | ||
| Optical sensor | Optical properties | 0.03~0.20 ppm | 15 min | High accuracy; affected by dust; expensive | [95,96] | ||
| Semi-conductor sensor | Changes in electrical properties generated by the adsorption of gas molecules on semiconductor materials | 50 ppm to 10 ppb | 8~131 s | Performance is related to the semiconductor material. | [97] | ||
1 LOD, limit of detection; TR, time resolution; OP, optical path; Ref., reference. 2 “-” means no data.
In summary, the results of offline formaldehyde detection are primarily influenced by chemical reagents and reaction conditions. By integrating various reagents and optimizing analytical conditions, rapid formaldehyde detection can be achieved for different scenarios. Spectroscopic online monitoring requires a stable light source to minimize environmental interference and an extended optical path to achieve high sensitivity. Most instruments are bulky, and optimized technologies such as CRDS and CRES feature complex systems. Gas sensors are portable and exhibit high selectivity for formaldehyde, but they are susceptible to environmental factors and highly dependent on the performance of sensor materials.
5. Simultaneous Analytical Methods for Multiple Atmospheric Carbonyl Compounds
Analytical methods for multiple carbonyl compounds are classified into online and offline techniques. In the 1970s and 1980s, HPLC-UV began to be used for offline detection of multiple carbonyl compounds. With advances in MS, HPLC-MS became a primary method in the 1990s, while canister-GC-MS enabled the detection of multiple VOC types. In the early 2000s, the development of various sampling methods led to significant advancements in GC-MS, and online monitoring technologies for multiple carbonyl compounds also began to emerge. By the 2010s, various online MS techniques were applied to the online monitoring of multiple carbonyl compounds (Figure 4).
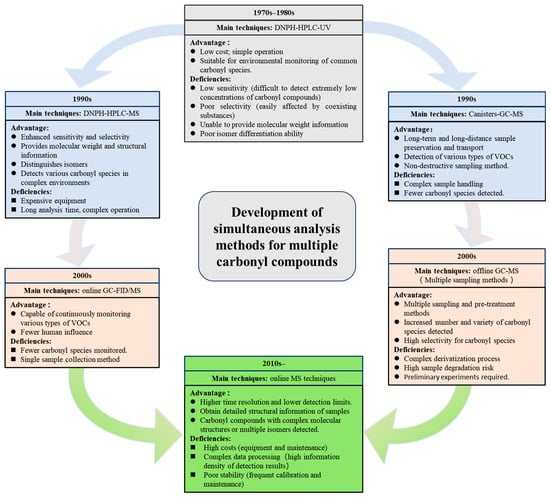
Figure 4.
Development of simultaneous analytical methods for multiple atmospheric carbonyl compounds.
5.1. Offline Analytical Methods for Multiple Carbonyl Compounds
Offline analytical methods for multiple carbonyl compounds are suitable for simultaneous multi-point sampling and for studying regional distribution patterns. These methods allow ample time for analytical preparation, enabling thorough qualitative and quantitative detection of target carbonyl compounds. The most commonly used offline methods include DNPH-HPLC-UV, GC-MS, and HPLC-MS techniques (Table 2).

Table 2.
Main offline analytical methods for multiple atmospheric carbonyl compounds.
5.1.1. DNPH-HPLC-UV
The DNPH-HPLC-UV technique is based on the reaction of carbonyl compounds with DNPH to form hydrazone derivatives, which are then eluted and analyzed using HPLC equipped with a UV detector [138]. This method’s high sensitivity, selectivity, and ease of use have led to its widespread adoption globally. In China, the “Ambient air—Determination of aldehyde and ketone compounds—High performance liquid chromatography (HJ 683-2014)” standard [139] follows the main guidelines from the U.S. EPA’s 1999 TO-11 method, making it suitable for detecting low-carbon atmospheric carbonyl compounds [42,140], although it has limitations in distinguishing between C3 [45] and C4 [141] carbonyls. The EPA’s 2016 TO-11 revision also removed unsaturated aldehydes from its analyte list. Researchers have found that using appropriate gradient elution conditions, column temperature, or specific chromatographic columns can enhance HPLC’s resolution of C3 and C4 carbonyls [142]. Possanzini et al. [143] demonstrated a secondary derivatization mechanism for alkenals (acrolein and crotonaldehyde) and optimized the mobile phase to separate these from other carbonyls. Wang et al. [131] selected appropriate columns and used a dual-gradient HPLC method with temperature and solvent control to address separation challenges for C3 (acrolein, acetone, and propanal) and C4 (crotonaldehyde, methacrolein, butanone, and butyraldehyde) compounds. Zhang et al. [144] improved separation of acrolein, acetone, and propanal using multiple mobile phases, while other researchers completed the detection of 13 OVOCs within 10 min by using specialized columns [145]. The 2020 Chinese standard, “Ambient air—Determination of aldehyde and ketone compounds—Solution absorption-High performance liquid chromatography (HJ 1154-2020)”, builds on the 2014 standard by enriching carbonyl compounds via solution absorption, followed by liquid extraction and concentration for the detection of 16 different carbonyl compounds [146]. Although the above DNPH-HPLC-UV techniques provides stable detection for common carbonyls, the number of detectable species remains limited due to detector constraints.
5.1.2. Chromatography Tandem MS
Chromatography tandem MS is currently the most widely used method in laboratories for detecting multiple carbonyl compounds, primarily including LC-MS and GC-MS techniques. Optimizing this technique allows for the differentiation of various types of carbonyl compounds and enables the detection of a broader range of species, although it often involves complex procedures [147]. Studies have found that the ionization source in MS significantly influences detection performance. When using electrospray ionization (ESI) in combination with the single-ion monitoring (SIM) mode, the detection limit is minimized [148]. The use of atmospheric pressure photoionization (APPI) allows for the detection of carbonyls without the need for dopants, reducing the potential interference caused by dopants [149]. The HPLC-MS/MS (tandem MS) method has been shown to differentiate between aldehydes and ketones, linear and branched structures, and unsaturated and aromatic carbonyl compounds [46]. Fourier transform ion cyclotron resonance MS (FTICR-MS) with nano-electrospray ionization (nano-ESI) allows for direct sample injection without the need for derivatization [150]. Zhang et al. [136] optimized sampling and analysis processes, using DNPH-HPLC/APCI-MS (atmospheric pressure chemical ionization) to detect 30 OVOCs, including mono-carbonyl, di-carbonyl, and oxygen-containing and heterocyclic carbonyl. Chi et al. [135] made customized adjustments to ESI and MS/MS parameters for different carbonyl compounds, achieving the detection of 32 carbonyl species using DNPH-HPLC-ESI-MS/MS. Xu et al. [137] developed a novel UHPLC-MS/MS-based analytical method to identify and quantify 47 carbonyl compounds with carbon numbers ranging from 1 to 13, including 28 aliphatic saturated mono-carbonyls, 8 aromatic mono-carbonyls, 8 other unsaturated mono-carbonyls, and 3 di-carbonyl compounds.
The canister sampling GC-MS method is the standard offline method for measuring VOCs as specified by the Ministry of Ecology and Environment, typically measuring only a limited number of carbonyl compounds, such as acetone, acrolein, and 2-butanone [151]. Some researchers have achieved the measurement of low-concentration carbonyl compounds in the C4–C8 range by adjusting various parameters of instruments and the column temperature program [39,42]. The Deans Switch central cutting device enables the simultaneous measurement of other VOCs and 13 carbonyl compounds in a single analysis run [123,124]. The use of supercritical fluid as a mobile phase has addressed the issue of limited peak capacity in GC-MS [125]. The collection efficiency of carbonyl compounds can be greatly enhanced by using sampling methods such as sampling tubes, sampling columns, denuders, and SPME fibers, as well as optimizing the sample pretreatment and analysis processes. Zou et al. [152] collected atmospheric samples using a Tenax-TA sampling tube coated with PFBHA and determined 14 mono-carbonylic compounds and 2 di-carbonyl compounds in the atmosphere using solvent extraction coupled with GC-MS. After collecting carbonyl compounds with PFPH tubing, pretreatment is required, typically involving solvent desorption, and this process can be complex [51]. Li et al. [34] developed a GC/MS method for the simultaneous determination of 20 carbonyl compounds by altering the solvent extraction reagent and found that formaldehyde was not completely separated due to significant human factors. The TD technique can significantly simplify the operation process and enable automated desorption, concentration, and analysis [52]. Chien et al. [126] used liquid-coated PFPH tubing combined with TD-GC-MS and multiple-bed adsorbent tubes to achieve simultaneous detection of carbonyl compounds and aromatic hydrocarbons. Wang et al. [153] used multi-bed absorption tubes combined with two-stage TD and GC-MS techniques to detect 28 OVOC species in Hong Kong, including aldehydes, ketones, alcohols, acrylates, acetate esters, and ethers, although the process was highly complex. Kahnt et al. [50] used an XAD-4/DNPH denuder for in situ derivatization on the surface, reducing the sample preparation steps, and detected 12 carbonyl compounds using HPLC/ESI-TOF-MS techniques. Temime and Brice [66] employed an XAD-4/PFBHA denuder-filter sampling method, combined with GC-MS, to detect gas- and particle-phase arbonyl compounds, identifying 23 aldehydes and ketones, with a distinct advantage in the analysis of isomers. Bourdin and Desauziers [62] and Poole et al. [128] used SPME fibers for in situ PFPH derivatization, significantly improving the time resolution for aldehyde species detection while reducing the sample preparation steps.
Quinone compounds are organic compounds that contain one or more carbonyl groups (C=O), typically consisting of one or more aromatic rings. Until the 2010s, only a few studies had reported the concentration and gas-particle distribution of environmental quinones. Few reports have been made on the spectroscopic detection techniques for quinones. As early as the 1950s, Fuson et al. [154] conducted an infrared spectroscopic study on various types of ketones, benzoquinones, and naphthoquinones, investigating the influence of carbonyl (C=O) stretching frequencies. They found that the molecular structure, conjugation effects, substituent effects, and ring strain all affect spectral detection. Spectroscopic techniques, such as LP-FTIR, detect fewer species compared with GC-MS techniques [155]. Compared with spectroscopic detection techniques, GC-MS is the most commonly used method. Due to the large differences in the properties of quinone species, some studies choose to use derivatization followed by CI or EI ionization for GC-MS detection to enhance analytical sensitivity [156]. However, not all quinones can be effectively derivatized. During the derivatization and analysis processes, thermally unstable quinones may undergo transformation and/or degradation [157]. Some studies have found that for certain compounds, the GC-MS measurements of derivatized and non-derivatized quinones showed no significant differences [158]. HPLC-MS/MS does not require derivatization of thermally unstable, non-volatile, or highly polar compounds, offering certain advantages [159]. dos Santos et al. [160] used cold-fiber SPME-GC-MS (CF-SPME-GC-MS) to achieve higher detection sensitivity and lower detection limits. Four quinone compounds (2-methyl-1,4-naphthoquinone, 2,6-di-tert-butyl-1,4-benzoquinone, methyl-1,4-benzoquinone, and 2,3-dimethylanthraquinone) were detected for the first time in ambient air. Jo et al. [161] developed a quantitative chemical ionization technique based on GC-MS and GC-MS/MS, which can be used for the quantitative analysis of oxidized polycyclic aromatic hydrocarbons (OPAHs) across different concentration ranges and is suitable for detecting various types of OPAHs.
In summary, the main offline analytical methods for multiple carbonyl compounds include DNPH-HPLC-UV and chromatographic tandem MS techniques. DNPH-HPLC-UV is a straightforward technique, and by optimizing gradient elution conditions and column temperature or selecting the appropriate chromatographic column, it can enable the detection of more than ten common carbonyl compounds. The optimization of chromatographic tandem MS techniques is always accompanied by improvements in the complex sampling, pretreatment, and analytical processes, which demand high expertise from the researchers. By optimizing chromatographic tandem MS, it is possible to distinguish between various types of carbonyl compounds (e.g., aldehydes and ketones, straight-chain and branched structures, and unsaturated aromatics; mono-carbonyl, di-carbonyl, oxygenated, and heterocyclic carbonyls; and carbonyl compounds, alcohols, acrylic esters, acetate esters, ethers, and quinones).
5.2. Online Analytical Methods for Multiple Carbonyl Compounds
Online analytical methods for multiple carbonyl compounds can provide higher time resolution, avoid measurement errors caused by target species’ reactions or volatilization during the measurement process, and obtain complete and detailed time series. The most commonly used online methods include online MS techniques, such as GC-FID/MS and RTR-MS (Table 3).

Table 3.
Main online analytical methods for multiple atmospheric carbonyl compounds.
5.2.1. GC-FID/MS
GC-FID/MS can achieve online monitoring of various types of VOCs but has limited detection capability for carbonyl compounds. Traditional online GC-FID/MS systems mainly use a dual-channel sampling, dual-column separation, and dual-detector systems [41]. Different types of atmospheric species are split into two streams at the channel inlet, separately sampled and analyzed by different detectors. The FID detector is primarily used for low-carbon hydrocarbons (C2–C5, with lower boiling points), while hydrocarbons from C5 to C12, halogenated hydrocarbons, and carbonyl compounds are detected by the MS detector. Typically, up to 12 carbonyl compound species can be detected [168]. The availability of additional standard gases for carbonyl compounds can expand the range of target species identified by GC-FID/MS [32]. The entire analysis process takes about 1 h. To capture rapid variations or fluctuations of environmental pollutants, Apel et al. [169] introduced a portable, fast-response GC-MS capable of high-frequency monitoring of C2–C4 carbonyl compounds on board aircraft within 5 min. However, this technique is still based on direct injection measurement without enrichment or efficient desorption of species. It remains insufficiently sensitive for trace compounds, and the range of detected species is still limited.
Researchers often optimize sampling and pretreatment methods to improve the detection performance of online GC-FID/MS technique. Apel et al. [162] utilized a custom three-stage preconcentration system combined with MS (SIM), enabling stable online monitoring of low-concentration carbonyl compounds. Li et al. [30] applied a cryogenic empty-tube freezing preconcentration technique combined with a GC-FID/MS analysis system for automated online monitoring. This method allowed flexible selection of the sampling flow rate, sampling time, and trapping temperature, achieving stable online monitoring of 13 carbonyl compounds. However, the reliance on refrigerants limits the applicability of these cryogenic preconcentration techniques in remote areas. Wang et al. [40] developed a refrigerant-free GC-FID/MS system, which was validated to accurately detect C3–C6 carbonyl compounds through comparison with results from other technologies.
The analysis of carbonyl compounds in aerosols is particularly challenging. Typically, chemical reagents are used for derivatization prior to GC/MS analysis, with specially designed in situ online aerosol collection devices and TD techniques being the primary sampling methods [170]. Williams et al. [171] used a thermal desorption aerosol gas chromatography system (TAG) for real-time, in situ measurement of organic and inorganic compounds in atmospheric aerosols. A small portion of organic aerosol mass (10–40%) was analyzed, and alcohol species derived from isoprene were detected. Hamilton et al. [163] used direct thermal desorption combined with comprehensive two-dimensional GC×GC-TOF/MS to analyze oxygenated components in organic aerosol samples. They detected linear-chain aldehydes from C5 to C9, ketones from C8 to C13, and some cyclic products such as furanone.
5.2.2. Online MS Techniques Without Chromatographic Separation
Online MS allows direct sample injection, minimizing potential human errors during sample collection and pretreatment [25], and provides high temporal resolution for a deeper understanding of chemical transformations. The development of ionization sources and mass analyzers is one of the driving forces behind advancements in MS and a key reason for its online application [172]. Electron ionization (EI) [173] is the most traditional form of hard ionization, widely used in the GC-MS technique. It produces numerous fragments, making accurate determination of molecular ion masses challenging [174]. Soft ionization methods primarily include chemical ionization (CI) [175], photoionization (PI) [176], and ESI [177]. These ionization methods offer high temporal resolution and enable the online detection of atmospheric carbonyl compounds [178].
PTR-MS is an online MS technique for detecting multiple carbonyl compounds without chromatographic separation. Its basic principle involves proton transfer reactions between target compounds in the environment and reagent ions, with the resulting product ions analyzed in a mass analyzer. Its detection limit and analysis time are typically lower than those of GC-FID/MS, but the method often requires extensive data analysis, heavily relying on machine learning and artificial intelligence [179]. Currently, PTR-MS primarily employs CI and can be equipped with various mass analyzers, including quadrupole (Q), ion trap (IT), and time-of-flight (TOF). PTR-Q-MS can detect compounds overlooked by GC-MS [180]. However, Q mass analyzers exhibit low transmission efficiency for heavier ions, resulting in reduced sensitivity for high-mass compounds [181]. PTR-IT-MS has been shown to analyze the entire mass spectrum almost simultaneously and distinguish ions with the same nominal mass [165], although it has a relatively high detection limit [164]. PTR-TOF-MS offers higher sensitivity and lower detection limits [166]. It has been successfully applied in airborne measurements [182] and outperforms various online spectroscopic methods in detecting glyoxal and methylglyoxal [183]. Advances in light sources have greatly driven the development of the PI technique. Synchrotron light sources can reduce or eliminate ion fragmentation [184], but both synchrotron and laser light sources [185] are complex, bulky, and expensive, limiting their application to laboratory-based fundamental research rather than online or field applications. The vacuum ultraviolet (VUV) PI-MS technique, characterized by minimal ion fragmentation, no need for sample pretreatment, and low polarity requirements for analytes [186,187], has been widely applied in the online monitoring of VOCs under various conditions [188]. Liu et al. [167] combined CI with VUV-PI-MS to develop a new method integrating a chemical ionization focusing ion source (CIFI) with TOF-MS, significantly improving ionization and ion transmission efficiency. CIFI-TOF-MS has been validated as a highly efficient and sensitive online monitoring technique, capable of real-time detection and analysis of 12 carbonyl compounds. The selected ion flow tube mass spectrometer (SIFT-MS) [189] can be used to detect formaldehyde and acetaldehyde and is capable of using multiple reagent ions simultaneously, with a detection limit for formaldehyde of less than 200 ppt/1 s.
In the analysis of particle-phase carbonyl compounds, the online use of TD can be affected by thermal decomposition during desorption and ion dissociation during the ionization process in the PTR-MS drift tube [190] and may not avoid surface collection artifacts [191]. Vogel et al. [192] employed a miniaturized, versatile aerosol concentration enrichment system (mVACES) to address the issue of particulate matter collection and applied it in the field. Additionally, several aerosol online collection devices, such as ACM [65] and CHARON [67], have been widely used in smoke chambers. Thompson et al. [193] utilized four different instruments to study the gas-particle distribution of carbonyl compounds, including FIGAERO (filter inlet for gases and aerosols)-ToF-CIMS, SV-TAG (semi-volatile thermal desorption aerosol gas chromatograph)/TD-PTR-MS, and NO3−-IMS (nitrate ion mobility spectrometry)-TOF-MS. They found that the mass spectrometry techniques with specific ion sources limited the range of measurable compounds and exhibited interference from different ion sources, making it difficult to recognize isomers.
In summary, the main online analytical methods for multiple carbonyl compounds include GC-FID/MS and other online MS techniques, such as PTR-MS and VUV-PI-MS. GC-FID/MS systems are primarily used for detecting various types of VOCs. By optimizing sampling and pretreatment methods, stable detection of certain carbonyl compounds can be achieved. Other online MS techniques can integrate diverse ionization sources, mass analyzers, and online sampling systems to enhance detection performance, achieving lower detection limits and higher temporal resolution.
6. Analytical Methods for Carbonyl Compounds in Atmospheric Photochemical Smog Chamber Simulation Studies
The smog chamber allows for precise control of added reagents and environmental conditions, gradually increasing the complexity of chemical reactions to simulate different environmental and chemical systems. Currently, the analysis of multi-functional products (including carbonyl compounds) formed during VOC photooxidation in the smog chamber is typically conducted using spectroscopic methods or independent MS. Spectroscopic methods are simple to operate and require no complex pre-treatment steps, but they can only detect species with specific optical properties. Klotz et al. [194] used the DOAS technique to detect photooxidation products of toluene, identifying benzaldehyde and methylphenol. Olariu [155] used FTIR to detect carbonyl compounds formed from reactions between OH radicals and phenol, ortho-, meta-, and para- methylphenol and first identified 1,2-dihydroxybenzene and 1,4-benzoquinone as new products of OH radical-induced phenol and methylphenol oxidation. Thalman et al. [183] demonstrated the differences in detecting di-carbonyls using seven different measurement techniques in smog chambers in the U.S. and Europe. They found that spectral detectors, such as BBCEAS, CE-DOAS, white-cell DOAS, FTIR, and LIP, were all affected by interference from water vapor, aerosols, or other gases in the environment, leading to poor stability.
PTR-MS is a powerful soft ionization technique that allows for the direct injection and quantification of photochemical oxidation products and precursor VOCs, providing additional insights into potential photochemical oxidation products [195]. Eichler et al. [67] used a CHARON-PTR-ToF-MS combination device to measure organic and ammonium components in submicron particles in real time, detecting carbonyl compound signals. Holzinger et al. [196] used TD-PTR-MS to detect products such as formaldehyde and formic acid during the reaction between ozone and terpenes and found that the desorption temperature of samples in the smog chamber was much lower than that of environmental samples, suggesting a significant discrepancy between the smog chamber experimental conditions and atmospheric conditions. Gkatzelis et al. [197] conducted a comparative study on the gas-particle partitioning of major biogenic oxidation products. In the experiment, different sampling inlets (ACM, CHARON, and TD units) were connected to the PTR-ToF-MS, and it was found that all three systems were capable of detecting aldehyde and ketone compounds. However, since PTR-MS provides molecular formulas rather than specific compound structures, there is a possibility that original chemical components may not be accurately identified due to isomeric compounds or fragments generated by thermal dissociation and ion fragmentation. Furthermore, as different thermal desorption temperatures and operational conditions affect ion fragmentation, the detection limits and time resolutions of each system also vary. TD has the lowest detection limit (10−3 ng/m3), while ACM has the highest detection limit (250 ng/m3). CHARON provides real-time measurements (10 s), while the sampling times for ACM and TD are 120 min and 240 min, respectively.
GC-MS can effectively identify isomeric information. Yu et al. [127] employed impinger -PFBHA-GC-MS in both indoor Teflon bag reactors (TBRs) and outdoor Teflon smog chambers to analyze the carbonyl compounds produced from the reactions between six alkylbenzenes and OH radicals. They detected aromatic aldehydes, quinones, di-carbonyls, and other carbonyl products, with a particular focus on distinguishing various isomeric forms of di-carbonyl products. Kourtchev et al. [198,199] utilized Denuder-PFBHA-GC-MS to study the reactions of (E)-β-farnesene with O3 and OH radicals in the gas phase, including the determination of rate coefficient and analysis of carbonyl products and distinguishing isomeric forms. These offline techniques separate the sampling and derivatization processes, resulting in significant time consumption. Borrás et al. [59] employed in situ dual derivatization SPME-GC-MS at EUPHORE to identify various compounds, including aldehydes, ketones, α-di-carbonyl compounds, hydroxy aldehydes, hydroxy ketones, and carboxylic acids. They applied this method to the ozonolysis of isoprene, tracking the formation of multi-functional oxygenated compounds. Spittler et al. [200] studied the dark reactions between NO3 radicals and two monoterpenes—limonene and α-pinene—and their role in SOA formation in the EUPHORE photoreactor facility and a large volume laboratory photoreactor, where the main carbonyl products, pinonaldehyde and endolim, were successfully detected and quantified using long-path FTIR, HPLC-UV/Vis, and GC-FID. Hohaus et al. [65] connected the ACM to a GC-MS/FID system and successfully detected several carbonyl compounds in SOA during β-pinene ozonolysis, including acetone, bicyclo[3,1,1]hept-3-ene-2-one, myrtanal, myrtenol, 1-hydroxynopinone, 3-oxonopinone, and 3,7-dihydroxynopinone. Although these techniques have undergone optimization in sampling systems and pretreatment processes, their temporal resolution remains inferior to that of PTR-MS technique.
Combining isomeric information from GC-MS with high-time-resolution data from PTR-ToF MS can expand and improve insights obtained from photochemical oxidation experiments. Gómez Alvarez et al. [201] employed a method combining SPME with PFBHA and used PTR-MS, GC-FID, and FTIR instruments to detect di-carbonyl compounds during the photochemical oxidation of toluene and benzene. Finja Löher et al. [202] constructed a Teflon atmospheric simulation chamber, which was connected to a PTR-ToF-MS and an SPME-GC-MS system. Under NOx-free conditions, they detected the first-generation products of toluene oxidation and compared their results with those of Borrás [59]. They found that different smog chamber devices (the Teflon chamber and the EUPHORE chamber) affected the experimental results for acrolein.
7. Problems and Challenges
In the ongoing development and optimization of various commonly used sampling techniques and analytical methods, these approaches have revealed distinct and significant problems and face different challenges for further development and optimization. Table 4 summarizes the main problems and challenges of commonly used sampling techniques and analytical methods.

Table 4.
Problems and challenges of analytical methods for atmospheric carbonyl compounds.
7.1. Sampling Methods
Direct sampling methods for atmospheric carbonyl compounds have limitations in simultaneous sampling of multiple carbonyl species. Tube sampling requires chemical derivatization to collect a broader range of carbonyl compounds, whereas canister sampling and cryogenic enrichment techniques are typically coupled with specific chromatographic systems and are suitable for sampling various VOCs (limited carbonyl species). Challenges in direct sampling of carbonyl compounds include developing novel adsorbents or efficient solvents to reduce reliance on derivatization in tube sampling, designing canisters made from new materials to enhance sample stability, and advancing cryogen-free enrichment techniques alongside optimizing cold trap devices.
Indirect sampling methods are often associated with significant sample loss and high time consumption. Furthermore, the selection of derivatization reagents, adsorbents, coating methods for reagents, and sampling devices is complex, requiring careful adjustment to meet diverse detection requirements and analytical objectives. Designing derivatization reagents with immediate derivatization, high derivative stability, and good sensitivity and selectivity, as well as rapidly determining sampling schemes, remains a significant challenge.
7.2. Analytical Methods of Formaldehyde
Fluorescence and spectrophotometric methods are limited to the detection of single species and exhibit low time resolution. The selection of reagents and optimization of analytical conditions often vary with environmental contexts, making multiple pre-experiments necessary to achieve accurate formaldehyde detection results.
Spectroscopic detection techniques are highly sensitive to environmental conditions, with overlapping or missing spectral signals being common issues. The development of spectroscopic instruments with stable selectivity is therefore critical. Formaldehyde monitoring devices based on chemical methods often involve multiple steps, requiring customized designs. Currently, high-precision spectrometers are typically bulky to meet the requirements for long optical paths, limiting their applicability for field monitoring. Although advanced CRDS and CRES achieve extended optical paths, their instruments are complex and prohibitively expensive. Gas sensors are greatly affected by the environment, and their performance is highly dependent on sensor materials. Developing new sensor materials is crucial.
7.3. Analytical Methods of Multiple Carbonyl Compounds
7.3.1. Offline Analytical Methods
The DNPH-HPLC-UV method suffers from significant sample loss during sampling and high background concentrations of carbonyl compounds. Additionally, there is no coordinated control between the sampling volume and the flow rate. The duration of sampling affects sample recovery, and derivatives may undergo isomerization, leading to the formation of isomers that compromise accurate quantification and result in lower reproducibility than expected. This method has limitations in the species it can detect, and the lack of standard derivatives restricts the identification of unknown compounds [203]. Therefore, optimizing sorbent tubes, studying the derivatization mechanism, developing new standard reference materials, and determining appropriate analytical conditions are crucial for improving the method.
Chromatography tandem MS provides structural information on unknown compounds but requires advanced experimental techniques. The MS may fail to detect peaks captured by other detectors, such as UV detectors, which can lead to underestimation [136]. Therefore, combining multiple detectors in experiments is essential. Different ion sources in MS require distinct operational adjustments for chromatographic coupling, and each ionization source has specific environmental requirements for sample analysis. Achieving optimal results often requires multiple pre-experiments to fine-tune experimental conditions. Currently, MS often uses SIM mode, which detects only a few selected ions, eliminating interference from other ions, but fails to provide a full spectrum, making the qualitative analysis of unknown compounds challenging. In contrast, the full scan mode enables the exploration of unknown compounds, which is crucial for detecting new carbonyl compounds, although it is challenging to operate.
7.3.2. Online Analytical Methods
During continuous operation, GC-FID/MS equipment may experience changes in the retention times of certain compounds due to factors such as decreased column efficiency and variations in the heating rates of preprocessing devices. Consequently, it is necessary to conduct weekly retention time checks for all target compounds to ensure accurate identification. The lack of available standard gases limits the ability of GC to identify and quantify various carbonyl compounds, often resulting in fewer detectable compounds compared with offline analytical methods. Currently, online systems rely on cryogenic enrichment techniques for carbonyl compounds, whereas alternative sampling methods remain unsuitable for GC-based online analysis. As a result, the online GC-FID/MS technique faces challenges related to instrument maintenance and the development of new standard gases and sampling methods.
The performance of ion sources in online MS can be influenced by various factors, such as temperature and humidity. Mass spectra are often complex, and simplifying them typically requires the use of high-precision instruments, which demand regular maintenance and calibration and are extremely costly, making them difficult for commercial use. MS often encounters multiple isomers or fragment ions, making the correct identification and differentiation of compounds a technical challenge. A thorough analysis of sample information requires the combined use of GC-MS and PTR-MS, integrating the separation advantages of GC with the qualitative capabilities of PTR-MS. However, the operation and data processing are complex, requiring the integration of artificial intelligence and machine learning techniques. In the analysis of the gas-particle distribution of carbonyl compounds, MS often requires specific sampling systems, which can affect the analytical performance.
7.4. Analytical Methods in Smog Chamber Simulation Studies
In situ detection techniques, such as CEAS, DOAS, FTIR, and LIP, are more suited for detection in simple environments due to the poor stability of their spectroscopic detectors under certain conditions. Online techniques such as PTR-MS may fail to accurately identify species composition due to isomers and fragmentation caused by thermal dissociation or ion dissociation. Offline techniques such as GC-MS provide better isomer differentiation capabilities but suffer from poor temporal resolution. The combination of PTR-MS and GC-MS integrates isomer information with the high temporal resolution, thereby expanding and refining the information obtained from photochemical oxidation experiments. However, the data processing and operation are complex, and the smog chamber itself may influence chemical reactions, leading to discrepancies compared with real atmospheric detection. Therefore, detecting carbonyl compounds in smog chambers faces challenges in both spectral detection in complex environments and the combined use of PTR-MS and GC-MS. This necessitates the continued development of new sampling systems and comparative studies with real atmospheric detection to characterize the effects of various smog chambers, materials, and other factors on the samples
8. Future Research Needs and Prospects
Future analytical technologies for atmospheric carbonyl compounds must meet the requirements of detecting more high-molecular-weight carbonyl species, adapting to diverse monitoring scenarios, and ensuring stable long-term operation. This will enable a more comprehensive understanding of the environmental behavior and distribution characteristics of carbonyl compounds. For offline detection, the focus should be on simplifying procedures and minimizing measurement errors caused by manual operations. For online monitoring, achieving a more stable operating environment, compact instrumentation, and the ability to detect a wider range of carbonyl compounds with high reliability is essential. For the detection in smog chambers, it is essential to achieve stable spectral detection and characterize the impact of various sampling system, as well as the shape, light source, and materials of the smog chamber, on the results of PTR-MS and GC-MS detection.
To meet the future demands of both offline detection and online monitoring technologies for atmospheric carbonyl compounds, research should prioritize the following five areas: ① Develop derivatization reagents and adsorbents with immediate reaction capabilities, high derivative stability, sensitivity, and selectivity to fundamentally improve the accuracy of offline detection results for carbonyl compounds. ② Achieve high levels of automation in sampling, pretreatment, and analysis, which are crucial for improving the temporal resolution of offline detection. ③ Conduct analytical research combining multiple detectors to extract more comprehensive information on carbonyl compounds in samples. ④ Develop additional standard gases for carbonyl compounds, stable light sources, and MS ion sources, along with designing precise detectors. These advancements will reduce the size of online monitoring instruments and ensure stable detection of carbonyl compounds across diverse environments. ⑤ Integrate artificial intelligence (AI) and machine learning (ML) technologies to process and analyze the extensive data generated by online monitoring instruments. ⑥ Continuously developing the sampling system for smog chambers and characterizing the differences in the shape, light source, and materials of various types of smog chambers are essential to minimize their impact on detection results.
9. Conclusions
Optimizing analytical methods for atmospheric carbonyl compounds has been an ongoing effort. Over the past few decades, researchers worldwide have refined sampling, pretreatment, and analysis processes to achieve adequate sensitivity, selectivity, temporal resolution, accuracy, and operability for both laboratory research and routine monitoring. Based on an extensive literature review and systematic analysis, the key conclusions are as follows:
(1) Direct sampling methods for atmospheric carbonyl compounds encompass tube sampling, canister sampling, and cryogenic enrichment techniques. These methods are effective for collecting low-reactivity, low-boiling-point, highly polar, and volatile low-molecular-weight carbonyl compounds. Indirect sampling methods primarily involve the use of derivatization techniques in combination with specific sampling devices to collect samples, suitable for carbonyl compound sampling in various environments and phases. The reagents, adsorbents, coating methods for the reagents, and sampling devices all need to be adjusted according to specific detection requirements and analytical objectives.
(2) Offline analytical methods for formaldehyde, including fluorescence and spectrophotometry, are highly sensitive to chemical reagents and reaction conditions. By combining different reagents and optimizing analytical conditions, these methods enable rapid detection of formaldehyde under varying scenarios. Online monitoring techniques for formaldehyde primarily include spectroscopic and sensor-based detection methods. Spectroscopic monitoring relies on stable light sources to reduce environmental interference and extended optical paths to achieve high sensitivity. However, these instruments are frequently bulky and complex. In contrast, gas sensors are portable and exhibit high selectivity for formaldehyde, but their performance is susceptible to environmental factors and strongly dependent on sensor materials.
(3) Offline analytical methods for multiple carbonyl compounds primarily include DNPH-HPLC-UV and chromatographic tandem MS. The DNPH-HPLC-UV method is simple to operate and provides reliable detection of over ten common carbonyl compounds. Advances in chromatographic tandem MS have been accompanied by improvements in complex sampling, pretreatment, and analytical methods, facilitating the differentiation of various types of carbonyl compounds. Online analytical methods for multiple carbonyl compounds primarily include GC-FID/MS and other online MS techniques, such as PTR-MS and VUV-PI-MS. GC-FID/MS systems are primarily employed for VOC analysis and can reliably detect a limited range of carbonyl compounds. Other online MS techniques can integrate various ionization sources, mass analyzers and online sampling systems to enhance performance, enabling improved isomer differentiation, lower detection limits, and shorter analysis times.
(4) Analytical methods for carbonyl compounds in smog chamber simulation studies primarily include spectroscopy, PTR-MS, and GC-MS. Spectral detectors are suitable for detection in simple environments. PTR-MS offers high time resolution, and GC-MS has strong isomer identification capabilities. The combination of PTR-MS and GC-MS can expand and improve the information obtained from detected samples. However, the smog chamber itself and the connected sampling system influence the chemical reactions, which in turn affect the detection results.
(5) Current offline analytical methods for carbonyl compounds heavily rely on the optimization of sampling, pretreatment, and analytical methods, requiring extensive time and advanced experimental skills for repeated pre-experiments. Online monitoring methods face challenges including limited stability and species coverage. In smog chamber simulation studies, the detection of carbonyl compounds is heavily influenced by both the sampling system and the chamber itself. Future detection methods should focus on improving environmental adaptability and automation while emphasizing the integration of multiple techniques and the impact of the sampling system and the smog chamber itself on the detection in smog chamber simulation studies. This approach aims to enhance the sensitivity, selectivity, temporal resolution, accuracy, and practicality of analytical methods.
Author Contributions
Conceptualization, X.G. and X.Y.; methodology, X.G. and Y.Y.; validation, X.Z.; formal analysis, X.G.; investigation, X.G. and Y.N.; resources, H.L.; data curation, X.G. and X.Y.; writing—original draft preparation, X.G.; writing—review and editing, W.W. and H.L.; visualization, X.G.; supervision, J.L. and Y.L.; project administration, J.B.; funding acquisition, R.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Ministry of Ecology and Environment of China under the project DQGG202121 and the Dongying Ecological and Environmental Bureau (2021DFKY-0779).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Atkinson, R. Atmospheric chemistry of VOCs and NOx. Atmos. Environ. 2000, 34, 2063–2101. [Google Scholar] [CrossRef]
- Zhang, Y.; Xue, L.; Dong, C.; Wang, T.; Mellouki, A.; Zhang, Q.; Wang, W. Gaseous carbonyls in China’s atmosphere: Tempo-spatial distributions, sources, photochemical formation, and impact on air quality. Atmos. Environ. 2019, 214, 116863. [Google Scholar] [CrossRef]
- Chen, T.; Xue, L.; Zheng, P.; Zhang, Y.; Liu, Y.; Sun, J.; Han, G.; Li, H.; Zhang, X.; Li, Y.; et al. Volatile organic compounds and ozone air pollution in an oil production region in northern China. Atmos. Chem. Phys. 2020, 20, 7069–7086. [Google Scholar] [CrossRef]
- Kroll, J.A.; Hansen, A.S.; Moller, K.H.; Axson, J.L.; Kjaergaard, H.G.; Vaida, V. Ultraviolet Spectroscopy of the Gas Phase Hydration of Methylglyoxal. ACS Earth Space Chem. 2017, 1, 345–352. [Google Scholar] [CrossRef]
- Mellouki, A.; Wallington, T.J.; Chen, J. Atmospheric Chemistry of Oxygenated Volatile Organic Compounds: Impacts on Air Quality and Climate. Chem. Rev. 2015, 115, 3984–4014. [Google Scholar] [CrossRef]
- Ling, Z.H.; Guo, H.; Chen, G.X.; Lam, S.H.M.; Fan, S.J. Formaldehyde and Acetaldehyde at Different Elevations in Mountainous Areas in Hong Kong. Aerosol Air Qual. Res. 2016, 16, 1868–1878. [Google Scholar] [CrossRef]
- Wang, C.; Huang, X.F.; Han, Y.; Zhu, B.; He, L.Y. Sources and Potential Photochemical Roles of Formaldehyde in an Urban Atmosphere in South China. J. Geophys. Res. Atmos. 2017, 122, 2169–8996. [Google Scholar] [CrossRef]
- Chou, C.C.K.; Tsai, C.Y.; Chang, C.C.; Lin, P.H.; Liu, S.C.; Zhu, T. Photochemical production of ozone in Beijing during the 2008 Olympic Games. Atmos. Chem. Phys. 2011, 11, 9825–9837. [Google Scholar] [CrossRef]
- Turpin, B.J.; Saxena, P.; Andrews, E. Measuring and simulating particulate organics in the atmosphere: Problems and prospects. Atmos. Environ. 2000, 34, 2983–3013. [Google Scholar] [CrossRef]
- Du, Z.; Mo, J.; Zhang, Y. Risk assessment of population inhalation exposure to volatile organic compounds and carbonyls in urban China. Env. Int. 2014, 73, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Delikhoon, M.; Fazlzadeh, M.; Sorooshian, A.; Baghani, A.N.; Golaki, M.; Ashournejad, Q.; Barkhordari, A. Characteristics and health effects of formaldehyde and acetaldehyde in an urban area in Iran. Environ. Pollut. 2018, 242, 938–951. [Google Scholar] [CrossRef] [PubMed]
- Flowers, L.; Broder, M.W.; Forsyth, C. Toxicological Review of Acetone; US Environmental Protection Agency: Washington, DC, USA, 2003. [Google Scholar]
- Li, L.; Jia, Q.; Zhang, Z. Advances in the study of crotonaldehyde toxicity. Chin. J. Ind. Hyg. Occup. Dis. 2016, 43, 758–761. [Google Scholar]
- Atkinson, R.; Arey, J. Atmospheric Degradation of Volatile Organic Compounds. Chem. Rev. 2003, 103, 4605–4638. [Google Scholar] [CrossRef]
- Zhang, K.; Duan, Y.; Huo, J.; Huang, L.; Wang, Y.; Fu, Q.; Wang, Y.; Li, L. Formation mechanism of HCHO pollution in the suburban Yangtze River Delta region, China: A box model study and policy implementations. Atmos. Environ. 2021, 267, 118755. [Google Scholar] [CrossRef]
- Bao, J.M.; Li, H.; Wu, Z.H.; Zhang, X.; Zhang, H.; Li, Y.F.; Qian, J.; Chen, J.H.; Deng, L.Q. Atmospheric carbonyls in a heavy ozone pollution episode at a metropolis in Southwest China: Character istics, health risk assessment, sources analysis. J. Environ. Sci. 2022, 113, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Chai, W.; Wang, M.; Li, J.; Tang, G.; Zhang, G.; Chen, W. Pollution characteristics, sources, and photochemical roles of ambient carbonyl compounds in summer of Beijing, China. Environ. Pollut. 2023, 336, 122403. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.H.; Wang, S.X.; Ying, Q.; Duan, L.; Xing, J.; Cao, J.Y.; Wu, D.; Li, X.X.; Xing, C.Z.; Yan, X.; et al. Importance of Wintertime Anthropogenic Glyoxal and Methylglyoxal Emissions in Beijing and Implications for Secondary Organic Aerosol Formation in Megacities. Environ. Sci. Technol. 2020, 54, 11809–11817. [Google Scholar] [CrossRef]
- Doussin, J.-F.; Fuchs, H.; Kiendler-Scharr, A.; Seakins, P.; Wenger, J. A Practical Guide to Atmospheric Simulation Chambers, 1st ed.; Springer Nature Switzerland AG: Cham, Switzerland, 2023; pp. 1–339. [Google Scholar]
- Liu, Q.; Gao, Y.; Huang, W.; Ling, Z.; Wang, Z.; Wang, X. Carbonyl compounds in the atmosphere: A review of abundance, source and their contributions to O3 and SOA formation. Atmos. Res. 2022, 274, 106184. [Google Scholar] [CrossRef]
- Tang, J.; Wang, X.; Sheng, G.; Fu, J. Advances in the analysis of formaldehyde and carbonyl compounds in the atmosphere. Chin. J. Anal. Chem. 2005, 33, 134–140. [Google Scholar]
- Nier, A.O. Some reflections on the early days of mass spectrometry at the university of minnesota. Int. J. Mass. Spectrom. Ion Process. 1990, 100, 1–13. [Google Scholar] [CrossRef]
- Nash, T. The colorimetric estimation of formaldehyde by means of the Hantzsch reaction. Biochem. J. 1953, 55, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, H.L.C.; de Andrade, M.V.; Pereira, P.A.P.; de Andrade, J.B. Spectrofluorimetric determination of formaldehyde in air after collection onto silica cartridges coated with Fluoral P. Microchem. J. 2004, 78, 15–20. [Google Scholar] [CrossRef]
- Yuan, B.; Koss, A.R.; Warneke, C.; Coggon, M.; Sekimoto, K.; de Gouw, J.A. Proton-Transfer-Reaction Mass Spectrometry: Applications in Atmospheric Sciences. Chem. Rev. 2017, 117, 13187–13229. [Google Scholar] [CrossRef]
- Chung, P.R.; Tzeng, C.T.; Ke, M.T.; Lee, C.Y. Formaldehyde Gas Sensors: A Review. Sensors 2013, 13, 4468–4484. [Google Scholar] [CrossRef]
- Wu, T.; Hu, R.; Xie, P.; Wang, J.; Liu, W. Research progress of atmospheric HCHO spectroscopy detection technology. J. Quantum Electron. 2021, 38, 699. [Google Scholar]
- Huang, M.; Ma, F.; Zhang, J.; Zhou, Y.; Wen, S.; Liu, Z. Research Progress of the Environmental Monitoring Analytical Methods of Aldehydes and Ketones Carbonyl Compounds. Sichuan Environ. 2023, 42, 300–305. [Google Scholar]
- Xu, Y.; Hui, L.; Zheng, P.; Liu, G.; Yu, J.Z.; Wang, Z. Monitoring techniques of airborne carbonyl compounds: Principles, performance and challenges. TrAC Trends Anal. Chem. 2023, 169, 117395. [Google Scholar] [CrossRef]
- Li, H.; Xiao, Q.; Han, C.; Liu, L.; Fan, X.; Chen, N.; Pan, Y. On-Line Monitoring of VOCs in Ambient Air Based on Cryo-Trapping-GC-MS-FID. Chin. Environ. Monit. 2017, 33, 100–110. [Google Scholar]
- MEE. Determination of Volatile Organic Compounds in Ambient Air—Adsorbent Tube Sampling—Thermal Desorption/Gas Chromatography-Mass Spectrometry: HJ 644-2013; China Environmental Science Press: Beijing, China, 2013. [Google Scholar]
- Hui, L.; Liu, X.; Tan, Q.; Feng, M.; An, J.; Qu, Y.; Zhang, Y.; Jiang, M. Characteristics, source apportionment and contribution of VOCs to ozone formation in Wuhan, Central China. Atmos. Environ. 2018, 192, 55–71. [Google Scholar] [CrossRef]
- Druzik, C.M.; Grosjean, D.; Van Neste, A.; Parmar, S.S. Sampling of Atmospheric Carbonyls with Small DNPH-Coated C18 Cartridges and Liquid Chromatography Analysis with Diode Array Detection. Int. J. Environ. Anal. Chem. 1990, 38, 495–512. [Google Scholar] [CrossRef]
- Li, J.; Feng, Y.L.; Xie, C.J.; Huang, J.; Yu, J.Z.; Feng, J.L.; Sheng, G.Y.; Fu, J.M.; Wu, M.H. Determination of gaseous carbonyl compounds by their pentafluorophenyl hydrazones with gas chromatography/mass spectrometry. Anal. Chim. Acta. 2009, 635, 84–93. [Google Scholar] [CrossRef] [PubMed]
- U.S. Environmental Protection Agency (EPA). Compendium of Methods for the Determination of Toxic Organic Compounds in Ambient Air. Second Edition. Compendium Method TO-14A: Determination of Volatile Organic Compounds (VOCs) in Ambient Air Using Specially Prepared Canisters with Subsequent Analysis by Gas Chromatography. EPA/625/R-96/010b.PU.S; EPA: Cincinnati, OH, USA, 1999. [Google Scholar]
- U.S. Environmental Protection Agency (EPA). Method TO-15A: Determination of Volatile Organic Compounds (VOCs) in Air Collected in Specially Prepared Canisters and Analyzed by Gas Chromatography–Mass Spectrometry (GC-MS); U.S. EPA: Cincinnati, OH, USA, 2019. [Google Scholar]
- Batterman, S.A.; Zhang, G.-Z.; Baumann, M. Analysis and stability of aldehydes and terpenes in electropolished canisters. Atmos. Environ. 1998, 32, 1647–1655. [Google Scholar] [CrossRef]
- Kelly, T.J.; Holdren, M.W. Applicability of canisters for sample storage in the determination of hazardous air pollutants. Atmos. Environ. 1995, 29, 2595–2608. [Google Scholar] [CrossRef]
- Xie, X.; Lu, X.; Zhang, S. Comparison on Monitoring Methods of Environmental Air Volatile Organic Compounds in Nanjing. China Environ. Monit. 2020, 36, 11–15. [Google Scholar]
- Wang, M.; Zeng, L.; Lu, S.; Shao, M.; Liu, X.; Yu, X.; Chen, W.; Yuan, B.; Zhang, Q.; Hu, M.; et al. Development and validation of a cryogen-free automatic gas chromatograph system (GC-MS/FID) for online measurements of volatile organic compounds. Anal. Methods 2014, 6, 9424–9434. [Google Scholar] [CrossRef]
- Li, X.; Lu, S.; Shao, M. Methods for Measurement of Oxygenated Volatile Organic Compounds in the Atmosphere. Peking. Univ. Sci. 2006, 42, 548–554. [Google Scholar]
- Liu, L.; Dong, Y.; Sun, X. Comparison of HPLC and GC-MS for the Determination of Aldehydes and Ketones in Ambient Air. Environ. Monit. Manag. Technol. 2023, 35, 45–48. [Google Scholar]
- Pal, R.; Kim, K.H. Experimental choices for the determination of carbonyl compounds in air. J. Sep. Sci. 2007, 30, 2708–2718. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Han, B.; Xia, L. The comparison between the MBTH and Acetylacetone spectrophotometric methods in Determination of Formaldehyde in Indoor Air. Energy Conserv. Environ. Prot. 2020, 06, 58–59. [Google Scholar]
- U.S. Environmental Protection Agency (EPA). Compendium of Methods for the Determination of Toxic Organic Compounds in Ambient Air. Second Edition. Compendium Method TO-11A: Determination of Formaldehyde in Ambient Air Using Adsorbent Cartridge Followed by High Performance Liquid Chromatography (HPLC) [Active Sampling Methodology]. EPA/625/R-96/010b; U.S. EPA: Cincinnati, OH, USA, 1999. [Google Scholar]
- Pang, X.; Lewis, A.C.; Hamilton, J.F. Determination of airborne carbonyls via pentafluorophenylhydrazine derivatisation by GC–MS and its comparison with HPLC method. Talanta 2011, 85, 406–414. [Google Scholar] [CrossRef]
- Arnts, R.R.; Tejada, S.B. 2,4-Dinitrophenylhydrazine-coated silica gel cartridge method for determination of formaldehyde in air: Identification of an ozone interference. Environ. Sci. Technol. 1989, 23, 1428–1430. [Google Scholar] [CrossRef]
- Williams, E.L.; Grosjean, D. Removal of atmospheric oxidants with annular denuders. Environ. Sci. Technol. 1990, 24, 811–814. [Google Scholar] [CrossRef]
- Ho, S.S.H.; Ho, K.F.; Liu, W.D.; Lee, S.C.; Dai, W.T.; Cao, J.J.; Ip, H.S.S. Unsuitability of using the DNPH-coated solid sorbent cartridge for determination of airborne unsaturated carbonyls. Atmos. Environ. 2011, 45, 261–265. [Google Scholar] [CrossRef]
- Kahnt, A.; Iinuma, Y.; Böge, O.; Mutzel, A.; Herrmann, H. Denuder sampling techniques for the determination of gas-phase carbonyl compounds: A comparison and characterisation of in situ and ex situ derivatisation methods. J. Chromatogr. B 2011, 879, 1402–1411. [Google Scholar] [CrossRef] [PubMed]
- Cecinato, A.; Di Palo, V.; Mabilia, R.; Possanzini, M. Pentafluorophenylhydrazine as a coating reagent for the HRGC-MS determination of semi-volatile carbonyl compounds in air. Chromatographia 2001, 54, 263–269. [Google Scholar] [CrossRef]
- Ho, S.S.H.; Yu, J.Z. Determination of airborne carbonyls: Comparison of a thermal desorption/GC method with the standard DNPH/HPLC method. Environ. Sci. Technol. 2004, 38, 862–870. [Google Scholar] [CrossRef] [PubMed]
- Apel, E.C.; Calvert, J.G.; Riemer, D.; Pos, W.; Zika, R.; Kleindienst, T.E.; Lonneman, W.A.; Fung, K.; Fujita, E.; Shepson, P.B.; et al. Measurements comparison of oxygenated volatile organic compounds at a rural site during the 1995 SOS Nashville Intensive. J. Geophys. Res. Atmos. 1998, 103, 22295–22316. [Google Scholar] [CrossRef]
- Lomonaco, T.; Romani, A.; Ghimenti, S.; Biagini, D.; Bellagambi, F.G.; Onor, M.; Salvo, P.; Fuoco, R.; Di Francesco, F. Determination of carbonyl compounds in exhaled breath by on-sorbent derivatization coupled with thermal desorption and gas chromatography-tandem mass spectrometry. J. Breath. Res. 2018, 12, 046004. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.S.H.; Yu, J.Z. Feasibility of Collection and Analysis of Airborne Carbonyls by On-Sorbent Derivatization and Thermal Desorption. Anal. Chem. 2002, 74, 1232–1240. [Google Scholar] [CrossRef]
- Cecinato, A.; Yassaa, N.; Di Palo, V.; Possanzini, M.; Cecinato, A. Observation of volatile and semi-volatile carbonyls in an Algerian urban environment using dinitrophenylhydrazine/silica-HPLC and pentafluorophenylhydrazine/silica-GC-MS. J. Environ. Monit. 2002, 4, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Arthur, C.L.; Pawliszyn, J. Solid phase microextraction with thermal desorption using fused silica optical fibers. Anal. Chem. 1990, 62, 2145–2148. [Google Scholar] [CrossRef]
- Schmarr, H.G.; Sang, W.; Ganß, S.; Fischer, U.; Köpp, B.; Schulz, C.; Potouridis, T. Analysis of aldehydes via headspace SPME with on-fiber derivatization to their O-(2,3,4,5,6-pentafluorobenzyl)oxime derivatives and comprehensive 2D-GC-MS. J. Sep. Sci. 2008, 31, 3458–3465. [Google Scholar] [CrossRef] [PubMed]
- Borrás, E.; Tortajada-Genaro, L.A.; Ródenas, M.; Vera, T.; Speak, T.; Seakins, P.; Shaw, M.D.; Lewis, A.C.; Muñoz, A. On-line solid phase microextraction derivatization for the sensitive determination of multi-oxygenated volatile compounds in air. Atmos. Meas. Tech. 2021, 14, 4989–4999. [Google Scholar] [CrossRef]
- Tumbiolo, S.; Gal, J.-F.; Maria, P.-C.; Zerbinati, O. Determination of benzene, toluene, ethylbenzene and xylenes in air by solid phase micro-extraction/gas chromatography/mass spectrometry. Anal. Bioanal. Chem. 2004, 380, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Ghiasvand, A.R.; Hajipour, S.; Heidari, N. Cooling-assisted microextraction: Comparison of techniques and applications. TrAC 2016, 77, 54–65. [Google Scholar] [CrossRef]
- Bourdin, D.; Desauziers, V. Development of SPME on-fiber derivatization for the sampling of formaldehyde and other carbonyl compounds in indoor air. Anal. Bioanal. Chem. 2014, 406, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Tobias, H.J.; Kooiman, P.M.; Docherty, K.S.; Ziemann, P.J. Real-Time Chemical Analysis of Organic Aerosols Using a Thermal Desorption Particle Beam Mass Spectrometer. Aerosol Sci. Technol. 2000, 33, 170–190. [Google Scholar] [CrossRef]
- Williams, B.J.; Goldstein, A.H.; Kreisberg, N.M.; Hering, S.V. An in-situ instrument for speciated organic composition of atmospheric aerosols: T hermal desorption a erosol GC/MS-FID (TAG). Aerosol Sci. Technol. 2006, 40, 627–638. [Google Scholar] [CrossRef]
- Hohaus, T.; Trimborn, D.; Kiendler-Scharr, A.; Gensch, I.; Laumer, W.; Kammer, B.; Andres, S.; Boudries, H.; Smith, K.A.; Worsnop, D. A new aerosol collector for quasi on-line analysis of particulate organic matter: The Aerosol Collection Module (ACM) and first applications with a GC/MS-FID. Atmos. Meas. Tech. 2010, 3, 1423–1436. [Google Scholar] [CrossRef]
- Temime, B.; Healy, R.M.; Wenger, J.C. A denuder-filter sampling technique for the detection of gas and particle phase carbonyl compounds. Environ. Sci. Technol. 2007, 41, 6514–6520. [Google Scholar] [CrossRef]
- Eichler, P.; Müller, M.; D’Anna, B.; Wisthaler, A. A novel inlet system for online chemical analysis of semi-volatile submicron particulate matter. Atmos. Meas. Tech. 2015, 8, 1353–1360. [Google Scholar] [CrossRef]
- Vairavamurthy, A.; Roberts, J.M.; Newman, L. Methods for Determination of Low-Molecular-Weight Carbonyl-Compounds in the Atmosphere. Atmos. Env. Part. A-Gen. Topics. 1992, 26, 1965–1993. [Google Scholar] [CrossRef]
- Dong, B.; Song, X.; Tang, Y.; Lin, W. A rapid and facile fluorimetric method for detecting formaldehyde. Sens. Actuators B: Chemical. 2016, 222, 325–330. [Google Scholar] [CrossRef]
- Li, Q.; Sritharathikhun, P.; Motomizu, S. Development of Novel Reagent for Hantzsch Reaction for the Determination of Formaldehyde by Spectrophotometry and Fluorometry. Anal. Sci. 2007, 23, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Chen, J.; Chen, J.; Liu, B.; Song, G. Application of spectroscopic detection technology in formaldehyde concentration measurement. J. Mudanjiang Univ. 2010, 19, 131–132+136. [Google Scholar]
- Huang, Y. Determination of formaldehyde in air by spectrophotometric method with phenol reagent and acetylacetone spectrophotometry. Environ. Dev. 2018, 30, 115–116. [Google Scholar]
- Wang, D.; Jiang, Y.; Li, P.; Li, L.; Liu, Q.; Sun, Y. Comparison of phenol reagent spectrophotometry and acetylacetone spectrophotometry for determining formaldehyde content in air. Chin. Med. Instrum. Inf. 2017, 23, 13–15. [Google Scholar]
- Ji, K.; Su, Y.; Xiao, Y.; Liang, K.; Wang, J.; Liu, P.; Zhang, K.; Zhao, L. Key factor of the determination of formaldehyde in low concentration by phenol reagent spectrophotometry. Chem. Ind. Progr. 2020, 39, 281–286. [Google Scholar]
- GB/T 18204.2-2014; Examination Methods for Public Place. Part 2: Chemical Pollutant. Standards Press of China: Beijing, China, 2014.
- Wang, Q. Influence factors of determination of formaldehyde concentration by AHMT spectrophotometry. Chem. Eng. 2018, 32, 34–37. [Google Scholar]
- Wei, K.; Ma, L.; Ma, G.; Ji, C.; Yin, M. A two-step responsive colorimetric probe for fast detection of formaldehyde in weakly acidic environment. Dye. Pigments. 2019, 165, 294–300. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Mubarak, A.T.; Marestani, Z.M.H.; Fawy, K.F. Highly sensitive and selective catalytic determination of formaldehyde and acetaldehyde. Talanta. 2008, 74, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas, L.M.; Brassington, D.J.; Allan, B.J.; Coe, H.; Alicke, B.; Platt, U.; Wilson, K.M.; Plane, J.M.C.; Penkett, S.A. Intercomparison of Formaldehyde Measurements in Clean and Polluted Atmospheres. J. Atmos. Chem. 2000, 37, 53–80. [Google Scholar] [CrossRef]
- Hak, C.; Pundt, I.; Trick, S.; Kern, C.; Platt, U.; Dommen, J.; Ordóñez, C.; Prévôt, A.S.H.; Junkermann, W.; Astorga-Lloréns, C.; et al. Intercomparison of four different in-situ techniques for ambient formaldehyde measurements in urban air. Atmos. Chem. Phys. 2005, 5, 2881–2900. [Google Scholar] [CrossRef]
- Catoire, V.; Bernard, F.; Mébarki, Y.; Mellouki, A.; Eyglunent, G.; Daële, V.; Robert, C. A tunable diode laser absorption spectrometer for formaldehyde atmospheric measurements validated by simulation chamber instrumentation. J. Environ. Sci. 2012, 24, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Kleine, D.; Mürtz, M.; Lauterbach, J.; Dahnke, H.; Urban, W.; Hering, P.; Kleinermanns, K. Atmospheric trace gas analysis with cavity ring-down spectroscopy. Isr. J. Chemistry. 2001, 41, 111–116. [Google Scholar] [CrossRef]
- Peltola, J.; Vainio, M.; Ulvila, V.; Siltanen, M.; Metsälä, M.; Halonen, L. Off-axis re-entrant cavity ring-down spectroscopy with a mid-infrared continuous-wave optical parametric oscillator. Appl. Phys. B 2012, 107, 839–847. [Google Scholar] [CrossRef]
- Motyka, K.; Mikuska, P. Continuous fluorescence determination of formaldehyde in air. Anal. Chim. Acta. 2004, 518, 51–57. [Google Scholar] [CrossRef]
- Xu, R.; Li, X.; Dong, H.; Wu, Z.; Chen, S.; Fang, X.; Gao, J.; Guo, S.; Hu, M.; Li, D.; et al. Measurement of gaseous and particulate formaldehyde in the Yangtze River Delta, China. Atmos. Environ. 2020, 224, 117114. [Google Scholar] [CrossRef]
- Jiang, J.; Zeng, L. The design and application of an online gaseous formaldehyde analyzer. Acta Sci. Circumst. 2018, 38, 3475–3481. [Google Scholar]
- Henry, S.B.; Kammrath, A.; Keutsch, F.N. Quantification of gas-phase glyoxal and methylglyoxal via the Laser-Induced Phosphorescence of (methyl)GLyOxal Spectrometry (LIPGLOS) Method. Atmos. Meas. Tech. 2012, 5, 181–192. [Google Scholar] [CrossRef]
- Volkamer, R.; Molina, L.T.; Molina, M.J.; Shirley, T.; Brune, W.H. DOAS measurement of glyoxal as an indicator for fast VOC chemistry in urban air. Geophys. Res. Lett. 2005, 32, L08806. [Google Scholar] [CrossRef]
- MacDonald, S.M.; Oetjen, H.; Mahajan, A.S.; Whalley, L.K.; Edwards, P.M.; Heard, D.E.; Jones, C.E.; Plane, J.M.C. DOAS measurements of formaldehyde and glyoxal above a south-east Asian tropical rainforest. Atmos. Chem. Phys. 2012, 12, 5949–5962. [Google Scholar] [CrossRef]
- Hong, Q.; Liu, C.; Hu, Q.; Zhang, Y.; Xing, C.; Ou, J.; Tan, W.; Liu, H.; Huang, X.; Wu, Z. Vertical distribution and temporal evolution of formaldehyde and glyoxal derived from MAX-DOAS observations: The indicative role of VOC sources. J. Environ. Sci. 2022, 122, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Thalman, R.; Volkamer, R. Inherent calibration of a blue LED-CE-DOAS instrument to measure iodine oxide, glyoxal, methyl glyoxal, nitrogen dioxide, water vapour and aerosol extinction in open cavity mode. Atmos. Meas. Tech. 2010, 3, 1797–1814. [Google Scholar] [CrossRef]
- Washenfelder, R.A.; Langford, A.O.; Fuchs, H.; Brown, S.S. Measurement of glyoxal using an incoherent broadband cavity enhanced absorption spectrometer. Atmos. Chem. Phys. 2008, 8, 7779–7793. [Google Scholar] [CrossRef]
- Flowerday, C.E.; Thalman, R.; Asplund, M.C.; Hansen, J.C.J.T. Broadband Cavity-Enhanced Absorption Spectroscopy (BBCEAS) Coupled with an Interferometer for On-Band and Off-Band Detection of Glyoxal. Toxics 2023, 12, 26. [Google Scholar] [CrossRef]
- An, C.; Yan, Y.; Gao, X.; Yan, X.; Ji, Y.; Shang, F.; Li, J.; Tan, L.; Gao, R.; Bi, F.; et al. A Comparative Investigation of the Characteristics of Nocturnal Ozone Enhancement Events and Their Effects on Ground-Level Ozone and PM2.5 in the Central City of the Yellow River Delta, China, in 2022 and 2023. Atmosphere 2024, 15, 475. [Google Scholar] [CrossRef]
- Fang, B.; Yang, N.; Wang, C.; Zhao, W.; Zhou, H.; Zhang, W. Highly sensitive portable laser absorption spectroscopy formaldehyde sensor using compact spherical mirror multi-pass cell. Sens. Actuators B Chem. 2023, 394, 134379. [Google Scholar] [CrossRef]
- Darder, M.d.M.; Bedoya, M.; Serrano, L.A.; Alba, M.Á.; Orellana, G. Fiberoptic colorimetric sensor for in situ measurements of airborne formaldehyde in workplace environments. Sens. Actuators B Chem. 2022, 353, 131099. [Google Scholar] [CrossRef]
- Yuan, Z.; Yang, C.; Meng, F. Strategies for Improving the Sensing Performance of Semiconductor Gas Sensors for High-Performance Formaldehyde Detection: A Review. Chemosensors. 2021, 9, 179. [Google Scholar] [CrossRef]
- Warneke, C.; Veres, P.; Holloway, J.S.; Stutz, J.; Tsai, C.; Alvarez, S.; Rappenglueck, B.; Fehsenfeld, F.C.; Graus, M.; Gilman, J.B.; et al. Airborne formaldehyde measurements using PTR-MS: Calibration, humidity dependence, inter-comparison and initial results. Atmos. Meas. Tech. 2011, 4, 2345–2358. [Google Scholar] [CrossRef]
- Platt, U.; Perner, D.; Pätz, H.W. Simultaneous measurement of atmospheric CH2O, O3, and NO2 by differential optical absorption. J. Geophys. Res. Oceans 1979, 84, 6329–6335. [Google Scholar] [CrossRef]
- Stutz, J.; Platt, U. Improving long-path differential optical absorption spectroscopy with a quartz-fiber mode mixer. Appl. Opt. 1997, 36, 1105–1115. [Google Scholar] [CrossRef]
- Grutter, M.; Flores, E.; Andraca-Ayala, G.; Báez, A. Formaldehyde levels in downtown Mexico City during 2003. Atmos. Environ. 2005, 39, 1027–1034. [Google Scholar] [CrossRef]
- Heckel, A.; Richter, A.; Tarsu, T.; Wittrock, F.; Hak, C.; Pundt, I.; Junkermann, W.; Burrows, J.P. MAX-DOAS measurements of formaldehyde in the Po-Valley. Atmos. Chem. Phys. 2005, 5, 909–918. [Google Scholar] [CrossRef]
- Tuazon, E.C.; Graham, R.A.; Winer, A.M.; Easton, R.R.; Pitts, J.N.; Hanst, P.L. A kilometer pathlength fourier-transform infrared system for the study of trace pollutants in ambient and synthetic atmospheres. Atmos. Environ (1967) 1978, 12, 865–875. [Google Scholar] [CrossRef]
- Suarez-Bertoa, R.; Clairotte, M.; Arlitt, B.; Nakatani, S.; Hill, L.; Winkler, K.; Kaarsberg, C.; Knauf, T.; Zijlmans, R.; Boertien, H.J.F. Intercomparison of ethanol, formaldehyde and acetaldehyde measurements from a flex-fuel vehicle exhaust during the WLTC. Fuel 2017, 203, 330–340. [Google Scholar] [CrossRef]
- Becker, K.H.; Schurath, U.; Tatarczyk, T. Fluorescence Determination of Low Formaldehyde Concentrations in Air by Dye Laser Excitation. Appl. Opt. 1975, 14, 310–313. [Google Scholar] [CrossRef]
- Möhlmann, G.R. Formaldehyde Detection in Air by Laser-Induced Fluorescence. Appl. Spectrosc. 1985, 39, 98–101. [Google Scholar] [CrossRef]
- Cazorla, M.; Wolfe, G.M.; Bailey, S.A.; Swanson, A.K.; Arkinson, H.L.; Hanisco, T.F. A new airborne laser-induced fluorescence instrument for in situ detection of formaldehyde throughout the troposphere and lower stratosphere. Atmos. Meas. Tech. 2015, 8, 541–552. [Google Scholar] [CrossRef]
- Hottle, J.R.; Huisman, A.J.; Digangi, J.P.; Kammrath, A.; Galloway, M.M.; Coens, K.L.; Keutsch, F.N. A Laser Induced Fluorescence-Based Instrument for In-Situ Measurements of Atmospheric Formaldehyde. Environ. Sci. Technol. 2009, 43, 790–795. [Google Scholar] [CrossRef] [PubMed]
- Clair, J.M.; Swanson, A.K.; Bailey, S.A.; Hanisco, T.F. CAFE: A new, improved nonresonant laser-induced fluorescence instrument for airborne in situ measurement of formaldehyde. Atmos. Meas. Tech. 2019, 12, 4581–4590. [Google Scholar] [CrossRef]
- Harris, G.W.; Mackay, G.I.; Iguchi, T.; Mayne, L.K.; Schiff, H.I. Measurements of formaldehyde in the troposphere by tunable diode laser absorption spectroscopy. J. Atmos. Chem. 1989, 8, 119–137. [Google Scholar] [CrossRef]
- Mackay, G.I.; Karecki, D.R.; Schiff, H.I. Tunable diode laser absorption measurements of H2O2 and HCHO during the Mauna Loa Observatory Photochemistry Experiment. J. Geophys. Res. Atmos. 1996, 101, 14721–14728. [Google Scholar] [CrossRef]
- Richter, D.; Fried, A.; Wert, B.P.; Walega, J.G.; Tittel, F.K. Development of a tunable mid-IR difference frequency laser source for highly sensitive airborne trace gas detection. Appl. Phys. B. 2002, 75, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Herndon, S.C.; Zahniser, M.S.; Nelson, D.D.; Shorter, J.; McManus, J.B.; Jiménez, R.; Warneke, C.; de Gouw, J.A. Airborne measurements of HCHO and HCOOH during the New England Air Quality Study 2004 using a pulsed quantum cascade laser spectrometer. J. Geophys. Res. Atmos. 2007, 112, D10. [Google Scholar] [CrossRef]
- McManus, J.B.; Zahniser, M.S.; Nelson, D.D. Dual quantum cascade laser trace gas instrument with astigmatic Herriott cell at high pass number. Appl. Opt. 2011, 50, A74–A85. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Yu, Y.; Li, C.; So, S.; Tittel, F.K. Ppb-level formaldehyde detection using a CW room-temperature interband cascade laser and a miniature dense pattern multipass gas cell. Opt. Express. 2015, 23, 19821–19830. [Google Scholar] [CrossRef] [PubMed]
- Fang, B.; Yang, N.N.; Zhao, W.X.; Wang, C.H.; Zhang, W.J.; Song, W.; Venables, D.S.; Chen, W.D. Improved spherical mirror multipass-cell-based interband cascade laser spectrometer for detecting ambient formaldehyde at parts per trillion by volume levels. Appl. Opt. 2019, 58, 8743–8750. [Google Scholar] [CrossRef]
- Rella, C.; Hoffnagle, J.; Kim-Hak, D. Quantification of atmospheric formaldehyde by near-infrared cavity ring-down spectroscopy. EGU Gen. Assem. Conf. Abstr. 2018, 20, 11101. [Google Scholar]
- Hoffnagle, J.; Fleck, D.; Rella, C.; Kim-Hak, D. Quantification of atmospheric formaldehyde by infrared absorption spectroscopy. EGU Gen. Assem. Conf. Abstr. 2017, 19, 8972. [Google Scholar]
- Liu, J.; Li, X.; Yang, Y.; Wang, H.; Wu, Y.; Lu, X.; Chen, M.; Hu, J.; Fan, X.; Zeng, L.; et al. An IBBCEAS system for atmospheric measurements of glyoxal and methylglyoxal in the presence of high NO2 concentrations. Atmos. Meas. Tech. 2019, 12, 4439–4453. [Google Scholar] [CrossRef]
- Liu, J.; Li, X.; Yang, Y.; Wang, H.; Kuang, C.; Zhu, Y.; Chen, M.; Hu, J.; Zeng, L.; Zhang, Y. Sensitive Detection of Ambient Formaldehyde by Incoherent Broadband Cavity Enhanced Absorption Spectroscopy. Anal. Chem. 2020, 92, 2697–2705. [Google Scholar] [CrossRef] [PubMed]
- Gorrotxategi-Carbajo, P.; Fasci, E.; Ventrillard, I.; Carras, M.; Maisons, G.; Romanini, D. Optical-feedback cavity-enhanced absorption spectroscopy with a quantum-cascade laser yields the lowest formaldehyde detection limit. Appl. Phys. B. 2013, 110, 309–314. [Google Scholar] [CrossRef]
- He, Q.; Zheng, C.; Lou, M.; Ye, W.; Wang, Y.; Tittel, F.K.J.O.E. Dual-feedback mid-infrared cavity-enhanced absorption spectroscopy for H2CO detection using a radio-frequency electrically-modulated interband cascade laser. Optics 2018, 26, 15436–15444. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Feng, S. Sampling by canister, combining with Agilent 5977B GC/MS to analysis 13 aldehyde and keystone volatile organic compounds in Air. Environ. Chem. 2019, 38, 697–700. [Google Scholar]
- Zhou, Z.; Feng, S. Analysis of 104 volatile organic compounds in the atmosphere using canister sampling-atmospheric preconcentration instrument coupled with Agilent 5977B single quadrupole GC-MS system. Environ. Chem. 2018, 37, 1869–1872. [Google Scholar]
- Cui, T.; Li, H.; Tang, S.; Liu, C.; Gao, X.; Li, Z.; Zhang, F.; Yang, G.; Jiang, W.; Liu, Z.; et al. Determination of Aldehydes and Ketones Compounds in Mainstream Cigarette Smoke by Supercritical Fluid Chromatography and Gas Chromatography—Mass Spectrometry. J. Anal. Test. 2018, 37, 766–771. [Google Scholar]
- Chien, Y.-C.; Yin, K.-G.; Chien, Y.-C. Simultaneous determination of airborne carbonyls and aromatic hydrocarbons using mixed sorbent collection and thermal desorption-gas chromatography/mass spectrometric analysis. J. Environ. Monit. 2009, 11, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Jeffries, H.E.; Sexton, K.G. Atmospheric photooxidation of alkylbenzenes—I. Carbonyl product analyses. Atmos. Environ. 1997, 31, 2261–2280. [Google Scholar] [CrossRef]
- Poole, J.J.; Grandy, J.J.; Gómez-Ríos, G.A.; Gionfriddo, E.; Pawliszyn, J. Solid Phase Microextraction On-Fiber Derivatization Using a Stable, Portable, and Reusable Pentafluorophenyl Hydrazine Standard Gas Generating Vial. Anal. Chem. 2016, 88, 6859–6866. [Google Scholar] [CrossRef]
- Aiello, M.; McLaren, R. Measurement of Airborne Carbonyls Using an Automated Sampling and Analysis System. Environ. Sci. Technol. 2009, 43, 8901–8907. [Google Scholar] [CrossRef] [PubMed]
- Huo, Y.; Qiu, X.; Zhan, S. Determination of 13 aldehyde and ketone compounds in air using ultra-high performance liquid chromatography. Environ. Chem. 2013, 32, 1823–1825. [Google Scholar]
- Wang, F.; Wang, T.; Fan, Z.; Wu, W.; Ma, W.; Bai, Z. Determination of Carbonyl Compounds in Ambient Air by Temperature/Solvent Double-Gradient High Performance Liquid Chromatography. Anhui Agric. Bull. 2015, 21, 23–25. [Google Scholar]
- Si, L.; Xing, G.; Wang, C.; Tan, L.; Chen, Y.; Yu, J.; Liu, F.; Yuan, M. Determination of aldehydes and ketones in ambient air using adsorbent cartridge followed by high performance liquid chromatography. Environ. Chem. 2019, 38, 2222–2228. [Google Scholar]
- Fan, Z.; Wang, F.; He, Y.; Ma, W. Simultaneous Determination of Multiple Carbonyl Compounds in Ambient Air. Environ. Monit. Manag. Technol. 2018, 30, 43–45. [Google Scholar]
- Tao, M.; Yin, L.; Fu, X. Determination of 16 Aldehydes and Ketones in Ambient Air by Solution Absorption-High Performance Liquid Chromatography. Sci. Technol. Innov. 2024, 2, 107–110. [Google Scholar]
- Chi, Y.; Feng, Y.; Wen, S.; Lu, H.; Yu, Z.; Zhang, W.; Sheng, G.; Fu, J. Determination of carbonyl compounds in the atmosphere by DNPH derivatization and LC-ESI-MS/MS detection. Talanta 2007, 72, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Kong, Y.; Cao, J.; Li, H.; Gao, R.; Zhang, Y.; Wang, K.; Li, Y.; Ren, Y.; Wang, W. A sensitive simultaneous detection approach for the determination of 30 atmospheric carbonyls by 2,4-dinitrophenylhydrazine derivatization with HPLC-MS technique and its preliminary application. Chemosphere 2022, 303, 134985. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Feng, X.; Chen, Y.; Zheng, P.; Hui, L.; Chen, Y.; Yu, J.Z.; Wang, Z. Development of an enhanced method for atmospheric carbonyls and characterizing their roles in photochemistry in subtropical Hong Kong. Sci. Total Environ. 2023, 896, 165135. [Google Scholar] [CrossRef] [PubMed]
- Fung, K.; Grosjean, D. Determination of nanogram amounts of carbonyls as 2,4-dinitrophenylhydrazones by high-performance liquid chromatography. Anal. Chem. 1981, 53, 168–171. [Google Scholar] [CrossRef]
- MEE. Ambient Air-Determination of Aldehyde and Ketone Compounds-High: HJ683-2014; China Environmental Science Press: Beijing, China, 2020. [Google Scholar]
- Guo, S.; Chen, M.; Tan, J. Seasonal and diurnal characteristics of atmospheric carbonyls in Nanning, China. Atmos. Res. 2016, 169, 46–53. [Google Scholar] [CrossRef]
- Possanzini, M.; DiPalo, V. Determination of olefinic aldehydes and other volatile carbonyls in air samples by DNPH-coated cartridges and HPLC. Chromatographia 1995, 40, 134–138. [Google Scholar] [CrossRef]
- Schulte-Ladbeck, R.; Lindahl, R.; Levin, J.-O.; Karst, U.; Schulte Ladbeck, R. Characterization of chemical interferences in the determination of unsaturated aldehydes using aromatic hydrazine reagents and liquid chromatography. J. Environ. Monit. 2001, 3, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Slemr, J. Determination of volatile carbonyl compounds in clean air. Fresenius J. Anal. Chem. 1991, 340, 672–677. [Google Scholar] [CrossRef]
- Zhang, H.; Hou, H.; Pang, Y.; Jiang, X.; Tang, G. HPLC Determination of Major Carbonyl Compounds in Mainstream ofCigarette Smoke with Solvent Trapping. Phys. Chem. Test. (Chem. Sect.) 2013, 49, 19–22. [Google Scholar]
- Liu, L.; Yuan, B.; Jin, Y. Analysis of 13 carbonyl compounds using ultra-high-performance liquid chromatography with a Carbonyl column. Environ. Chem. 2013, 32, 715–716. [Google Scholar]
- MEE. Determination of Aldehydes and Ketones in Ambient Air by Solution Absorption-High Performance Liquid Chromatography: HJ 1154-2020; China Environmental Science Press: Beijing, China, 2020. [Google Scholar]
- Castellani, F.; Antonucci, A.; Pindinello, I.; Protano, C.; Vitali, M. Determination of Carbonyl Compounds in Different Work Environments: Comparison between LC-UV/DAD and LC–MS/MS Detection Methods. Int. J. Environ. Res. Public Health. 2022, 19, 12052. [Google Scholar] [CrossRef] [PubMed]
- Van den Bergh, V.; Coeckelberghs, H.; Vankerckhoven, H.; Compernolle, F.; Vinckier, C. Study of the carbonyl products of terpene/OH radical reactions: Detection of the 2,4-DNPH derivatives by HPLC-MS. Anal. BioAnal. Chem. 2004, 379, 484–494. [Google Scholar] [CrossRef] [PubMed]
- van Leeuwen, S.M.; Hendriksen, L.; Karst, U. Determination of aldehydes and ketones using derivatization with 2,4-dinitrophenylhydrazine and liquid chromatography–atmospheric pressure photoionization-mass spectrometry. J. Chromatogr. A 2004, 1058, 107–112. [Google Scholar] [CrossRef]
- Li, M.; Biswas, S.; Nantz, M.H.; Higashi, R.M.; Fu, X.-A. Preconcentration and Analysis of Trace Volatile Carbonyl Compounds. Anal. Chem. 2012, 84, 1288–1293. [Google Scholar] [CrossRef] [PubMed]
- MEE. Determination of 65 Volatile Organic Compounds in Ambient Air by Canister Sampling and Gas Chromatography-Mass Spectrometry: HJ 759–2023; China Environmental Science Press: Beijing, China, 2023. [Google Scholar]
- ZOU, T.; FENG, Y.; FU, Z.; MU, C.; ZHAI, J. Determination of mono- and dicarbonyls in the atmosphere using gas chromatography/mass spectrometry after PFBHA derivatization. ASC 2012, 32, 718–2724. [Google Scholar]
- Fuson, N.; Josien, M.-L.; Shelton, E.M. An Infrared Spectroscopic Study of the Carbonyl Stretching Frequency in Some Groups of Ketones and Quinones. J. Am. Chem. Soc. 1954, 76, 2526–2533. [Google Scholar] [CrossRef]
- Wang, F.; Ho, S.S.H.; Man, C.L.; Qu, L.; Wang, Z.; Ning, Z.; Ho, K.F. Characteristics and sources of oxygenated VOCs in Hong Kong: Implications for ozone formation. Sci. Total Environ. 2024, 912, 169156. [Google Scholar] [CrossRef] [PubMed]
- Olariu, R.I.; Klotz, B.; Barnes, I.; Becker, K.H.; Mocanu, R. FT–IR study of the ring-retaining products from the reaction of OH radicals with phenol, o-, m-, and p-cresol. Atmos. Environ. 2002, 36, 3685–3697. [Google Scholar] [CrossRef]
- Delgado-Saborit, J.M.; Alam, M.S.; Godri Pollitt, K.J.; Stark, C.; Harrison, R.M. Analysis of atmospheric concentrations of quinones and polycyclic aromatic hydrocarbons in vapour and particulate phases. Atmos. Environ. 2013, 77, 974–982. [Google Scholar] [CrossRef]
- Chung, M.Y.; Lazaro, R.A.; Lim, D.; Jackson, J.; Lyon, J.; Rendulic, D.; Hasson, A.S. Aerosol-Borne Quinones and Reactive Oxygen Species Generation by Particulate Matter Extracts. Environ. Sci. Technol. 2006, 40, 4880–4886. [Google Scholar] [CrossRef] [PubMed]
- Sousa, E.T.; Cardoso, M.P.; Silva, L.A.; de Andrade, J.B. Direct determination of quinones in fine atmospheric particulate matter by GC–MS. Microchem. J. 2015, 118, 26–31. [Google Scholar] [CrossRef]
- Wnorowski, A.; Charland, J.-P. Profiling quinones in ambient air samples collected from the Athabasca region (Canada). Chemosphere 2017, 189, 55–66. [Google Scholar] [CrossRef]
- Dos Santos, R.R.; Cardeal, Z.d.L.; Menezes, H.C. Phase distribution of polycyclic aromatic hydrocarbons and their oxygenated and nitrated derivatives in the ambient air of a Brazilian urban area. Chemosphere 2020, 250, 126223. [Google Scholar] [CrossRef] [PubMed]
- Jo, J.; Lee, J.-Y.; Jang, K.-S.; Matsuki, A.; Natsagdorj, A.; Ahn, Y.-G. Development of Quantitative Chemical Ionization Using Gas Chromatography/Mass Spectrometry and Gas Chromatography/Tandem Mass Spectrometry for Ambient Nitro- and Oxy-PAHs and Its Applications. Molecules 2023, 28, 775. [Google Scholar] [CrossRef] [PubMed]
- Apel, E.C.; Riemer, D.D.; Hills, A.; Baugh, W.; Orlando, J.; Faloona, I.; Tan, D.; Brune, W.; Lamb, B.; Westberg, H.; et al. Measurement and interpretation of isoprene fluxes and isoprene, methacrolein, and methyl vinyl ketone mixing ratios at the PROPHET site during the 1998 Intensive. J. Geophys. Res. Atmos. 2002, 107, 4034. [Google Scholar] [CrossRef]
- Hamilton, J.F.; Webb, P.J.; Lewis, A.C.; Hopkins, J.R.; Smith, S.; Davy, P. Partially oxidised organic components in urban aerosol using GCXGC-TOF/MS. Atmos. Chem. Phys. 2004, 4, 1279–1290. [Google Scholar] [CrossRef]
- Prazeller, P.; Palmer, P.T.; Boscaini, E.; Jobson, T.; Alexander, M. Proton transfer reaction ion trap mass spectrometer. Rapid Commun. Mass Spectrom. 2003, 17, 1593–1599. [Google Scholar] [CrossRef] [PubMed]
- Warneke, C.; Rosén, S.; Lovejoy, E.R.; de Gouw, J.A.; Fall, R. Two additional advantages of proton-transfer ion trap mass spectrometry. Rapid Commun. Mass Spectrom. 2003, 18, 133–134. [Google Scholar] [CrossRef]
- Graus, M.; Müller, M.; Hansel, A. High resolution PTR-TOF: Quantification and formula confirmation of VOC in real time. J. Am. Soc. Mass Spectrom. 2010, 21, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Guo, Y.; Li, M.; Li, J.; Yang, D.; Hou, K. Development and Application of a Chemical Ionization Focusing Integrated Ionization Source TOFMS for Online Detection of OVOCs in the Atmosphere. Molecules 2023, 28, 6600. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Li, X.; Yang, S.; Yu, X.; Zhou, S.; Yang, Y.; Chen, S.; Dong, H.; Liao, K.; Chen, Q.; et al. Spatiotemporal variation, sources, and secondary transformation potential of volatile organic compounds in Xi’an, China. Atmos. Chem. Phys. 2021, 21, 4939–4958. [Google Scholar] [CrossRef]
- Apel, E.C.; Hills, A.J.; Lueb, R.; Zindel, S.; Eisele, S.; Riemer, D.D. A fast-GC/MS system to measure C2 to C4 carbonyls and methanol aboard aircraft. J. Geophys. Res. 2003, 108, 8794. [Google Scholar] [CrossRef]
- Waterman, D.; Horsfield, B.; Leistner, F.; Hall, K.; Smith, S. Quantification of Polycyclic Aromatic Hydrocarbons in the NIST Standard Reference Material (SRM1649A) Urban Dust Using Thermal Desorption GC/MS. Anal. Chem. 2000, 72, 3563–3567. [Google Scholar] [CrossRef]
- Williams, B.J.; Zhang, Y.; Zuo, X.; Martinez, R.E.; Walker, M.J.; Kreisberg, N.M.; Goldstein, A.H.; Docherty, K.S.; Jimenez, J.L. Organic and inorganic decomposition products from the thermal desorption of atmospheric particles. Atmos. Meas. Tech. 2016, 9, 1569–1586. [Google Scholar] [CrossRef]
- Maher, S.; Jjunju, F.P.M.; Taylor, S. Colloquium: 100 years of mass spectrometry: Perspectives and future trends. Rev. Mod. Phys. 2015, 87, 113–135. [Google Scholar] [CrossRef]
- Buiarelli, F.; Canepari, S.; Di Filippo, P.; Perrino, C.; Pomata, D.; Riccardi, C.; Speziale, R. Extraction and analysis of fungal spore biomarkers in atmospheric bioaerosol by HPLC–MS–MS and GC–MS. Talanta 2013, 105, 142–151. [Google Scholar] [CrossRef]
- Mirsaleh-Kohan, N.; Robertson, W.D.; Compton, R.N. Electron ionization time-of-flight mass spectrometry: Historical review and current applications. Mass Spectrom. Rev. 2008, 27, 237–285. [Google Scholar] [CrossRef]
- Bertram, T.H.; Kimmel, J.R.; Crisp, T.A.; Ryder, O.S.; Yatavelli, R.L.N.; Thornton, J.A.; Cubison, M.J.; Gonin, M.; Worsnop, D.R. A field-deployable, chemical ionization time-of-flight mass spectrometer. Atmos. Meas. Tech. 2011, 4, 1471–1479. [Google Scholar] [CrossRef]
- Adam, T.; Zimmermann, R. Determination of single photon ionization cross sections for quantitative analysis of complex organic mixtures. Anal. BioAnal. Chem. 2007, 389, 1941–1951. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, M.; Fenn, J.B. Electrospray ion source. Another variation on the free-jet theme. J. Phys. Chem. 1984, 88, 4451–4459. [Google Scholar] [CrossRef]
- Awad, H.; Khamis, M.M.; El-Aneed, A. Mass Spectrometry, Review of the Basics: Ionization. Appl. Spectrosc. Rev. 2014, 50, 158–175. [Google Scholar] [CrossRef]
- Zhong, S.; Zhang, K.; Bagheri, M.; Burken, J.G.; Gu, A.; Li, B.; Ma, X.; Marrone, B.L.; Ren, Z.J.; Schrier, J.; et al. Machine Learning: New Ideas and Tools in Environmental Science and Engineering. Environ. Sci. Technol. 2021, 55, 12741–12754. [Google Scholar] [CrossRef]
- Kim, S.; Karl, T.; Guenther, A.; Tyndall, G.; Orlando, J.; Harley, P.; Rasmussen, R.; Apel, E. Emissions and ambient distributions of Biogenic Volatile Organic Compounds (BVOC) in a ponderosa pine ecosystem: Interpretation of PTR-MS mass spectra. Atmos. Chem. Phys. 2010, 10, 1759–1771. [Google Scholar] [CrossRef]
- Steinbacher, M.; Dommen, J.; Ammann, C.; Spirig, C.; Neftel, A.; Prevot, A.S.H. Performance characteristics of a proton-transfer-reaction mass spectrometer (PTR-MS) derived from laboratory and field measurements. Int. J. Mass Spectrom. 2004, 239, 117–128. [Google Scholar] [CrossRef]
- Müller, M.; Mikoviny, T.; Feil, S.; Haidacher, S.; Hanel, G.; Hartungen, E.; Jordan, A.; Märk, L.; Mutschlechner, P.; Schottkowsky, R.; et al. A compact PTR-ToF-MS instrument for airborne measurements of volatile organic compounds at high spatiotemporal resolution. Atmos. Meas. Tech. 2014, 7, 3763–3772. [Google Scholar] [CrossRef]
- Thalman, R.; Baeza-Romero, M.T.; Ball, S.M.; Borrás, E.; Daniels, M.J.S.; Goodall, I.C.A.; Henry, S.B.; Karl, T.; Keutsch, F.N.; Kim, S.; et al. Instrument intercomparison of glyoxal, methyl glyoxal and NO2 under simulated atmospheric conditions. Atmos. Meas. Tech. 2015, 8, 1835–1862. [Google Scholar] [CrossRef]
- Zhou, Z.; Guo, H.; Qi, F. Recent developments in synchrotron vacuum ultraviolet photoionization coupled to mass spectrometry. TrAC Trends Anal. Chem. 2011, 30, 1400–1409. [Google Scholar] [CrossRef]
- Lipson, R.H.; Dimov, S.S.; Wang, P.; Shi, Y.J.; Mao, D.M.; Hu, X.K.; Vanstone, J. VACUUM ULTRAVIOLET AND EXTREME ULTRAVIOLET LASERS: PRINCIPLES, INSTRUMENTATION, AND APPLICATIONS. Instrum. Sci. Technol. 2000, 28, 85–118. [Google Scholar] [CrossRef]
- Yang, B.; Zhang, H.; Shu, J.; Ma, P.; Zhang, P.; Huang, J.; Li, Z.; Xu, C. A VUV-excited and CHCl/HO-amplified ionization-coupled mass spectrometry for oxygenated organics analysis. Anal. Chem. 2017, 90, 1301–1308. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Li, Q.; Hua, L.; Chen, P.; Hu, F.; Wan, N.; Li, H. Highly selective and sensitive online measurement of trace exhaled HCN by acetone-assisted negative photoionization time-of-flight mass spectrometry with in-source CID. Anal. Chim. Acta. 2020, 1111, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Dang, M.; Liu, R.; Dong, F.; Liu, B.; Hou, K. Vacuum ultraviolet photoionization on-line mass spectrometry: Instrumentation developments and applications. TrAC Trends Anal. Chem. 2022, 149, 116542. [Google Scholar] [CrossRef]
- Zogka, A.G.; Romanias, M.N.; Thevenet, F. Formaldehyde and glyoxal measurement deploying a selected ion flow tube mass spectrometer (SIFT-MS). Atmos. Meas. Tech. 2022, 15, 2001–2019. [Google Scholar] [CrossRef]
- Gkatzelis, G.I.; Tillmann, R.; Hohaus, T.; Müller, M.; Eichler, P.; Xu, K.-M.; Schlag, P.; Schmitt, S.H.; Wegener, R.; Kaminski, M.; et al. Comparison of three aerosol chemical characterization techniques utilizing PTR-ToF-MS: A study on freshly formed and aged biogenic SOA. Atmos. Meas. Tech. 2018, 11, 1481–1500. [Google Scholar] [CrossRef]
- Timkovsky, J.; Dusek, U.; Henzing, J.S.; Kuipers, T.L.; Röckmann, T.; Holzinger, R. Offline thermal-desorption proton-transfer-reaction mass spectrometry to study composition of organic aerosol. J. Aerosol Sci. 2015, 79, 1–14. [Google Scholar] [CrossRef]
- Vogel, A.L.; Äijälä, M.; Brüggemann, M.; Ehn, M.; Junninen, H.; Petäjä, T.; Worsnop, D.R.; Kulmala, M.; Williams, J.; Hoffmann, T. Online atmospheric pressure chemical ionization ion trap mass spectrometry (APCI-IT-MSn) for measuring organic acids in concentrated bulk aerosol—A laboratory and field study. Atmos. Meas. Tech. 2013, 6, 431–443. [Google Scholar] [CrossRef]
- Thompson, S.L.; Yatavelli, R.L.N.; Stark, H.; Kimmel, J.R.; Krechmer, J.E.; Day, D.A.; Hu, W.; Isaacman-VanWertz, G.; Yee, L.; Goldstein, A.H.; et al. Field intercomparison of the gas/particle partitioning of oxygenated organics during the Southern Oxidant and Aerosol Study (SOAS) in 2013. Aerosol Sci. Technol. 2016, 51, 30–56. [Google Scholar] [CrossRef]
- Klotz, B.; Sørensen, S.; Barnes, I.; Becker, K.H.; Etzkorn, T.; Volkamer, R.; Platt, U.; Wirtz, K.; Martín-Reviejo, M. Atmospheric oxidation of toluene in a large-volume outdoor photoreactor: In situ determination of ring-retaining product yields. J. Phys. Chem. A 1998, 102, 10289–10299. [Google Scholar] [CrossRef]
- Romano, A.; Hanna, G.B. Identification and quantification of VOCs by proton transfer reaction time of flight mass spectrometry: An experimental workflow for the optimization of specificity, sensitivity, and accuracy. J. Mass Spectrom. 2018, 53, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Holzinger, R.; Williams, J.; Herrmann, F.; Lelieveld, J.; Donahue, N.M.; Röckmann, T. Aerosol analysis using a Thermal-Desorption Proton-Transfer-Reaction Mass Spectrometer (TD-PTR-MS): A new approach to study processing of organic aerosols. Atmos. Chem. Phys. 2010, 10, 2257–2267. [Google Scholar] [CrossRef]
- Gkatzelis, G.I.; Hohaus, T.; Tillmann, R.; Gensch, I.; Müller, M.; Eichler, P.; Xu, K.-M.; Schlag, P.; Schmitt, S.H.; Yu, Z.; et al. Gas-to-particle partitioning of major biogenic oxidation products: A study on freshly formed and aged biogenic SOA. Atmos. Chem. Phys. 2018, 18, 12969–12989. [Google Scholar] [CrossRef]
- Kourtchev, I.; Bejan, I.; Sodeau, J.R.; Wenger, J.C. Gas-phase reaction of (E)-β-farnesene with ozone: Rate coefficient and carbonyl products. Atmos. Environ. 2009, 43, 3182–3190. [Google Scholar] [CrossRef]
- Kourtchev, I.; Bejan, I.; Sodeau, J.R.; Wenger, J.C. Gas phase reaction of OH radicals with (E)-β-farnesene at 296±2 K: Rate coefficient and carbonyl products. Atmos. Environ. 2012, 46, 338–345. [Google Scholar] [CrossRef]
- Spittler, M.; Barnes, I.; Bejan, I.; Brockmann, K.J.; Benter, T.; Wirtz, K. Reactions of NO3 radicals with limonene and α-pinene: Product and SOA formation. Atmos. Environ. 2006, 40, 116–127. [Google Scholar] [CrossRef]
- Gómez Alvarez, E.; Viidanoja, J.; Muñoz, A.; Wirtz, K.; Hjorth, J. Experimental Confirmation of the Dicarbonyl Route in the Photo-oxidation of Toluene and Benzene. Environ. Sci. Technol. 2007, 41, 8362–8369. [Google Scholar] [CrossRef] [PubMed]
- Löher, F.; Borrás, E.; Muñoz, A.; Nölscher, A.C. Characterization of a new Teflon chamber and on-line analysis of isomeric multifunctional photooxidation products. Atmos. Meas. Tech. 2024, 17, 4553–4579. [Google Scholar] [CrossRef]
- Komenda, M.; Schaub, A.; Koppmann, R. Description and characterization of an on-line system for long-term measurements of isoprene, methyl vinyl ketone, and methacrolein in ambient air. J. Chromatogr. A 2003, 995, 185–201. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).