Phytotoxicity Testing of Atmospheric Polycyclic Aromatic Hydrocarbons
Abstract
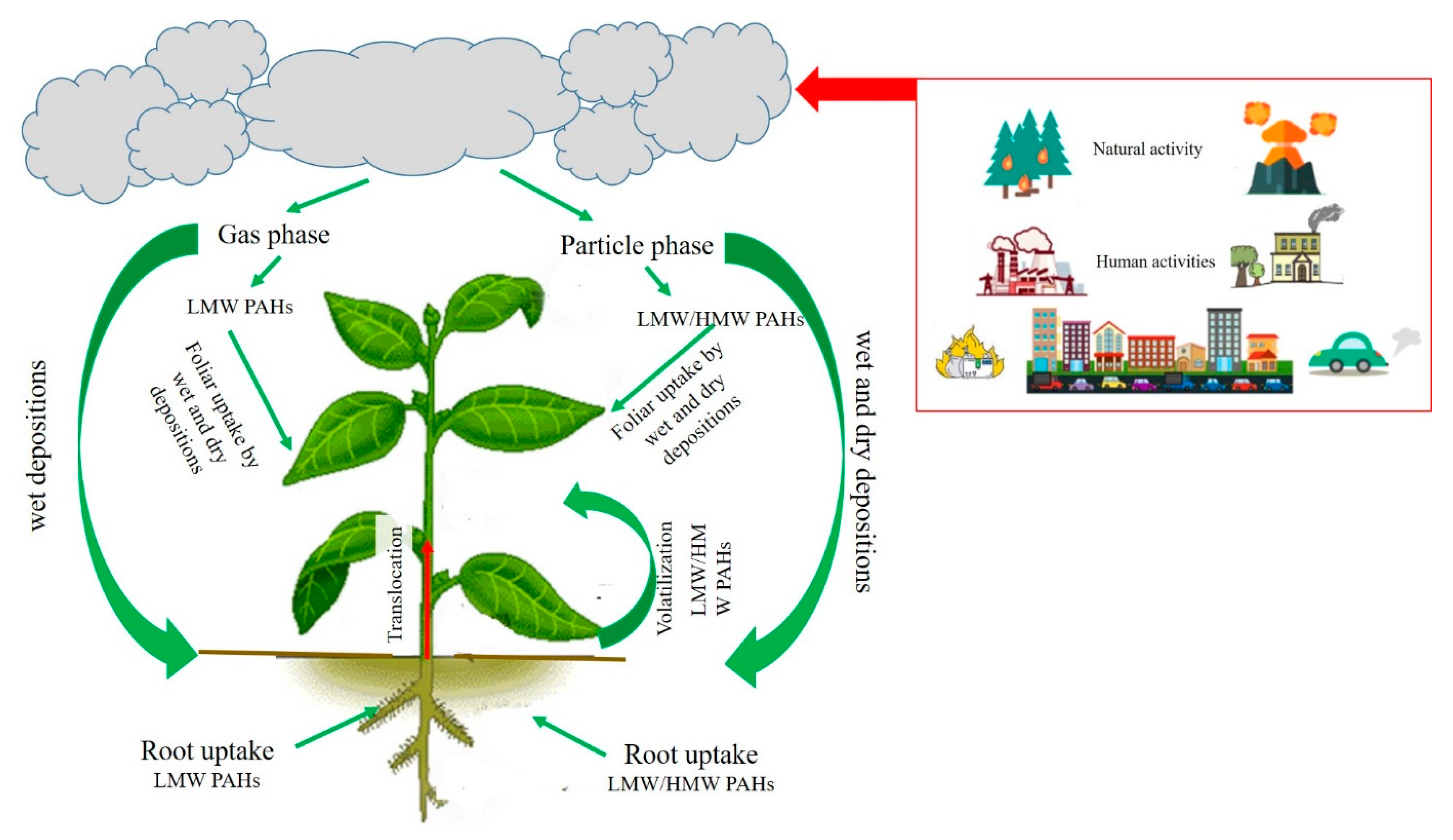
1. Introduction
1.1. PAHs in the Atmosphere
1.2. Exposure Pathways and Uptake Mechanisms
2. Toxic Effects
3. Bioindication vs. Ecotoxicology
4. Reported Tests
4.1. Fumigation Chambers
4.2. Direct Foliar Treatment
4.3. Phytotoxicity Testing of Photomodified PAHs
4.4. Uptake via Roots
4.5. Phytotoxic Effects on Freshwater Species
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Zhang, P.; Zhou, Y.; Chen, Y.; Yu, M.; Xia, Z. Construction of an atmospheric PAH emission inventory and health risk assessment in Jiangsu, China. Air Qual. Atmos. Health 2023, 16, 629–640. [Google Scholar] [CrossRef]
- Liu, J.; Jia, J.; Grathwohl, P. Dilution of concentrations of PAHs from atmospheric particles, bulk deposition to soil: A review. Environ. Geochem. Health 2022, 44, 4219–4234. [Google Scholar] [CrossRef] [PubMed]
- Samek, L.; Furman, L.; Mikrut, M.; Regiel-Futyra, A.; Macyk, W.; Stochel, G.; van Eldik, R. Chemical composition of submicron and fine particulate matter collected in Krakow, Poland. Consequences for the APARIC project. Chemosphere 2017, 187, 430–439. [Google Scholar] [CrossRef]
- Sarti, E.; Pasti, L.; Scaroni, I.; Casali, P.; Cavazzini, A.; Rossi, M. Determination of nalkanes, PAHs and nitro-PAHs in PM2.5 and PM1 sampled in the surroundings of a municipal waste incinerator. Atmos. Environ. 2017, 149, 12–23. [Google Scholar] [CrossRef]
- Wilson, W.E.; Suh, H.H. Fine particles and coarse particles: Concentration relationships relevant to epidemiologic studies. J. Air Waste Manag. Assoc. 1997, 47, 1238–1249. [Google Scholar] [CrossRef]
- Desalme, D.; Binet, P.; Epron, D.; Bernard, N.; Gilbert, D.; Toussaint, M.L.; Plain, C.; Chiapusio, G. Atmospheric phenanthrene pollution modulates carbon allocation in red clover (Trifolium pratense L.). Environ. Pollut. 2011, 159, 2759–2765. [Google Scholar] [CrossRef]
- Huang, S.; Dai, C.; Zhou, Y.; Peng, H.; Yi, K.; Qin, P.; Luo, S.; Zhang, X. Comparisons of three plant species in accumulating polycyclic aromatic hydrocarbons (PAHs) from the atmosphere: A review. Environ. Sci. Pollut. Res. 2018, 25, 16548–16566. [Google Scholar] [CrossRef]
- Lehndorff, E.; Schwark, L. Biomonitoring of air quality in the Cologne Conurbation using pine needles as a passive sampler—Part II: Polycyclic aromatic hydrocarbons (PAH). Atmos. Environ. 2004, 38, 3793–3808. [Google Scholar] [CrossRef]
- Hubai, K.; Kováts, N.; Eck-Varanka, B. Urban Gardening—How Safe Is It? Urban Sci. 2024, 8, 91. [Google Scholar] [CrossRef]
- Xiong, T.-T.; Leveque, T.; Austruy, A.; Goix, S.; Schreck, E.; Dappe, V.; Sobanska, S.; Foucault, Y.; Dumat, C. Foliar uptake and metal(loid) bioaccessibility in vegetables exposed to particulate matter. Environ. Geochem. Health 2014, 36, 897–909. [Google Scholar] [CrossRef]
- Shi, T.; Tian, K.; Bao, H.; Liu, X.; Wu, F. Variation in foliar uptake of polycyclic aromatic hydrocarbons in six varieties of winter wheat. Environ. Sci. Pollut. Res. 2017, 24, 27215–27224. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Liang, B.; Chen, L.; Zhu, Y.; Gao, M.; Chen, J.; Wang, F.; Chen, Y.; Tian, M. Atmospheric wet and dry depositions of polycyclic aromatic compounds in a megacity of Southwest China. Environ. Res. 2022, 204, 112151. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, M.; Li, Y.; Liu, Y.; Li, S.; Ge, R. Dry and wet deposition of polycyclic aromatic hydrocarbons and comparison with typical media in urban system of Shanghai, China. Atmos. Environ. 2016, 144, 175–181. [Google Scholar] [CrossRef]
- Škrdlíková, L.; Landlová, L.; Klánová, J.; Lammel, G. Wet deposition and scavenging efficiency of gaseous and particulate phase polycyclic aromatic compounds at a central European suburban site. Atmos. Environ. 2011, 45, 4305–4312. [Google Scholar] [CrossRef]
- Giráldez, P.; Aboal, J.R.; Fernández, J.Á.; Di Guardo, A.; Terzaghi, E. Plant-air partition coefficients for thirteen urban conifer tree species: Estimating the best gas and particulate matter associated PAH removers. Environ. Pollut. 2022, 315, 120409. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.; Xu, Y.; Rodgers, T.F.; Ablimit, M.; Li, J.; Tan, F. Identifying the contributions of root and foliage gaseous/particle uptakes to indoor plants for phthalates, OPFRs and PAHs. Sci. Total Environ. 2023, 883, 163644. [Google Scholar] [CrossRef]
- Kulhánek, A.; Trapp, S.; Sismilich, M.; Jankl, J.; Zimová, M. Crop-specific human exposure assessment for polycyclic aromatic hydrocarbons in Czech soils. Sci. Total Environ. 2005, 339, 71–80. [Google Scholar] [CrossRef]
- Lin, H.; Tao, S.; Zuo, Q.; Coveney, R.M. Uptake of polycyclic aromatic hydrocarbons by maize plants. Environ. Pollut. 2007, 148, 614–619. [Google Scholar] [CrossRef]
- Jia, J.; Bi, C.; Zhang, J.J.; Chen, Z. Atmospheric deposition and vegetable uptake of polycyclic aromatic hydrocarbons (PAHs) based on experimental and computational simulations. Atmos. Environ. 2019, 204, 135–141. [Google Scholar] [CrossRef]
- Anand, P.; Mina, U.; Khare, M.; Kumar, P.; Kota, S.H. Air pollution and plant health response-current status and future directions. Atmos. Pollut. Res. 2022, 13, 101508. [Google Scholar] [CrossRef]
- Oksanen, E.; Kontunen-Soppela, S. Plants have different strategies to defend against air pollutants. Curr. Opin. Environ. Sci. Health 2021, 19, 100222. [Google Scholar] [CrossRef]
- Ali, I.; Liu, B.; Farooq, M.A.; Islam, F.; Azizullah, A.; Yu, C.; Su, W.; Gan, Y. Toxicological effects of bisphenolA on growth and antioxidant defense system in Oryza sativa as revealed by ultrastructure analysis. Ecotoxicol. Environ. Safe 2016, 124, 277–284. [Google Scholar] [CrossRef] [PubMed]
- OECD. Test No. 227: Terrestrial Plant Test: Vegetative Vigour Test; OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 2006. [Google Scholar] [CrossRef]
- Duffner, A.; Moser, T.; Candolfi, M.P. Feasibility of assessing vegetative and generative endpoints of crop- and non- crop terrestrial plant species for non-target terrestrial plant (NTTP) regulatory testing under greenhouse conditions. PLoS ONE 2020, 15, e0230155. [Google Scholar] [CrossRef]
- Storch-Böhm, R.F.; Somensi, C.A.; Cotelle, S.; Deomar-Simões, M.J.; Poyer-Radetski, L.; Dalpiaz, F.L.; Pimentel-Almeida, W.; Férard, J.-F.; Radetski, C.M. Sensitivity of different parameters for selection of higher plants in urban afforestation: Exposure of Guabiroba (Campomanesia xanthocarpa O. Berg.) to diesel engine exhaust. Environ. Pollut. 2020, 265, 114675. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Chen, J.; Wang, W.; Zhu, L. Mechanism of growth inhibition mediated by disorder of chlorophyll metabolism in rice (Oryza sativa) under the stress of three polycyclic aromatic hydrocarbons. Chemosphere 2023, 329, 138554. [Google Scholar] [CrossRef]
- Jajoo, A.; Mekala, N.R.; Tomar, R.S.; Grieco, M.; Tikkanen, M.; Aro, E.M. Inhibitory effects of polycyclic aromatic hydrocarbons (PAHs) on photosynthetic performance are not related to their aromaticity. J. Photochem. Photobiol. B 2014, 137, 151–155. [Google Scholar] [CrossRef]
- Mukherjee, A.; Agrawal, M. Use of GLM approach to assess the responses of tropical trees to urban air pollution in relation to leaf functional traits and tree characteristics. Ecotoxicol. Environ. Safe 2018, 152, 42–54. [Google Scholar] [CrossRef]
- Middleton, J.T.; Kendrick, J.B., Jr.; Schwalm, H.W. Injury to herbaceous plants by smog or air pollution. Plant Dis. Rep. 1950, 34, 245–252. [Google Scholar]
- Roth, H.P.; Swenson, E.A. Physiological studies of irritant aspects of atmospheric pollution. In Report to Los Angeles County Department of Air Pollution; University of Southern California: Los Angeles, CA, USA, 1947. [Google Scholar]
- Tomar, R.S.; Singh, B.; Jajoo, A. Effects of organic pollutants on photosynthesis. In Photosynthesis, Productivity, and Environmental Stress; Ahmad, P., Ahanger, M.A., Alyemeni, M.N., Alam, P., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2019; pp. 1–26. [Google Scholar]
- Rabe, R.; Kreeb, K.H. Enzyme activities and chlorophyll and protein content in plants as indicators of air pollution. Environ. Pollut. 1979, 19, 119–137. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Extraction of phtosynthetic tissues: Chlorophylls and carotenoids. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.2.1–F4.2.6. [Google Scholar]
- Tomar, R.S.; Jajoo, A. Fluoranthene, a polycyclic aromatic hydrocarbon, inhibits light as well as dark reactions of photosynthesis in wheat (Triticum aestivum). Ecotoxicol. Environ. Safe 2014, 109, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Arikan, B.; Yildiztugay, E.; Ozfidan-Konakci, C. Responses of salicylic acid encapsulation on growth, photosynthetic attributes and ROS scavenging system in Lactuca sativa exposed to polycyclic aromatic hydrocarbon pollution. Plant Physiol. Biochem. 2023, 203, 108026. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Singh, S.N. Biochemical and ultrastructural changes in plant foliage exposed to auto-pollution. Environ. Monit. Assess. 2006, 120, 585–602. [Google Scholar] [CrossRef]
- Husen, A. Morpho-anatomical, physiological, biochemical and molecular responses of plants to air pollution. In Harsh Environment and Plant Resilience: Molecular and Functional Aspects; Springer: Berlin/Heidelberg, Germany, 2021; pp. 203–234. [Google Scholar]
- Wu, D.; Zhang, F.; Lou, W.; Li, D.; Chen, J. Chemical characterization and toxicity assessment of fine particulate matters emitted from the combustion of petrol and diesel fuels. Sci. Total Environ. 2017, 605–606, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Lv, J.; Dong, X.; Du, N.; Piao, F. Gamma-aminobutyric acid improves phenanthrene phytotoxicity tolerance in cucumber through the glutathione-dependent system of antioxidant defense. Ecotoxicol. Environ. Safe 2021, 217, 112254. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Li, Z.; Chen, J.; Dong, Y.; Qu, K.; Guo, T.; Wang, F.; Liu, A.; Chen, S.; Li, X. Reactive oxygen species signaling in melatonin-mediated plant stress response. Plant Physiol. Biochem. 2024, 207, 108398. [Google Scholar] [CrossRef]
- Krzyszczak, A.; Dybowski, M.; Jośko, I.; Kusiak, M.; Sikora, M.; Czech, B. The antioxidant defense responses of Hordeum vulgare L. to polycyclic aromatic hydrocarbons and their derivatives in biochar-amended soil. Environ. Pollut. 2022, 294, 118664. [Google Scholar] [CrossRef]
- Bali, S.; Kaur, P.; Jamwal, V.L.; Gandhi, S.G.; Sharma, A.; Ohri, P.; Bhardwaj, R.; Ali, M.A.; Ahmad, P. Seed priming with jasmonic acid counteracts root knot nematode infection in tomato by modulating the activity and expression of antioxidative enzymes. Biomolecules 2020, 10, 98. [Google Scholar] [CrossRef]
- Mathé, C.; Barre, A.; Jourda, C.; Dunand, C. Evolution and Expression of Class III Peroxidases. Arch. Biochem. Biophys. 2010, 500, 58–65. [Google Scholar] [CrossRef]
- Hatami, M.; Ghorbanpour, M. Metal and metal oxide nanoparticles-induced reactive oxygen species: Phytotoxicity and detoxification mechanisms in plant cell. Plant Physiol. Biochem. 2024, 213, 108847. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Weisman, D.; Ye, Y.B.; Cui, B.; Huang, Y.H.; Colón-Carmona, A.; Wang, Z.H. An oxidative stress response to polycyclic aromatic hydrocarbon exposure is rapid and complex in Arabidopsis thaliana. Plant Sci. 2009, 176, 375–382. [Google Scholar] [CrossRef]
- Fränzle, O. Complex bioindication and environmental stress assessment. Ecol. Indic. 2006, 6, 114–136. [Google Scholar] [CrossRef]
- Falla, J.; Laval-Gilly, P.; Henryon, M.; Morlot, D.; Ferard, J.-F. Biological air quality monitoring: A review. Environ. Monit. Assess. 2000, 64, 627–644. [Google Scholar] [CrossRef]
- Doucette, W.J.; Shunthirasingham, S.; Dettenmaier, E.M.; Zaleski, R.T.; Fantke, P.; Arnot, J.A. A Review of Measured Bioaccumulation Data on Terrestrial Plants for Organic Chemicals: Metrics, Variability, and the Need for Standardized Measurement Protocols. Environ. Toxicol. Chem. 2018, 37, 21–33. [Google Scholar] [CrossRef]
- Van Leeuwen, C.J. Ecotoxicological effects. In Risk Assessment of Chemicals: An Introduction; Springer: Berlin/Heidelberg, Germany, 1995; pp. 175–237. [Google Scholar]
- Dellinger, M.; Carvan, M.J.; Klingler, R.H.; McGraw, J.E.; Ehlinger, T. An exploratory analysis of stream teratogenicity and human health using zebrafish whole-sediment toxicity test. Challenges 2014, 5, 75–97. [Google Scholar] [CrossRef]
- Gartiser, S.; Heisterkamp, I.; Schoknecht, U.; Burkhardt, M.; Ratte, M.; Ilvonen, O.; Brauer, F.; Brückmann, J.; Dabrunz, A.; Egeler, P.; et al. Results from a round robin test for the ecotoxicological evaluation of construction products using two leaching tests and an aquatic test battery. Chemosphere 2017, 175, 138–146. [Google Scholar] [CrossRef]
- Hanson, M.; Wolff, B.; Green, J.; Kivi, M.; Panter, G.; Warne, M.; Ågerstrand, M.; Sumpter, J. How we can make ecotoxicology more valuable to environmental protection. Sci. Total Environ. 2017, 578, 228–235. [Google Scholar] [CrossRef]
- Keith, L.H. The source of U.S. EPA’s sixteen PAH priority pollutants. Polycycl. Aromat. Comp. 2015, 35, 147–160. [Google Scholar] [CrossRef]
- Honour, S.L.; Bell, J.N.B.; Ashenden, T.W.; Cape, J.N.; Power, S.A. Responses of herbaceous plants to urban air pollution: Effects on growth, phenology and leaf surface characteristics. Environ. Pollut. 2009, 157, 1279–1286. [Google Scholar] [CrossRef]
- Bell, J.N.B.; Honour, S.L.; Power, S.A. Effects of vehicle exhaust emissions on urban wild plant species. Environ. Pollut. 2011, 159, 1984–1990. [Google Scholar] [CrossRef] [PubMed]
- Haagen-Smit, A.J.; Darley, E.F.; Zaitlin, M.; Hull, H.; Noble, W. Investigation on Injury to Plants from Air Pollution in the Los Angeles Area. Plant Physiol. 1952, 27, 18–34. [Google Scholar] [CrossRef] [PubMed]
- Wedding, J.B.; Carlson, R.W.; Stukel, J.J.; Bazzaz, F.A. Aerosol deposition on plant leaves. Environ. Sci. Technol. 1975, 9, 151–153. [Google Scholar] [CrossRef]
- Grattan, S.R.; Maas, E.V.; Ogata, G. Foliar uptake and injury from saline aerosol. J. Environ. Qual. 1981, 10, 406–409. [Google Scholar] [CrossRef]
- Shann, J.R.; Adriano, D.C. Design and Assessment of a Chamber to Expose Plants to Simulated Aerosol and Rain. Environ. Pollut. 1988, 54, 63–74. [Google Scholar] [CrossRef]
- Viskari, E.-L.; Surakka, J.; Pasanen, P.; Mirme, A.; Kössi, S.; Ruuskanen, J.; Holopainen, J.K. Responses of spruce seedlings (Picea abies) to exhaust gas under laboratory conditions—I plant–insect interactions. Environ. Pollut. 2000, 107, 89–98. [Google Scholar] [CrossRef]
- Paull, N.J.; Irga, P.J.; Torpy, F.R. Active green wall plant health tolerance to diesel smoke exposure. Environ. Pollut. 2018, 240, 448–456. [Google Scholar] [CrossRef]
- Desalme, D.; Binet, P.; Bernard, N.; Gilbert, D.; Toussaint, M.L.; Chiapusio, G. Atmospheric phenanthrene transfer and effects on two grassland species and their root symbionts: A microcosm study. Environ. Exp. Bot. 2011, 71, 146–151. [Google Scholar] [CrossRef]
- Slaski, J.J.; Archambault, D.J.; Li, X. Physiological tests to measure impacts of gaseous polycyclic aromatic hydrocarbons (PAHs) on cultivated plants. Commun. Soil Sci. Plan. 2002, 33, 3227–3239. [Google Scholar] [CrossRef]
- Kummerová, M.; Váňová, L. Chlorophyll fluorescence as an indicator of fluoranthene phototoxicity. Plant Soil Environ. 2007, 53, 430–436. [Google Scholar] [CrossRef]
- Váňová, L. The Use of in vitro Cultures for Effect Assessment of Persistent Organic Pollutants on Plants. Ph.D. Thesis, Masaryk University, Brno, Czech Republic, 2009; p. 151. [Google Scholar]
- Vánová, L.; Kummerová, M.; Klemš, M.; Zezulka, S. Fluoranthene influences endogenous abscisic acid level and primary photosynthetic processes in pea (Pisum sativum L.) plants in vitro. Plant Growth Regul. 2009, 57, 39–47. [Google Scholar] [CrossRef]
- Kreslavski, V.D.; Brestic, M.; Zharmukhamedov, S.K.; Lyubimov, V.Y.; Lankin, A.V.; Jajoo, A.; Allakhverdiev, S.I. Mechanisms of inhibitory effects of polycyclic aromatic hydrocarbons in photosynthetic primary processes in pea leaves and thylakoid preparations. Plant Biol. 2017, 19, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Che, X.; Zhang, Z.; Li, Y.; Gao, H.; Zhao, S. The mechanisms by which phenanthrene affects the photosynthetic apparatus of cucumber leaves. Chemosphere 2017, 168, 1498–1505. [Google Scholar] [CrossRef]
- Babula, P.; Vodicka, O.; Adam, V.; Kummerova, M.; Havel, L.; Hosek, J.; Provaznik, I.; Skutkova, H.; Beklova, M.; Kizek, R. Effect of fluoranthene on plant cell model: Tobacco BY-2 suspension culture. Environ. Exp. Bot. 2012, 78, 117–126. [Google Scholar] [CrossRef]
- Oguntimehin, I.; Nakatani, N.; Sakugawa, H. Phytotoxicities of fluoranthene and phenanthrene deposited on needle surfaces of the evergreen conifer, Japanese red pine (Pinus densiflora Sieb. et Zucc.). Environ. Pollut. 2008, 154, 64–271. [Google Scholar] [CrossRef] [PubMed]
- Oguntimehin, I.; Sakugawa, H. Fluoranthene fumigation and exogenous scavenging of reactive oxygen intermediates (ROI) in evergreen Japanese red pine seedlings (Pinus densiflora Sieb. et. Zucc.). Chemosphere 2008, 72, 747–754. [Google Scholar] [CrossRef]
- Oguntimehin, I.; Eissa, F.; Sakugawa, H. Negative effects of fluoranthene on the ecophysiology of tomato plants (Lycopersicon esculentum Mill) Fluoranthene mists negatively affected tomato plants. Chemosphere 2010, 78, 877–884. [Google Scholar] [CrossRef]
- Oguntimehin, I.; Kondo, H.; Sakugawa, H. The use of Sunpatiens (Impatiens spp.) as a bioindicator of some simulated air pollutants–Using an ornamental plant as bioindicator. Chemosphere 2010, 81, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Oguntimehin, I.; Bandai, S.; Sakugawa, H. Mannitol can mitigate negative effects of simulated acid mist and fluoranthene in juvenile Japanese red pine (P. densiflora Sieb. et Zucc.). Environ. Pollut. 2013, 174, 78–84. [Google Scholar] [CrossRef]
- Khpalwak, W.; Abdel-Dayem, S.M.; Sakugawa, H. Individual and combined effects of fluoranthene, phenanthrene, mannitol and sulfuric acid on marigold (Calendula officinalis). Ecotoxicol. Environ. Safe 2018, 148, 834–841. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Wang, M.M.; Zhou, Y.H.; Xia, X.J.; Mao, W.H.; Shi, K.; Yu, J.Q. The growth, photosynthesis and antioxidant defense responses of five vegetable crops to phenanthrene stress. Ecotoxicol. Environ. Safe 2012, 80, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Ahammed, G.J.; Li, X.; Xia, X.-J.; Shi, K.; Zhou, Y.-H.; Yu, J.-Q. Enhanced photosynthetic capacity and antioxidant potential mediate brassinosteriod-induced phenanthrene stress tolerance in tomato. Environ. Pollut. 2015, 201, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.F.; Fang, G.C.; Chen, J.C.; Wu, Y.S. Atmospheric polycyclic aromatic hydrocarbons (PAHs) in Asia: A review from 1999 to 2004. Environ. Pollut. 2006, 142, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Park, S.S.; Kim, Y.J.; Kang, C.H. Atmospheric polycyclic aromatic hydrocarbons in Seoul, Korea. Atmos. Environ. 2002, 36, 2917–2924. [Google Scholar] [CrossRef]
- Huang, X.D.; Zeiler, L.F.; Dixon, D.G.; Greenberg, B.M. Photoinduced Toxicity of PAHs to the Foliar Regions of Brassica napus (Canola) and Cucumbis sativus (Cucumber) in Simulated Solar Radiation. Ecotoxicol. Environ. Safe 1996, 35, 190–197. [Google Scholar] [CrossRef]
- Kováts, N.; Horváth, E.; Eck-Varanka, B.; Csajbók, E.; Hoffer, A. Adapting the Vegetative Vigour Terrestrial Plant Test for assessing ecotoxicity of aerosol samples. Environ. Sci. Pollut. Res. 2017, 24, 15291–15298. [Google Scholar] [CrossRef]
- Boutin, C.; Aya, K.L.; Carpenter, D.; Thomas, P.J.; Rowland, O. Phytotoxicity testing for herbicide regulation: Shortcomings in relation to biodiversity and ecosystem services in agrarian systems. Sci. Total Environ. 2012, 415, 79–92. [Google Scholar] [CrossRef]
- Carpenter, C.; Boutin, C.; Allison, J.E. Effects of chlorimuron ethyl on terrestrial and wetland plants: Levels of, and time to recovery following sublethal exposure. Environ. Pollut. 2013, 172, 275–282. [Google Scholar] [CrossRef]
- Kováts, N.; Hubai, K.; Diósi, D.; Sainnokhoi, T.A.; Hoffer, A.; Tóth, Á.; Teke, G. Sensitivity of typical European roadside plants to atmospheric particulate matter. Ecol. Indic. 2021, 124, 107428. [Google Scholar] [CrossRef]
- Hubai, K.; Székely, O.; Teke, G.; Kováts, N. Is essential oil production influenced by air pollution in Ocimum basilicum L.? Biochem. Syst. Ecol. 2021, 96, 104248. [Google Scholar] [CrossRef]
- Kummerová, M.; Váňová, L.; Krulová, J.; Zezulka, S. The use of physiological characteristics for comparison of organic compounds phytotoxicity. Chemosphere 2008, 71, 2050–2059. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Q.; Lin, H.; Zhang, X.L.; Li, Q.L.; Liu, S.Z.; Tao, S. A two-compartment exposure device for foliar uptake study. Environ. Pollut. 2006, 143, 126–128. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, J.K.; Wieczorek, Z.J. Phytotoxicity and accumulation of anthracene applied to the foliage and sandy substrate in lettuce and radish plants. Ecotoxicol. Environ. Safe 2007, 66, 369–377. [Google Scholar] [CrossRef]
- Wieczorek, J.; Sienkiewicz, S.; Pietrzak, M.; Wieczorek, Z. Uptake and phytotoxicity of anthracene and benzo[k]fluoranthene applied to the leaves of celery plants (Apium graveolens var. secalinum L.). Ecotoxicol. Environ. Safe 2015, 115, 19–25. [Google Scholar]
- Daresta, B.E.; Italiano, F.; de Gennaro, G.; Trotta, M.; Tutino, M.; Veronico, P. Atmospheric particulate matter (PM) effect on the growth of Solanum lycopersicum cv. Roma plants. Chemosphere 2015, 119, 37–42. [Google Scholar] [CrossRef]
- Mondal, N.K.; Panja, D.; Das, C.; Dey, U.; Das, K. Impacts of vehicle exhaust black and soot on germination of gram seed (Cicer arietinum L.). Commun. Plant Sci. 2014, 4, 1–9. [Google Scholar]
- Sharma, B.M.; Melymuk, L.; Bharat, G.K.; Přibylová, P.; Sáňka, O.; Klánová, J.; Nizzetto, L. Spatial gradients of polycyclic aromatic hydrocarbons (PAHs) in air, atmospheric deposition, and surface water of the Ganges River basin. Sci. Total Environ. 2018, 627, 1495–1504. [Google Scholar] [CrossRef]
- Wang, W. Literature review on duckweed toxicity testing. Environ. Res. 1990, 52, 7–22. [Google Scholar] [CrossRef]
- ISO 20079; Water Quality—Determination of the Toxic Effect of Water Constituents and Waste Water on Duckweed (Lemna minor)—Duckweed Growth Inhibition Test. International Organization for Standardization: Geneva, Switzerland, 2005.
- OECD. Test No. 221: Lemna sp. Growth Inhibition Test; OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 2006. [Google Scholar] [CrossRef]
- G3. E-1415-91: 1–10; ASTM–American Society for Testing and Materials. Standard Guide for Conducting Toxicity Tests with Lemna gibba. ASTM: West Conshohocken, PA, USA, 1991.
- SS 02 82 13; SIS—Swedish Standards Institute. Water Quality–Determination of Growth Inhibition (7d) of Lemna minor Duckweed. SIS: Stockholm, Sweden, 1995; 15p.
- USEPA—United States Environmental Protection Agency. Aquatic plant toxicity test using Lemna spp. In Tiers I and II. Ecological Effects Test Guidelines OPPTS 850.4400; USEPA: Washington, DC, USA, 1996; pp. 96–156. [Google Scholar]
- Eck-Varanka, B.; Kováts, N.; Hubai, K.; Sainnokhoi, T.A. Assessing the effect of glyphosate toxicity on Lemna minor in different temperature regimes. Pollutants 2023, 3, 451–460. [Google Scholar] [CrossRef]
- Scherr, C.; Simon, M.; Spranger, J.; Baumgartner, S. Test system stability and natural variability of a Lemna gibba L. bioassay. PLoS ONE 2008, 3, e3133. [Google Scholar] [CrossRef]
- Huang, X.-D.; Dixon, D.-G.; Greenberg, B.M. Impacts of UV radiation and photomodification ont he toxicity of PAHs to the higher plant Lemna gibba (Duckweed). Environ. Toxicol. Chem. 1993, 12, 1067–1077. [Google Scholar]
- Ren, L.; Huang, X.-D.; McConkey, B.J.; Dixon, D.G.; Greenberg, B.M. Photoinduced toxicity of three polycyclic aromatc hydrocarbons (Fluoranthene, Pyrene and Naphtalanene) to the duckweed Lemna gibba L. G-3. Ecotoxicol. Environ. Safe 1994, 28, 160–171. [Google Scholar]
- Huang, X.D.; Dixon, D.G.; Greenberg, B.M. Increased Polycyclic Aromatic Hydrocarbon Toxicity Following Their Photomodification in Natural Sunlight: Impacts on the Duckweed Lemna gibba L. G-3. Ecotoxicol. Environ. Safe 1995, 32, 194–200. [Google Scholar] [CrossRef] [PubMed]
| Basic Information |
|---|
| 1. Test compound |
| Name |
| Supplier |
| Purity |
| In case of complex samples, source, and collection method |
| 2. Experimental design and conditions |
| Description of treatment method |
| Exposure levels/concentrations tested |
| Number of replicates |
| Number and type of controls |
| General test conditions |
| Duration of exposure |
| 3. Test organism |
| Taxonomic name (including varieties if applicable) |
| Source |
| Cultivation, conditioning |
| Age |
| Control performance criteria |
| 4. End-points |
| End-points clearly defined and quantified |
| End-points statistically evaluated |
| Component | Test Organism | Treatment Method | Reference |
|---|---|---|---|
| FLT | pea (Pisum sativum L.) | Immersion | Kummerová and Vánová 2007 [65] Váňová 2009 [66] Vánová et al., 2009 [67] |
| NAP, PHE, FLT | pea (Pisum sativum L.) | Immersion | Kreslavski et al., 2017 [68] |
| PHE | cucumber (Cucumis sativus L.) | Immersion | Jin et al., 2017 [69] |
| FLT | tobacco BY-2 cell culture | Suspension culture | Babula et al., 2012 [70] |
| FLU, PHE | Japanese red pine (Pinus densiflora Sieb. et Zucc.) | Spraying | Oguntimehin et al., 2008 [71] |
| FLU | Japanese red pine (Pinus densiflora Sieb. et Zucc.) | Spraying | Oguntimehin and Sakugawa 2008 [72] |
| FLT | cherry tomato (Lycopersicon esculentum Mill) | Spraying | Oguntimehin et al., 2010a [73] |
| FLT | sunpatiens (Impatiens spp.) | Spraying | Oguntimehin et al., 2010b [74] |
| FLT | Japanese red pine (Pinus densiflora Sieb. et Zucc.) | Spraying | Oguntimehin et al., 2013 [75] |
| FLT, PHE | pot marigold (Calendula officinalis L.) | Spraying | Khpalwak et al., 2018 [76] |
| PHE | pakchoi (Brassica rapa cv. chinensis), cucumber (Cucumis sativus cv. Jinyan4), flowering Chinese cabbage (Brassica campestris L. ssp. chinensis var. purpurea Bailey), tomato (Solanum lycopersicum L. cv. Hezuo903) lettuce (Lactuca sativa L.). | Spraying | Ahammed et al., 2012 [77] |
| PHE | tomato (Solanum lycopersicum L. cv. Hezuo903) | Spraying | Ahammed et al., 2015 [78] |
| ANT, B(a)P, B(a)A, FLU, PHE, PYR | canola (Brassica napus L.), cucumber (Cucumis sativus L.) | Spraying | Huang et al., 1996 [81] |
| Urban PM extract | cucumber (Cucumis sativus L.) | Spraying | Kováts et al., 2017 [82] |
| Diesel emission extract | wild parsnip (Pastinaca sativa L.) common daisy (Bellis perennis L.) common thistle (Cirsium arvense (L.) Scop.) common sowthistle (Sonchus oleraceus L.) common dandelion (Taraxacum officinale F.H. Wigg.) soapwort (Saponaria officinalis L.) goosefoot (Chenopodium album L.) white clover (Trifolium repens L.) Mediterranean sage (Salvia aethiopis L.) hoary plantain (Plantago media L.) common sorrel (Rumex acetosa L.) meadow buttercup (Ranunculus acris L.) wood avens (Geum urbanum L.) | Spraying | Kováts et al., 2021 [85] |
| Diesel emission extract | sweet basil (Ocimum basilicum L.) | Spraying | Hubai et al., 2021 [86] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tumurbaatar, S.; Kováts, N.; Hubai, K. Phytotoxicity Testing of Atmospheric Polycyclic Aromatic Hydrocarbons. Atmosphere 2024, 15, 1143. https://doi.org/10.3390/atmos15091143
Tumurbaatar S, Kováts N, Hubai K. Phytotoxicity Testing of Atmospheric Polycyclic Aromatic Hydrocarbons. Atmosphere. 2024; 15(9):1143. https://doi.org/10.3390/atmos15091143
Chicago/Turabian StyleTumurbaatar, Selenge, Nora Kováts, and Katalin Hubai. 2024. "Phytotoxicity Testing of Atmospheric Polycyclic Aromatic Hydrocarbons" Atmosphere 15, no. 9: 1143. https://doi.org/10.3390/atmos15091143
APA StyleTumurbaatar, S., Kováts, N., & Hubai, K. (2024). Phytotoxicity Testing of Atmospheric Polycyclic Aromatic Hydrocarbons. Atmosphere, 15(9), 1143. https://doi.org/10.3390/atmos15091143






